Introduction
In 2022, 20 million new cases of cancer and 9.7
million cancer-related mortality occurred worldwide (1). According to the Global Report 2022,
2,041,910 new cases of cancer and 618,120 cancer-related mortality
cases were predicted in the United States in 2025 (2). Treating cancer remains a major
challenge. Early diagnosis and prevention of distant metastasis
remain difficult in the clinical diagnosis and treatment of cancer
(3). Therefore, additional in-depth
investigation of cancer pathogenesis and the identification of
molecular markers for early diagnosis are essential for the
clinical diagnosis and treatment of cancer. Recent studies on
microRNAs (miRNAs) have reported that they can serve important
roles in cancer development. Specifically, they can function either
as tumor suppressors or oncogenes in the proliferation, metastasis
and invasion of cancer. In turn, they can be associated with the
diagnosis, prognosis and treatment of cancer (4,5).
miRNA is a single-stranded non-coding RNA molecule
that is ~22 nucleotides in length, which is widely found in
eukaryotes (6). miRNA mainly
negatively regulate gene expression by binding to the
3′-untranslated regions (UTR) of target mRNAs, which leads to the
degradation or translational repression of the latter (7). This process involves several steps: i)
In the nucleus, miRNA genes are transcribed into primary miRNAs by
RNA polymerase II, where primary miRNA are further processed into
precursor miRNA by the Drosha enzyme-DiGeorge syndrome critical
region 8 complex and transported to the cytoplasm (8); ii) in the cytoplasm, the precursor
miRNA is sheared by the Dicer enzyme into a mature double-stranded
miRNA of ~22 nucleotides in length, where one strand of the mature
double-stranded miRNA (the guide strand) is subsequently loaded
into the Argonaute protein to form an RNA-induced silencing
complex; and iii) this complex binds to the 3′-UTR of the target
mRNA through sequence complementation, resulting in the degradation
of the mRNA and the formation of an RNA-induced silencing complex.
In this manner, mRNA degradation and translational repression is
achieved (8).
A number of studies have shown that miRNA-214-3p
expression is dysregulated in different cancers, where it can
regulate their progression by inhibiting the expression of its
target genes (9,10). In the present review, the role of
miRNA-214-3p in different types of cancer was summarized, whilst
also analyzing its potential effects on cancer chemotherapy,
targeted therapy and radiotherapy. In addition, the present review
aimed to analyze the potential of miRNA-214-3p as a biomarker and
its delivery strategy.
miRNA-214-3p regulation in cancer
Competitive endogenous RNA
(ceRNA)
Long-stranded non-coding RNA (lncRNA) and circular
RNAs (circRNA), the expression of which has also been found to be
dysregulated in cancer, can competitively bind to miRNA-214-3p
through the ceRNA mechanism, in turn promoting the expression of
its downstream target mRNAs. The circRNA nuclear factor IX (NFIX)
can bind to and inhibit the expression of miRNA-214-3p to
upregulate the expression of TP53 regulation of apoptosis inhibitor
1, which promotes lung cancer progression (11). This finding suggests that the
circRNA NFIX can target miRNA-214-3p to act as an oncogene in lung
cancer. In addition, circRNA 0038718 can target and inhibit
miRNA-214-3p function, which in turn inhibits breast cancer cell
proliferation and invasion (12),
suggesting that circRNA 0038718 can target miRNA-214-3p to serve as
a tumor suppressor.
DNA methylation
DNA methylation is an important epigenetic
modification that can regulate miRNA expression (13). In Pediatric central nervous system
germ cell tumors, the expression of miRNA-214-3p is negatively
correlated with its methylation status (14). Further studies revealed that the
expression of miRNA-214-3p is significantly upregulated when the
DNA demethylating agent 5-aza-2′-deoxycytidine was added (14).
Transcription factors
Twist1 is a transcription factor, which is a
transcription factor with a highly conserved basic helix-loop-helix
motif (15). In ovarian cancer,
Twist1 can positively regulate the level of miRNA-214-3p expression
(16).
Others
In medullary thyroid carcinoma, the expression of
miRNA-214-3p is downregulated under hypoxia (17).
Role of miRNA-214-3p in different types of
cancers
In humans, miRNA-214 is mapped to chromosome 1q24.3,
where the amplification of 1q24.3 has been associated with the
histological typing of liposarcoma (18). miRNA-214 cyclic precursor is sheared
by the Dicer enzyme to form miRNA-214-3p and miRNA-214-5p (19). miRNA-214-3p has been shown to be
involved in lung cancer (11,20),
nasopharyngeal carcinoma (21),
esophageal cancer (22,23), gallbladder cancer (24), colorectal cancer (25,26),
cervical cancer (9,27), endometrial cancer (28), prostate cancer (29,30),
leukaemia (31), medullary thyroid
cancer (17), retinoblastoma
(32) and Ewing sarcoma of bone
(33), where they appear to mainly
serve the role of tumor suppressor. In liver cancer (34–36),
gastric cancer (37,38), pancreatic cancer (39–41),
breast cancer (42,43), ovarian cancer (44,45),
renal cell carcinoma (46,47), glioma (48,49)
and osteosarcoma (50,51), miRNA-214-3p was found to mediate a
dual role of both oncogene and tumor suppressor. By contrast, in
bladder cancer (52) miRNA-214-3p
was found to function as an oncogene. miRNA-214-3p can regulate
various cellular processes, such as tumor cell proliferation and
metastasis, by targeting downstream target genes (Fig. 1). However, miRNA-214-3p itself can
also be regulated by various lncRNAs and circRNAs (Fig. 2). Table
I summarizes the role of miRNA-214-3p in different types of
cancers.
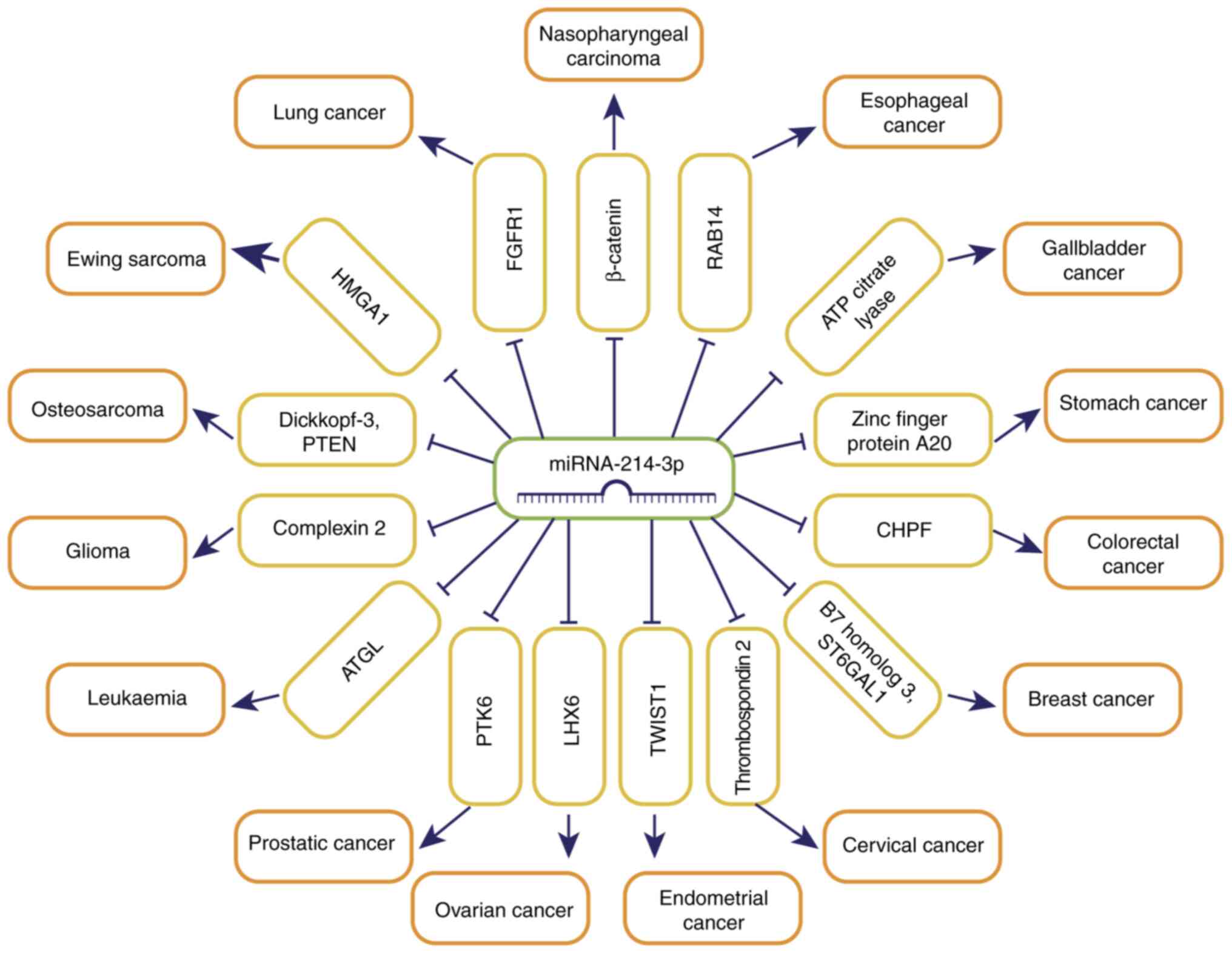 | Figure 1.miRNA-214-3p regulates tumor
progression by targeting downstream target genes. miRNA, microRNA;
FGFR1, fibroblast growth factor receptor 1; RAB14, Ras-related
protein 14; CHPF, Chondroitin polymerizing factor; ST6GAL1,
β-galactoside α-2,6-sialyltransferase 1; LHX6, LIM homeobox domain
6; PTK6, Protein Tyrosine Kinase 6; ATGL, Adipose triglyceride
lipase; HMGA1, high mobility group AT-hook 1. |
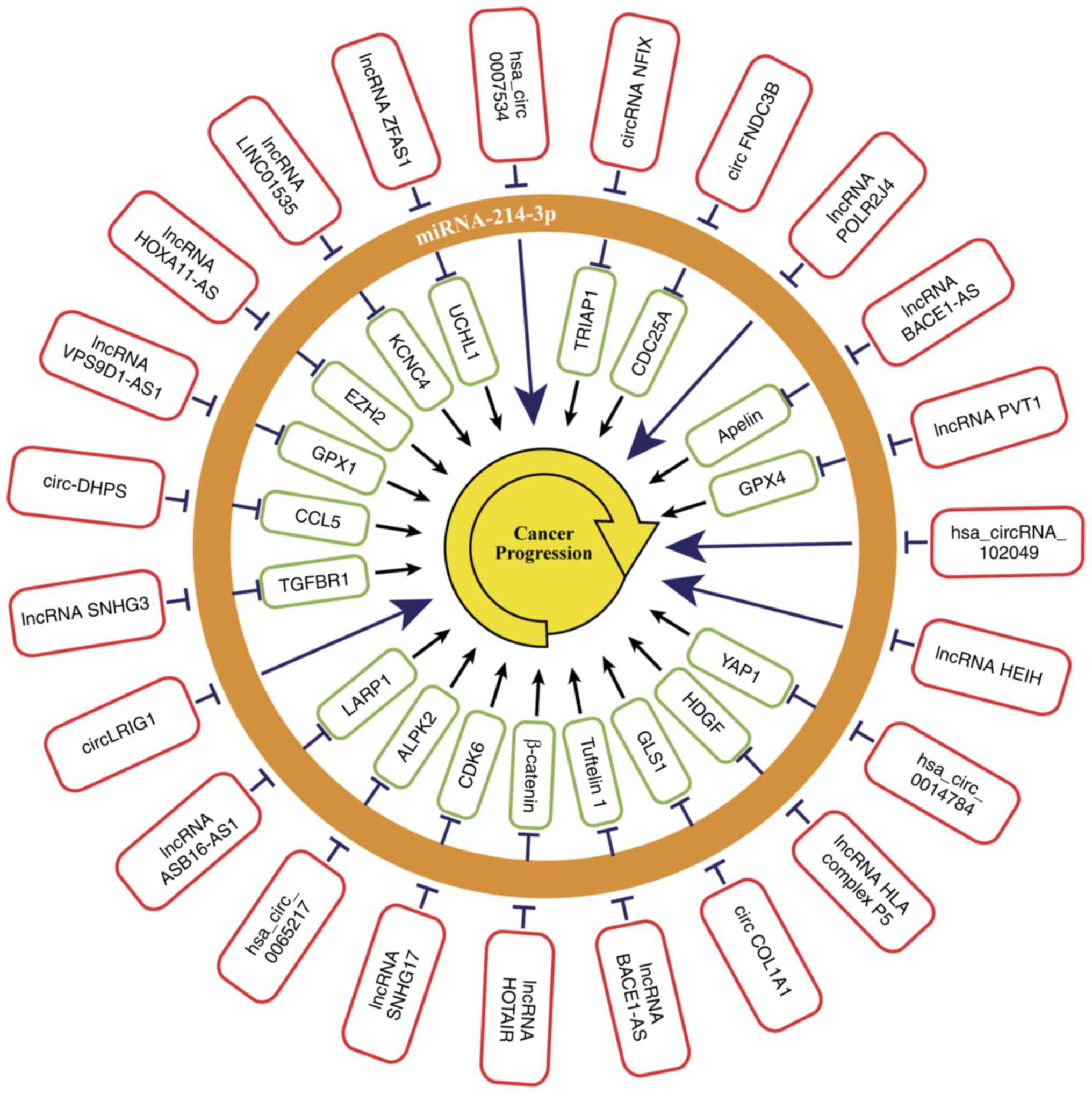 | Figure 2.miRNA-214-3p is regulated by a
variety of lncRNA and circRNA that affect miRNA-214-3p or
downstream target gene expression, which in turn regulates cancer
progression. miRNA, microRNA; circRNA, circular RNA; lncRNA, long
non-coding RNA; circRNA NFIX, circRNA nuclear factor IX; TRIAP1,
TP53 regulation of apoptosis inhibitor 1; circFNDC3B, Circ RNA
