Introduction
Lung cancer (LC) is among the most prevalent and
lethal malignancies worldwide, ranking as the leading cause of
cancer-related mortality (1).
Early-stage LC is often asymptomatic and difficult to detect,
resulting in delayed diagnosis. For patients diagnosed at advanced
stages, the five-year survival rate remains dismally low at ~6%
(2). Globally, ~1.6 million new LC
cases are reported annually (3). In
men, LC constitutes the primary cause of both cancer incidence and
mortality, whereas among women, its incidence ranks second only to
breast and colorectal cancers (4).
LC poses a significant public health burden, with major risk
factors including exogenous environmental exposures such as tobacco
exposure, occupational and environmental carcinogens, and air
pollution, as well as endogenous biological factors such as genetic
predisposition, chronic lung disease, and hormonal and metabolic
factors (5,6). Despite substantial advancements in
diagnostic and therapeutic modalities in recent years, the overall
prognosis of LC remains poor, particularly for patients with
advanced disease (7,8).
Chemoprevention, defined as the use of natural or
synthetic agents to prevent, inhibit, delay, or reverse
carcinogenesis, has attracted considerable attention as a strategy
to combat LC (9). Although various
candidate chemo-preventive agents such as vitamin E, isotretinoin
and aspirin have been evaluated, none have demonstrated definitive
clinical efficacy (10). Natural
bioactive compounds, characterized by diverse pharmacological
activities and relatively low toxicity, have emerged as promising
candidates in cancer prevention and treatment, thereby becoming a
central focus of chemoprevention research (11). Ursolic acid (UA; PubChem Compound
ID: 64945) is a pentacyclic triterpenoid compound
(C30H48O3) widely distributed in
various plants, including calendula, lavender, oregano, Melaleuca,
sage, apple, rosemary and pear. It has garnered significant
scientific interest due to its diverse biological activities
(12,13). Previous studies have revealed that
UA possesses multifaceted biological activities, including
anti-inflammatory (14),
antioxidant (15), and anti-obesity
effects (16). Additionally, UA
exhibits therapeutic potential in a variety of conditions,
including diabetes (17), liver
diseases (18), cardiovascular
diseases (19), gastrointestinal
disorders (20) and
neurodegenerative diseases (21).
Of particular note is its remarkable anticancer potential (22). UA has been demonstrated to inhibit
tumor cell proliferation, induce apoptosis, and suppress tumor
invasion and metastasis across multiple cancer types, including
breast (23), pancreatic (24), prostate (25), colorectal (26), cervical (27) and renal cancers (28).
In LC chemoprevention, UA exerts its effects through
multiple molecular mechanisms. Although extensive preclinical
evidence supports the potential role of UA in LC prevention, the
precise molecular pathways remain to be fully elucidated. Moreover,
challenges related to the bioavailability, safety, and clinical
efficacy of UA must be addressed to facilitate its translational
application. The present review aims to comprehensively summarize
the chemo-preventive effects and molecular mechanisms of UA in LC,
thereby providing new insights and a theoretical framework for its
future application in LC prevention and therapy.
UA and its derivatives
UA is a pentacyclic triterpenoid compound that
naturally occurs either as triterpene saponins or in its free acid
form (29,30). It is also referred to as urson,
malol, prunol, or 3β-3-hydroxy-urs-12-en-28-oic acid (31). UA has the molecular formula
C30H48O3, a molecular weight of
456.68 g/mol, and a melting point ranging from 283–285°C (12,32).
Its chemical structure comprises five rings-four six-membered and
one five-membered-along with multiple hydroxyl and carboxyl
functional groups. This distinctive molecular framework confers a
broad spectrum of biological activities, including
anti-inflammatory, antioxidant, antimicrobial and anticancer
effects (33–35). Numerous studies have demonstrated
that UA exerts potent anticancer effects across various
malignancies (36,37). Its mechanisms of action extend
beyond conventional pathways such as apoptosis induction and
proliferation inhibition, encompassing unique molecular targets and
biological processes. Importantly, UA selectively targets cancer
stem cells (CSCs), impairing their self-renewal capacity, which is
critical for tumor initiation and recurrence (38). Moreover, UA exhibits
context-dependent modulation of autophagy, a process highly
relevant to cancer therapy. At low concentrations, UA activates the
AMP-activated protein kinase/mammalian target of rapamycin pathway,
inducing protective autophagy that facilitates the clearance of
damaged cellular components and enhances chemoresistance.
Conversely, at high concentrations, UA inhibits autophagy by
blocking autophagosome-lysosome fusion, thereby promoting
autophagic cell death and augmenting its anticancer efficacy.
Beyond autophagy regulation, UA exerts precise anticancer effects
by modulating glycolytic metabolism (39), inducing structural modifications
(40), and remodeling the tumor
microenvironment (41). These
multifaceted actions underscore UA as a promising candidate for
cancer therapy.
To improve the pharmacological activity and
therapeutic potential of UA, various derivatives have been
synthesized through chemical modifications targeting its hydroxyl,
carboxyl and cyclic backbone moieties. Most reported UA derivatives
fall into two principal categories: modifications at C-3/C-28,
C-11, C-17 and C-28 positions, and structural alterations involving
C-2/C-3 and the A-ring (13,42).
Representative examples include UA-piperazine-dithio-carbamate
ruthenium (II) polypyridyl complexes, which induce antitumor
activity via necrosis (43);
corosolic acid (CA), a natural UA derivative that inhibits cancer
cell proliferation through β-catenin downregulation (44); UA232, which promotes apoptosis by
inducing cell cycle arrest and endoplasmic reticulum (ER) stress
(45); and compound 17, a
UA-derived small molecule that triggers cancer cell death through
macropinocytosis (46).
Collectively, these findings suggest that UA derivatives exert
anticancer effects through multi-target and multi-pathway
mechanisms, enhancing the pharmacological potency and drug-like
properties of UA. This not only reinforces its therapeutic efficacy
but also offers novel insights for the development of innovative
anticancer agents.
Chemo-preventive effects of UA on LC
Induction of autophagy and apoptosis
in LC cells
Apoptosis plays a pivotal role in tumor development,
progression and metastasis, yet cancer cells often evade apoptotic
mechanisms to sustain survival (47). Induction of apoptosis facilitates
the elimination of potentially harmful or precancerous cells,
whereas inhibition of apoptotic pathways contributes to
uncontrolled cell proliferation and malignant transformation
(48). UA primarily induces
apoptosis in LC cells through mitochondria-dependent (MD) pathways,
ER stress pathways, and modulation of associated signaling cascades
(Fig. 1).
