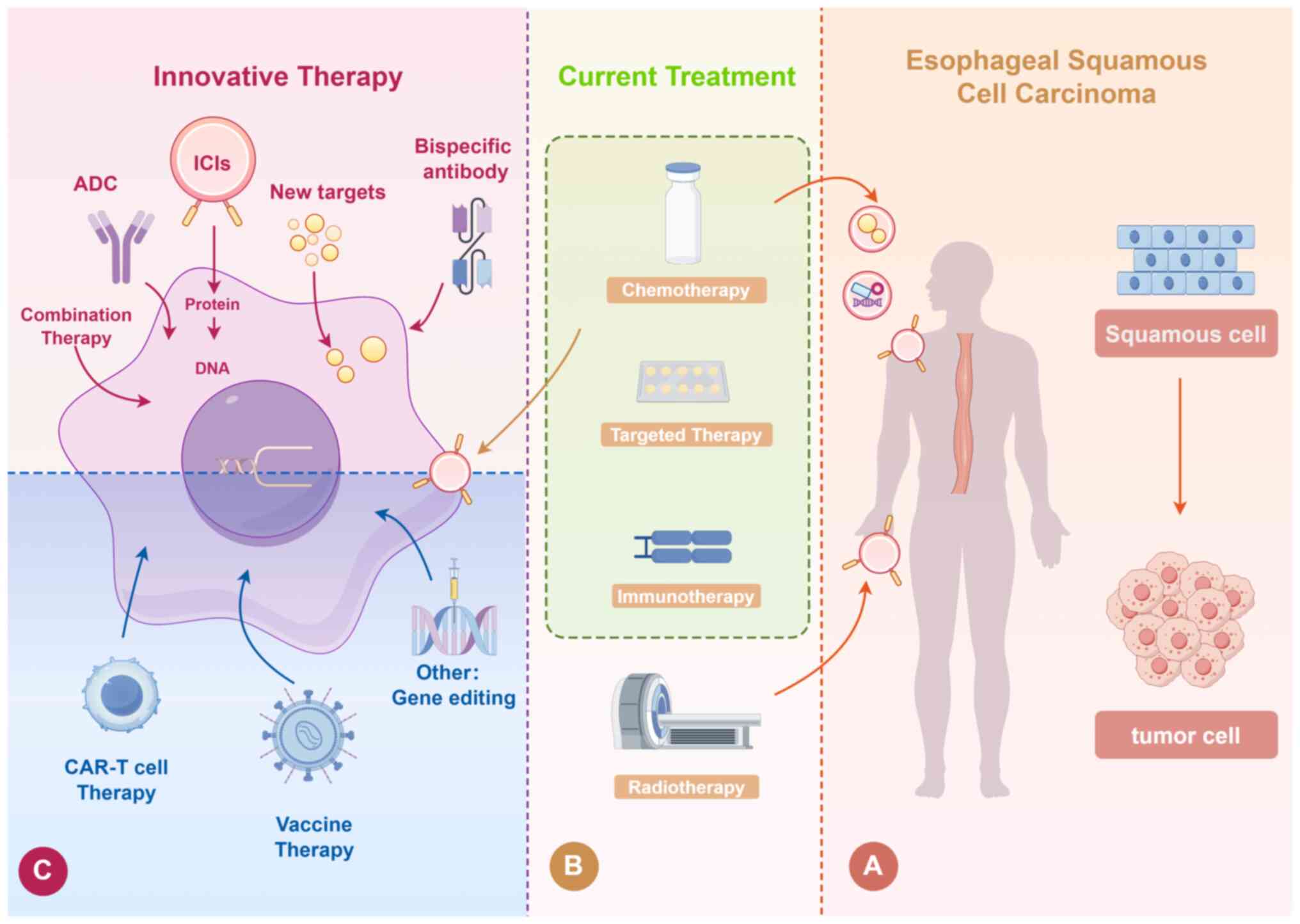
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Jokhadze N, Das A and Dizon DS: Global
cancer statistics: A healthy population relies on population
health. CA Cancer J Clin. 74:224–226. 2024.PubMed/NCBI
|
|
3
|
Decker A and Quante M: Esophageal cancer:
New developments in prevention and therapy. Dtsch Med Wochenschr.
