Introduction
In 2022, gastric cancer (GC) was reported to be the
fifth most common cancer worldwide, with ~968,000 new cases
diagnosed globally (1). Although
various treatment modalities have been developed for GC and other
cancer types, including surgery, chemotherapy, radiotherapy,
immunotherapy and targeted therapy, therapeutic outcomes remain
suboptimal, particularly in the advanced stages (2). Surgery is the primary treatment option
for early-stage GC; however, its efficacy diminishes in locally
advanced or metastatic disease (3).
Chemotherapy is widely used in advanced settings; however, it is
often associated with severe systemic toxicities that limit
long-term application (4).
Radiotherapy is primarily used for palliative symptom control or as
an adjuvant treatment; however, it has limited effectiveness in
improving overall survival (OS) in metastatic GC (5,6).
Immunotherapy has shown promising efficacy in certain subgroups,
such as in patients with high microsatellite instability or
Epstein-Barr virus-positive GC; however, response rates remain low
in unselected populations (7,8).
Traditional Chinese medicine, including herbal formulations and
acupuncture, has been integrated into supportive cancer care to
alleviate treatment-related side effects and to improve quality of
life, although robust evidence from large-scale randomized
controlled trials supporting its direct antitumor efficacy is still
lacking (9–13). Biomarker-driven targeted therapies
are effective in patients with specific molecular alterations;
however, they offer no benefit to most patients with GC lacking
these targets (14). Consequently,
despite the diversity of available treatments, the annual number of
GC-related deaths remains as high as 660,000 globally (15), indicating the urgent need to develop
more effective and better-tolerated therapeutic strategies for
GC.
Claudin 18.2 (CLDN18.2) is highly and specifically
expressed in GC (16,17), making it a primary target for
precision therapy (18). Beyond its
role as a surface biomarker, CLDN18.2 contributes to gastric
oncogenesis through multifaceted mechanisms (19). In normal gastric mucosa, CLDN18.2 is
exclusively localized to the tight junctions of polarized
epithelial cells; however, in gastric cancer, neoplastic cells
frequently lose their polarity, leading to the mislocalization of
CLDN18.2, characterized by its diffuse presence over the entire
cell membrane rather than being confined to the apical junction
complex (17). Its aberrant
expression and mislocalization in GC disrupt tight junction
integrity and epithelial polarity, thereby enhancing tumor cell
invasion and metastasis (20,21).
Furthermore, CLDN18.2 is implicated in pro-tumorigenic signaling,
potentially through interactions with integrins to activate
FAK/SRC, PI3K/AKT and MAPK/ERK pathways, which promote cell
proliferation, survival and migration (22). This protein also influences the
tumor immune microenvironment (23,24);
high CLDN18.2 expression is associated with altered immune cell
infiltration (such as increased CD68+ macrophage levels)
and may contribute to an immunosuppressive context, potentially
affecting responses to immunotherapy (25,26).
These biological roles provide a strong rationale for targeting
CLDN18.2, as its inhibition may not only deliver cytotoxic agents
directly to tumor cells but also counteract its functional
oncogenic drivers.
Based on existing data, the relationship between
CLDN18.2 expression and OS in patients with GC or gastroesophageal
junction cancer is complex and remains controversial. It has been
indicated that a CLDN18.2-negative status may be associated with a
longer OS (27,28), whereas other studies have reported
that CLDN18.2 positivity is linked to improved OS (26,29).
However, several studies have found no notable association between
CLDN18.2 expression and OS or histopathological subtypes (30–32). A
2021 meta-analysis of six studies concluded that there was no
significant difference in OS between CLDN18.2-positive and
CLDN18.2-negative groups (33).
Therefore, CLDN18.2 is generally not recommended as an independent
prognostic biomarker for OS in patients with GC (20). Instead, CLDN18.2 has a more
consistent, evidence-based role as a predictive biomarker,
indicating the potential benefits of targeted therapy.
In recent years, studies on targeted therapies
against CLDN18.2, including monoclonal antibodies (mAbs),
bispecific antibodies, antibody-drug conjugates (ADCs) and
radionuclide-drug conjugates (RDCs) (34–36),
have advanced rapidly. Among these, zolbetuximab, a CLDN18.2 mAb,
has demonstrated limited efficacy in clinical trials (37–39).
However, ADCs and RDCs developed based on CLDN18.2 mAb, such as
CLDN18.2–307-ADC (40) and
[177Lu]Lu-TST001, have shown marked antitumor effects
and controllable safety profiles (41).
Both ADCs and RDCs achieve precise treatment through
specifically targeting tumor cells (42,43).
ADCs deliver cytotoxic drugs directly to tumor cells using mAbs to
recognize tumor cell-surface antigens with high specificity,
thereby minimizing toxicity to normal tissues (44). By contrast, RDCs utilize
tumor-targeting molecules labeled with radioactive isotopes to
provide precise radiation therapy, and demonstrate strong cytotoxic
effects against local refractory lesions or metastatic sites
(45). However, both classes of
drugs face inherent challenges; ADCs may experience reduced
efficacy owing to the development of resistance, whereas RDCs may
cause radiation-related side effects (46).
Existing data have indicated that ADCs and RDCs
demonstrate superior antitumor efficacy compared with CLDN18.2
mAbs, with controllable safety profiles (47). However, studies comparing the
antitumor efficacy and safety of ADCs and RDCs, as well as those
exploring their combination are lacking. To the best of our
knowledge, the current study is the first to systematically compare
the antitumor efficacy and safety of ADCs, RDCs and their
sequential combination regimens. The findings of the present study
may provide preclinical evidence to guide future clinical
applications of ADCs and RDCs. Specifically, identifying the
treatment modality or combination sequence that exhibits superior
efficacy and safety will be invaluable for designing clinical
trials and selecting therapeutic strategies for patients with
CLDN18.2-positive tumors. This comparative strategy may also serve
as a paradigm for evaluating novel targeted conjugates beyond
CLDN18.2. Therefore, the present study aimed to utilize ADC
(SYSA1801) and RDC ([177Lu]Lu-DOTA-SYSA1801mAb) agents
derived from the same CLDN18.2 mAb (SYSA1801mAb) and to compare
their efficacy and safety in the treatment of CLDN18.2-positive GC.
In addition, the potential of their combined treatment strategies
was evaluated.
Materials and methods
Cell culture
The human GC cell line NUGC-4-CLDN18.2 was purchased
from Chengdu Besidi Biotechnology Co., Ltd. This cell line was
generated by the vendor through lentiviral transduction to stably
express CLDN18.2, and its expression was confirmed by them. in
addition, the cell line was authenticated by short tandem repeat
(STR) profiling (Genetic Testing Biotechnology Co., Ltd.). The STR
profile matched the reference profile for NUGC-4 (DSMZ database,
http://www.dsmz.de) at all eight core loci and
amelogenin. The NUGC-4-CLDN18.2 cells were cultured in Roswell Park
Memorial Institute 1640 medium (Gibco; Thermo Fisher Scientific,
Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.), 1% penicillin-streptomycin and 0.5%
puromycin. Cultures were maintained at 37°C in a 5% CO2
incubator.
Animal models
Female BALB/c-nu mice (n=48; age, 6–8 weeks; weight,
18–22 g) were purchased from SPF Biotechnology Co., Ltd. and housed
under specific pathogen-free (SPF) conditions. The housing
environment was maintained at a temperature of 22±2°C, 50±10%
humidity, under a 12/12-h light/dark cycle. Mice had ad
libitum access to a standard SPF rodent diet and water. For the
subcutaneous xenograft model, 1×107 NUGC-4-CLDN18.2
cells in 100 µl PBS were injected into the right dorsal flank of
each mouse (48,49). Tumor-bearing mice were then
allocated to the following studies: 30 mice for the therapeutic
efficacy experiment (6 groups; n=5), 15 mice for biodistribution
studies (n=3 mice per time point across five time points) and 3
mice for positron emission tomography-computed tomography (PET/CT)
imaging. The experiment was initiated when tumor volumes reached
~300 mm3. Humane endpoint criteria were strictly
followed, and mice were euthanized immediately if the tumor volume
exceeded 2,000 mm3 or if there was a 20% decrease in
body weight relative to the baseline (day 0). All animal
experiments were approved by the Animal Ethics Committee of
Mianyang Central Hospital (ethical approval no. S20240210;
Mianyang, China).
Source of SYSA1801mAb
SYSA1801mAb is a fully human mAb targeting CLDN18.2,
which was developed by and sourced from CSPC Pharmaceutical
Holdings Group Ltd. Detailed information regarding antibody
production, plasmid structure and amino acid sequences remains
confidential. SYSA1801mAb was used to generate both diagnostic and
therapeutic radioconjugates. For PET imaging, the antibody was
conjugated to DFO and labeled with 89Zr to form
[89Zr]Zr-DFO-SYSA1801mAb. For biodistribution studies
and therapeutic purposes, the antibody was conjugated to DOTA and
labeled with 177Lu to form the therapeutic
radioconjugate [177Lu]Lu-DOTA-SYSA1801mAb (referred to
as RDC).
Flow cytometry of cell lines
First, 5×104 cells/well were pre-seeded
in a 96-well U-bottom cell culture plate, and were centrifuged at
200 × g for 5 min at 4°C. The pellets were then resuspended and
washed twice with ice cold fluorescence-activated cell sorting
(FACS) buffer (95% PBS and 5% FBS). Subsequently, the cells were
incubated in 50 µl anti-human CLDN18.2 mAb (SYSA1801mAb) for 60 min
at 4°C. The concentration of the antibody added to the first well
was 15 µg/ml, followed by twelve serial two-fold dilutions. Then,
the cells were incubated in the dark for 30 min at 4°C with a
R-phycoerythrin-conjugated goat anti-human IgG secondary antibody
(1:1,000; cat. no. 12499882; eBioscience; Thermo Fisher Scientific,
Inc.). After incubation, the cells were washed twice with FACS
buffer, as aforementioned. The cells were resuspended in 200 µl
FACS buffer for analysis in a FACSCalibur flow cytometer (Beckman
Coulter, Inc.). Data for 3,000 cells were collected and analyzed
using the BD FlowJo software (version v2.1.0; BD Biosciences).
