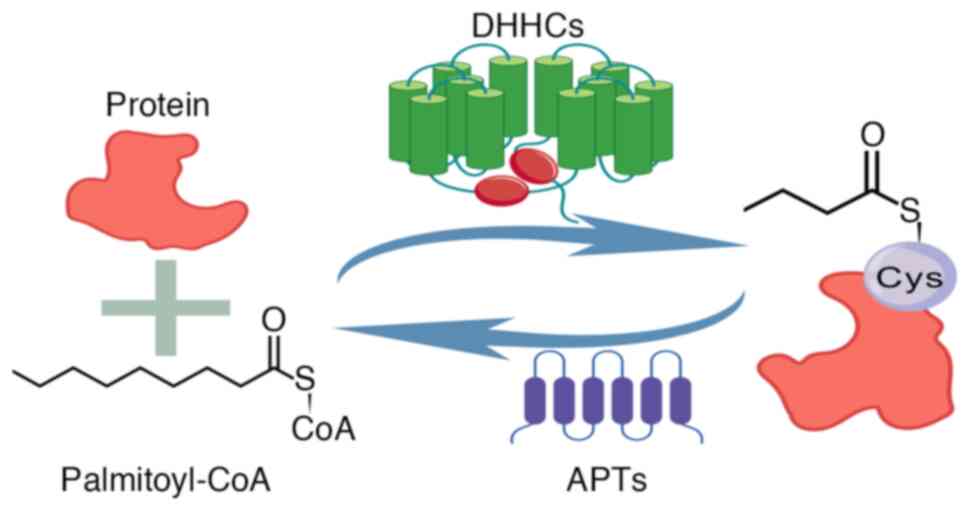
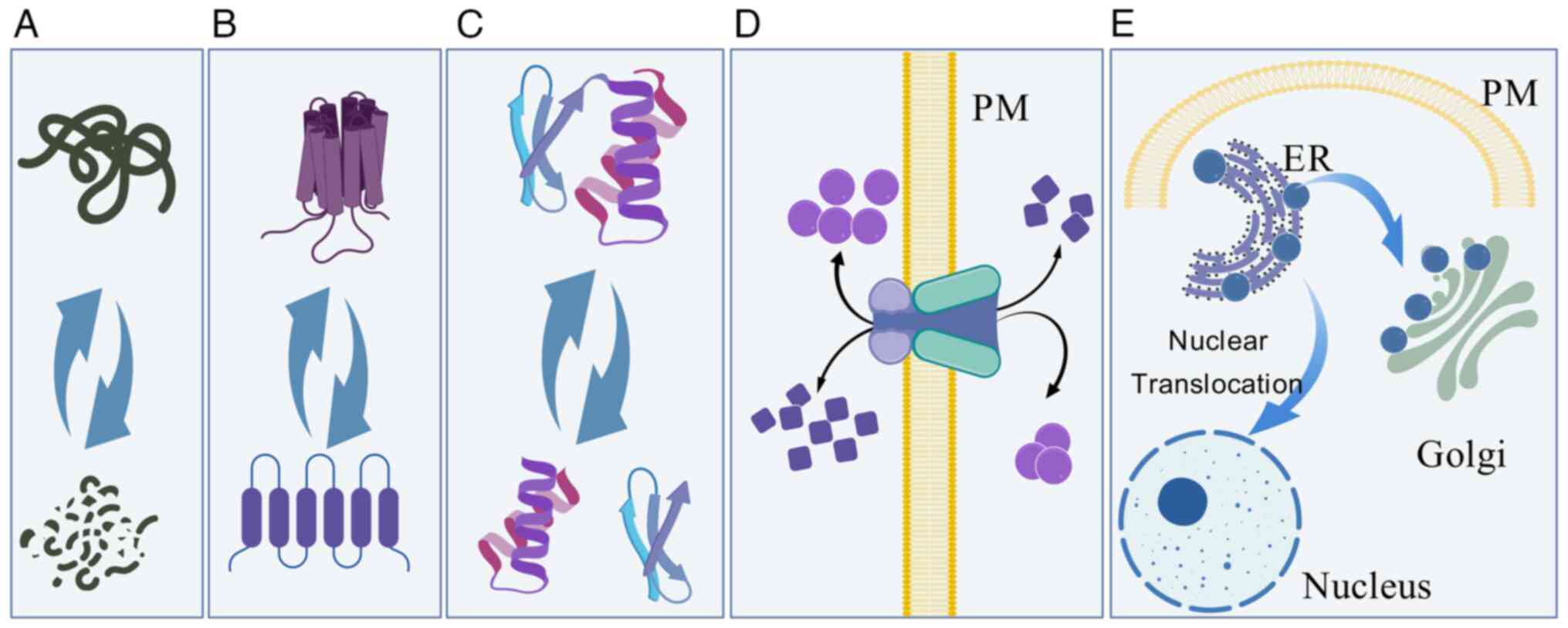
|
1
|
Kiri S and Ryba T: Cancer, metastasis, and
the epigenome. Mol Cancer. 23:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu YT, Che Y, Qiu HL, Xia HX, Feng YZ,
Deng JY, Yuan Y and Tang QZ: ADP-ribosylation: An emerging
direction for disease treatment. Ageing Res Rev. 94:1021762024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He Y, Song T, Ning J, Wang Z, Yin Z, Jiang
P, Yuan Q, Yu W and Cheng F: Lactylation in cancer: Mechanisms in
tumour biology and therapeutic potentials. Clin Transl Med.