149:1329–1334. 2024.(In German). PubMed/NCBI
|
|
4
|
Al-Nattah S, Matkovic E, Schwalbe M and
Matkowskyj KA: Pathologic features of esophageal and gastric
malignancies. Cancer Treat Res. 192:19–48. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang YX, Wu JH, Zhao YQ, Sui WN, Tian T,
Han WX and Ni J: An atlas on risk factors for gastrointestinal
cancers: A systematic review of Mendelian randomization studies.
Prev Med. 189:1081472024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ogura N, Yamamoto S and Kato K: Progress
in second-line antibody therapies for advanced esophageal squamous
cell carcinoma. Exp Opin Biol Ther. 24:503–509. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mitamura A, Tsujinaka S, Nakano T, Sawada
K and Shibata C: Treatment strategies for locoregional recurrence
in esophageal squamous-cell carcinoma: An updated review. Cancers
(Basel). 16:25392024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eisner DC: Esophageal cancer: Treatment
advances and need for screening. JAAPA. 37:19–24. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ajani JA, D'Amico TA, Bentrem DJ, Cooke D,
Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, et al:
Esophageal and esophagogastric junction cancers, version 2.2023,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 21:393–422. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ku GY: Systemic therapy for esophageal
cancer: Chemotherapy. Chin Clin Oncol. 6:492017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Famurewa AC, Prabhune NM and Prabhu S:
Natural product mitigation of ferroptosis in platinum-based
chemotherapy toxicity: Targeting the underpinning oxidative
signaling pathways. J Pharm Pharmacol. 77:1–17. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Moraes FCA, de Almeida Barbosa AB, Sano
VKT, Kelly FA and Burbano RMR: Pharmacogenetics of DPYD and
treatment-related mortality on fluoropyrimidine chemotherapy for
cancer patients: A meta-analysis and trial sequential analysis. BMC
Cancer. 24:12102024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen L, Kato K, Kim SB, Ajani JA, Zhao K,
He Z, Yu X, Shu Y, Luo Q, Wang J, et al: Tislelizumab versus
chemotherapy as second-line treatment for advanced or metastatic
esophageal squamous cell carcinoma (RATIONALE-302): A randomized
phase III study. J Clin Oncol. 40:3065–3076. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jung HA, Adenis A, Lee J, Park SH, Maeng
CH, Park S, Ahn HK, Shim YM, Penel N and Im YH: Nomogram to predict
treatment outcome of fluoropyrimidine/platinum-based chemotherapy
in metastatic esophageal squamous cell carcinoma. Cancer Res Treat.
45:285–294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hategan M, Cook N, Prewett S, Hindmarsh A,
Qian W and Gilligan D: Trimodality therapy and definitive
chemoradiotherapy for esophageal cancer: A single-center experience
and review of the literature. Dis Esophagus. 28:612–618. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
van Kleef JJ, Ter Veer E, van den Boorn
HG, Schokker S, Ngai LL, Prins MJ, Mohammad NH, van de Poll-Franse
LV, Zwinderman AH, van Oijen MG, et al: Quality of life during
palliative systemic therapy for esophagogastric cancer: Systematic
review and meta-analysis. J Natl Cancer Inst. 112:12–29.
2020.PubMed/NCBI
|
|
17
|
Arora M, Singh AK, Kumar A, Singh H,
Pathak P, Grishina M, Yadav JP, Verma A and Kumar P: Semisynthetic
phytochemicals in cancer treatment: A medicinal chemistry
perspective. RSC Med Chem. 15:3345–3370. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang Y and Ando Y: UGT1A1 testing for the
risk of nanoliposomal irinotecan-related toxicity. J Clin Oncol.