Synthesis of SYSA1801mAb-DFO
SYSA1801mAb stock solution was added to an
ultrafiltration tube [molecular weight cut-off (MWCO), 30 kDa;
MilliporeSigma] and centrifuged at 14,000 × g for 10 min at 4°C to
remove the solvent from the stock solution. A suitable volume of
buffer A solution (Na2CO3-NaHCO3,
pH 9.5, 0.15 M) was added, and the mixture was immediately
centrifuged (14,000 × g for 10 min at 4°C). This procedure was
repeated three times. The purified antibody was then transferred to
a 1.5-ml centrifuge tube, 10 times the molar mass ratio of
p-NCS-Bz-DFO [20 nmol/µl, dissolved in dimethyl sulfoxide (DMSO)]
was added, gently blown on and mixed well. The mixture was then
incubated at a constant temperature of 37°C on a shaker for 1 h at
70 rpm. Subsequently, the antibody was transferred to an
ultrafiltration tube. Following the addition of buffer C solution
(NaOAc-Ac, pH 5.5, 0.5 M), it was centrifuged at 14,000 × g for 10
min at 4°C, a process that was repeated three times to remove the
solvent.
Synthesis of SYSA1801mAb-DOTA
SYSA1801mAb was added to an ultrafiltration tube
(MWCO, 30 kDa; Chelex 100 sodium form), and the solvent in the
stock solution was removed by centrifugation at 14,000 × g for 10
min at 4°C. A suitable volume of buffer A solution
(Na2CO3-NaHCO3, pH 9.5, 0.15 M)
was added, and the mixture was immediately centrifuged (14,000 × g
for 10 min at 4°C). This procedure was repeated three times. The
purified antibody was then transferred to a 1.5-ml centrifuge tube
and 10 times the molar mass ratio of p-NCS-Bz-DOTA (20 nmol/µl,
dissolved in DMSO) was added. The mixture was incubated at a
constant temperature of 37°C on a shaker for 1 h at 70 rpm.
Subsequently, the antibody was transferred to an ultrafiltration
tube. Following the addition of buffer C solution (NaOAc-Ac, pH
5.5, 0.5 M), it was centrifuged at 14,000 × g for 10 min at 4°C, a
procedure that was repeated three times to remove the solvent.
Synthesis of
[89Zr]Zr-DFO-SYSA1801mAb
High-purity yttrium foil (purity >99.99%,
specification: 11×16×0.65 mm; Suzhou Changyou Gas Co., Ltd.) was
procured as the target material and then irradiated with a
cyclotron to induce nuclear reactions. After irradiation, the
target material was subjected to acid dissolution and purification
to obtain 89Zr with acceptable radioactivity and
chemical purity in the form of [89Zr]Zr-oxalate.
[89Zr]Zr-oxalate was added to the centrifuge tube, and
the pH was adjusted to ~7.0 with solution B (0.5 M
Na2CO3) and left at room temperature for 3
min. SYSA1801mAb-DFO was then added to the solution at a 1:2
labeling ratio, and 2-[4-(2-hydroxyethyl) piperazin-1-yl]
ethanesulfonic acid solution was added to the centrifuge tube
according to solution B and total volume of 89Zr. The
mixture was reacted in a metal bath at 37°C for 90 min, shaking the
solution by hand every 10 min. At the end of the reaction, the
labeling rate of the samples was determined using thin-layer
chromatography (TLC) (50).
Synthesis of
[177Lu]Lu-DOTA-SYSA1801mAb
To synthesize [177Lu]Lu-DOTA-SYSA1801mAb,
SYSA1801mAb-DOTA and 177LuCl3 (sourced from
the Institute of Nuclear Physics and Chemistry, China Academy of
Engineering Physics, Mianyang, China) were combined at a 1:2 ratio
and incubated the mixture for 1 h at 42°C in a metal bath. After
the reaction, the labeling rate of the samples was determined using
TLC. Microfiber cellophane (cat. no. SG10001; iTLC-SG-Glass
microfiber chromatography paper impregnated with silica gel;
Agilent Technologies, Inc.) was cut into long strips of paper (10
cm long and 1.5 cm wide), and a marked line was drawn 1.5 cm from
the bottom as a scale for spiking. Subsequently, 2 µl of the
radioactive sample was pipetted to the marked line of the
microfiber glassine strip, which was placed into a sodium citrate
unfolding system after spotting (sodium citrate-citric acid, 0.5 M,
pH 5.5). The reaction mixture was analyzed by high-performance
liquid chromatography (HPLC) using an Agilent 1260 Infinity II
system (Agilent Technologies, Inc.) to determine the radiochemical
purity. The separation was performed on an Agilent XDB-C18
reversed-phase analytical column (4.6×250 mm, 5 µm; Agilent
Technologies, Inc.) maintained at 25°C. A sample volume of 30 µl
was injected. The mobile phase consisted of water with 0.1%
trifluoroacetic acid (TFA) and acetonitrile with 0.1% TFA. The
purification method was as follows: The column was initially
conditioned and washed with 0.1% TFA in water, then eluted with a
linear gradient of 13 to 33% acetonitrile (with 0.1% TFA) in water
over 20 min at a flow rate of 1 ml/min. Radio-HPLC detection was
performed using a γ-radiation detector for 177Lu, and
ultraviolet-HPLC detection was carried out at 254 nm
absorption.
In vitro stability
To determine in vitro stability, 10 µl
[177Lu]Lu-DOTA-SYSA1801mAb was added separately to 15 µl
saline, and to 15 µl 1640 medium containing 10% FBS. The mixture
was left at room temperature, and the labeling rate was determined
by TLC at 4, 24, 48, 96 and 168 h for the two groups.
Binding assays
[177Lu]Lu-DOTA-SYSA1801mAb was prepared
as aforementioned and the radiochemical purity of
[177Lu]Lu-DOTA-SYSA1801mAb was >99%. For the
subsequent experiment, NUGC-4-CLDN18.2 cells (1×105
cells/well) were placed into a 100-µl centrifuge tube. The total
binding of [177Lu]Lu-DOTA-SYSA1801mAb was determined by
adding it to cell suspensions at concentrations of 0.025–10.000 nM.
Non-specific binding was determined by adding 50X half maximal
effective concentration (EC50) cold SYSA1801mAb to
NUGC-4-CLDN18.2 cell mixtures at one concentration. The cells were
incubated with 5 µCi [177Lu]Lu-DOTA-SYSA1801mAb for 1 h
at room temperature and washed three times with FACS buffer (95%
PBS and 5% FBS). Bound and unbound radioactive fractions were
collected and measured using a gamma counter (GC-1500; ANHUI USTC
Zonkia Scientific Instruments Co., Ltd.). Saturation binding
capacity (Bmax) and EC50 values were
calculated using PRISM v9.0 (Dotmatics).
PET/CT imaging
Tumor-bearing mice were sedated with isoflurane
(2.0–2.5% in 2 l/min air) for intravenous injection of 4 MBq (104
µg) [89Zr]Zr-DFO-SYSA1801mAb for PET/CT imaging. During
the uptake periods (4, 24, 48, 96, 144 and 192 h post-injection),
the mice were housed individually in a temperature-controlled room
with free access to food and water, minimizing stress-related
metabolic changes. Before PET/CT, the mice were re-anesthetized,
placed on a heated imaging bed (37±0.5°C), and monitored to
maintain stable body temperature and respiration (respiratory rate:
60–80 breaths/min). Imaging was performed using Super Nova
micro-PET/CT [PINGSENG Healthcare (Kunshan) Inc]. Spherical volumes
of interest were delineated to encompass the tumor, major organs
(the liver, spleen, kidney and heart), and background region (the
thigh skeletal muscles). Decay-corrected injected activity,
measured via pre- and post-injection weighing, and mouse body
weight, recorded before the imaging, were used to calculate the
percentage of injected dose per gram of tissue (%ID/g). This
methodology was used to evaluate the target specificity of
[89Zr]Zr-DFO-SYSA1801mAb.
Biodistribution
Each tumor-bearing mouse was injected with 0.8 MBq
(10 µg) [177Lu]Lu-DOTA-SYSA1801mAb via the tail vein. As
aforementioned, the mice were euthanized at selected time points
(4, 24, 48, 96 and 144 h post-injection), with three mice per time
point. Subsequently, major tissues and organs, including the heart,
liver, spleen, lungs, kidneys, skeletal muscle (thigh muscle),
femur (bone), whole blood, stomach, pancreas and intestines, were
collected. All of the samples were blotted dry with a filter paper
and weighed using an electronic balance.
The radioactivity of each sample was measured using
an automated gamma counter (GC-1500; ANHUI USTC Zonkia Scientific
Instruments Co., Ltd.) with a counting time of 10 sec. Background
radioactivity was subtracted to correct the raw counts. The total
injected radioactivity was determined by measuring the
radioactivity of the injection syringe before and after
administration (to account for residual radioactivity in the
syringe). Tissue uptake was expressed as the %ID/g, calculated
using the following formula: %ID/g=(corrected tissue radioactivity
count/total injected radioactivity count) × (1/tissue wet weight)
×100. Mouse biodistribution data (%ID/g) at multiple time points
were converted to human organ %ID using the %kg/g scaling approach.
