Esophageal cancer (EC), one of the major
malignancies of the digestive system, poses a pronounced global
health burden. According to the most recent global cancer
statistics, EC ranked as the 11th most commonly diagnosed cancer
and the 7th leading cause of cancer-related mortalities worldwide
in 2022 (1). Epidemiological data
indicate that ~511,000 new cases and 445,000 mortalities occurred
globally in that year, underscoring the substantial clinical and
public health challenges associated with this disease (1). Historically, EC is classified into two
main subtypes: Esophageal squamous cell carcinoma (ESCC) and
esophageal adenocarcinoma (EAC). ESCC is primarily associated with
lifestyle risk factors such as tobacco smoking and alcohol
consumption, while EAC is more commonly associated with
gastroesophageal reflux disease and obesity (2). ESCC accounts for >90% of EC cases
worldwide (3). These subtypes
exhibit distinct molecular profiles: ESCC is characterized by
frequent increased expression of CCND1, SOX2 and/or TP63, whereas
EAC more often involves amplification of ERBB2, VEGFA and members
of the GATA gene family (GATA4/6) (4). Although the overall incidence of EC
has shown a downward trend, the prognosis remains poor (5). This is mainly due to the asymptomatic
nature of early-stage disease, the lack of specific diagnostic
biomarkers and the limitations of current detection methods. As a
result, the majority of patients are diagnosed at an advanced
stage, with a 5-year overall survival (OS) rate of <20%
(6). The advent of immune
checkpoint inhibitors (ICIs) has ushered in a new era in EC
therapy, particularly monoclonal antibodies targeting programmed
cell death protein 1 (PD-1) and its ligand (PD-L1). These agents
have demonstrated promising efficacy across multiple malignancies,
including melanoma, non-small cell lung cancer, renal cell
carcinoma, head and neck squamous cell carcinoma and several types
of gastroesophageal cancer (7).
Clinical studies have shown that PD-1/PD-L1
inhibitors markedly improve OS in advanced EC, with particularly
notable benefits in the ESCC subtype (8,9).
Currently approved or investigational anti-PD-1/PD-L1 agents
include nivolumab, pembrolizumab, JS001, SHR-1210, durvalumab and
SHR-1316 (10). However, the
response rate to PD-1/PD-L1 blockade remains limited. Emerging
evidence indicates that immunosuppressive signaling within the
tumor microenvironment (TME) carries out a key role in limiting
efficacy, primarily by persistently impairing T cell function
through aberrant activation of inhibitory pathways (11). In fact, immunosuppression represents
the primary source of resistance to immunotherapy (12). To comprehensively decipher the
molecular landscape of tumors, researchers have extensively
integrated multi-dimensional data, including genomics,
transcriptomics, proteomics and metabolomics, to deeply explore the
intricate interactions between tumor and immune cells within the
TME, thereby providing direction for personalized cancer
immunotherapy. In this context, combination therapies are widely
regarded as a key strategy to overcome resistance to immunotherapy.
Among them, unleashing the therapeutic potential of ICIs in
combination with targeted therapy, cytokines, metabolic
immunotherapies, chemotherapy, radiotherapy and multifunctional
nanoparticles has gained recognition (13). However, although a study has shown
that anti-PD-L1 inhibitors combined with radiotherapy can elicit
systemic antitumor immunity by remodeling the immune
microenvironment in mouse models of ESCC, the role of the spleen
and tumor-draining lymph nodes in radioimmunotherapy remains to be
elucidated. Moreover, to the best of our knowledge, these studies
have not thoroughly analyzed the relationship between PD-1
expression and CD8+ T-cell function (14).
It is noteworthy that the complex composition of
different types of cancer and TMEs collectively shapes the
multidimensional mechanisms of immune regulation. Therefore,
determining the optimal combinations between immunotherapies and
TME-modulating agents remains a major challenge. The present review
aims to systematically dissect the complex immunosuppressive
network mechanisms in ESCC based on an in-depth analysis of its TME
and further discuss the translational potential of combination
immunotherapy in this setting.
Driven by breakthroughs in immunological theory, T
lymphocytes, as central effector cells, have been widely used in
tumor immunotherapy (15). PD-1 was
first discovered in 1992 and is functionally recognized as a key
molecule regulating both adaptive and innate immune responses
(16).
The PD-1 gene, PDCD1, is located on human chromosome
2q37 and encodes a 288 amino acid type I transmembrane glycoprotein
belonging to the CD28 immunoglobulin superfamily (16,17).
PD-1 is highly expressed on the surface of activated T cells and is
also dynamically distributed across various antigen presenting
cells (APCs), including B cells, macrophages, dendritic cells (DCs)
and monocytes. Its primary biological function is to negatively
regulate T cell effector activity, carrying out a key role in
maintaining immune homeostasis, preventing autoimmunity and
modulating anti-tumor immune responses (18,19).
PD-1 exerts its signaling through interaction with two specific
ligands, PD-L1 and PD-L2. PD-L1 is widely expressed on APCs,
activated lymphocytes and a variety of tumor cells, whereas PD-L2
expression is more restricted and is primarily observed on
activated DCs and macrophages (20). Notably, aberrant overexpression of
PD-L1 in the TME represents a major mechanism of immune evasion.
Its expression is directly regulated by abnormal activation of the
nuclear factor erythroid 2-related factor 2 (NRF2) signaling
pathway and is positively associated with tumor node-metastasis
staging, lymphatic metastasis and poor prognosis (19). At the molecular level, PD-L1 binds
to PD-1 on T cells, effectively suppressing the proliferation of
PD-1+ immune cells, thereby dampening anti-tumor immunity and
facilitating tumor progression (21,22).
In normal tissues, PD-L1 displays tissue-specific
expression patterns, primarily localized on the surface of
CD4+Foxp3+ regulatory T cells (Tregs). By
inhibiting the phosphorylation cascade of the mTOR/AKT signaling
pathway, PD-L1 promotes Treg development, maintains their
homeostasis and supports their immunosuppressive functions
(23). During the malignant
transformation of esophageal tissues, PD-L1 expression undergoes
considerable reprogramming. From precancerous lesions such as
Barrett's esophagus and low-/high-grade intraepithelial neoplasia
to invasive gastroesophageal junction adenocarcinoma, the PD-L1
positivity rate increases progressively (24). Among them, IFN-γ can directly induce
PD-L1 expression in esophageal epithelial cells via activation of
the JAK/STAT signaling axis, thereby establishing a molecular
barrier to immune surveillance (25,26).
Clinical data show that ~40% of patients with EC exhibit PD-L1
overexpression, and its expression level is positively associated
with disease progression. In cohorts treated with ICIs, the
objective response rate (ORR) in EC ranges from 9.9 to 33.3%,
further confirming the clinical value of PD-L1 as a therapeutic
target (27). Importantly, PD-L1
expression is negatively associated with both progression-free
survival (PFS) and OS in patients with EC, supporting its potential
as an independent prognostic biomarker (28). These findings define the dual role
of PD-L1 in EC: It acts both as a key driver of immune evasion and
as a predictive biomarker for immune therapy response. This dual
function provides a molecular basis for the development of
stratified treatment strategies.
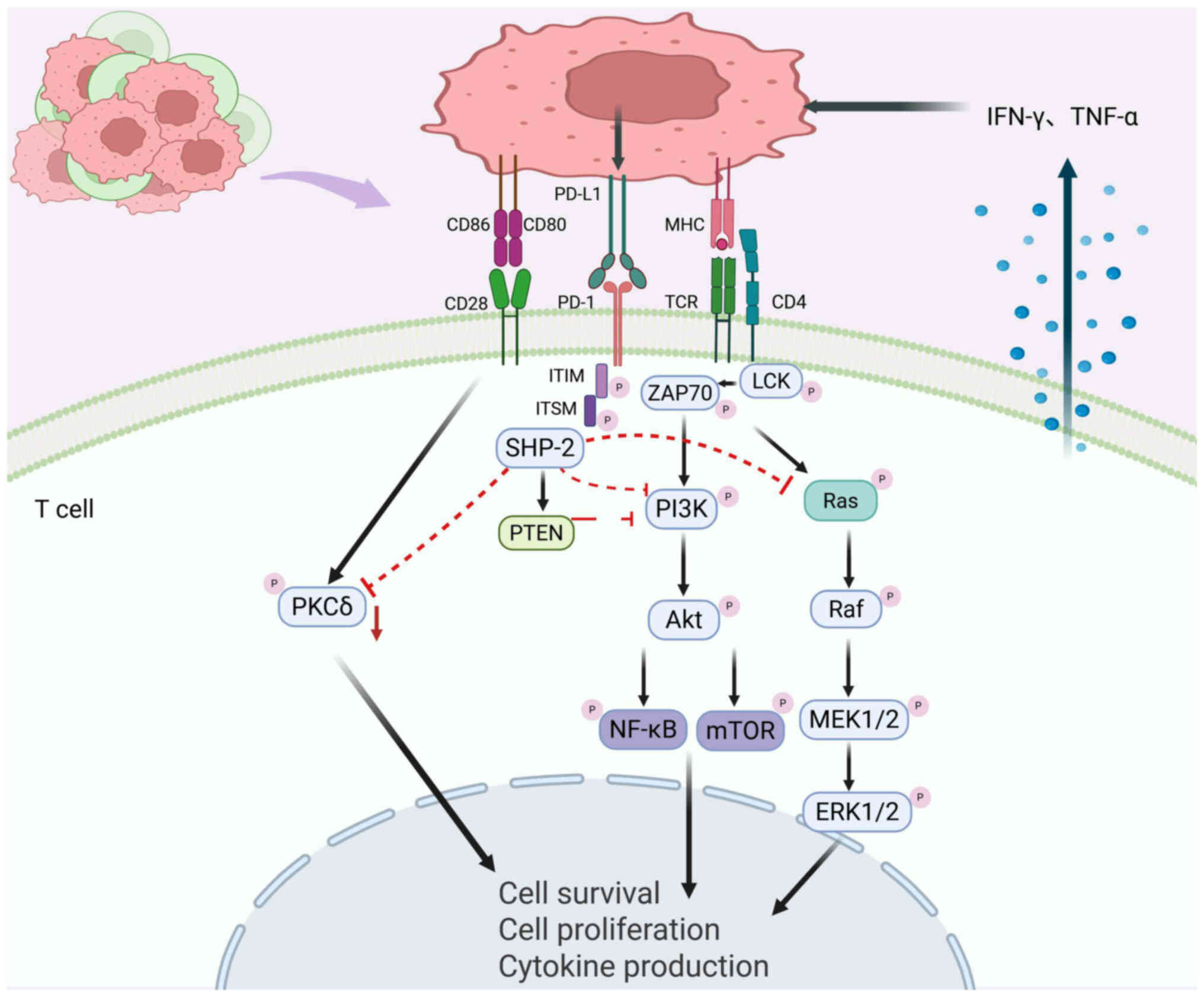
Evidence has confirmed that the PD-1/PD-L1 axis
mediates immunosuppressive effects by directly interfering with T
cell receptor (TCR) signaling. In the TME, PD-1 binding on
activated T cells to its PD-L1 ligand triggers phosphorylation of
tyrosine residues within the immunoreceptor tyrosine switch motif
(ITSM). This conformational change recruits SH2 domain tyrosine
phosphatases SHP-1 and SHP-2 to ITSM. These phosphatases inhibit
the phosphorylation cascade of spleen tyrosine kinase and
phosphoinositide 3-kinase (PI3K), ultimately inhibiting PI3K/Akt
signaling. This results in downregulation of the anti-apoptotic
protein Bcl-xL, initiation of apoptosis in T lymphocytes and
suppression of T cell proliferation, survival and effector
function, thus establishing a key molecular basis for tumor immune
escape (21,29).