Fibronectin Type III Domain Containing 3B; CDC25A, cell division
cycle 25 homologue A; lncRNA POLR2J4, lncRNA RNA polymerase II
subunit J4 (pseudogene); lncRNA BACE1-AS, lncRNA β-secretase
1 antisense RNA; lncRNA PVT1, lncRNA plasmacytoma variant
translocation 1; GPX4, glutathione peroxidase 4; lncRNA HEIH,
hepatocellular carcinoma upregulated EZH2-associated lncRNA; YAP1,
yes-associated protein 1; HDGF, hepatoma-derived growth factor;
circ COL1A1, circ collagen, type I, α1; GLS1, glutaminase 1; lncRNA
HOTAIR, lncRNA HOX transcript antisense intergenic RNA; lncRNA
SNHG17, Small nucleolar RNA host gene 17; CDK6, cyclin-dependent
kinase 6; ALPK2, α-Protein Kinase 2; lncRNA ASB16-AS1, lncRNA
ankyrin repeat And SOCS Box-Containing 16 antisense RNA1; LARP1,
La-related protein 1; circLRIG1, circ Leucine-rich repeats and
immunoglobulin-like domains 1; lncRNA SNHG3, lncRNA small nucleolar
RNA host gene 3; TGFBR1, transforming growth factor β receptor 1;
circ-DHPS, circular RNA-deoxyhypusine synthase; CCL5, C-C motif
chemokine ligand 5; lncRNA VPS9D1-AS1, lncRNA VPS9D1 antisense RNA
1; GPX1, glutathione peroxidase 1; lncRNA HOXA11-AS, lncRNA HOXA11
antisense RNA; EZH2, enhancer of zeste homolog 2; KCNC4, Potassium
Voltage-Gated Channel Subfamily C Member 4; LncRNA ZFAS1, LncRNA
zinc finger antisense 1; UCHL1, Ubiquitin carboxyterminal hydrolase
L1. |
 | Table I.Summary of the role of miRNA-214-3p
in different types of cancers. |
Table I.
Summary of the role of miRNA-214-3p
in different types of cancers.
| Type | Upstream
regulator | Target | Biological
function | Role | (Refs.) |
|---|
| Lung cancer | circRNA NFIX | TRIAP1 | Inhibits tumor cell
proliferation and promotes apoptosis | Tumor
suppressor | (11) |
|
| - | FGFR1 | Inhibits tumor cell
proliferation, migration and invasion | Tumor
suppressor | (20) |
| Nasopharyngeal | - | β-catenin | Inhibits tumor cell
proliferation Carcinoma | Tumor
suppressor | (21) |
| Esophageal
cancer | circRNA FNDC3B | CDC25A | Inhibits tumor cell
proliferation, migration and invasion | Tumor
suppressor | (22) |
|
| - | RAB14 | Inhibits tumor cell
growth, migration and invasion | Tumor
suppressor | (23) |
| Liver cancer | LncRNA
BACE1-AS | Apelin | Inhibits tumor cell
proliferation, migration, invasion and promotes apoptosis | Tumor
suppressor | (34) |
|
| LncRNA PVT1 | GPX4 | Inhibits tumor cell
viability and promotes iron death | Tumor
suppressor | (35) |
|
|
Hsa_circRNA_102049 | - | Inhibits cellular
sensitivity to sorafenib | Oncogene | (36) |
| Gallbladder
cancer | - | ATP citrate
lyase | Inhibits tumor cell
proliferation and migration | Tumor
suppressor | (24) |
| Gastric cancer | - | Zinc finger protein
A20 | Attenuating the
anti-angiogenic effect of apatinib on tumor cells | Oncogene | (37) |
|
| LncRNA HEIH | - | Inhibits tumor cell
proliferation, migration and invasion | Tumor
suppressor | (38) |
| Pancreatic
cancer | Hsa_circ_0014
784 | YAP1 | Inhibits tumor cell
proliferation, migration, EMT and tumor angiogenesis | Tumor
suppressor | (39) |
|
| LncRNA HLA complex
P5 | HDGF | Inhibits tumor cell
proliferation, migration and invasion | Tumor
suppressor | (40) |
|
| - | - | Promoting stellate
cell proliferation in pancreatic cancer | Oncogene | (41) |
| Colorectal
cancer | LncRNA
BACE1-AS | Tuftelin 1 | Inhibits tumor cell
invasion, migration and liver metastasis | Tumor
suppressor | (25) |
|
| - | CHPF | Inhibits glycolysis
and promotes cellular iron death in tumor cells | Tumor
suppressor | (26) |
| Breast cancer | - | B7 homolog 3 | Enhancing the tumor
immune microenvironment and inhibiting tumor cell
proliferation | Tumor
suppressor | (42) |
|
| - | ST6GAL1 | Promote tumor cell
viability, migration and invasion | Oncogene | (43) |
| Cervical
cancer | - | Thrombosp ondin
2 | Inhibits tumor cell
viability, invasion and metastasis | Tumor
suppressor | (9) |
|
| LncRNA HOTAIR | β-catenin | Inhibits tumor cell
proliferation and promotes apoptosis | Tumor
suppressor | (27) |
| Endometrial
carcinoma |
| TWIST1 | Inhibits tumor cell
migration, invasion and EMT | Tumor
suppressor | (28) |
| Ovarian cancer | LncRNA SNHG17 | CDK6 | Inhibits tumor cell
growth | Tumor
suppressor | (44) |
|
| - | LHX6 | Promote tumor cell
proliferation and inhibit apoptosis | Oncogene | (45) |
| Renal cell
cancer | Hsa_circ_0065
217 | ALPK2 | Inhibits tumor cell
proliferation and invasion | Tumor
suppressor | (46) |
|
| LncRNA
ASB16-AS1 | LARP1 | Promotes tumor cell
proliferation, migration and invasion | Oncogene | (47) |
| Bladder cancer | CircRNA LRIG1 | - | Promotes tumor cell
growth, migration, invasion and inhibits apoptosis | Oncogene | (52) |
| Prostate
cancer | circRNA DHPS | CCL5 | Inhibiting
osteoblastic metastasis of tumor cells | Tumor
suppressor | (29) |
|
| - | PTK6 | Inhibits tumor cell
growth and EMT | Tumor
suppressor | (30) |
| Leukaemia | - | ATGL | Inhibits tumor cell
growth | Tumor
suppressor | (31) |
|
| LncRNA
HOXA11-AS | EZH2 | Inhibits tumor cell
growth and metastasis | Tumor
suppressor | (48) |
| Glioma |
| Complexin 2 | Inhibits tumor cell
sensitivity to temozolomide | Oncogene | (49) |
|
| - | Dickkopf-3 | Promotes tumor cell
viability, migration, invasion and inhibits apoptosis | Oncogene | (50) |
| Osteosarcoma | LncRNA
LINC01535 | KCNC4 | Inhibits tumor cell
proliferation, migration, invasion and promotes apoptosis | Tumor
suppressor | (51) |
| Medullary thyroid
cancer | LncRNA ZFAS1 | UCHL1 | Inhibits tumor cell
proliferation and invasion | Tumor
suppressor | (17) |
| Retinoblastoma |
Hsa_circ_0007534 | - | Inhibits tumor cell
viability, proliferation, colony formation and promotes
apoptosis | Tumor
suppressor | (32) |
| Ewing sarcoma | - | HMGA1 | Inhibits tumor cell
growth and migration | Tumor
suppressor | (33) |
Lung cancer
Lung cancer accounts for ~12.4% of all cancers and
can be divided into small-cell lung cancer and non-small cell lung
cancer (NSCLC), with NSCLC accounting for 80–85% of all cases
(1). miRNA-214-3p is typically
downregulated in NSCLC, inhibition of which can promote cell
proliferation and inhibit apoptosis in NSCLC (11). Fibroblast growth factor receptor 1
(FGFR1) is a member of FGFR that promotes cancer metastasis and
drug resistance through epithelial-mesenchymal transition (EMT)
(53). miRNA-214-3p can directly
target the 3′-UTR of FGFR1 to inhibit EMT and the Wnt/MAPK/AKT
pathways in NSCLC cells, which in turn inhibits cell proliferation,
migration and invasion (20). These
studies highlight the role of miRNA-214-3p in inhibiting the
progression of lung cancer.
