Introduction
Hepatocellular carcinoma (HCC) is the leading cause
of liver cancer, accounting for an overwhelming 90% of all cases
(1). This stark reality highlights
the urgent need for proactive prevention, early detection, and
treatment measures to combat this formidable disease. The treatment
options for patients with HCC depend on their clinical stage
(2). Due to the difficulties in
early detection and the rapid progression of the disease, numerous
patients are diagnosed at advanced stages, and chemotherapeutic
agents are the primary form of systemic therapy for these patients
(1).
Sorafenib and regorafenib are oral multi-kinase
inhibitor that targets several tyrosine kinases, including
serine-threonine kinases Raf-1 and B-Raf, which are involved in the
MAPK/ERK pathway (3,4). They are also found to inhibit the
platelet-derived growth factor receptor and the vascular
endothelial growth factor receptors (VEGFR-2/3) (5). Sorafenib and regorafenib have been
approved by the Food and Drug Administration as the first-line and
second-line treatments, respectively, to help control tumor
angiogenesis and progression in patients with advanced HCC
(6,7). While sorafenib and regorafenib are the
main systemic therapies for advanced HCC, their effectiveness and
application are frequently hindered by severe adverse reactions and
the common development of drug resistance (8). Grasping the intricate mechanisms
behind this resistance is essential for devising effective
strategies to overcome it.
Prolonged use of antitumor drugs can frequently lead
to the development of acquired resistance, ultimately undermining
the effectiveness of these vital treatments. Multiple critical
pathways have been identified that lead to acquired resistance to
sorafenib in HCC (9). Importantly,
inhibiting T-cell attack in the tumor microenvironment (TME) plays
a significant role in promoting drug resistance (10). Programmed death-ligand-1 (PD-L1) is
an immune checkpoint protein found on numerous cancer cells that
suppresses anticancer immunity by interacting with PD-1 on T cells
(11). Overexpression of PD-L1 is
found to increase the drug resistance in cisplatin-resistant small
cell lung cancer cells, enzalutamide-resistant prostate cancer, and
sorafenib-resistant HCC (12–14).
Accordingly, PD-1-PD-L1 blockade has been shown to foster
CD8+ T-cell infiltration and therapeutic efficacy of
sorafenib and regorafenib in tumor-bearing mouse models (15,16).
Despite the critical role of PD-L1 in promoting resistance to
sorafenib and regorafenib in HCC, the mechanisms underlying PD-L1
upregulation in HCC cells following these drug treatments remain
incompletely understood (17).
IL-33 is a member of the IL-1 cytokine family and is
widely expressed in various cell types, primarily localized in the
nucleus to regulate gene transcription (18). Importantly, IL-33 is able to be
released as an alarmin to activate innate and adaptive immune
responses by binding its specific receptor ST2L in response to cell
stress, pathogen infection, tissue injury and necrotic cell death
(19–21). When IL-33 binds to ST2L, this
signaling triggers the MyD88-dependent pathways and subsequently
induces the activation of activator protein-1 and NF-κB
transcription factors, leading to inflammatory gene expression
(22,23). This IL-33/ST2L signaling has been
observed in accelerating multiple types of cancer progression,
including HCC (24,25). In HCC, IL-33/ST2L signaling has been
shown to enhance the stemness of HCC cells and alter the immune
responses within the TME, leading to accelerated HCC progression
(25–27). Notably, our current findings
indicate that lung cancer cells treated with cisplatin can release
IL-33, creating a positive feedback loop through IL-33/ST2L that
limits the efficacy of cisplatin therapy (28). However, it is unclear whether
IL-33/ST2L signaling participates in the development of acquired
resistance to sorafenib or regorafenib in HCC.
In the present study, it was found that sorafenib
and regorafenib treatments induced cell senescence in HCC and
promoted the secretion of IL-33 through an IL-33/ST2L positive
feedback loop. The secreted IL-33 enhanced PD-L1 expression in HCC
cells by activating NF-κB pathways in response to these treatments.
Blockage of the IL-33 signaling pathway using anti-IL-33 or
anti-ST2L antibodies alongside sorafenib resulted in significant
tumor growth reduction in HCC-bearing mice, decreased tumor PD-L1
expression, and increased CD8+ T cell infiltration.
However, the increased efficacy was not observed in
immunocompromised mice, indicating the crucial role of T cells in
the reduced efficacy of sorafenib due to IL-33. The current
findings highlight how IL-33 may undermine the effectiveness of
sorafenib and regorafenib, suggesting that targeting the IL-33/ST2L
axis could enhance therapeutic outcomes in HCC.
Materials and methods
Cell lines and culture
Human hepatoma cell lines Huh-7 cells were obtained
from the Bioresource Collection and Research Center.
ML-15a is a mouse hepatoma cell line that was developed
from ML-1 cells after being adapted through five generations in
BALB/c mice. It originates from the parental ML-1 hepatoma cell
line, which was established using liver cells from BALB/c mice that
were transformed with the hepatitis B virus X (HBx) gene (29). It was noted that ML-1 is not
registered in the Cellosaurus database and therefore does not have
an assigned CVCL number. Huh7 and ML-15a cells were
maintained in Dulbecco's Modified Eagle's Medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine
serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Gibco; Thermo Fisher Scientific, Inc.) and
cultured at 37°C with 5% CO2 in a humidified
incubator.
Cell death assay
Huh-7 cells (5×103 cells/well) were
seeded in 96-well plates and treated with sorafenib (cat. no.
SC-220125; Santa Cruz Biotechnology) or regorafenib (cat. no.
SC-477163; Santa Cruz Biotechnology) at the indicated
concentrations for 24 to 96 h. Cell death was assessed using the
FITC Annexin V Apoptosis Detection Kit with propidium iodide (cat.
no. 640914; BioLegend, Inc.) according to the manufacturer's
instructions. Stained cells were analyzed by a CytoFLEX flow
cytometer (Beckman Coulter, Inc.), and the acquired data were
processed with CytExpert software (version 2.3; Beckman Coulter,
Inc.).
Western blot analysis
Cells were harvested and lysed with Cell Lysis
Buffer (cat. no. 9803; Cell Signaling Technology, Inc.)
supplemented with protease and phosphatase inhibitors. A total of
30 µg of protein lysates, quantified using the Bradford assay, was
separated on 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis and transferred onto polyvinyl difluoride
membranes. Membranes were then blocked with 5% skimmed milk in TBST
(TBS containing 0.1% Tween-20) for 1 h at room temperature and
incubated with specific primary antibodies overnight at 4°C. The
antibodies used were as follows: anti-IL-33 (1:1,000; cat. no.
LG3314; Leadgene Biomedical), anti-p16 (1:2,000; cat. no. ab81278;
Abcam), anti-p21 (1:1,000; cat. no. 2947S; Cell Signaling
Technology, Inc.), anti-PD-L1 (1:1,000; cat. no. 13684S; Cell
Signaling Technology, Inc.) and anti-β-actin (1:5,000; cat. no.
ab8226-20; Abcam). The membranes were then washed and incubated
with horseradish peroxidase-conjugated anti-mouse (1:5,000; cat.
no. GTX213111-01; GeneTex, Inc.) or anti-rabbit (1:5,000; cat. no.
GTX213110-01; GeneTex, Inc.) antibodies for 1 h at room
temperature. Protein expression was visualized by enhanced
chemiluminescence treatment using Western Lightning Plus-ECL
(PerkinElmer, Inc.) and Amersham™ Imager 600 (GE Healthcare).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using the
Quick-RNA™ MiniPrep kit (cat. no. R1055; Zymo Research), and then 1
µg of total RNA was reverse transcribed into cDNA using M-MLV
Reverse Transcriptase (cat. no. LDG0006RF; Leadgene Biomedical)
with random primers under the following conditions: 25°C for 10 min
(primer annealing), 42°C for 60 min (cDNA synthesis), and 70°C for
10 min (enzyme inactivation). qPCR was performed with the FastStart
Universal SYBR Green Master (Rox) (cat. no. 04913850001; Roche
Diagnostics) in the StepOnePlus™ Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). PCR amplification was
performed with an initial denaturation at 95°C for 10 min, followed
by 40 cycles consisting of denaturation at 95°C for 15 sec and
combined annealing/extension at 60°C for 1 min. Gene expression
levels were normalized to ACTB, and relative expression was
calculated using the 2−ΔΔCq method (30). The following primer sequences were
used: IL33 (orward, 5′-CAAAGAAGTTTGCCCCATGT-3′ and reverse,
5′-AAGGCAAAGCACTCCACAGT-3′; CD274 forward,
5′-AAACAATTAGACCTGGCTG-3′ and reverse, 5′-TCTTACCACTCAGGACTTG-3′;
and ACTB forward, 5′-AAGGAGAAGCTGTGCTACGTCGC-3′ and reverse,
5′-AGACAGCACTGTGTTGGCGTACA-3′.
