Introduction
Thyroid cancer is the most common malignant
endocrine tumor, with an increasing incidence, especially among
women (1). Differentiated thyroid
cancer (DTC), which includes papillary thyroid cancer (PTC) and
follicular subtypes, accounts for 95% of all cases (2). The standard treatment for
intermediate- and high-risk DTC is total thyroidectomy, followed by
postoperative monitoring of serum thyroglobulin (Tg) and Tg
antibody (Tg/Ab) levels to detect residual or recurrent disease
(3,4). Oral administration of radioactive
iodine (131I) is used when the Tg and Tg/Ab levels
exceed standard levels. Conventional radioisotope therapy using
131I increases serum thyroid-stimulating hormone (TSH)
levels by thyroid hormone withdrawal (THW), promoting
131I accumulation in the remaining DTC tissues (5). However, because THW-induced
hypothyroidism increases the risk of complications, and the number
of patients waiting for treatment is increasing, recombinant human
TSH (rhTSH), which does not require THW treatment and
hospitalization, is recommended (3). However, serum Tg levels are often
disturbed by Tg/Ab interference, and this is observed in
one-quarter of all patients with DTC (6–8).
Therefore, researchers are exploring metabolic and genetic
alterations as complementary biomarkers to Tg to improve the
assessment of disease status. However, no reliable standard test
has been developed (8–10). Additionally, morphology and
functionality can be observed using radiological imaging
techniques, such as positron emission tomography/computed
tomography. However, the levels or presence of Tg and Tg/Ab cannot
be visualized using imaging modalities (11). The lack of association between serum
Tg levels and antitumor efficacy in Tg/Ab-positive patients makes
it difficult to continue 131I therapy (12) and novel therapeutic biomarkers are
needed to address this challenge. Mass spectrometry has been used
to explore the possibility of using serum metabolomic analysis to
quantify metabolites in biological samples (13). Blood metabolites in patients with
DTC are likely to include various diagnostic biomarkers, such as
altered levels of amino acids (leucine, valine, lysine and
tyrosine), lactate, citrate and lipids, including
lysophosphatidylcholine and sphingomyelins, according to previous
metabolomic studies (14–17). If Tg/Ab is detected as positive
during 131I therapy in combination with rhTSH, the
identification of a novel metabolite that can complement Tg will
allow treatment optimization. Therefore, the present study aimed to
elucidate the molecular mechanisms of metabolomic alterations in
patients with DTC treated with 131I and to identify
Tg/Ab-independent biomarker candidates using a TPC-1 thyroid cancer
cell model. Although the clinical sample size was limited, the
present study was designed as a proof-of-concept to provide
preliminary evidence supported by experimental validation.
Materials and methods
Analysis of patients with thyroid
cancer
The present study was approved by the Committee of
Medical Ethics of the Hirosaki University Graduate School of Health
Sciences (approval no. 2021–050; Hirosaki, Japan). To protect the
rights and privacy of the participants, written informed consent
was obtained after providing a detailed verbal explanation, and all
collected data were anonymized and handled in accordance with
ethical guidelines. The present study included 3 patients (2 female
patients and 1 male patient; median age, 58 years; age range, 45–81
years) with DTC who received 131I internal therapy with
rhTSH (Thyrogen®; Sanofi S.A.) between April 2022 and
December 2024 at Aomori Rosai Hospital (Hachinohe, Japan) (Table I). The inclusion criteria were as
follows: Age ≥18 years; histologically confirmed differentiated
thyroid carcinoma; completion of total thyroidectomy; planned
adjuvant 131I therapy with rhTSH stimulation; and
provision of informed consent. The exclusion criteria were:
Presence of medullary or anaplastic thyroid carcinoma; uncontrolled
severe comorbidities (such as renal failure or severe cardiac
disease); prior radioiodine therapy within 12 months; and inability
to comply with the study procedures. During treatment, clinical
information and blood samples were collected. Routine blood tests,
including complete blood counts (red blood cells, white blood cells
and platelets) and thyroid-related parameters [TSH, free
triiodothyronine (FT3), free thyroxine (FT4), Tg and Tg/Ab], were
performed at the clinical laboratory of Aomori Rosai Hospital at
three timepoints: Before 131I administration (baseline),
on the day of 131I administration (day 0) and 30 days
after treatment (day 30), consistent with the measurements shown in
Fig. 1.
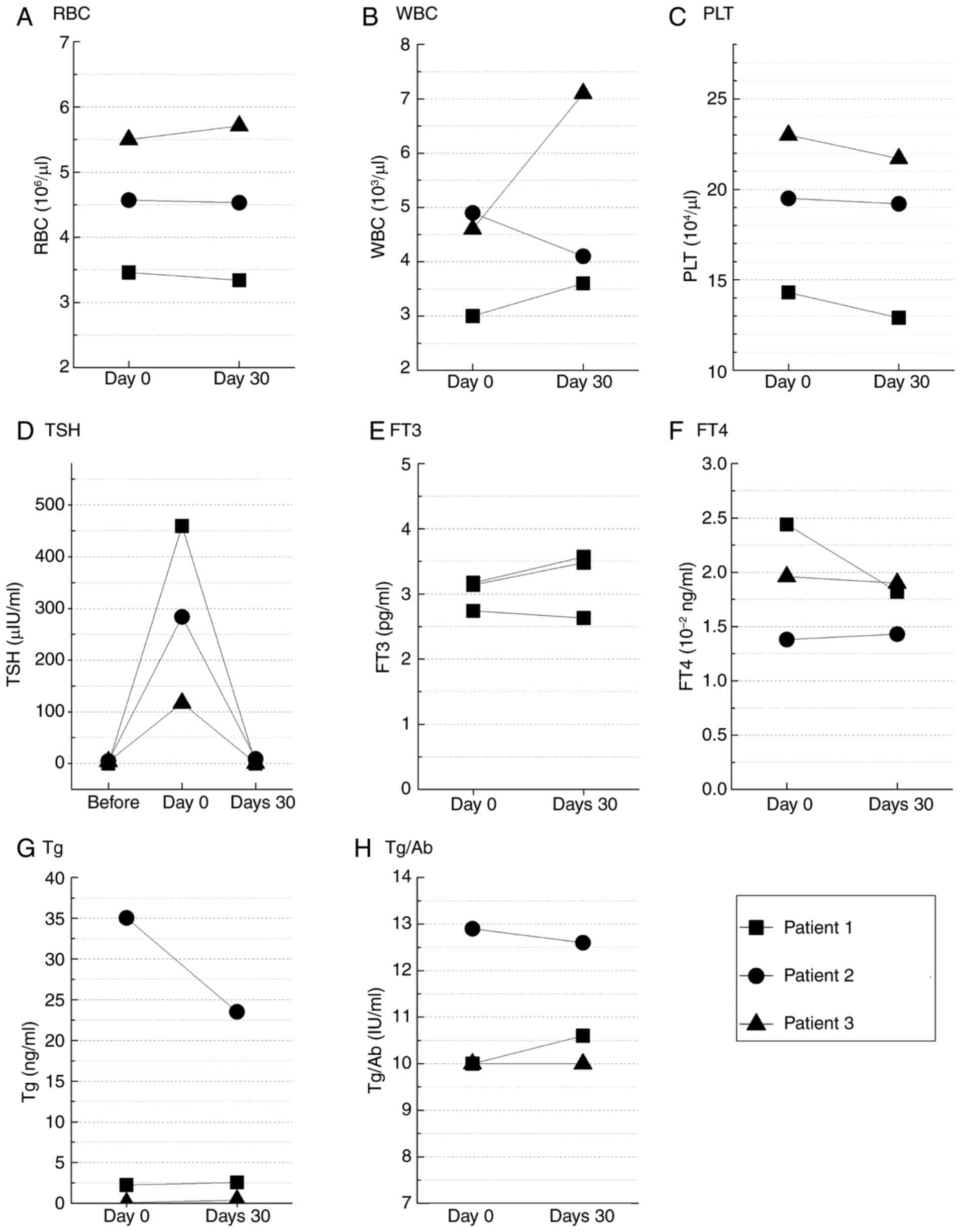 | Figure 1.Blood biomarkers measured before
131I administration (baseline), on the day of
131I administration (day 0) and 30 days after treatment
(day 30) in 3 patients with differentiated thyroid cancer after
thyroidectomy. (A) RBC, (B) WBC, (C) PLT, (D) TSH, (E) FT3, (F)
FT4, (G) Tg and (H) Tg/Ab. Statistical analysis was performed using
the Wilcoxon signed-rank test for paired comparisons involving two
timepoints. For TSH, comparisons across three timepoints were
performed using the Friedman test. No significant differences were
observed in any of the biomarkers. Normal reference ranges: RBC,
4.0–5.5×106/µl; WBC, 4.0–9.0×103/µl; PLT,
15–35×104/µl; TSH, 0.4–4.0 µIU/ml; FT3, 2.3–4.1 pg/ml;
FT4, 0.9–1.7×10−2 ng/ml; Tg, <30 ng/ml; Tg/Ab, <40
IU/ml. FT3, free triiodothyronine; FT4, free thyroxine; PLT,
platelets; RBC, red blood cells; Tg, thyroglobulin; Tg/Ab,
thyroglobulin antibody; TSH, thyroid-stimulating hormone; WBC,
white blood cells. |
 | Table I.Clinical characteristics of the study
population. |
Table I.