Nasopharyngeal cancer (NPC)
Nasopharyngeal cancer (NPC) is a malignant
epithelial tumor that has a subtle onset and is prone to metastasis
(54). Although radiotherapy
combined with chemotherapy for NPC can produce a 5-year survival
rate of 85–90%, but recurrence and metastasis can still occur in
8–10% patients (55). Therefore, it
is important to assess its pathogenesis and novel therapeutic
targets for NPC. Bisphenol A was previously found to induce NPC
cell proliferation by inhibiting the expression of miRNA-214-3p,
leading to the upregulation of β-catenin expression (21). These findings indicate that
miRNA-214-3p can function as a tumor suppressor in NPC.
Esophageal cancer
Esophageal cancer is a common tumor occurring in the
digestive system (1). CircRNA
fibronectin type III domain-containing 3B (circFNDC3B) can sponge
miRNA-214-3p to upregulate the expression of cell division cycle 25
homologue A (CDC25A), which promotes esophageal cancer cell
proliferation, migration and invasion (22). Therefore, restoring the expression
of miRNA-214-3p or targeting the circFNDC3B/miRNA-214-3p/CDC25A
axis may be a promising therapeutic approach for esophageal cancer
(22). Additionally, miRNA-214-3p
expression was observed to be significantly lower in esophageal
cancer cells and tissues compared with that in non-malignant
oesophageal tissues (23). In
addition, overexpression of miRNA-214-3p was able to inhibit the
ability of esophageal cancer cells to proliferate, migrate and
invade, thereby exerting an oncogenic effect by targeting
Ras-related protein 14 (23). These
findings suggest the tumor-suppressive role of miRNA-214-3p in
esophageal cancer, where that restoring its expression may be a
viable therapeutic approach.
Liver cancer
Hepatocellular carcinoma (HCC) is the most common
primary liver cancer (56). Its
incidence is the highest in Asia, where it accounts for ~72% of all
global cases (56). Ji et al
(57) shown that the expression of
miRNA-214-3p is reduced in HCC cells and tissues compared with that
in normal tissues, cells and non-hepatitis B virus-infected cells.
miRNA-214-3p is a downstream target of the lncRNA polymerase (RNA)
II subunit J4, pseudogene β-secretase (BACE1) antisense RNA
(BACE1-AS) and plasmacytoma variant translocation 1, which serve as
oncogenes by promoting the progression of HCC. The overexpression
of miRNA-214-3p can counteract the oncogenic effects of these three
aforementioned lncRNAs. This suggests the tumor suppressor role of
miRNA-214-3p in HCC (34,35,37).
However, hsa_circRNA_102049 can bind to miRNA-214-3p to upregulate
reelin expression, which promoted the sensitivity of HCC cells to
sorafenib (36). Therefore,
miRNA-214-3p may yet serve as an oncogene and a tumor suppressor in
HCC. This dual-identity appear likely to be associate with whether
its upstream regulators and downstream target genes are oncogenes
or tumor suppressors.
Gallbladder cancer
Gallbladder cancer is a highly aggressive malignancy
that is prone to liver metastasis and lymphatic metastasis
(58). Liu et al (24) previously reported that human
umbilical cord mesenchymal stem cells-derived exosomal miRNA-214-3p
can targets ATP citrate lyase whilst downregulating the expression
of glucose transporters 1, which then inhibits the proliferation
and migration of gallbladder cancer cells (24). These findings indicate that stem
cell exosomes may be a treatment option for gallbladder cancer.
Gastric cancer
Gastric cancer is a malignant tumor that is subtle
and prone to metastasis (2). In
gastric cancer vascular endothelial cells, exosomal miRNA-214-3p
can reverse the anti-angiogenic effect of apatinib by inhibiting
the ferroptosis pathway toward (through zinc finger protein A20)
(37). However, Jiang et al
(38) found opposite trends in the
same cancer. Specifically, the lncRNA hepatocellular carcinoma
up-regulated EZH2 (HEIH) can bind to miRNA-214-3p, where the
overexpression of miRNA-214-3p can reverse the effects of lncRNA
HEIH in promoting the proliferation, migration and invasion of
gastric cancer cells, highlighting the possible anticancer effects
of miRNA-214-3p (38). These
studies suggest that miRNA-214-3p can serve as both an oncogene and
a tumor suppressor in gastric cancer, which may be associated with
the cancer secretion of exosomes for carcinogenesis.
Pancreatic cancer
Pancreatic cancer is one of the leading causes of
cancer-related mortality (59).
Yes-associated protein 1 (YAP1) is a downstream target of the
hsa_circ_0014784/miRNA-214-3p axis, where silencing the
miRNA-214-3p was found to promote YAP1 expression, which in turn
promoted pancreatic cancer cell proliferation, migration, EMT and
tumor angiogenesis (39).
Anti-tumor cell angiogenesis is also important to improve therapy
in pancreatic cancer. Inhibiting the expression of vascular
endothelial growth factor receptor (VEGFR)-2 can inhibit pancreatic
cancer invasion (60). In addition,
the lncRNA human leukocyte antigen complex P5 can competitively
bind to miRNA-214-3p to upregulate the expression of
hepatoma-derived growth factor, which can promote pancreatic cancer
proliferation, migration and invasion (40). Both of the aforementioned studies
therefore support the anticancer role of miRNA-214-3p in pancreatic
cancer.
However, another previous study (41) revealed that miRNA-214-3p can promote
the proliferation of pancreatic cancer stellate cells and that the
activation of stellate cells can results in the release of various
cytokines (such as hepatocyte growth factor, basic fibroblast
growth factor and IL-8) to promote the progression of pancreatic
cancer (41,61,62).
Therefore, miRNA-214-3p may yet have dual roles as both an oncogene
and a tumor suppressor in pancreatic cancer.
Colorectal cancer
The major cause of death from colorectal cancer is
metastasis (63). The lncRNA BACE1
antisense RNA (BACE1-AS)/miRNA-214-3p/Tuftelin 1 axis and the circ
collagen type Iα1/miRNA-214-3p/glutaminase 1 axis are potential
treatment targets for colorectal cancer, whereby silencing
miRNA-214-3p expression was observed to promote colorectal cancer
progression (25,64). Chondroitin polymerizing factor
(CHPF) is a type II transmembrane protein that can promote the
progression of colorectal cancer (65). miRNA-214-3p can directly bind to the
3′-UTR of CHPF to inhibit its expression, which in turn increases
ferrous iron and reactive oxygen species levels to inhibit cellular
glycolysis. This resulted in ferroptosis in colorectal cancer cells
(26). These findings suggest the
existence of a therapeutic miRNA-214-3p/CHPF pathway. Therefore,
miRNA-214-3p likely functions as a tumor suppressor in colorectal
cancer.
Breast cancer
Breast cancer is a common malignancy in women, From
1990 to 2019, the global incidence of breast cancer in young women
increased from 89,174 to 168,776 cases (66). miRNA-214-3p was previously found to
promote the killing of breast cancer cells by CD8+ T cells and
natural killer cells by targeting B7 homolog 3 (42). These findings indicate that
miRNA-214-3p can inhibit the progression of breast cancer cells by
favoring a more hostile tumor microenvironment. In another study,
berberine was reported to inhibit the proliferation, migration and
invasion of triple-negative breast cancer cells (67). The mechanism involved the
upregulation of miRNA-214-3p expression by berberine, which
suppressed secretin expression (67). These studies support the cancer
suppressive role of miRNA-214-3p.
However, Tao et al (43) reported that miRNA-214-3p expression
is elevated in both triple-negative breast cancer cells and tissues
comparison with that in their normal adjacent tissues.
miRNA-214-3p, which targets ST6 β-galactoside
α-2,6-sialyltransferase 1, can increase breast cancer cell
viability, migration and invasion (43). The dual role of miRNA-214-3p in
breast cancer may be associated with the complex network of
molecular interactions and different functions of downstream target
genes in cancer cells.
Cervical cancer
In total, 760,000 new cases of cervical cancer and
411,000 mortality cases are predicted to occur globally in 2030
(68). miRNA-214-3p was found to be
expressed at low levels in patients with cervical cancer, where
this low expression of miRNA-214-3p was suggested to promote its
progression (9). Mechanistically,
miRNA-214-3p can directly target thrombospondin 2 to inhibit
cervical cancer cell viability, invasion and metastasis (9). In addition, the lncRNA HOX transcript
antisense intergenic RNA can sponge miRNA-214-3p to upregulate
β-catenin expression, which promoted cervical cancer cell
proliferation and inhibited apoptosis (27). This suggests that miRNA-214-3p serve
the role of a tumor suppressor in cervical cancer.
Endometrial cancer
Endometrial cancer is a malignancy of the female
reproductive system (69). TWIST1
is an EMT-associated transcription factor that can promote
metastasis and maintains the stemness of cancer stem cells
(70). Fang et al (28) found that compared with that in
normal tissues and human endometrial epithelial cells, miRNA-214-3p
expression in endometrial cancer cells and tissue is downregulated
and that the overexpression of miRNA-214-3p can target TWIST1 to
inhibit endometrial cancer cell migration, invasion and EMT
(28). In addition, miRNA-214-3p is
inhibited by the lncRNA nuclear paraspeckle assembly transcript 1
(NEAT1), leading to the upregulation of high mobility group A1,
c-Myc and MMP-9 expression. This in turn promoted the
proliferation, invasion and metastasis of endometrial cancer cells
(71). These studies indicate that
miRNA-214-3p likely functions as a tumor suppressor in endometrial
cancer.
Ovarian Cancer
Ovarian cancer is a malignant tumor of the female
reproductive system, where its 5-year survival rate is <50%.
Therefore, it can gravely threaten the lives and health of women
(72). Liu et al (73) revealed that the lncRNA NEAT1 can
bind to miRNA-214-3p to promote angiogenesis and metastasis in
ovarian cancer cells (73).
Furthermore, miRNA-214-3p can be suppressed by the lncRNA small
nucleolar RNA host gene 17 (SNHG17). Specifically, since
cyclin-dependent kinase 6 (CDK6) is a downstream target of
miRNA-214-3p, CDK6 was found to mediate the pro-carcinogenic role
of lncRNA SNHG17 in ovarian cancer (44). These studies suggest the tumor
suppressor role of miRNA-214-3p.
However, Yang et al (45) previously reported a positive
association between serum exosomal miRNA-214-3p levels and
pathological malignancy degree of ovarian cancer. In patients with
ovarian cancer, miRNA-214-3p expression was increased ~7.9-fold in
borderline tissues, 21.8-fold in low-grade serous ovarian cancer
tissues and 31.8-fold in platinum-sensitive high-grade serous
ovarian cancer tissues compared with that in benign tissues
(45). The lncRNA X-inactive
specific transcript (XIST) can also serve an tumor suppressor role
in ovarian cancer by inhibiting the expression of miRNA-214-3p,
such that the overexpression of miRNA-214-3p can reverse the
anticancer effect of lncRNA XIST (74). Overall, miRNA-214-3p may yet serve a
dual-role as an oncogene and a tumor suppressor in ovarian
cancer.