14:e700702024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Millán-Zambrano G, Burton A, Bannister AJ
and Schneider R: Histone post-translational modifications-cause and
consequence of genome function. Nat Rev Genet. 23:563–580. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xia L, Mei J, Huang M, Bao D, Wang Z and
Chen Y: O-GlcNAcylation in ovarian tumorigenesis and its
therapeutic implications. Transl Oncol. 51:1022202025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang J, Song C and Zhan X: The role of
protein acetylation in carcinogenesis and targeted drug discovery.
Front Endocrinol (Lausanne). 13:9723122022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang H and Han W: Protein
Post-translational modifications in head and neck cancer. Front
Oncol. 10:5719442020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Goldtzvik Y, Sen N, Lam SD and Orengo C:
Protein diversification through Post-translational modifications,
alternative splicing, and gene duplication. Curr Opin Struct Biol.
81:1026402023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janssen SM and Lorincz MC: Interplay
between chromatin marks in development and disease. Nat Rev Genet.
23:137–153. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hu D, Li Y, Wang X, Zou H, Li Z, Chen W,
Meng Y, Wang Y, Li Q, Liao F, et al: Palmitoylation of NLRP3
modulates inflammasome activation and inflammatory bowel disease
development. J Immunol. 213:481–493. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu D, Aji G, Li G, Li Y, Fang W, Zhang S,
Yu R, Jiang S, Gao X, Jiang Y, et al: ZDHHC18 promotes renal
fibrosis development by regulating HRAS palmitoylation. J Clin
Invest. 135:e1802422025. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan S and Chen R: Pathological implication
of protein Post-translational modifications in cancer. Mol Aspects
Med. 86:1010972022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou X, Wang Y, Li X, Zhou J, Yang W, Wang
X, Jiao S, Zuo W, You Z, Ying W, et al: O-GlcNAcylation regulates
the stability of transferrin receptor (TFRC) to control the
ferroptosis in hepatocellular carcinoma cells. Redox Biol.
73:1031822024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen L, Liu S and Tao Y: Regulating tumor
suppressor genes: Post-translational modifications. Signal
Transduct Target Ther. 5:902020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kumari S, Gupta R, Ambasta RK and Kumar P:
Emerging trends in post-translational modification: Shedding light
on Glioblastoma multiforme. Biochim Biophys Acta Rev Cancer.
1878:1889992023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Macek B, Forchhammer K, Hardouin J,
Weber-Ban E, Grangeasse C and Mijakovic I: Protein
post-translational modifications in bacteria. Nat Rev Microbiol.