For each organ, the scaled human %ID(t) was fitted with a
bi-exponential model; the area under the fitted curve was
integrated to infinity to derive the cumulated activity, which was
then normalized by the administered activity (A0) to
yield the time-integrated activity coefficient: τ=(1/A0)
∫0^∞ A_org(t) dt, reported in MBq·h/MBq.
ADC drug details
SYSA1801 (also known as EO3021; sourced from CSPC
Pharmaceutical Holdings Group Ltd.) is an ADC drug that targets
CLDN18.2. It consists of an mAb (SYSA1801mAb) that targets CLDN18.2
and monomethyl auristatin E (MMAE), conjugated via a cleavable
linker with a drug-to-antibody ratio (DAR) of 2. Detailed synthesis
information is confidential. SYSA1801 is currently undergoing phase
I clinical trials for the treatment of advanced solid tumors,
demonstrating promising antitumor efficacy and manageable safety
(NCT05009966).
Therapeutic dosing rationale and
schedule
Dose selection
In BALB/c nude tumor-bearing mouse models, prior
studies established 11.1 MBq as an appropriate dose for
177Lu-labeled full-length mAb for therapeutic use
(51,52). At this dose, the RDC demonstrated
favorable tolerability, manageable toxicity and effective tumor
growth suppression. Therefore, 11.1 MBq was selected as the single
administration dose of RDC in the present study.
Antibody mass equivalence
To isolate payload effects, an identical mAb
(SYSA1801mAb; 50 nmol/kg) was administered to RDC, ADC and mAb
groups. This enabled a direct comparison of 177Lu with
cytotoxic drug efficacy under equivalent target engagement. For RDC
preparation, a 1:2 antibody-to-177Lu mass ratio was
utilized, where 11.1 MBq RDC contained 150 µg antibody. To maintain
antibody dose equivalence, both the ADC and mAb groups contained
150 µg per administration. The dosing for each group was as
follows: [177Lu]Lu-DOTA-SYSA1801mAb (11.1 MBq, 50
nmol/kg SYSA1801mAb), SYSA1801 (50 nmol/kg) and SYSA1801mAb (50
nmol/kg).
Grouping and intervention of
tumor-bearing mice
Tumor-bearing mice were randomized into six groups
(n=5/group), with interventions administered on days 0 and 14 as
follows: The RDC group, 11.1 MBq (50 nmol/kg)
[177Lu]Lu-DOTA-SYSA1801mAb on days 0 and 14; the ADC
group, 50 nmol/kg SYSA1801 on days 0 and 14; the ADC→RDC group, 50
nmol/kg SYSA1801 on day 0 and 11.1 MBq (50 nmol/kg)
[177Lu]Lu-DOTA-SYSA1801mAb on day 14; the RDC→ADC group,
11.1 MBq (50 nmol/kg) [177Lu]Lu-DOTA-SYSA1801mAb on day
0 and 50 nmol/kg SYSA1801 on day 14; the mAb group, 50 nmol/kg
SYSA1801mAb on days 0 and 14; the normal saline (NS) group, NS on
days 0 and 14. Tumor-bearing mice were administered injections
though the tail vein, with a dose volume of 200 µl/mouse. A
schematic diagram of grouping and intervention for the
tumor-bearing mice is shown in Fig.
1A.
Monitoring and subsequent testing
Tumor size and body weight of the mice were
monitored starting from day 0 (first injection day). Tumor size was
measured using a digital caliper, with the volume calculated as
(length × width2)/2 (length, longest diameter of the
tumor; width, shortest diameter perpendicular to the length), and
body weight was measured using an electronic balance. Both
parameters were assessed on Tuesday and Friday after the first
injection. The tumor growth inhibition rate (TGI%) was calculated
as follows: TGI%=[1-(ΔT/ΔC)] ×100, where ΔT is the change in mean
tumor volume of the treatment group from baseline, and ΔC is the
change in mean tumor volume of the control group from baseline.
The study endpoints were set as follows: i) Tumor
volume reaching 2,000 mm3 (triggering euthanasia); or
ii) a 20% decrease in body weight relative to the baseline weight
on day 0. Mice that did not meet these criteria were considered
survivors for the purpose of the study. At these endpoints, the
mice were anesthetized via inhalation of 5% isoflurane, and 500–800
µl blood was collected from the orbital venous plexus. Mice that
reached predefined humane endpoints underwent blood collection from
the orbital venous plexus and subsequent immediate euthanasia.
Animals were first anesthetized via inhalation of 5%
isoflurane in an induction chamber. Upon loss of consciousness, the
mice were transferred to a euthanasia chamber and exposed to 100%
CO2 at a flow rate displacing 40% of the chamber
volume/min until respiratory arrest occurred. Death was
subsequently confirmed by cervical dislocation. A portion of the
blood sample was immediately used for routine hematological tests
using an automated hematology analyzer (Mira BF; Shanghai RuiYu
Biotech Co., Ltd.), and the remaining blood samples were
centrifuged at 3,000 × g for 10 min at 4°C to separate the serum,
which was used for subsequent hepatorenal function tests using an
automated biochemistry analyzer (BS-360S; Shenzhen Mindray
Bio-Medical Electronics Co., Ltd.). Subsequently, major organs (the
heart, liver, spleen, lung and kidney) were harvested, fixed in 4%
paraformaldehyde for 24 h at room temperature, embedded in
paraffin, and sectioned into 5-µm slices. The slices were stained
with hematoxylin for 8 min and eosin for 3 min at room temperature,
and observed under a light microscope (CX-31; Olympus Corporation)
to evaluate histopathological changes (such as inflammatory cell
infiltration, tissue necrosis and cellular degeneration), and to
assess drug-induced hematological and organ toxicity.
Statistical analysis
Statistical analyses were performed using IBM SPSS
Statistics for Windows (version 22.0; IBM Corp). For comparisons
involving >2 groups, one-way analysis of variance was conducted,
followed by Tukey's post hoc test for multiple comparisons where
appropriate. P<0.05 was considered to indicate a statistically
significant difference. Survival analysis was performed using
Kaplan-Meier curves and differences between groups were assessed
using the log-rank (Mantel-Cox) test. For the pre-specified
pairwise comparisons among the four key treatment groups (RDC, ADC,
ADC→RDC, RDC→ADC), a Bonferroni correction for multiple testing was
applied. A total of six comparisons were conducted, resulting in a
corrected significance threshold of α=0.0083. Statistical
significance for these pairwise comparisons was declared only when
P<0.0083.
Results
Cell transfection and animal
models
Flow cytometry confirmed a stable high expression of
CLDN18.2 in the NUGC-4-CLDN18.2 cells (Fig. 1B). Finally, a subcutaneous positive
GC xenograft model was established using NUGC-4-CLDN18.2 cells for
subsequent animal experiments. When the NUGC-4-CLDN18.2 ×enografts
had grown to a volume of 732.75±197.96 mm3, the
therapeutic experiment was initiated.
Binding and stability
The integrity and radiochemical purity of
[177Lu]Lu-DOTA-SYSA1801mAb were determined to be >99%
by HPLC using an Agilent 1260 Infinity II HPLC system (Fig. 1C). Subsequent incubation in NS and
1640 medium (containing 10% FBS) for up to 7 days showed no
degradation of [177Lu]Lu-DOTA-SYSA1801mAb, as confirmed
by radiolabeled TLC (Fig. 1E).
Radioligand-binding assays were conducted using NUGC-4-CLDN18.2
cells. The Bmax of [177Lu]Lu-DOTA-SYSA1801mAb
to NUGC-4-CLDN18.2 cells (1×05 cells) was 1,149±48.16
nmol, and the equilibrium dissociation constant was 24.36±3.5
nmol/l (Fig. 1D).
PET/CT imaging of
[89Zr]Zr-DFO-SYSA1801mAb
PET/CT imaging revealed that 4 h post-injection,
[89Zr]Zr-DFO-SYSA1801mAb accumulated in the tumor, with
a clear tumor outline visible at 24 h post-injection (Fig. 2A). Over time, radioactive uptake in
the tumor gradually increased. The maximum uptake of
[89Zr]Zr-DFO-SYSA1801mAb at the tumor site was observed
at 48 h, with an uptake of ~4.94±1.06%ID/g (n=3) (Fig. 2B). Region of interest was further
defined and radioactive uptake in the tumor, heart (blood), liver,
spleen, muscle and kidneys was assessed. Radioactive uptake in
non-target organs, such as the heart (blood), liver and kidneys,
gradually decreased over time.
![(A) Positron emission
tomography-computed tomography images showing tumor-specific
accumulation of [89Zr]Zr-DFO-SYSA1801mAb in
tumor-bearing mice over time, in contrast to that in other
non-target tissues. (B) Quantitative region of interest analysis of
the tumor, heart, liver, spleen, kidney, and muscle in
tumor-bearing mice at 4, 24, 48, 96, 144 and 192 h after injection
of [89Zr]Zr-DFO-SYSA1801mAb. (C) Biodistribution results
at 4, 24, 48, 96 and 144 h after injection of
[177Lu]Lu-DOTA-SYSA1801mAb. (D) Tumor-to-blood, (E)
tumor-to-liver and (F) tumor-to-kidney ratios at 4, 24, 48, 96 and
144 h after injection of [177Lu]Lu-DOTA-SYSA1801mAb.