Renal cell carcinoma (RCC)
RCC accounts for ~2% of all cancer diagnoses
worldwide (75). The most common
histopathological type of RCC is clear renal cell carcinoma
(75). Previous studies have shown
that hsa_circ_0065217 can inhibit miRNA-214-3p to upregulate
α-protein kinase 2 expression, which then promotes the
proliferation and invasion of RCC (46). However, lncRNA ankyrin repeat and
SOCS box-containing 16-AS1 can inhibit the proliferation, migration
and invasion of clear renal cell carcinoma cells by sponging
miRNA-214-3p to upregulate the expression of LA-related protein 1
(47). The dual role of
miRNA-214-3p in RCC may be associated with its different upstream
regulators and different downstream targets.
Bladder cancer
Bladder cancer is a common urological malignancy,
ranking fourth in cancer incidence among men in the United States
in 2024 (69). The expression of
miRNA-214-3p was found to be significantly upregulated in bladder
cancer tissues (52). Circ
leucine-rich repeats and Ig-like domains protein 1 can target
miRNA-214-3p to increase E-cadherin expression, which downregulates
the protein expression levels of N-cadherin and Vimentin. This in
turn inhibited the proliferation, migration and invasion of bladder
cancer cells and promoted their apoptosis (52). These findings indicate that
miRNA-214-3p serves an oncogenic role in bladder cancer.
Prostate cancer
Androgen deprivation therapy is the main treatment
for patients with advanced prostate cancer (76). Knocking down androgen receptor
expression was revealed to upregulate the expression of circular
RNA-deoxyhypusine synthase (circ-DHPS) in prostate cancer cells
(29). Circ-DHPS can bind to
miRNA-214-3p to upregulate the expression of C-C motif chemokine
ligand 5, thereby promoting the metastasis of prostate cancer cells
to osteoblasts (29). In addition,
the lncRNA small nucleolar RNA host gene 3 (SNHG3) can
competitively bind to miRNA-214-3p, which upregulates the
expression of TGF-β receptor 1 and activates its signaling pathway,
in turn promoting the bone metastasis of prostate cancer cells
(77). These studies emphasize the
anticancer role of miRNA-214-3p in prostate cancer.
Leukemia
Leukemia is a hematological malignancy that includes
acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL)
and chronic myeloid leukemia (78).
miRNA-214-3p is typically expressed at low levels in T-cell ALL
(T-ALL), which may be associated with the development of T-ALL
(79). The lncRNA VPS9D1 antisense
RNA 1 (VPS9D1-AS1) can bind to miRNA-214-3p to upregulate
glutathione peroxidase 1 expression, which in turn promotes the
proliferation of ALL cells whilst inhibiting apoptosis (80). Additionally, miRNA-214-3p can
inhibit adipose triglyceride lipase expression, downregulate
peroxisome proliferator-activated receptor α expression and inhibit
the production of diacylglycerol and free fatty acids, thereby
inhibiting the proliferation of AML cells (31). These studies suggest that
miRNA-214-3p primarily functions as a tumor suppressor in
leukemia.
Glioma
Glioma is a common malignancy of the central nervous
system (81). The lncRNA homeobox
A11 (HOXA11)-AS has been reported to be highly expressed in
glioblastoma, which is associated with poor prognosis (48). The lncRNA HOXA11-AS can bind to
miRNA-214-3p to upregulate the expression of enhancer of zeste
homolog 2, which in turn promotes the proliferation and metastasis
of glioma cells (48). However, the
expression of miRNA-214-3p is elevated in temozolomide-resistant
glioma compared with that in temozolomide-sensitive tissues
(49). In particular, inhibiting
the expression of miRNA-214-3p was found to promote the sensitivity
of glioma cells to temozolomide, which inhibited cell proliferation
and promoted apoptosis. The underlying mechanism was associated
with miRNA-214-3p targeting Complexin 2 (49). Therefore, the miRNA-214-3p duality
in glioma is likely to be dependent on its upstream regulators and
corresponding downstream targets.
Osteosarcoma
Osteosarcoma is high-grade malignant bone tumor that
commonly develops in adolescents and has a poor prognosis, with
rapid proliferation, high mortality and high chances of disability
(82). miRNA-214-3p was observed to
be highly expressed in osteosarcoma tissues and cells (50). It can promote the viability,
migration and invasion of osteosarcoma cells whilst inhibiting
apoptosis by binding to the 3′-UTR of Dickkopf-3 and activating the
Wnt/β-catenin/lymphoid enhancer-binding factor 1 signaling pathway
(50). Li et al (83) previously found that miRNA-214-3p can
also target PTEN, enhancing osteosarcoma cell viability and
inhibiting apoptosis. By contrast, the long intergenic non-coding
RNA 01535 can inhibit the expression of miRNA-214-3p to upregulate
potassium voltage-gated channel subfamily C member 4 expression,
which promoted cell proliferation, migration and invasion, whilst
inhibiting apoptosis (51). These
studies suggest that miRNA-214-3p also has a dual role as both an
oncogene and a tumor suppressor in osteosarcoma.
Other cancers
LncRNA zinc finger antisense 1 has been demonstrated
to serve as an miRNA-214-3p sponge to upregulate ubiquitin
carboxyterminal hydrolase L1 expression, which promoted the
proliferation and invasion of medullary thyroid cancer cells
(17). In retinoblastoma, elevated
levels of miRNA-214-3p may serve a tumor-suppressing effect
(32). In another study, osthole
(Chinese Herbal Extract of Cnidium Officinale) was found to inhibit
the PI3K/AKT/mTOR pathway by decreasing hsa_circ_0007534 expression
and increasing the level of miRNA-214-3p expression, which
suppressed the viability, proliferation and colony formation
ability of retinoblastoma cells and promoted their apoptosis
(32). Additionally, miRNA-214-3p
can repress high mobility group AT-hook 1 expression and inhibit
the proliferation and migration of bone Ewing sarcoma cells
(33).
miRNA-214-3p as a biomarker
With technological advancements, the detection and
quantification of miRNA have become more efficient (84), such that a multitude of studies have
identified miRNA-214-3p as having the potential to serve as a
biomarker for cancer diagnosis, prognosis and therapeutic response
(Table II) (10,45,85).
 | Table II.miRNA-214-3p as biomarkers. |
Table II.
miRNA-214-3p as biomarkers.
| Type | Expression | Indication | (Refs.) |
|---|
| Lung cancer | Down | Low expression is
associated with poor prognosis | (10) |
| Prostate
cancer | Down | Low expression is
associated with poor prognosis | (30) |
| Ovarian cancer | Up | Positive
association between serum exosomal miRNA-214-3p levels and
pathological malignancy degree of ovarian cancer | (45) |
| Osteosarcoma | Up | High expression is
associated with poor prognosis | (50) |
| Breast cancer | Up | High expression
positively associates with chemotherapy resistance | (85) |
Diagnostic biomarkers
Yang et al (45) revealed that miRNA-214-3p is highly
expressed in exosomes of ovarian cancer tissues. However,
miRNA-214-3p expression was increased 7.9-fold in borderline
tissues, 21.8-fold in low-grade serous ovarian cancer tissues and
31.8-fold in platinum-sensitive high-grade serous ovarian cancer
tissues compared with that in benign tissues (45). Receiver operating characteristic
curve results in another previous study suggested that four miRNAs
(including miRNA-214-3p) can be used to distinguish between
patients with HCC and non-HCC patients. In addition, miRNA-214-3p
alone could distinguish between patients with HCC and patients with
chronic hepatitis B or normal healthy individuals, but could not
accurately distinguish between patients with HCC and those with
cirrhosis, which may be due to the smaller sample size of patients
with cirrhosis in that particular study (86). These findings suggest that
miRNA-214-3p can be used as a diagnostic biomarker for ovarian and
liver cancers.
Prognostic biomarkers
Low expression of miRNA-214-3p has been shown to be
associated with poor prognosis in patients with colorectal cancer
(87), liver cancer (88), prostate cancer (30) and lung cancer (10). By contrast, in NPC, high expression
of miRNA-214-3p is associated with tumor recurrence and metastasis
(89). Unfortunately, this study
(89) did not go further to
overexpress or knockdown the expression of miRNA-214-3p in NPC
cells. Therefore, the effect of miRNA-214-3p expression on various
processes, such as proliferation, migration and apoptosis in NPC
cells, was not investigated (89).
In another previous study, a Cox regression model consisting of
miRNA-199a-3p, miRNA-214-3p and three clinicopathological factors
was used to predict overall survival in patients undergoing radical
cystectomy, where the hazard ratio (95% CI) for miRNA-214-3p is
3.30 (1.11–9.77), P=0.031 (90).
Therefore, clinicians can predict the prognosis of patients with
colorectal cancer, liver cancer, prostate cancer, lung cancer, NPC
and bladder cancer based on the level of expression of
miRNA-214-3p, which may be beneficial for the timely intervention
in these patients.
Treatment response biomarkers
The therapeutic response can affect the prognosis
and survival of patients with cancer. Therefore, it is necessary to
identify cancer therapeutic response biomarkers. Xing et al
(85) previously found that high
expression of miRNA-214-3p was associated with chemotherapy
resistance in breast cancer, where a logistic regression signature
consisting of five miRNAs, including miRNA-214-3p, was more stable
compared with each single miRNA at accurately predicting
chemotherapy resistance in breast cancer (AUC=0.839; 95% CI,
0.730–0.949) (85). miRNA-214-3p
may therefore have clinical potential as a treatment response
biomarker for improving the individualized cancer treatment
protocols.
miRNA-214-3p in chemotherapy, targeted
therapy and radiotherapy
Chemotherapy, and radiotherapy are important tools
in cancer treatment. Drug resistance poses a major obstacle and is
primary cause of cancer recurrence and poor prognosis (91). Several studies have previously
suggested that miRNAs can serve a key role in regulating drug
resistance to cancer chemotherapy and radiotherapy (92–96),
to which miRNA-214-3p is no exception (Table III).
 | Table III.Effect of miRNA-214-3p on cancer
chemotherapy. |
Table III.