Clinical characteristics of the study
population.
| Patient no. | Sex | Age, years | TNM
classification | Thyroid hormone
replacement after thyroidectomy | External
radiotherapy | 131I
administration |
|---|
| 1 | F | 81 |
T4aN0M0 | Liothyronine | 50 Gy/25 fr | 1.11 GBq |
| 2 | F | 58 |
rT0N0Mx |
| 40 Gy/20 fr + |
|
| 3 | M | 45 |
pT1aN1aM0 |
| 20 Gy/10 fr |
|
|
|
|
|
|
| (boost) |
|
Serum metabolomic analysis
Peripheral blood was collected from patients with
DTC using serum separation tubes (BD Biosciences) at two time
points: Before the administration of 131I (day 0) and 30
days after administration (day 30), both under Thyrogen
stimulation. To minimize the influence of recent dietary intake,
patients were instructed to fast for ≥6 h prior to blood
collection. The collected sample tubes were stored in a deep
freezer (−80°C) until analysis. Serum metabolomic analysis was
performed using liquid chromatography-mass spectrometry (LC-MS) and
flow injection analysis-mass spectrometry (FIA-MS), and the
AbsoluteIDQ P180 kit (Biocrates, Inc.) was used to quantify
metabolites. The analysis was performed using the method described
in our previous study (18). The
target metabolites were phenyl isothiocyanate-derivatized and
analyzed based on internal standards for quantitation. LC-MS and
FIA-MS were performed in positive ion mode using electrospray
ionization using a high-performance liquid chromatography system
(ExionLC™ AD; SCIEX) combined with a QTRAP 6500+ triple
quadruple ion trap hybrid mass spectrometer system (SCIEX) operated
with Analyst® 1.6.3 software (SCIEX). LC-MS was used to
measure amino acids and biogenic amines, and the multiple reaction
monitoring (MRM) conditions are shown in Table SI. The MS measurement conditions
were as follows: Curtain gas, 45 psi; collision gas, 6 psi; ion
spray voltage, 5,500 V; temperature, 500°C; ion source gas 1, 40
psi; and ion source gas 2, 50 psi. LC separation was performed on a
Zorbax Eclipse XDB-C18 column (3×100 mm; 3.5 µm; Agilent
Technologies, Inc.) at 40°C. The mobile phases consisted of solvent
A (water with 0.2% formic acid) and solvent B (acetonitrile with
0.2% formic acid). The flow rate was set at 0.5 ml/min. The
gradient program was as follows: 0–0.5 min, 0% B; 0.5–5.5 min,
linear increase to 95% B; 5.5–6.5 min, held at 95% B; 6.5–7 min,
returned to 0% B and equilibrated for the next injection for 1.5
min. FIA-MS measurements included carnitines and acylcarnitines,
hydroxy- and dicarboxyacylcarnitines, sphingomyelins and
hydroxysphingomyelins, diacyl phosphatidylcholine, acyl-alkyl
phosphatidylcholine, lysophosphatidylcholine, and sugar as targets.
The MRM conditions for measuring these metabolites are shown in
Table SII. The MS settings were
the same as those for LC-MS, except that the temperature was 175°C.
The FIA-MS flow program was as follows: 0–1.6 min, 0.03 ml/min;
1.6–2.4 min, linear increase to 0.2 ml/min; 2.4–2.8 min, held at
0.2 ml/min; and 2.8–3.0 min, returned to 0.03 ml/min. The injection
volume was 20 µl. As with LC-MS, FIA-MS quantification was
performed using isotopically labeled internal standards provided in
the AbsoluteIDQ P180 kit to ensure accuracy and reproducibility.
All metabolites were identified and quantified using isotopically
labeled internal standards and multiple reaction monitoring as
optimized and provided by Biocrates, Inc. Table II shows the measurable metabolites
and their abbreviations. Amino acids and biogenic amines were
quantified using LC-MS, whereas acylcarnitines,
phosphatidylcholines, lysophosphatidylcholines, sphingomyelins and
hexose were analyzed by FIA-MS. Among these, Fig. 2 highlights representative
metabolites from each category that showed statistically
significant or consistent changes before and after 131I
administration. The metabolomics datasets of LC-MS and FIA-MS
generated in the present study have been deposited in MetaboBank
(https://www.ddbj.nig.ac.jp/metabobank/index-e.html)
under the accession numbers MTBKS257 and MTBKS258, respectively.
For pathway enrichment analysis, metabolites that showed
significant changes before and after 131I administration
were analyzed using MetaboAnalyst 6.0 (https://www.metaboanalyst.ca/) with pathway annotation
based on the Kyoto Encyclopedia of Genes and Genomes (KEGG)
database (https://www.genome.jp/kegg/).
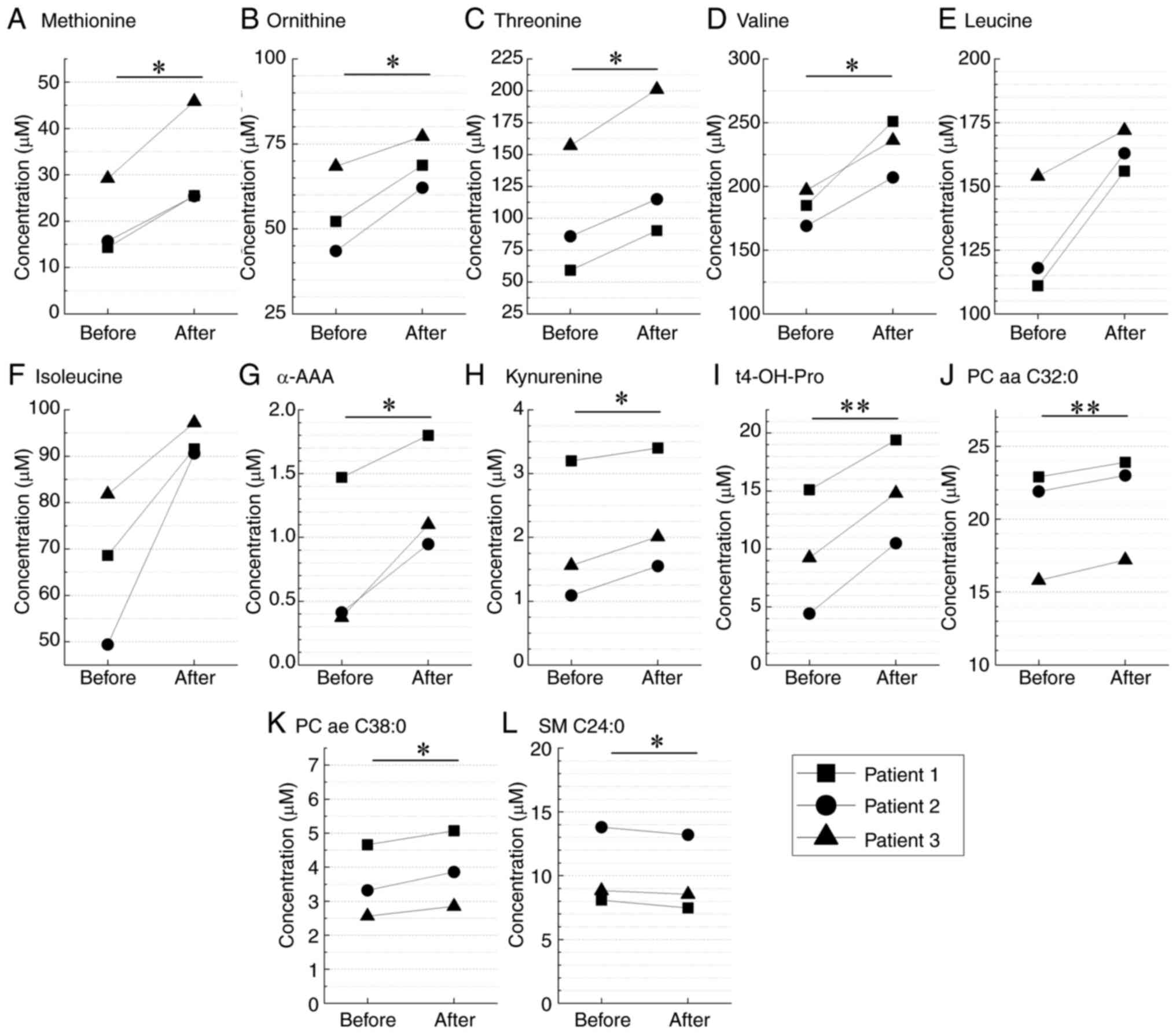 | Figure 2.Quantification of serum metabolites
using mass spectrometry. Analysis of the serum samples collected
from patients with differentiated thyroid cancer before (day 0) and
after 131I administration (day 30). The levels of 12
metabolites, namely (A) methionine, (B) ornitine, (C) threonine,
(D) valine, (E) leucine, (F) isoleucine, (G) α-AAA, (H) kynurenine,
(I) t4-OH-Pro, (J) PC aa C32:0, (K) PC ae C38:0 and (L) SM C24:0,
are shown. *P<0.05 and **P<0.01 (Wilcoxon signed-rank test).
α-AAA, α-aminoadipic acid; PC aa C32:0, phosphatidylcholine type aa
C32:0; PC ae C38:0, phosphatidylcholine type ae C38:0; SM C24:0,
sphingomyelin type C24:0; t4-OH-Pro, trans-4-hydroxyprolin. |
 | Table II.List of target metabolites measured
with the AbsoluteIDQ P180 kit. |
Table II.