Data are presented as the mean ± SD. Statistical differences across
the different time points for each ratio were determined by one-way
analysis of variance followed by Tukey's post hoc test for multiple
comparisons. *P<0.05, **P<0.01, ***P<0.001. %DI/g,
percentage of injected dose per gram of tissue; mAb, monoclonal
antibody.](/article_images/or/55/1/or-55-01-09009-g01.jpg) | Figure 2.(A) Positron emission
tomography-computed tomography images showing tumor-specific
accumulation of [89Zr]Zr-DFO-SYSA1801mAb in
tumor-bearing mice over time, in contrast to that in other
non-target tissues. (B) Quantitative region of interest analysis of
the tumor, heart, liver, spleen, kidney, and muscle in
tumor-bearing mice at 4, 24, 48, 96, 144 and 192 h after injection
of [89Zr]Zr-DFO-SYSA1801mAb. (C) Biodistribution results
at 4, 24, 48, 96 and 144 h after injection of
[177Lu]Lu-DOTA-SYSA1801mAb. (D) Tumor-to-blood, (E)
tumor-to-liver and (F) tumor-to-kidney ratios at 4, 24, 48, 96 and
144 h after injection of [177Lu]Lu-DOTA-SYSA1801mAb.
Data are presented as the mean ± SD. Statistical differences across
the different time points for each ratio were determined by one-way
analysis of variance followed by Tukey's post hoc test for multiple
comparisons. *P<0.05, **P<0.01, ***P<0.001. %DI/g,
percentage of injected dose per gram of tissue; mAb, monoclonal
antibody. |
Biodistribution of
[177Lu]Lu-DOTA-SYSA1801mAb
Biodistribution results indicated that
[177Lu]Lu-DOTA-SYSA1801mAb effectively targeted tumors
in the NUGC-4-CLDN18.2 model. Over time, tumor uptake increased,
reaching a peak at 48 h (16.44±3.13%ID/g) (Fig. 2C). Liver uptake peaked at 24 h
(12.06±2.63%ID/g) and kidney uptake peaked at 4 h (4.96±1.66%ID/g).
As time progressed, uptake in the liver and kidneys gradually
decreased. Following blood circulation,
[177Lu]Lu-DOTA-SYSA1801mAb was rapidly cleared from the
bloodstream. The concentration of radioactivity in the blood
decreased by over 80% during the period from 4 and 48 h
post-injection. Between 4 and 96 h post-injection, the
tumor-to-blood ratio gradually increased, reaching a maximum ratio
of 31.62±17.13 (Fig. 2D).
Tumor-to-liver and tumor-to-kidney ratios exhibited fluctuating
trends, with the highest values observed at 48 h (Fig. 2E and F). Dosimetry results are
presented in Table I.
 | Table I.Estimates of time-integrated activity
coefficients for human organs from mouse biodistribution data. |
Table I.
Estimates of time-integrated activity
coefficients for human organs from mouse biodistribution data.
| Source organ | Kinetics value,
MBq-h/MBq |
|---|
| Adrenal glands | 0 |
| Brain | 0 |
| Esophagus | 0 |
| Eyes | 0 |
| Gallbladder
contents | 0 |
| Left colon | 0 |
| Small
intestine | 0.777 |
| Stomach
contents | 0.268 |
| Right colon | 0 |
| Rectum | 0 |
| Heart contents | 1.07×10¹ |
| Heart wall | 0.566 |
| Kidneys | 0.246 |
| Liver | 1.02×10¹ |
| Lungs | 1.32 |
| Pancreas | 0.059 |
| Prostate | 0 |
| Salivary
glands | 0 |
| Red bone
marrow | 0 |
| Cortical bone | 1.70×10¹ |
| Trabecular
bone | 0 |
| Spleen | 0.31 |
| Testes | 0 |
| Thymus | 0 |
| Thyroid | 0 |
| Urinary bladder
contents | 0 |
| Total body | 0 |
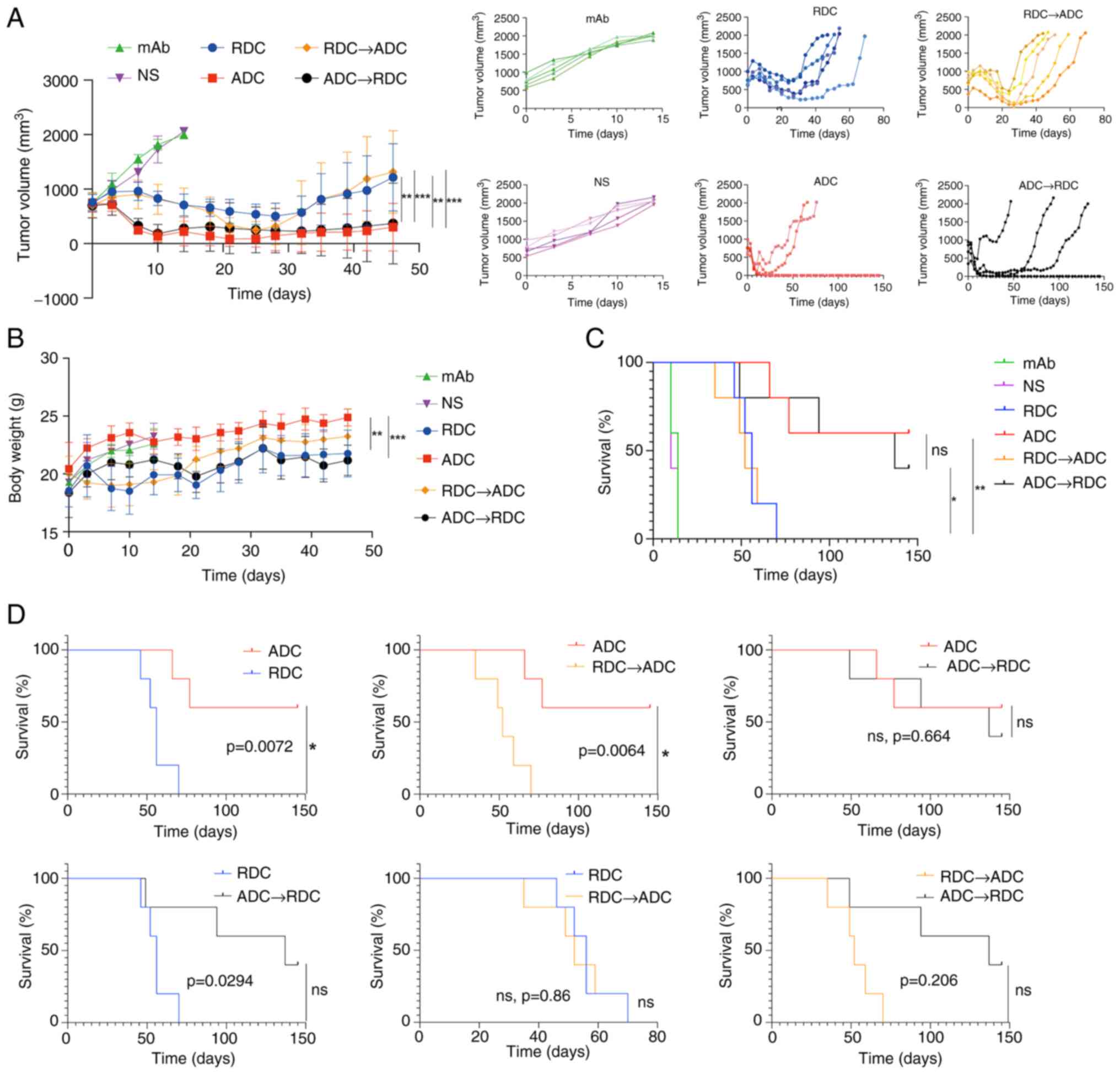
Effect of treatment
In the NUGC-4-CLDN18.2 model, the NS and mAb groups
reached humane endpoints by day 14 post-treatment. The average
tumor volume in the mAb group was 1,906.58±71.11 mm3
(TGI%=4.57%), while in the NS group, it was 1,997.88±71.11
mm3, with no significant difference between the two
groups (Figs. 3A and S1). A total of 14 days after the first
administration, in the RDC monotherapy group, the average tumor
volume was 686.86±153.12 mm3 (TGI%=105.32%), in the ADC
group, it was 222.49±236.45 mm3 (TGI%=139.80%), in the
RDC→ADC group, it was 713.65±271.28 mm3 (TGI%=100.60%),
and in the ADC→RDC group, it was 278.79±381.72 mm3
(TGI%=137.93%). Significant differences in tumor volume were
observed between some treatment groups, and the detailed pairwise
comparisons are presented in Table
SI. On day 46 post-treatment, the average tumor volume in the
RDC group was 871.63±503.97 mm3, in the ADC group, it
was 303.05±439.34 mm3, in the RDC→ADC group, it was
941.74±803.32 mm3, and in the ADC→RDC group, it was
248.31±474.01 mm3. Significant differences in tumor
volume were observed between the following pairs: RDC and ADC, RDC
and ADC→RDC, ADC and RDC→ADC, and RDC→ADC and ADC→RDC. On day 14
post-treatment, the mice in the NS and mAb groups showed a gradual
increase in body weight, whereas the mice in the other treatment
groups experienced an initial weight decrease followed by a slow
increase (Fig. 3B). Significant
differences in weight were observed between some of the groups.
Specifically, the ADC→RDC group showed a statistically significant
difference compared with the ADC group, and the RDC group also
differed significantly from the ADC group.
On day 46 post-treatment, the body weight of the
mice in the RDC, ADC, ADC→RDC and RDC→ADC groups showed fluctuating
increments, with no significant difference in body weight among the
treatment groups (Table SII). On
day 145 post-treatment, complete remission (CR) rate in the ADC
group was 60%, with the OS rate also at 60%. In the ADC→RDC group,
the CR rate was 40% and the OS rate was 40%, whereas the OS rate in
the other groups was 0%. Statistically significant differences in
OS rates were observed between the ADC and RDC groups, as well as
between the ADC and RDC→ADC groups (Fig. 3C and D).