Cell proliferation analysis
Huh-7 cells (2×105 cells/well) were
seeded in 6-well plates and treated with sorafenib (1 and 10 µM) or
regorafenib (10 and 20 µM) at the indicated concentrations for 5 to
7 days. At the end of treatment, cells were trypsinized, collected,
and stained with 0.4% Trypan Blue solution (cat. no. T8154;
MilliporeSigma) to assess cell viability. Viable (unstained) and
non-viable (stained) cells were counted using a hemocytometer under
a light microscope. Cell proliferation was determined by
quantifying the number of viable cells at each time point.
Senescence-associated β-galactosidase
(SA-β-gal) staining
SA-β-gal activity was detected using the Senescence
β-Galactosidase Staining Kit (cat. no. 9860; Cell Signaling
Technology, Inc.) according to the manufacturer's instructions.
Briefly, Huh-7 cells were treated with sorafenib (10 µM) or
regorafenib (20 µM) for 4 days. After treatment, cells were washed
with PBS and fixed with 1X Fixative Solution for 10-15 min at room
temperature. Following two washes with PBS, cells were incubated
with β-galactosidase staining solution (pH 6.0) at 37°C in a sealed
container without CO2 for 1 to 2 days. SA-β-gal-positive
cells were observed and imaged using an Olympus BX61 light
microscope (Olympus Corporation).
Flow cytometry
To detect surface expression of PD-L1 on Huh-7
cells, the cells were harvested and washed with PBS. The prepared
cells were incubated with APC-conjugated anti-human PD-L1 antibody
(1:200; cat. no. 393609; BioLegend, Inc.) in staining buffer (2%
FBS and 0.1% sodium azide in PBS). The staining was performed for
30 min on ice in the dark, followed by flow cytometric analysis.
For immunophenotyping of tumor-infiltrating immune cells isolated
from HCC-bearing mice, single-cell suspensions were incubated with
fluorochrome-conjugated monoclonal antibodies in staining buffer
for 30 min on ice in the dark. The following antibodies were used:
FITC anti-mouse CD8a antibody (cat. no. 553031; BD Biosciences), PE
anti-mouse CD4 antibody (cat. no. 553049; BD Biosciences) and PE
anti-mouse CD152 (CTLA-4) antibody (cat. no. 106305; BioLegend,
Inc.). The mean fluorescence intensity and the proportion of cells
within a specific gate were detected using a CytoFLEX (Beckman
Coulter, Inc.) and analyzed using CytExpert software (Beckman
Coulter, Inc.).
HCC-bearing subcutaneous mouse
model
A total of 36 male BALB/c mice and 20 male nude mice
(8 weeks-old; weighing 25 g) were used. Mice were housed under
standard conditions with a temperature of 22±2°C, relative humidity
of 50±5%, a 12/12-h light/dark cycle, and ad libitum access
to food and water. All animal experiments were conducted in
accordance with National Cheng Kung University institutional
guidelines for care and use of laboratory animals (approval no.
107130; Tainan, Taiwan). To establish the subcutaneous tumor model,
1×106 ML-15a cells were injected into the
flank of BALB/c or nude mice. Mice harboring HCC allografts were
treated with IgG (10 µg/g), sorafenib (10 µg/g), α-IL-33 (10 µg/g;
cat. no. LG3314; Leadgene Biomedical), and/or α-ST2L (10 µg/g; cat.
no. LGT2105; Leadgene Biomedical) via intraperitoneal (i.p.)
injection. Mice received daily i.p. injections of sorafenib from
day 7 to day 27. For both monotherapy and combination therapy
groups, α-IL-33 or α-ST2L antibodies were administered
intraperitoneally on days 7, 14 and 21 post-inoculation. Tumor
volume was measured every four days starting on day 5
post-inoculation using a digital caliper and calculated using the
formula: V=(length2 × width)/2. For HCC-bearing nude
mice, mice received daily i.p. injections of sorafenib (10 µg/g)
from day 4 to day 23, and/or α-IL-33 (10 µg/g) on days 4, 11, and
18. On day 25 or 29 after subcutaneous injection, the HCC-bearing
mice were sacrificed, and their solid tumors were removed to
determine the tumor weight and infiltrated phenotypes of immune
cells by flow cytometry. In the present study, mice were euthanized
by carbon dioxide (CO2) inhalation, with CO2
introduced into the chamber at a displacement rate of 30% of the
chamber volume per min, in accordance with institutional animal
care guidelines. Death was confirmed by the absence of respiration
and heartbeat, followed by cervical dislocation as a secondary
method. All animal experiments were performed between 2020 and
2021.
Immunohistochemistry assay
Formalin-fixed, paraffin-embedded tissue sections
(4-µm thick) of mouse HCC tumors were deparaffinized with xylene
and rehydrated through a graded series of ethanol solutions.
Antigen retrieval was performed by heating the sections in 10 mM
citrate buffer (pH 6.0) at 95-100°C for 10 min. Endogenous
peroxidase activity was blocked using 3% hydrogen peroxide for 15
min at room temperature. After washing, the sections were blocked
with blocking reagent for 1 h at room temperature and then
incubated with primary antibodies against IL-33 (1:200; cat. no.
LG3314; Leadgene Biomedical) and PD-L1 (1:200; cat. no. 13684S;
Cell Signaling Technology, Inc.) at 4°C overnight. The
immunoreactions were revealed using the secondary antibody of
OneStep Polymer HRP anti-mouse/rat/rabbit Detection System (cat.
no. GTX83398; GeneTex, Inc.) and using DAB as chromogen. Nuclei
were counterstained with Mayer's hematoxylin (cat. no. HMM125;
ScyTek Laboratories Inc.), and slides were subsequently dehydrated
and mounted for microscopic analysis.
Enzyme-linked immunosorbent assay
(ELISA) assay of IL-6 concentration
Huh7 cells were treated with sorafenib (10 µM) or
regorafenib (20 µM) for 72 h, and the culture supernatants were
collected. Human IL-6 levels were quantified using a human IL-6
DuoSet ELISA kit (cat. no. DY206; R&D Systems, Inc.) according
to the manufacturer's instructions. Optical density (OD) was
measured at 450 nm using a SpectraMax iD5 Multi-Mode Microplate
Reader (Molecular Devices). The IL-6 concentrations were calculated
from a standard curve (Fig.
S2).
Correlation analysis of IL33 and CD274
expression in patients with liver hepatocellular carcinoma
(LIHC)
The correlation between IL33 and CD274
expression in LIHC was analyzed using the TIMER2.0 web server
(http://timer.cistrome.org/) (31), which integrates RNA-seq data from
The Cancer Genome Atlas (TCGA; http://portal.gdc.cancer.gov). Spearman's correlation
analysis was performed to assess the association between
IL33 and CD274 mRNA expression levels in the
TCGA-LIHC cohort (Fig. S3).
Statistical analysis
Data are presented as the mean ± standard deviations
(SD). Data were analyzed using one-way ANOVA followed by Tukey's
post hoc test with GraphPad Prism 5 software (GraphPad Software
Inc.; Dotmatics). P<0.05 was considered to indicate a
statistically significant difference.
Results
Sorafenib and regorafenib trigger an
IL-33/ST2L positive feedback loop in senescent HCC cells
Our current findings showed that cisplatin-treated
lung cancer cells can release IL-33 to cause an IL-33/ST2L positive
feedback loop to limit cisplatin therapeutic efficacy (28). IL-33/ST2L signaling has been
implicated in promoting the progression of HCC (26,32).