List of target metabolites measured
with the AbsoluteIDQ P180 kit.
| Metabolite
class | Number of analysis
targets | Abbreviations of
analysis objects |
|---|
| Amino acids and
biogenic amines | 42 | Ala, Arg, Asn, Asp,
Cit, Gln, Glu, Gly, His, Ile, Leu, Lys, Met, Orn, Phe, Pro, Ser,
Thr, Trp, Tyr, Val, Ac-Orn, ADMA, α-AAA, c4-OH-Pro, carnosine,
creatinine, DOPA, dopamine, histamine, kynurenine, Met-SO,
nitro-Tyr, PEA, putrescine, SDMA, serotonin, spermidine, spermine,
t4-OH-Pro, taurine, total DMA |
| Carnitines and
acylcarnitines | 26 | C0, C2, C3, C3:1,
C4, C4:1, C5, C5:1, C6 (C4:1-DC), C6:1, C8, C9, C10, C10:1, C10:2,
C12, C12:1, C14, C14:1, C14:2, C16, C16:1, C16:2, C18, C18:1,
C18:2 |
| Hydroxy- and
dicarboxyacylcarnitines | 14 | C3-DC (C4-OH),
C3-OH, C5-DC (C6-OH), C5-M-DC, C5-OH (C3-DC-M), C5:1-DC, C7-DC,
C12-DC, C14:1-OH, C14:2-OH, C16-OH, C16:1-OH, C16:2-OH,
C18:1-OH |
| Sphingomyeline and
hydroxysphingomyelins | 15 | SM (OH) C14:1, SM
(OH) C16:1, SM (OH) C22:1, SM (OH) C22:2, SM (OH) C24:1, SM C16:0,
SM C16:1, SM C18:0, SM C18:1, SM C20:2, SM C22:3, SM C24:0, SM
C24:1, SM C26:0, SM C26:1 |
| Diacyl
phosphatidylcholine | 38 | PC aa C24:0, PC aa
C26:0, PC aa C28:1, PC aa C30:0, PC aa C30:2, PC aa C32:0, PC aa
C32:1, PC aa C32:2, PC aa C32:3, PC aa C34:1, PC aa C34:2, PC aa
C34:3, PC aa C34:4, PC aa C36:0, PC aa C36:1, PC aa C36:2, PC aa
C36:3, PC aa C36:4, PC aa C36:5, PC aa C36:6, PC aa C38:0, PC aa
C38:1, PC aa C38:3, PC aa C38:4, PC aa C38:5, PC aa C38:6, PC aa
C40:1, PC aa C40:2, PC aa C40:3, PC aa C40:4, PC aa C40:5, PC aa
C40:6, PC aa C42:0, PC aa C42:1, PC aa C42:2, PC aa C42:4, PC aa
C42:5, PC aa C42:6 |
| Acyl-alkyl
phosphatidylcholine | 38 | PC ae C30:0, PC ae
C30:1, PC ae C30:2, PC ae C32:1, PC ae C32:2, PC ae C34:0, PC ae
C34:1, PC ae C34:2, PC ae C34:3, PC ae C36:0, PC ae C36:1, PC ae
C36:2, PC ae C36:3, PC ae C36:4, PC ae C36:5, PC ae C38:0, PC ae
C38:1, PC ae C38:2, PC ae C38:3, PC ae C38:4, PC ae C38:5, PC ae
C38:6, PC ae C40:1, PC ae C40:2, PC ae C40:3, PC ae C40:4, PC ae
C40:5, PC ae C40:6, PC ae C42:0, PC ae C42:1, PC ae C42:2, PC ae
C42:3, PC ae C42:4, PC ae C42:5, PC ae C44:3, PC ae C44:4, PC ae
C44:5, PC ae C44:6 |
|
Lysophosphatidylcholine | 14 | lysoPC a C14:0,
lysoPC a C16:0, lysoPC a C16:1, lysoPC a C17:0, lysoPC a C18:0,
lysoPC a C18:1, lysoPC a C18:2, lysoPC a C20:3, lysoPC a C20:4,
lysoPC a C24:0, lysoPC a C26:0, lysoPC a C26:1, lysoPC a C28:0,
lysoPC a C28:1 |
| Sugar | 1 | H1 |
| Total | 188 | - |
Cell culture for thyroid cancer cell
model
The TPC-1 human thyroid papillary carcinoma cell
line was obtained from Merck KGaA. The cells were cultured in
RPMI-1640 (Nacalai Tesque, Inc.) containing 10% heat-inactivated
fetal bovine serum (Biowest), 2 mM L-glutamine (MilliporeSigma) and
1% penicillin/streptomycin in a humidified atmosphere at 37°C with
5% CO2.
Exposure to ionizing radiation (IR)
for the basic experiment
TPC-1 cells were irradiated using an X-ray generator
(MBR-1520R-3; Hitachi Power Solutions Co., Ltd.) at 1 Gy/min (150
kVp; 20 mA) with 0.5-mm aluminum and 0.3-mm copper filters. The
radiation dose was monitored using a thimble ionization chamber.
Handling of ¹3¹I in vitro requires specialized
radiation safety measures and licensing, which can impede
exploratory studies. Therefore, X-rays were chosen as a practical
and controllable radiation source to model general cellular
responses to IR independently of sodium/iodide symporter
(NIS)-mediated uptake. Based on clonogenic survival assays, an 8 Gy
dose was selected for irradiation to ensure ~1% survival of TPC-1
cells. This dose was chosen to induce measurable biological effects
relevant to therapeutic radiation exposure while maintaining
sufficient cell viability for downstream analyses. Although
clinical radioiodine therapy doses vary depending on remnant size
and uptake, the 8 Gy dose provides a reproducible and biologically
meaningful model to study cellular radiation responses. Clinical
absorbed doses to thyroid remnants and metastases are typically
much higher (commonly cited targets, ~300 Gy for remnants and ~80
Gy for metastases) (19,20). Cell viability was assessed using a
trypan blue exclusion assay. Briefly, 20 µl cell suspension was
mixed with 20 µl trypan blue solution (0.4%; 2-fold dilution;
Nacalai Tesque, Inc.) at room temperature. The cells were gently
mixed and incubated for <3 min to allow dye penetration into
nonviable cells. The viable (unstained) and nonviable
(blue-stained) cells were then counted using a Bürker-Türk
hemocytometer (Erma, Inc.) under a light microscope (IX71; Olympus
Corporation) without fixation.
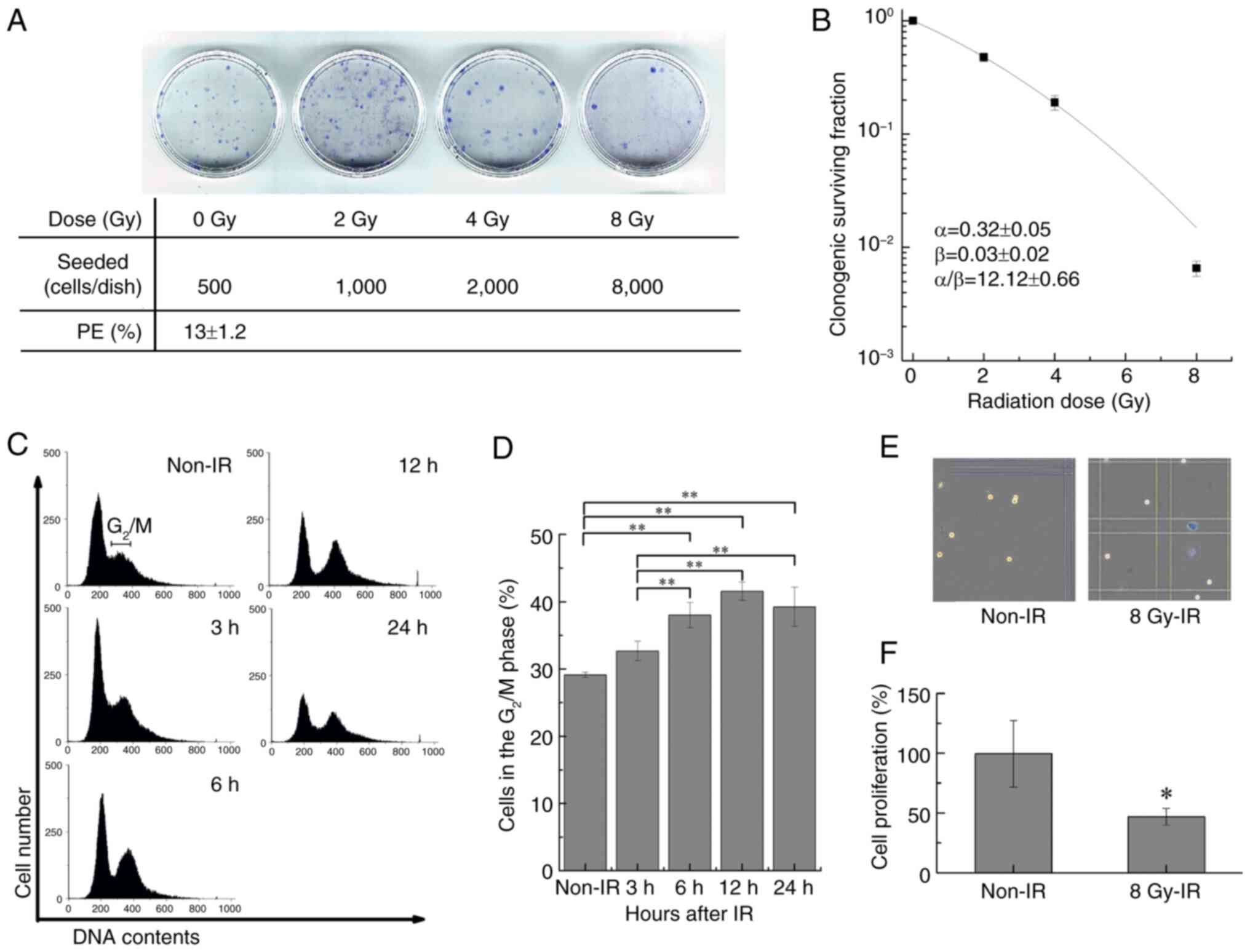
Clonogenic potency assay for TPC-1
cells
Colony-forming cells were counted using a clonogenic
potency assay. The cells were plated at a density of 500–8,000
cells/dish in a 35-mm diameter culture dish, irradiated with 0 to 8
Gy of IR and incubated for 6–9 days in a humidified atmosphere at
37°C with 5% CO2. The irradiation was delivered at a
dose rate of 1 Gy/min, and the exposure time was adjusted according
to the prescribed dose. Subsequently, the dishes were fixed with
−20°C cold methanol for 15 min and stained with Giemsa solution
(Muto Pure Chemicals Co., Ltd.) at room temperature for 30 min.
Colonies containing >50 cells were manually counted under an
inverted microscope (IX71; Olympus Corporation). The cell survival
rate was calculated relative to the nonirradiated controls and
plating efficiency, as described previously (21).
Cell cycle distribution analysis
The cell cycle distribution was analyzed using flow
cytometry. The harvested cells were treated with precooled (−20°C)
70% ethanol for 10 min on ice and stored at −20°C until measured.