Toxicity of treatment
To comprehensively evaluate the potential adverse
effects of these treatments, hematological, biochemical and
histopathological analyses were conducted. Hematological analysis
revealed elevated white blood cell counts in all treatment groups
relative to the NS control group (Fig.
4A), with detailed results of pairwise comparisons between the
groups provided in Table SIII.
This elevation might suggest an inflammatory response or stress
induced by treatment, which warrants further exploration of the
impact on the immune system. Red blood cell counts showed minimal
differences across groups, with a significant difference observed
only between the ADC and mAb groups, and between the mAb and
ADC→RDC sequential therapy groups. No significant differences were
found in platelet counts among the groups. Levels of hepatocellular
injury markers, alanine and aspartate aminotransferases exhibited
only minor fluctuations across the treatment groups, with no
significant differences observed (Fig.
4C). However, a significant decrease in albumin levels was
noted in all the treatment groups, except the mAb group, compared
with those in the NS group (P<0.05), suggesting a potential
impairment of hepatic synthetic function, which is a known side
effect of certain chemotherapeutic and targeted agents (51). More pronounced toxicological
profiles were observed in the kidneys. Compared with those in the
NS group, blood urea nitrogen (BUN), creatinine (Cr) and uric acid
levels were elevated to varying degrees in the treatment groups,
with relatively greater changes in the combination treatment and
RDC groups (Fig. 4C; Table SIII). This consistent pattern
suggests a potential impairment of the glomerular filtration rate
and renal function.
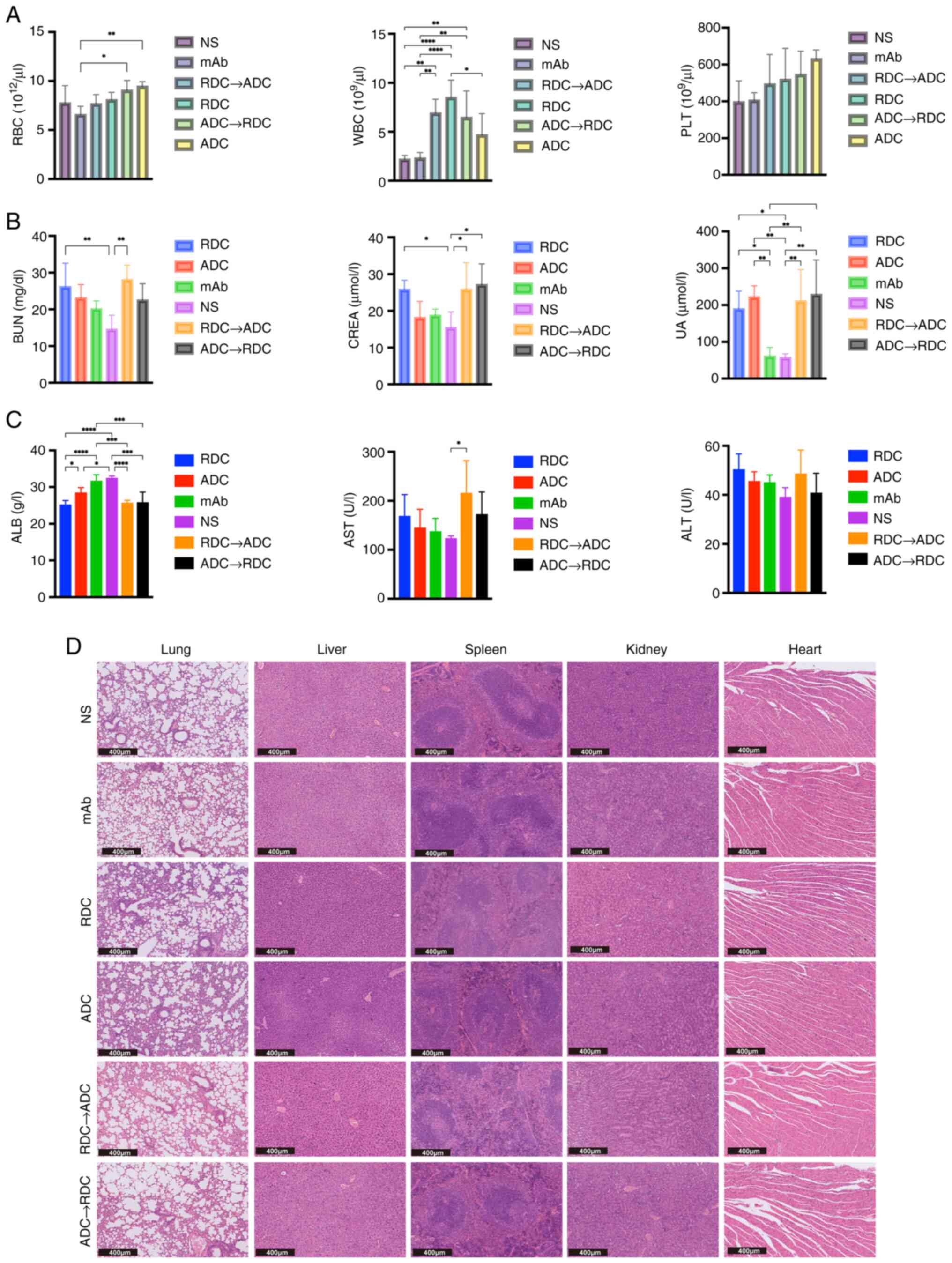 | Figure 4.(A) WBC, RBC and PLT counts of each
treatment group at the end of treatment (n=5 mice/group). (B) BUN,
CREA and UA levels in each treatment group at the end of treatment.
(C) ALB, AST and ALT levels in each treatment group at the end of
treatment. (D) Representative hematoxylin and eosin staining of
tissues collected from euthanized mice from each treatment group.
Statistical differences across the groups were determined by
one-way analysis of variance followed by Tukey's post hoc test for
multiple comparisons. *P<0.05, **P<0.01, ***P<0.001,
****P<0.0001. ADC, antibody-drug conjugate; ALB, albumin; ALT
alanine amino transferase; AST, aspartate aminotransferase; BUN,
blood urea nitrogen; CREA, creatinine; mAb, monoclonal antibody;
NS, normal saline; PLT, platelet; RBC, red blood cell; RDC,
radionuclide-drug conjugate; UA, uric acid; WBC, white blood
cell. |
Histopathological examination provided evidence for
the biochemical findings (Fig. 4D).
Although the architecture of the heart, lungs and liver remained
intact across all the groups, notable changes were observed in the
kidneys and spleen. Specifically, the mice treated with RDC and
combination therapy exhibited partial damage and deformation of
glomeruli and renal tubules, consistent with renal function
deterioration, as indicated by elevated BUN and Cr levels.
Disruption of the splenic follicular structure was also observed in
these groups. These changes were markedly milder in the ADC and mAb
monotherapy groups compared with those in the RDC and combination
therapy groups. Cardiac, pulmonary and hepatic tissues remained
largely intact across all the groups, with no signs of notable
drug-induced injury.
Overall, the toxicity profile indicated that RDC and
the combination therapy were associated with controllable but
notable nephrotoxicity and potential effects on hepatic synthetic
function. The absence of marked histopathological damage in the
heart and lungs suggests favorable cardiotoxicity and pulmonary
toxicity safety profiles.
Discussion
To the best of our knowledge, the present study is
the first preclinical experiment comparing the efficacy and safety
of ADCs, RDCs and their sequential combinations targeting the same
receptor. The current study systematically evaluated the
therapeutic efficacy of CLDN18.2-targeted ADC, RDC and various
sequential combination therapies in a preclinical GC mouse model.
The results showed that the ADC group was significantly associated
with improved key efficacy endpoints, such as OS and CR rates,
compared with those in both the RDC and RDC→ADC groups, alongside a
more favorable safety profile. Although the survival benefit of the
ADC→RDC combination treatment did not significantly differ from
that of ADC monotherapy, the combination therapy group exhibited
higher blood toxicity, and liver and kidney damage. These findings
suggest that ADC monotherapy may have superior efficacy and safety
in the current preclinical model. From a mechanistic perspective,
the therapeutic advantage of ADCs primarily arises from their
ability to precisely deliver cytotoxic drugs via antibody-targeted
delivery (53). Additionally, the
cleavable linker of ADCs can generate a ‘bystander effect’, which
helps eliminate nearby antigen-negative cells (54). In the present study, a 60% CR rate
was maintained for 5 months following ADC monotherapy, which is
closely associated with high stability of the CLDN18.2 antigen
during the treatment period. However, existing clinical studies
have indicated that prolonged use of ADCs may lead to acquired
resistance through mechanisms such as abnormal antigen
internalization or lysosomal escape (55,56).
The absence of such acquired resistance in the current study may be
linked to the relatively low dosing frequency (only two
administrations) and short observation period in the current study.
Future studies should assess the long-term effects of treatment on
drug resistance.
In the combination therapy strategy, the tumor CR
rate in the ADC→RDC group was higher than that in the RDC→ADC
group, with a 40% difference. Although the sample size (n=5/group)
in the current study was limited, resulting in a low statistical
power, and this difference did not reach statistical significance
after correction for multiple comparisons, the magnitude of the
observed trend and effect size still suggests that the sequence of
administration may be a potential factor influencing antitumor
efficacy. This finding provides a valuable preliminary hypothesis
for future large-scale studies. Furthermore, based on previous
literature, it may be hypothesized that administering ADC first
could create a favorable condition for subsequent RDC therapy
through several potential mechanisms: i) Rapidly reducing tumor
burden by downregulating hypoxia-inducible factor 1α expression,
which alleviates the tumor hypoxic microenvironment (57), thereby enhancing the sensitivity of
the tumor to radiotherapy (58);
ii) ADC-induced G2/M phase cell cycle arrest, which
makes tumor cells more sensitive during radiotherapy (59); and iii) conversely, if RDC therapy
is used first, it may induce radiation-induced fibrosis, which
increases the hydraulic pressure of the tumor stroma, hindering the
intratumoral penetration of ADC drugs (60). These potential mechanisms may
explain the efficacy trend observed in the current study.