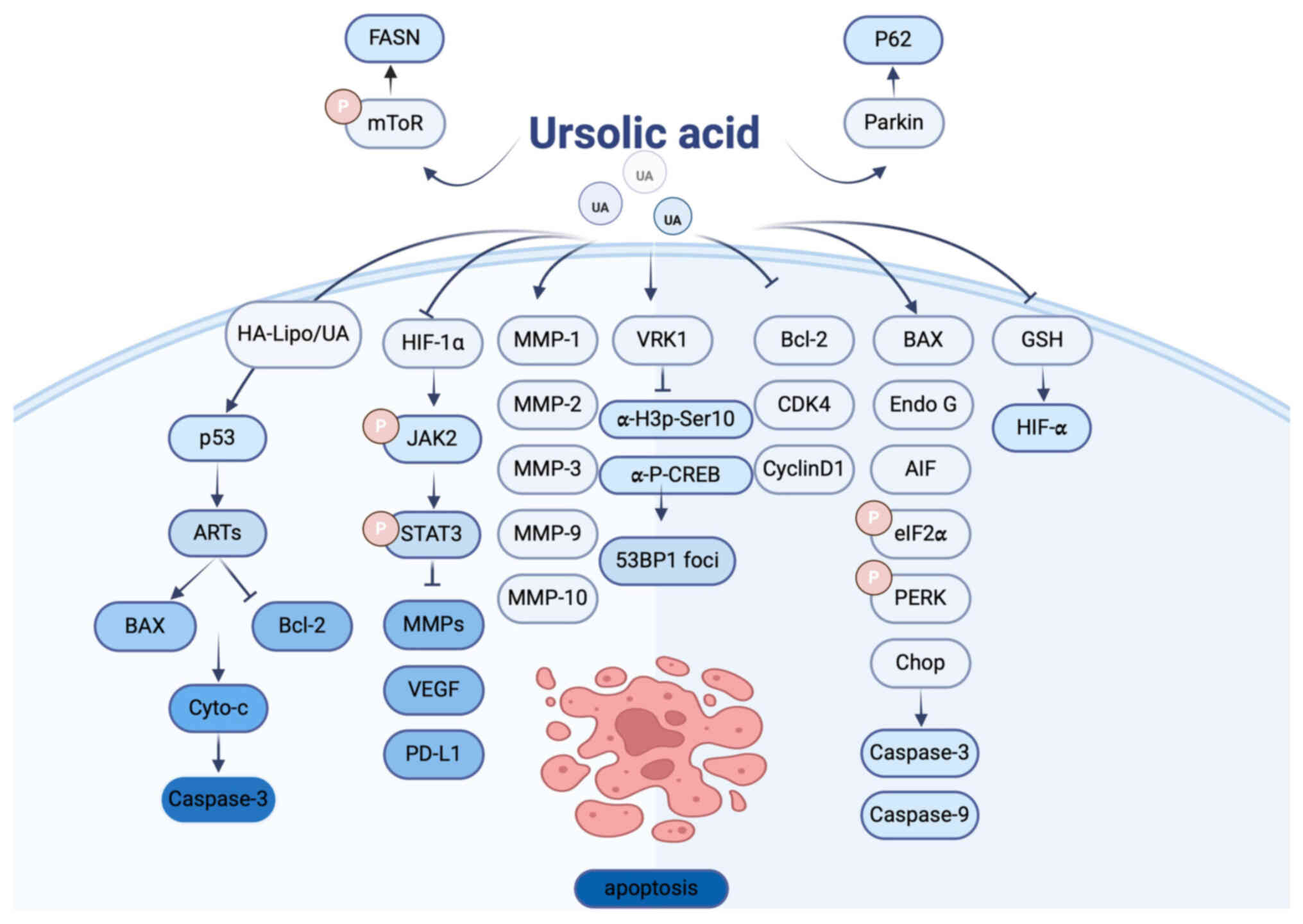 | Figure 1.Molecular targets regulated by UA and
their effects on cellular autophagy and apoptosis. UA, ursolic
acid; HA-Lipo/UA, hyaluronic acid-liposome UA; GSH, glutathione;
MMP, matrix metalloproteinase; ARTS, apoptosis-related protein in
the TGF-β signaling pathway; HIF, hypoxia-inducible factor; PD-L1,
programmed death-ligand 1; VRK1, vaccinia-related kinase 1; Chop,
C/EBP-homologous protein. Figure created using Adobe Illustrator
2024 (v28.0), Adobe Inc. |
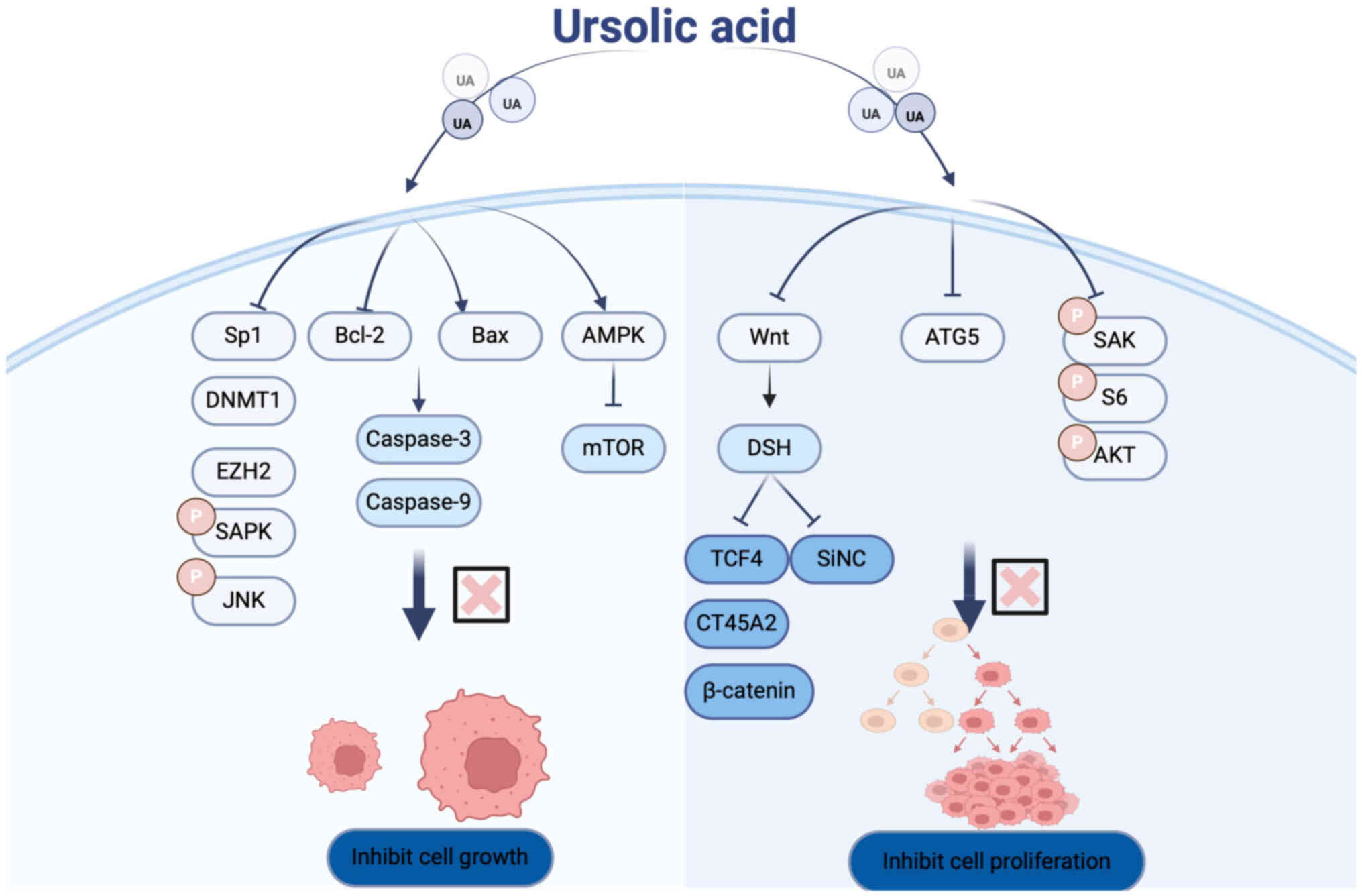
Multiple studies have demonstrated that UA inhibits
the proliferation of non-small cell LC (NSCLC) cells in a dose- and
time-dependent manner while promoting apoptosis. UA activates
caspase-3 and caspase-9, downregulates the anti-apoptotic protein
Bcl-2, and upregulates the pro-apoptotic protein Bax, collectively
inducing apoptotic cell death in LC cells (49). Matrix metalloproteinases (MMPs), a
family of zinc-dependent endopeptidases, mediate extracellular
matrix (ECM) remodeling, which is implicated in various
pathological processes (50).
Notably, upregulation of MMP gene expression has been associated
with apoptosis induction in NSCLC cells. Lai et al (51) reported that treatment of H460 cells
with UA for 24 h led to increased expression of MMP-1, −2, −3, −9,
and −10, concomitant with activation of caspase-3, nuclear
morphological changes, and DNA fragmentation, thereby promoting
apoptosis. Additionally, Gou et al (45) demonstrated that the UA derivative
UA232 induces apoptosis in A549 and H460 cells by causing G0/G1
phase cell cycle arrest and upregulating C/EBP-homologous protein,
which triggers apoptosis via the ER stress pathway. Consistently,
UA has been shown to exert anticancer effects in both A549 and H460
cells by inducing G0/G1 arrest and apoptosis, thereby suppressing
tumor growth and progression (52).
Mitochondria are essential organelles involved in
regulating cellular functions such as autophagy and apoptosis under
stress conditions (53). Chen et
al (54) observed that UA
increases the expression of apoptosis-inducing factor and
endonuclease G in NCI-H292 cells, promoting their release via the
MD pathway and triggering apoptosis. To address UA's limited
solubility and lack of tumor specificity, Ma et al (55) developed a hyaluronic acid-liposome
UA delivery system (HA-Lipo/UA). Their results showed that
HA-Lipo/UA upregulated apoptosis-related protein in the TGF-β
signaling pathway and p53 expression in A549 cells, activated
caspase-3, and enhanced mitochondrial apoptosis, suggesting a
promising strategy for targeted LC therapy.
Mitophagy, a selective autophagic process that
degrades damaged mitochondria via lysosomal pathways, is another
mechanism by which UA exerts anticancer effects. Castrejon-Jiménez
et al (56) reported that UA
induces mitophagy in A549 cells through a Parkin-independent
mechanism, leading to p62 overexpression and subsequent apoptosis.
Furthermore, Song et al (57) demonstrated that UA significantly
enhances radiosensitivity in NSCLC cells, especially in
radiation-resistant cells overexpressing hypoxia-inducible factor 1
alpha (HIF-1α). By reducing the glutathione ratio and
downregulating HIF-1α in H1299/M-HIF-1α cells, UA increases
radiosensitivity and promotes radiation-induced cell death.
