|
1
|
Zhang Z, Yue P, Lu T, Wang Y, Wei Y and
Wei X: Role of lysosomes in physiological activities, diseases, and
therapy. J Hematol Oncol. 14:792021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Savini M, Zhao Q and Wang MC: Lysosomes:
Signaling hubs for metabolic sensing and longevity. Trends Cell
Biol. 29:876–887. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hraběta J, Belhajová M, Šubrtová H, Merlos
Rodrigo MA, Heger Z and Eckschlager T: Drug sequestration in
lysosomes as one of the mechanisms of chemoresistance of cancer
cells and the possibilities of its inhibition. Int J Mol Sci.
21:43922020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamagishi T, Sahni S, Sharp DM, Arvind A,
Jansson PJ and Richardson DR: P-glycoprotein mediates drug
resistance via a novel mechanism involving lysosomal sequestration.
J Biol Chem. 288:31761–31771. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Seebacher N, Lane DJ, Richardson DR and
Jansson PJ: Turning the gun on cancer: Utilizing lysosomal
P-glycoprotein as a new strategy to overcome multi-drug resistance.
Free Radic Biol Med. 96:432–445. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhitomirsky B and Assaraf YG: Lysosomes as
mediators of drug resistance in cancer. Drug Resist Updat.
24:23–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu H and Ren D: Lysosomal physiology. Annu
Rev Physiol. 77:57–80. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kendall RL and Holian A: The role of
lysosomal ion channels in lysosome dysfunction. Inhal Toxicol.
33:41–54. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu M and Dong XP: Endolysosomal TRPMLs in
cancer. Biomolecules. 11:652021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bargal R, Avidan N, Ben-Asher E, Olender
Z, Zeigler M, Frumkin A, Raas-Rothschild A, Glusman G, Lancet D and
Bach G: Identification of the gene causing mucolipidosis type IV.
Nat Genet. 26:118–123. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Santoni G, Maggi F, Amantini C, Marinelli
O, Nabissi M and Morelli MB: Pathophysiological role of transient
receptor potential mucolipin channel 1 in calcium-mediated
stress-induced neurodegenerative diseases. Front Physiol.
11:2512020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Soyombo AA, Tjon-Kon-Sang S, Rbaibi Y,
Bashllari E, Bisceglia J, Muallem S and Kiselyov K: TRP-ML1
regulates lysosomal pH and acidic lysosomal lipid hydrolytic
activity. J Biol Chem. 281:7294–7301. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Di Paola S, Scotto-Rosato A and Medina DL:
TRPML1: The Ca(2+)retaker of the lysosome. Cell Calcium.
69:112–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Qi J, Li Q, Xin T, Lu Q, Lin J, Zhang Y,
Luo H, Zhang F, Xing Y, Wang W, et al: MCOLN1/TRPML1 in the
lysosome: A promising target for autophagy modulation in diverse
diseases. Autophagy. 20:1712–1722. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medina DL, Di Paola S, Peluso I, Armani A,
De Stefani D, Venditti R, Montefusco S, Scotto-Rosato A, Prezioso
C, Forrester A, et al: Lysosomal calcium signalling regulates
autophagy through calcineurin and TFEB. Nat Cell Biol. 17:288–299.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim SW, Kim DH, Park KS, Kim MK, Park YM,
Muallem S, So I and Kim HJ: Palmitoylation controls trafficking of
the intracellular Ca2+ channel MCOLN3/TRPML3 to regulate
autophagy. Autophagy. 15:327–340. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SW, Kim MK, Hong S, Choi A, Choi JH,
Muallem S, So I, Yang D and Kim HJ: The intracellular
Ca2+ channel TRPML3 is a PI3P effector that regulates
autophagosome biogenesis. Proc Natl Acad Sci USA.