Effect of miRNA-214-3p on cancer
chemotherapy.
| Chemotherapeutic
drugs | Cancer | Role | Target | (Refs.) |
|---|
|
| Pediatric central
nervous system germ cell tumors | Induced cisplatin
resistance | Bcl2-like 11 | (14) |
|
| Ovarian cancer | Reduced cisplatin
sensitivity | - | (74) |
| Cisplatinum | Oral squamous cell
carcinoma | Inhibits cisplatin
resistance | PIM1 | (93) |
|
| Esophageal
cancer | Enhanced cisplatin
sensitivity | Survivin and
CUG-BP1 | (94) |
| Vincristine and
carboplatin | Retinoblastoma | Promotes
vincristine and carboplatin sensitivity | ABCB1 and XIAP | (96) |
| Temozolomide | Glioblastoma | Inhibits
temozolomide resistance | MGMT | (95) |
| Temozolomide | Glioma | Promotes
temozolomide resistance | CPLX2 | (49) |
| Gemcitabine | Pancreatic
cancer | Inhibits
gemcitabine resistance | HDGF | (40) |
Platinum-based chemotherapeutics
Common platinum-based chemotherapeutic agents
include cisplatin, carboplatin and oxaliplatin. Wang et al
(93) previously reported that
miRNA-214-3p can inhibit cisplatin resistance in oral squamous cell
carcinoma cells by targeting PIM1, a key promoter of
hypoxia-induced chemotherapy resistance (97). In pediatric central nervous system
germ cell tumor cells, high expression of miRNA-214-3p was found to
promote cisplatin resistance through a mechanism associated with
targeting BCL2-like 11, a pro-apoptotic protein, leading to the
suppression of apoptosis (14). In
esophageal cancer, miRNA-214-3p can bind to the 3′-UTR of survivin
and embryo deadenylation element-binding protein, an antiapoptotic
protein, to upregulate the expression of caspase-3 and promote
sensitivity to cisplatin (94). In
addition, miRNA-214-3p was observed to target ATP-binding cassette
subfamily B member 1 and X-linked inhibitor of apoptosis protein
(an antiapoptotic protein), promoting the sensitivity of
retinoblastoma to vincristine and carboplatin (96). These studies collectively suggest
that the anticancer efficacy of platinum-based chemotherapeutic
agents can be promoted by either increasing or decreasing the
expression of miRNA-214-3p. The mechanism by which miRNA-214-3p
regulates platinum-based chemotherapeutic agent sensitivity may be
associated with regulation of the tumor microenvironment and the
targeting of apoptotic proteins, since tumor microenvironment and
inactivation of the apoptotic pathways tended to cause chemotherapy
resistance in tumors (98).
Other chemotherapeutic agents
Liu et al (40) previously revealed that miRNA-214-3p
can target hepatoma-derived growth factor and promote the
sensitivity of pancreatic cancer cells to gemcitabine (40). In glioma, miRNA-214-3p was found to
promote resistance to temozolomide by targeting complexin 2
(49). Notably, in glioblastoma,
miRNA-214-3p can target O6-methylguanine-DNA methyltransferase to
reverse glioblastoma resistance to temozolomide (95).
Radiotherapy
Mesenchymal stem cell extracellular vesicular
miRNA-214-3p can ameliorate thoracic vascular injury, inflammatory
response and pulmonary fibrosis after radiotherapy, which in turn
attenuates lung injury caused by radiotherapy (99). Therefore, administering radiotherapy
for treating thoracic malignancies whilst increasing miRNA-214-3p
expression was proposed to be a strategy to attenuate
radiotherapy-induced lung injury (99).
Targeted therapy
Through the in-depth study of the molecular
mechanisms of tumor progression, targeted therapy has advanced
considerably and is becoming one of the primary modes of cancer
treatment (100). However,
acquired resistance poses a major dilemma for targeted therapeutics
(101). A previous study revealed
that miRNA-214-3p can target transducin (β)-like 1 X-linked
receptor 1 to promote the sensitivity of prostate cancer cells to
nituzumab (102). In addition,
miRNA-214-3p can target zinc finger protein A20 and inhibit the
anti-vascular effects of apatinib on gastric cancer vascular
endothelial cells (37).
Strategies for delivering miRNA-214-3p
Although miRNA have potential for disease therapy,
difficulties exist that limit the efficiency of their delivery.
miRNAs are readily degraded by some nucleases (103–105). Furthermore, their negative charge,
high molecular mass and hydrophilicity of nucleic acids renders it
difficult to cross cell membranes (106). Therefore, delivering miRNAs to
target cells efficiently and accurately remains a considerable
challenge for miRNA anti-tumor therapy. Viral and non-viral
nanocarrier systems have been developed for delivering
miRNA-214-3p, with non-viral carrier systems including exosomes and
nanocarrier systems.
Exosome carriers
Exosomes are nanoscale vesicles secreted by cells,
with diameters of 30–100 nm. They can transfer biologically active
components, such as proteins, miRNAs and mRNA, to recipient cells
(107). When exosomes interact
with surrounding cells, cell surface receptors are activated, where
vesicle contents can be translocated to the corresponding cells.
The lipid bilayer membrane of exosomes also prevents cargoes, such
as miRNAs, from being degraded, thereby exerting a regulatory
effect on target cells (107). Liu
et al (24) previously
reported that exosomes released from human umbilical cord
mesenchymal stem cells contained miRNA-214-3p. After adding
exosomes to gallbladder cancer cells, miRNA-214-3p can inhibit cell
proliferation by suppressing the expression of facilitative glucose
transporter 1 and ATP-citrate lyase (24).
However, in cases of exosomes of tumor origin that
can mediate miRNA expression, they may promote cancer progression
(108). Wang et al
(37) revealed that exosomes
secreted by gastric cancer contain miRNA-214-3p, which can be taken
up and enter vascular endothelial cells to increase their own
miRNA-214-3p expression, in turn increasing glutathione expression
and decreasing lipid reactive oxygen species production. This
culminated in reversing the anti-angiogenic effects of apatinib
(37).
It must be acknowledged that the functional role of
exosome-associated miRNA-214-3p is likely to be context-dependent,
potentially either promoting or inhibiting tumor progression
depending on its origin and target.
Viral vectors
Viral vectors commonly used to deliver miRNA
include lentiviruses, adenoviruses, adeno-associated viruses and
retroviruses (109). Phatak et
al (23) previously used
lentiviral vectors to deliver miRNA-214-3p into esophageal cancer
cells, which was followed by a significant increase in the
expression of miRNA-214-3p, which then targeted RAB14 to inhibit
the migration and invasion of esophageal cancer cells. Furthermore,
colorectal cancer cells with low miRNA-214-3p expression were
constructed using a lentiviral vector system to deliver an
inhibitor of miRNA-214-3p (87).
miRNA-214-3p inhibition promoted colorectal cancer cell
proliferation and metastasis, whereas overexpression of
miRNA-214-3p reversed this process (87). Although lentiviral vectors are
effective methods for delivering miRNA, their immunogenicity and
potential induction of mutations cannot be ignored. Viruses bind to
cell surface receptors through their envelope proteins (110). Lentiviruses are capable of
delivering vectors to both normal and tumor cells. Consequently,
standard lentiviral vectors lack tumor specificity and may infect
both healthy and malignant cells (110,111). However, when the selective
targeting of tumor cells is desired, this can be achieved by
modifying the viral receptor, either through pseudotyping or the
incorporation of receptor-specific ligands. Miletic et al
(112) designed lentiviral vectors
pseudotyped with lymphocytic choriomeningitis virus glycoproteins
that selectively targeted G62 human glioma cells (112). Therefore, viral delivery systems
for delivering miRNA-214-3p into cancer cells for anticancer
therapy remain desirable, but studies on this topic remain
limited.
Nanoparticles (NPs) vectors
Liposomes, inorganic nanoparticles and polymer
nanoparticles are commonly used carriers for delivering molecules
(113). Delivering nanoparticles
and cargoes specifically to tumor tissues remains a major challenge
in the nanoparticle transport field. NPs can bind specifically to
receptors overexpressed on cancer cells by targeting ligands
(surface functionalization of ligands, such as antibodies, peptides
and small molecules), ensuring the selective accumulation of
nanoparticles in the tumor microenvironment (114). Folate receptor is frequently
overexpressed in cancer, rendering it a common target for liposomal
nanoparticle delivery systems (115). Rong et al (116) previously developed lactobionic
acid-modified liposomal nanoparticles that efficiently delivered
sialic acid and miRNA-145 specifically to HCC cells expressing
salivary acid glycoprotein receptors, which promote apoptosis with
negligible side effects. Therefore, engineering the nanoparticle
ligands to specifically target tumors would facilitate NP and the
cargo accumulation at the tumor site.
A representative 3D DNA nanostructured material
known as tetrahedral framework nucleic acid (tFNA) consists of four
single-stranded DNAs of equal lengths, which has reported
advantages of satisfactory biocompatibility, editability, high
stability, low biotoxicity and ease of preparation (117). Survivin is an inhibitor of
apoptosis that is highly expressed only in tumor cells and
embryonic cells, whilst being largely undetectable in normal
tissues. Therefore, it was proposed as an anticancer target
(118). Li et al (119) previously modified miRNA-214-3p to
one of the vertices of tFNA and synthesized tFNA-miRNA-214-3p,
which was shown to target survivin in NSCLC cells and induce the
mitochondrial apoptotic pathway, in turn promoting apoptosis
(119). Although the delivery of
miRNAs using nanoparticles is a promising strategy for treating
cancer, its limitations cannot be ignored. It is difficult for
liposomes to load high quantities of therapeutic drugs into a lipid
matrix (120). Additionally,
polymer nanoparticles encapsulated with molecular drugs are
difficult allow prolonged release, and the biodegradation of
polymers can become cytotoxic (120). Similarly, the low solubility and
toxicity of inorganic nanoparticles remain major challenges that
need to be addressed (121).
Clinical significance and future
challenges
Abnormal proliferation, metastasis and invasion of
tumor cells are the three main features of cancer that can affect
the prognosis and treatment options for patients. Therefore, the
inhibition of tumor cell proliferation, metastasis and invasion is
key to halting cancer progression. However, the role of
miRNA-214-3p in cancer has been receiving attention. In lung
cancer, miRNA-214-3p was found to target FGFR1 to inhibit cell
proliferation, metastasis and invasion (20). Overexpression of miRNA-214-3p can
suppress drug resistance in retinoblastoma, which in turn promoted
apoptosis (96). Conversely, as an
oncogene, high expression of miRNA-214-3p can promote the
proliferation, migration and invasion of bladder cancer cells
(52). In addition, miRNA-214-3p
can affect the sensitivity of cancer cells to chemotherapy,
radiotherapy and targeted therapy. Therefore, regulating the
expression of miRNA-214-3p during cancer treatment can inhibit
cancer progression. miRNA-214-3p can also be used as a biomarker
for cancer diagnosis, prognosis and therapeutic response, which may
prove beneficial for early the diagnosis and personalized treatment
of cancer. The effective systemic delivery of miRNA-214-3p would
then avoid enzymatic hydrolysis and enables its stable expression
in tumor cells.
However, the role of miRNA-214-3p in cancer faces
challenges. The complexity of upstream regulators and downstream
targets of miRNA-214-3p allows it to serve a dual role as an
oncogene and a tumor suppressor in cancers dependent on the type
involved. The interactions among the multiple downstream target
genes of miRNA-214-3p warrant further investigated. Viral vectors
may also induce an immune response, leading to cytotoxic damage
after entering host cells. Additionally, the extensive nature of
miRNA regulation leads to unexpected regulatory effects that may
cause off-target effects and trigger the development of other
diseases (such as leukemia) (122). Therefore, the clinical application
of miRNA therapy may have promise at this stage, but its efficacy
and safety require further investigation.