DNA damage repair (DDR) is critical for maintaining
genomic stability (58). A strong
correlation exists between DNA damage and the initiation of cell
death pathways (59). When DNA
repair mechanisms are compromised, apoptosis acts as a safeguard to
eliminate damaged cells (60).
Vaccinia-related kinase 1 (VRK1), a mitotic kinase frequently
overexpressed in lung adenocarcinoma, belongs to the nuclear
serine-threonine chromatin kinase family (61). UA has been shown to bind the
catalytic domain of VRK1, inhibiting its kinase activity. This
interference impairs VRK1-mediated 53BP1 foci formation, thereby
disrupting DDR and contributing to the therapeutic efficacy of UA
against LC (62).
Inhibition of LC cell invasion and
migration
Tumor metastasis is a major contributor to the high
morbidity and mortality associated with cancer (63). Dysregulation of cancer cell
migration critically influences the ability of tumor cells to
detach from the primary site and invade surrounding tissues,
thereby facilitating metastasis (63).
Proteases play a vital role in cancer progression
not only by mediating ECM remodeling but also by regulating the
bioavailability of growth factors, pro-angiogenic factors, and
cytokines, which collectively promote tumor development through
both direct and indirect mechanisms (64). However, the clinical application of
UA is limited by its poor water solubility and insufficient
tumor-targeting capability (65).
To address these challenges, Xu et al (66) developed a novel multifunctional
nanoparticle formulation [HA-modified UA and astragaloside IV
(AS-IV)-loaded polydopamine nanoparticles (NPs;
UA/(AS-IV)@PDA-HA)]. This delivery system significantly enhanced
the cytotoxicity of UA and improved its anti-metastatic efficacy in
NSCLC cells. Furthermore, UA has been shown to inhibit LC cell
invasion in a concentration-dependent manner, with notable
suppression of cell migration observed at concentrations between 4
and 16 µmol/l (67).
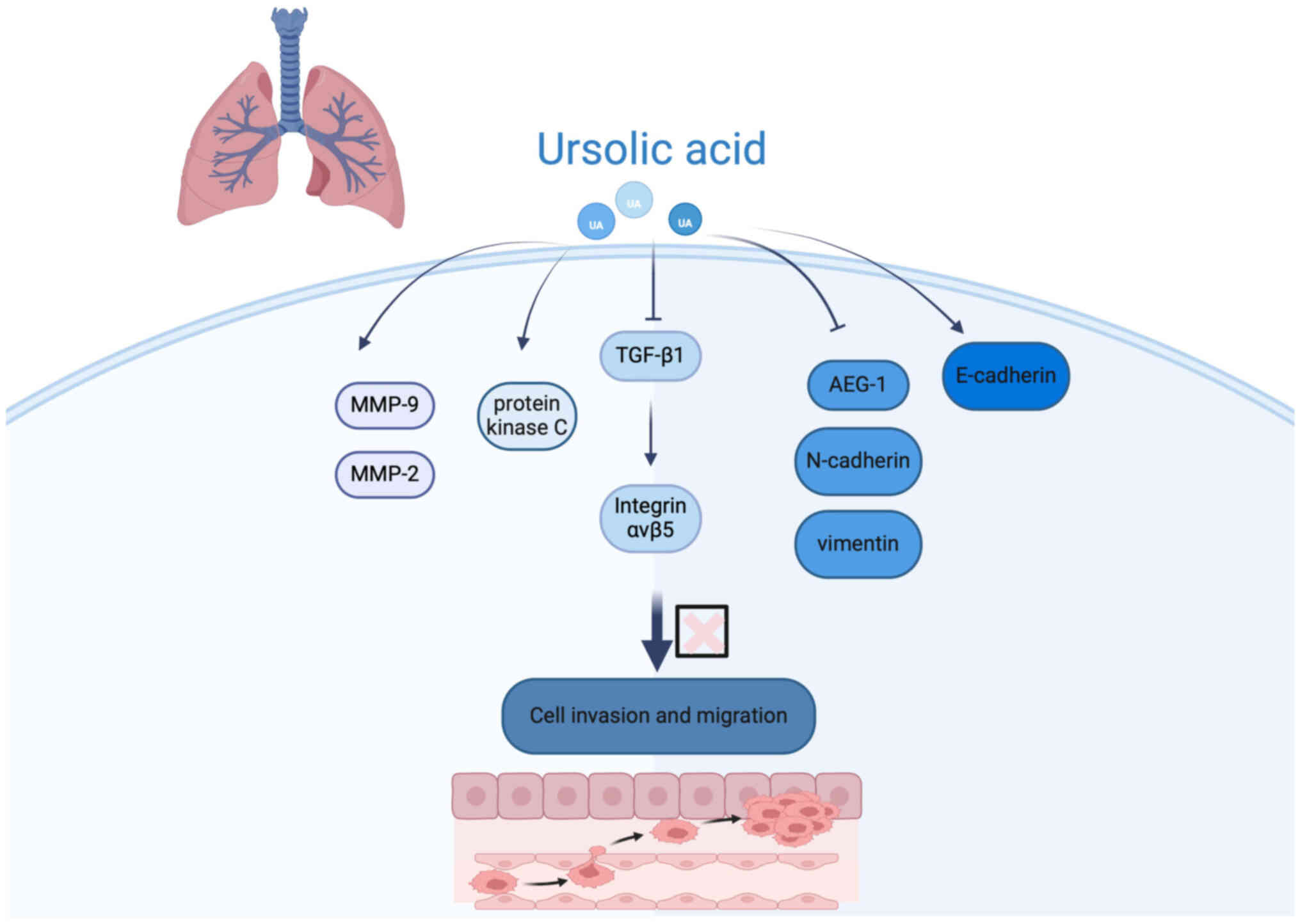
Epithelial-mesenchymal transition (EMT) is a
fundamental cellular program involved in embryonic development,
tissue repair and stem cell plasticity, but it also contributes
pathologically to fibrosis and cancer metastasis by enhancing
cellular motility and invasiveness (68). Experimental studies demonstrated
that UA reduces EMT induction in H1975 LC cells triggered by
transforming growth factor-β1, thereby inhibiting metastatic
potential (69). Additionally,
investigations in human NSCLC A549 cells revealed that UA
suppresses EMT by inhibiting the nuclear factor
Kappa-light-chain-enhancer of activated B Cells (NF-κB) signaling
pathway, downregulating astrocyte-elevated gene-1 (AEG-1), and
modulating key EMT markers-upregulating epithelial marker
E-cadherin while downregulating mesenchymal markers N-cadherin and
vimentin. This mechanism effectively curtails tumor cell migration
and invasion, underscoring the promise of UA as an anti-metastatic
agent in LC treatment (Fig. 2)
(70).
Inhibition of LC cell proliferation
and growth
Cell proliferation is fundamental to the normal
development and homeostasis of multicellular organisms.
Dysregulated cell proliferation is a hallmark of tumorigenesis and
can also contribute to congenital malformations (71).
Cancer/Testis Antigen Family 45 Member A2 (CT45A2)
is implicated as an oncogene in NSCLC and is associated with
Epidermal Growth Factor Receptor (EGFR) T790 mutations. The
antitumor effects of UA in NSCLC predominantly depend on CT45A2
expression in H1975 cells. UA inhibits CT45A2 transcription by
targeting the β-catenin/transcription factor 4 (TCF4) signaling
pathway. Specifically, UA negatively regulates the
β-catenin/TCF4/CT45A2 axis, thereby suppressing the proliferation
of H1975 cells (72).
Autophagy-related gene 5 (ATG5) plays a pivotal role in autophagy
by participating in the formation of autophagic complexes and
autophagosomes, thus amplifying the autophagic response and
offering potential therapeutic avenues (73,74).
Mutations in ATG5 have been linked to various pathologies. Notably,
inhibition of ATG5 via chloroquine (CQ) or siRNA enhances UA's
anti-proliferative effects by suppressing autophagy, suggesting
that autophagy may serve as a protective mechanism in cancer cells
under UA treatment (75).
Cell proliferation involves the biosynthesis of
macromolecules such as proteins, lipids and nucleic acids, as well
as an increase in cell size and mass. Aberrant regulation of cell
proliferation and division contribute to tumor development. Wu
et al (76) demonstrated
that UA suppresses NSCLC cell proliferation by inducing
phosphorylation of stress-activated protein kinase/c-Jun N-terminal
kinase and subsequently downregulating Sp1 expression. Furthermore,
Way et al (49) reported
that UA inhibits LC cell proliferation through modulation of key
signaling proteins, including mTOR and AMPK. Their findings
indicate that UA activates AMPK in a dose- and time-dependent
manner, which in turn suppresses mTOR activity-a central regulator
of protein synthesis and cellular proliferation (Fig. 3).