However, whether HCC cells can release IL-33 in response to stress
induced by sorafenib and regorafenib remains unclear. To
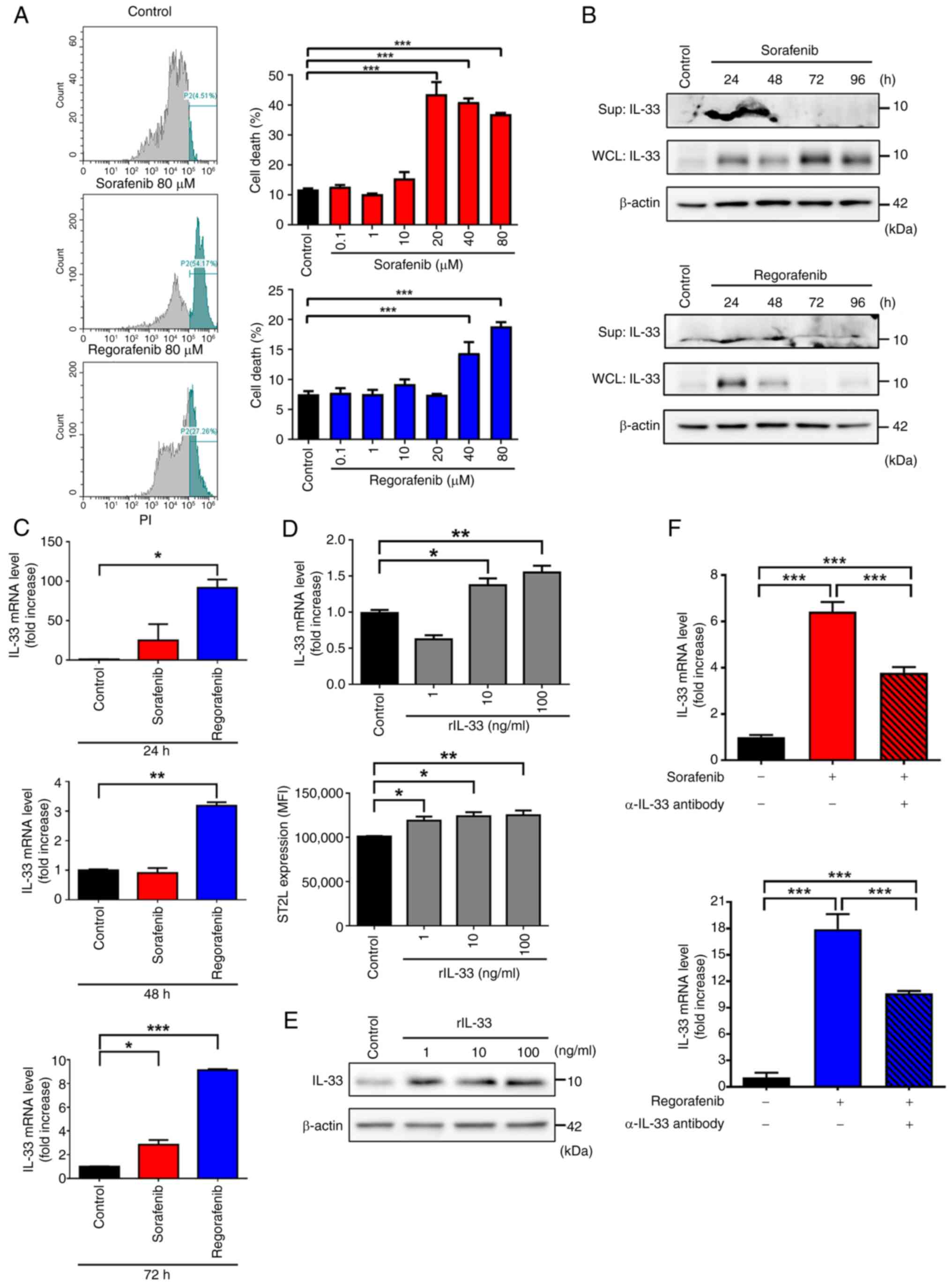
investigate this, Huh-7 cells were treated with various doses of
sorafenib and regorafenib. Significant cytotoxicity was observed at
concentrations exceeding 20 µM for sorafenib and 40 µM for
regorafenib (Fig. 1A). To avoid the
passive release of IL-33 due to cell death, Huh-7 cells were
treated with 10 µM sorafenib and 20 µM regorafenib in the following
experiments. As shown in Fig. 1B,
both sorafenib and regorafenib induced the release of IL-33 in
Huh-7 cells at 24 and 48 h post-treatment. Interestingly, a notable
increase in the cytosolic protein level of IL-33 and the mRNA level
of IL-33 was observed in both sorafenib and regorafenib-treated
Huh-7 cells (Fig. 1B and C). Since
an IL-33 positive feedback loop has been previously identified in
macrophages and lung cancer cells (28,33),
it was next tested whether this loop occurs in sorafenib and
regorafenib-treated Huh-7 cells. To examine this, the mRNA levels
of IL33 and ST2L were first measured in recombinant
IL-33 (rIL-33)-treated Huh-7 cells. Similar to previous findings,
rIL-33 treatment upregulated the mRNA levels of IL33 and
protein levels of surface ST2L in Huh-7 cells (Fig. 1D). Additionally, increased protein
levels of IL-33 were also observed following rIL-33 treatment
(Fig. 1E). Furthermore, blocking
IL-33 signaling with a neutralizing anti-IL-33 (α-IL-33) antibody
abolished the upregulation of IL-33 induced by sorafenib and
regorafenib in Huh-7 cells (Fig.
1F), indicating that sorafenib and regorafenib trigger an
IL-33/ST2L positive feedback loop in HCC cells. Notably, a recent
study pointed out that IL-33 was highly induced in senescent
hepatic stellate cells within the TME of HCC (27). Given that sorafenib and regorafenib
treatment can lead to cellular senescence in HCC cells (34), it was hypothesized that IL-33 is
induced in senescent HCC cells triggered by these treatments. To
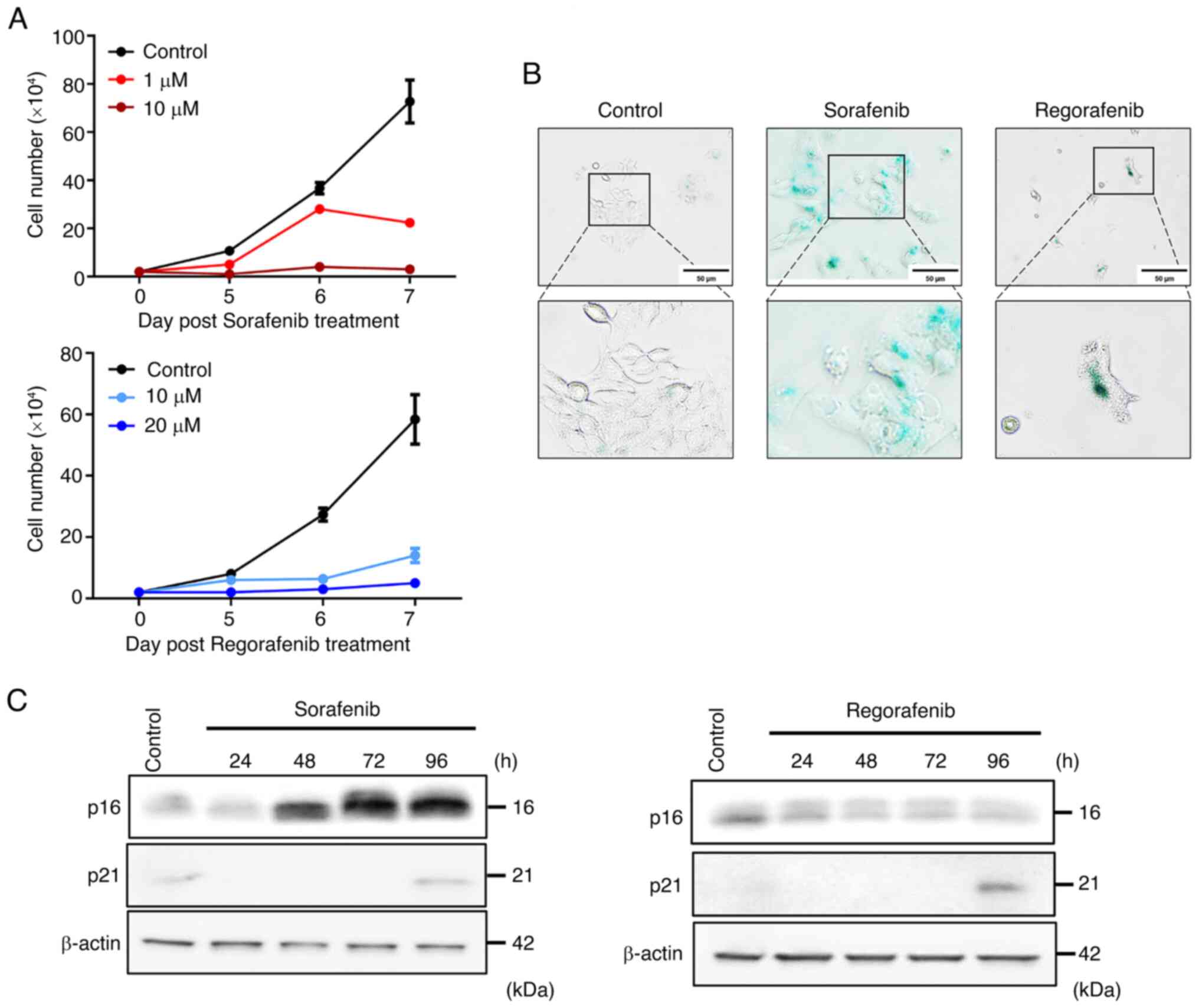
examine whether sorafenib and regorafenib can cause cell growth
inhibition, a characteristic of cellular senescence, Huh-7 cells
were treated with various concentrations of sorafenib and
regorafenib for 24 to 96 h. Significant cell growth arrest was
observed in Huh-7 cells treated with 10 µM sorafenib and 20 µM
regorafenib (Fig. 2A).