43:3572024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Y, Zhang B, Xu J, Wang X, Tang J and
Huang J: Phase I study of liposomal irinotecan (LY01610) in
patients with advanced esophageal squamous cell carcinoma. Cancer
Chemother Pharmacol. 88:403–414. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai X, Tao L, Wang J, Wu W, Bian W, Dai X
and Chen S: Efficacy and safety of irinotecan combined with
raltitrexed or irinotecan monotherapy for salvage chemotherapy of
esophageal squamous cell cancer: A prospective, open label,
randomized phase II study. Cancer Med. 12:16108–16118. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakajima M, Muroi H, Kikuchi M, Kubo T,
Takise S, Ihara K, Nakagawa M, Morita S, Nakamura T, Yamaguchi S
and Kojima K: Strategy treatment of cT4b esophageal squamous cell
carcinoma using docetaxel, cisplatin, and 5-fluorouracil.
Anticancer Res. 42:3725–3733. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yazdani F, Mottaghi-Dastjerdi N, Shahbazi
B, Ahmadi K, Ghorbani A, Soltany-Rezaee-Rad M, Montazeri H,
Khoshdel F and Guzzi PH: Identification of key genes and pathways
involved in T-DM1-resistance in OE-19 esophageal cancer cells
through bioinformatics analysis. Heliyon. 10:e374512024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tchelebi LT and Goodman KA:
Esophagogastric cancer: The current role of radiation therapy.
Hematol Oncol Clin North Am. 38:569–583. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen S, Wang J, Hu W and Xu Y: Comparative
evaluation of dosimetric quality and treatment efficiency for
halcyon, TrueBeam, and TomoTherapy in cervical-thoracic esophageal
cancer radiotherapy. Technol Cancer Res Treat.
23:153303382412933212024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Prasanna PGS, Ahmed MM, Hong JA and
Coleman CN: Best practices and novel approaches for the preclinical
development of drug-radiotherapy combinations for cancer treatment.
Lancet Oncol. 25:e501–e511. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hokamura N, Fukagawa T, Fukushima R,
Kiyokawa T, Horikawa M, Kumata Y, Suzuki Y and Midorikawa H:
Pembrolizumab plus cisplatin and fluorouracil as induction
chemotherapy followed by definitive chemoradiotherapy for patients
with cT4 and/or supraclavicular lymph node metastasis (M1Lym) of
esophageal squamous cell carcinoma. Surg Today. 54:1410–1413. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu S, Wen J, Yang H, Li Q, Chen Y, Zhu C,
Fang W, Yu Z, Mao W, Xiang J, et al: Recurrence patterns after
neoadjuvant chemoradiotherapy compared with surgery alone in
oesophageal squamous cell carcinoma: Results from the multicenter
phase III trial NEOCRTEC5010. Eur J Cancer. 138:113–121. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang K, Wang Q, Cao J, Fan C, Shen W,
Xiao Q, Ge X, Zhang T, Liu X, Chen X, et al: Tislelizumab plus
concurrent chemoradiotherapy versus concurrent chemoradiotherapy
for elderly patients with inoperable locally advanced esophageal
squamous cell carcinoma: A multicenter, randomized,
parallel-controlled, phase II clinical trial. BMC Cancer.
25:3472025. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang W, Ye L, Li H, Mao W and Xu X:
Targeting esophageal carcinoma: Molecular mechanisms and clinical
studies. MedComm (2020). 5:e7822024. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gouda MA, Thein KZ and Hong DS:
Tissue-Agnostic targeting of neurotrophic tyrosine receptor kinase
fusions: Current approvals and future directions. Cancers (Basel).
16:33952024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dean L, Kane M, Pratt VM, Scott SA,
Pirmohamed M, Esquivel B, Kattman BL and Malheiro AJ: Dabrafenib
therapy and BRAF genotype. Medical Genetics Summaries (eds.).
National Center for Biotechnology Information (US); Bethesda (MD):
2012, PubMed/NCBI
|
|
32
|
Chen MF, Repetto M, Wilhelm C and Drilon
A: RET Inhibitors in RET fusion-positive lung cancers: Past,
present, and future. Drugs. 84:1035–1053. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu L, Li B, Wan G, Wang Y, Zhu J, Liang L,
Leng X, He W, Peng L, Han Y, et al: Toripalimab plus chemotherapy
and radiotherapy for treatment-naive advanced esophageal squamous
cell carcinoma: A single-arm phase 2 trial. Nat Commun.