RNase I was added to the sample tube at 37°C for 20 min (5 µg/ml;
Merck KGaA) to remove internal RNAs. The cells were then stained
with PI (40 µg/ml; FUJIFILM Wako Pure Chemical Corporation) in the
dark at room temperature for 2 min. Cell cycle distribution
analysis was performed using Cell Lab Quanta™ Sc MPL
(Beckman Coulter, Inc.) and analyzed with Kaluza software (version
2.1; Beckman Coulter, Inc.).
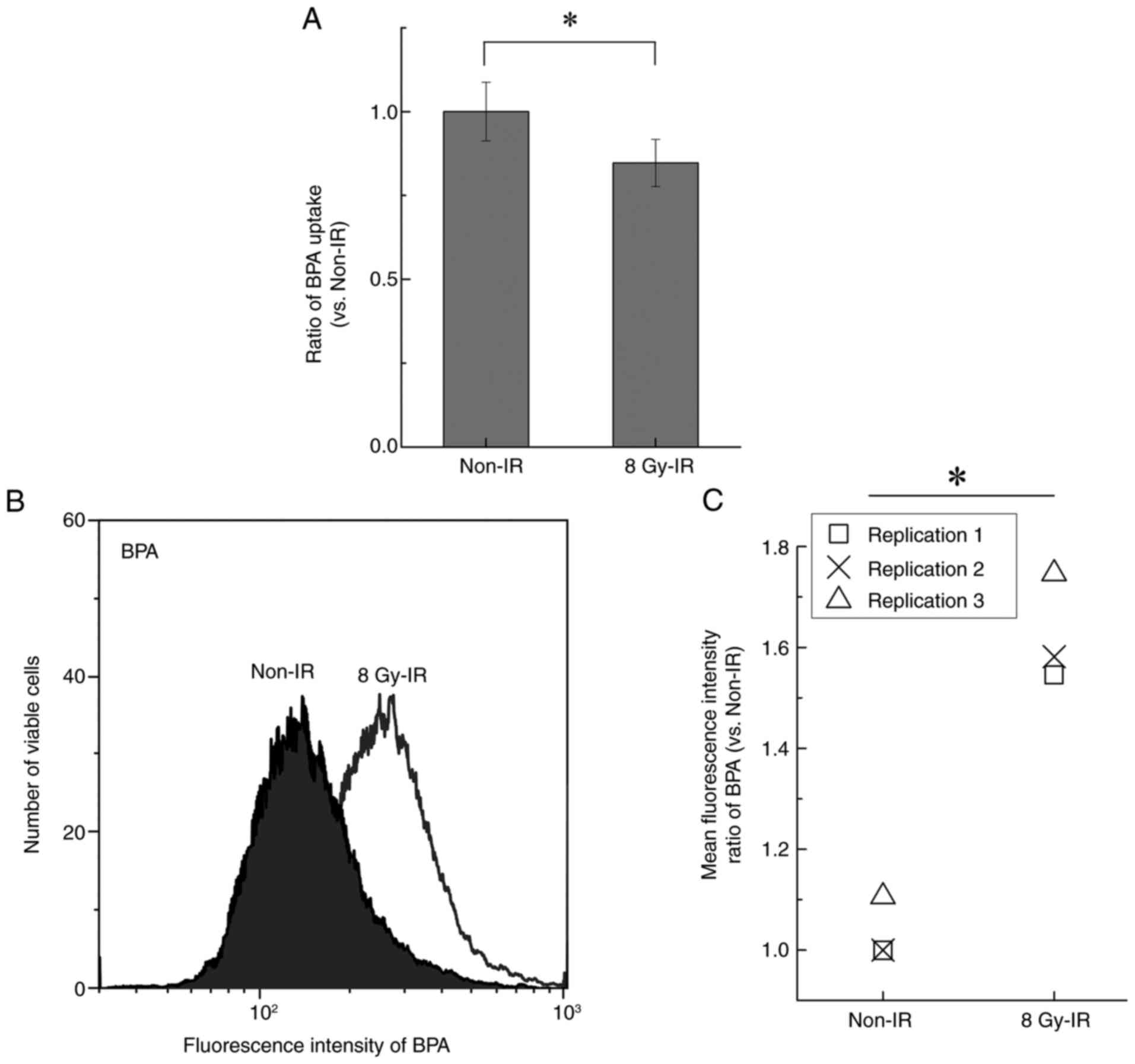
Amino acid uptake assay
Amino acid uptake into cells was analyzed using a
boronophenylalanine (BPA) uptake assay kit (cat. no. 342-09893;
Dojindo Laboratories, Inc.). BPA is an amino acid analog. The cells
exposed to IR were plated in a 96-well microplate and incubated for
12 h at 37°C in a 5% CO2 incubator. Reagents were added
according to the manufacturer's instructions. The fluorescence of
the samples (Ex350/Em455) was first measured
using a microplate reader (TriStar LB 941; Berthold Technologies
GmbH & Co. KG). After this, the incubated cells were harvested
and analyzed by flow cytometry (Aria SORP; BD Biosciences) using
FACS Diva™ software (ver.6.0; BD Biosciences) to assess
BPA uptake at the single-cell level. This assay utilizes a
cell-permeable fluorescent probe that binds to intracellular amino
acid analogs. Upon uptake of BPA via amino acid transporters such
as solute carrier family 7 member 5 [L-type amino acid transporter
type 1 (LAT1)], the probe emits fluorescence (λex, 360
nm; λem, 460 nm), allowing quantification of transporter
activity in viable individual cells.
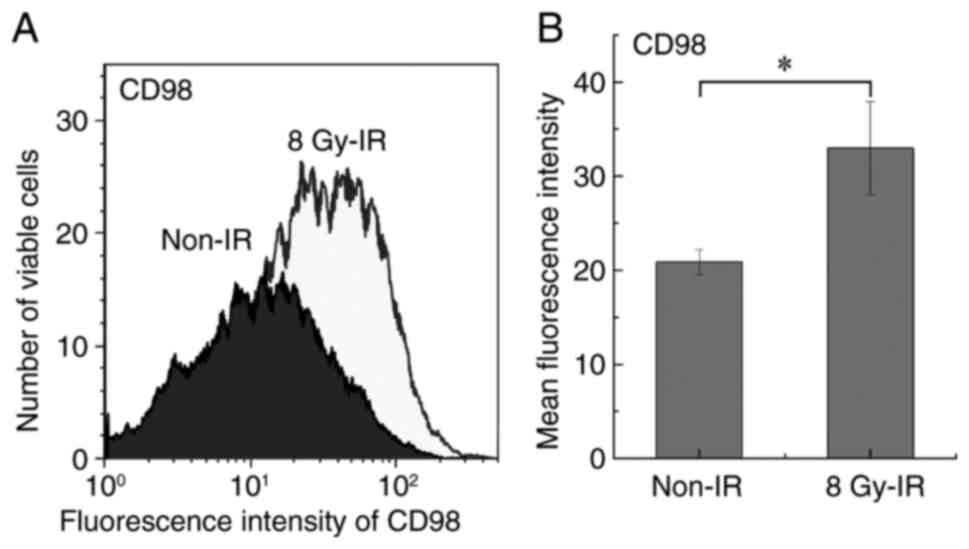
CD98 cell surface antigen
expression
The expression levels of the CD98 cell surface
antigen were analyzed using double staining with PI and CD98-FITC.
The harvested cells were suspended in Hanks' balanced salt solution
at 4°C. CD98-FITC (5 µl per 1×106 cells in 100 µl
staining volume; cat. no. 315603; BioLegend, Inc.) was added to the
sample tubes and these were incubated for 20 min on ice (~0°C) in
the dark. After washing with Hanks' balanced salt solution, the
samples were stained with PI (40 µg/ml; FUJIFILM Wako Pure Chemical
Corporation) for 3 min at room temperature. Cellular fluorescence
was measured using Cell Lab Quanta™ Sc MPL (Beckman
Coulter, Inc.) and analyzed with Kaluza software (version 2.1;
Beckman Coulter, Inc.).
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the cells using the
RNeasy® Plus Mini Kit (Qiagen, Inc.). The concentration
and quality were confirmed using the NanoDrop system (Thermo Fisher
Scientific, Inc.). cDNAs were synthesized to analyze mRNA
expression using ReverTra Ace qPCR RT Master Mix (Toyobo Co., Ltd.)
according to the manufacturer's instructions. The primer sequences
for LAT1, solute carrier family 7 member 8 [L-type amino
acid transporter type 2 (LAT2)] and solute carrier family 3
member 2 (CD98hc) were designed based on the gene sequences
obtained from the National Center for Biotechnology Information
database (https://www.ncbi.nlm.nih.gov/gene/). The mRNA
expression levels were evaluated using RT-qPCR with the Power
SYBR™ Green PCR Master Mix (Thermo Fisher Scientific,
Inc.) and SmartCycler® II (Takara Bio, Inc.). The
thermocycling conditions were: 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 1 min. The relative
expression of each target mRNA was determined using the
2−ΔΔCq method (22). The
accession numbers were as follows: LAT1, NM_003486.7;
LAT2, NM_001267036.1; CD98hc, NM_001012662.3; and
β-2-microglobulin (B2M), NM_004048.4. The oligonucleotide
primer sets used for RT-qPCR were designed using Primer3 software
(version 4.1.0) (23) and supplied
by Eurofins Genomics (Table III).
B2M was selected as the housekeeping gene for normalization
based on the Minimum Information for Publication of Quantitative
Real-Time PCR Experiments guidelines (24).
 | Table III.Sequences of human LAT1, LAT2,
CD98hc and B2M primers used for reverse
transcription-quantitative PCR. |
Table III.