Additionally, a significant increase in SA-β-galactosidase
expression was observed in sorafenib and regorafenib-treated Huh-7
cells (Fig. 2B). It has been
reported that cellular senescence is mainly regulated by
p16INK4/CDKN2 and p21WAF1/Cip1 pathways
(35). The present results revealed
that sorafenib induced upregulation of p16, while regorafenib
increased p21 expression post-treatment compared with the control
group (Fig. 2C). These findings
collectively indicate that sorafenib and regorafenib trigger an
IL-33/ST2L positive feedback loop in senescent HCC cells.
Sorafenib and regorafenib treatments
lead to PD-L1 upregulation via IL-33 signaling in HCC cells
It has been reported that IL-33/ST2L signaling can
induce PD-L1 expression, leading to immune escape in oral squamous
cell carcinoma (36). Notably,
elevated PD-L1 expression in patients with HCC correlates with poor
survival and resistance to sorafenib (14). It was investigated whether sorafenib
and regorafenib treatments increase PD-L1 expression via IL-33
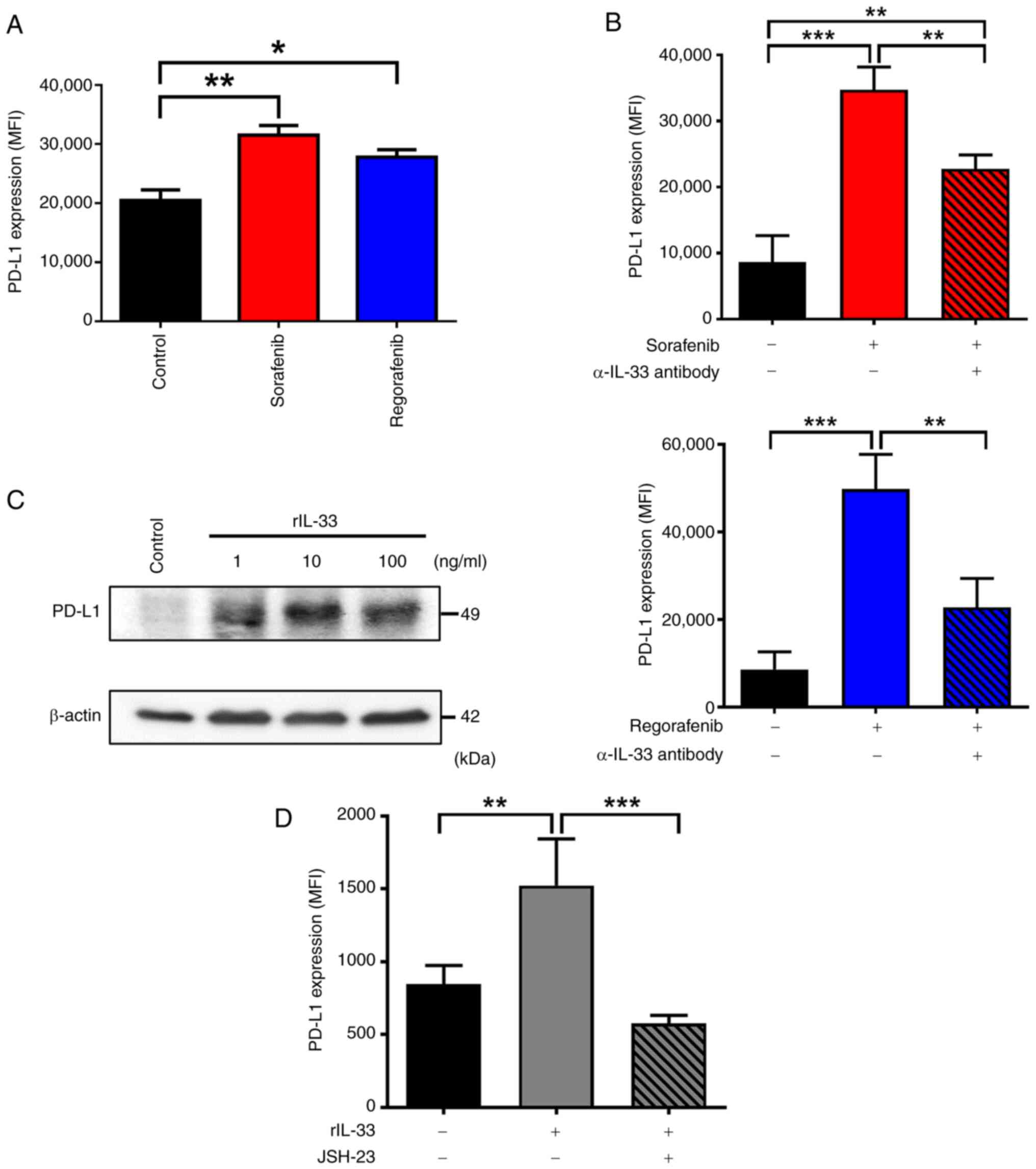
release. As shown in Fig. 3A, both
sorafenib and regorafenib enhanced PD-L1 expression on the plasma
membrane of Huh-7 cells. Blocking IL-33 signaling with a
neutralizing α-IL-33 antibody abolished the upregulation of PD-L1
induced by sorafenib and regorafenib in Huh-7 cells (Fig. 3B), suggesting that IL-33 released
from these drug-treated cells induces PD-L1 expression. To further
examine whether IL-33 induces PD-L1 upregulation in HCC cells,
Huh-7 cells were treated with rIL-33 protein. The immunoblot
results revealed that protein levels of PD-L1 increased following
stimulation with rIL-33 (Fig. 3C).
Additionally, elevated PD-L1 on the surface of rIL-33-treated Huh-7
cells was also detected (Fig. 3D),
confirming that IL-33 is capable of inducing PD-L1 expression in
HCC cells. The regulation of PD-L1 expression mainly involves the
participation of transcriptional regulators, including NF-κB
(37). Given that our current
findings revealed that NF-κB mediates IL-33-dependent actions in
the TME (28), it was examined
whether IL-33 induced PD-L1 expression via NF-κB in HCC cells. The
current data showed that rIL-33-induced upregulation of PD-L1 was
significantly abolished in the presence of NF-κB inhibitor
(Fig. 3D). These data collectively
indicated that sorafenib and regorafenib treatments lead to PD-L1
upregulation via IL-33 signaling in HCC cells.