15:71162024. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ooki A, Osumi H, Chin K, Watanabe M and
Yamaguchi K: Potent molecular-targeted therapies for advanced
esophageal squamous cell carcinoma. Ther Adv Med Oncol.
15:175883592211383772023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Alsina M and Fleitas-Kanonnikoff T: Immune
checkpoint inhibitors for first-line treatment of advanced
esophageal squamous cell carcinoma. Med. 5:1038–1040. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato K, Cho BC, Takahashi M, Okada M, Lin
CY, Chin K, Kadowaki S, Ahn MJ, Hamamoto Y, Doki Y, et al:
Nivolumab versus chemotherapy in patients with advanced oesophageal
squamous cell carcinoma refractory or intolerant to previous
chemotherapy (ATTRACTION-3): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:1506–1517. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kato K, Shah MA, Enzinger P, Bennouna J,
Shen L, Adenis A, Sun JM, Cho BC, Özgüroğlu M, Kojima T, et al:
KEYNOTE-590: Phase III study of first-line chemotherapy with or
without pembrolizumab for advanced esophageal cancer. Future Oncol.
15:1057–1066. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kato K, Doki Y, Chau I, Xu J, Wyrwicz L,
Motoyama S, Ogata T, Kawakami H, Hsu CH, Adenis A, et al: Nivolumab
plus chemotherapy or ipilimumab versus chemotherapy in patients
with advanced esophageal squamous cell carcinoma (CheckMate 648):
29-month follow-up from a randomized, open-label, phase III trial.
Cancer Med. 13:e72352024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thuss-Patience P and Stein A:
Immunotherapy in squamous cell cancer of the esophagus. Curr Oncol.
29:2461–2471. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Paz-Manrique R, Pinto JA and Moreno HL:
Antibody-Drug conjugates in breast cancer: Toward a molecular
perspective into clinical practice. JCO Precis Oncol.
8:e24001732024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chalouni C and Doll S: Fate of
antibody-drug conjugates in cancer cells. J Exp Clin Cancer Res.
37:202018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Colombo R, Tarantino P, Rich JR, LoRusso
PM and de Vries EGE: The journey of antibody-drug conjugates:
Lessons learned from 40 years of development. Cancer Discov.
14:2089–2108. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Patel MR, Doi T, Koyama T, Falchook GS,
Friedman CF, Piha-Paul SA, Gutierrez M, Awad MM, Mattour AH, Sarih
T, et al: 690P Ifinatamab deruxtecan (I-DXd; DS-7300) in patients
with advanced solid tumors: Updated clinical and biomarker results
from a phase I/II study. ESMO. 34:S481–S482
|
|
44
|
Ma Y, Yang Y, Huang Y, Fang W, Xue J, Meng
X, Fan Y, Fu S, Wu L, Zheng Y, et al: A B7H3-targeting
antibody-drug conjugate in advanced solid tumors: A phase 1/1b
trial. Nat Med. 31:1949–1957. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang J, Liu R, Gao S, Yang H, Chen J,
Yuan F, Liu J, Guo H, Zhang S, Li X, et al: 9MW2821, a nectin-4
antibody-drug conjugate (ADC), in patients with advanced solid
tumor: Results from a phase 1/2a study. J Clin Oncol. 42 no 16
supp:2024.
|
|
46
|
Bardia A, Messersmith WA, Kio EA, Berlin
JD, Vahdat L, Masters GA, Moroose R, Santin AD, Kalinsky K, Picozzi
V, et al: Sacituzumab govitecan, a Trop-2-directed antibody-drug
conjugate, for patients with epithelial cancer: Final safety and
efficacy results from the phase I/II IMMU-132-01 basket trial. Ann
Oncol. 32:746–756. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chang L, Lu Z, Ji Y, Sun M, Wen Q, Gao SG,
Ma XL, Zhu J, Zhong D, Guo Q, et al: 1426P BL-B01D1, an EGFR × her3
bispecific antibody-drug conjugate (ADC), in patients with locally
advanced or metastatic esophageal squamous cell carcinoma (ESCC).
