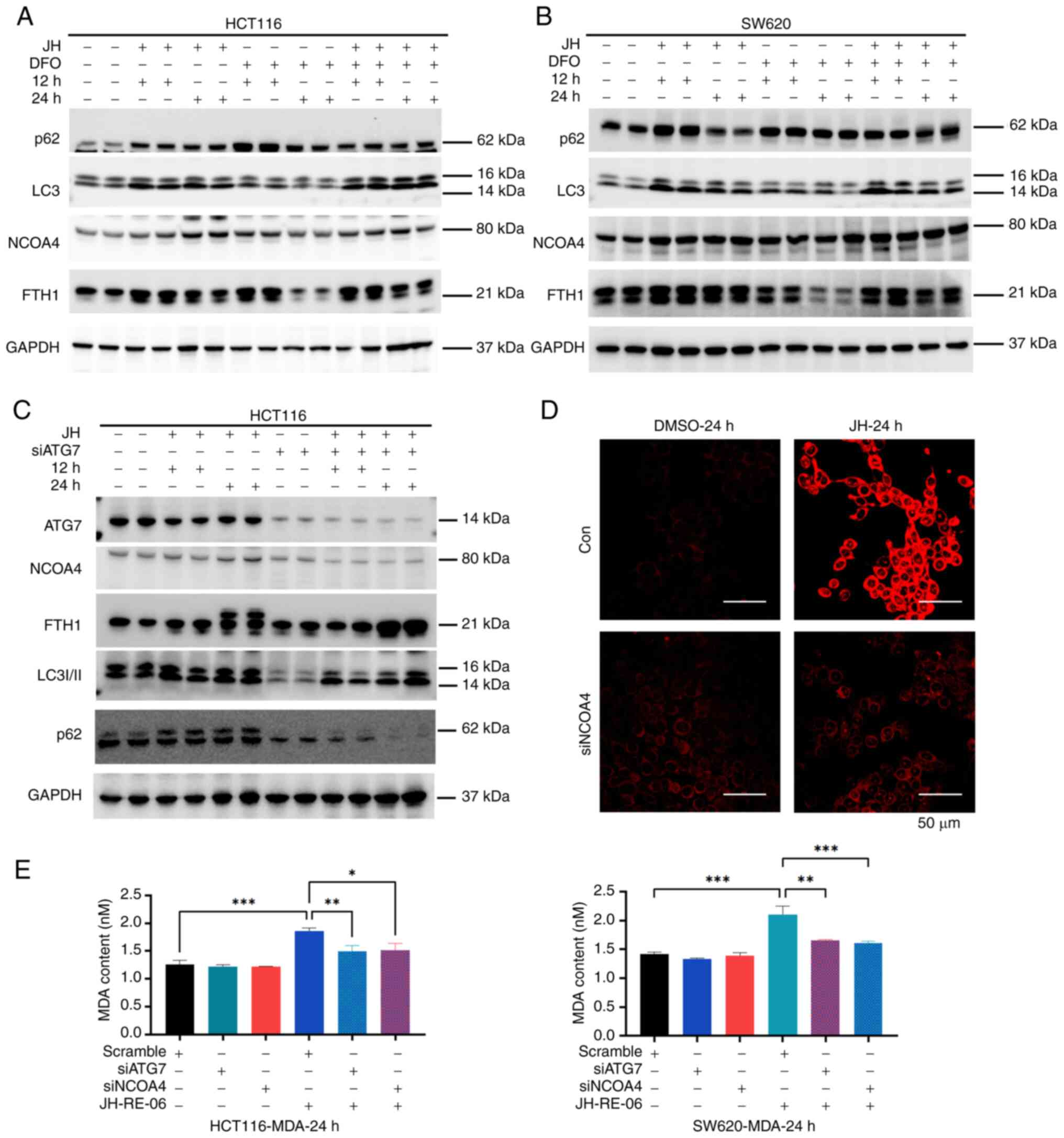
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Liu Z, Xu Y, Xu G, Baklaushev VP,
Chekhonin VP, Peltzer K, Ma W, Wang X, Wang G and Zhang C: Nomogram
for predicting overall survival in colorectal cancer with distant
metastasis. BMC Gastroenterol. 21:1032021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Veenstra CM and Krauss JC: Emerging
systemic therapies for colorectal cancer. Clin Colon Rectal Surg.
31:179–191. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Berti M, Cortez D and Lopes M: The
plasticity of DNA replication forks in response to clinically
relevant genotoxic stress. Nat Rev Mol Cell Biol. 21:633–651. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang W and Gao Y: Translesion and repair
DNA polymerases: Diverse structure and mechanism. Annu Rev Biochem.