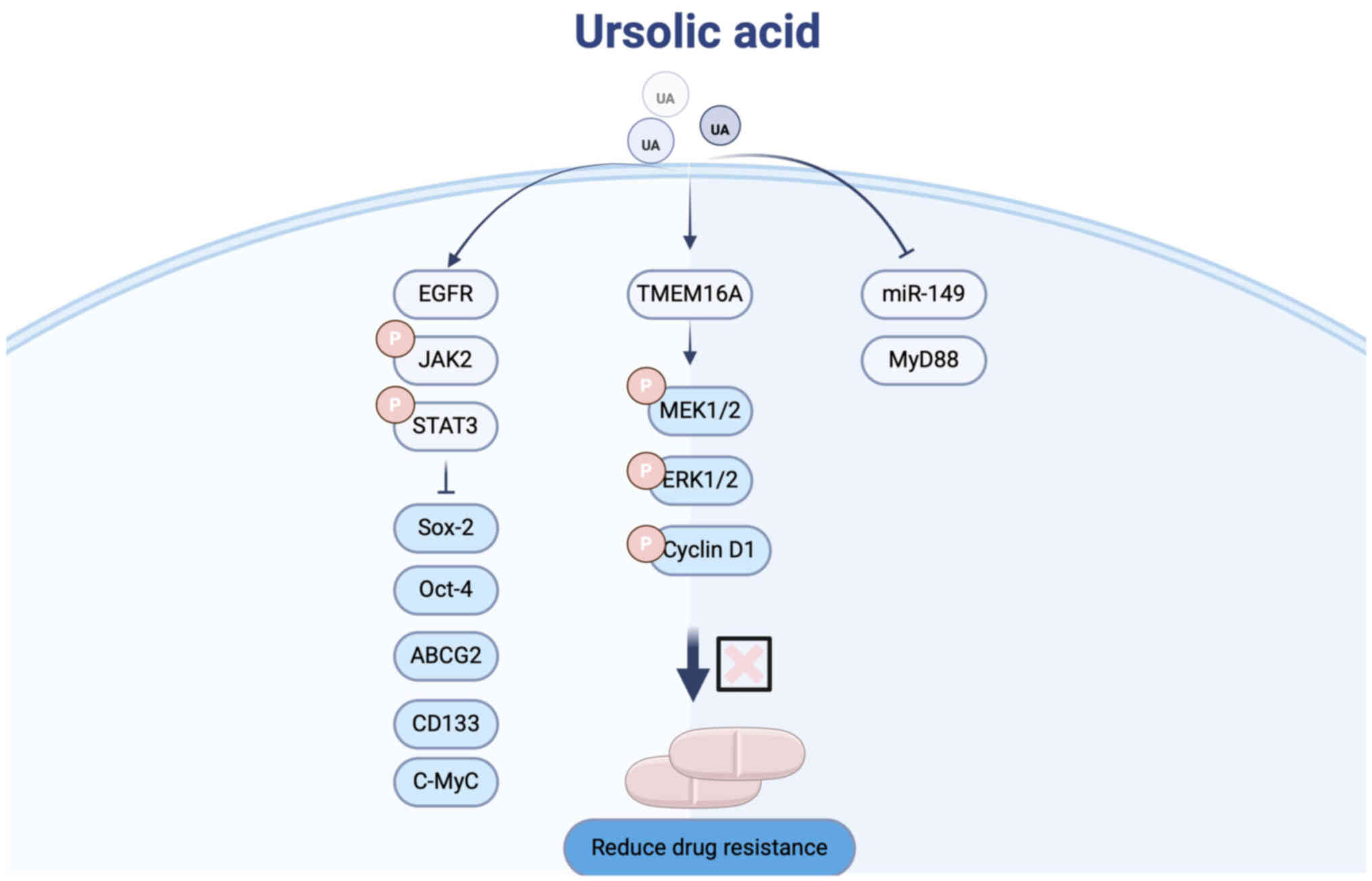
Combination with other anticancer
drugs
Chemotherapy remains a cornerstone in the treatment
of LC (77,78). However, the emergence of drug
resistance constitutes a significant barrier to successful
chemotherapy outcomes (79).
Cisplatin resistance, in particular, is a major cause of
therapeutic failure in patients with LC (80). Recently, UA has attracted increasing
attention for its potential to overcome cisplatin resistance in LC
(Fig. 4).
Fan et al (81) utilized the A549 human LC cell line
as a model for cisplatin-resistant cells and demonstrated that UA
treatment inhibited the activation of the Janus kinase 2/signal
transducer and activator of transcription 3 (Jak2/Stat3) signaling
pathway. This inhibition led to downregulation of
pluripotency-associated transcription factors and suppression of
CSC enrichment, thereby attenuating chemoresistance.
To improve UA delivery and targeting, Li et
al (82) developed a
hydrogel-based drug delivery system co-loaded with UA and
cisplatin. This platform enhances the targeting of UA to LC cells
by facilitating its binding to the LC-specific membrane protein
TMEM16A, effectively inhibiting lung adenocarcinoma progression.
The hydrogel system thus provides a promising strategy to
potentiate UA's therapeutic efficacy in resistant LC.
Furthermore, Chen et al (83) identified the miRNA-149-5p/MyD88
signaling axis as a critical mediator of chemoresistance in NSCLC
cells. Their study revealed that UA suppresses this signaling
pathway, thereby reducing cancer stemness, overcoming chemotherapy
resistance, and ultimately enhancing treatment efficacy in LC.
Novel drug carriers
Currently, most anticancer drugs have the drawback
of poor targeting, which can cause irreversible damage to the body
during treatment. New drug delivery systems aimed at ensuring drug
safety and efficient therapy may become key to enhancing the
efficacy of anticancer drugs (84).
Research indicates that nanoparticle-based drug delivery systems
(Nano-DDS) could potentially resolve the challenges associated with
drug administration in cancer therapy (85).
In an experiment by Wu et al (79), a biomimetic red blood cell membrane
(RBCM) nano-carrier, UA-loaded NPs coated with RBCM, was developed.
This novel biomimetic drug delivery platform enhanced the tumor
targeting, stability and biocompatibility of the UA NPs. It
addressed the clinical limitations of UA and improved its
bioavailability. This innovative biomimetic delivery system
significantly improved the anticancer efficacy in advanced NSCLC
model, offering a promising strategy for enhancing UA-based
treatments. In addition, Xu et al (66) developed a HA-modified
UA/AS-IV-loaded polydopamine (PDA) nanomedicine
(UA/(AS-IV)@PDA-HA). This nanomedicine effectively
enhanced the water solubility and targeting ability of UA. It
allows UA to specifically bind to the overexpressed cluster of
differentiation 44 on the surface of NSCLC cells. Moreover, the
modified nanomedicine significantly improved the cytotoxicity
mediated by UA and enhanced its ability to inhibit NSCLC cell
proliferation and metastasis. These findings suggest that UA has
great potential as an effective targeted anticancer drug for LC
therapy. Novel drug delivery systems have greatly enhanced the
ability of UA to target LC, enabling precise control that reduces
systemic toxicity and improves anticancer efficacy. However, the
integration and interaction between targeted therapies and UA in
addiction-related oncogenes of NSCLC remain unclear and require
further investigation.
Pharmacokinetics of UA
According to the Biopharmaceutics Classification
System (BCS), UA is classified as a Class IV drug, characterized by
low solubility and low permeability. Although UA demonstrates
unique advantages in the treatment of cancers such as LC, its
pharmacological efficacy is limited. Due to its lipophilic nature,
UA exhibits poor oral bioavailability and has difficulty
penetrating biological membranes, thereby reducing its therapeutic
effectiveness against tumors (86).
To overcome these challenges, Antonio et al (87) developed chitosan (CS)-modified
polylactic acid NPs encapsulating UA. Their study showed that this
nanoparticle system reduced the average particle size and promoted
the amorphous transformation of UA, enabling sustained release.
This significantly reduced cytotoxicity in tumor cells and enhanced
UA's oral absorption, clearance rate and metabolic efficiency in
rat models. Furthermore, Yang et al (88) demonstrated that amorphous UA NPs
prepared using the supercritical antisolvent technique effectively
improved UA's supersaturation, solubility and absorption. In
another study, Yu et al (89) formulated a supramolecular
co-amorphous system combining UA and piperine. Through differential
scanning calorimetry and scanning electron microscopy, it was
confirmed that the co-amorphous system significantly enhanced UA's
oral bioavailability and solubility in physiological media compared
to its crystalline form.
Regarding drug absorption, high-performance liquid
chromatography-mass spectrometry was used to analyze the tissue
distribution of UA in rats 1 h after oral administration. The study
revealed that UA concentrations were highest in the lungs, followed
by the spleen, liver, brain, heart and kidneys, in descending order
(90). Additionally, Wang et
al (91) found that a novel
CS-coated UA liposome achieved significantly higher accumulation at
tumor sites in mice compared with conventional UA liposomes and
free UA. These findings offer new insights and strategies for
enhancing UA's therapeutic efficacy against LC.