ESMO. 35:S8892024.
|
|
48
|
Ma Y, Huang Y, Zhao Y, Zhao S, Xue J, Yang
Y, Fang W, Guo Y, Han Y, Yang K, et al: BL-B01D1, a first-in-class
EGFR-HER3 bispecific antibody-drug conjugate, in patients with
locally advanced or metastatic solid tumours: A first-in-human,
open-label, multicentre, phase 1 study. Lancet Oncol. 25:901–911.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fantacuzzi M, Carradori S, Giampietro L,
Maccallini C, De Filippis B, Amoroso R and Ammazzalorso A: A novel
life for antitumor combretastatins: Recent developments of hybrids,
prodrugs, combination therapies, and antibody-drug conjugates. Eur
J Med Chem. 281:1170212024. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li CL, Ma XY and Yi P: Bispecific
antibodies, immune checkpoint inhibitors, and antibody-drug
conjugates directing antitumor immune responses: Challenges and
prospects. Cell Biochem Funct. 42:e700112024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong MH, Heo SG, Lee YG, Kim HS, Park KU,
Kim HG, Ko YH, Chung IJ, Min YJ, Kim MK, et al: Phase 2 study of
afatinib among patients with recurrent and/or metastatic esophageal
squamous cell carcinoma. Cancer. 126:4521–4531. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Liu R, Liu L, Zhao C, Bai Y, Zheng Y,
Zhang S, Li N, Yang J, Fan Q, Wang X, et al: Larotinib in patients
with advanced and previously treated esophageal squamous cell
carcinoma with epidermal growth factor receptor overexpression or
amplification: An open-label, multicenter phase 1b study. BMC
Gastroenterol. 21:3982021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lorenzen S, Schuster T, Porschen R,
Al-Batran SE, Hofheinz R, Thuss-Patience P, Moehler M, Grabowski P,
Arnold D, Greten T, et al: Cetuximab plus cisplatin-5-fluorouracil
versus cisplatin-5-fluorouracil alone in first-line metastatic
squamous cell carcinoma of the esophagus: A randomized phase II
study of the Arbeitsgemeinschaft Internistische Onkologie. Ann
Oncol. 20:1667–1673. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Moehler M, Maderer A, Thuss-Patience PC,
Brenner B, Meiler J, Ettrich TJ, Hofheinz RD, Al-Batran SE, Vogel
A, Mueller L, et al: Cisplatin and 5-fluorouracil with or without
epidermal growth factor receptor inhibition panitumumab for
patients with non-resectable, advanced or metastatic oesophageal
squamous cell cancer: A prospective, open-label, randomised phase
III AIO/EORTC trial (POWER). Ann Oncol. 31:228–235. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Suntharalingam M, Winter K, Ilson D,
Dicker AP, Kachnic L, Konski A, Chakravarthy AB, Anker CJ, Thakrar
H, Horiba N, et al: Effect of the addition of cetuximab to
paclitaxel, cisplatin, and radiation therapy for patients with
esophageal cancer: The NRG oncology RTOG 0436 phase 3 randomized
clinical trial. JAMA Oncol. 3:1520–1528. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Crosby T, Hurt CN, Falk S, Gollins S,
Mukherjee S, Staffurth J, Ray R, Bashir N, Bridgewater JA, Geh JI,
et al: Chemoradiotherapy with or without cetuximab in patients with
oesophageal cancer (SCOPE1): A multicentre, phase 2/3 randomised
trial. Lancet Oncol. 14:627–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kotani D, Yamaguchi K, Kato K, Hara H,
Miura A, Satoh T, Komine K, Kunisaki C, Yabusaki H and Hagiwara A:
A phase 2, open-label study of amivantamab in patients with
previously treated advanced or metastatic gastric or esophageal
cancer. J Clin Oncol. 42:3632024. View Article : Google Scholar
|
|
58
|
Lorenc P, Sikorska A, Molenda S, Guzniczak
N, Dams-Kozlowska H and Florczak A: Physiological and
tumor-associated angiogenesis: Key factors and therapy targeting
VEGF/VEGFR pathway. Biomed Pharmacother. 180:1175852024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Katari V, Dalal K, Adapala RK, Guarino BD,
Kondapalli N, Paruchuri S and Thodeti CK: A TRP to pathological
angiogenesis and vascular normalization. Compr Physiol.