Conclusions
In the present review, the role of miRNA-214-3p in
a range of cancers was summarized. The expression of miRNA-214-3p
can be affected by ceRNA, transcription factors, DNA methylation
and hypoxic conditions. miRNA-214-3p can serve as a tumor
suppressor in the majority of cancer types. In liver cancer,
gastric cancer, pancreatic cancer, breast cancer, ovarian cancer,
renal cell cancer, glioma and osteosarcoma, miRNA-214-3p can also
function as both an oncogene and a tumor suppressor. However, in
bladder cancer, miRNA-214-3p functions as an oncogene.
Additionally, miRNA-214-3p can serve as a biomarker for the
diagnosis, prognosis and therapeutic response of some types of
cancer. The expression of miRNA-214-3p can also affect sensitivity
of chemotherapy, radiotherapy and targeted therapy. Finally, the
systemic delivery strategy of miRNA-214-3p holds promise for
miRNA-based therapies for cancer. These findings provide novel
ideas for cancer treatment and drug development.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Guangxi Science and
Technology Programme Project (grant no. GuiKe AB20297002), Guangxi
University of Traditional Chinese Medicine Gui Pai Traditional
Chinese Medicine Inheritance Innovation Team Funding Project (grant
no. 2022B004) and Guangxi Natural Science Foundation (grant no.
2023GXNSFBA026066).
Availability of data and materials
Not applicable.
Authors' contributions
ZC wrote the manuscript and constructed figures and
tables. YL and LL reviewed and edited the manuscript. SL and HQ
analyzed the literature. XD and YQ conceptualized the review and
oversaw the process. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Siegel RL, Kratzer TB, Giaquinto AN, Sung
H and Jemal A: Cancer statistics, 2025. CA Cancer J Clin. 75:10–45.
2025.PubMed/NCBI
|
|
3
|
Jamal MH and Khan MN: Developments in
pancreatic cancer emerging therapies, diagnostic methods, and
epidemiology. Pathol Res Pract. 271:1560122025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fu Z, Zhou Y, Zhang Y, Zhou Z, Yu Y, Yuan
C, Dong J and Duan S: MicroRNA-325: A comprehensive exploration of
its multifaceted roles in cancer pathogenesis and therapeutic
implications (Review). Oncol Lett. 28:4592024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ameya KP, Ashikha Shirin Usman PP and
Sekar D: Navigating the tumor landscape: VEGF, MicroRNAs, and the
future of cancer treatment. Biochim Biophys Acta Gene Regul Mech.
1868:1950912025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Barbagallo D, Ponti D, Bassani B, Bruno A,
Pulze L, Akkihal SA, George-William JN, Gundamaraju R and
Campomenosi P: MiR-223-3p in cancer development and cancer drug
resistance: Same coin, different faces. Int J Mol Sci. 25:81912024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi JY, Seok HJ, Lee DH, Kwon J, Shin US,
Shin I and Bae IH: miR-1226-5p is involved in radioresistance of
colorectal cancer by activating M2 macrophages through suppressing
IRF1. J Transl Med. 22:9802024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hill M and Tran N: miRNA interplay:
Mechanisms and consequences in cancer. Dis Model Mech.
14:dmm0476622021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Xu J, Hua F, Wang Y, Fang G, Zhang
H and Wu X: MiR-214-3p suppresses cervical cancer cell metastasis
by downregulating THBS2. Cell Mol Biol (Noisy-le-grand).
69:195–200. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu T, Yang Y, Li Z and Lu S:
MicroRNA-214-3p inhibits the stem-like properties of lung squamous
cell cancer by targeting YAP1. Cancer Cell Int. 20:4132020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu G, Shi H, Zheng H, Kong W, Cheng X and
Deng L: Circular RNA NFIX functions as an oncogene in non-small
cell lung cancer by modulating the miR-214-3p/TRIAP1 axis. Clin
Respir J. 18:e138012024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ren J, Chen W, Zhou Y, Sun J and Jiang G:
The novel circRNA circ_0045881 inhibits cell proliferation and
invasion by targeting mir-214-3p in triple-negative breast cancer.
BMC Cancer. 24:2782024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhingardeve S, Sagvekar P, Desai S,
Mangoli V, Jagtap R and Mukherjee S: The regulatory interplay
between miRNA and DNA methylation orchestrates vital ovarian
functions and associated traits in PCOS. Gene. 940:1491652025.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsieh TH, Liu YR, Chang TY, Liang ML, Chen
HH, Wang HW, Yen Y and Wong TT: Global DNA methylation analysis
reveals miR-214-3p contributes to cisplatin resistance in pediatric
intracranial nongerminomatous malignant germ cell tumors. Neuro
Oncol. 20:519–530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mirjat D, Kashif M and Roberts CM: Shake
it up baby now: The changing focus on TWIST1 and epithelial to
mesenchymal transition in cancer and other diseases. Int J Mol Sci.
24:175392023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yin G, Chen R, Alvero AB, Fu HH, Holmberg
J, Glackin C, Rutherford T and Mor G: TWISTing stemness,
inflammation and proliferation of epithelial ovarian cancer cells
through MIR199A2/214. Oncogene. 29:3545–3553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen W, Wang S, Wei D, Zhai L, Liu L, Pan
C, Han Z, Liu H, Zhong W and Jiang X: LncRNA ZFAS1 promotes
invasion of medullary thyroid carcinoma by enhancing EPAS1
expression via miR-214-3p/UCHL1 axis. J Cell Commun Signal.
18:e120212024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tap WD, Eilber FC, Ginther C, Dry SM,
Reese N, Barzan-Smith K, Chen HW, Wu H, Eilber FR, Slamon DJ and
Anderson L: Evaluation of well-differentiated/de-differentiated
liposarcomas by high-resolution oligonucleotide array-based
comparative genomic hybridization. Genes Chromosomes Cancer.
50:95–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hou D, Wu Q, Wang S, Pang S, Liang H, Lyu
H, Zhou L, Wang Q and Hao L: Knockdown of miR-214 alleviates renal
interstitial fibrosis by targeting the regulation of the
PTEN/PI3K/AKT Knockdown of miR-214 alleviates renal interstitial
fibrosis by targeting the regulation of the PTEN/PI3K/AKT
signalling pathway. Oxid Med Cell Longev. 2022:75539282022.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Y, Li Z, Yuan H, Ji W, Wang K, Lu T,
Yu Y, Zeng Q, Li F, Xia W and Lu S: Reciprocal regulatory mechanism
between miR-214-3p and FGFR1 in FGFR1-amplified lung cancer.
Oncogenesis. 8:502019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zeng W: Bisphenol A triggers the
malignancy of nasopharyngeal carcinoma cells via activation of
Wnt/β-catenin pathway. Toxicol In Vitro. 66:1048812020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang J, Li X, Duan C and Jia Y: CircFNDC3B
knockdown restrains the progression of oesophageal squamous cell
carcinoma through miR-214-3p/CDC25A axis. Clin Exp Pharmacol
Physiol. 49:1209–1220. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phatak P, Burrows WM, Creed TM, Youssef M,
Lee G and Donahue JM: MiR-214-3p targets Ras-related protein 14
(RAB14) to inhibit cellular migration and invasion in esophageal
Cancer cells. BMC Cancer. 22:12652022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu L, Xiao W, Yang Z, Wang Q and Yi J:
Human umbilical cord mesenchymal stem cell-derived exosomal
miR-214-3p regulates the progression of gallbladder cancer by
regulating ACLY/GLUT1. Adv Clin Exp Med. 33:499–510. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang X, Liu Y, Zhou M, Yu L and Si Z: m6A
modified BACE1-AS contributes to liver metastasis and stemness-like
properties in colorectal cancer through TUFT1 dependent activation
of Wnt signaling. J Exp Clin Cancer Res. 42:3062023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yun ZY, Wu D, Wang X, Huang P and Li N:
MiR-214-3p overexpression-triggered chondroitin polymerizing factor
(CHPF) inhibition modulates the ferroptosis and metabolism in colon
cancer. Kaohsiung J Med Sci. 40:244–254. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhou Y, Wang Y, Lin M, Wu D and Zhao M:
LncRNA HOTAIR promotes proliferation and inhibits apoptosis by
sponging miR-214-3p in HPV16 positive cervical cancer cells. Cancer
Cell Int. 21:4002021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fang YY, Tan MR, Zhou J, Liang L, Liu XY,
Zhao K and Bao EC: miR-214-3p inhibits epithelial-to-mesenchymal
transition and metastasis of endometrial cancer cells by targeting
TWIST1. Onco Targets Ther. 12:9449–9458. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang Z, Chen JQ, Liu TJ, Chen YL, Ma ZK,
Fan YZ, Wang ZX, Xu S, Wang K, Wang XY, et al: Knocking down AR
promotes osteoblasts to recruit prostate cancer cells by altering
exosomal circ-DHPS/miR-214-3p/CCL5 pathway. Asian J Androl.
26:195–204. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cagle P, Smith N, Adekoya TO, Li Y, Kim S,
Rios-Colon L, Deep G, Niture S, Albanese C, Suy S, et al: Knockdown
of microRNA-214-3p promotes tumor growth and epithelial-mesenchymal
transition in prostate cancer. Cancers (Basel). 13:58752021.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Yuan S, Wu W, Zhou J, Zhang P, Li D,
Zhang Y and Lou S: The hsa-miR-214-3p/ATGL axis regulates aberrant
lipolysis to promote acute myeloid leukemia progression via PPARα
in vitro. Biochem Biophys Res Commun. 608:73–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lv X, Yang H, Zhong H, He L and Wang L:
Osthole exhibits an antitumor effect in retinoblastoma through
inhibiting the PI3K/AKT/mTOR pathway via regulating the
hsa_circ_0007534/miR-214-3p axis. Pharm Biol. 60:417–426. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
De Feo A, Pazzaglia L, Ciuffarin L,
Mangiagli F, Pasello M, Simonetti E, Pellegrini E, Ferrari C,
Bianchi G, Spazzoli B and Scotlandi K: miR-214-3p is commonly
downregulated by EWS-FLI1 and by CD99 and its restoration limits
ewing sarcoma aggressiveness. Cancers (Basel). 14:17622022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tian Q, Yan X, Yang L, Liu Z, Yuan Z and
Zhang Y: Long non-coding RNA BACE1-AS plays an oncogenic role in
hepatocellular carcinoma cells through miR-214-3p/APLN axis. Acta
Biochim Biophys Sin (Shanghai). 53:1538–1546. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He GN, Bao NR, Wang S, Xi M, Zhang TH and
Chen FS: Ketamine induces ferroptosis of liver cancer cells by
targeting lncRNA PVT1/miR-214-3p/GPX4. Drug Des Devel Ther.