In terms of drug metabolism and excretion, research
on UA remains relatively limited. Available studies indicate that
UA is primarily metabolized in the human body by the hepatic
cytochrome P450 (CYP450) enzyme system, along with phase II
conjugation reactions. In Caco-2 cells, CYP3A4 and CYP2C9 play key
roles in UA metabolism, and the PXR-RXRα pathway has been shown to
significantly upregulate CYP2C9 expression, thereby promoting UA
biotransformation (92).
In summary, although UA shows promising
pharmacokinetic behavior, further studies are needed to fully
elucidate its absorption, metabolism and elimination profiles,
which will be essential for advancing its clinical application.
Conclusion and outlook
LC, a malignant tumor originating from lung tissue,
is one of the most common cancers worldwide and a leading cause of
cancer-related mortality (93,94).
UA plays a significant role in the treatment of LC, with mechanisms
that include inhibiting cell proliferation, invasion and migration,
and reducing drug resistance. These effects involve signaling
pathways such as β-catenin, β-catenin/TCF4/CT45A2, NF-κB,
Jak2/Stat3, and the regulation of related genes such as ATG5,
CT45A2, MMP and AEG-1, as well as the expression of proteins such
as Bcl-2, Bax, upregulation of E-cadherin, downregulation of
N-cadherin, vimentin, mTOR and sp1, alongside the involvement of
proteases and kinases such as caspase-3, caspase-9, urokinase,
cathepsin B, AMPK and VRK1 (Table
I).
 | Table I.Mechanism of action of ursolic acid
in lung cancer. |
Table I.
Mechanism of action of ursolic acid
in lung cancer.
| First author/s,
year | Disease | Real modules
(Animal/Cell/Patient) | Possible
mechanisms | Targets | Doses | Treatment
period | (Refs.) |
|---|
| Kang et al,
2021 | Lung cancer | Cell: A549,
H460 | Inducing apoptosis,
inhibiting | MMP2↓, MMP3↓, | 10 µM, | 24 h | (52) |
|
|
|
| cell proliferation,
and concen- | MMP9↓, PD-L1↓, | 20 µM |
|
|
|
|
|
| trating on blocking
the cell cycle. | CDK4↓, CCND1↓ |
|
|
|
|
|
|
|
| CCNE1↓,
CDKN1A↑, |
|
|
|
|
|
|
|
| CDKN1B↑,
VEGF↓, |
|
|
|
|
|
|
|
| pSTAT3↓ |
|
|
|
| Way et al,
2014 |
| Cell: A549,
H460 | Induces apoptosis
and inhibits | Caspase-3↑,
Caspase-9↑, | 30 µM | 24; 48 h | (49) |
|
|
|
| cell
proliferation. | Bax↑, Bcl-2↓,
p-mTOR↓, |
|
|
|
|
|
|
|
| FASN↓ |
|
|
|
| Lai et al,
2007 |
| Cell: H460 | Induces apoptosis
of tumor cells. | MMP-1↑,
MMP-2↑, | 10 µM | 24 h | (51) |
|
|
|
|
| MMP-3↑,
MMP-9↑, |
|
|
|
|
|
|
|
| MMP-10↑,
Caspase-3↑ |
|
|
|
| Gou et al,
2020 |
| Cell: H460,
A549 | Induces apoptosis
by activating | CHOP↑, Cyclin
D1↓, | 0-50 µM | 24, 48, | (45) |
|
|
|
| the endoplasmic
reticulum stress | CDK4↓p-PERK↑, |
| 72 h |
|
|
|
|
| pathway, arresting
the cell cycle. | p-eIF2α↑,
eIF2α↑ |
|
|
|
| Chen et al,
2019 |
| Cell: NCI-H292 | Induces apoptosis
through | AIF↑, Endo G↑, | 3, 6, 9, 12 | Respectively | (54) |
|
|
|
|
mitochondrial-dependent | BCL-Xs↑ | and 15 µM | 24, 48 h |
|
|
|
|
| pathway. |
|
|
|
|
| Ma et al,
2023 |
| Cell: A549 | Induces
mitochondrial apoptosis, | ARTS↑, p53↑, | 20 µg/ml | 24 h | (55) |
|
|
|
| create HA-Lipo/UA
delivery | ascapase-3↑, |
|
|
|
|
|
|
| system. | Cyto-C↑, Bcl-2↓,
Bax↑ |
|
|
|
| Castrejon- |
| Cell: A549 | Induces apoptosis,
induces | P62↑, Parkin↑ | 10 µg/ml | 24, 48 h | (56) |
| Jimenez et
al, 2019 |
|
| mitochondrial
phagocytosis. |
|
|
|
|
| Song et al,
2017 |
| Cell: H1299 | Induces cell death,
enhances cell radiosensitivity. | GSH↓, HIF-1α↓ | 50, 80 µg/ml | 24 h | (57) |
| Kim et al,
2015 |
| Cell: A549 | Induces apoptosis
and impedes | 53BP1 foci↓, | 50 µM | 12 h | (62) |
|
|
|
| DNA damage repair
capability. | α-p-CREB↓, |
|
|
|
|
|
|
|
| α-H3 p-Ser10↓ |
|
|
|
| Lamouille et
al, |
| Cell: HNBE, | Reduces cell
invasion, reduce | MMP-9↑,
MMP-2↑, | 2, 4, 8, | 48 h | (68) |
| 2014 |
| A549, H3255 | cell migration,
reduces cell | protein kinase
C↓ | 16 µMol/l |
|
|
|
|
| and Calu-6 | activity. |
|
|
|
|
| Liu et al,
2013 |
| Cell: H1975 | Inhibits cell
invasion and | EMT↓, MMP-2↓, | 0, 1, 5, 10, | 24 h | (70) |
|
|
|
| migration. | MMP-9↓,
Integrin | 15, 20, 25, |
|
|
|
|
|
|
| αvβ5↓ | 50, 100 ng/l |
|
|
| Jesudason et
al, |
| Cell: A549 and | Inhibits cell
metastasis and EMT | AEG-1↓,
E-cadherin↑, | 5, 10, 20, 30, | 24 h | (71) |
| 2000 |
| H1975 |
| N-cadherin↓,
vimentin↓ | 40 µM |
|
|
| Higgins et
al, |
| Cell: PC9,
H1299, | Inhibits cell
proliferation. | Sp1↓, DNMT1↓,
EZH2↓, | 30 µM | 48 h | (77) |
| 2009 |
| A549, H1650, H358
and H1975 |
| SAPK/JNK↓ |
|
|
|
| Way et al,
2014 |
| Cell: A549,
H460 | Inhibits cell
proliferation. | AMPK↑, mTOR↓, | 30 µM | 24, 48 h | (49) |
|
|
|
|
| Caspase-3↓,
Caspase-9↓, |
|
|
|
|
|
|
|
| Bcl-2↓, Bax↑ |
|
|
|
| Changotra et
al, |
| Cell: H1975 | Inhibits cell
proliferation. | CT45A2↓,
transcription | 25 µM | 24, 48, 72 h | (73) |
| 2022 |
|
|
|
factor/β-catenin↓, |
|
|
|
|
|
|
|
| TCF4↓, siNC↓ |
|
|
|
| Wu et al, 2015 |
| Cell: H460,
H1975, | Inhibits cell
proliferation and | ATG5↓, p-SAK↓, | 10 µM, | 24, 48, 72 h | (76) |
|
|
| A549, H1299, H520,
H82 and H446 | autophagy-related
genes. | p-S6↓, p-AKT↓ | 15 µM |
|
|
| Li et al, 2024 |
| Cell: A549 | Reduces cell
resistance and | Oct-4↓,
Sox-2↓, | 10, 20, 30, | 48 h | (82) |
|
|
|
| inhibits the
enrichment of tumor stem cells. | c-Myc↓, Jak2↑,
Stat3↑ | 40 µM |
|
|
| Chen et al,
2020 |
| Cell: LA795 | Inhibits neural
pathways, induces | TMEM16A↓, | 5, 10, 15, 20, | 24, 48, 72 h | (83) |
|
|
|
| DNA damage and cell
membrane | p-MEK1/2↓, | 30, 50 µM |
|
|
|
|
|
| damage, and design
hydrogel | p-ERK1/2↓, Cyclin
D1↓ |
|
|
|
|
|
|
| drug delivery
systems to enhance |
|
|
|
|
|
|
|
| targeting
ability. |
|
|
|
|
| Perez- |
| Cell: NSCLC,
A549 | Reduces chemical
resistance, | miR-149↓,
MyD88↓ | 5, 10, 20 µM | 24, 48, 72 h | (84) |
| Herrero et
al, |
|
| inhibits miRNA,
weakens |
|
|
|
|
| 2015 |
|
| stemness of
cells. |
|
|
|
|
Due to the limited water solubility, poor targeting
ability, and drug resistance of UA (95), the development of novel DDS,
including Nano-DDS and hydrogel-based delivery systems, has greatly
addressed these issues. These innovations significantly enhance the
delivery efficiency, targeting ability and bioavailability of UA,
improving the effectiveness of monotherapy and boosting the
antitumor effect through synergistic strategies. Furthermore, Ram
Kumar Pandian et al (96)
found that polyhydroxybutyrate NPs could deliver UA, enhancing its
stability and bioactivity, thus improving the treatment of tumors.