119:e22000851192022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paroutis P, Touret N and Grinstein S: The
pH of the secretory pathway: Measurement, determinants, and
regulation. Physiology (Bethesda). 19:207–215. 2004.PubMed/NCBI
|
|
19
|
Miao Y, Li G, Zhang X, Xu H and Abraham
SN: A TRP channel senses lysosome neutralization by pathogens to
trigger their expulsion. Cell. 161:1306–1319. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gerasimenko JV, Tepikin AV, Petersen OH
and Gerasimenko OV: Calcium uptake via endocytosis with rapid
release from acidifying endosomes. Curr Biol. 8:1335–1338. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martina JA, Lelouvier B and Puertollano R:
The calcium channel mucolipin-3 is a novel regulator of trafficking
along the endosomal pathway. Traffic. 10:1143–1156. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liang J, Bi G, Sui Q, Zhao G, Zhang H,
Bian Y, Chen Z, Huang Y, Xi J, Shi Y, et al: Transcription factor
ZNF263 enhances EGFR-targeted therapeutic response and reduces
residual disease in lung adenocarcinoma. Cell Rep. 43:1137712024.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patro R, Duggal G, Love MI, Irizarry RA
and Kingsford C: Salmon provides fast and bias-aware quantification
of transcript expression. Nat Methods. 14:417–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Robinson MD, McCarthy DJ and Smyth GK:
edgeR: A Bioconductor package for differential expression analysis
of digital gene expression data. Bioinformatics. 26:139–140. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chu VT, Gottardo R, Raftery AE, Bumgarner
RE and Yeung KY: MeV+R: Using MeV as a graphical user interface for
Bioconductor applications in microarray analysis. Genome Biol.
9:R1182008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kurien BT and Scofield RH: Western
blotting: An introduction. Methods Mol Biol. 1312:17–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ramalingam SS, Vansteenkiste J, Planchard
D, Cho BC, Gray JE, Ohe Y, Zhou C, Reungwetwattana T, Cheng Y,
Chewaskulyong B, et al: Overall survival with osimertinib in
untreated, EGFR-mutated advanced NSCLC. N Engl J Med. 382:41–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ohashi K, Maruvka YE, Michor F and Pao W:
Epidermal growth factor receptor tyrosine kinase
inhibitor-resistant disease. J Clin Oncol. 31:1070–1080. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Halaby R: Influence of lysosomal
sequestration on multidrug resistance in cancer cells. Cancer Drug
Resist. 2:31–42. 2019.PubMed/NCBI
|
|
33
|
Zhitomirsky B and Assaraf YG: Lysosomal
sequestration of hydrophobic weak base chemotherapeutics triggers
lysosomal biogenesis and lysosome-dependent cancer multidrug
resistance. Oncotarget. 6:1143–1156. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu M, Li X, Zhang T, Liu Z and Zhao Y:
Identification of a nine-gene signature and establishment of a
prognostic nomogram predicting overall survival of pancreatic
cancer. Front Oncol. 9:9962019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim HJ, Li Q, Tjon-Kon-Sang S, So I,
Kiselyov K and Muallem S: Gain-of-function mutation in TRPML3
causes the mouse Varitint-Waddler phenotype. J Biol Chem.
282:36138–36142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim HJ, Soyombo AA, Tjon-Kon-Sang S, So I
and Muallem S: The Ca(2+) channel TRPML3 regulates membrane
trafficking and autophagy. Traffic. 10:1157–1167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim MS, Yang SH and Kim MS: TRPML3
enhances drug resistance in non-small cell lung cancer cells by
promoting Ca2+-mediated lysosomal trafficking. Biochem
Biophys Res Commun. 627:152–159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Christensen KA, Myers JT and Swanson JA:
pH-dependent regulation of lysosomal calcium in macrophages. J Cell
Sci. 115:599–607. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morgan AJ, Platt FM, Lloyd-Evans E and
Galione A: Molecular mechanisms of endolysosomal Ca2+ signalling in
health and disease. Biochem J. 439:349–374. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shen D, Wang X, Li X, Zhang X, Yao Z,
Dibble S, Dong XP, Yu T, Lieberman AP, Showalter HD and Xu H: Lipid
storage disorders block lysosomal trafficking by inhibiting a TRP
channel and lysosomal calcium release. Nat Commun. 3:7312012.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Araujo MEG, Liebscher G, Hess MW and
Huber LA: Lysosomal size matters. Traffic. 21:60–75. 2020.