14:5389–5406. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carmeliet P and Jain RK: Principles and
mechanisms of vessel normalization for cancer and other angiogenic
diseases. Nat Rev Drug Discov. 10:417–427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Meng X, Wang J, Xia J, Wu T, Luo Z, Hong
Y, Lu P, Guo Y, Ji Y, Zhang M, et al: Efficacy and safety of
camrelizumab plus apatinib in patients with advanced esophageal
squamous cell carcinoma previously treated with immune checkpoint
inhibitors (CAP 02 Re-challenge): A single-arm, phase II study. Eur
J Cancer. 212:1143282024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Huang J, Xiao J, Fang W, Lu P, Fan Q, Shu
Y, Feng J, Zhang S, Ba Y, Zhao Y, et al: Anlotinib for previously
treated advanced or metastatic esophageal squamous cell carcinoma:
A double-blind randomized phase 2 trial. Cancer Med. 10:1681–1689.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ho JN, Byun SS, Kim D, Ryu H and Lee S:
Dasatinib induces apoptosis and autophagy by suppressing the
PI3K/Akt/mTOR pathway in bladder cancer cells. Investig Clin Urol.
65:593–602. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang S, Liu C, Yang C, Jin Y, Cui Q, Wang
D, Ge T, He G, Li W, Zhang G, et al: PI3K/AKT/mTOR and
PD-1/CTLA-4/CD28 pathways as key targets of cancer immunotherapy
(Review). Oncol Lett. 28:5672024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kojima T, Kato K, Hara H, Takahashi S,
Muro K, Nishina T, Wakabayashi M, Nomura S, Sato A, Ohtsu A and Doi
T: Phase II study of BKM120 in patients with advanced esophageal
squamous cell carcinoma (EPOC1303). Esophagus. 19:702–710. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Angerilli V, Fornaro L, Pepe F, Rossi SM,
Perrone G, Malapelle U and Fassan M: FGFR2 testing in
cholangiocarcinoma: Translating molecular studies into clinical
practice. Pathologica. 115:71–82. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Meric-Bernstam F, Bahleda R, Hierro C,
Sanson M, Bridgewater J, Arkenau HT, Tran B, Kelley RK, Park JO,
Javle M, et al: Futibatinib, an irreversible FGFR1-4 inhibitor, in
patients with advanced solid tumors harboring FGF/FGFR aberrations:
A phase I dose-expansion study. Cancer Discov. 12:402–415. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xue Y and Zhai J: Strategy of combining
CDK4/6 inhibitors with other therapies and mechanisms of
resistance. Int J Clin Exp Pathol. 17:189–207. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
O'Hara MH, Jegede O, Dickson MA, DeMichele
AM, Piekarz R, Gray RJ, Wang V, McShane LM, Rubinstein LV, Patton
DR, et al: Phase 2 study of palbociclib in patients with tumors
with CDK4 or CDK6 amplification: Results from the NCI-MATCH
ECOG-ACRIN trial (EAY131) subprotocol Z1C. Clin Cancer Res.
31:56–64. 2024. View Article : Google Scholar
|
|
70
|
Rodon J, Prenen H, Sacher A,
Villalona-Calero M, Penel N, El Helali A, Rottey S, Yamamoto N,
Ghiringhelli F, Goebeler ME, et al: First-in-human study of AMG
193, an MTA-cooperative PRMT5 inhibitor, in patients with
MTAP-deleted solid tumors: Results from phase I dose exploration.