17:651–664. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhong Q, Xiao X, Qiu Y, Xu Z, Chen C,
Chong B, Zhao X, Hai S, Li S, An Z, et al: Protein
posttranslational modifications in health and diseases: Functions,
regulatory mechanisms, and therapeutic implications. MedComm
(2020). 4:e2612023. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Z, Xiao M, Mo Y, Wang H, Han Y, Zhao
X, Yang X, Liu Z and Xu B: Emerging roles of protein palmitoylation
and its modifying enzymes in cancer cell signal transduction and
cancer therapy. Int J Biol Sci. 18:3447–3457. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei F, Wang Y, Yao J, Mei L, Huang X, Kong
H, Chen J, Chen X, Liu L, Wang Z, et al: ZDHHC7-mediated
S-palmitoylation of ATG16L1 facilitates LC3 lipidation and
autophagosome formation. Autophagy. 20:2719–2737. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Li S, Jiang L, Wu K, Chen S, Su L,
Liu C, Liu P, Luo W, Zhong S and Li Z: Palmitic acid accelerates
endothelial cell injury and cardiovascular dysfunction via
palmitoylation of PKM2. Adv Sci (Weinh). 12:e24128952025.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang N, Liu J, Guo R, Yan L, Yang Y, Shi
C, Zhang M, Shan B, Li W, Gu J and Xu D: Palmitoylation licenses
RIPK1 kinase activity and cytotoxicity in the TNF pathway. Mol
Cell. 84:4419–4435.e10. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li W, Li F, Zhang X, Lin HK and Xu C:
Insights into the post-translational modification and its emerging
role in shaping the tumor microenvironment. Signal Transduct Target
Ther. 6:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Linder ME and Deschenes RJ:
Palmitoylation: Policing protein stability and traffic. Nat Rev Mol
Cell Biol. 8:74–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ko PJ and Dixon SJ: Protein palmitoylation
and cancer. EMBO Rep. 19:e466662018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan Z, Hao Y, Huo Y, Cao F, Li L, Xu J,
Song Y and Yang K: Modulators for palmitoylation of proteins and
small molecules. Eur J Med Chem. 271:1164082024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao D, Huang Y, Liu D, Zhang H, Shi X, Li
X and Luo P: The role of s-palmitoylation in neurological diseases:
Implication for zDHHC family. Front Pharmacol. 14:13428302023.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mitchell DA, Vasudevan A, Linder ME and
Deschenes RJ: Protein palmitoylation by a family of DHHC protein
S-acyltransferases. J Lipid Res. 47:1118–1127. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Stix R, Lee CJ, Faraldo-Gómez JD and
Banerjee A: Structure and mechanism of DHHC protein
acyltransferases. J Mol Biol. 432:4983–4998. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin Z, Agarwal S, Tan S, Shi H, Lu X, Tao
Z, Dong X, Wu X, Zhao JC and Yu J: Palmitoyl acyltransferase ZDHHC7
inhibits androgen receptor and suppresses prostate cancer.
Oncogene. 42:2126–2138. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohno Y, Kihara A, Sano T and Igarashi Y:
Intracellular localization and tissue-specific distribution of
human and yeast DHHC Cysteine-rich domain-containing proteins.
Biochim Biophys Acta. 1761:474–483. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tang B, Kang W, Dong Q, Qin Z, Duan L,
Zhao X, Yuan G and Pan Y: Research progress on S-palmitoylation
modification mediated by the ZDHHC family in glioblastoma. Front
Cell Dev Biol. 12:14137082024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li X, Shen L, Xu Z, Liu W, Li A and Xu J:
Protein palmitoylation modification during viral infection and
detection methods of palmitoylated proteins. Front Cell Infect
Microbiol. 12:8215962022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Li Y and Wu L: Protein
S-palmitoylation modification: Implications in tumor and tumor
immune microenvironment. Front Immunol. 15:13374782024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Yan D, Liu J, Tang D and Chen X:
Protein modification and degradation in ferroptosis. Redox Biol.
75:1032592024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dennis K and Heather LC:
Post-translational palmitoylation of metabolic proteins. Front
Physiol. 14:11228952023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fhu CW and Ali A: Protein lipidation by
palmitoylation and myristoylation in cancer. Front Cell Dev Biol.
9:6736472021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan Y, Li P, Li J, Zhao Q, Chang Y and He
X: Protein lipidation in health and disease: Molecular basis,
physiological function and pathological implication. Signal
Transduct Target Ther. 9:602024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y and Yang W: Proteome-Scale analysis
of protein S-acylation comes of age. J Proteome Res. 20:14–26.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gu M, Jiang H, Tan M, Yu L, Xu N, Li Y, Wu
H, Hou Q and Dai C: Palmitoyltransferase DHHC9 and acyl protein
thioesterase APT1 modulate renal fibrosis through regulating
β-catenin palmitoylation. Nat Commun. 14:66822023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Heakal Y, Woll MP, Fox T, Seaton K,
Levenson R and Kester M: Neurotensin receptor-1 inducible
palmitoylation is required for efficient receptor-mediated
mitogenic-signaling within structured membrane microdomains. Cancer
Biol Ther. 12:427–435. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Greenlee JD, Lopez-Cavestany M,
Ortiz-Otero N, Liu K, Subramanian T, Cagir B and King MR:
Oxaliplatin resistance in colorectal cancer enhances TRAIL
sensitivity via death receptor 4 upregulation and lipid raft
localization. Elife. 10:e677502021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhou B, Hao Q, Liang Y and Kong E: Protein
palmitoylation in cancer: Molecular functions and therapeutic
potential. Mol Oncol. 17:3–26. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhu J, Cao X, Chen Z, Lai B, Xi L, Zhang
J, Zhu S, Qi S, Liang Y, Cao F, et al: Inhibiting S-palmitoylation
arrests metastasis by relocating Rap2b from plasma membrane in
colorectal cancer. Cell Death Dis. 15:6752024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Barylko B, Mao YS, Wlodarski P, Jung G,
Binns DD, Sun HQ, Yin HL and Albanesi JP: Palmitoylation controls
the catalytic activity and subcellular distribution of
phosphatidylinositol 4-kinase II{alpha}. J Biol Chem.