The immunosuppressive function of PD-1 is mediated
through multiple regulatory pathways: i) Inhibition of the
RAS-ERK1/2 signaling cascade blocks T cell cycle progression; ii)
downregulation of protein kinase C δ activity reduces the secretion
of cytokines such as IL-2, weakening the immune response; iii)
activation of PTEN inhibits TCR-induced PI3K/AKT signaling
(17). Additionally, inflammatory
cytokines such as interferon-γ (IFN-γ) and tumor necrosis factor-α
(TNF-α) in the TME can markedly upregulate PD-L1 expression on
immune and stromal cells, creating a positive feedback loop that
reinforces immune suppression and enhances tumor immune evasion
(30) (Fig. 1). Unlike cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4), which primarily
regulates early T cell activation in lymph nodes, PD-1 primarily
functions at later stages in peripheral tissues, where it inhibits
effector T cell activity through multifaceted mechanisms. This
spatial and temporal distinction suggests that the PD-1/PD-L1 axis
carries out a central role in the effector phase of tumor immune
escape (31).
T cell exhaustion serves as a key driver of immune
evasion in ESCC. Although PD-1 blockade can partially reverse the
dysfunctional state of exhausted T cells, this therapeutic effect
is generally transient and limited within the complex
immunosuppressive TME of ESCC (32). This persistent exhausted state is
reinforced by multiple mechanisms within the TME. For instance,
fibroblast-derived growth factor (FGF)-2 exacerbates T cell
exhaustion by upregulating Sprouty RTK signaling antagonist 1
(33). Concurrently, non-coding
RNAs carry out pivotal regulatory roles: The long non-coding RNA
FOXP4-AS1 stabilizes PD-L1 via the deubiquitinase
ubiquitin-specific protease (USP) 10, thereby suppressing
CD8+ T cell viability, promoting their exhaustion and
consequently driving immune evasion (34). Meanwhile, the circular RNA circNF1
upregulates PD-L1 expression through dual mechanisms, activating
the IL-6-induced JAK-STAT3 pathway and inhibiting USP7-mediated
deubiquitination of annexin A1, thereby enhancing PD-L1-mediated
immunosuppression (35). Beyond
direct regulation of T cells, their interactions with myeloid cells
are key. In patients with ESCC with stable disease,
PD-1+ CD8+ T cells frequently interact with
macrophages; targeting the iron-sulfur cluster assembly enzyme in
macrophages can repolarize them, enhance CD8+ T cell
cytotoxicity and synergize with anti-PD-1 therapy (36). Furthermore, melanoma-associated
antigen C3 amplifies IFN-γ signaling and upregulates PD-L1 by
binding to IFN-γ receptor 1 and enhancing its interaction with
STAT1, while also promoting epithelial-mesenchymal transition,
ultimately leading to immune evasion (37).
Finally, the CD47-SIRPα axis, a key regulator of
innate immune surveillance, promotes immune evasion by delivering a
‘don't eat me’ signal that inhibits macrophage phagocytosis of
tumor cells. Elevated SIRPα expression is associated with poor
prognosis in patients with ESCC, potentially due to impaired
phagocytic function and subsequent suppression of antitumor
immunity (38–40). The PD-1/PD-L1 axis represents a core
mechanism of tumor immune evasion. Although PD-1 blockade can
partially reverse T cell exhaustion, its efficacy is often
constrained and prone to resistance within the multi-mechanistic,
multidimensional immunosuppressive microenvironment of ESCC.
Therefore, in-depth dissection of this regulatory network and the
subsequent development of rational combination targeting strategies
are key directions for overcoming immunotherapy resistance in
ESCC.
Beyond its well-known role in inhibiting anti-tumor
immunity by binding PD-1 to immune cells, tumor-intrinsic PD-L1
signaling also shapes tumor immunogenicity. Surface PD-L1 may
engage PD-1 on tumor cells themselves, promoting proliferation
through enhanced phosphorylation of ribosomal protein S6. Cytosolic
PD-L1 regulates mRNA stability of DNA damage response (DDR) genes
and activates key pathways such as MAPK/ERK and STAT3 through
protein interactions. Nuclear PD-L1 may modulate transcription of
immunogenicity-related genes or influence chromosomal stability
through mechanisms such as sister chromatid cohesion (29).
Deficiencies in DDR are commonly observed in ESCC,
and DDR alterations may influence mutational processes and immune
cell infiltration (41). Increased
expression of DDR genes contributes to ESCC progression and
suppresses the tumor immune microenvironment, while targeting DDR
pathways has demonstrated potent efficacy in inducing immune
activation and improving patient outcomes (42). Moreover, a study investigating the
impact of DDR on the TME have confirmed that wild-type p53
overexpression suppresses DNA damage pathways and reduces PD-L1
expression in prostate cancer (43). Additionally, it has been suggested
that oxidative stress may induce DNA damage signaling that
upregulates PD-L1 expression. Both activation of DNA damage
signaling and DDR deficiencies carry out important roles in
promoting PD-L1 expression (44).
Notably, DNA damage can stimulate PD-L1 mRNA expression, leading to
increased PD-L1 levels on the cell surface. For example, DNA
double-strand breaks (DSBs) activate the STAT1/3-IRF1 signaling
axis, leading to PD-L1 upregulation (45). In addition, DSBs induced by
chemotherapy and/or radiotherapy may activate DDR. While excessive
DNA damage leads to tumor cell death, it also promotes the release
of damage-associated molecular patterns, which in turn elicit
anti-tumor immune responses. These observations highlight the
strong potential of targeting DDR pathways in cancer immunotherapy.
Combining DDR-targeted agents with ICIs represents a promising
therapeutic strategy. For example, colorectal cancer with mismatch
repair deficiency exhibit high sensitivity to anti-PD-1 checkpoint
blockade therapy (46). A subset of
patients with ESCC harbor DDR gene mutations. These patients with
DDR gene alterations show considerable associations with
immunological biomarkers, suggesting the potential feasibility of
combining DDR-targeting agents with immunotherapy for DDR-deficient
patients in future therapeutic strategies (47).
TME acts as a key mediator of the dynamic interplay
between tumor cells and host immune defense. Accumulating evidence
suggests that resistance to ICIs stems primarily from the intricate
crosstalk within the tumor-TME network (48). The following sections of the present
review aim to systematically explore the multifaceted regulatory
mechanisms of the PD-1/PD-L1 axis in ESCC TMEs, with particular
attention to T and natural killer (NK) cell exhaustion,
immunosuppression, angiogenesis, tertiary lymphoid structures (TLS)
and tumor-driving mutations. This exploration holds promise for
identifying potential strategies to improve therapeutic outcomes in
EC.
The exhausted state in NK cells is characterized by
the loss of cytotoxic capacity and cytokine production (49). In patients with ESCC, PD-1
expression is markedly elevated on the surface of NK cells, and
this expression is negatively associated with patient prognosis
(50). Subsets of
PD-1+NK cells exhibit stronger functional activity than
PD-1−counterparts, suggesting that this subpopulation
may represent NK cells with the highest effector potential. This
observation helps explain why, despite PD-1 being expressed on only
a small proportion of NK cells, its interaction with PD-L1 on tumor
cells can still effectively suppress NK cell responses (51). Similar to regulatory mechanisms in T
cells, the PD-1/PD-L1 axis impairs NK cell activation thresholds,
proliferation capacity and cytokine secretion, ultimately leading
to an exhausted NK phenotype and weakened anti-tumor immune
surveillance (52).
Mechanistically, this inhibition is mediated primarily by blocking
the PI3K/AKT phosphorylation cascade (50).
In addition, T cell immunoreceptor with Ig and ITIM
domains (TIGIT) is often co-expressed with PD-1 on
tumor-infiltrating T and NK cells, forming a synergistic inhibitory
network. A study has confirmed that high TIGIT expression
associates with an exhausted state in tumor-infiltrating NK cells
(53). Experimental evidence
indicates that TIGIT promotes lymphocyte exhaustion through two
mechanisms: i) Inhibition of AKT pathway activation and ii)
inhibition of forkhead box protein O1 phosphorylation (54). Notably, blocking TIGIT can
effectively reverse exhaustion of NK cells within the TME and
restore their effector function (55). In a study based on peripheral
samples from surgically resected patients with ESCC, PD-1
expression revealed a notable positive association with TIGIT
levels (53). Correspondingly, an
ESCC clinical trial demonstrated that the combination of the
TIGIT-targeting antibody vibostolimab with pembrolizumab exhibited
encouraging clinical activity without requiring biomarker
prescreening; particularly, PD-L1 high-expressing patients, defined
by a combined positive score ≥10, achieved higher ORR (53). Both TIGIT and T cell immunoglobulin
and mucin domain-3 (Tim-3) are associated with poor survival
outcomes in ESCC (56). Similarly,
Tim-3, which is upregulated on NK cells across various types of
cancer, is a hallmark of lymphocyte exhaustion. In vitro
inhibition of Tim-3 not only enhances NK cell cytotoxicity but also
improves tumor cell killing efficiency (57). Research has confirmed that
functionally impaired Tim-3+ NK cells in EC associates
with poor patient prognosis, and the formation of this cellular
subset is mechanistically driven by TNF-α through NF-κB signaling
pathway-mediated TIM-3 expression (58). Multiple TIM-3-targeting
immunotherapeutic agents are currently undergoing phase 1/2
clinical trials, including Cobolimab, Sabatolimab, BGB-A425,
BC3402, TQB2618, NB002, AZD7789, LB1410 and INCAGN02390 (59). PD-1+NK cells in the TME
frequently exhibit a PD-1+/Tim-3+
double-positive depleted phenotype, suggesting potential
synergistic suppression (60).
Although substantial fundamental mechanistic
research supports TIM-3 as a key target for improving NK cell
function and enhancing cancer immunotherapy efficacy, with its
individual blockade or combination with PD-1 inhibitors considered
to hold considerable potential (59,61,62).
The field still contains debatable complexities worthy of deeper
investigation. This controversy is highlighted by an in
vitro study by Astaneh et al (49), which reached contrasting
conclusions. In early-stage chronic lymphocytic leukemia,
researchers found that pre-treatment of NK cells with anti-PD-1 and
anti-Tim-3 blocking antibodies failed to effectively restore or
enhance NK cell functional characteristics, while also not
promoting the production of key pro-inflammatory cytokines TNF-α
and IFN-γ. In summary, these studies reveal the complex
interactions among PD-1, TIM-3 and TIGIT in driving NK cell
dysfunction in ESCC. These findings not only elucidate the
multidimensional regulatory mechanisms of NK cell exhaustion but
also provide a theoretical foundation for developing PD-1 inhibitor
combination therapies with TIM-3 or TIGIT blockade, demonstrating
notable translational potential. However, the efficacy of
TIM-3/PD-1 co-targeting strategies should not be simplistically
generalized, as their effectiveness highly depends on specific
tumor types and disease stages. Future research should validate
this strategy in ESCC and other cancer types across different
disease stages, while further clarifying the intrinsic mechanisms
of treatment resistance.