View Article : Google Scholar : PubMed/NCBI
|















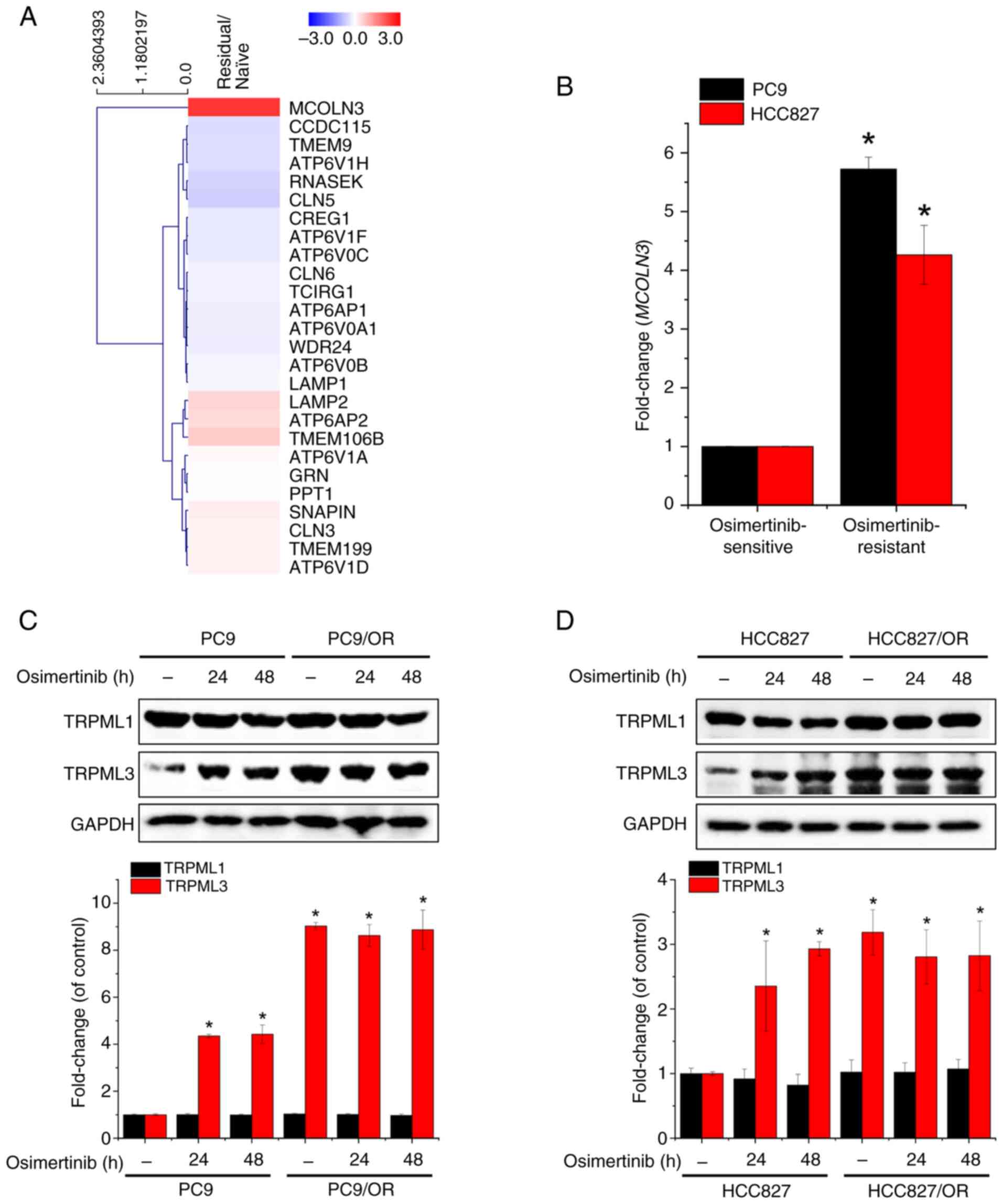
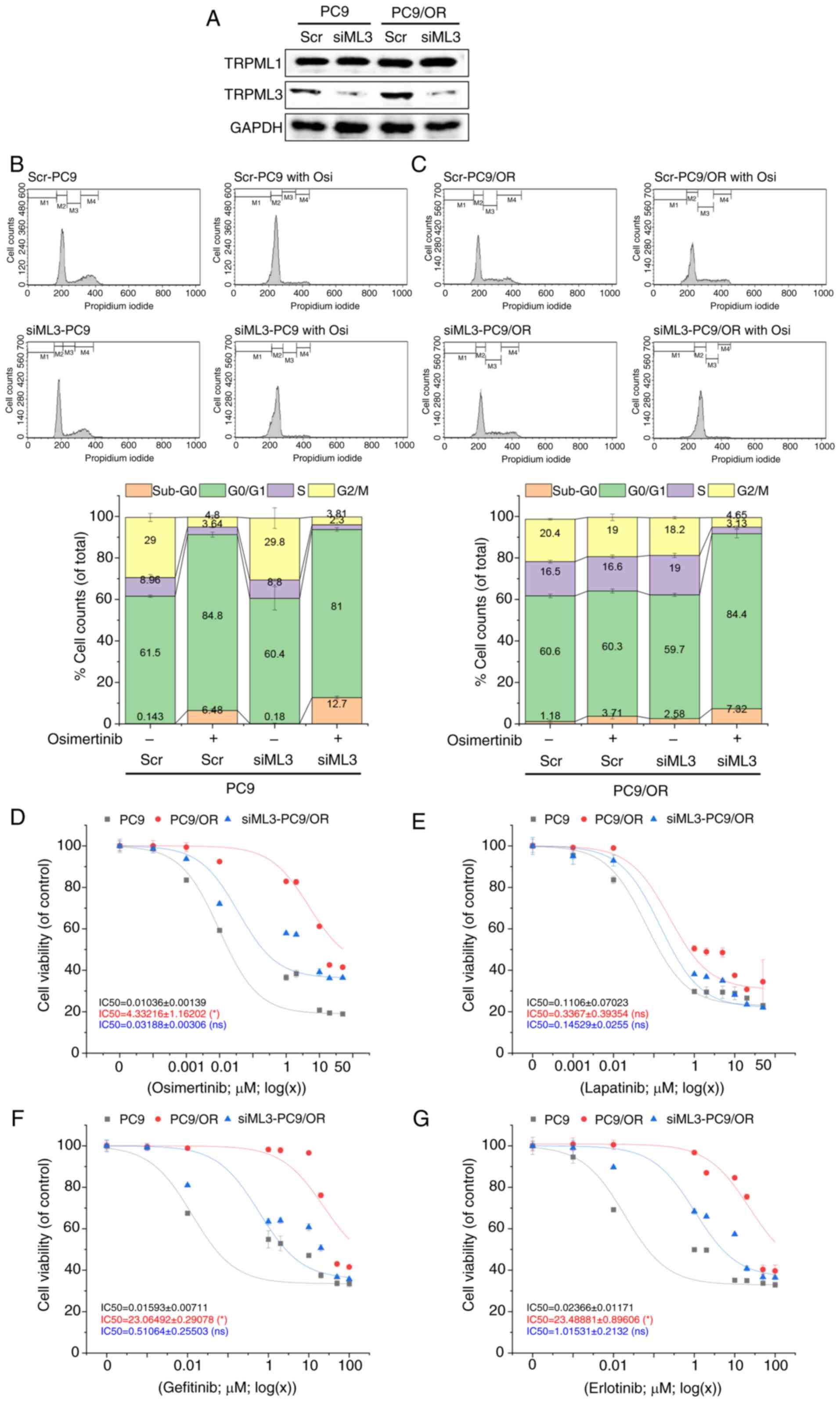
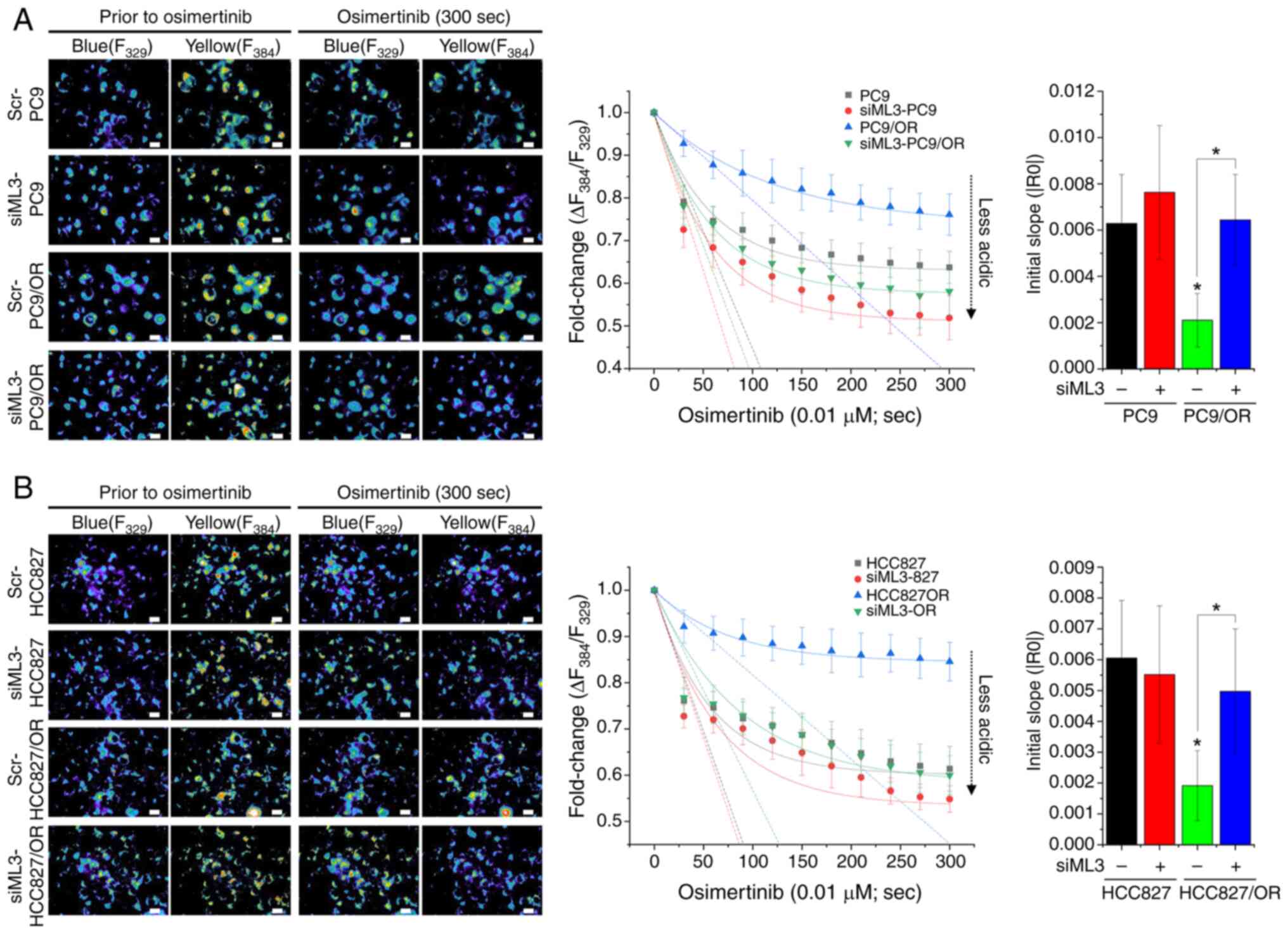
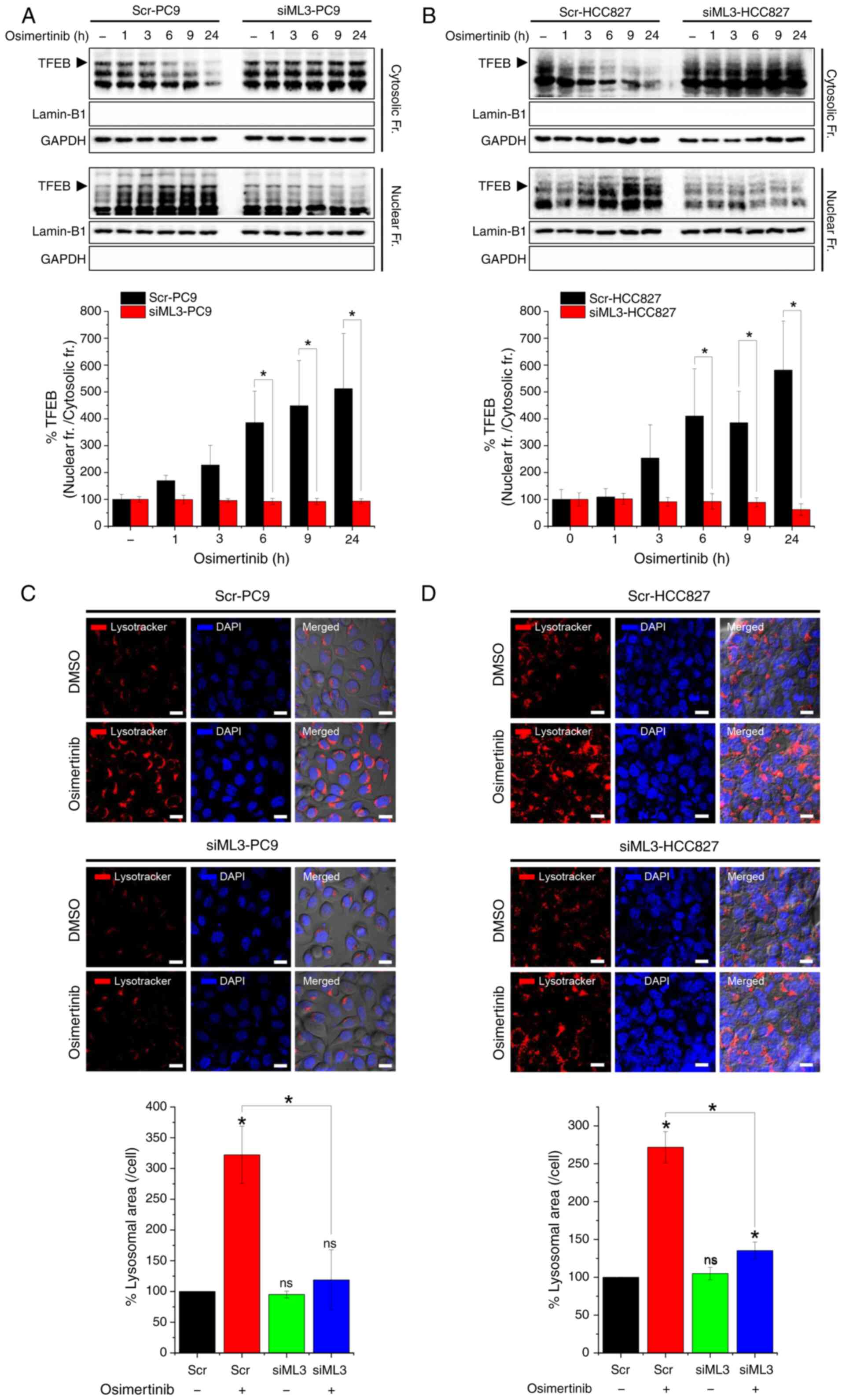
![Osimertinib-induced
Ca2+i oscillations depend on lysosomal
Ca2+ release mediated by TRPML3.
[Ca2+]i measured using Fura-2/AM in cells