Blockage of IL-33 or ST2L enhances the
antitumor immune responses and therapeutic efficacy of sorafenib in
HCC-bearing mice
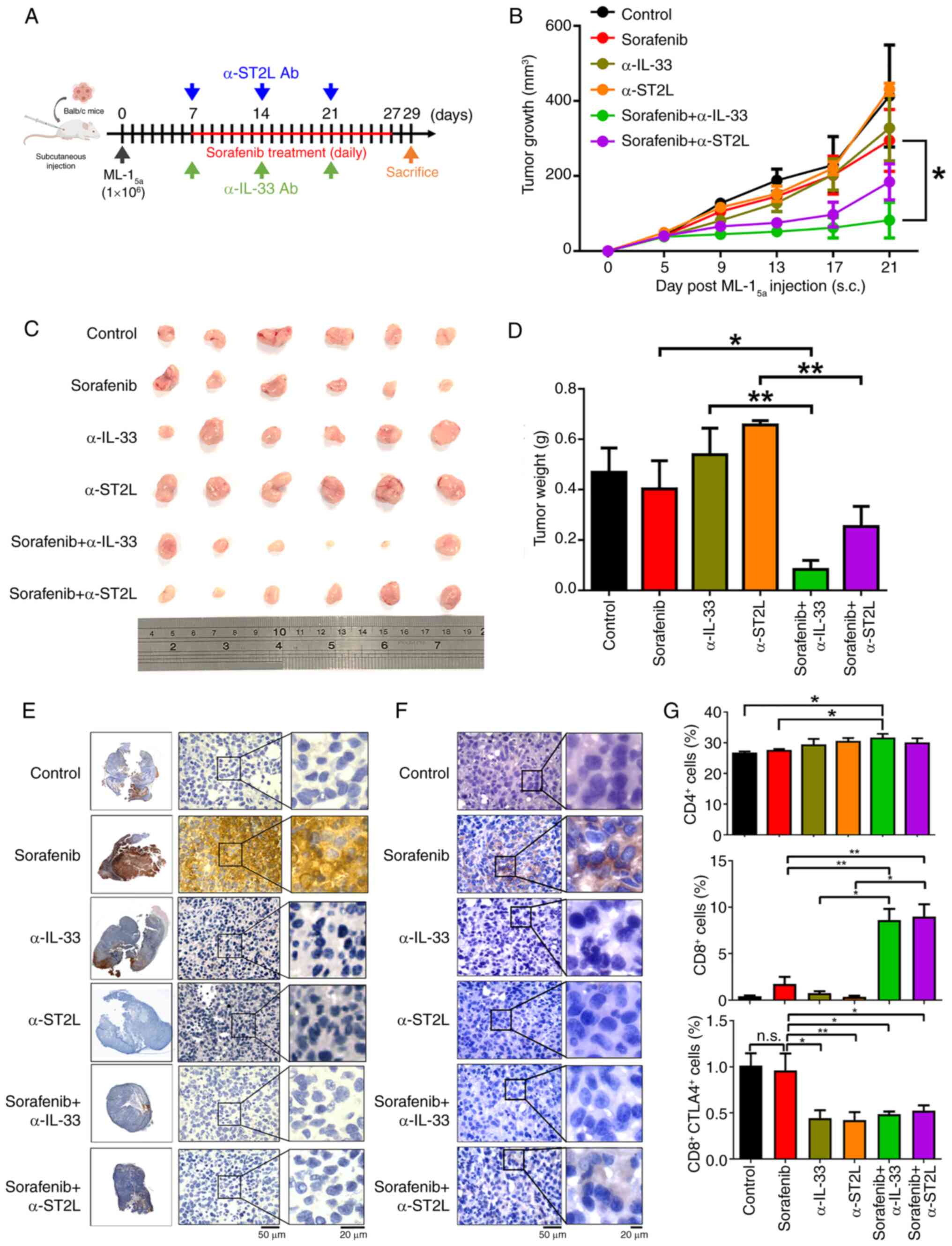
Next, it was examined whether blocking IL-33/ST2L
signaling could enhance the therapeutic efficacy of sorafenib in
HCC-bearing mice. To test this hypothesis, low-dose sorafenib
treatment (10 mg/kg) (38) was
combined with α-IL-33 or α-ST2L neutralizing antibodies (10 mg/kg)
in a subcutaneous HCC mouse model by inoculating a mouse HCC cell
line ML-15a in BALB/c mice (39) (Fig.
4A). To assess whether these combination treatments could cause
significant toxicity in mice, body weights and serum levels of
glutamic oxaloacetic transaminase (GOT), glutamic pyruvic
transaminase (GPT), albumin, blood urea nitrogen (BUN) and
creatinine were monitored. The results revealed no significant
changes in these parameters in mice undergoing the combined
treatment compared with control mice (Fig. S1A and B), indicating that no
significant toxicity was induced by these treatments. In Fig. 4C, the maximum tumor observed
measured 9 mm in length and 13 mm in width, corresponding to a
calculated tumor volume of 526.5 mm3 and a maximum
diameter of 13 mm. It was found that single treatments with
sorafenib, α-IL-33, or α-ST2L neutralizing antibodies in
HCC-bearing mice showed no significant differences in tumor size,
growth rate, and weight compared with control groups. Notably,
combined treatment with sorafenib and either α-IL-33 or α-ST2L
neutralizing antibodies resulted in a remarkable reduction in tumor
size, growth rate and weight (Fig.
4B-D). These data indicated that blocking IL-33/ST2L can
enhance the antitumor efficacy of sorafenib in HCC. Additionally,
consistent with in vitro findings, sorafenib treatment
induced elevated expression of IL-33 as well as PD-L1 in HCC
tumors, which was inhibited by combined treatments with α-IL-33 or
α-ST2L neutralizing antibodies (Fig. 4E
and F). Given that IL-33/ST2L signaling-induced PD-L1
expression can lead to immune escape (36), the tumor-infiltrating lymphocytes
were analyzed 21 days after HCC inoculation. A slight increase was
observed in tumor-infiltrating CD4+ T cells in HCC
tumors treated with a combination of sorafenib and α-IL-33
neutralizing antibodies compared with other treatment groups. It is
worth noting that a significant increase in tumor-infiltrating
CD8+ T cells was observed in HCC tumors treated with
sorafenib combined with either α-IL-33 or α-ST2L neutralizing
antibodies compared with other treatment groups. Furthermore, a
reduction in CTLA4 expression on tumor-infiltrating CD8+
T cells was observed in HCC tumors following treatment with α-IL-33
or α-ST2L neutralizing antibodies (Fig.
4G). These findings suggested that combining sorafenib
treatment with α-IL-33 or α-ST2L neutralizing antibodies enhances
the activation and infiltration of CD8+ T cells into the
TME of HCC tumors, leading to increased therapeutic efficacy of
sorafenib.
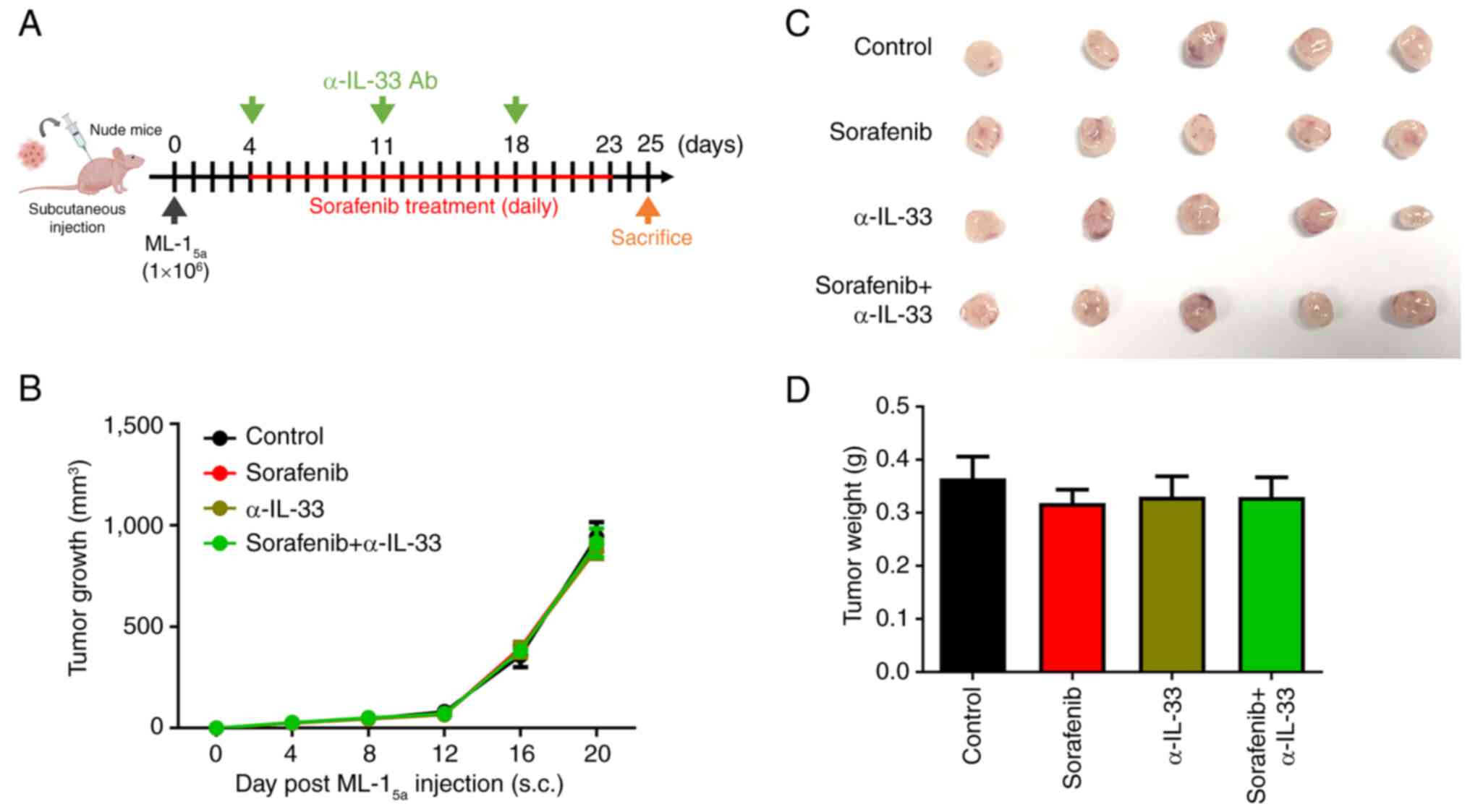
α-IL-33 neutralizing antibodies
enhancing the antitumor activity of sorafenib depend on T
cells
Since it was observed that combining sorafenib with
an α-IL-33 antibody increased tumor-infiltrated T cells, it was
examined whether the α-IL-33 neutralizing antibody enhances the
antitumor activity of sorafenib through T cells. To test this
hypothesis, an HCC model in nude mice was established by
subcutaneously implanting ML-15a cells and then treating
them with either sorafenib alone or in combination with the α-IL-33
neutralizing antibody (Fig. 5A).