In the ESCC microenvironment, PD-1 expression on NK
cells is dynamically regulated by various cytokines. Experimental
data show that IL-12 and IL-15 markedly upregulate PD-1 expression
in patient-derived NK cells in a time-dependent manner (50). Notably, TGF-β, a key inhibitory
cytokine for NK cells, promotes NK cell exhaustion by upregulating
the expression of the inhibitory receptor PD-1 on their surface
(63,64). Mechanistically, abnormal
accumulation of TGF-β in the TME results from the coordinated
secretion by multiple cell types: In addition to tumor
cell-autonomous secretion (65),
immunosuppressive subpopulations such as cancer-associated
fibroblasts (CAFs), myeloid-derived suppressor cells (MDSCs),
tumor-associated macrophages (TAMs) and Tregs collectively form a
cascading TGF-β secretion network, shaping a hostile
microenvironment that promotes NK cell dysfunction (63,66). A
mechanistic study by Thangaraj et al (67) demonstrated that in tumor models with
high TGF-β expression, effective blockade of the TGF-β signaling
pathway is a prerequisite for restoring NK cell anti-tumor
activity. It is noteworthy that elevated TGF-β in the TME
contributes to cancer immune evasion. The PD-1 and TGF-β pathways
function independently yet interactively, collectively facilitating
immune escape by tumor cells. Co-blockade of TGF-β and PD-L1 has
been validated to enhance the efficacy of PD-L1 monoclonal
antibodies and overcome therapeutic resistance (68). Furthermore, bispecific
anti-PD-(L)1/TGF-β inhibitors can reinvigorate the effector
functions of CD8+ T and NK cells, hinder Treg expansion and
increase M1 macrophage density. Additionally, anti-PD-(L)1/TGF-β
inhibitor therapy can be safely administered in combination with
vaccination, radiotherapy and chemotherapy (69). Research by Lucarini et al
(70) demonstrated that low-dose
mitoxantrone combined with anti-TGF-β and PD-1 blockade therapy
enhances anti-tumor immunity by remodeling the tumor immune
landscape and overcoming the immunosuppressive microenvironment in
aggressive neuroblastoma.
These findings validate the central role of the
TGF-β-PD-1 regulatory axis in NK cell exhaustion and offer an
experimental basis for targeting the TME to restore immune
competence. They also offer theoretical guidance for exploring the
therapeutic potential of dual TGF-β and PD-1/PD-L1 blockade in ESCC
immunotherapy.
Impaired metabolic functioning of NK cells is a
major factor contributing to their reduced activity, and the TME
carries out a key role in this process. The extensive consumption
of nutrients (such as glucose and glutamine) and the continuous
release of TGF-β in the TME inhibit NK cell glycolysis and
mitochondrial oxidative phosphorylation (OXPHOS), leading to
decreased functional activity (61,71).
Additionally, a study has confirmed that tumor cells in the TME can
secrete TGF-β, which further induces the upregulation of
fructose-1,6-bisphosphatase expression in NK cells, thereby
inhibiting glycolysis and ultimately impairing NK cell cytotoxicity
and vitality (72). Beyond the
reduced glucose concentration, other characteristic factors of the
TME also suppress NK cell function, such as hypoxia and acidic pH
(61). Research has shown that in
hypoxic prostate tumors, hypoxia-inducible factor 1α (HIF-1α)
mediates the overexpression of miR-224, which suppresses NCR1
signaling, markedly reducing NK cell cytotoxicity (73). Moreover, elevated expression levels
of HIF-1α in patients with ESCC are associated with poor prognosis
(74). Based on this association,
HIF-1α could serve as a potential biomarker for predicting the
prognosis of ESCC (75).
Additionally, PD-L1 is not only expressed on tumor
cells in the TME but also on T cells and NK cells. HIF-1α, as a
response element of the PD-L1 promoter, can promote and sustain
PD-L1 expression, further enhancing its function through a positive
feedback mechanism (76). Research
by Ding et al (77)
demonstrated that in gliomas, hypoxia-induced overexpression of
HIF-1α promotes PD-L1 expression, confirming the positive
association between the two. In their study, the combination of
anti-PD-L1 antibody and HIF-1α inhibitors in a mouse glioma model
considerably increased the infiltration of immune cells (CD4+ T,
CD8+ T and CD11c+ DC) into the tumor while reducing PD-L1
expression, suggesting that this combination strategy could
effectively reverse the immunosuppressive microenvironment in
tumors. Furthermore, Shurin et al (78) suggested that HIF-1α inhibition has a
synergistic anti-tumor effect with anti-PD-1 therapy. In a mouse
melanoma model, inhibiting HIF-1α transcriptional activity led to
increased infiltration of NK cells and CTLs mediated by CCL2 and
CCL5, markedly enhancing the anti-tumor effect of PD-1 blockade
antibodies.
T cell exhaustion is a dynamic process characterized
by a gradual transition from stem-like self-renewal to terminal
exhaustion. Persistent expression of PD-1 inhibitory receptor on T
cells is a hallmark of exhaustion (79). As previously mentioned, one of the
key signaling pathways targeted by PD-1 is the TCR signaling
pathway. In exhausted T cells, the function of the TCR signaling
pathway is impaired (80).
In ESCC TMEs, IL-2 levels are notably increased when
compared with normal tissues (81).
IL-2 promotes CD8+ T cell proliferation and its decline is one of
the key markers of T cell exhaustion. However, despite the decline
in IL-2 levels, IL-2 receptor β (IL-2Rβ) associated with PD-1
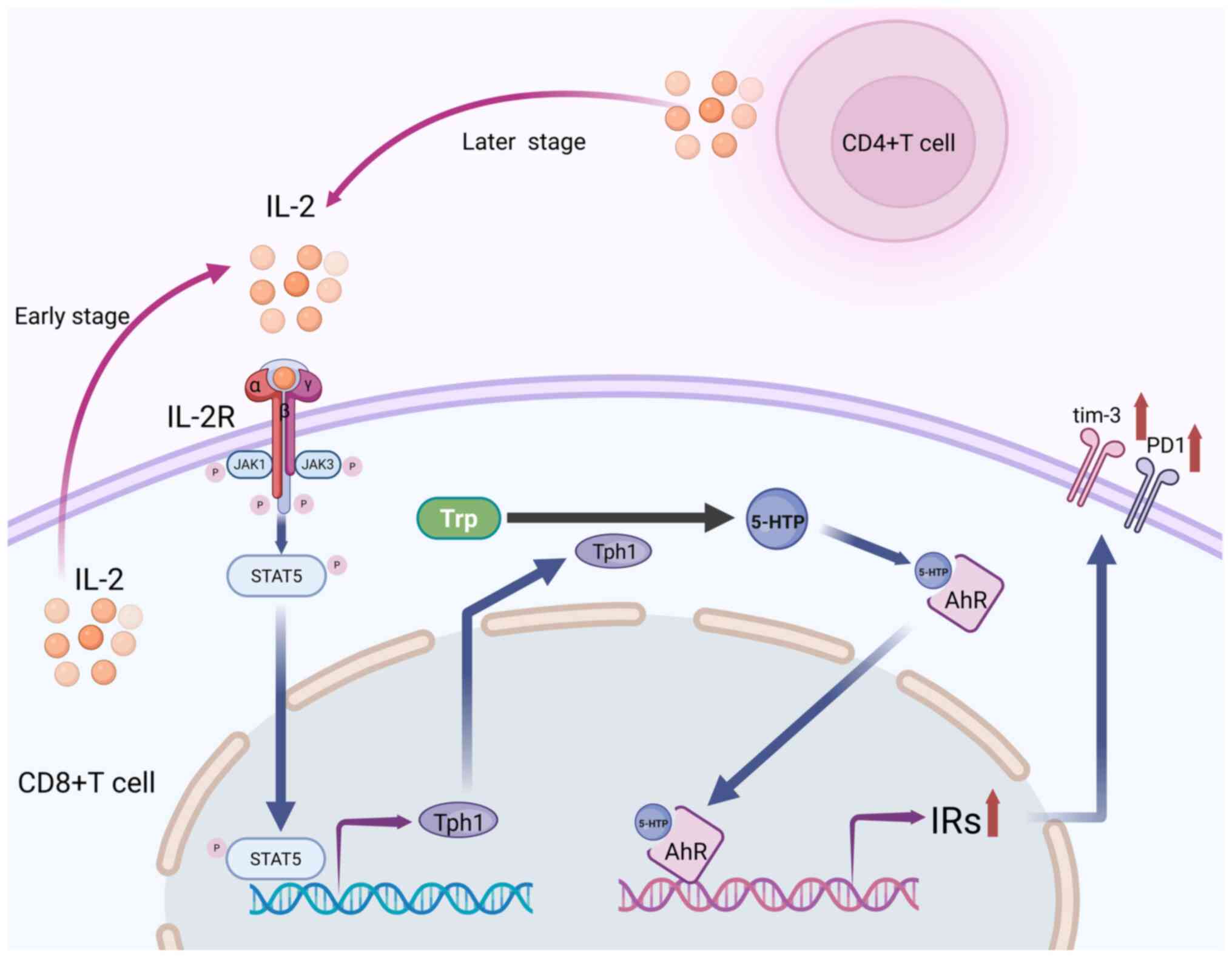
expression is upregulated in exhausted CD8+ T cells (79). In the early stages of tumor growth,
IL-2 induces CD8+ T cell differentiation through autocrine
signaling. In the mid to late stages of tumor growth, CD4+ T cells
continuously secrete IL-2, which further acts on CD8+ T cells. The
mechanism is that IL-2 continuously activates STAT5 in CD8+ T
cells, inducing the expression of tryptophan hydroxylase 1 (TPH1),
which catalyzes the conversion of tryptophan to 5-hydroxytryptophan
(5-HTP). The 5-HTP then activates AHR nuclear translocation,
leading to coordinated upregulation of inhibitory receptors such as
PD-1 and TIM-3, while inhibiting cytokine and effector molecule
production, ultimately leading to T cell exhaustion (82,83)
(Fig. 2). A study by Hashimoto
et al (84) showed that
PD-1+TCF-1+ CD8+ T cells (precursors of exhausted CD8+ T cells) are
not fate-locked to the exhausted path and their differentiation can
be regulated by IL-2 signaling. The researchers suggested that
PD-1+IL-2 combination therapy could markedly alter the
differentiation program of PD-1+TCF-1+ stem-like CD8+ T cells.
To address the clinical toxicity associated with
IL-2 therapy, antibody-cytokine fusion proteins such as PD1-IL2v
have been developed. These conjugates enhance the cytotoxic and
proliferative functions of stem-like precursor exhausted CD8+ T
cells (85). Accordingly, the
antibody-cytokine fusion protein eciskafusp alfa (PD1-IL2v) has
been engineered, wherein the PD-1 antibody delivers an IL-2R
agonist to PD-1-expressing T cells via a fused monomeric IL-2
variant (IL-2v), providing cis-binding that reportedly generates
superior effector populations (86). However, integrating both mechanisms
into a single molecule presents considerable challenges due to
competing requirements for PD-1 engagement and IL-2 receptor
signaling. Researchers including Mure et al (87) have developed a novel bispecific
antibody-cytokine fusion protein, ANV600, which delivers IL-2Rβγ
agonists specifically to PD-1+ cells while preserving the binding
site for PD-1 checkpoint inhibitors. This approach provides a new
strategy with enhanced antitumor activity and reduced toxicity. By
targeting PD-1, ANV600 selectively expands tumor antigen-specific
CD8+ T cells, particularly progenitor exhausted (Tpex) and
cytotoxic exhausted subsets, while sparing Treg and NK cells.