284:9994–10003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang Z, Li X, Yang F, Chen C, Liu P, Ren
Y, Sun P, Wang Z, You Y, Zeng YX, et al: DHHC9-mediated GLUT1
S-palmitoylation promotes glioblastoma glycolysis and
tumorigenesis. Nat Commun. 12:58722021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen S, Han C, Miao X, Li X, Yin C, Zou J,
Liu M, Li S, Stawski L, Zhu B, et al: Targeting MC1R
depalmitoylation to prevent melanomagenesis in redheads. Nat
Commun. 10:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Jin Q, Qi D, Zhang M, Qu H, Dong Y, Sun M
and Quan C: CLDN6 inhibits breast cancer growth and metastasis
through SREBP1-mediated RAS palmitoylation. Cell Mol Biol Lett.
29:1122024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ali A, Levantini E, Teo JT, Goggi J,
Clohessy JG, Wu CS, Chen L, Yang H, Krishnan I, Kocher O, et al:
Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated
non-small cell lung cancer. EMBO Mol Med. 10:e83132018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Sosa L, Petiti JP, Picech F, Chumpen S,
Nicola JP, Perez P, De Paul A, Valdez-Taubas J, Gutierrez S and
Torres AI: The ERα membrane pool modulates the proliferation of
pituitary tumours. J Endocrinol. 240:229–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cuiffo B and Ren R: Palmitoylation of
oncogenic NRAS is essential for leukemogenesis. Blood.
115:3598–3605. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sun Y, Zhang H, Meng J, Guo F, Ren D, Wu H
and Jin X: S-palmitoylation of PCSK9 induces sorafenib resistance
in liver cancer by activating the PI3K/AKT pathway. Cell Rep.
40:1111942022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Sada R, Kimura H, Fukata Y, Fukata M,
Yamamoto H and Kikuchi A: Dynamic palmitoylation controls the
microdomain localization of the DKK1 receptors CKAP4 and LRP6. Sci
Signal. 12:eaat95192019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang Q, Yang X, Wu J, Ye S, Gong J, Cheng
WM, Luo Z, Yu J, Liu Y, Zeng W, et al: Reprogramming of palmitic
acid induced by dephosphorylation of ACOX1 promotes β-catenin
palmitoylation to drive colorectal cancer progression. Cell Discov.
9:262023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yuan M, Chen X, Sun Y, Jiang L, Xia Z, Ye
K, Jiang H, Yang B, Ying M, Cao J, et al: ZDHHC12-mediated
claudin-3 S-palmitoylation determines ovarian cancer progression.
Acta Pharm Sin B. 10:1426–1439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Mo Y, Han Y, Chen Y, Fu C, Li Q, Liu Z,
Xiao M and Xu B: ZDHHC20 mediated S-palmitoylation of fatty acid
synthase (FASN) promotes hepatocarcinogenesis. Mol Cancer.
23:2742024. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li M, Zhang L and Chen CW: Diverse roles
of protein palmitoylation in cancer progression, immunity,
stemness, and beyond. Cells. 12:22092023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Gang X, Yan J, Li X, Shi S, Xu L, Liu R,
Cai L, Li H and Zhao M: Immune checkpoint inhibitors rechallenge in
non-small cell lung cancer: Current evidence and future directions.
Cancer Lett. 604:2172412024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
De Martin E, Fulgenzi C, Celsa C,
Laurent-Bellue A, Torkpour A, Lombardi P, D'Alessio A and Pinato
DJ: Immune checkpoint inhibitors and the liver: Balancing
therapeutic benefit and adverse events. Gut. 74:1165–1177. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Song J, Zhu J, Jiang Y, Guo Y, Liu S, Qiao
Y, Du Y and Li J: Advancements in immunotherapy for gastric cancer:
Unveiling the potential of immune checkpoint inhibitors and
emerging strategies. Biochim Biophys Acta Rev Cancer.