The results showed that the combined treatment did not
significantly reduce tumor size, growth rate, or tumor weights in
the HCC-bearing nude mice compared with the sorafenib-only group
(Fig. 5B-D). In Fig. 5C, the largest tumor had a length of
1.3 cm and a width of 1.3 cm, corresponding to a calculated volume
of 1,098.5 mm3. These data suggested that the α-IL-33
neutralizing antibody enhances the antitumor activity of sorafenib
by regulating T cell-mediated immunity.
Discussion
When cells experience stress or damage, IL-33 is
rapidly released into the cytoplasm, where it serves as an alarmin
by binding to ST2L. This IL-33/ST2L interaction has been shown to
promote cancer progression, while its role in regulating tumor cell
resistance to therapy is just beginning to be investigated. IL-33
has been found to facilitate resistance to Fluorouracil (5-FU) in
murine melanoma cells by triggering polyploidy and inducing immune
exhaustion (40). Our previous
study consistently revealed that IL-33, which is released from lung
cancer cells treated with cisplatin, enhances M2 macrophage
function, thereby limiting the efficacy of the treatment (28). Additionally, a current study
demonstrated that the IL-33/ST2L axis can activate the
non-homologous end joining pathway, contributing to DNA damage
resistance in lung cancer response to cisplatin or doxorubicin
(41). In line with these findings,
it was demonstrated that IL-33 plays a significant role in
enhancing resistance to the anticancer drugs sorafenib and
regorafenib in HCC. Taken together, these results suggest that
targeting the IL-33/ST2L pathway could be beneficial in improving
the effectiveness of these treatments. While IL-33 can be released
from non-necrotic HCC cells in response to sorafenib and
regorafenib, little is known about how it is mobilized and released
into the extracellular space. Recently, several mechanisms based on
non-classical secretion appear to be involved in IL-33 secretion.
It has been found that IL-33 can be cosecreted with exosomes
through the neutral sphingomyelinase-2-dependent multivesicular
endosome pathway in primary human airway basal cells (42). Additionally, current studies
demonstrated that IL-33 release from allergen-stimulated lung
epithelial cells and senescent hepatic stellate cells depends on
gasdermin D pores, whose generation is independent of or dependent
on caspase-1 and caspase-11, respectively (27,43).
Understanding whether IL-33 release from sorafenib- and
regorafenib-treated HCC cells occurs via gasdermin D pores or
exosomal pathways will be important in future studies.
Numerous chemotherapeutic drugs can induce
senescence in cancer cells and TME, stimulating immunosurveillance
to eliminate tumor cells. However, they can also lead to chronic
inflammation and drug resistance, primarily through a set of
factors known as senescence-associated secretory phenotypes
(SASPs), including pro-inflammatory IL-1 family cytokines (44). In fact, as one of the IL-1 family
cytokines, IL-33 has currently been observed in SASPs. For example,
IL-33 is released from senescent hepatic stellate cells as the
SASPs to promote obesity-associated HCC (27). In radiation-induced skin injury,
IL-33 secreted by senescent skin cells inhibits macrophage
phagocytosis ability to clear these cells (45). Furthermore, IL-33-induced cellular
senescence results in kidney cell damage through the secretion of
IL-33-containing SASPs (46). Based
on these studies, the present findings suggested that treatments
with sorafenib and regorafenib induce cellular senescence in HCC
cells and release IL-33 as part of an SASP, which ultimately limits
the effectiveness of these drugs. To further investigate the
interaction between IL-33 and other SASP factors, the secretion of
IL-6, a prominent SASP cytokine, was measured in sorafenib- and
regorafenib-treated Huh7 cells. However, it was found that IL-6
levels were below the detection threshold in all groups, indicating
that IL-6 might not play a significant role as a SASP factor
interacting with IL-33 in our model (Fig. S2). Nevertheless, the potential for
IL-33 to cross-talk with other components of the SASPs cannot be
ruled out.
Overexpression of PD-L1 is observed in
sorafenib-resistant HCC cells and tissues, which is linked to the
facilitation of sorafenib resistance (47). Research using sorafenib-resistant
HCC cell lines has demonstrated that c-Met upregulates PD-L1
through the MAPK/NF-κB p65 signaling cascade, thereby contributing
to sorafenib resistance (48). The
elevated levels of PD-L1 can activate the STAT3/DNA
methyltransferase 1 pathway to trigger sorafenib resistance or
promote epithelial-mesenchymal transition (EMT) in these resistant
HCC cell lines (49,50). However, upregulation of PD-L1 is
also observed in non-resistant HCC cells upon sorafenib exposure.
For example, the induction of PD-L1 and EMT in sorafenib-treated
non-resistant HCC cells is reported, while combined inhibition of
EMT and PD-L1 enhances the sensitivity of HCC cells to sorafenib
(51). In the present study, it was
observed that IL-33 mediates the upregulation of PD-L1 in
non-resistant HCC cells when exposed to sorafenib and regorafenib.
These observations collectively suggest an early induction of PD-L1
expression in HCC cells following these drug treatments.
Additionally, our mechanistic study identified a positive feedback
loop involving the IL-33/ST2L/NF-κB axis, which contributes to the
induction of PD-L1 in HCC cells. Our findings are consistent with
earlier observations that IL-33 promotes PD-L1 expression in murine
acute myeloid leukemia and oral squamous cell carcinoma (36,52).
Importantly, the analysis of data from The Cancer Genome Atlas data
on Liver Hepatocellular Carcinoma revealed a significant positive
correlation between IL33 and CD274 mRNA expression
(Spearman's rho=0.349, P=4.78×10−12) (Fig. S3). This finding supports the
observations of the present study that IL-33 may play a role in
inducing PD-L1 upregulation in human HCC. Notably, tumor stromal
cells, in addition to tumor cells, have also been shown to produce
IL-33. Research has revealed that both IL-33 and its receptor ST2
are highly expressed in the microvascular vessels of the TME in
colorectal cancer to regulate tumor angiogenesis and progression
(53). Cancer-associated
fibroblast-derived IL-33 can promote breast cancer cell metastasis
by modifying the immune microenvironment at the metastatic niche
toward type 2 inflammation (54).
Thus, the current findings do not exclude the possibility of IL-33
produced by tumor-stromal cells due to sorafenib and
regorafenib.