Furthermore, ANV600 produces additive effects when combined with
either pembrolizumab or nivolumab.
In summary, T cell exhaustion represents a key
immunosuppressive phenomenon in the TME, particularly prominent
during the pathogenesis and progression of ESCC. PD1-IL2v molecules
such as ANV600 and AWT020, which modulate T cell status by
targeting PD-1, provide novel insights and strategic approaches for
ameliorating T cell exhaustion in ESCC, enhancing anti-tumor immune
responses and improving survival outcomes.
Exhausted T cells exhibit notable heterogeneity in
phenotype and function, mainly classified into two major subsets:
Tpex cells and terminally exhausted T cells. Tpex cells have a
stem-like exhaustion phenotype characterized by markers such as
PD-1, CD127, chemokine receptor CXCR5 and high levels of TCF-1
(encoded by Tcf7). Tpex cells rely primarily on mitochondrial fatty
acid oxidation and OXPHOS for metabolism. However, prolonged
antigen stimulation leads to changes in mitochondrial structure,
which in turn impairs both glycolysis and OXPHOS function (91). Furthermore, a reduction in mitophagy
or impaired OXPHOS can lead to the accumulation of reactive oxygen
species in mitochondria and increased accumulation of damaged
mitochondria, which causes the loss of mitochondrial membrane
potential and depolarization, ultimately leading to mitochondrial
dysfunction, increased hypoxic stress, induced epigenetic
reprogramming and irreversible terminal T cell exhaustion (92,93).
The PD-1/PD-L1 axis carries out a particularly key role in T cell
exhaustion, particularly in regulating mitochondrial function. The
PD-1 signaling pathway inhibits phosphorylation of dynamin-related
protein 1 by modulating extracellular protein kinases 1/2 and mTOR
pathways, blocking mTOR-mediated mitochondrial fission, thereby
reducing mitochondrial numbers and weakening their function. This
process ultimately suppresses T cell activation, proliferation and
infiltration (94). Current
research indicates that immune-related pathways and mitochondrial
function are associated with immunotherapy sensitivity in ESCC
(95). To overcome the
immunosuppressive TME, characterized by hypoxia and lipid raft
formation, which considerably suppress T cell infiltration and
function, Chen et al (96)
developed a stable nano-formulation named Abstatin. This
formulation combines partially denatured albumin with fluvastatin,
effectively mitigating mitochondrial respiratory suppression and
disrupting lipid raft stability in non-small cell lung cancer
cellular and animal models. Notably, Abstatin notably enhanced the
efficacy of anti-PD-1 therapy with minimal toxicity, demonstrating
promising clinical safety prospects. This approach, which
integrates cellular metabolic regulation with immune sensitization,
broadens the potential applications of immunotherapy and offers
novel insights for ESCC treatment. Similarly, in manganese-based
STING-activating cancer immunotherapy, efficacy is limited by T
cell exhaustion, with mitochondrial dysfunction being a key
contributing factor. A study shows that spermidine can enhance
mitochondrial function in T cells, thereby providing a viable
strategy to reverse T cell exhaustion in manganese-based
immunotherapy (97). Additionally,
supplementation with nicotinamide riboside has been shown to
improve T cell mitochondrial function, consequently enhancing their
responsiveness to PD-1 inhibitors (98). Recent evidence further emphasizes
mitochondria as key hubs for enhancing anti-tumor immune responses
in ‘immune desert’ tumors (99).
For instance, targeted induction of mitochondrial DNA release can
activate the cyclic GMP-AMP synthase-stimulator of interferon genes
(STING) pathway, thereby sensitizing tumors to ICI therapy
(100). This opens new
perspectives for enhancing tumor immunogenicity in ESCC.
In addition, various amino acids regulate the
PD-1/PD-L1 signaling axis through different mechanisms. For
example, glutamine is abundant in EC cells and other tumor cells,
glutamine deficiency lowers intracellular glutathione levels in
tumor cells, thereby upregulating PD-L1 expression to suppress T
cells. Additionally, glutamine depletion irreversibly inhibits T
cell proliferation and cytokine secretion, creating a dual
inhibitory effect (101,102). In tryptophan metabolism, IDO+ DCs
activate Tregs, upregulate PD-L1 on DCs and enhance immune
suppression. Multiple amino acid deficiencies inhibit mTOR/Akt
signaling, activate the FOXO-PD-1 pathway in Tregs and form a
PD-1-PTEN-PI3K/Akt negative feedback loop, further reinforcing the
immune suppression state (101).
Serum metabolomic analyses indicate that elevated serum
L-tryptophan levels are associated with reduced risk of ESCC
(103). Dysregulated tryptophan
metabolism conversely promotes progression and metastasis (103). While metabolism carries out a
pivotal role in tumorigenesis and cancer progression, the
regulatory mechanisms of tryptophan metabolism within the EC
microenvironment remain largely unknown. Finally, from the emerging
perspective of metabolic intervention and immune sensitization,
serine metabolism reveals another mechanism. Serine occupies a
central position in maintaining metabolic homeostasis of cancer
cells. Research by Saha et al (104) confirmed that restricting both
endogenous and exogenous serine supply in colorectal cancer cells
induces mitochondrial dysfunction, which unexpectedly enhances
tumor sensitivity to PD-1 ICIs. This discovery not only reveals a
novel immune mechanism underlying the anti-tumor effects of serine
deprivation but also demonstrates the pronounced potential of
targeting amino acid metabolism to reverse immunotherapy
resistance. It outlines a blueprint for transitioning ESCC
treatment from ‘immune monotherapy’ to ‘multidimensional
immune-metabolic combination therapy’. Future clinical
breakthroughs in ESCC will likely emerge from combination therapies
designed against its specific metabolic vulnerabilities. By
precisely intervening in the ‘metabolic battle’ within the TME, the
full anti-tumor potential of the immune system can be unveiled and
improve patient survival outcomes.
In the TME, Treg cells, MDSCs, TAMs-M2 type,
neutrophils and CAFs cooperate to promote immune suppression
through cytokine networks (105).
In ESCC, Treg cells are central to the TME, maintaining immune
homeostasis while exerting immune suppressive effects (106). Tregs suppress immune cell function
through the secretion of immunosuppressive cytokines, such as
IL-10, IL-35 and TGF-β, and through direct cell-to-cell contact
(107). The PD-1/PD-L1 signaling
axis carries out a key role in regulating Treg differentiation and
function, specifically by reducing STAT activation in Th1 cells
through SHP-1/2 phosphatases, promoting Th1 cell to Treg
conversion. In addition, Tregs may affect the efficacy of
PD-1/PD-L1 blockade therapy (108). Additionally, the expression of
PD-1 can suppress the immunosuppressive capacity of Tregs and
promote the production of IFN-γ. Conversely, PD-L1, promotes the
conversion of naïve CD4+ T cells to Tregs by downregulating Akt,
mTOR and ERK2, and upregulating PTEN (108).
Among these secreted proteins, TGF-β can impair the
cytotoxicity of effector T cells or NK cells, while upregulating
immune checkpoints on regulatory immune cells (such as TAMs and
MDSCs) and Tregs (109). TGF-β not
only stimulates Treg differentiation and expansion but also
converts normal T cells into Tregs (107,110). Notably, blocking MDSC-derived
TGF-β can enhance the efficacy of PD-L1/PD-1 inhibition therapy and
induce an immune response from MAGE-A3+ specific CD8+ T cells in
ESCC. Therefore, combining T cell-based therapies with dual
blockade of the PD-1/PD-L1 and TGF-β signaling pathways may be a
promising cancer treatment strategy (111).
As another important immunosuppressive secretory
factor in TME, IL-6 is overexpressed in both ESCC and EAC, and its
levels associate positively with disease progression. IL-6 carries
out a key role in ESCC by upregulating PD-L1 expression (112). IL-6 inhibits DC function in
antigen presentation by activating STAT3, thereby inhibiting tumor
immune response (113). In
addition, other cytokines carry out a key role in T cell
exhaustion. For example, IL-6 recruits MDSCs in the TME, which
inhibit T cell activity via IFN-γ and promote partial CD8+ T cell
exhaustion. IL-6 released by MDSCs also interacts with IL-6/STAT1
and is associated with multidrug resistance (114). In addition, activated fibroblasts
secrete IL-6, which further promotes ESCC cell proliferation and
enhances cisplatin resistance (115). The mechanism involves IL-6
secreted by CAFs upregulating the expression of CXCR7 via the
STAT3/NF-κB pathway, thereby promoting chemotherapy resistance
(116). More importantly, IL-6
expression was positively associated with PD-L1 expression levels.
Metformin downregulates PD-L1 expression by inhibiting the
IL-6/JAK2/STAT3 signaling pathway in ESCC, thereby enhancing
antitumor immune responses (117).
Furthermore, radiation therapy considerably upregulates PD-L1
expression levels via the IL-6/STAT3 signaling pathway, suggesting
that combining anti-PD-1/PD-L1 immunotherapy with radiation therapy
may improve the treatment outcomes (118). Based on clinical and experimental
evidence, drugs targeting IL-6 signaling provide a reasonable
theoretical basis for combination therapy of patients with patients
with ICIs (119). As a
tumor-derived and systemic immune checkpoint, IL-6 can evade the
killing effects of tumor-reactive CD8+ T cells activated by ICI
therapy through tumor hijacking mechanisms. Therefore, combining
IL-6 signaling pathway inhibitors with ICIs is well supported by
clinical evidence (120).
In ESCC, IL-6 carries out a particularly prominent
role: It not only serves as a pivotal factor shaping the
immunosuppressive microenvironment but also possesses clear
clinical predictive value. Specifically, IL-6 overexpression may
serve as a potential biomarker for predicting response to PD-1
inhibitors (121); concurrently,
elevated serum IL-6 levels markedly associate with increased risk
of immune-related adverse events and poor overall patient prognosis
(122). These findings
collectively establish the key position of IL-6 in ESCC
immunotherapy and provide further rationale for the clinical
application of combination blockade strategies in this patient
population.
In the TME, various innate immune cells participate
in angiogenesis regulation through multiple molecular mechanisms.
Macrophages, N2-type neutrophils, MDSCs and masT cells not only
directly promote the formation of new blood vessels by secreting
VEGF, but also mediate the release of VEGF-A through the secretion
of MMP-9. Additionally, MDSCs produce FGF-2 and Bv8 molecules,
which, together with FGF-2, IL-8, TGF-β and TNF-α secreted by masT
cells, form a complex pro-angiogenesis signaling regulatory network
that carries out a key role in the TME (126). In turn, VEGF can inhibit the
maturation, differentiation and antigen presenting abilities of
professional APCs, such as DCs, NK cells and T cells, while
enhancing the immunosuppressive functions of Treg cells, TAMs and
MDSCs (48). Notably, VEGF
inhibition has been shown to increase the number of
tumor-infiltrating lymphocytes (127). In addition, other mediators of
angiogenesis, such as platelet-derived growth factor and FGF
signaling pathways, also carry out roles in regulating
angiogenesis, tumor growth and metastasis. Therefore, when VEGF
signaling is blocked, compensatory mechanisms may come into play,
suggesting that anti-angiogenesis therapy needs to block not only
VEGF/VEGFR signaling pathways but also other key pathways involved
in angiogenesis and tumor growth (128).