1880:1892772025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong
W, Yi M and Xiang B: Regulatory mechanisms of PD-1/PD-L1 in
cancers. Mol Cancer. 23:1082024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bardhan K, Anagnostou T and Boussiotis VA:
The PD1:PD-L1/2 pathway from discovery to clinical implementation.
Front Immunol. 7:5502016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang S, Wang HY, Tao X, Chen Z, Levental
I and Lin X: Palmitoylation of PD-L1 regulates its membrane
orientation and immune evasion. Langmuir. 41:5170–5178. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang Z, Ren C, Xiao R, Ma S, Liu H, Dou
Y, Fan Y, Wang S, Zhan P, Gao C, et al: Palmitoylation of TIM-3
promotes immune exhaustion and restrains antitumor immunity. Sci
Immunol. 9:eadp73022024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang J, Wang Y, Jiang X, Xu M, Wang M,
Wang R, Zheng B, Chen M, Ke Q and Long J: Unleashing the power of
immune checkpoints: Post-translational modification of novel
molecules and clinical applications. Cancer Lett. 588:2167582024.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yao H, Lan J, Li C, Shi H, Brosseau JP,
Wang H, Lu H, Fang C, Zhang Y, Liang L, et al: Inhibiting PD-L1
palmitoylation enhances T-cell immune responses against tumours.
Nat Biomed Eng. 3:306–317. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shi X, Zhao X, He Y, Zhang L, Zheng X, Qin
X, Li K, Li J, Wang Y, Dai L and Li X: Posttranslational remodeling
micelle reverses cell-surface and exosomal PD-L1 immunosuppression
in tumors resistant to PD-L1 antibody therapy. J Control Release.
384:1139612025. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cuyàs E, Pedarra S, Verdura S, Pardo MA,
Espin Garcia R, Serrano-Hervás E, Llop-Hernández À, Teixidor E,
Bosch-Barrera J, López-Bonet E, et al: Fatty acid synthase (FASN)
is a tumor-cell-intrinsic metabolic checkpoint restricting T-cell
immunity. Cell Death Discov. 10:4172024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Huang J, Tsang WY, Fang XN, Zhang Y, Luo
J, Gong LQ, Zhang BF, Wong CN, Li ZH, Liu BL, et al: FASN
inhibition decreases MHC-I degradation and synergizes with PD-L1
checkpoint blockade in hepatocellular carcinoma. Cancer Res.
84:855–871. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee TA, Tsai EY, Liu SH, Hsu Hung SD,
Chang SJ, Chao CH, Lai YJ, Yamaguchi H and Li CW:
Post-translational modification of PD-1: Potential targets for
cancer immunotherapy. Cancer Res. 84:800–807. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang G, Jiang P, Tang W, Wang Y, Qiu F,
An J, Zheng Y, Wu D, Zhou J, Neculai D, et al: CPT1A induction
following epigenetic perturbation promotes MAVS palmitoylation and
activation to potentiate antitumor immunity. Mol Cell.