Ann Oncol. 35:1138–1147. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu L, Lu Z, Hu X, Su T, Su L and Pu H:
Clinical significance of YAP1 and TAZ in esophageal squamous cell
carcinoma. Medicine. 100:e265972021. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Song S, Li Y, Xu Y, Ma L, Pizzi MP, Jin J,
Scott AW, Huo L, Wang Y, Lee JH, et al: Targeting hippo coactivator
YAP1 through BET bromodomain inhibition in esophageal
adenocarcinoma. Mol Oncol. 14:1410–1426. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Ning J, Wang Y and Tao Z: The complex role
of immune cells in antigen presentation and regulation of T-cell
responses in hepatocellular carcinoma: Progress, challenges, and
future directions. Front Immunol. 15:14838342024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Xu H, Yang Y, Yan Y, Li M, Wu S, Cao L,
Chen W, Luo H and He Y: Safety and efficacy of rechallenge with
immune checkpoint inhibitors in advanced solid tumor: A systematic
review and meta-analysis. Cancer Med. 13:e703242024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hossain MA: A comprehensive review of
immune checkpoint inhibitors for cancer treatment. Int
Immunopharmacol. 143:1133652024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Su X, Li J, Xu X, Ye Y, Wang C, Pang G,
Liu W, Liu A, Zhao C and Hao X: Strategies to enhance the
therapeutic efficacy of anti-PD-1 antibody, anti-PD-L1 antibody and
anti-CTLA-4 antibody in cancer therapy. J Transl Med. 22:7512024.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Lu T, Xu R, Wang CH, Zhao JY, Peng B, Wang
J and Zhang LY: Identification of tumor antigens and immune
subtypes of esophageal squamous cell carcinoma for mRNA vaccine
development. Front Genet. 13:8531132022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Martínez-Pérez A, Granda-Díaz R,
Aguilar-García C, Sordo-Bahamonde C and Gonzalez S: Deciphering
LAG-3: Unveiling molecular mechanisms and clinical advancements.
Biomark Res. 12:1262024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang J, Wang L, Guo H, Kong S, Li W, He
Q, Ding L and Yang B: The role of Tim-3 blockade in the tumor
immune microenvironment beyond T cells. Pharmacol Res.
209:1074582024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
van Laarhoven HWM and Derks S: The success
of anti-PD-1 with chemotherapy for esophageal squamous cell
carcinoma. Cell Rep Med. 4:1009902023. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang ZX, Cui C, Yao J, Zhang Y, Li M, Feng
J, Yang S, Fan Y, Shi J, Zhang X, et al: Toripalimab plus
chemotherapy in treatment-naïve, advanced esophageal squamous cell
carcinoma (JUPITER-06): A multi-center phase 3 trial. Cancer Cell.
40:277–288. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
He M, Wang Z, Lu J, Bai Y, Mao T, Wang J,
Fan Q, Zhang Y, Zhao K, Chen Z, et al: Final analysis of
camrelizumab plus chemotherapy for untreated advanced or metastatic
esophageal squamous cell carcinoma: The ESCORT-1st trial. Med.
5:1137–1149. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Xu M, Pu Y, Jiang Y, Liu H, Feng Y, Qian
C, Dai N and Li M: 1542P Updated results of a phase II clinical
trial: Paclitaxel and carboplatin plus PD-1 blockades combined with
anlotinib as first-line treatment for advanced esophageal cancer.
ESMO. 34:S8662023.
|
|
84
|
Meng X, Wu T, Hong Y, Fan Q, Ren Z, Guo Y,
Yang X, Shi P, Yang J, Yin X, et al: Camrelizumab plus apatinib as
second-line treatment for advanced oesophageal squamous cell
carcinoma (CAP 02): A single-arm, open-label, phase 2 trial. Lancet
Gastroenterol Hepatol. 7:245–253. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhang B, Qi L, Wang X, Xu J, Liu Y, Mu L,
Wang X, Bai L and Huang J: Phase II clinical trial using
camrelizumab combined with apatinib and chemotherapy as the
first-line treatment of advanced esophageal squamous cell
carcinoma. Cancer Commun (Lond). 40:711–720. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang R, Ling Y, Chen B, Zhu Y, Hu Y, Liu
M, Yang Y, Zhang L, Lv Y, Liu S, et al: Long-term survival and
post-hoc analysis of toripalimab plus definitive chemoradiotherapy