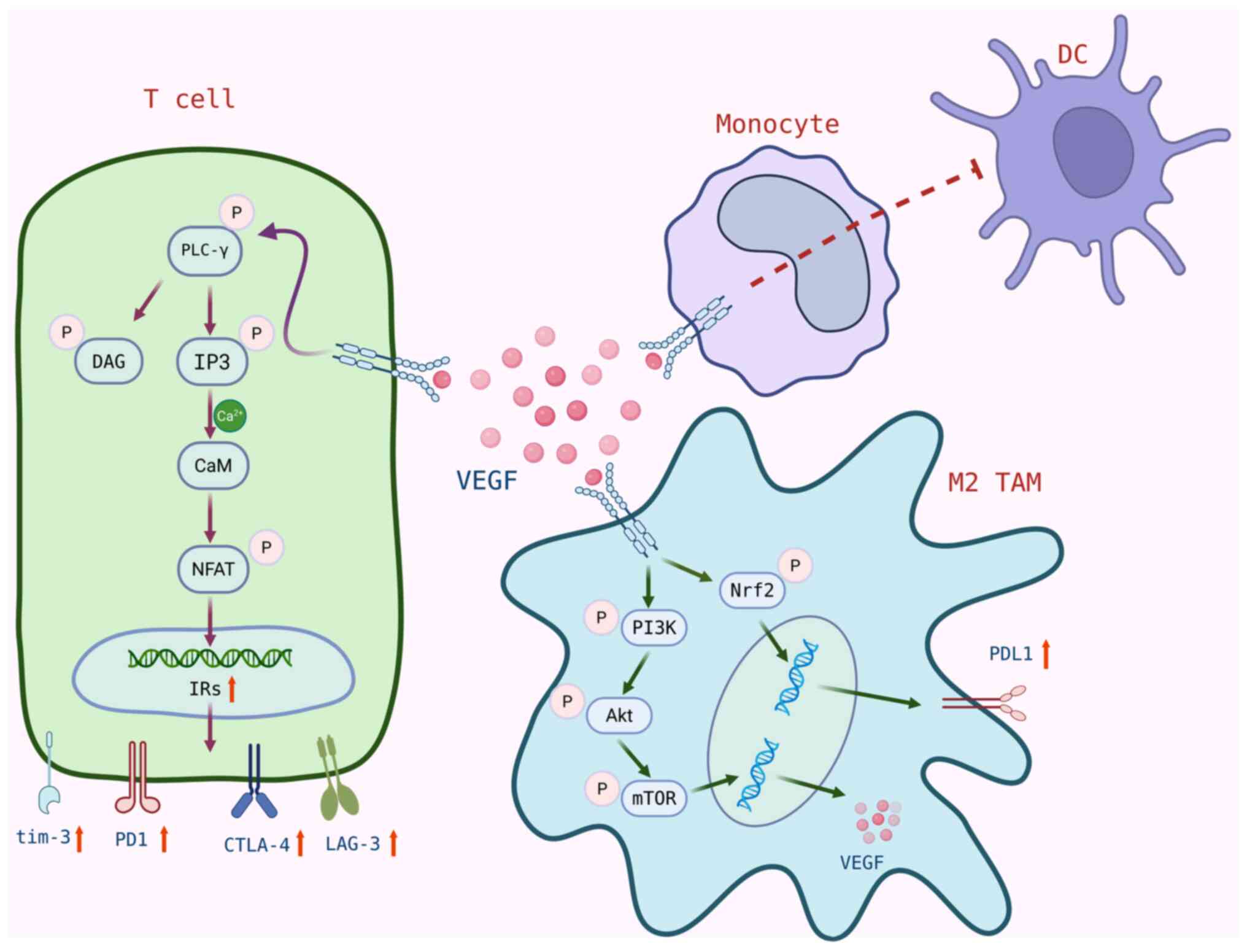
Most importantly, VEGF expression shows notable
association with EC progression and prognosis, and contributes to
improved survival outcomes in advanced ESCC and EAC (129). VEGF increases the expression of
PD-1 and other immune checkpoints (such as CTLA-4, tim-3 and Lag-3)
on CD8+ T cells via the VEGFR-2-PLCγ-calmodulin
phosphatase-activated T cell nuclear factor NFAT pathway, thereby
promoting CD8+ T cell exhaustion and facilitating tumor immune
evasion (130,131). Additionally, high expression of
VEGFR-2 is considered a poor prognostic factor in patients with
ESCC, with activation of the Akt/mTOR signaling pathway carrying
out a key role in the tumorigenesis, progression and prognosis of
ESCC cells (132). Upon activation
of VEGFR-2 on M2-type TAMs, VEGF secretion is induced via the
Akt/mTOR/PI3K pathway and PD-L1 expression is upregulated by
activation of Nrf2 (133). The
binding of VEGF/VEGFR2 also inhibits the differentiation of
monocytes into DCs and reduces DC maturation and antigen-presenting
function through inhibition of the NF-κB-mediated pathway, driving
immune evasion and promoting PD-L1 expression on DCs (134). Studies have shown that blocking
PD-L1 can reactivate DC function and enhance anticancer T cell
immune responses (135) (Fig. 3). These findings demonstrate that
anti-angiogenic agents can effectively enhance tumor
immunogenicity, thereby improving responses to immunotherapy. Zhang
et al (PMID: 31704735) reported that VEGF-A induces the
expression of the transcription factor TOX in T cells, driving
specific exhaustion transcription programs within T cells. Through
combined in vitro, ex vivo and in vivo mouse
experiments, they demonstrated that a combination of PD-1 and
VEGF-A blockade could restore the anti-tumor function of T cells
(136).
Substantial clinical investigations have been
conducted in this area. For instance, Hou et al in a
retrospective propensity-matched cohort study of 135 patients with
metastatic clear cell renal cell carcinoma, found that anti-PD1
therapy following anti-angiogenic treatment considerably improved
OS and PFS (137). In a separate
study, Lochrin et al (138)
retrospectively analyzed the clinical characteristics, treatment
and outcomes of patients with mucosal melanoma treated with Axtinib
(a selective VEGF inhibitor) with or without anti-PD-1 therapy at a
single US referral center between 2018 and 2021. They found that
78% of patients received Axtinib combined with PD-1 inhibitors,
with a median treatment duration of 3.2 months. The overall
response rate was 13%, disease control rate was 26%, median PFS was
3.2 months and median OS was 8.2 months. Overall, this regimen was
well-tolerated. Additionally, a phase II study demonstrated that
neoadjuvant toripalimab combined with Axtinib shows promise in
reducing inferior vena cava tumor thrombus staging and decreasing
the need for extensive surgery in patients with clear cell renal
cell carcinoma and inferior vena cava tumor thrombus, while also
establishing the safety profile of this combination therapy
(139). Furthermore, Atkins et
al (140) conducted a
non-randomized phase 1b clinical trial in treatment-naive patients
with advanced renal cell carcinoma, showing that the combination of
Axtinib (a selective VEGF inhibitor) and pembrolizumab (an
anti-PD-1 therapy) was safe, well-tolerated and exhibited some
anti-tumor activity. However, trials of this scale are insufficient
to demonstrate substantial clinical benefit, and further validation
in larger trials may be required. Notably, a previous case analysis
reported a patient with ESCC who experienced disease recurrence
after chemoradiotherapy and achieved a durable complete remission
confirmed by PET/CT evaluation following treatment with the PD-1
inhibitor camrelizumab combined with the anti-angiogenic agent
apatinib, with manageable treatment-related toxicity (141). However, a study in other cancer
types such as metastatic renal cell carcinoma, such as the
combination of lenvatinib with pembrolizumab compared with
sunitinib, did not show notable improvement in OS (137). These results suggest that the
clinical efficacy of combining immunotherapy with anti-angiogenic
strategies may be heterogeneous across different tumor types, drug
combinations and patient populations. Additionally, both
anti-angiogenic drugs and ICIs have their own side effect profiles,
and adverse events become more complex with combination therapy
(142). The synergistic mechanisms
between ICIs and anti-angiogenic agents have not been fully
elucidated, and identifying potential beneficiary populations for
ICI combined with anti-angiogenic therapy in ESCC and other types
of cancer remains a key clinical challenge.
TLS have a positive prognostic value in the majority
of solid tumors, carrying out a important role in creating a
microenvironment with a unique cytokine profile (143). TLS is characterized by its
temporary formation at tumor sites, rather than in secondary
lymphoid organs, and these ectopic lymphoid tissues consist mainly
of follicular DCs, B cell zones, T cell zones and high endothelial
venules (144). TLS formation
begins with the release of CXCL13 and IL-7 by stromal cells
(143). Deguchi et al
(144) showed that high TLS
presence was associated with improved prognosis in patients with
early-stage ESCC, but no notable difference was observed in
advanced cases at stage III or higher. This may be due to the
increased presence of Treg cells and M2 macrophages, which exert
immunosuppressive functions in the vicinity of TLS or locally
within the tumor. In addition, studies have found that in ESCC
immunotherapy, the maturity of TLS is markedly associated with
improved prognosis, with mature TLS (MTLS) being an independent
prognostic factor for ESCC (145,146). MTLS contribute to improved
treatment outcomes in patents with ESCC who undergo various
neoadjuvant therapies, and T cells reactivated by PD-1 inhibitors
within TLS may promote TLS maturation through interactions and
activation with B cells. As TLS matures, both humoral and adaptive
anti-tumor immune responses are effectively activated. MTLS
accelerates the spread of T cells from TLS to tumor sites,
enhancing tumor cell elimination (146).
In gastric cancer, the presence of tissue-resident
memory T (Trm) cells or TLS is associated with a favorable
prognosis (147). Hu et al
(148) demonstrated that activated
B cells enhanced the secretion of CXCL13 and granzyme B by
CD103+CD8+ Trm cells. Furthermore, the presence of TLS and
CXCL13+CD103+CD8+ Trm cells was associated with a tumor necrosis
factor receptor 2-dependent effective response to PD-1 inhibitor
treatment and CD103+CD8+Trm cells in the high TLS group showed
markedly increased PD-1 expression levels. Additionally, Zhang
et al (149) identified the
presence of HLA-A+ TLS through multiplex immunohistochemistry and
showed that as TLS matured, the resident cells within TLS gradually
expressed HLA-A. Spatially distinct tumor-infiltrating lymphocyte
(TIL)-T cells and single-cell RNA-sequencing data from 60 ESCC
tumor tissues showed through digital spatial analysis that as TLS
matured, CXCL13-expressing exhausted TIL-T cells within TLS were
reactivated and features of antigen-presenting machinery were
upregulated. Further experiments by Zhang et al (149) confirmed the presence of HLA-A+ TLS
in ESCC tumor tissues and HLA-A+ TLS, along with their major cell
components, TIL-Ts and TIL-Bs, were associated with clinical
benefits from ICI treatment in ESCC. These observations suggest
that integrating tumor mutational burden (TMB) with PD-L1
expression, TLS maturity and other multidimensional parameters may
improve ESCC patient stratification and therapeutic
decision-making. This implies that combining ICIs with agents
targeting these inhibitory cells could potentially alleviate
microenvironmental suppression and restore or enhance the
anti-tumor function of TLS, thereby offering promising prospects
for improving treatment outcomes in patients with advanced
ESCC.