The present findings revealed that blocking the
IL-33 signaling pathway with anti-IL-33 or anti-ST2L antibodies
alongside sorafenib significantly reduced tumor growth in mice with
HCC. Notably, this enhanced effect was abolished in athymic nude
mice. This suggests that IL-33 promotes the acquired resistance to
sorafenib in HCC-bearing mice mainly by regulating immune responses
rather than directly affecting HCC cell growth. In fact,
accumulated evidence has revealed that the IL-33/ST2 signaling
pathway is a potent regulator of the TME. It recruits various
immune cell populations that can either promote tumor malignancy or
induce tumor regression. This occurs by activating suppressor
immune cells, such as tumor-associated macrophages (TAM),
regulatory T cells (Tregs), and CD4+ Th2 cells, to
inhibit antitumor immunity. Consequently, this pathway plays a
significant role in tumor growth and metastasis (55). Notably, current research indicates
that sorafenib treatment results in the polarization of TAM into M2
macrophages and promotes Treg differentiation, consequently
suppressing the immune response and diminishing the efficacy of
sorafenib to HCC (56,57). It will be important in future
studies to understand whether IL-33 activates TAM and Tregs to
trigger immunosuppressive responses and hence reduce the efficacy
of sorafenib and regorafenib to HCC.
HCC is a primary liver cancer that is increasingly
common. For patients with advanced HCC, targeted therapies such as
sorafenib and regorafenib are recommended. While these treatments
can improve survival rates for patients with HCC, the development
of acquired resistance often leads to a poor response to these
therapies. In the present study, it was revealed that treatments
with sorafenib and regorafenib initiate a positive feedback loop
involving IL-33 and ST2L in senescent HCC cells. The IL-33 that is
secreted enhances the expression of PD-L1 in HCC cells by
activating NF-κB pathways in response to these treatments. When the
IL-33 signaling pathway was blocked using anti-IL-33 or anti-ST2L
antibodies in combination with sorafenib, a significant reduction
in tumor growth was observed in mice with HCC. This was accompanied
by decreased PD-L1 expression in tumors and increased infiltration
of CD8+ T cells. Importantly, the enhanced efficacy of
sorafenib against HCC by inhibiting IL-33 was dependent on T cells.
The present findings suggested that IL-33 may diminish the
effectiveness of sorafenib and regorafenib, indicating that
targeting the IL-33/ST2L axis could improve therapeutic outcomes
for HCC. Future studies should extend these findings by evaluating
the efficacy and toxicity of IL-33/ST2L inhibitors in higher-order
animal models and by investigating their use in combination with
other HCC therapies, such as immunotherapy. These approaches will
be essential to determine the translational potential of targeting
the IL-33/ST2L axis in HCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Ministry of Science and
Technology of Taiwan (grant nos. MOST 109-2622-B-006-002-CC1 and
MOST 110-2622-B-006- 006-CC1), the National Science and Technology
Council of Taiwan (grant no. NSTC 112-2320-B-006-047) and Chia-Yi
Christian Hospital (grant no. CYC111005).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YXL and HL confirm the authenticity of all the raw
data. YXL, HL, CCC and CPC were responsible for study conception
and design, and methodology development. YXL, WCL and BCZ performed
the experiments and collected the data. WCL, BCZ, CCC and CPC
carried out data analysis and interpretation. YXL, HL, WCL, CCC and
CPC wrote and/or revised the manuscript. YCW and SWW provided
administrative, technical, or material support and critical
revisions. CCC and CPC conducted project administration and overall
supervision. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures followed institutional guidelines and
were approved by the Institutional Animal Care and Use Committee of
National Cheng Kung University (approval no. 107130; Tainan,
Taiwan).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript or to generate images, and subsequently,
the authors revised and edited the content produced by the
artificial intelligence tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilhelm SM, Carter C, Tang L, Wilkie D,
McNabola A, Rong H, Chen C, Zhang X, Vincent P, McHugh M, et al:
BAY 43-9006 exhibits broad spectrum oral antitumor activity and
targets the RAF/MEK/ERK pathway and receptor tyrosine kinases
involved in tumor progression and angiogenesis. Cancer Res.
64:7099–7109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schroeder B, Li Z, Cranmer LD, Jones RL
and Pollack SM: Targeting gastrointestinal stromal tumors: The role
of regorafenib. Onco Targets Ther. 9:3009–3016. 2016.PubMed/NCBI
|
|
5
|
Mody K and Abou-Alfa GK: Systemic therapy
for advanced hepatocellular carcinoma in an evolving landscape.
Curr Treat Options Oncol. 20:32019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the Asia-Pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu S, Du Y, Ma H, Liang Q, Zhu X and Tian
J: Preclinical comparison of regorafenib and sorafenib efficacy for
hepatocellular carcinoma using multimodality molecular imaging.
Cancer Lett. 453:74–83. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huang A, Yang XR, Chung WY, Dennison AR
and Zhou J: Targeted therapy for hepatocellular carcinoma. Signal
Transduct Target Ther. 5:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ladd AD, Duarte S, Sahin I and Zarrinpar
A: Mechanisms of drug resistance in HCC. Hepatology. 79:926–940.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang W, Hong X, Xiao Y, Wang H and Zeng
X: Sorafenib resistance and therapeutic strategies in
hepatocellular carcinoma. Biochim Biophys Acta Rev Cancer.
1880:1893102025. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goodman A, Patel SP and Kurzrock R:
PD-1-PD-L1 immune-checkpoint blockade in B-cell lymphomas. Nat Rev
Clin Oncol. 14:203–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan F, Pang J, Peng Y, Molina JR, Yang P
and Liu S: Elevated cellular PD1/PD-L1 expression confers acquired
resistance to cisplatin in small cell lung cancer cells. PLoS One.
11:e01629252016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bishop JL, Sio A, Angeles A, Roberts ME,
Azad AA, Chi KN and Zoubeidi A: PD-L1 is highly expressed in
enzalutamide resistant prostate cancer. Oncotarget. 6:234–242.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li D, Sun FF, Wang D, Wang T, Peng JJ,
Feng JQ, Li H, Wang C, Zhou DJ, Luo H, et al: Programmed death
ligand-1 (PD-L1) regulated by NRF-2/MicroRNA-1 regulatory axis
enhances drug resistance and promotes tumorigenic properties in
sorafenib-resistant hepatoma cells. Oncol Res. 28:467–481. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kikuchi H, Matsui A, Morita S, Amoozgar Z,
Inoue K, Ruan Z, Staiculescu D, Wong JSL, Huang P, Yau T, et al:
Increased CD8+ T-cell infiltration and efficacy for multikinase
inhibitors after PD-1 blockade in hepatocellular carcinoma. J Natl
Cancer Inst. 114:1301–1305. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shigeta K, Matsui A, Kikuchi H, Klein S,
Mamessier E, Chen IX, Aoki S, Kitahara S, Inoue K, Shigeta A, et
al: Regorafenib combined with PD1 blockade increases CD8 T-cell
infiltration by inducing CXCL10 expression in hepatocellular
carcinoma. J Immunother Cancer. 8:e0014352020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Q, Han J, Yang Y and Chen Y: PD-1/PD-L1
checkpoint inhibitors in advanced hepatocellular carcinoma
immunotherapy. Front Immunol. 13:10709612022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cayrol C and Girard JP: Interleukin-33
(IL-33): A nuclear cytokine from the IL-1 family. Immunol Rev.