15:3965–3978. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang S, Liu D, Wei H, Hua Y, Shi G and
Qiao J: The hsa_circRNA_102049 mediates the sorafenib sensitivity
of hepatocellular carcinoma cells by regulating Reelin gene
expression. Bioengineered. 13:2272–2284. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang W, Wang T, Zhang Y, Deng T, Zhang H
and Ba YI: Gastric cancer secreted miR-214-3p inhibits the
anti-angiogenesis effect of apatinib by suppressing ferroptosis in
vascular endothelial cells. Oncol Res. 32:489–502. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang L, Zhang L, Chen Q, Qiao S, Zhou F
and Han M: LncRNA HEIH promotes cell proliferation, migration and
invasion by suppressing miR-214-3p in gastric carcinoma. J Biochem.
169:535–542. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu B, Gong Y, Jiang Q, Wu S, Han B, Chen
F, Lin Q, Wang P and Yang D: Hsa_circ_0014784-induced YAP1 promoted
the progression of pancreatic cancer by sponging miR-214-3p. Cell
Cycle. 22:1583–1596. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu Y, Wang J, Dong L, Xia L, Zhu H, Li Z
and Yu X: Long noncoding RNA HCP5 regulates pancreatic cancer
gemcitabine (GEM) resistance by sponging Hsa-miR-214-3p to target
HDGF. Onco Targets Ther. 12:8207–8216. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kuninty PR, Bojmar L, Tjomsland V, Larsson
M, Storm G, Östman A, Sandström P and Prakash J: MicroRNA-199a and
−214 as potential therapeutic targets in pancreatic stellate cells
in pancreatic tumor. Oncotarget. 7:16396–16408. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lu Y, Wang K, Peng Y, Chen M, Zhong L,
Huang L, Cheng FU, Sheng X, Yang X, Ouyang M, et al: Hsa-miR-214-3p
inhibits breast cancer cell growth and improves the tumor immune
microenvironment by downregulating B7H3. Oncol Res. 33:103–121.
2024.PubMed/NCBI
|
|
43
|
Tao Y, Zhao Z, Ma J, Dong L, Liang Y, Li
S, Mao Y, Li Y and Zhang Y: MiR-214-3p regulates the viability,
invasion, migration and EMT of TNBC cells by targeting ST6GAL1.
Cytotechnology. 71:1155–1165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pan X, Guo Z, Chen Y, Zheng S, Peng M,
Yang Y and Wang Z: STAT3-Induced lncRNA SNHG17 exerts oncogenic
effects on ovarian cancer through regulating CDK6. Mol Ther Nucleic
Acids. 22:38–49. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang C, Kim HS, Park SJ, Lee EJ, Kim SI,
Song G and Lim W: Inhibition of miR-214-3p aids in preventing
epithelial ovarian cancer malignancy by increasing the expression
of LHX6. Cancers (Basel). 11:19172019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan JS, Chen Q, Li YL and Gao YQ:
Hsa_circ_0065217 promotes growth and metastasis of renal cancer
through regulating the miR-214-3p-ALPK2 axis. Cell Cycle.
20:2519–2530. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li M, Yin B, Chen M, Peng J, Mu X, Deng Z,
Xiao J, Li W and Fan J: Downregulation of the lncRNA ASB16-AS1
Decreases LARP1 expression and promotes clear cell renal cell
carcinoma progression via miR-185-5p/miR-214-3p. Front Oncol.
10:6171052021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Xu C, He T, Li Z, Liu H and Ding B:
Regulation of HOXA11-AS/miR-214-3p/EZH2 axis on the growth,
migration and invasion of glioma cells. Biomed Pharmacother.
95:1504–1513. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peng Q, Wang L, Wang S, Wang C and Xue Z:
MicoRNA-214-3p: a key player in CPLX2-mediated inhibition on
temozolomide resistance in glioma. Neurol Res. 44:879–887. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu S, Chang J, Ruan H, Zhi W, Wang X, Zhao
F, Ma X, Sun X, Liang Q, Xu H, et al: Cantharidin inhibits
osteosarcoma proliferation and metastasis by directly targeting
miR-214-3p/DKK3 axis to inactivate β-catenin nuclear translocation
and LEF1 translation. Int J Biol Sci. 17:2504–2522. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Yao X, Wu L, Gu Z and Li J: LINC01535
promotes the development of osteosarcoma through modulating
miR-214-3p/KCNC4 Axis. Cancer Manag Res. 12:5575–5585. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cheng S, Li C, Liu L, Liu X, Li M, Zhuo J,
Wang J, Zheng W and Wang Z: Dysregulation and antimetastatic
function of circLRIG1 modulated by miR-214-3p/LRIG1 axis in bladder
carcinoma. Biol Direct. 19:202024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Clement MS, Gammelgaard KR, Nielsen AL and
Sorensen BS: Epithelial-to-mesenchymal transition is a resistance
mechanism to sequential MET-TKI treatment of MET-amplified EGFR-TKI
resistant non-small cell lung cancer cells. Transl Lung Cancer Res.
9:1904–1914. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Wang K, Yu H, Guo S, Sun G, Cao H, Xing D,
Li D and Yan A: CAPRIN1/TYMS/MTHFD2 axis promotes EMT process in
nasopharyngeal carcinoma development. Int J Biochem Cell Biol.
185:1067842025. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jin Y, Wang Z, Liang Y, Jiang Y, Yuan F
and Zhang T: miRNA-22-3p inhibits cell viability and metastasis of
nasopharyngeal carcinoma by targeting FOXP1. Oncol Lett. 29:962024.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Petrick JL, Florio AA, Znaor A, Ruggieri
D, Laversanne M, Alvarez CS, Ferlay J, Valery PC, Bray F and
McGlynn KA: International trends in hepatocellular carcinoma
incidence, 1978–2012. Int J Cancer. 147:317–330. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Ji Y, Chen X, Liu X, Huang J and Liu P:
lncRNA POLR2J4 plays a biomarker role in Hepatitis B virus-related
hepatocellular carcinoma through regulating miR-214-3p. Turk J
Gastroenterol. 35:787–794. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen K, Feng X, Shi Y, Li XL, Shi ZR and
Lan X: Complete response of gallbladder cancer treated with
gemcitabine and cisplatin chemotherapy combined with durvalumab: A
case report and review of literature. World J Gastrointest Oncol.
17:984332025. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Park W, Chawla A and O'Reilly EM:
Pancreatic cancer: A review. JAMA. 326:851–862. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Grobbelaar C, Steenkamp V and Mabeta P:
Vascular endothelial growth factor receptors in the vascularization
of pancreatic tumors: Implications for prognosis and therapy. Curr
Issues Mol Biol. 47:1792025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Sigirli S and Karakas D: Fibrotic
fortresses and therapeutic frontiers: Pancreatic stellate cells and
the extracellular matrix in pancreatic cancer. Cancer Med.
14:e707882025. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y and Xue R: Pancreatic stellate cell:
Update on molecular investigations and clinical translation in
pancreatic cancer. Int J Cancer. 156:1672–1685. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Rodger EJ, Gimenez G, Ajithkumar P,
Stockwell PA, Almomani S, Bowden SA, Leichter AL, Ahn A, Pattison
S, McCall JL, et al: An epigenetic signature of advanced colorectal
cancer metastasis. iScience. 26:1069862023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Liu J and Zhang X, Yang M and Zhang X:
CircCOL1A1 promotes proliferation, migration, and invasion of
colorectal cancer (CRC) cells and glutamine metabolism through GLS1
up-regulation by sponging miR-214-3p. J Cancer Res Clin Oncol.
150:2112024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhao J, Tang X and Zhu H: Chondroitin
polymerizing factor (CHPF) promotes the progression of colorectal
cancer through ASB2-mediated ubiquitylation of SMAD9. Histol
Histopathol. 39:1493–1503. 2024.PubMed/NCBI
|
|
66
|
Yuan M, Zhu Y, Ren Y, Chen L, Dai X, Wang
Y, Huang Y and Wang H: Global burden and attributable risk factors
of breast cancer in young women: Historical trends from 1990 to
2019 and forecasts to 2030 by sociodemographic index regions and
countries. J Glob Health. 14:041422024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu C, Li J, Hua Y, Wang J, Wang K and Sun
J: Berberine inhibits the expression of SCT through miR-214-3p
stimulation in breast cancer cells. Evid Based Complement Alternat
Med. 2020:28171472020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li Z, Liu P, Yin A, Zhang B, Xu J, Chen Z,
Zhang Z, Zhang Y, Wang S, Tang L, et al: Global landscapeof
cervical cancer incidence and mortality in 2022 andpredictions to
2030: The urgent need to address inequalities incervical cancer.
Int J Cancer. 157:288–297. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49.
2024.PubMed/NCBI
|
|
70
|
Wang N, Yin J, You N, Zhu W, Guo N, Liu X,
Zhang P, Huang W, Xie Y, Ren Q and Ma X: Twist family BHLH
transcription factor 1 is required for the maintenance of leukemia
stem cell in MLL-AF9+ acute myeloid leukemia. Haematologica.
109:84–97. 2024.PubMed/NCBI
|
|
71
|
Wang J, Zhao X, Guo Z, Ma X, Song Y and
Guo Y: Regulation of NEAT1/miR-214-3p on the growth, migration and
invasion of endometrial carcinoma cells. Arch Gynecol Obstet.
295:1469–1475. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Huang W, Meng H, Xu Y, Huang L and Lou G:
Olaparib promotes FABP4 expression and reduces antitumor effect in
ovarian cancer cells with a BRCA1 mutation. Oncol Lett. 29:672024.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Liu Y, Li Y, Wu Y, Zhao Y, Hu X and Sun C:
The long non-coding RNA NEAT1 promotes the progression of human
ovarian cancer through targeting miR-214-3p and regulating
angiogenesis. J Ovarian Res. 16:2192023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wang C, Qi S, Xie C, Li C, Wang P and Liu
D: Upregulation of long non-coding RNA XIST has anticancer effects
on epithelial ovarian cancer cells through inverse downregulation
of hsa-miR-214-3p. J Gynecol Oncol. 29:e992018. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Padala SA and Barsouk A, Thandra KC,
Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A:
Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Liang H, Zhou B, Li P, Zhang X, Zhang S,
Zhang Y, Yao S, Qu S and Chen J: Stemness regulation in prostate
cancer: Prostate cancer stem cells and targeted therapy. Ann Med.