In another study by Sharma et al (97), HA-UA conjugates were used to prepare
PTX-loaded HA-UA NPs, which significantly inhibited tumor cell
proliferation in experimental models, offering new ideas and
methods for combined chemotherapy delivery. If these two delivery
systems are applied to the treatment of LC, they might yield
unforeseen effects and become a new direction for future research.
In addition, smoking as an important factor affecting LC is
detrimental to the whole process of carcinogenesis. It has been
identified that polycyclic aromatic hydrocarbons in cigarettes can
affect the metabolism of drugs by key drug-metabolizing enzymes of
cytochrome P450 and isoforms of the glucuronosyltransferase family,
shortening the duration of drug action by accelerating the
metabolism of related drugs and thus affecting therapeutic efficacy
(98). In addition, Zevin et
al (99) found that smoking
significantly upregulated CYP2E1 activity, increasing the rate of
metabolizing drugs while activating carcinogens and increasing the
risk of disease. Whether smoking affects the metabolism and
utilization of natural active ingredients, such as UA, has not been
confirmed by relevant experiments, and the authors will further
explore this issue in future studies.
Although UA shows remarkable therapeutic potential
in LC treatment, there are still some limitations: First, low
bioavailability: Its poor water solubility results in low
absorption and bioavailability in the body. Second, lack of
clinical trial data: Most studies on UA in LC remain at the
laboratory stage, lacking large-scale clinical trials to support
its safety and efficacy. In particular, clinical evidence for UA
combination chemotherapy in non-small cell carcinoma remains low
and a distant dream. Third, Dose and administration challenges: In
clinical application, determining the appropriate dose is crucial.
A very low dose may result in ineffective treatment, while a very
high dose could cause toxicity. Further experimental research is
needed to optimize dosing and administration methods. Fourth, drug
interactions: UA may interact with other drugs, influencing their
metabolism and therapeutic effects. Identifying interactions with
other drugs is one of the future research directions. Fifth, side
effects and toxicity: While UA is considered a relatively safe
natural compound, high doses or prolonged use may still produce
toxicity and side effects, which need further investigation. Sixth,
individual differences: Patients may have different responses to UA
based on factors such as genetic background, tumor type, stage and
overall health. Hence, it is essential to avoid generalizing
treatment approaches.
In conclusion, while UA has made significant
progress in non-clinical research, some issues and limitations
remain. In the future, the authors will focus research on the
cultivation of real animal cells, to deepen the analysis of their
multi-target regulatory networks and develop efficient delivery
systems, to promote the clinical translation of UA and its
derivatives, and ultimately to realize the leap from cellular
evidence to human disease intervention (Fig. 5).
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Program of the
Shandong Provincial Natural Science Foundation (grant no.
ZR2023QH079).
Availability of data and materials
Not applicable.
Authors' contributions
ZL wrote the manuscript and drew the pictures. QC
and ZC collected and organized literature. TP and JB proofread the
manuscript. FM are fully responsible for the study designing,
research fields, drafting, and finalizing the manuscript. All
authors read and approved the final version of the manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frydrychowicz M, Kuszel Ł, Dworacki G and
Budna-Tukan J: MicroRNA in lung cancer-a novel potential way for
early diagnosis and therapy. J Appl Genet. 64:459–477. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Juang YR, Ang L and Seow WJ: Predictive
performance of risk prediction models for lung cancer incidence in
Western and Asian countries: A systematic review and meta-analysis.
Sci Rep. 15:42592025. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang M and Ma C: LSR promotes cell
proliferation and invasion in lung cancer. Comput Math Methods Med.
2021:66519072021.PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
5
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI
|
|
6
|
Pallis AG and Syrigos KN: Lung cancer in
never smokers: Disease characteristics and risk factors. Crit Rev
Oncol Hematol. 88:494–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jones GS and Baldwin DR: Recent advances
in the management of lung cancer. Clin Med (Lond). 18 (Suppl
2):S41–S46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim RB: End-of-life care in patients with
advanced lung cancer. Ther Adv Respir Dis. 10:455–467. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Koh YC, Ho CT and Pan MH: Recent advances
in cancer chemoprevention with phytochemicals. J Food Drug Anal.
28:14–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Similie D, Minda D, Bora L, Kroškins V,
Lugiņina J, Turks M, Dehelean CA and Danciu C: An update on
pentacyclic triterpenoids ursolic and oleanolic acids and related
derivatives as anticancer candidates. Antioxidants (Basel).
13:9522024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pironi AM, de Araújo PR, Fernandes MA,
Salgado HRN and Chorilli M: Characteristics, biological properties
and analytical methods of ursolic acid: A review. Crit Rev Anal
Chem. 48:86–93. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mlala S, Oyedeji AO, Gondwe M and Oyedeji
OO: Ursolic acid and its derivatives as bioactive agents.
Molecules. 24:27512019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashyap D, Sharma A, Tuli HS, Punia S and
Sharma AK: Ursolic acid and oleanolic acid: Pentacyclic terpenoids
with promising Anti-inflammatory activities. Recent Pat Inflamm
Allergy Drug Discov. 10:21–33. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liobikas J, Majiene D, Trumbeckaite S,
Kursvietiene L, Masteikova R, Kopustinskiene DM, Savickas A and
Bernatoniene J: Uncoupling and antioxidant effects of ursolic acid
in isolated rat heart mitochondria. J Nat Prod. 74:1640–1644. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jayaprakasam B, Olson LK, Schutzki RE, Tai
MH and Nair MG: Amelioration of obesity and glucose intolerance in
high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in
Cornelian cherry (Cornus mas). J Agric Food Chem. 54:243–248. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yu SG, Zhang CJ, Xu XE, Sun JH, Zhang L
and Yu PF: Ursolic acid derivative ameliorates
Streptozotocin-induced diabestic bone deleterious effects in mice.
Int J Clin Exp Pathol. 8:3681–3690. 2015.PubMed/NCBI
|
|
18
|
Sundaresan A, Radhiga T and Pugalendi KV:
Effect of ursolic acid and Rosiglitazone combination on hepatic
lipid accumulation in high fat diet-fed C57BL/6J mice. Eur J
Pharmacol. 741:297–303. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Erdmann J, Kujaciński M and Wiciński M:
Beneficial effects of ursolic acid and its derivatives-focus on
potential biochemical mechanisms in cardiovascular conditions.
Nutrients. 13:39002021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shi Y, Leng Y, Liu D, Liu X, Ren Y, Zhang
J and Chen F: Research advances in protective effects of ursolic
acid and oleanolic acid against gastrointestinal diseases. Am J
Chin Med. 49:413–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen C, Ai Q, Shi A, Wang N, Wang L and
Wei Y: Oleanolic acid and ursolic acid: Therapeutic potential in
neurodegenerative diseases, neuropsychiatric diseases and other
brain disorders. Nutr Neurosci. 26:414–428. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sandhu SS, Rouz SK, Kumar S, Swamy N,
Deshmukh L, Hussain A, Haque S and Tuli HS: Ursolic acid: A
pentacyclic triterpenoid that exhibits anticancer therapeutic
potential by modulating multiple oncogenic targets. Biotechnol
Genet Eng Rev. 39:729–759. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Iqbal J, Abbasi BA, Ahmad R, Mahmood T,
Kanwal S, Ali B, Khalil AT, Shah SA, Alam MM and Badshahet H:
Ursolic acid a promising candidate in the therapeutics of breast
cancer: Current status and future implications. Biomed
Pharmacother. 108:752–756. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin JH, Xu JJ, Liu XF, Liu WH, Wang YF, Li
ZS, Wang LW and Wang W: Ursolic acid promotes apoptosis, autophagy,
and chemosensitivity in gemcitabine-resistant human pancreatic
cancer cells. Phytother Res. 34:2053–2066. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meng Y, Lin ZM, Ge N, Zhang DL, Huang J
and Kong F: Ursolic acid induces apoptosis of prostate cancer cells
via the PI3K/Akt/mTOR pathway. Am J Chin Med. 43:1471–1486. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang H, Zhang X, Ma X and Wang X: Ursolic
acid in colorectal cancer: Mechanisms, current status, challenges,
and future research directions. Pharmacol Rep. 77:72–86. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo JL, Han T, Bao L, Li XM, Ma JQ and
Tang LP: Ursolic acid promotes the apoptosis of cervical cancer
cells by regulating endoplasmic reticulum stress. J Obstet Gynaecol
Res. 45:877–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cai C, Zhi Y, Xie C, Geng S, Sun F, Ji Z,
Zhang P, Wang H and Tang J: Ursolic acid-downregulated long
noncoding RNA ASMTL-AS1 inhibits renal cell carcinoma growth via
binding to HuR and reducing vascular endothelial growth factor
expression. J Biochem Mol Toxicol. 37:e233892023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Namdeo P, Gidwani B, Tiwari S, Jain V,
Joshi V, Shukla SS, Pandey RK and Vyas A: Therapeutic potential and
novel formulations of ursolic acid and its derivatives: An updated
review. J Sci Food Agric. 103:4275–4292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma X, Bai Y, Liu K, Han Y, Zhang J, Liu Y,
Hou X, Hao E, Hou Y and Bai G: Ursolic acid inhibits the
cholesterol biosynthesis and alleviates high fat Diet-induced
hypercholesterolemia via irreversible inhibition of HMGCS1 in vivo.