281:154–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shlomovitz I, Erlich Z, Speir M, Zargarian
S, Baram N, Engler M, Edry-Botzer L, Munitz A, Croker BA and Gerlic
M: Necroptosis directly induces the release of full-length
biologically active IL-33 in vitro and in an inflammatory disease
model. FEBS J. 286:507–522. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cayrol C and Girard JP: IL-33: An alarmin
cytokine with crucial roles in innate immunity, inflammation and
allergy. Curr Opin Immunol. 31:31–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cayrol C: IL-33, an alarmin of the IL-1
family involved in allergic and non allergic inflammation: Focus on
the mechanisms of regulation of its activity. Cells. 11:1072021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Weber A, Wasiliew P and Kracht M:
Interleukin-1 (IL-1) pathway. Sci Signal. 3:cm12010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Griesenauer B and Paczesny S: The
ST2/IL-33 axis in immune cells during inflammatory diseases. Front
Immunol. 8:4752017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pisani LF, Teani I, Vecchi M and
Pastorelli L: Interleukin-33: Friend or foe in gastrointestinal
tract cancers? Cells. 12:14812023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao R, Yu Z, Li M and Zhou Y:
Interleukin-33/ST2 signaling promotes hepatocellular carcinoma cell
stemness expansion through activating c-Jun N-terminal kinase
pathway. Am J Med Sci. 358:279–288. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang W, Wu J, Ji M and Wu C: Exogenous
interleukin-33 promotes hepatocellular carcinoma growth by
remodelling the tumour microenvironment. J Transl Med. 18:4772020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamagishi R, Kamachi F, Nakamura M,
Yamazaki S, Kamiya T, Takasugi M, Cheng Y, Nonaka Y, Yukawa-Muto Y,
Thuy LTT, et al: Gasdermin D-mediated release of IL-33 from
senescent hepatic stellate cells promotes obesity-associated
hepatocellular carcinoma. Sci Immunol. 7:eabl72092022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yang YE, Hu MH, Zeng YC, Tseng YL, Chen
YY, Su WC, Chang CP and Wang YC: IL-33/NF-κB/ST2L/Rab37
positive-feedback loop promotes M2 macrophage to limit
chemotherapeutic efficacy in lung cancer. Cell Death Dis.
15:3562024. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen SH, Hu CP, Lee CK and Chang C: Immune
reactions against hepatitis B viral antigens lead to the rejection
of hepatocellular carcinoma in BALB/c mice. Cancer Res.
53:4648–4651. 1993.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res 48 (W1). W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan X, Liu J, Li M, Liang Y, Liu Z, Lao M
and Fang M: The association of serum IL-33/ST2 expression with
hepatocellular carcinoma. BMC Cancer. 23:7042023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Akimoto M, Hayashi JI, Nakae S, Saito H
and Takenaga K: Interleukin-33 enhances programmed oncosis of
ST2L-positive low-metastatic cells in the tumour microenvironment
of lung cancer. Cell Death Dis. 7:e20572016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang C, Wang H, Lieftink C, du Chatinier
A, Gao D, Jin G, Jin H, Beijersbergen RL, Qin W and Bernards R:
CDK12 inhibition mediates DNA damage and is synergistic with
sorafenib treatment in hepatocellular carcinoma. Gut. 69:727–736.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Roger L, Tomas F and Gire V: Mechanisms
and regulation of cellular senescence. Int J Mol Sci. 22:131732021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhao M, He Y, Zhu N, Song Y, Hu Q, Wang Z,
Ni Y and Ding L: IL-33/ST2 signaling promotes constitutive and
inductive PD-L1 expression and immune escape in oral squamous cell
carcinoma. Br J Cancer. 128:833–843. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin X, Kang K, Chen P, Zeng Z, Li G, Xiong
W, Yi M and Xiang B: Regulatory mechanisms of PD-1/PD-L1 in
cancers. Mol Cancer. 23:1082024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jian C, Fu J, Cheng X, Shen LJ, Ji YX,
Wang X, Pan S, Tian H, Tian S, Liao R, et al: Low-dose sorafenib
acts as a mitochondrial uncoupler and ameliorates nonalcoholic
steatohepatitis. Cell Metab. 31:12062020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shiau DJ, Kuo WT, Davuluri GVN, Shieh CC,
Tsai PJ, Chen CC, Lin YS, Wu YZ, Hsiao YP and Chang CP:
Hepatocellular carcinoma-derived high mobility group box 1 triggers
M2 macrophage polarization via a TLR2/NOX2/autophagy axis. Sci Rep.
10:135822020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kudo-Saito C, Miyamoto T, Imazeki H, Shoji
H, Aoki K and Boku N: IL33 is a key driver of treatment resistance
of cancer. Cancer Res. 80:1981–1990. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Luo H, Liu L, Liu X, Xie Y, Huang X, Yang
M, Shao C and Li D: Interleukin-33 (IL-33) promotes DNA
damage-resistance in lung cancer. Cell Death Dis. 16:2742025.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Katz-Kiriakos E, Steinberg DF, Kluender
CE, Osorio OA, Newsom-Stewart C, Baronia A, Byers DE, Holtzman MJ,
Katafiasz D, Bailey KL, et al: Epithelial IL-33 appropriates
exosome trafficking for secretion in chronic airway disease. JCI
Insight. 6:e1361662021.PubMed/NCBI
|
|
43
|
Chen W, Chen S, Yan C, Zhang Y, Zhang R,
Chen M, Zhong S, Fan W, Zhu S, Zhang D, et al: Allergen
protease-activated stress granule assembly and gasdermin D
fragmentation control interleukin-33 secretion. Nat Immunol.
23:1021–1030. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dong Z, Luo Y, Yuan Z, Tian Y, Jin T and
Xu F: Cellular senescence and SASP in tumor progression and
therapeutic opportunities. Mol Cancer. 23:1812024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu G, Chen Y, Dai S, Wu G, Wang F, Chen
W, Wu L, Luo P and Shi C: Targeting the NLRP3 in macrophages
contributes to senescence cell clearance in radiation-induced skin
injury. J Transl Med. 23:1962025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen L, Gao C, Yin X, Mo L, Cheng X, Chen
H, Jiang C, Wu B, Zhao Y, Li H, et al: Partial reduction of
interleukin-33 signaling improves senescence and renal injury in
diabetic nephropathy. MedComm (2020). 5:e7422024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Zhao X, Ma X, Yuan Z and Hu M:
KCNQ1OT1 contributes to sorafenib resistance and programmed
death-ligand-1-mediated immune escape via sponging miR-506 in
hepatocellular carcinoma cells. Int J Mol Med. 46:1794–1804.
2020.PubMed/NCBI
|
|
48
|
Xu R, Liu X, Li A, Song L, Liang J, Gao J
and Tang X: c-Met up-regulates the expression of PD-L1 through
MAPK/NF-κBp65 pathway. J Mol Med (Berl). 100:585–598. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liu J, Liu Y, Meng L, Liu K and Ji B:
Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib
in human hepatocellular carcinoma. Oncol Rep. 38:899–907. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Xu GL, Ni CF, Liang HS, Xu YH, Wang WS,
Shen J, Li MM and Zhu XL: Upregulation of PD-L1 expression promotes
epithelial-to-mesenchymal transition in sorafenib-resistant
hepatocellular carcinoma cells. Gastroenterol Rep (Oxf). 8:390–398.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shrestha R, Prithviraj P, Bridle KR,
Crawford DHG and Jayachandran A: Combined inhibition of
TGF-β1-induced EMT and PD-L1 silencing re-sensitizes hepatocellular
carcinoma to sorafenib treatment. J Clin Med. 10:18892021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Qin L, Dominguez D, Chen S, Fan J, Long A,
Zhang M, Fang D, Zhang Y, Kuzel TM and Zhang B: Exogenous IL-33
overcomes T cell tolerance in murine acute myeloid leukemia.
Oncotarget. 7:61069–61080. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Cui G, Qi H, Gundersen MD, Yang H,
Christiansen I, Sørbye SW, Goll R and Florholmen J: Dynamics of the
IL-33/ST2 network in the progression of human colorectal adenoma to
sporadic colorectal cancer. Cancer Immunol Immunother. 64:181–190.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Shani O, Vorobyov T, Monteran L, Lavie D,
Cohen N, Raz Y, Tsarfaty G, Avivi C, Barshack I and Erez N:
Fibroblast-derived IL33 Facilitates breast cancer metastasis by
modifying the immune microenvironment and driving type 2 immunity.
Cancer Res. 80:5317–5329. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yeoh WJ, Vu VP and Krebs P: IL-33 biology
in cancer: An update and future perspectives. Cytokine.
157:1559612022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang Q, Cui M, Wang J, Zhao Y, Yin W, Liao
Z, Liang Y, Jiang Z, Li Y, Guo J, et al: Circulating mitochondrial
DNA promotes M2 polarization of tumor associated macrophages and
HCC resistance to sorafenib. Cell Death Dis. 16:1532025. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Shen Y, Wang H, Ma Z, Hao M, Wang S, Li J,
Fang Y, Yu L, Huang Y, Wang C, et al: Sorafenib promotes treg cell
differentiation to compromise its efficacy via VEGFR/AKT/Foxo1
signaling in hepatocellular carcinoma. Cell Mol Gastroenterol
Hepatol. 19:1014542025. View Article : Google Scholar : PubMed/NCBI
|