57:24420672025. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Xi X, Hu Z, Wu Q, Hu K, Cao Z, Zhou J,
Liao J, Zhang Z, Hu Y, Zhong X and Bao Y: High expression of small
nucleolar RNA host gene 3 predicts poor prognosis and promotes bone
metastasis in prostate cancer by activating transforming growth
factor-beta signaling. Bioengineered. 13:1895–1907. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Chen Y, Yin Z, Westover KD, Zhou Z and Shu
L: Advances and challenges in RAS signaling targeted therapy in
leukemia. Mol Cancer Ther. 24:33–46. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
He Z, Liao Z, Chen S, Li B, Yu Z, Luo G,
Yang L, Zeng C and Li Y: Downregulated miR-17, miR-29c, miR-92a and
miR-214 may be related to BCL11B overexpression in T cell acute
lymphoblastic leukemia. Asia Pac J Clin Oncol. 14:e259–e265. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiao S, Xu N, Ding Q, Huang S, Zha Y and
Zhu H: LncRNA VPS9D1-AS1 promotes cell proliferation in acute
lymphoblastic leukemia through modulating GPX1 expression by
miR-491-5p and miR-214-3p evasion. Biosci Rep. 40:BSR201934612020.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Elguindy MM, Young JS, Ho WS and Lu RO:
Co-evolution of glioma and immune microenvironment. J Immunother
Cancer. 12:e0091752024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Benjamin AS and Nayak S: Iron oxide
nanoparticles coated with bioactive materials: a viable
theragnostic strategy to improve osteosarcoma treatment. Discov
Nano. 20:182025. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Li K, Shen H, Lu M, Chen J, Yin Q and Li
P: Formononetin inhibits osteosarcoma cell proliferation and
promotes apoptosis by regulating the miR-214-3p/phosphatase and
tensin homolog pathway. Transl Cancer Res. 9:4914–4921. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Xiong X, Dang W, Luo R, Long Y, Tong C,
Yuan L and Liu B: A graphene-based fluorescent nanoprobe for
simultaneous imaging of dual miRNAs in living cells. Talanta.
225:1219472021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Xing AY, Wang B, Li YH, Chen X, Wang YW,
Liu HT and Gao P: Identification of miRNA signature in breast
cancer to predict neoadjuvant chemotherapy response. Pathol Oncol
Res. 27:16097532021. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jin Y, Wong YS, Goh BKP, Chan CY, Cheow
PC, Chow PKH, Lim TKH, Goh GBB, Krishnamoorthy TL, Kumar R, et al:
Circulating microRNAs as potential diagnostic and prognostic
biomarkers in hepatocellular carcinoma. Sci Rep. 9:104642019.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhou Z, Wu L, Liu Z, Zhang X, Han S, Zhao
N, Bao H, Yuan W, Chen J, Ji J and Shu X: MicroRNA-214-3p targets
the PLAGL2-MYH9 axis to suppress tumor proliferation and metastasis
in human colorectal cancer. Aging (Albany NY). 12:9633–9657. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li Y, Li Y, Chen Y, Xie Q, Dong N, Gao Y,
Deng H, Lu C and Wang S: MicroRNA-214-3p inhibits proliferation and
cell cycle progression by targeting MELK in hepatocellular
carcinoma and correlates cancer prognosis. Cancer Cell Int.
17:1022017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Wang J, Xu Y, Wang J and Ying H:
Circulating miR-214-3p predicts nasopharyngeal carcinoma recurrence
or metastasis. Clin Chim Acta. 503:54–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ecke TH, Stier K, Weickmann S, Zhao Z,
Buckendahl L, Stephan C, Kilic E and Jung K: miR-199a-3p and
miR-214-3p improve the overall survival prediction of
muscle-invasive bladder cancer patients after radical cystectomy.
Cancer Med. 6:2252–2262. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kuang L, Wang P, Zhou L and Li Y:
Transformation of lung adenocarcinoma to small cell lung cancer
following osimertinib treatment: A case report and literature
review. Anticancer Drugs. 36:253–259. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Maurya N, Meena A and Luqman S: Role of
microRNAs in lung oncogenesis: Diagnostic implications, resistance
mechanisms, and therapeutic strategies. Int J Biol Macromol 318 (Pt
1). 1442612025. View Article : Google Scholar
|
|
93
|
Wang X, Li H and Shi J: LncRNA HOXA11-AS
promotes proliferation and cisplatin resistance of oral squamous
cell carcinoma by suppression of miR-214-3p expression. Biomed Res
Int. 2019:86451532019.PubMed/NCBI
|
|
94
|
Phatak P, Byrnes KA, Mansour D, Liu L, Cao
S, Li R, Rao JN, Turner DJ, Wang JY and Donahue JM: Overexpression
of miR-214-3p in esophageal squamous cancer cells enhances
sensitivity to cisplatin by targeting survivin directly and
indirectly through CUG-BP1. Oncogene. 35:2087–2097. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Lan T, Quan W, Yu DH, Chen X, Wang ZF and
Li ZQ: High expression of LncRNA HOTAIR is a risk factor for
temozolomide resistance in glioblastoma via activation of the
miR-214/β-catenin/MGMT pathway. Sci Rep. 14:262242024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yang L, Zhang L, Lu L and Wang Y:
miR-214-3p regulates multi-drug resistance and apoptosis in
retinoblastoma cells by targeting ABCB1 and XIAP. Onco Targets
Ther. 13:803–811. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen J, Kobayashi M, Darmanin S, Qiao Y,
Gully C, Zhao R, Yeung SC and Lee MH: Pim-1 plays a pivotal role in
hypoxia-induced chemoresistance. Oncogene. 28:2581–2592. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chaudhary B, Arya P, Sharma V, Kumar P,
Singla D and Grewal AS: Targeting anti-apoptotic mechanisms in
tumour cells: Strategies for enhancing Cancer therapy. Bioorg Chem.
159:1083882025. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Lei X, He N, Zhu L, Zhou M, Zhang K, Wang
C, Huang H, Chen S, Li Y, Liu Q, et al: Mesenchymal stem
cell-derived extracellular vesicles attenuate radiation-induced
lung injury via miRNA-214-3p. Antioxid Redox Signal. 35:849–862.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shen G, Liu Z, Wang M, Zhao Y, Liu X, Hou
Y, Ma W, Han J, Zhou X, Ren D, et al: Neoadjuvant apatinib addition
to sintilimab and carboplatin-taxane based chemotherapy in patients
with early triple-negative breast cancer: The phase 2 NeoSAC trial.
Signal Transduct Target Ther. 10:412025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tian Z, Cen L, Wei F, Dong J, Huang Y, Han
Y, Wang Z, Deng J and Jiang Y: EGFR mutations in non-small cell
lung cancer: Classification, characteristics and resistance to
third-generation EGFR-tyrosine kinase inhibitors (Review). Oncol
Lett. 30:3752025. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Hu S, Zhou Q, Lu Q, Guo X, Wang Y and Duan
YX: miR-199a/214 cluster enhances prostate cancer sensitiveness to
nimotuzumab via targeting TBL1XR1. Kaohsiung J Med Sci.
39:1178–1189. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Seo Y, Rhim J and Kim JH: RNA-binding
proteins and exoribonucleases modulating miRNA in cancer: The enemy
within. Exp Mol Med. 56:1080–1106. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kang E and Kortylewski M: Lipid
nanoparticle-mediated delivery of miRNA mimics to myeloid cells.
Methods Mol Biol. 2691:337–350. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Qian Y, Zhu D, Xu Q, Wang Y, Chen X, Hua
W, Xi J and Lu F: PAMAM/miR-144 nanocarrier system inhibits the
migration of gastric cancer by targeting mTOR signal transduction
pathway. Colloids Surf B Biointerfaces. 249:1144922025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Tanno T, Zhang P, Bailey C, Wang Y,
Ittiprasert W, Devenport M, Zheng P and Liu Y: A novel
aptamer-based small RNA delivery platform and its application to
cancer therapy. Genes Dis. 10:1075–1089. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zeng H, Guo S, Ren X, Wu Z, Liu S and Yao
X: Current strategies for exosome cargo loading and targeting
delivery. Cells. 12:14162023. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
He L, Chen Q and Wu X: Tumour-derived
exosomal miR-205 promotes ovarian cancer cell progression through
M2 macrophage polarization via the PI3K/Akt/mTOR pathway. J Ovarian
Res. 18:282025. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Lundstrom K: Are viral vectors any good
for RNAi antiviral therapy? Viruses. 12:11892020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Arduini A, Katiyar H and Liang C: Progress
in pseudotyping lentiviral vectors towards cell-specific gene
delivery in vivo. Viruses. 17:8022025. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Gutierrez-Guerrero A, Cosset FL and
Verhoeyen E: Lentiviral vector pseudotypes: Precious tools to
improve gene modification of hematopoietic cells for research and
gene therapy. Viruses. 12:10162020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Miletic H, Fischer YH, Neumann H, Hans V,
Stenzel W, Giroglou T, Hermann M, Deckert M and Von Laer D:
Selective transduction of malignant glioma by lentiviral vectors
pseudotyped with lymphocytic choriomeningitis virus glycoproteins.
Hum Gene Ther. 15:1091–1100. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Roszkowski S and Durczynska Z: Advantages
and limitations of nanostructures for biomedical applications. Adv
Clin Exp Med. 34:447–456. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Kim B, Shin J, Wu J, Omstead DT, Kiziltepe
T, Littlepage LE and Bilgicer B: Engineering peptide-targeted
liposomal nanoparticles optimized for improved selectivity for
HER2-positive breast cancer cells to achieve enhanced in vivo
efficacy. J Control Release. 322:530–541. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dinakar YH, Karole A, Parvez S, Jain V and
Mudavath SL: Folate receptor targeted NIR cleavable liposomal
delivery system augment penetration and therapeutic efficacy in
breast cancer. Biochim Biophys Acta Gen Subj. 1867:1303962023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Rong J, Liu T, Yin X, Shao M, Zhu K, Li B,
Wang S, Zhu Y, Zhang S, Yin L, et al: Co-delivery of camptothecin
and MiR-145 by lipid nanoparticles for MRI-visible targeted therapy
of hepatocellular carcinoma. J Exp Clin Cancer Res. 43:2472024.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fan Q, Sun B and Chao J: Advancements in
engineering tetrahedral framework nucleic acids for biomedical
innovations. Small Methods. 9:e24013602025. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Siragusa G, Tomasello L, Giordano C and
Pizzolanti G: Survivin (BIRC5): Implications in cancer therapy.
Life Sci. 350:1227882024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Li S, Sun Y, Tian T, Qin X, Lin S, Zhang
T, Zhang Q, Zhou M, Zhang X, Zhou Y, et al: MicroRNA-214-3p
modified tetrahedral framework nucleic acids target survivin to
induce tumour cell apoptosis. Cell Prolif. 53:e127082020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Hassan AAA, Ramadan E, Kristó K, Regdon G
Jr and Sovány T: Lipid-polymer hybrid nanoparticles as a smart drug
delivery system for peptide/protein delivery. Pharmaceutics.
17:7972025. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sonkar C, Ranjan R and Mukhopadhyay S:
Inorganic nanoparticle-based nanogels and their biomedical
applications. Dalton Trans. 54:6346–6360. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Howe SJ, Mansour MR, Schwarzwaelder K,
Bartholomae C, Hubank M, Kempski H, Brugman MH, Pike-Overzet K,
Chatters SJ, de Ridder D, et al: Insertional mutagenesis combined
with acquired somatic mutations causes leukemogenesis following
gene therapy of SCID-X1 patients. J Clin Invest. 118:3143–3150.
2008. View Article : Google Scholar : PubMed/NCBI
|
