Sequences of human LAT1, LAT2,
CD98hc and B2M primers used for reverse
transcription-quantitative PCR.
| Name | Sequence
(5′-3′) |
|---|
|
LAT1-Forward |
TACTTCACCACCCTGTCCAC |
|
LAT1-Reverse |
TGGAGGATGTGAACAGGGAC |
|
LAT2-Forward |
ATTGAGCTGCTAACCCTGGT |
|
LAT2-Reverse |
AGGAGAGAGTAGCCAGGGAA |
|
CD98hc-Forward |
TCTTGATTGCGGGGACTAAC |
|
CD98hc-Reverse |
GCCTTGCCTGAGACAAACTC |
|
B2M-Forward |
TTTCATCCATCCGACATTGA |
|
B2M-Reverse |
CCTCCATGATGCTGCTTACA |
Statistical analysis
Statistical analysis was performed using R software
(version 4.4.0; http://www.r-project.org/). Cell cycle analysis was
conducted using Kaluza software (version 2.1; Beckman Coulter,
Inc.) to identify sub-G1, G0/G1, S
and G2/M phases. The clonogenic survival fraction (F)
was fitted to the equation F(D)=exp
(−αD-βD2), where D is the dose in Gy and α
and β are fitting coefficients, using the
Levenberg-Marquardt algorithm. For small sample sizes (n=3), the
Wilcoxon signed-rank test was used for paired comparisons involving
two timepoints (such as metabolomic data before vs. after
131I administration). For TSH levels measured at three
timepoints (before rhTSH, day 0 and day 30), the Friedman test was
applied to account for repeated measures across multiple
timepoints. This test was chosen as a non-parametric alternative to
repeated-measures ANOVA suitable for small sample sizes and
non-normal distributions. For comparisons between two independent
groups with normal distribution and equal variance, an unpaired
Student's t-test was used. For data with unequal variance, Welch's
t-test was applied. For comparisons among multiple groups with
normal distribution and equal variance, one-way ANOVA was used,
followed by the Tukey-Kramer post hoc test for pairwise
comparisons. For comparisons of mRNA expression levels among
multiple radiation dose groups, one-way ANOVA followed by the
Tukey-Kramer post hoc test was used. Data are presented as the mean
± SD. The number of independent experiments ranged between 3 to 10.
P<0.05 or false discovery rate (FDR) <0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics during
131I therapy
Of the 3 patients with DTC included in the present
study, 2 were female and 1 was male. The patients were diagnosed
with PTC (Table I). Additionally,
the patients had no previous medical history of other thyroid
diseases. Thyrogen was administered twice, 2 days and 1 day before
131I administration, and its accumulation in the
residual tumor was observed using scintillation scanning during
treatment. Antitumor effects were observed in all patients 6 months
after the initial prescription of Thyrogen, as evaluated based on
the medical records of the patients (data not shown). No
significant hemocytopenia was observed in terms of the numbers of
red blood cells, white blood cells and platelets within 30 days
after 131I therapy (Fig.
1A-C). Serum TSH levels increased in all patients, reaching a
maximum of 105–455 µIU/ml, from before Thyrogen administration to
just prior to 131I administration. By day 30 after
administration, TSH levels had returned to the same range as before
131I administration (Fig.
1D). FT3 and FT4 before and after treatment remained at 2.6–3.6
pg/ml and 1.4–2.4×10−2 ng/ml, respectively, which were
confirmed to be normal values (Fig. 1E
and F). Conversely, the levels of Tg/Ab and Tg in the serum
showed individual variations between patients (patient 1: Tg/Ab,
10–10.6 IU/ml; Tg, 2.24 ng/ml; patient 2: Tg/Ab, 12.6–12.9 IU/ml;
Tg, 23.5–35.1 ng/ml; patient 3: Tg/Ab, 10 IU/ml; Tg, 0.07–0.39
ng/ml; Fig. 1G and H).
Patient metabolomic analysis
Metabolomic analysis was performed using patient
serum to explore serum metabolites specifically altered by
131I radiotherapy following Thyrogen stimulation for
DTC. Of the 188 types of target metabolomic analysis data obtained
from mass spectrometry, 168 were highly reliable. Among them, the
levels of nine metabolites that changed before and after
131I administration were increased, and the levels of
one metabolite were decreased (Fig.
2). Furthermore, enrichment analysis based on the KEGG database
using MeatboAnalyst6.0 identified valine, leucine and isoleucine
biosynthesis as a significant metabolic pathway (FDR, 0.0392; data
not shown). Valine, leucine and isoleucine are essential amino
acids referred to as branched-chain amino acids (BCAAs) and are
involved in the nitrogen supply to cancer (25). In all cases, BCAA levels tended to
increase after 131I administration compared with before
administration (Fig. 2). However,
statistical significance was observed only for valine, whereas the
increases in leucine and isoleucine were not significant. Given the
small sample size (n=3), statistical comparisons were performed
using the Wilcoxon signed-rank test. The consistent upward trend
across patients supports the robustness of this exploratory
observation.
Radiosensitivity of TPC-1 cells
The TPC-1 cell line was used to examine whether the
serum metabolic changes observed in patients with DTC could be
recapitulated at the cellular level. Clonogenic potency and the
cell cycle were analyzed to confirm the radiosensitivity of TPC-1
cells. The plating efficiency for TPC-1 cell colony formation was
13±1.2% (Fig. 3A), the parameters
in the linear-quadratic model were α=0.32±0.05 Gy−1,
β=0.03±0.02 Gy−2 and α/β=12.12±0.66, and the survival
rate was <1% at 8 Gy (Fig. 3B).
The cell cycle phase distribution analysis of cells exposed to 8 Gy
IR showed a time-dependent increase in cells in the G2/M
phase for 12 h. A significant increase was observed at 6–24 h
compared with the non-irradiated control (Fig. 3C and D). The number of viable cells
after 12 h of exposure decreased to ~50% compared with that of the
non-irradiated group (Fig. 3E).
Quantitation of BCAA uptake under IR
exposure
Similar to BCAAs, BPA is taken up by cells via LAT1
(26,27). Changes in BPA uptake were evaluated
to determine the BCAA uptake ability of irradiated TPC-1 cells.
After 12 h of exposure to 8 Gy IR, intracellular BPA levels
significantly decreased to ~80% of those in the non-irradiated
control group, corresponding to an ~20% reduction (Fig. 4A). Conversely, single-cell analysis
by flow cytometry revealed that the levels of intracellular BPA
following exposure to IR were significantly upregulated ~1.58 times
compared with the non-irradiated control (Fig. 4B and C).
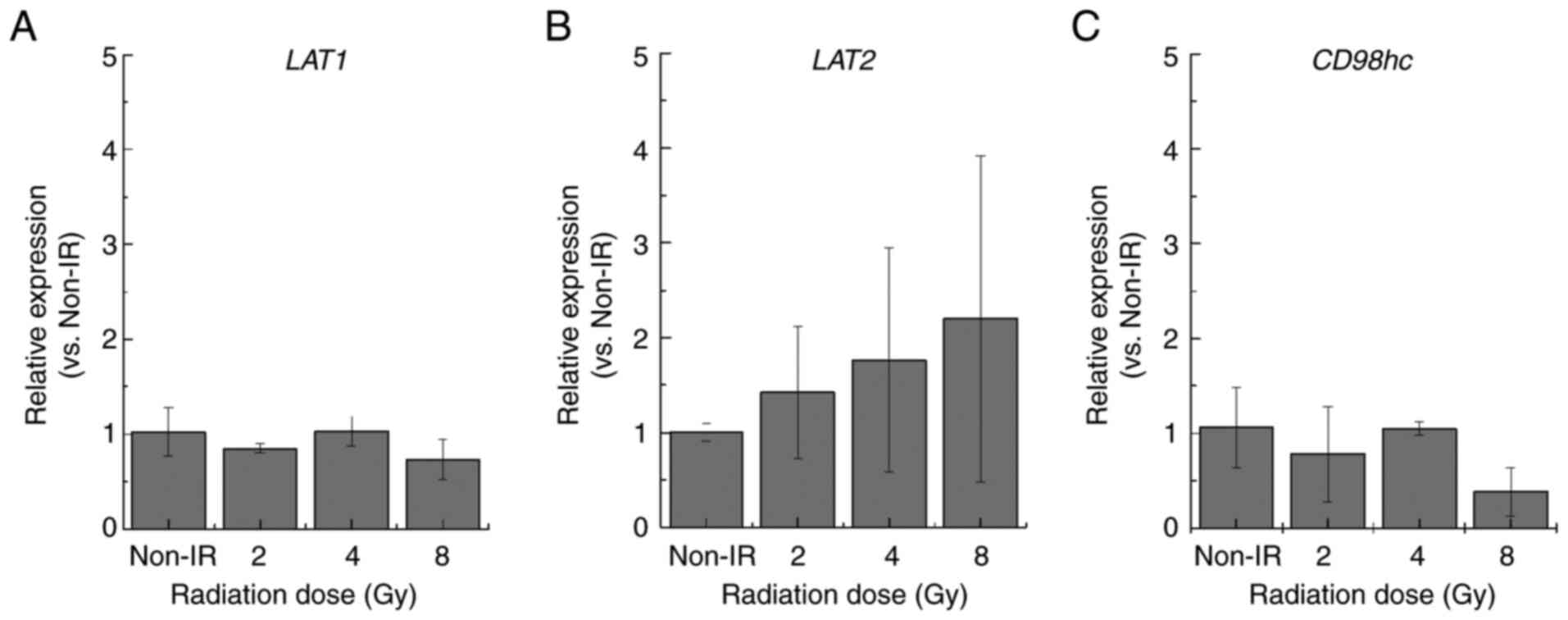
CD98 expression
LAT1 forms a complex with CD98hc on the cell
membrane and triggers amino acid uptake (28). The flow cytometry analysis of TPC-1
cells exposed to 8 Gy IR revealed significant upregulation of the
CD98 cell surface antigen compared with the non-irradiated control
(Fig. 5). However, RT-qPCR analysis
revealed that the expression levels of the related mRNAs LAT1,
LAT2 and CD98hc were similar to those in the
non-irradiated control group (Fig.
6).