87:239–261. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hicks JK, Chute CL, Paulsen MT, Ragland
RL, Howlett NG, Gueranger Q, Glover TW and Canman CE: Differential
roles for DNA polymerases eta, zeta, and REV1 in lesion bypass of
intrastrand versus interstrand DNA cross-links. Mol Cell Biol.
30:1217–1230. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharma S, Hicks JK, Chute CL, Brennan JR,
Ahn JY, Glover TW and Canman CE: REV1 and polymerase ζ facilitate
homologous recombination repair. Nucleic Acids Res. 40:682–691.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baranovskiy AG, Lada AG, Siebler HM, Zhang
Y, Pavlov YI and Tahirov TH: DNA polymerase delta and zeta switch
by sharing accessory subunits of DNA polymerase delta. J Biol Chem.
287:17281–17287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mellor C, Nassar J, Šviković S and Sale
JE: PRIMPOL ensures robust handoff between on-the-fly and
post-replicative DNA lesion bypass. Nucleic Acids Res. 52:243–258.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taglialatela A, Leuzzi G, Sannino V,
Cuella-Martin R, Huang JW, Wu-Baer F, Baer R, Costanzo V and Ciccia
A: REV1-Polzeta maintains the viability of homologous
recombination-deficient cancer cells through mutagenic repair of
PRIMPOL-dependent ssDNA gaps. Mol Cell. 81:4008–4025. e72021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nayak S, Calvo JA, Cong K, Peng M,
Berthiaume E, Jackson J, Zaino AM, Vindigni A, Hadden MK and Cantor
SB: Inhibition of the translesion synthesis polymerase REV1
exploits replication gaps as a cancer vulnerability. Sci Adv.
6:eaaz78082020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nayak S, Calvo JA and Cantor SB: Targeting
translesion synthesis (TLS) to expose replication gaps, a unique
cancer vulnerability. Expert Opin Ther Targets. 25:27–36. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin X and Howell SB: DNA mismatch repair
and p53 function are major determinants of the rate of development
of cisplatin resistance. Mol Cancer Ther. 5:1239–1247. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sasatani M, Xi Y, Kajimura J, Kawamura T,
Piao J, Masuda Y, Honda H, Kubo K, Mikamoto T, Watanabe H, et al:
Overexpression of Rev1 promotes the development of
carcinogen-induced intestinal adenomas via accumulation of point
mutation and suppression of apoptosis proportionally to the Rev1
expression level. Carcinogenesis. 38:570–578. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu N, Zhao Y, Mi M, Lu Y, Tan Y, Fang X,
Weng S and Yuan Y: REV1: A novel biomarker and potential
therapeutic target for various cancers. Front Genet. 13:9979702022.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wojtaszek JL, Chatterjee N, Najeeb J,
Ramos A, Lee M, Bian K, Xue JY, Fenton BA, Park H, Li D, et al: A
small molecule targeting mutagenic translesion synthesis improves
chemotherapy. Cell. 178:152–159.e11. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chatterjee N, Whitman MA, Harris CA, Min
SM, Jonas O, Lien EC, Luengo A, Vander Heiden MG, Hong J, Zhou P,
et al: REV1 inhibitor JH-RE-06 enhances tumor cell response to
chemotherapy by triggering senescence hallmarks. Proc Natl Acad Sci
USA. 117:28918–28921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Jie X, Xing B, Wu Z, Yang X, Rao
X, Xu Y, Zhou D, Dong X, Zhang T, et al: REV1 promotes lung
tumorigenesis by activating the Rad18/SERTAD2 axis. Cell Death Dis.