Phytomedicine. 103:1542332022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fernández-Hernández A, Martinez A, Rivas
F, García-Mesa JA and Parra A: Effect of the solvent and the sample
preparation on the determination of triterpene compounds in
two-phase olive-mill-waste samples. J Agric Food Chem.
63:4269–4275. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu J: Pharmacology of oleanolic acid and
ursolic acid. J Ethnopharmacol. 49:57–68. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ikeda Y, Murakami A and Ohigashi H:
Ursolic acid: An anti- and pro-inflammatory triterpenoid. Mol Nutr
Food Res. 52:26–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang X, Xiong W, Wang X, Qin L, Zhong M,
Liu Y, Xiong Y, Yi X, Wang X and Zhang H: Ursolic acid attenuates
cholestasis through NRF2-mediated regulation of UGT2B7 and
BSEP/MRP2. Naunyn Schmiedebergs Arch Pharmacol. 397:2257–2267.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luan M, Wang H, Wang J, Zhang X, Zhao F,
Liu Z and Meng Q: Advances in Anti-inflammatory activity, mechanism
and therapeutic application of ursolic acid. Mini Rev Med Chem.
22:422–436. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Ursolic acid in cancer prevention and
treatment: Molecular targets, pharmacokinetics and clinical
studies. Biochem Pharmacol. 85:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Khwaza V, Oyedeji OO and Aderibigbe BA:
Ursolic Acid-based derivatives as potential anti-cancer agents: An
update. Int J Mol Sci. 21:59202020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang DY, Sp N, Jang KJ, Jo ES, Bae SW and
Yang YM: Antitumor effects of natural bioactive ursolic acid in
embryonic cancer stem cells. J Oncol. 2022:67372482022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang S, Chang X, Zhang J, Li J, Wang N,
Yang B, Pan B, Zheng Y, Wang X, Ou H and Wang Z: Ursolic acid
inhibits breast cancer metastasis by suppressing glycolytic
metabolism via activating SP1/Caveolin-1 signaling. Front Oncol.
11:7455842021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Panda SS, Thangaraju M and Lokeshwar BL:
Ursolic acid analogs as potential therapeutics for cancer.
Molecules. 27:89812022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang N, Liu S, Shi S, Chen Y, Xu F, Wei X
and Xu Y: Solubilization and delivery of Ursolic-acid for
modulating tumor microenvironment and regulatory T cell activities
in cancer immunotherapy. J Control Release. 320:168–178. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen H, Gao Y, Wang A, Zhou X, Zheng Y and
Zhou J: Evolution in medicinal chemistry of ursolic acid
derivatives as anticancer agents. Eur J Med Chem. 92:648–655. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Jiang H, Wei JH, Lin CY, Liang GB, He RJ,
Huang RZ, Ma XL, Huang GB and Zhang Y: Ursolic
acid-piperazine-dithiocarbamate ruthenium(II) polypyridyl complexes
induced necroptosis in MGC-803 cells. Metallomics. 14:mfac0722022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim JH, Kim YH, Song GY, Kim DE, Jeong YJ,
Liu KH, Chung YH and Oh S: Ursolic acid and its natural derivative
corosolic acid suppress the proliferation of APC-mutated colon
cancer cells through promotion of beta-catenin degradation. Food
Chem Toxicol. 67:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gou W, Luo N, Wei H, Wu H, Yu X, Duan Y,
Bi C, Ning H, Hou W and Li Y: Ursolic acid derivative UA232 evokes
apoptosis of lung cancer cells induced by endoplasmic reticulum
stress. Pharm Biol. 58:707–715. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sun L, Li B, Su X, Chen G, Li Y, Yu L, Li
L and Wei W: An ursolic acid derived small molecule triggers cancer
cell death through hyperstimulation of macropinocytosis. J Med
Chem. 60:6638–6648. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu X, Qi M, Li X, Wang J and Wang M:
Curcumin: A natural organic component that plays a Multi-faceted
role in ovarian cancer. J Ovarian Res. 16:472023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nedopekina DA, Gubaidullin RR, Odinokov
VN, Maximchik PV, Zhivotovsky B, Bel'skii YP, Khazanov VA,
Manuylova AV, Gogvadze V and Spivak AY: Mitochondria-targeted
betulinic and ursolic acid derivatives: Synthesis and anticancer
activity. Medchemcomm. 8:1934–1945. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Way TD, Tsai SJ, Wang CM, Ho CT and Chou
CH: Chemical constituents of Rhododendron formosanum show
pronounced growth inhibitory effect on non-small-cell lung
carcinoma cells. J Agric Food Chem. 62:875–884. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Abdel-Hamid NM and Abass SA: Matrix
metalloproteinase contribution in management of cancer
proliferation, metastasis and drug targeting. Mol Biol Rep.
48:6525–6538. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lai MY, Leung HW, Yang WH, Chen WH and Lee
HZ: Up-regulation of matrix metalloproteinase family gene
involvement in ursolic acid-induced human lung non-small carcinoma
cell apoptosis. Anticancer Res. 27:145–153. 2007.PubMed/NCBI
|
|
52
|
Kang DY, Sp N, Lee JM and Jang KJ:
Antitumor effects of ursolic acid through mediating the inhibition
of STAT3/PD-L1 signaling in Non-small cell lung cancer cells.
Biomedicines. 9:2972021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Nunnari J and Suomalainen A: Mitochondria:
In sickness and in health. Cell. 148:1145–1159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen CJ, Shih YL, Yeh MY, Liao NC, Chung
HY, Liu KL, Lee MH, Chou PY, Hou HY, Chou JS and Chung JG: Ursolic
acid induces apoptotic cell death through AIF and endo G release
through a Mitochondria-dependent pathway in NCI-H292 human lung
cancer cells in vitro. In Vivo. 33:383–391. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ma T, Zhou J, Li J and Chen Q: Hyaluronic
Acid-modified liposomes for ursolic Acid-targeted delivery treat
lung cancer based on p53/ARTS-mediated mitochondrial apoptosis.
Iran J Pharm Res. 22:e1317582023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Castrejón-Jiménez NS, Leyva-Paredes K,
Baltierra-Uribe SL, Castillo-Cruz J, Campillo-Navarro M,
Hernández-Pérez AD, Luna-Angulo AB, Chacón-Salinas R, Coral-Vázquez
RM, Estrada-García I, et al: Ursolic and oleanolic acids induce
mitophagy in A549 human lung cancer cells. Molecules. 24:34441019.
View Article : Google Scholar
|
|
57
|
Song B, Zhang Q, Yu M, Qi X, Wang G, Xiao
L, Yi Q and Jin W: Ursolic acid sensitizes radioresistant NSCLC
cells expressing HIF-1α through reducing endogenous GSH and
inhibiting HIF-1α. Oncol Lett. 13:754–762. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Smith G, Alholm Z, Coleman RL and Monk BJ:
DNA damage repair Inhibitors-combination therapies. Cancer J.
27:501–505. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang JY: DNA damage and apoptosis. Cell
Death Differ. 8:1047–1048. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kaina B: DNA damage-triggered apoptosis:
Critical role of DNA repair, double-strand breaks, cell
proliferation and signaling. Biochem Pharmacol. 66:1547–1554. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Campillo-Marcos I, García-González R,
Navarro-Carrasco E and Lazo PA: The human VRK1 chromatin kinase in
cancer biology. Cancer Lett. 503:117–128. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim SH, Ryu HG, Lee J, Shin J, Harikishore
A, Jung HY, Kim YS, Lyu HN, Oh E, Baek NI, et al: Ursolic acid
exerts anti-cancer activity by suppressing Vaccinia-related kinase
1-mediated damage repair in lung cancer cells. Sci Rep.