Discussion
In the present study, serum metabolomic analysis
focusing on patients with DTC and single-cell analysis using TPC-1
cells were conducted to identify Tg/Ab-independent biomarkers that
indicate the effect of thyroid cancer ablation using
131I administration under Thyrogen therapy. On day 30
post-treatment, only 1 case showed a response based on Tg levels;
however, an increase in BCAA levels, particularly valine, was
observed independently of Tg/Ab fluctuations. In the DTC cell
model, exposure to 8 Gy IR altered BPA uptake, a leucine analog
used to evaluate BCAA transport, by viable cells. While a decrease
in overall BPA uptake was observed in the total cell population,
the surviving viable cells exhibited relatively increased uptake
compared with non-irradiated controls. This apparent discrepancy
reflects the fact that IR induced substantial cell death, thereby
reducing the absolute uptake at the population level, whereas the
residual viable cells responded to radiation-induced stress with
enhanced amino acid transporter activity. Several studies have
reported a similar phenomenon. Bo et al (29) reported that LAT1-mediated amino acid
uptake in pancreatic and lung cancer cells was enhanced under
conditions of both a high dose rate (such as increased radiation
flux or greater dose delivery per unit time) and a high accumulated
dose, suggesting that transporter activity is influenced by both
the dose rate and the cumulative radiation dose. Additionally,
their cells exhibited increased radiosensitivity due to the
inhibition of LAT1 expression. The present study demonstrated BPA
uptake in TPC-1 cells was predominantly downregulated following
exposure to 8 Gy IR, although a relative increase was observed in
the surviving viable cell fraction. LAT1 overexpression has been
reported to increase BCAA uptake in hepatocellular carcinoma
(30,31). While LAT1 functions as a transporter
only when complexed with CD98hc on the cell membrane (32), forming a functional heterodimer
essential for plasma membrane localization and activity (28), quantitative assessment of this
heterodimer via western blotting using whole-cell lysates remains
challenging, as membrane-bound and intracellular protein fractions
cannot be distinguished, and therefore, do not accurately reflect
surface expression (33,34). By contrast, flow cytometry allows
for the direct evaluation of membrane-localized LAT1, which is
closely associated with CD98hc-mediated transport activity
(35,36). Therefore, flow cytometry was used to
evaluate functional upregulation of LAT1/CD98hc at the cell
surface. Nevertheless, further studies incorporating membrane
protein isolation and direct analysis of CD98hc expression are
warranted to fully validate the mechanistic role of this
transporter system in response to irradiation.
Yoshida et al (37) and Matsuya et al (38) reported that the BPA uptake in tumor
cells was highest in the G2/M phase compared with other
cell cycle phases. These findings and those of the present study
indicate that the cellular uptake and serum concentration of BCAAs
are involved in LAT1 expression and cell cycle distribution.
Conversely, elevated blood BCAA levels have been reported in
patients with obesity, insulin resistance and cardiovascular
disease (39,40). However, the 3 DTC cases in the
present study did not have these conditions. High LAT1 expression
has been reported in PTC (41).
Based on these findings, the upregulation of serum valine (one of
the BCAAs) after 131I treatment with Thyrogen, as shown
in the patient analysis in the present study, may reflect the fact
that valine was not taken up by the tumor. Although leucine and
isoleucine also showed an increasing trend, these changes were not
statistically significant. These BCAA changes may reflect reduced
tumor burden, although direct histopathological confirmation is
lacking. In the present in vitro experiments, an X-ray dose
of 8 Gy was selected based on the clonogenic survival curve of
TPC-1 cells, which showed ~1% survival at this dose. This dose was
sufficient to elicit measurable biological responses, including
changes in amino acid uptake, and is commonly used in mechanistic
in vitro studies of DNA damage, cell cycle arrest and
radiosensitization (21,42,43).
Typically, 30–80 Gy are absorbed by the residual thyroid tissue in
patients receiving 131I (1.11 GBq) under Thyrogen
stimulation depending on remnant size, iodine uptake and kinetics,
as calculated using the International Commission on Radiological
Protection Publication 106 (44).
However, administration of such high doses is not feasible in cell
culture due to excessive cytotoxicity (45). Therefore, the use of 8 Gy in the
present study represents a biologically relevant and experimentally
practical model for evaluating the cellular response to therapeutic
radioiodine exposure.
Although 131I is clinically relevant, its
uptake relies on the functional NIS, which is minimally expressed
in certain thyroid cancer cells, including some differentiated
thyroid carcinoma cell lines (3,4).
Consequently, 131I-based radiation delivery in such
in vitro models would be highly variable and non-uniform,
reflecting differences in remnant tissue uptake and iodine kinetics
observed clinically (5,19,20).
By contrast, X-rays provide consistent and uniform exposure in cell
culture, making them a practical alternative to assess general
radiation-induced stress responses (21,42).
While X-rays do not replicate the β-particle emissions or
NIS-mediated intracellular effects of 131I, the present
approach offers a feasible and relevant model to evaluate
LAT1/CD98hc-mediated responses to cytotoxic stress. Future studies
using NIS-overexpressing cells or direct 131I labeling
will be necessary to clarify 131I-specific mechanisms.
However, the biological effects of 131I, primarily
through β-particle emissions and active uptake via NIS, are not
fully recapitulated by X-ray irradiation (3,5,19,20).
The use of TPC-1 cells, which express minimal NIS, limits direct
131I uptake, rendering 131I exposure
inconsistent and technically challenging for in vitro
assays. X-ray irradiation, by contrast, provides a uniform,
controllable and reproducible source of IR that induces cytotoxic
stress independently of NIS-mediated uptake (21,42).
Additionally, the handling of 131I in cell culture
requires specialized radiation safety measures, licensing and
infrastructure, which could delay exploratory mechanistic studies.
Therefore, X-rays were selected as a practical alternative to model
general IR-induced stress and to investigate the regulation of
amino acid transporter activity, particularly LAT1/CD98hc, under
controlled conditions. This is a limitation of the present study,
and future studies using NIS-overexpressing cells or direct
131I labeling are needed to elucidate
131I-specific mechanisms.
Exposure of TPC-1 cells to IR induced the expression
of the CD98 surface antigen, which may assist in increasing BCAA
uptake. BCAAs are essential amino acids mostly supplied through the
diet and taken up into tumors by the LAT1-CD98hc complex expressed
on the cell membrane (23,27,46).
Le Bricon et al (47)
performed a qualitative analysis using immunohistochemical methods
and reported no association between LAT1-positive cancer and BPA
uptake. In the present study, the expression levels of the CD98
surface antigen and related mRNAs were quantitatively examined,
which is a novel finding. Furthermore, the mRNA expression levels
of LAT1, LAT2 and CD98hc did not change under
exposure to IR based on RT-qPCR. Although LAT1 and
LAT2 mRNA levels were unchanged, membrane or total protein
expression was not assessed due to methodological limitations.
Future studies involving membrane protein isolation are needed to
clarify whether surface expression of LAT1/LAT2 is altered by
irradiation. These findings indicate that the increase in BCAA
uptake following IR is a temporary response driven by LAT1 protein
already present in the cells, rather than by newly transcribed
LAT1 mRNA. However, further studies are needed to elucidate
its molecular mechanism. Furthermore, the interpretation of the
increase in serum BCAA levels remains speculative, as
histopathological confirmation of the tumor response was not
performed in the present study, which analyzed patient samples
collected with clinical follow up after obtaining informed consent
and ethics approval. The observed changes in BCAA levels may
reflect either the release of metabolites from damaged tumor cells
or systemic metabolic shifts associated with reduced tumor burden.
Although elevated serum BCAA levels may reflect reduced tumor
uptake due to LAT1 downregulation, this interpretation should be
made with caution, since BCAA levels are also influenced by dietary
intake, gastrointestinal absorption, hepatic metabolism and
systemic inflammation, including cytokine activity (such as TNF-α),
which affects amino acid utilization and appetite (39,40).
Furthermore, while LAT1 was upregulated in PTC in previous studies
(48,49), its overall contribution to systemic
BCAA regulation remains unclear. In the present cases, there was no
clinical evidence of recurrence; thus, BCAA changes may reflect
systemic metabolic adaptations rather than direct tumor shrinkage.
The present in vitro data suggested that LAT1/CD98-mediated
BCAA uptake represents a mechanism of stress adaptation in
surviving cells; however, validation with tissue-level evidence is
warranted. This limitation should be addressed in a future
prospective cohort study.
Previous studies have demonstrated that LAT1
expression was upregulated in aggressive variants of DTC and was
associated with increased tumor cell proliferation and
dedifferentiation, particularly in BRAF-mutant PTC (48,49).
This upregulation facilitates increased uptake of essential amino
acids, such as BCAAs, supporting tumor metabolism and growth
(25,34,35).
In this context, the elevated serum BCAA levels observed after
131I treatment may reflect reduced tumor burden and
decreased LAT1-mediated amino acid uptake, rather than a mere
passive bystander effect. Although the present findings did not
establish causality, the consistent increase in serum BCAA
concentrations following radioiodine therapy in all patients
suggests potential utility as a surrogate biomarker for treatment
efficacy. Furthermore, preclinical studies have demonstrated that
LAT1 inhibition suppresses tumor growth in thyroid carcinoma
(48,49) and hepatocellular carcinoma (30), and has also been implicated in
several other cancer types (25,35).