Previous studies have shown that ADC and RDC
monotherapies demonstrate relatively good efficacy in
CLDN18.2-targeted therapy (61,62).
For example, [177Lu]Lu-TST001 has exhibited marked
efficacy and low short-term toxicity in preclinical GC models
(41), although no long-term
toxicity has been observed. In the current study, a 5-month
long-term observation revealed that although RDC treatment
effectively inhibited tumor growth, it was associated with
relatively high hematological and visceral toxicity. This suggests
that CLDN18.2-RDC therapy requires further optimization to reduce
side effects. Moreover, in a previous study, CLDN18.2–307-ADC
induced sustained tumor regression in cell-derived xenograft and
patient-derived xenograft models with manageable toxicity (40). These findings are consistent with
the results of this study, where a 60% CR rate was observed in the
ADC treatment group, and the tumors remained stable after
regression without recurrence. However, the prolonged use of ADC
may result in resistance issues, including target antigen
downregulation, antigen loss or mutations, leading to a gradual
reduction in therapeutic efficacy (63). Therefore, the potential efficacy of
the ADC→RDC combination therapy in resistant patients is of
particular importance. By initially using ADC to reduce tumor
burden and improve the microenvironment, subsequent RDC treatment
may eliminate residual tumor cells more effectively, thereby
lowering recurrence risk (64).
This treatment strategy is especially suitable for ADC-resistant
patients for whom RDC may serve as an effective adjunct
therapy.
The National Medical Products Administration has
approved CLDN18.2 mAb (zolbetuximab) in combination with
fluoropyrimidine and platinum-based chemotherapy as a first-line
treatment for locally advanced unresectable or metastatic
CLDN18.2-positive, human epidermal growth factor receptor 2
(HER2)-negative gastric or gastroesophageal junction
adenocarcinoma. However, in the present study, the mAb-CLDN18.2
monotherapy group did not show any significant antitumor efficacy.
This outcome may be attributed to the relatively low doses
administered. The conventional clinical dose is 800
mg/m2 (65,66), whereas the dose used in the current
study was only 50 nmol/kg. At such a low dose, the antibody may not
achieve sufficient target engagement or elicit a robust immune
response to produce antitumor effects as monotherapy. Future
studies should consider using higher doses in order to observe more
pronounced therapeutic outcomes.
The present study has several design limitations
that warrant further discussion. First, owing to experimental
constraints, the pharmacokinetic characteristics and maximum
tolerated doses of different treatment regimens were not
systematically compared, which may affect the generalizability of
the conclusions regarding efficacy and safety. To control for core
variables, a uniform dose of 150 µg (50 nmol/kg) mAb was used as a
baseline. This decision was based on the following considerations:
First, the fixed DAR (DAR=2) of ADCs is structurally consistent
with the radionuclide-to-antibody labeling ratio (value=2) of RDCs.
Second, prior studies have shown that 1.1 MBq
177Lu-labeled full antibodies (150 µg) effectively
suppress tumor growth with good tolerability (51,52).
Although there are differences in the mechanisms of action between
ADCs and RDCs (67,68), an equal antibody dosage ensures
comparability in target occupancy, thereby providing a basis for a
fair evaluation of the antitumor activity of different carrier
systems. Although the absence of pharmacokinetic parameters limits
the depth of mechanistic interpretation, the cross-modal drug
comparison paradigm and sequential strategy established in the
present study remain valuable and may provide insights for future
studies.
Second, the present study used only a single
conventional tumor model and did not account for the potential
effect of different GC subtypes or resistance models on treatment
outcomes. Tumor heterogeneity suggests that a single model may not
fully capture the diversity in clinical treatments. Therefore,
future studies should incorporate multi-model validation,
particularly the use of resistance models, to enhance the
generalizability of the findings. Nevertheless, although the
results obtained from a single model may not be broadly applicable,
the favorable outcomes observed with both ADC and ADC→RDC
sequential combination therapies in the current study offer
valuable insights and guidance for subsequent validation in
resistance models and the development of treatment strategies for
advanced clinical patients.
Third, all efficacy and toxicity observations were
derived from the NUGC-4-CLDN18.2 ×enograft model, which differs
fundamentally from human GC in several key aspects: i) Tumor
microenvironment: The murine model lacks the complex human tumor
microenvironment that regulates drug response and toxicity profiles
in patients. ii) Pharmacokinetic and pharmacodynamic disparities:
Interspecies differences in drug metabolism, distribution and
elimination may notably alter treatment outcomes. iii) CLDN18.2
expression heterogeneity: Human GC demonstrates variable CLDN18.2
expression patterns (such as focal vs. diffuse and high vs. low)
and potentially treatment-modifying co-mutations (including in HER2
and TP53), whereas the NUGC-4-CLDN18.2 model represents only a
single CLDN18.2-overexpressing cell line, thereby limiting the
generalizability of the findings across different patient
subgroups.
Fourth, the study included multiple groups, but the
sample size was limited (n=5/group), which resulted in a low
statistical power. After correction for multiple comparisons, some
intergroup differences in the survival analysis did not reach
statistical significance, and thus, these specific findings were
only presented as trends. Future studies will require a larger
sample size to validate these findings.
Finally, the current study did not explore the
potential acquired resistance mechanisms to ADC therapy, which is
directly relevant to the development of subsequent treatment
strategies for patients with resistance in clinical settings. These
limitations underscore the need for cautious interpretation of the
results and emphasize the requirement for future clinical studies
to establish the comparative efficacy and safety of ADC and RDC
strategies in patients with CLDN18.2-positive GC.
In conclusion, the present preclinical findings
suggest that ADC monotherapy may demonstrate superior efficacy and
safety profiles when compared with RDC monotherapy. Sequential
combination therapy starting with ADC appears to be more favorable
than that which starts with RDC. Although ADC→RDC sequential
therapy did not significantly outperform ADC monotherapy in this
model, it may serve as an effective subsequent treatment
strategy.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was financially supported by the Key
Laboratory of Nuclear Technology (grant nos. 2021HYX001 and 2022
HYX015), the Mianyang Science and Technology Bureau (Minyang
Science and Technology Program; grant no. 2023ZYDF073) and the
Mianyang Municipal Finance Bureau (grant no.
miancaijian2022-186).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XD, XY and YY conceived and designed the study. HD,
XH, BL, LR, JW, MT, DW, YZhu, JL and XY performed the experiments.
HD, XH, BL YZha, TD, and XY analyzed the data. HD was a major
contributor in writing the manuscript. XD and XY assume overall
responsibility for the manuscript. XD and HD confirm the
authenticity of all the raw data. XD, HD, YZha and TD were
responsible for supervision and funding acquisition. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethical
Review Board of Mianyang Central Hospital (approval no.
S20240210).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CLDN18.2
|
claudin 18.2
|
|
ADC
|
antibody-drug conjugate
|
|
RDC
|
radionuclide-drug conjugate
|
|
ALB
|
albumin
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
BUN
|
blood urea nitrogen
|
|
CREA
|
creatinine
|
|
UA
|
uric acid
|
|
WBC
|
white blood cells
|
|
RBC
|
red blood cells
|
|
PLT
|
platelets
|
|
OS
|
overall survival
|
|
CR
|
complete remission
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal AL: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sexton RE, Al Hallak MN, Diab M and Azmi
AS: Gastric cancer: A comprehensive review of current and future
treatment strategies. Cancer Metastasis Rev. 39:1179–1203. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smyth EC, Nilsson M, Grabsch HI, van
Grieken NC and Lordick F: Gastric cancer. Lancet. 396:635–648.
2020. View Article : Google Scholar
|
|
4
|
Ling Q, Huang ST, Yu TH, Liu HL, Zhao LY,
Chen XL, Liu K, Chen XZ, Yang K, Hu JK, et al: Optimal timing of
surgery for gastric cancer after neoadjuvant chemotherapy: A
systematic review and meta-analysis. World J Surg Oncol.
21:3772023. View Article : Google Scholar
|
|
5
|
Merchant SJ, Kong W, Mahmud A, Booth CM
and Hanna TP: Palliative radiotherapy for esophageal and gastric
cancer: Population-based patterns of utilization and outcomes in
Ontario, Canada. J Palliat Care. 38:157–166. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Haggstrom L, Chan WY, Nagrial A, Chantrill
LA, Sim HW, Yip D and Chin V: Chemotherapy and radiotherapy for
advanced pancreatic cancer. Cochrane Database Syst Rev.
12:CD0110442024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yan SY and Fan JG: Application of immune
checkpoint inhibitors and microsatellite instability in gastric
cancer. World J Gastroenterol. 30:2734–2739. 2024. View Article : Google Scholar
|
|
8
|
Nie Y, Schalper KA and Chiang A:
Mechanisms of immunotherapy resistance in small cell lung cancer.