83:4370–4385.e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Du W, Hua F, Li X, Zhang J, Li S, Wang W,
Zhou J, Wang W, Liao P, Yan Y, et al: Loss of optineurin drives
cancer immune evasion via Palmitoylation-dependent IFNGR1 lysosomal
sorting and degradation. Cancer Discov. 11:1826–1843. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tohumeken S, Baur R, Böttcher M, Stoll A,
Loschinski R, Panagiotidis K, Braun M, Saul D, Völkl S, Baur AS, et
al: Palmitoylated proteins on AML-Derived extracellular vesicles
promote Myeloid-derived suppressor cell differentiation via
TLR2/Akt/mTOR signaling. Cancer Res. 80:3663–3676. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Lin Z, Huang K, Guo H, Jia M, Sun Q, Chen
X, Wu J, Yao Q, Zhang P, Vakal S, et al: Targeting ZDHHC9
potentiates anti-programmed death-ligand 1 immunotherapy of
pancreatic cancer by modifying the tumor microenvironment. Biomed
Pharmacother. 161:1145672023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang Q, Wang J, Yu D, Zhang Q, Hu H, Xu M,
Zhang H, Tian S, Zheng G, Lu D, et al: Benzosceptrin C induces
lysosomal degradation of PD-L1 and promotes antitumor immunity by
targeting DHHC3. Cell Rep Med. 5:1013572024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Martin-Perez M, Urdiroz-Urricelqui U,
Bigas C and Benitah SA: The role of lipids in cancer progression
and metastasis. Cell Metab. 34:1675–1699. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Parton RG and Simons K: The biology of
lipids. Cold Spring Harb Perspect Biol. 16:a0417132024. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Domingues N, Pires J, Milosevic I and
Raimundo N: Role of lipids in interorganelle communication. Trends
Cell Biol. 35:46–58. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li Y, Pan Y, Zhao X, Wu S, Li F, Wang Y,
Liu B, Zhang Y, Gao X, Wang Y and Zhou H: Peroxisome
proliferator-activated receptors: A key link between lipid
metabolism and cancer progression. Clin Nutr. 43:332–345. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Ye L, Wen X, Qin J, Zhang X, Wang Y, Wang
Z, Zhou T, Di Y and He W: Metabolism-regulated ferroptosis in
cancer progression and therapy. Cell Death Dis. 15:1962024.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tufail M, Jiang CH and Li N: Altered
metabolism in cancer: Insights into energy pathways and therapeutic
targets. Mol Cancer. 23:2032024. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Bian X, Liu R, Meng Y, Xing D, Xu D and Lu
Z: Lipid metabolism and cancer. J Exp Med. 218:e202016062021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Gu Q, Wang Y, Yi P and Cheng C:
Theoretical framework and emerging challenges of lipid metabolism
in cancer. Semin Cancer Biol. 108:48–70. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ruan C, Meng Y and Song H: CD36: An
emerging therapeutic target for cancer and its molecular
mechanisms. J Cancer Res Clin Oncol. 148:1551–1558. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xu S, Chaudhary O, Rodríguez-Morales P,
Sun X, Chen D, Zappasodi R, Xu Z, Pinto A, Williams A, Schulze I,
et al: Uptake of oxidized lipids by the scavenger receptor CD36
promotes lipid peroxidation and dysfunction in CD8(+) T cells in
tumors. Immunity. 54:1561–1577.e7. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wang Z, Chao Z, Wang Q, Zou F, Song T, Xu
L, Ning J and Cheng F: EXO1/P53/SREBP1 axis-regulated lipid
metabolism promotes prostate cancer progression. J Transl Med.
22:1042024. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jin HR, Wang J, Wang ZJ, Xi MJ, Xia BH,
Deng K and Yang JL: Lipid metabolic reprogramming in tumor
microenvironment: From mechanisms to therapeutics. J Hematol Oncol.
16:1032023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Fatima S, Hu X, Gong RH, Huang C, Chen M,
Wong H, Bian Z and Kwan HY: Palmitic acid is an intracellular
signaling molecule involved in disease development. Cell Mol Life
Sci. 76:2547–2557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao Z, Wang J, Kong W, Newton MA, Burkett
WC, Sun W, Buckingham L, O'Donnell J, Suo H, Deng B, et al:
Palmitic acid exerts Anti-tumorigenic activities by modulating
cellular stress and lipid droplet formation in endometrial cancer.
Biomolecules. 14:6012024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Annevelink CE, Sapp PA, Petersen KS,
Shearer GC and Kris-Etherton PM: Diet-derived and diet-related
endogenously produced palmitic acid: Effects on metabolic
regulation and cardiovascular disease risk. J Clin Lipidol.
17:577–586. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Murru E, Manca C, Carta G and Banni S:
Impact of dietary palmitic acid on lipid metabolism. Front Nutr.
9:8616642022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Tan Y, Huang Z, Jin Y, Wang J, Fan H, Liu
Y, Zhang L, Wu Y, Liu P, Li T, et al: Lipid droplets sequester
palmitic acid to disrupt endothelial ciliation and exacerbate
atherosclerosis in male mice. Nat Commun. 15:82732024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Jeong DW, Park JW, Kim KS, Kim J, Huh J,
Seo J, Kim YL, Cho JY, Lee KW, Fukuda J, et al:
Palmitoylation-driven PHF2 ubiquitination remodels lipid metabolism
through the SREBP1c axis in hepatocellular carcinoma. Nat Commun.