TMB, as a biomarker for predicting responses to
anti-PD-1 therapy, represents the total number of mutations in all
coding regions of the tumor genome (150). Multiple studies have shown that
TMB is relatively high in locally advanced or metastatic ESCC and
that TMB is markedly associated with ORR and OS (151,152). Immunologically ‘hot’ tumors
typically exhibit (TMB-H) (TMB-H), which implies a higher load of
neoantigens and upregulation of PD-L1 expression, thereby promoting
increased TILs (153). For
example, in cancer types such as melanoma and colorectal cancer,
CD8+ TIL-T cell levels are positively associated with neoantigen
load and tumors with TMB-H are more responsive to immune checkpoint
blockade (ICB) (154). TMB-H can
optimize the spatial distribution of T cells by promoting APCs to
present neoantigens, thereby activating T cells and enhancing their
infiltration into the TME. As such, TMB was initially proposed as a
predictive biomarker for ICB responses (155,156).
However, subsequent research has shown that TMB-H is
not universally applicable to all solid tumors, including some
cases of ESCC, and its predictive value is limited. This
inconsistency can be attributed to notable differences in immune
cell infiltration density and immune activity between low-TMB and
high-TMB subtypes in these types of cancer (155,157). Concurrent chemoradiotherapy (CCRT)
can reduce TMB and induce non-specific inflammation in ESCC cells.
When ICIs are administered before or in combination with CCRT, they
may initiate and reactivate immune cells, triggering a more robust
immune response (158).
PD-1/PD-L1 ICIs have brought transformative
advances in the treatment of EC, yet their limited efficacy
highlights the need for a deeper understanding of complex
regulatory networks within the TME. The central role of the
PD-1/PD-L1 axis in mediating immune evasion in the EC has been
elucidated, conversely, PD-L1 impairs T cell function by promoting
exhaustion and apoptosis through TCR signaling; on the other hand,
the synergistic reinforcement of multiple mechanisms within the TME
leads to persistent immune evasion in ESCC, collectively resulting
in limited response rates and frequent resistance to PD-1/PD-L1
monotherapy. The TME in ESCC exhibits high heterogeneity and
multidimensional immunosuppressive characteristics, comprising
various immune cells (such as Tregs, MDSCs and TAMs) and their
secreted factors (such as TGF-β and IL-6), non-coding RNAs (such as
FOXP4-AS1 and circNF1), metabolic reprogramming (such as amino acid
metabolism and mitochondrial dysfunction), innate immune
checkpoints (such as CD47-SIRPα), angiogenic factors (such as VEGF)
and the maturation status of TLS. These multifaceted
immunosuppressive components interact and function synergistically,
forming an intricate immunosuppressive network that ultimately
undermines the therapeutic efficacy of PD-1/PD-L1 single-agent
targeting. Given the heterogeneity and complexity of the TME,
single ICB is unlikely to achieve curative outcomes. Future
research should focus on systematically characterizing the dynamic
regulatory networks within the TME. Rapid translation of these
fundamental discoveries into clinical benefits is key,
necessitating the promotion of more prospective clinical trials
exploring novel combination therapies and utilizing advanced models
such as patient-derived organoids for preclinical efficacy
evaluation. This includes exploring intelligent combinations of
PD-1/PD-L1 inhibitors with other targeted agents, such as
co-targeting additional immune checkpoints in the TME (such as
TIGIT and TIM-3), antagonizing key immunosuppressive factors (such
as TGF-β and IL-6), synergizing with anti-angiogenic drugs and
leveraging DDR pathway-targeting agents to enhance tumor
immunogenicity. Concurrently, developing targeted drug delivery
systems for specific TME components, employing IL-2 signaling to
regulate the differentiation of stem-like exhausted T cells and
creating novel PD1-IL2v fusion proteins (such as nanoformulations),
along with in-depth investigation of resistance mechanisms in
combination therapies and integration of multi-omics data with
clinical variables to build personalized immunotherapy decision
models, hold promise for gradually dismantling the immune defense
network of ESCC and offering patients more durable and effective
treatment options.
Not applicable.
This work was supported by Natural Science Foundation of Guizhou
Province [grant no. Qiankeheji-zk(2025) General Program 412].
Not applicable.
HL contributed to conceptualization, project
administration, writing-original draft, writing-review and editing.
LQ contributed to investigation, supervision, writing-review and
editing. ZLin contributed to investigation, writing-review and
editing. ZLi contributed to conceptualization, funding acquisition,
supervision, writing-review and editing. Data authentication not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263.
2024.PubMed/NCBI
|
|
2
|
Li N and Sohal D: Current state of the
art: Immunotherapy in esophageal cancer and gastroesophageal
junction cancer. Cancer Immunol Immunother. 72:3939–3952. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu S, Li K, Wang K, Liu G, Han Y, Peng L,
Chen L and Leng X: Global trends of esophageal cancer among
individuals over 60 years: An epidemiological analysis from 1990 to
2050 based on the global burden of disease study 1990–2021. Oncol
Rev. 19:16160802025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cancer Genome Atlas Research Network;
Analysis Working Group; Asan University; BC Cancer Agency; Brigham
and Women's Hospital; Broad Institute; Brown University; Case
Western Reserve University; Dana-Farber Cancer Institute; Duke
University; Greater Poland Cancer Centre, et al, . Integrated
genomic characterization of oesophageal carcinoma. Nature.
541:1692017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao Z, Wang H, Li Y, Ye S, Lin J, Li T,
Leng J, Jiang Y, Bie M and Li L: The global burden and trends of
esophageal cancer caused by smoking among men from 1990 to 2021 and
projections to 2040: An analysis of the Global Burden of Disease
2021. Eur J Med Res. 30:10432025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li J, Xu J, Zheng Y, Gao Y, He S, Li H,
Zou K, Li N, Tian J, Chen W and He J: Esophageal cancer:
Epidemiology, risk factors and screening. Chin J Cancer Res.
33:535–547. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Havel JJ, Chowell D and Chan TA: The
evolving landscape of biomarkers for checkpoint inhibitor
immunotherapy. Nat Rev Cancer. 19:133–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu W, Huo G and Chen P: Efficacy of
PD-1/PD-L1 inhibitors in advanced gastroesophageal cancer based on
characteristics: A meta-analysis. Immunotherapy. 15:751–771. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beshr MS, Shembesh RH, Salama AH, Chenfouh
I, Alfaqaih SM, Khashan A, Kara AO, Abuajamieh M, Basheer E, Ansaf
ZA, et al: PD-1/PD-L1 inhibitors in advanced, unresectable
esophageal squamous-cell carcinoma: A meta-analysis of their
effects across patient subgroups. Crit Rev Oncol Hematol.
215:1048762025. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiao R, Luo H, Xu W and Ge H: Immune
checkpoint inhibitors in esophageal squamous cell carcinoma:
Progress and opportunities. Onco Targets Ther. 12:6023–6032. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thommen DS and Schumacher TN: T cell
dysfunction in cancer. Cancer Cell. 33:547–562. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang X, He J, Ding G, Tang Y and Wang Q:
Overcoming resistance to PD-1 and CTLA-4 blockade mechanisms and
therapeutic strategies. Front Immunol. 16:16886992025. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandal K, Barik GK and Santra MK:
Overcoming resistance to anti-PD-L1 immunotherapy: Mechanisms,
combination strategies, and future directions. Molecular Cancer.
24:2462025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin Z, Zhang H, Zhang K, Yue J, Tang R,
Wang Y, Deng Q and Yu Q: Impacts of combining PD-L1 inhibitor and
radiotherapy on the tumour immune microenvironment in a mouse model
of esophageal squamous cell carcinoma. BMC Cancer. 25:4742025.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oh DY, Fong L, Newell EW, Turk MJ, Chi H,
Chang HY, Satpathy AT, Fairfax B, Silva-Santos B and Lantz O:
Toward a better understanding of T cells in cancer. Cancer Cell.
39:1549–1552. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kaushik I, Ramachandran S, Zabel C,
Gaikwad S and Srivastava SK: The evolutionary legacy of immune
checkpoint inhibitors. Semin Cancer Biol. 86:491–498. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Wang J, Deng X, Xiong F, Ge J,
Xiang B, Wu X, Ma J, Zhou M, Li X, et al: Role of the tumor
microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol
Cancer. 18:102019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bagchi S, Yuan R and Engleman EG: Immune
checkpoint inhibitors for the treatment of cancer: Clinical impact
and mechanisms of response and resistance. Annu Rev Pathol Mech
Dis. 16:223–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Yang Y, Zhao H, Tian Z, Cao Q, Li
Y, Gu Y, Song Q, Hu X, Jin M and Jiang X: Correlation of PD-L1
expression with CD8+ T cells and oxidative stress-related molecules
NRF2 and NQO1 in esophageal squamous cell carcinoma. J Pathol Clin
Res. 10:e123902024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mei Z, Huang J, Qiao B and Lam AK: Immune
checkpoint pathways in immunotherapy for head and neck squamous
cell carcinoma. Int J Oral Sci. 12:162020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiang Y, Chen M, Nie H and Yuan Y: PD-1
and PD-L1 in cancer immunotherapy: Clinical implications and future
considerations. Hum Vaccin Immunother. 15:11112019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Chen Z, Li Y, Zhao W, Wu J and
Zhang Z: PD-1/PD-L1 checkpoint inhibitors in tumor immunotherapy.
Front Pharmacol. 12:7317982021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Teng F, Kong L and Yu J: PD-L1
expression in human cancers and its association with clinical
outcomes. Onco Targets Ther. 9:5023–5039. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saller JJ, Mora LB, Nasir A, Mayer Z,
Shahid M and Coppola D: Expression of DNA Mismatch repair proteins,
PD1 and PDL1 in Barrett's neoplasia. Cancer Genomics Proteomics.
19:145–150. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Karstens KF, Kempski J, Giannou AD,
Pelczar P, Steglich B, Steurer S, Freiwald E, Woestemeier A,
Konczalla L, Tachezy M, et al: Anti-inflammatory microenvironment
of esophageal adenocarcinomas negatively impacts survival. Cancer
Immunol Immunother. 69:1043–1056. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mimura K, Teh JL, Okayama H, Shiraishi K,
Kua LF, Koh V, Smoot DT, Ashktorab H, Oike T, Suzuki Y, et al:
PD-L1 expression is mainly regulated by interferon gamma associated
with JAK-STAT pathway in gastric cancer. Cancer Sci. 109:43–53.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu J, Yin Z, Yang L, Wu F, Fan J, Huang Q,
Jin Y and Yang G: Evidence that dysplasia related microRNAs in
Barrett's esophagus target PD-L1 expression and contribute to the
development of esophageal adenocarcinoma. Aging (Albany NY).