Furthermore, LAT1 blockade has been shown to sensitize cancer cells
to radiation (29). As summarized
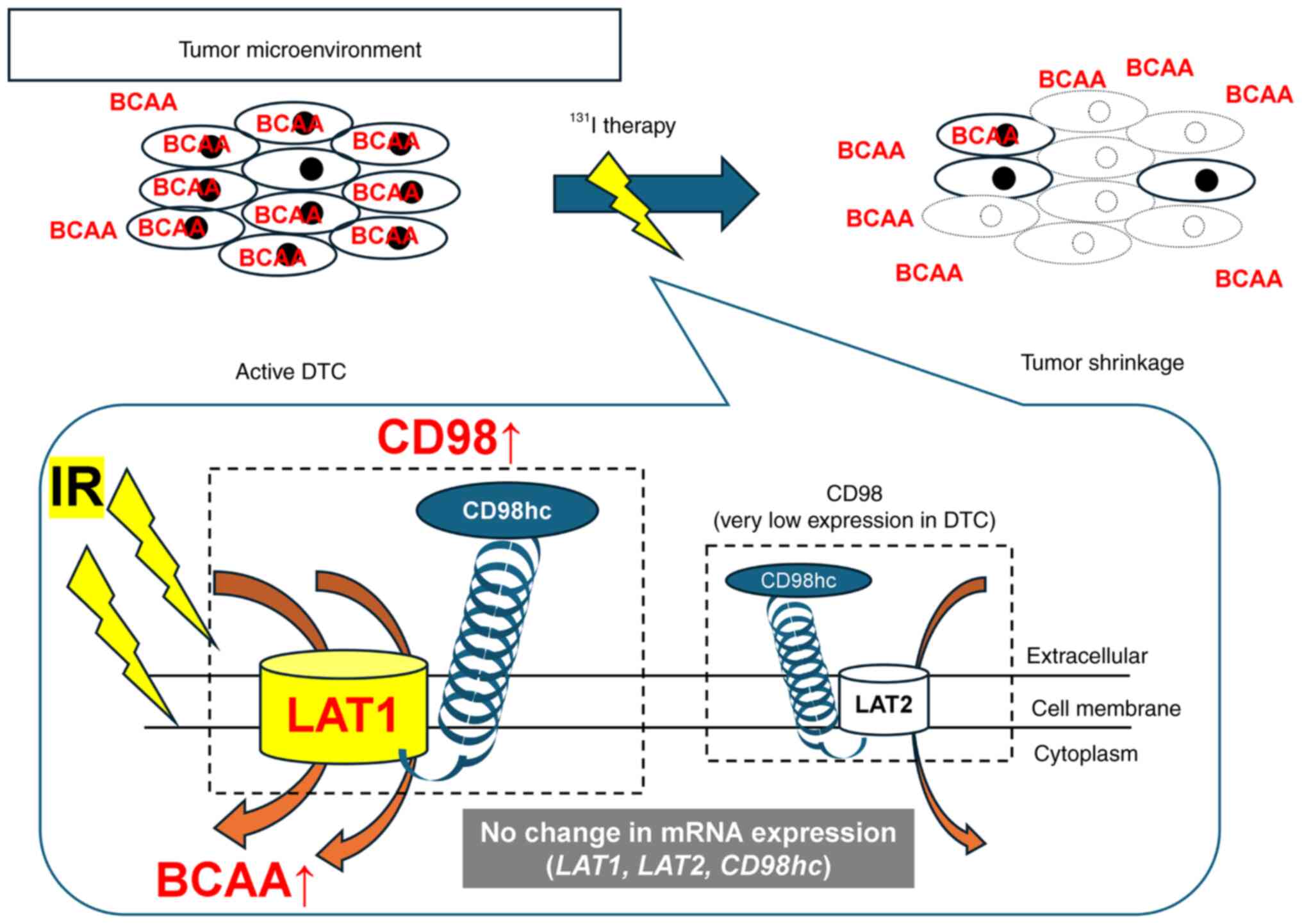
in Fig. 7, BCAAs are transported
into tumor cells via the LAT1/CD98 complex, IR exposure can
transiently increase BCAA uptake in surviving cells, and reduced
tumor uptake after 131I therapy may elevate serum BCAA
levels. This integrative mechanism highlights the link between LAT1
activity, amino acid metabolism and treatment response. These
observations raise the possibility that LAT1 inhibition could
potentiate the therapeutic efficacy of 131I in DTC,
especially in tumors with high LAT1 expression (Fig. 7). Further studies are needed to
evaluate the translational potential of combining LAT1 inhibitors
with radioiodine therapy.
The present study has several limitations. First,
the number of patients was small (n=3), which inevitably reduced
the statistical power of the clinical findings. Nevertheless, in
the absence of prior data on fluctuations in serum BCAA levels in
Tg/Ab-positive DTC, this work was designed as a proof-of-concept
study. The observed clinical trends were supported by mechanistic
experiments using the TPC-1 cell line, which reinforced the
biological relevance of BCAA metabolism as a potential surrogate
marker of the efficacy of radioiodine therapy. Second, dietary
intake of BCAAs was not assessed, which could affect the
interpretation of serum metabolite levels. Although all patients
fasted for ≥6 h before serum sampling to minimize acute
postprandial effects, the influence of habitual dietary intake
cannot be completely excluded. To address this, standardized
dietary questionnaires or controlled dietary interventions should
be incorporated in future studies to clarify the extent to which
serum BCAA levels reflect tumor-related metabolic changes rather
than nutritional variability. Future studies are also needed to
determine whether elevated serum BCAA levels following radioiodine
therapy reflect direct release from dying tumor cells, systemic
metabolic adaptations due to reduced tumor burden or altered
transporter activity in surviving cells. Analysis of associations
with tumor histopathology, imaging-based assessments of tumor
volume and LAT1/CD98hc expression in clinical specimens will be
critical to establish mechanistic links. Additionally, prospective
cohort studies with larger sample sizes and standardized dietary
assessments are warranted to validate BCAAs as a reliable biomarker
for therapeutic efficacy in DTC. Despite unchanged mRNA levels of
LAT1, LAT2 and CD98hc following IR, flow cytometry
demonstrated increased CD98hc surface expression, suggesting
post-transcriptional regulation, such as membrane translocation.
Notably, the present study assessed mRNA levels at a limited number
of time points, which were selected based on feasibility and
previous reports (42,43). It is possible that transient
fluctuations in gene expression at other time points were missed.
Furthermore, radiation-induced gene expression is known to be both
dose- and time-dependent (42,43).
Further studies are needed to clarify the temporal dynamics of
LAT1/CD98hc regulation by evaluating a broader range of radiation
doses and time points, and to investigate the mechanistic basis of
membrane translocation and its functional consequences in amino
acid transport activity.
In patients who are Tg/Ab-positive, monitoring of
serum Tg levels can be unreliable due to antibody interference
(6–8). The present results suggested that BCAA
levels may serve as a complementary biomarker to Tg, providing
additional information independent of Tg/Ab status. However,
further validation in larger cohorts is necessary to determine
whether BCAA monitoring could eventually replace or supplement Tg
measurements in clinical practice.
In conclusion, the present findings suggested that
an increased extracellular concentration of BCAAs following
131I therapy may reflect a decrease in residual DTC
tissue, and thus, may serve as a clinically useful surrogate marker
of therapeutic efficacy, particularly in patients for whom
traditional Tg-based monitoring is unreliable.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mrs. Miyu Miyazaki
(Scientific Research Facility Center of Hirosaki University
Graduate School of Medicine, Hirosaki, Aomori, Japan) for help with
the mass spectrometry, and Mrs. Suzuna Doi (Department of Radiation
Science, Hirosaki University Graduate School of Health Sciences,
Hirosaki, Aomori, Japan) for her assistance with fluorescence
measurements using a microplate reader.
Funding
The present study was supported by JSPS KAKENHI, Grants-in-Aid
for Scientific Research (B) (project no. 21H02861/23K21419) and
Takeda Science Foundation 2023.
Availability of data and materials
The metabolomics data generated in the present study
may be found in the MetaboBank database under accession numbers
MTBKS257 and MTBKS258 or at the following URLs: https://ddbj.nig.ac.jp/public/metabobank/study/MTBKS257/
and https://ddbj.nig.ac.jp/public/metabobank/study/MTBKS258/.
All other data generated in the present study are included in the
figures and/or tables of this article.
Authors' contributions
AK, YM, AW and SM designed the study, drafted the
manuscript and actively participated in its revision. AK, YT, RS,
YM and SM examined and analyzed the experimental data. YT, YM, AW
and SM oversaw the study, and provided final approval of the
version submitted and published. YM and SM confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Committee of
Medical Ethics of the Hirosaki University Graduate School of Health
Sciences (approval no. 2021-050; Hirosaki, Japan). Written informed
consent was obtained from all participants after providing a
detailed verbal explanation, and all collected data were anonymized
and handled in accordance with ethical guidelines.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI
|
|
2
|
Nabhan F, Dedhia PH and Ringel MD: Thyroid
cancer, recent advances in diagnosis and therapy. Int J Cancer.
149:984–992. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Japan Associations of Endocrine Surgery
Task Force on the Guidelines for Thyroid Tumors (2024), . Clinical
practice guidelines on the management of thyroid tumors 2024. Japan
Assoc Endocr Surg. 41 (Suppl 2):S23–S42. 2024.
|
|
5
|
Hänscheid H, Lassmann M, Luster M, Thomas
SR, Pacini F, Ceccarelli C, Ladenson PW, Wahl RL, Schlumberger M,
Ricard M, et al: Iodine biokinetics and dosimetry in radioiodine
therapy of thyroid cancer: Procedures and results of a prospective
international controlled study of ablation after rhTSH or hormone
withdrawal. J Nucl Med. 47:648–654. 2006.PubMed/NCBI
|
|
6
|
Stanojevic M, Savin S, Cvejic D, Djukic A,
Jeremic M and Zivancević Simonovic S: Comparison of the influence
of thyroglobulin antibodies on serum thyroglobulin values from two
different immunoassays in post surgical differentiated thyroid
carcinoma patients. J Clin Lab Anal. 23:341–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Spencer CA, Takeuchi M, Kazarosyan M, Wang
CC, Guttler RB, Singer PA, Fatemi S, LoPresti JS and Nicoloff JT:
Serum thyroglobulin autoantibodies: Prevalence, influence on serum
thyroglobulin measurement, and prognostic significance in patients
with differentiated thyroid carcinoma. J Clin Endocrinol Metab.