13:1102022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dobin A, Davis CA, Schlesinger F, Drenkow
J, Zaleski C, Jha S, Batut P, Chaisson M and Gingeras TR: STAR:
Ultrafast universal RNA-seq aligner. Bioinformatics. 29:15–21.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mizuno H, Kitada K, Nakai K and Sarai A:
PrognoScan: A new database for meta-analysis of the prognostic
value of genes. BMC Med Genomics. 2:182009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sharma S, Shah NA, Joiner AM, Roberts KH
and Canman CE: DNA polymerase ζ is a major determinant of
resistance to platinum-based chemotherapeutic agents. Mol
Pharmacol. 81:778–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gao M, Monian P, Pan Q, Zhang W, Xiang J
and Jiang X: Ferroptosis is an autophagic cell death process. Cell
Res. 26:1021–1032. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hou W, Xie Y, Song X, Sun X, Lotze MT, Zeh
HJ III, Kang R and Tang D: Autophagy promotes ferroptosis by
degradation of ferritin. Autophagy. 12:1425–1428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Radulescu S, Brookes MJ, Salgueiro P,
Ridgway RA, McGhee E, Anderson K, Ford SJ, Stones DH, Iqbal TH,
Tselepis C and Sansom OJ: Luminal iron levels govern intestinal
tumorigenesis after Apc loss in vivo. Cell Rep. 2:270–282. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu Q, Wei W, Wu D, Huang F, Li M, Li W,
Yin J, Peng Y, Lu Y, Zhao Q and Liu L: Blockade of GCH1/BH4 axis
activates ferritinophagy to mitigate the resistance of colorectal
cancer to erastin-induced ferroptosis. Front Cell Dev Biol.
10:8103272022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xie K, Doles J, Hemann MT and Walker GC:
Error-prone translesion synthesis mediates acquired
chemoresistance. Proc Natl Acad Sci USA. 107:20792–20797. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bruno PM, Liu Y, Park GY, Murai J, Koch
CE, Eisen TJ, Pritchard JR, Pommier Y, Lippard SJ and Hemann MT: A
subset of platinum-containing chemotherapeutic agents kills cells
by inducing ribosome biogenesis stress. Nat Med. 23:461–471. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sekimoto T, Oda T, Kurashima K, Hanaoka F
and Yamashita T: Both high-fidelity replicative and low-fidelity
Y-family polymerases are involved in DNA rereplication. Mol Cell
Biol. 35:699–715. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang G, Wang JJ, Zhi-Min Z, Xu XN, Shi F
and Fu XL: Targeting critical pathways in ferroptosis and enhancing
antitumor therapy of Platinum drugs for colorectal cancer. Sci
Prog. 106:3685042211471732023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fakouri NB, Durhuus JA, Regnell CE,
Angleys M, Desler C, Hasan-Olive MM, Martín-Pardillos A,
Tsaalbi-Shtylik A, Thomsen K, Lauritzen M, et al: Rev1 contributes
to proper mitochondrial function via the PARP-NAD+-SIRT1-PGC1alpha
axis. Sci Rep. 7:124802017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Anugula S, Li Z, Li Y, Hendriksen A,
Christensen PB, Wang L, Monk JM, de Wind N, Bohr VA, Desler C, et
al: Rev1 deficiency induces a metabolic shift in MEFs that can be
manipulated by the NAD(+) precursor nicotinamide riboside. Heliyon.
9:e173922023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Federico G, Carrillo F, Dapporto F,
Chiariello M, Santoro M, Bellelli R and Carlomagno F: NCOA4 links
iron bioavailability to DNA metabolism. Cell Rep. 40:1112072022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu H, Liu Q, Shan X, Gao W and Chen Q: ATM
orchestrates ferritinophagy and ferroptosis by phosphorylating
NCOA4. Autophagy. 19:2062–2077. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang MJ, Song ML, Zhang Y, Yang XM, Lin
HS, Chen WC, Zhong XD, He CY, Li T, Liu Y, et al: SNS alleviates
depression-like behaviors in CUMS mice by regluating dendritic
spines via NCOA4-mediated ferritinophagy. J Ethnopharmacol.
312:1163602023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kuno S and Iwai K: Oxygen modulates iron
homeostasis by switching iron sensing of NCOA4. J Biol Chem.
299:1047012023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bellelli R, Castellone MD, Guida T,
Limongello R, Dathan NA, Merolla F, Cirafici AM, Affuso A, Masai H,
Costanzo V, et al: NCOA4 transcriptional coactivator inhibits
activation of DNA replication origins. Mol Cell. 55:123–137. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li J, Zheng C, Mai Q, Huang X, Pan W, Lu
J, Chen Z, Zhang S, Zhang C, Huang H, et al: Tyrosine catabolism
enhances genotoxic chemotherapy by suppressing translesion DNA
synthesis in epithelial ovarian cancer. Cell Metab.
35:2044–2059.e8. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Y, Feng X, Wu Z, Yang Y, Rao X, Meng
R, Zhang S, Dong X, Xu S, Wu G and Jie X: USP9X-mediated REV1
deubiquitination promotes lung cancer radioresistance via the
action of REV1 as a Rad18 molecular scaffold for cystathionine
γ-lyase. J Biomed Sci. 31:552024. View Article : Google Scholar : PubMed/NCBI
|