14:63702023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang J, Hao JW, Wang X, Guo H, Sun HH, Lai
XY, Liu LY, Zhu M, Wang HY, Li YF, et al: DHHC4 and DHHC5
facilitate fatty acid uptake by palmitoylating and targeting CD36
to the plasma membrane. Cell Rep. 26:209–221.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shan J, Li X, Sun R, Yao Y and Sun Y,
Kuang Q, Dai X and Sun Y: Palmitoyltransferase ZDHHC6 promotes
colon tumorigenesis by targeting PPARγ-driven lipid biosynthesis
via regulating lipidome metabolic reprogramming. J Exp Clin Cancer
Res. 43:2272024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Qu M, Zhou X, Wang X and Li H:
Lipid-induced S-palmitoylation as a vital regulator of cell
signaling and disease development. Int J Biol Sci. 17:4223–4237.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Fan JJ and Huang X: Ion channels in
cancer: Orchestrators of electrical signaling and cellular
crosstalk. Rev Physiol Biochem Pharmacol. 183:103–133. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Li Y, Fu J and Wang H: Advancements in
targeting ion channels for the treatment of neurodegenerative
diseases. Pharmaceuticals (Basel). 17:14622024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Hu M, Feng X, Liu Q, Liu S, Huang F and Xu
H: The ion channels of endomembranes. Physiol Rev. 104:1335–1385.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Shi Q, Yang Z, Yang H, Xu L, Xia J, Gu J,
Chen M, Wang Y, Zhao X, Liao Z, et al: Targeting ion channels:
Innovative approaches to combat cancer drug resistance.
Theranostics. 15:521–545. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Yuan S, Sun R, Shi H, Chapman NM, Hu H,
Guy C, Rankin S, Kc A, Palacios G, Meng X, et al: VDAC2 loss
elicits tumour destruction and inflammation for cancer therapy.
Nature. 640:1062–1071. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Sobradillo D, Hernández-Morales M, Ubierna
D, Moyer MP, Núñez L and Villalobos C: A reciprocal shift in
transient receptor potential channel 1 (TRPC1) and stromal
interaction molecule 2 (STIM2) contributes to Ca2+ remodeling and
cancer hallmarks in colorectal carcinoma cells. J Biol Chem.
289:28765–28782. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Prevarskaya N, Skryma R and Shuba Y: Ion
channels and the hallmarks of cancer. Trends Mol Med. 16:107–121.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Zhang L, Gu H, Li X, Wang Y, Yao S, Chen
X, Zheng L, Yang X, Du Q, An J, et al: Pathophysiological role of
ion channels and transporters in hepatocellular carcinoma. Cancer
Gene Ther. 31:1611–1618. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Ji R, Chang L, An C and Zhang J:
Proton-sensing ion channels, GPCRs and calcium signaling regulated
by them: Implications for cancer. Front Cell Dev Biol.
12:13262312024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Panda S, Chatterjee O, Roy L and
Chatterjee S: Targeting Ca2+ signaling: A new arsenal against
cancer. Drug Discov Today. 27:923–934. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gao SH, Wang GZ, Wang LP, Feng L, Zhou YC,
Yu XJ, Liang F, Yang FY, Wang Z, Sun BB, et al: Mutations and
clinical significance of calcium voltage-gated channel subunit
alpha 1E (CACNA1E) in non-small cell lung cancer. Cell Calcium.
102:1025272022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lu F, Chen H, Zhou C, Liu S, Guo M, Chen
P, Zhuang H, Xie D and Wu S: T-type Ca2+ channel expression in
human esophageal carcinomas: A functional role in proliferation.
Cell Calcium. 43:49–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Taylor JT, Huang L, Pottle JE, Liu K, Yang
Y, Zeng X, Keyser BM, Agrawal KC, Hansen JB and Li M: Selective
blockade of T-type Ca2+ channels suppresses human breast cancer
cell proliferation. Cancer Lett. 267:116–124. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Zhang L, Ren C, Liu J, Huang S, Wu C and
Zhang J: Development and therapeutic implications of small
molecular inhibitors that target calcium-related channels in tumor
treatment. Drug Discov Today. 29:1039952024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Banderali U, Jayanthan A, Hoeksema KA,
Narendran A and Giles WR: Ion channels in pediatric CNS Atypical
Teratoid/Rhabdoid Tumor (AT/RT) cells: Potential targets for novel
therapeutic agents. J Neurooncol. 107:111–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Shipston MJ: Ion channel regulation by
protein S-acylation. J Gen Physiol. 143:659–678. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhu Z, Zhou X, Du H, Cloer EW, Zhang J,
Mei L, Wang Y, Tan X, Hepperla AJ, Simon JM, et al: STING
suppresses mitochondrial VDAC2 to govern RCC growth independent of
innate immunity. Adv Sci (Weinh). 10:e22037182023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Cassinelli S, Viñola-Renart C,
Benavente-Garcia A, Navarro-Pérez M, Capera J and Felipe A:
Palmitoylation of voltage-gated ion channels. Int J Mol Sci.