83:1121–1127. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Petrovic I, LoPresti J, Fatemi S,
Gianoukakis A, Burman K, Gomez-Lima CJ, Nguyen CT and Spencer CA:
Influence of thyroglobulin autoantibodies on thyroglobulin levels
measured by different methodologies: IMA, LC-MS/MS, and RIA. J Clin
Endocrinol Metab. 109:3254–3263. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cararo Lopes E, Sawant A, Moore D, Ke H,
Shi F, Laddha S, Chen Y, Sharma A, Naumann J, Guo JY, et al:
Integrated metabolic and genetic analysis reveals distinct features
of human differentiated thyroid cancer. Clin Transl Med.
13:e12982023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deja S, Dawiskiba T, Balcerzak W,
Orczyk-Pawiłowicz M, Głód M, Pawełka D and Młynarz P: Follicular
adenomas exhibit a unique metabolic profile. ¹H NMR studies of
thyroid lesions. PLoS One. 8:e846372013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abraham T and Schöder H: Thyroid
cancer-indications and opportunities for positron emission
tomography/computed tomography imaging. Semin Nucl Med. 41:121–138.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo L, Zhang Y, Li H, Li S and Guo X:
Clinical analysis of influencing factors on therapeutic effect of
radioactive iodine therapy in thyroglobulin antibodies positive
patients with papillary thyroid cancer. J Nucl Med. 63 (Suppl
2):S30062022.
|
|
13
|
Clish CB: Metabolomics: An emerging but
powerful tool for precision medicine. Cold Spring Harb Mol Case
Stud. 1:a0005882015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yu S, Liu C, Hou Y, Li J, Guo Z, Chen X,
Zhang L, Peng S, Hong S, Xu L, et al: Integrative metabolomic
characterization identifies plasma metabolomic signature in the
diagnosis of papillary thyroid cancer. Oncogene. 41:2422–2430.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jiang N, Zhang Z, Chen X, Zhang G, Wang Y,
Pan L, Yan C, Yang G, Zhao L, Han J and Xue T: Plasma lipidomics
profiling reveals biomarkers for papillary thyroid cancer
diagnosis. Front Cell Dev Biol. 9:6822692021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu J, Hu S, Miccoli P, Zeng Q, Liu S, Ran
L and Hu C: Non-invasive diagnosis of papillary thyroid
microcarcinoma: A NMR-based metabolomics approach. Oncotarget.
7:81768–81777. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Razavi SA, Mahmanzar M, Nobakht M Gh BF,
Zamani Z, Nasiri S and Hedayati M: Plasma metabolites analysis of
patients with papillary thyroid cancer: A preliminary untargeted
1H NMR-based metabolomics. J Pharm Biomed Anal.
241:1159462024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Monzen S, Tatara Y, Mariya Y, Chiba M,
Wojcik A and Lundholm L: HER2-positive breast cancer that resists
therapeutic drugs and ionizing radiation releases
sphingomyelin-based molecules to circulating blood serum. Mol Clin
Oncol. 13:702020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Maxon HR, Thomas SR, Hertzberg VS,
Kereiakes JG, Chen IW, Sperling MI and Saenger EL: Relation between
effective radiation dose and outcome of radioiodine therapy for
thyroid cancer. N Engl J Med. 309:937–941. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schlesinger T, Flower MA and McCready VR:
Radiation dose assessments in radioiodine (131I) therapy. 1. The
necessity for in vivo quantitation and dosimetry in the treatment
of carcinoma of the thyroid. Radiother Oncol. 14:35–41. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Franken NAP, Rodermond HM, Stap J, Haveman
J and van Bree C: Clonogenic assay of cells in vitro. Nat Protoc.
1:2315–2319. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Untergasser A, Cutcutache I, Koressaar T,
Ye J, Faircloth BC, Remm M and Rozen SG: Primer3-new capabilities
and interfaces. Nucleic Acids Res. 40:e1152012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanai Y: Amino acid transporter LAT1
(SLC7A5) as a molecular target for cancer diagnosis and
therapeutics. Pharmacol Ther. 230:1079642022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wittig A, Sauerwein WA and Coderre JA:
Mechanisms of transport of p-borono-phenylalanine through the cell
membrane in vitro. Radiat Res. 153:173–180. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wongthai P, Hagiwara K, Miyoshi Y,
Wiriyasermkul P, Wei L, Ohgaki R, Kato I, Hamase K, Nagamori S and
Kanai Y: Boronophenylalanine, a boron delivery agent for boron
neutron capture therapy, is transported by ATB0,+, LAT1 and LAT2.
Cancer Sci. 106:279–286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan R, Zhao X, Lei J and Zhou Q: Structure
of the human LAT1-4F2hc heteromeric amino acid transporter complex.
Nature. 568:127–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bo T, Kobayashi S, Inanami O, Fujii J,
Nakajima O, Ito T and Yasui H: LAT1 inhibitor JPH203 sensitizes
cancer cells to radiation by enhancing radiation-induced cellular
senescence. Transl Oncol. 14:1012122021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Namikawa M, Kakizaki S, Kaira K, Tojima H,
Yamazaki Y, Horiguchi N, Sato K, Oriuchi N, Tominaga H, Sunose Y,
et al: Expression of amino acid transporters (LAT1, ASCT2 and xCT)
as clinical significance in hepatocellular carcinoma. Hepatol Res.
45:1014–1022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ericksen RE, Lim SL, McDonnell E, Shuen
WH, Vadiveloo M, White PJ, Ding Z, Kwok R, Lee P, Radda GK, et al:
Loss of BCAA catabolism during carcinogenesis enhances mTORC1
activity and promotes tumor development and progression. Cell
Metab. 29:1151–1165.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanai Y, Segawa H, Miyamoto K, Uchino H,
Takeda E and Endou H: Expression cloning and characterization of a
transporter for large neutral amino acids activated by the heavy
chain of 4F2 antigen (CD98). J Biol Chem. 273:23629–23632. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fotiadis D, Kanai Y and Palacín M: The
SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med.
34:139–158. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Napolitano L, Scalise M, Galluccio M,
Pochini L, Albanese LM and Indiveri C: LAT1 is the transport
competent unit of the LAT1/CD98 heterodimeric amino acid
transporter. Int J Biochem Cell Biol. 67:25–33. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Häfliger P and Charles RP: The L-type
amino acid transporter LAT1-an emerging target in cancer. Int J Mol
Sci. 20:24282019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang J, Xu Y, Li D, Fu L, Zhang X, Bao Y
and Zheng L: Review of the correlation of LAT1 with diseases:
mechanism and treatment. Front Chem. 8:5648092020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yoshida F, Matsumura A, Shibata Y,
Yamamoto T, Nakauchi H, Okumura M and Nose T: Cell cycle dependence
of boron uptake from two boron compounds used for clinical neutron
capture therapy. Cancer Lett. 187:135–141. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsuya Y, Sato T, Kusumoto T, Yachi Y,
Seino R, Miwa M, Ishikawa M, Matsuyama S and Fukunaga H: Cell-cycle
dependence on the biological effects of boron neutron capture
therapy and its modification by polyvinyl alcohol. Sci Rep.
14:166962024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shah SH, Crosslin DR, Haynes CS, Nelson S,
Turer CB, Stevens RD, Muehlbauer MJ, Wenner BR, Bain JR, Laferrère
B, et al: Branched-chain amino acid levels are associated with
improvement in insulin resistance with weight loss. Diabetologia.
55:321–330. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
McGarrah RW and White PJ: Branched-chain
amino acids in cardiovascular disease. Nat Rev Cardiol. 20:77–89.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wittig A, Sheu-Grabellus SY, Collette L,
Moss R, Brualla L and Sauerwein W: BPA uptake does not correlate
with LAT1 and Ki67 expressions in tumor samples (results of EORTC
trial 11001). Appl Radiat Isot. 69:1807–1812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao H, Zhuang Y, Li R, Liu Y, Mei Z, He
Z, Zhou F and Zhou Y: Effects of different doses of X-ray
irradiation on cell apoptosis, cell cycle, DNA damage repair and
glycolysis in HeLa cells. Oncol Lett. 17:42–54. 2019.PubMed/NCBI
|
|
43
|
Keam S, MacKinnon KM, D'Alonzo RA, Gill S,
Ebert MA, Nowak AK and Cook AM: Effects of photon radiation on DNA
damage, cell proliferation, cell survival, and apoptosis of murine
and human mesothelioma cell lines. Adv Radiat Oncol. 7:1010132022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
ICRP, . Radiation dose to patients from
radiopharmaceuticals. Addendum 3 to ICRP Publication 53. ICRP
Publication 106. Approved by the Commission in October 2007. Ann
ICRP. 38:1–197. 2008.PubMed/NCBI
|
|
45
|
Du J, Ren W, Liu W, Zhou Y, Li Y, Lai Q,
Liu X, Chen T, Liu W, Chen Z, et al: Experimental study of
iodine-131 labeling of a novel tumor-targeting peptide, TFMP-Y4, in
the treatment of hepatocellular carcinoma with internal
irradiation. BMC Cancer. 25:2452025. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan R, Li Y, Müller J, Zhang Y, Singer S,
Xia L, Zhong X, Gertsch J, Altmann KH and Zhou Q: Mechanism of
substrate transport and inhibition of the human LAT1-4F2hc amino
acid transporter. Cell Discov. 7:162021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Le Bricon T, Cynober L, Field CJ and
Baracos VE: Supplemental nutrition with ornithine
alpha-ketoglutarate in rats with cancer-associated cachexia:
Surgical treatment of the tumor improves efficacy of nutritional
support. J Nutr. 125:2999–3010. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Häfliger P, Graff J, Rubin M, Stooss A,
Dettmer MS, Altmann KH, Gertsch J and Charles RP: The LAT1
inhibitor JPH203 reduces growth of thyroid carcinoma in a fully
immunocompetent mouse model. J Exp Clin Cancer Res. 37:2342018.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Enomoto K, Sato F, Tamagawa S, Gunduz M,
Onoda N, Uchino S, Muragaki Y and Hotomi M: A novel therapeutic
approach for anaplastic thyroid cancer through inhibition of LAT1.
Sci Rep. 9:146162019. View Article : Google Scholar : PubMed/NCBI
|