Cancer Drug Resist. 7:552024.
|
|
9
|
Xu W, Li B, Xu M, Yang T and Hao X:
Traditional Chinese medicine for precancerous lesions of gastric
cancer: A review. Biomed Pharmacother. 146:1125422022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Y, Huang T, Wang L, Wang Y, Liu Y, Bai
J, Wen X, Li Y, Long K and Zhang H: Traditional Chinese Medicine in
the treatment of chronic atrophic gastritis, precancerous lesions
and gastric cancer. J Ethnopharmacol. 337:1188122025. View Article : Google Scholar
|
|
11
|
Dai Z, Tan C, Wang J, Wang Q, Wang Y, He
Y, Peng Y, Gao M, Zhang Y, Liu L, et al: Traditional Chinese
medicine for gastric cancer: An evidence mapping. Phytother Res.
38:2707–2723. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao S, Xu T, Wang W, Li J, Shan Y, Wang Y
and Tan H: Polysaccharides from Anemarrhena asphodeloides Bge, the
extraction, purification, structure characterization, biological
activities and application of a traditional herbal medicine. Int J
Biol Macromol. 311:1434972025. View Article : Google Scholar
|
|
13
|
Gao S, Wang Y, Shan Y, Wang W, Li J and
Tan H: Rhizoma Coptidis polysaccharides: Extraction, separation,
purification, structural characteristics and bioactivities. Int J
Biol Macromol. 320:1456772025. View Article : Google Scholar
|
|
14
|
Wang G, Huang Y, Zhou L, Yang H, Lin H,
Zhou S, Tan Z and Qian J: Immunotherapy and targeted therapy as
first-line treatment for advanced gastric cancer. Crit Rev Oncol
Hematol. 198:1041972024. View Article : Google Scholar
|
|
15
|
Zhang X, Yang L, Liu S, Cao LL, Wang N, Li
HC and Ji JF: Interpretation on the report of global cancer
statistics 2022. Zhonghua Zhong Liu Za Zhi. 46:710–721. 2024.(In
Chinese).
|
|
16
|
Inamoto R, Takahashi N and Yamada Y:
Claudin18.2 in advanced gastric cancer. Cancers (Basel).
15:57422023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sahin U, Koslowski M, Dhaene K, Usener D,
Brandenburg G, Seitz G, Huber C and Türeci O: Claudin-18 splice
variant 2 is a pan-cancer target suitable for therapeutic antibody
development. Clin Cancer Res. 14:7624–7634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Baek JH, Park DJ, Kim GY, Cheon J, Kang
BW, Cha HJ and Kim JG: Clinical implications of claudin18.2
expression in patients with gastric cancer. Anticancer Res.
39:6973–6979. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bunga OD and Danilova NV: Claudin-18.2 and
gastric cancer: From physiology to carcinogenesis. Arkh Patol.
86:92–99. 2024.(In Russian). View Article : Google Scholar
|
|
20
|
Mathias-Machado MC, de Jesus VHF, Jácome
A, Donadio MD, Aruquipa MPS, Fogacci J, Cunha RG, da Silva LM and
Peixoto RD: Claudin 18.2 as a new biomarker in gastric cancer-what
should we know? Cancers (Basel). 16:6792024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakayama I, Qi C, Chen Y, Nakamura Y, Shen
L and Shitara K: Claudin 18.2 as a novel therapeutic target. Nat
Rev Clin Oncol. 21:354–369. 2024. View Article : Google Scholar
|
|
22
|
Tojjari A, Idrissi YA and Saeed A:
Emerging targets in gastric and pancreatic cancer: Focus on claudin
18.2. Cancer Lett. 611:2173622024. View Article : Google Scholar
|
|
23
|
Seckin Y, Arici S, Harputluoglu M, Yonem
O, Yilmaz A, Ozer H, Karincaoglu M and Demirel U: Expression of
claudin-4 and beta-catenin in gastric premalignant lesions. Acta
Gastroenterol Belg. 72:407–412. 2009.PubMed/NCBI
|
|
24
|
Konno H, Lin T, Wu R, Dai X, Li S, Wang G,
Chen M, Li W, Wang L, Sun BC, et al: ZL-1211 exhibits robust
antitumor activity by enhancing ADCC and activating NK
Cell-mediated inflammation in CLDN18.2-High and -Low expressing
gastric cancer models. Cancer Res Commun. 2:937–950. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kubota Y, Kawazoe A, Mishima S, Nakamura
Y, Kotani D, Kuboki Y, Bando H, Kojima T, Doi T, Yoshino T, et al:
Comprehensive clinical and molecular characterization of claudin
18.2 expression in advanced gastric or gastroesophageal junction
cancer. ESMO Open. 8:1007622023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castillo DR, Guo M, John Kim K II, Zhong
H, Brar G, Wu S, Mannan R, Cecilia Lau S, Xing Y and Peter Wu S:
Association of Claudin18.2 expression, PD-L1, and prognostic
implications in gastric and gastroesophageal junction cancers. J
Clin Oncol. 43:e160942025. View Article : Google Scholar
|
|
27
|
Wang C, Wang Y, Chen J, Wang Y, Pang C,
Liang C, Yuan L and Ma Y: CLDN18.2 expression and its impact on
prognosis and the immune microenvironment in gastric cancer. BMC
Gastroenterol. 23:2832023. View Article : Google Scholar
|
|
28
|
Rha SY, Kwon WS, Lee CK, Jung M, Shin S
and Kim H, Park S, Yoo JH and Kim H: Co-expressing pattern of
multiple biomakers and dynamic change of Claudin18.2 expression
after systemic chemotherapy in advanced gastric cancer. J Clin
Oncol. 43:40372025. View Article : Google Scholar
|
|
29
|
Lordick F, Van Cutsem E, Shitara K, Xu RH,
Ajani JA, Shah MA, Oh M, Ganguli A, Chang L, Rhoten S, et al:
Health-related quality of life in patients with CLDN18.2-positive,
locally advanced unresectable or metastatic gastric or
gastroesophageal junction adenocarcinoma: Results from the
SPOTLIGHT and GLOW clinical trials. ESMO Open. 9:1036632024.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kim HD, Choi E, Shin J, Lee IS, Ko CS, Ryu
MH and Park YS: Clinicopathologic features and prognostic value of
claudin 18.2 overexpression in patients with resectable gastric
cancer. Sci Rep. 13:200472023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pellino A, Brignola S, Riello E, Niero M,
Murgioni S, Guido M, Nappo F, Businello G, Sbaraglia M, Bergamo F,
et al: Association of CLDN18 protein expression with
clinicopathological features and prognosis in advanced gastric and
gastroesophageal junction adenocarcinomas. J Pers Med. 11:10952021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Waters R, Sewastjanow-Silva M, Yamashita
K, Abdelhakeem A, Iwata KK, Moran D, Elsouda D, Guerrero A, Pizzi
M, Vicentini ER, et al: Retrospective study of claudin 18 isoform 2
prevalence and prognostic association in gastric and
gastroesophageal junction adenocarcinoma. JCO Precis Oncol.
8:e23005432024. View Article : Google Scholar
|
|
33
|
Ungureanu BS, Lungulescu CV, Pirici D,
Turcu-Stiolica A, Gheonea DI, Sacerdotianu VM, Liliac IM, Moraru E,
Bende F and Saftoiu A: Clinicopathologic relevance of claudin 18.2
expression in gastric cancer: A Meta-analysis. Front Oncol.
11:6438722021. View Article : Google Scholar
|
|
34
|
Gao J, Wang Z, Jiang W, Zhang Y, Meng Z,
Niu Y, Sheng Z, Chen C, Liu X, Chen X, et al: CLDN18.2 and 4-1BB
bispecific antibody givastomig exerts antitumor activity through
CLDN18.2-expressing tumor-directed T-cell activation. J Immunother
Cancer. 11:e0067042023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Optimism Surrounds Claudin 18.2 ADC.
Cancer Discov. 14:122024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li D, Ding L, Chen Y, Wang Z, Zeng Z, Ma
X, Huang H, Li H, Qian X, Yang Z and Zhu H: Exploration of
radionuclide labeling of a novel scFv-Fc fusion protein targeting
CLDN18.2 for tumor diagnosis and treatment. Eur J Med Chem.
266:1161342024. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sahin U, Türeci Ö, Manikhas G, Lordick F,
Rusyn A, Vynnychenko I, Dudov A, Bazin I, Bondarenko I, Melichar B,
et al: FAST: A randomised phase II study of zolbetuximab (IMAB362)
plus EOX versus EOX alone for first-line treatment of advanced
CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma.
Ann Oncol. 32:609–619. 2021. View Article : Google Scholar
|
|
38
|
Shitara K, Kawazoe A, Hirakawa A,
Nakanishi Y, Furuki S, Fukuda M, Ueno Y, Raizer J and Arozullah A:
Phase 1 trial of zolbetuximab in Japanese patients with CLDN18.2+
gastric or gastroesophageal junction adenocarcinoma. Cancer Sci.
114:1606–1615. 2023. View Article : Google Scholar
|
|
39
|
Klempner SJ, Lee KW, Shitara K, Metges JP,
Lonardi S, Ilson DH, Fazio N, Kim TY, Bai LY, Moran D, et al:
ILUSTRO: Phase II multicohort trial of zolbetuximab in patients
with advanced or metastatic claudin 18.2-Positive gastric or
gastroesophageal junction adenocarcinoma. Clin Cancer Res.
29:3882–3891. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
O'Brien NA, McDermott MSJ, Zhang J, Gong
KW, Lu M, Hoffstrom B, Luo T, Ayala R, Chau K, Liang M, et al:
Development of a novel CLDN18.2-directed monoclonal antibody and
antibody-drug conjugate for treatment of CLDN18.2-positive cancers.
Mol Cancer Ther. 22:1365–1375. 2023. View Article : Google Scholar
|
|
41
|
Zeng Z, Li L, Tao J, Liu J, Li H, Qian X,
Yang Z and Zhu H: [177Lu]Lu-labeled anti-claudin-18.2
antibody demonstrated radioimmunotherapy potential in gastric
cancer mouse xenograft models. Eur J Nucl Med Mol Imaging.