for oesophageal squamous cell carcinoma: Insights from the
EC-CRT-001 phase II trial. EClinicalMedicine. 75:1028062024.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wu L, Wang Y, Li B, Wan G, Liang L, Li T,
Lang J and Wang Q: Toripalimab in combination with induction
chemotherapy and subsequent chemoradiation as first-line treatment
in patients with advanced/metastatic esophageal carcinoma: Protocol
for a single-arm, prospective, open-label, phase II clinical trial
(TR-EAT). Front Oncol. 12:8788512022. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Xu X, Sun Z, Liu Q, Zhang Y, Shen L, Zhang
C, Lin H, Hu B, Rong L, Chen H, et al: Neoadjuvant
chemoradiotherapy combined with sequential perioperative
toripalimab in locally advanced esophageal squamous cell cancer. J
Immunother Cancer. 12:e0086312024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
To SY, Lee CH, Chen YH, Hsu CL, Yang HW,
Jiang YS, Wen YL, Chen IW and Kao LT: Psoriasis risk with immune
checkpoint inhibitors. JAMA Dermatol. 161:31–38. 2024. View Article : Google Scholar
|
|
90
|
Noseda R, Bedussi F, Giunchi V, Fusaroli
M, Raschi E and Ceschi A: Reporting of late-onset immune-related
adverse events with immune checkpoint inhibitors in VigiBase. J
Immunother Cancer. 12:e0099022024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Yue G, Tang J, Zhang L, Niu H, Li H and
Luo S: CD276 suppresses CAR-T cell function by promoting tumor cell
glycolysis in esophageal squamous cell carcinoma. J Gastrointest
Oncol. 12:38–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Cheng C, Cui H, Liu H, Wu Y, Ding N, Weng
Y, Zhang W and Cui Y: Role of epidermal growth factor
receptor-specific CAR-T cells in the suppression of esophageal
squamous cell carcinoma. Cancers. 14:60212022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang L, Wang X, Wu Y, Wang J, Zhou W, Wang
J, Guo H, Zhang N, Zhang L, Hu X, et al: A novel microenvironment
regulated system CAR-T (MRS.CAR-T) for immunotherapeutic treatment
of esophageal squamous carcinoma. Cancer Lett. 568:2163032023.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Shi H, Yu F, Mao Y, Ju Q, Wu Y, Bai W,
Wang P, Xu R, Jiang M and Shi J: EphA2 chimeric antigen
receptor-modified T cells for the immunotherapy of esophageal
squamous cell carcinoma. J Thorac Dis. 10:2779–2788. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Makino T, Miyata H, Yasuda T, Kitagawa Y,
Muro K, Park JH, Hikichi T, Hasegawa T, Igarashi K, Iguchi M, et
al: A phase 3, randomized, double-blind, multicenter,
placebo-controlled study of S-588410, a five-peptide cancer vaccine
as an adjuvant therapy after curative resection in patients with
esophageal squamous cell carcinoma. Esophagus. 21:447–455. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Iinuma H, Fukushima R, Inaba T, Tamura J,
Inoue T, Ogawa E, Horikawa M, Ikeda Y, Matsutani N, Takeda K, et
al: Phase I clinical study of multiple epitope peptide vaccine
combined with chemoradiation therapy in esophageal cancer patients.
J Transl Med. 12:842014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yasuda T, Nishiki K, Hiraki Y, Kato H,
Iwama M, Shiraishi O, Yasuda A, Shinkai M, Kimura Y, Sukegawa Y, et
al: Phase II adjuvant cancer-specific vaccine therapy for
esophageal cancer patients curatively resected after preoperative
therapy with pathologically positive nodes; possible significance
of tumor immune microenvironment in its clinical effects. Ann Surg.
275:e155–e162. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Katsuda M, Iwahashi M, Matsuda K, Miyazawa
M, Nakamori M, Nakamura M, Naka T, Ojima T, Iida T and Yamaue H:
Peptide vaccine therapy with TLR-9 agonist for patients with
esophageal squamous cell carcinoma. Gan To Kagaku Ryoho.
38:1942–1944. 2011.(In Japanese). PubMed/NCBI
|
|
99
|
Wang B, Peng X, Li J, Wang Y, Chen L, Wu
M, Zhang Y, Wang W, Feng D, Tang S, et al: Personalized mRNA
vaccine combined with PD-1 inhibitor therapy in a patient with
advanced esophageal squamous cell carcinoma. Am J Cancer Res.
14:3896–3904. 2024. View Article : Google Scholar : PubMed/NCBI
|