5:145702015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Han T, Kang D, Ji D, Wang X, Zhan W, Fu M,
Xin HB and Wang JB: How does cancer cell metabolism affect tumor
migration and invasion? Cell Adh Migr. 7:395–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Affara NI, Andreu P and Coussens LM:
Delineating protease functions during cancer development. Methods
Mol Biol. 539:1–32. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Liu J: Oleanolic acid and ursolic acid:
Research perspectives. J Ethnopharmacol. 100:92–94. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Xu F, Li M, Que Z, Su M, Yao W, Zhang Y,
Luo B, Li Y, Zhang Z and Tian J: Combined chemo-immuno-photothermal
therapy based on ursolic acid/astragaloside IV-loaded hyaluronic
acid-modified polydopamine nanomedicine inhibiting the growth and
metastasis of non-small cell lung cancer. J Mater Chem B.
11:3453–3472. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Huang CY, Lin CY, Tsai CW and Yin MC:
Inhibition of cell proliferation, invasion and migration by ursolic
acid in human lung cancer cell lines. Toxicol In Vitro.
25:1274–1280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ruan JS, Zhou H, Yang L, Wang L, Jiang ZS,
Sun H and Wang SM: Ursolic acid attenuates TGF-β1-induced
epithelial-mesenchymal transition in NSCLC by targeting integrin
αVβ5/MMPs signaling. Oncol Res. 27:593–600. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liu K, Guo L, Miao L, Bao W, Yang J, Li X,
Xi T and Zhao W: Ursolic acid inhibits epithelial-mesenchymal
transition by suppressing the expression of astrocyte-elevated
gene-1 in human nonsmall cell lung cancer A549 cells. Anticancer
Drugs. 24:494–503. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jesudason EC, Connell MG, Fernig DG, Lloyd
DA and Losty PD: Cell proliferation and apoptosis in experimental
lung hypoplasia. J Pediatr Surg. 35:129–133. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yang K, Chen Y, Zhou J, Ma L, Shan Y,
Cheng X, Wang Y, Zhang Z, Ji X, Chen L, et al: Ursolic acid
promotes apoptosis and mediates transcriptional suppression of
CT45A2 gene expression in non-small-cell lung carcinoma harbouring
EGFR T790M mutations. Br J Pharmacol. 176:4609–4624. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Changotra H, Kaur S, Yadav SS, Gupta GL,
Parkash J and Duseja A: ATG5: A central autophagy regulator
implicated in various human diseases. Cell Biochem Funct.
40:650–667. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Baeva ME and Camara-Lemarroy C: The role
of autophagy protein Atg5 in multiple sclerosis. Mult Scler Relat
Disord. 79:1050292023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wang M, Yu H, Wu R, Chen ZY, Hu Q, Zhang
YF, Gao SH and Zhou GB: Autophagy inhibition enhances the
inhibitory effects of ursolic acid on lung cancer cells. Int J Mol
Med. 46:1816–1826. 2020.PubMed/NCBI
|
|
76
|
Wu J, Zhao S, Tang Q, Zheng F, Chen Y,
Yang L, Yang X, Li L, Wu W and Hann SS: Activation of SAPK/JNK
mediated the inhibition and reciprocal interaction of DNA
methyltransferase 1 and EZH2 by ursolic acid in human lung cancer
cells. J Exp Clin Cancer Res. 34:992015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Higgins MJ and Ettinger DS: Chemotherapy
for lung cancer: The state of the art in 2009. Expert Rev
Anticancer Ther. 9:1365–1378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Souhami RL: Defining the role of
chemotherapy in non-small-cell lung cancer. Ann Oncol. 6:317–318.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wu T, Yan D, Hou W, Jiang H, Wu M, Wang Y,
Chen G, Tang C, Wang Y and Xu H: Biomimetic red blood cell
membrane-Mediated nanodrugs loading ursolic acid for targeting
NSCLC therapy. Cancers (Basel). 14:45202022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shen M, Xu Z, Xu W, Jiang K, Zhang F, Ding
Q, Xu Z and Chen Y: Inhibition of ATM reverses EMT and decreases
metastatic potential of cisplatin-resistant lung cancer cells
through JAK/STAT3/PD-L1 pathway. J Exp Clin Cancer Res. 38:1492019.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fan L, Wang X, Cheng C, Wang S, Li X, Cui
J, Zhang B and Shi L: Inhibitory effect and mechanism of ursolic
acid on Cisplatin-induced resistance and stemness in human lung
cancer A549 cells. Evid Based Complement Alternat Med.
2023:13073232023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li S, Guo X, Liu H, Chen Y, Wan H, Kang X,
Qin J and Guo S: Ursolic acid, an inhibitor of TMEM16A, co-loaded
with cisplatin in hydrogel drug delivery system for multi-targeted
therapy of lung cancer. Int J Biol Macromol. 277:1345872024.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen Q, Luo J, Wu C, Lu H, Cai S, Bao C,
Liu D and Kong J: The miRNA-149-5p/MyD88 axis is responsible for
ursolic Acid-mediated attenuation of the stemness and
chemoresistance of non-small cell lung cancer cells. Environ
Toxicol. 35:561–569. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Pérez-Herrero E and Fernández-Medarde A:
Advanced targeted therapies in cancer: Drug nanocarriers, the
future of chemotherapy. Eur J Pharm Biopharm. 93:52–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fu S, Li G, Zang W, Zhou X, Shi K and Zhai
Y: Pure drug Nano-assemblies: A facile carrier-free nanoplatform
for efficient cancer therapy. Acta Pharm Sin B. 12:92–106. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Jinhua W: Ursolic acid: Pharmacokinetics
process in vitro and in vivo, a mini review. Arch Pharm (Weinheim).
352:e18002222019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Antonio E, Dos Reis Antunes Junior O,
Marcano RGDJV, Diedrich C, da Silva Santos J, Machado CS, Khalil NM
and Mainardes RM: Chitosan modified poly (lactic acid)
nanoparticles increased the ursolic acid oral bioavailability. Int
J Biol Macromol. 172:133–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Yang L, Sun Z, Zu Y, Zhao C, Sun X, Zhang
Z and Zhang L: Physicochemical properties and oral bioavailability
of ursolic acid nanoparticles using supercritical anti-solvent
(SAS) process. Food Chem. 132:319–325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Yu D, Kan Z, Shan F, Zang J and Zhou J:
Triple strategies to improve oral bioavailability by fabricating
coamorphous forms of ursolic acid with piperine: Enhancing
Water-solubility, permeability, and inhibiting cytochrome P450
isozymes. Mol Pharm. 17:4443–4462. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chen Q, Luo S, Zhang Y and Chen Z:
Development of a liquid chromatography-mass spectrometry method for
the determination of ursolic acid in rat plasma and tissue:
Application to the pharmacokinetic and tissue distribution study.
Anal Bioanal Chem. 399:2877–2884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Wang M, Zhao T, Liu Y, Wang Q, Xing S, Li
L, Wang L, Liu L and Gao D: Ursolic acid liposomes with chitosan
modification: Promising antitumor drug delivery and efficacy. Mater
Sci Eng C Mater Biol Appl. 71:1231–1240. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Lei P, Li Z, Hua Q, Song P, Gao L, Zhou L
and Cai Q: Ursolic acid alleviates neuroinflammation after
intracerebral hemorrhage by mediating microglial pyroptosis via the
NF-κB/NLRP3/GSDMD pathway. Int J Mol Sci. 24:147712023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sun K, Wu H, Zhu Q, Gu K, Wei H, Wang S,
Li L, Wu C, Chen R, Pang Y, et al: Global landscape and trends in
lifetime risks of haematologic malignancies in 185 countries:
Population-based estimates from GLOBOCAN 2022. EClinicalMedicine.
83:1031932025. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
95
|
Wu BY and Parks LM: Chemical studies on
ursolic acid. J Am Pharm Assoc Am Pharm Assoc. 42:603–606. 1953.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Ram Kumar Pandian S, Kunjiappan S, Pavadai
P, Sundarapandian V, Chandramohan V and Sundar K: Delivery of
ursolic acid by polyhydroxybutyrate nanoparticles for cancer
therapy: In silico and in vitro studies. Drug Res (Stuttg).
72:72–81. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sharma R, Yadav V, Jha S, Dighe S and Jain
S: Unveiling the potential of ursolic acid modified hyaluronate
nanoparticles for combination drug therapy in triple negative
breast cancer. Carbohydr Polym. 338:1221962024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
O'Malley M, King AN, Conte M, Ellingrod VL
and Ramnath N: Effects of cigarette smoking on metabolism and
effectiveness of systemic therapy for lung cancer. J Thorac Oncol.
9:917–926. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zevin S and Benowitz NL: Drug interactions
with tobacco smoking. An update. Clin Pharmacokinet. 36:425–438.
1999. View Article : Google Scholar : PubMed/NCBI
|