51:1221–1232. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dumontet C, Reichert JM, Senter PD,
Lambert JM and Beck A: Antibody-drug conjugates come of age in
oncology. Nat Rev Drug Discov. 22:641–661. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu X, Zhu Y, Deng X, Kong F, Xi C, Luo Q
and Zhu X: Development of a supermolecular Radionuclide-drug
conjugate system for integrated radiotheranostics for Non-small
cell lung cancer. J Med Chem. 67:11152–11167. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chau CH, Steeg PS and Figg WD:
Antibody-drug conjugates for cancer. Lancet. 394:793–804. 2019.
View Article : Google Scholar
|
|
45
|
Zhou L, Lu Y, Liu W, Wang S, Wang L, Zheng
P, Zi G, Liu H, Liu W and Wei S: Drug conjugates for the treatment
of lung cancer: From drug discovery to clinical practice. Exp
Hematol Oncol. 13:262024. View Article : Google Scholar
|
|
46
|
Khongorzul P, Ling CJ, Khan FU, Ihsan AU
and Zhang J: Antibody-drug conjugates: A comprehensive review. Mol
Cancer Res. 18:3–19. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou KI, Strickler JH and Chen H:
Targeting Claudin-18.2 for cancer therapy: Updates from 2024 ASCO
annual meeting. J Hematol Oncol. 17:732024. View Article : Google Scholar
|
|
48
|
Yeh CL, Pai MH, Li CC, Tsai YL and Yeh SL:
Effect of arginine on angiogenesis induced by human colon cancer:
In vitro and in vivo studies. J Nutr Biochem. 21:538–543. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Kinjo K, Kizaki M, Muto A, Fukuchi Y,
Umezawa A, Yamato K, Nishihara T, Hata J, Ito M, Ueyama Y, et al:
Arsenic trioxide (As2O3)-induced apoptosis and differentiation in
retinoic acid-resistant acute promyelocytic leukemia model in
hGM-CSF-producing transgenic SCID mice. Leukemia. 14:431–438. 2000.
View Article : Google Scholar
|
|
50
|
Yao Y, Ren Y, Hou X, Zhu J, Ma X, Liu S,
Liu T, Zhang Q, Ma X, Yang Z, et al: Construction and preclinical
evaluation of a zirconium-89 labelled monoclonal antibody targeting
PD-L2 in lung cancer. Biomed Pharmacother. 168:1156022023.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Du H, Hao X, Lin B, Tang M, Wang D, Yang
X, Wang J, Qin L, Yang Y and Du X: 177Lu-labeled anticlaudin 6
monoclonal antibody for targeted therapy in esophageal cancer. J
Nucl Med. 66:377–384. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng L, Li C, Yang X, Liu J, Wang G, Zhou
Z, Zhu X, Gong J and Yang J: GD2-targeted theranostics of
neuroblastoma with [64Cu]Cu/[177Lu]Lu-hu3F8.
Eur J Nucl Med Mol Imaging. 52:1764–1777. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Drago JZ, Modi S and Chandarlapaty S:
Unlocking the potential of antibody-drug conjugates for cancer
therapy. Nat Rev Clin Oncol. 18:327–344. 2021. View Article : Google Scholar
|
|
54
|
Baah S, Laws M and Rahman KM:
Antibody-Drug Conjugates-A tutorial review. Molecules. 26:29432021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Chen YF, Xu YY, Shao ZM and Yu KD:
Resistance to antibody-drug conjugates in breast cancer: mechanisms
and solutions. Cancer Commun (Lond). 43:297–337. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Díaz-Rodríguez E, Gandullo-Sánchez L,
Ocaña A and Pandiella A: Novel ADCs and strategies to overcome
resistance to Anti-HER2 ADCs. Cancers (Basel). 14:1542021.
View Article : Google Scholar
|
|
57
|
Jain RK: Normalizing tumor
microenvironment to treat cancer: bench to bedside to biomarkers. J
Clin Oncol. 31:2205–2218. 2013. View Article : Google Scholar
|
|
58
|
Beckers C, Pruschy M and Vetrugno I: Tumor
hypoxia and radiotherapy: A major driver of resistance even for
novel radiotherapy modalities. Semin Cancer Biol. 98:19–30. 2024.
View Article : Google Scholar
|
|
59
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Straub JM, New J, Hamilton CD, Lominska C,
Shnayder Y and Thomas SM: Radiation-induced fibrosis: Mechanisms
and implications for therapy. J Cancer Res Clin Oncol.
141:1985–1994. 2015. View Article : Google Scholar
|
|
61
|
Yamamoto K, Nakayama I and Shitara K: A
new molecular targeted agent for gastric Cancer-The Anti-Claudin
18.2 antibody, zolbetuximab. Gan To Kagaku Ryoho. 51:1111–1118.
2024.(In Japanese). PubMed/NCBI
|
|
62
|
Liu Y, Wang X, Zhang N, He S, Zhang J, Xu
X and Song S: Utility of 131I-HLX58-Der for the precision
treatment: Evaluation of a preclinical
radio-antibody-drug-conjugate approach in mouse models. Int J
Nanomedicine. 20:723–739. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chang HL, Schwettmann B, McArthur HL and
Chan IS: Antibody-drug conjugates in breast cancer: Overcoming
resistance and boosting immune response. J Clin Invest.
133:e1721562023. View Article : Google Scholar
|
|
64
|
Jiao J, Qian Y, Lv Y, Wei W, Long Y, Guo
X, Buerliesi A, Ye J, Han H, Li J, et al: Overcoming limitations
and advancing the therapeutic potential of antibody-oligonucleotide
conjugates (AOCs): Current status and future perspectives.
Pharmacol Res. 209:1074692024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shah MA, Shitara K, Ajani JA, Bang YJ,
Enzinger P, Ilson D, Lordick F, Van Cutsem E, Gallego Plazas J,
Huang J, et al: Zolbetuximab plus CAPOX in CLDN18.2-positive
gastric or gastroesophageal junction adenocarcinoma: The
randomized, phase 3 GLOW trial. Nat Med. 29:2133–2141. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Shitara K, Shah MA, Lordick F, Van Cutsem
E, Ilson DH, Klempner SJ, Kang YK, Lonardi S, Hung YP, Yamaguchi K,
et al: Zolbetuximab in gastric or gastroesophageal junction
adenocarcinoma. N Engl J Med. 391:1159–1162. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hammood M, Craig AW and Leyton JV: Impact
of endocytosis mechanisms for the receptors targeted by the
currently approved Antibody-drug conjugates (ADCs)-A necessity for
future ADC research and development. Pharmaceuticals (Basel).
14:6742021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang S, Wang X, Gao X, Chen X, Li L, Li
G, Liu C, Miao Y, Wang R and Hu K: Radiopharmaceuticals and their
applications in medicine. Signal Transduct Target Ther. 10:12025.
View Article : Google Scholar : PubMed/NCBI
|















![(A) Flow chart of the experimental
design. Created with BioRender.com. (B) Flow cytometry was used to
detect CLDN18.2 expression in NUGC-4-CLDN18.2 cells. Blue line:
Anti-SYSA1801mAb (PE); red line: isotype control (PE). (C)
Radiochemical purity of [177Lu]Lu-DOTA-SYSA1801mAb
determined by HPLC was >99%. Upper panel: Radio-HPLC
(177Lu γ-radiation detection); lower panel:
Ultraviolet-HPLC (254 nm absorption detection). (D) Specific
binding of [177Lu]Lu-DOTA-SYSA1801mAb in NUGC-4-CLDN18.2
cells. Left panel: Saturation binding curve plotted on a
logarithmic concentration scale for calculation of the equilibrium
dissociation constant (Kd). Right panel: Saturation binding curve
plotted on a linear concentration scale for determination of the
maximum binding capacity (Bmax). (E) Stability of
[177Lu]Lu-DOTA-SYSA1801mAb detected by thin-layer
chromatography in normal saline and 1640 (10% FBS). ADC,
antibody-drug conjugate; CLDN18.2, claudin 18.2; EC50,
half maximal effective concentration; FBS, fetal bovine serum;
HPLC, high-performance liquid chromatography; mAb, monoclonal
antibody; RDC, radionuclide-drug conjugate.](/article_images/or/55/1/or-55-01-09009-g00.jpg)
![(A) Positron emission
tomography-computed tomography images showing tumor-specific
accumulation of [89Zr]Zr-DFO-SYSA1801mAb in
tumor-bearing mice over time, in contrast to that in other
non-target tissues. (B) Quantitative region of interest analysis of
the tumor, heart, liver, spleen, kidney, and muscle in
tumor-bearing mice at 4, 24, 48, 96, 144 and 192 h after injection
of [89Zr]Zr-DFO-SYSA1801mAb. (C) Biodistribution results
at 4, 24, 48, 96 and 144 h after injection of
[177Lu]Lu-DOTA-SYSA1801mAb. (D) Tumor-to-blood, (E)
tumor-to-liver and (F) tumor-to-kidney ratios at 4, 24, 48, 96 and
144 h after injection of [177Lu]Lu-DOTA-SYSA1801mAb.
Data are presented as the mean ± SD. Statistical differences across
the different time points for each ratio were determined by one-way
analysis of variance followed by Tukey's post hoc test for multiple
comparisons. *P<0.05, **P<0.01, ***P<0.001. %DI/g,
percentage of injected dose per gram of tissue; mAb, monoclonal
antibody.](/article_images/or/55/1/or-55-01-09009-g01.jpg)