23:93572022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhao L, Zhang C, Luo X, Wang P, Zhou W,
Zhong S, Xie Y, Jiang Y, Yang P, Tang R, et al: CD36 palmitoylation
disrupts free fatty acid metabolism and promotes tissue
inflammation in non-alcoholic steatohepatitis. J Hepatol.
69:705–717. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Li W, Liu J, Yu T, Lu F, Miao Q, Meng X,
Xiao W, Yang H and Zhang X: ZDHHC9-mediated Bip/GRP78
S-palmitoylation inhibits unfolded protein response and promotes
bladder cancer progression. Cancer Lett. 598:2171182024. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jeyifous O, Lin EI, Chen X, Antinone SE,
Mastro R, Drisdel R, Reese TS and Green WN: Palmitoylation
regulates glutamate receptor distributions in postsynaptic
densities through control of PSD95 conformation and orientation.
Proc Natl Acad Sci USA. 113:E8482–E8491. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chamberlain LH and Shipston MJ: The
physiology of protein S-acylation. Physiol Rev. 95:341–376. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zheng S, Que X, Wang S, Zhou Q, Xing X,
Chen L, Hou C, Ma J, An P, Peng Y, et al: ZDHHC5-mediated NLRP3
palmitoylation promotes NLRP3-NEK7 interaction and inflammasome
activation. Mol Cell. 83:4570–4585.e7. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Gök C, Plain F, Robertson AD, Howie J,
Baillie GS, Fraser NJ and Fuller W: Dynamic palmitoylation of the
Sodium-calcium exchanger modulates its structure, affinity for
Lipid-ordered domains, and inhibition by XIP. Cell Rep.
31:1076972020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen B, Sun Y, Niu J, Jarugumilli GK and
Wu X: Protein lipidation in cell signaling and diseases: Function,
regulation, and therapeutic opportunities. Cell Chem Biol.
25:817–831. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Rebecca VW, Nicastri MC, Fennelly C, Chude
CI, Barber-Rotenberg JS, Ronghe A, McAfee Q, McLaughlin NP, Zhang
G, Goldman AR, et al: PPT1 Promotes tumor growth and is the
molecular target of chloroquine derivatives in cancer. Cancer
Discov. 9:220–229. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Chen X, Li H, Fan X, Zhao C, Ye K, Zhao Z,
Hu L, Ma H, Wang H and Fang Z: Protein palmitoylation regulates
cell survival by modulating XBP1 activity in glioblastoma
multiforme. Mol Ther Oncolytics. 17:518–530. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Lan T, Delalande C and Dickinson BC:
Inhibitors of DHHC family proteins. Curr Opin Chem Biol.
65:118–125. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Yang Q, Qin T, An T, Wu H, Xu G, Xiang J,
Lei K, Zhang S, Xia J, Su G, et al: Novel PORCN inhibitor WHN-88
targets Wnt/β-catenin pathway and prevents the growth of Wnt-driven
cancers. Eur J Pharmacol. 945:1756282023. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Kharbanda A, Walter DM, Gudiel AA, Schek
N, Feldser DM and Witze ES: Blocking EGFR palmitoylation suppresses
PI3K signaling and mutant KRAS lung tumorigenesis. Sci Signal.
13:eaax23642020. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang C, Zhang Y, Dong Y, Zi R, Wang Y,
Chen Y, Liu C, Wang J, Wang X, Li J, et al: Non-alcoholic fatty
liver disease promotes liver metastasis of colorectal cancer via
fatty acid synthase dependent EGFR palmitoylation. Cell Death
Discov. 10:412024. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Lemonidis K, Salaun C, Kouskou M,
Diez-Ardanuy C, Chamberlain LH and Greaves J: Substrate selectivity
in the zDHHC family of S-acyltransferases. Biochem Soc Trans.
45:751–758. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Balasubramanian A, Hsu AY, Ghimire L,
Tahir M, Devant P, Fontana P, Du G, Liu X, Fabin D, Kambara H, et
al: The palmitoylation of gasdermin D directs its membrane
translocation and pore formation during pyroptosis. Sci Immunol.
9:eadn14522024. View Article : Google Scholar : PubMed/NCBI
|