12:17062–17078. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baba Y, Nomoto D, Okadome K, Ishimoto T,
Iwatsuki M, Miyamoto Y, Yoshida N and Baba H: Tumor immune
microenvironment and immune checkpoint inhibitors in esophageal
squamous cell carcinoma. Cancer Sci. 111:3132–3141. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kornepati AVR, Vadlamudi RK and Curiel TJ:
Programmed death ligand 1 signals in cancer cells. Nat Rev Cancer.
22:174–189. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ohaegbulam KC, Assal A, Lazar-Molnar E,
Yao Y and Zang X: Human cancer immunotherapy with antibodies to the
PD-1 and PD-L1 pathway. Trends Mol Med. 21:24–33. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
pathways: Similarities, differences, and implications of their
inhibition. Am J Clin Oncol. 39:98–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Zhang RY, Xu YF, Yue BT, Zhang JY
and Wang F: Unmasking immune checkpoint resistance in esophageal
squamous cell carcinoma: Insights into the tumor microenvironment
and biomarker landscape. World J Gastrointest Oncol. 17:1094892025.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen QY, Li YN, Wang XY, Zhang X, Hu Y, Li
L, Suo DQ, Ni K, Li Z, Zhan JR, et al: Tumor Fibroblast-Derived
FGF2 regulates expression of SPRY1 in esophageal Tumor-infiltrating
T cells and plays a role in T-cell exhaustion. Cancer Res.
80:5583–5596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shen GY, Zhang Y, Huang RZ, Huang ZY, Yang
LY, Chen DZ and Yang SB: FOXP4-AS1 promotes CD8+ T cell exhaustion
and esophageal cancer immune escape through USP10-stabilized PD-L1.
Immunol Res. 72:766–775. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang C, Ju C, Du D, Zhu P, Yin J, Jia J,
Wang X, Xu X, Zhao L, Wan J, et al: CircNF1 modulates the
progression and immune evasion of esophageal squamous cell
carcinoma through dual regulation of PD-L1. Cell Mol Biol Lett.
30:372025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Luo J, Zhang X, Liang Z, Zhuang W, Jiang
M, Ma M, Peng S, Huang S, Qiao G, Chen Q, et al: ISCU-p53 axis
orchestrates macrophage polarization to dictate immunotherapy
response in esophageal squamous cell carcinoma. Cell Death Dis.
16:4622025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wu Q, Zhang W, Wang Y, Min Q, Zhang H,
Dong D and Zhan Q: MAGE-C3 promotes cancer metastasis by inducing
epithelial-mesenchymal transition and immunosuppression in
esophageal squamous cell carcinoma. Cancer Commun (Lond).
41:1354–1372. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li J, Ozawa Y, Mozumi T, Jiang K, Taniyama
Y, Sato C, Okamoto H, Ishida H, Ujiie N, Ohnuma S, et al:
Expression of cluster of differentiation 47 (CD47) and signal
regulatory protein alpha (SIRPα) as prognostic biomarkers and
potentially therapeutic targets in esophageal squamous cell
carcinoma. Esophagus. Sep 10–2025.doi: 10.1007/s10388-025-01152-5
(Epub ahead of print). View Article : Google Scholar
|
|
39
|
Koga N, Hu Q, Sakai A, Takada K, Nakanishi
R, Hisamatsu Y, Ando K, Kimura Y, Oki E, Oda Y and Mori M: Clinical
significance of signal regulatory protein alpha (SIRPα) expression
in esophageal squamous cell carcinoma. Cancer Sci. 112:3018–3028.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao CL, Yu S, Wang SH, Li SG, Wang ZJ and
Han SN: Characterization of cluster of differentiation 47
expression and its potential as a therapeutic target in esophageal
squamous cell cancer. Oncol Lett. 15:2017–2023. 2018.PubMed/NCBI
|
|
41
|
Yuan H, Qing T, Zhu S, Yang X, Wu W, Xu K,
Chen H, Jiang Y, Zhu C, Yuan Z, et al: The effects of altered DNA
damage repair genes on mutational processes and immune cell
infiltration in esophageal squamous cell carcinoma. Cancer Med.
12:10077–10090. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei Z, Zhao N, Kuang L, Cong J, Zheng S,
Li Y and Liu Z: DNA/RNA-binding protein KIN17 supports esophageal
cancer progression via resolving noncanonical STING activation
induced by R-loop. Signal Transduct Target Ther. 10:2562025.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang H, Lu G, Hu Y, Yang Q, Jiang J and
Xu M: Wild-type p53 overexpression inhibits DNA damage pathways and
reduces PD-L1 expression in prostate cancer. J Immunother. Aug
12–2025.doi: 10.1097/CJI.0000000000000573 (Epub ahead of print).
View Article : Google Scholar
|
|
44
|
Jiang M, Jia K, Wang L, Li W, Chen B, Liu
Y, Wang H, Zhao S, He Y and Zhou C: Alterations of DNA damage
response pathway: Biomarker and therapeutic strategy for cancer
immunotherapy. Acta Pharm Sin B. 11:2983–2994. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sato H, Niimi A, Yasuhara T, Permata TBM,
Hagiwara Y, Isono M, Nuryadi E, Sekine R, Oike T, Kakoti S, et al:
DNA double-strand break repair pathway regulates PD-L1 expression
in cancer cells. Nat Commun. 8:17512017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch-repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen G, Zhu YJ, Chen J, Miao F, Wu N, Song
Y, Mao BB, Wang SZ, Xu F and Chen ZM: Mutational landscape of DNA
damage response deficiency-related genes and its association with
immune biomarkers in esophageal squamous cell carcinoma. Neoplasma.
69:1314–1321. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Genova C, Dellepiane C, Carrega P,
Sommariva S, Ferlazzo G, Pronzato P, Gangemi R, Filaci G, Coco S
and Croce M: Therapeutic implications of tumor microenvironment in
lung cancer: Focus on immune checkpoint blockade. Front Immunol.
12:7994552022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Astaneh M, Rezazadeh H, Hossein-Nataj H,
Shekarriz R, Zaboli E, Shabani M and Asgarian-Omran H: Tim-3 and
PD-1 blocking cannot restore the functional properties of natural
killer cells in early clinical stages of chronic lymphocytic
leukemia: An in vitro study. J Cancer Res Ther. 18:704–711. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu Y, Cheng Y, Xu Y, Wang Z, Du X, Li C,
Peng J, Gao L, Liang X and Ma C: Increased expression of programmed
cell death protein 1 on NK cells inhibits NK-cell-mediated
anti-tumor function and indicates poor prognosis in digestive
cancers. Oncogene. 36:6143–6153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hsu J, Hodgins JJ, Marathe M, Nicolai CJ,
Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph
HE, Thompson TW, et al: Contribution of NK cells to immunotherapy
mediated by PD-1/PD-L1 blockade. J Clin Invest. 128:4654–4668.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Beldi-Ferchiou A, Lambert M, Dogniaux S,
Vély F, Vivier E, Olive D, Dupuy S, Levasseur F, Zucman D, Lebbé C,
et al: PD-1 mediates functional exhaustion of activated NK cells in
patients with Kaposi sarcoma. Oncotarget. 7:72961–72977. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chuang CH, Guo JC, Kato K and Hsu CH:
Exploring novel immunotherapy in advanced esophageal squamous cell
carcinoma: Is targeting TIGIT an answer? Esophagus. 22:139–147.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chu X, Tian W, Wang Z, Zhang J and Zhou R:
Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy:
Mechanisms and Clinical Trials. Mol Cancer. 22:932023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Sivori S, Pende D, Quatrini L, Pietra G,
Della Chiesa M, Vacca P, Tumino N, Moretta F, Mingari MC, Locatelli
F and Moretta L: NK cells and ILCs in tumor immunotherapy. Mol
Aspects Med. 80:1008702021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yan C, Ma X, Guo Z, Wei X, Han D, Zhang T,
Chen X, Cao F, Dong J, Zhao G, et al: Time-spatial analysis of T
cell receptor repertoire in esophageal squamous cell carcinoma
patients treated with combined radiotherapy and PD-1 blockade.
Oncoimmunology. 11:20256682022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Portale F and Di Mitri D: NK Cells in
cancer: Mechanisms of dysfunction and therapeutic potential. Int J
Mol Sci. 24:95212023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zheng Y, Li Y, Lian J, Yang H, Li F, Zhao
S, Qi Y, Zhang Y and Huang L: TNF-α-induced Tim-3 expression marks
the dysfunction of infiltrating natural killer cells in human
esophageal cancer. J Transl Med. 17:1652019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Yan Z, Wang C, Wu J, Wang J and Ma T:
TIM-3 teams up with PD-1 in cancer immunotherapy: Mechanisms and
perspectives. Mol Biomed. 6:272025. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sivori S, Vacca P, Del Zotto G, Munari E,
Mingari MC and Moretta L: Human NK cells: Surface receptors,
inhibitory checkpoints, and translational applications. Cell Mol
Immunol. 16:430–441. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Gemelli M, Noonan DM, Carlini V, Pelosi G,
Barberis M, Ricotta R and Albini A: Overcoming resistance to
checkpoint inhibitors: Natural killer cells in Non-small cell lung
cancer. Front Oncol. 12:8864402022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Wang L, Chen Z, Liu G and Pan Y:
Functional crosstalk and regulation of natural killer cells in
tumor microenvironment: Significance and potential therapeutic
strategies. Genes Dis. 10:990–1004. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang H, Wang J and Li F: Modulation of
natural killer cell exhaustion in the lungs: The key components
from lung microenvironment and lung tumor microenvironment. Front
Immunol. 14:12869862023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hu W, Wang G, Huang D, Sui M and Xu Y:
Cancer immunotherapy based on natural killer cells: Current
progress and new opportunities. Front Immunol. 10:12052019.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Shimasaki N, Coustan-Smith E, Kamiya T and
Campana D: Expanded and armed natural killer cells for cancer
treatment. Cytotherapy. 18:1422–1434. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Marofi F, Abdul-Rasheed OF, Rahman HS,
Budi HS, Jalil AT, Yumashev AV, Hassanzadeh A, Yazdanifar M,
Motavalli R, Chartrand MS, et al: CAR-NK cell in cancer
immunotherapy; A promising frontier. Cancer Sci. 112:3427–3436.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Thangaraj JL, Coffey M, Lopez E and
Kaufman DS: Disruption of TGF-β signaling pathway is required to
mediate effective killing of hepatocellular carcinoma by human
iPSC-derived NK cells. Cell Stem Cell. 31:1327–1343.e5. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li T, Wang X, Niu M, Wang M, Zhou J, Wu K
and Yi M: Bispecific antibody targeting TGF-β and PD-L1 for
synergistic cancer immunotherapy. Front Immunol. 14:11969702023.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Karami Z, Mortezaee K and Majidpoor J:
Dual anti-PD-(L)1/TGF-β inhibitors in cancer immunotherapy-Updated.
Int Immunopharmacol. 122:1106482023. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Lucarini V, Melaiu O, D'Amico S, Pastorino
F, Tempora P, Scarsella M, Pezzullo M, De Ninno A, D'Oria V, Cilli
M, et al: Combined mitoxantrone and anti-TGFβ treatment with PD-1
blockade enhances antitumor immunity by remodelling the tumor
immune landscape in neuroblastoma. J Exp Clin Cancer Res.