treated with 0.01 µM osimertinib in the absence of extracellular
Ca2+. (A and B) Osimertinib-induced
Ca2+i mobilization in (A) PC9 and (B) PC9/OR
cells. (C) Effects of ML-SI1, a TRPML channel blocker, on
osimertinib-induced Ca2+i oscillations. (D-F)
Ca2+i oscillations in PC9 cells transfected
with scrambled RNA (D; scr), TRPML1-targeting siRNA (E; siML1), or
TRPML3-targeting siRNA (F; siML3), following osimertinib treatment.
Each line represents Ca2+i mobilization in a
single cell. (G) Statistical analysis of Ca2+ spike
frequencies (*P<0.05, n=6). (H and I) Lysosomal Ca2+
release in (H) PC9 and (I) HCC827 cells expressing TRPML3-GCaMP6
and treated with 0.01 µM osimertinib in the absence of
extracellular Ca2+. ML-SA1 was used as a positive
control. Lines indicate changes in GFP intensity (F488)
in individual cells, reflecting TRPML3-mediated lysosomal
Ca2+ release. F340/380 and F488
values were normalized to the mean and are presented as
F340/380-norm and F488-norm, respectively.
TRPML, transient receptor potential mucolipin; si-, small
interfering; scr, scrambled.](/article_images/or/54/3/or-54-03-08946-g03.jpg)