41:3262022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Viel S, Marçais A, Guimaraes FS, Loftus R,
Rabilloud J, Grau M, Degouve S, Djebali S, Sanlaville A, Charrier
E, et al: TGF-β inhibits the activation and functions of NK cells
by repressing the mTOR pathway. Sci Signal. 9:ra192016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jia H, Yang H, Xiong H and Luo KQ: NK cell
exhaustion in the tumor microenvironment. Front Immunol.
14:13036052023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Wu Q, You L, Nepovimova E, Heger Z, Wu W,
Kuca K and Adam V: Hypoxia-inducible factors: Master regulators of
hypoxic tumor immune escape. J Hematol Oncol. 15:772022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Semenza GL: Intratumoral hypoxia and
mechanisms of immune evasion mediated by Hypoxia-inducible factors.
Physiology (Bethesda). 36:73–83. 2021.PubMed/NCBI
|
|
75
|
Zhao X, Tang YP, Wang CY, Wu JX and Ye F:
Prognostic values of STAT3 and HIF-1α in esophageal squamous cell
carcinoma. Eur Rev Med Pharmacol Sci. 23:3351–3357. 2019.PubMed/NCBI
|
|
76
|
Bai R and Cui J: Burgeoning exploration of
the role of natural killer cells in Anti-PD-1/PD-L1 therapy. Front
Immunol. 13:8869312022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ding X, Wang L, Zhang XD, Xu JL, Li PF,
Liang H, Zhang XB, Xie L, Zhou ZH, Yang J, et al: The relationship
between expression of PD-L1 and HIF-1α in glioma cells under
hypoxia. J Hematol Oncol. 14:922021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shurin MR and Umansky V: Cross-talk
between HIF and PD-1/PD-L1 pathways in carcinogenesis and therapy.
J Clin Invest. 132:e1594732022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jiang W, He Y, He W, Wu G, Zhou X, Sheng
Q, Zhong W, Lu Y, Ding Y, Lu Q, et al: Exhausted CD8+T cells in the
tumor immune microenvironment: New pathways to therapy. Front
Immunol. 11:6225092021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Su X, Zhang M, Zhu H, Cai J, Wang Z, Xu Y,
Wang L, Shen C and Cai M: Mechanisms of T-cell depletion in tumors
and advances in clinical research. Biol Proced Online. 27:52025.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xin Z, Wenyu F and Shenhua X:
Clinicopathologic significance of cytokine levels in esophageal
squamous cell carcinoma. Hepatogastroenterology. 57:1416–1422.
2010.PubMed/NCBI
|
|
82
|
Liu Y, Zhou N, Zhou L, Wang J, Zhou Y,
Zhang T, Fang Y, Deng J, Gao Y, Liang X, et al: IL-2 regulates
tumor-reactive CD8+ T cell exhaustion by activating the aryl
hydrocarbon receptor. Nat Immunol. 22:358–369. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Kwon B: The two faces of IL-2: A key
driver of CD8+ T-cell exhaustion. Cell Mol Immunol. 18:1641–1643.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Hashimoto M, Araki K, Cardenas MA, Li P,
Jadhav RR, Kissick HT, Hudson WH, McGuire DJ, Obeng RC, Wieland A,
et al: PD-1 combination therapy with IL-2 modifies CD8+ T cell
exhaustion program. Nature. 610:173–181. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Fusi I, Serger C, Herzig P, Germann M,
Sandholzer MT, Oelgarth N, Schwalie PC, Don L, Vetter VK, Koelzer
VH, et al: PD-1-targeted cis-delivery of an IL-2 variant induces a
multifaceted antitumoral T cell response in human lung cancer. Sci
Transl Med. 17:eadr37182025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Hutchinson LG, Lewin TD, Lauener L,
Martin-Facklam M, Muecke M, Teichgraeber V and Codarri Deak L:
PD-1-Cis IL-2R agonism determines the predicted pharmacological
dose range for the immunocytokine eciskafusp alfa (PD1-IL2v). CPT
Pharmacometrics Syst Pharmacol. Sep 27–2025.doi: 10.1002/psp4.70112
(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Murer P, Petersen L, Egli N, Salazar U,
Neubert P, Zurbach A, Rau A, Stocker C, Reichenstein D, Katopodis A
and Huber C: ANV600 is a novel PD-1 targeted IL-2Rβγ agonist that
selectively expands tumor antigen-specific T cells and potentiates
PD-1 checkpoint inhibitor therapy. J Immunother Cancer.
13:e0119052025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ye F, Huang J, Cheng X, Chen SC, Huang F,
Huang WC, Hua B, Li E, Jiang J, Lin H, et al: AWT020: A novel
fusion protein harnessing PD-1 blockade and selective IL-2
Cis-activation for enhanced anti-tumor immunity and diminished
toxicity. Front Immunol. 16:15374662025. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Gadwa J, Amann M, Bickett TE, Knitz MW,
Darragh LB, Piper M, Van Court B, Bukkapatnam S, Pham TT, Wang XJ,
et al: Selective targeting of IL2Rβγ combined with radiotherapy
triggers CD8- and NK-mediated immunity, abrogating metastasis in
HNSCC. Cell Rep Med. 4:1011502023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Piper M, Hoen M, Darragh LB, Knitz MW,
Nguyen D, Gadwa J, Durini G, Karakoc I, Grier A, Neupert B, et al:
Simultaneous targeting of PD-1 and IL-2Rβγ with radiation therapy
to inhibit pancreatic cancer growth and metastasis. Cancer Cell.
41:950–969.e6. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liu Y, Wang T, Ma W, Jia Z, Wang Q, Zhang
M, Luo Y and Sun H: Metabolic reprogramming in the tumor
microenvironment: Unleashing T cell stemness for enhanced cancer
immunotherapy. Front Pharmacol. 14:13277172023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kang K, Lin X, Chen P, Liu H, Liu F, Xiong
W, Li G, Yi M, Li X, Wang H and Xiang B: T cell exhaustion in human
cancers. Biochim Biophys Acta Rev Cancer. 1879:1891622024.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhu Y, Tan H, Wang J, Zhuang H, Zhao H and
Lu X: Molecular insight into T cell exhaustion in hepatocellular
carcinoma. Pharmacol Res. 203:1071612024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hu Y, Zhang Y, Shi F, Yang R, Yan J, Han T
and Guan L: Reversal of T-cell exhaustion: Mechanisms and
synergistic approaches. Int Immunopharmacol. 138:1125712024.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ma F, Li Y, Xiang C, Wang B, Lv J, Wei J,
Qin Z, Pu Y, Li K, Teng H, et al: Proteomic characterization of
esophageal squamous cell carcinoma response to immunotherapy
reveals potential therapeutic strategy and predictive biomarkers. J
Hematol Oncol. 17:112024. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chen N, Li Z, Liu H, Jiang A, Zhang L, Yan
S, He W, Yang J and Liu T: Enhancing PD-1 blockade in NSCLC:
Reprogramming tumor immune microenvironment with albumin-bound
statins targeting lipid rafts and mitochondrial respiration. Bioact
Mater. 49:140–153. 2025.PubMed/NCBI
|
|
97
|
Chen N, Yang Y, Fan L, Cai Y, Yin W, Yang
Z, Zhao Y, Chen S, Zhi H, Xue L, et al: The STING-activating
nanofactory relieves T cell exhaustion in Mn-based tumor
immunotherapy by regulating mitochondrial dysfunction. J
Nanobiotechnology. 23:4032025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yu YR, Imrichova H, Wang H, Chao T, Xiao
Z, Gao M, Rincon-Restrepo M, Franco F, Genolet R, Cheng WC, et al:
Disturbed mitochondrial dynamics in CD8+ TILs reinforce T cell
exhaustion. Nat Immunol. 21:1540–1551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ren L, Wan J, Li X, Yao J, Ma Y, Meng F,
Zheng S, Han W and Wang H: Mitochondrial rewiring with
small-molecule drug-free nanoassemblies unleashes anticancer
immunity. Nat Commun. 15:76642024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhong N, Zu Z, Lu Y, Sha X, Li Y, Liu Y,
Lu S, Luo X, Zhou Y, Tao J, et al: Mitochondria-targeted
manganese-based mesoporous silica nanoplatforms trigger cGAS-STING
activation and sensitize anti PD-L1 therapy in triple-negative
breast cancer. Acta Biomaterialia. 199:374–386. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Zheng Y, Yao Y, Ge T, Ge S, Jia R, Song X
and Zhuang A: Amino acid metabolism reprogramming: Shedding new
light on T cell anti-tumor immunity. J Exp Clin Cancer Res.
42:2912023. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Guo ZX, Ma JL, Zhang JQ, Yan LL, Zhou Y,
Mao XL, Li SW and Zhou XB: Metabolic reprogramming and
immunological changes in the microenvironment of esophageal cancer:
Future directions and prospects. Front Immunol. 16:15248012025.
View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Wu Z, Liu Z, Wang Y, Teng G, Li X, Lu T,
Hu F, Wu S, Ma G and Zhang H: A comprehensive analysis of the
tryptophan metabolism-related gene signature to predict the
prognosis of esophageal squamous cell carcinoma based on
multi-omics. Front Mol Biosci. 12:16135392025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Saha S, Ghosh M, Li J, Wen A, Galluzzi L,
Martinez LA and Montrose DC: Serine depletion promotes antitumor
immunity by activating mitochondrial DNA-mediated cGAS-STING
signaling. Cancer Res. 84:2645–2659. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Ho T and Msallam R: Tissues and tumor
microenvironment (TME) in 3D: Models to shed light on
immunosuppression in cancer. Cells. 10:8312021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhang P, Dong S, Sun W, Zhong W, Xiong J,
Gong X, Li J, Lin H and Zhuang Y: Deciphering Treg cell roles in
esophageal squamous cell carcinoma: A comprehensive prognostic and
immunotherapeutic analysis. Front Mol Biosci. 10:12775302023.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Wang WL, Chang WL, Yang HB, Chang IW, Lee
CT, Chang CY, Lin JT and Sheu BS: Quantification of tumor
infiltrating Foxp3+ regulatory T cells enables the identification
of high-risk patients for developing synchronous cancers over upper
aerodigestive tract. Oral Oncol. 51:698–703. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Cai J, Wang D, Zhang G and Guo X: The role
Of PD-1/PD-L1 axis in treg development and function: Implications
for cancer immunotherapy. Onco Targets Ther. 12:8437–8445. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Huang TX and Fu L: The immune landscape of
esophageal cancer. Cancer Commun (Lond). 39:792019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Gao Y, You M, Fu J, Tian M, Zhong X, Du C,
Hong Z, Zhu Z, Liu J, Markowitz GJ, et al: Intratumoral stem-like
CCR4+ regulatory T cells orchestrate the immunosuppressive
microenvironment in HCC associated with hepatitis B. J Hepatol.
76:148–159. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen X, Wang L, Li P, Song M, Qin G, Gao
Q, Zhang Z, Yue D, Wang D, Nan S, et al: Dual TGF-β and PD-1
blockade synergistically enhances MAGE-A3-specific CD8+ T cell
response in esophageal squamous cell carcinoma. Int J Cancer.
143:2561–2574. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Chen MF, Chen PT, Chen WC, Lu MS, Lin PY
and Lee KD: The role of PD-L1 in the radiation response and
prognosis for esophageal squamous cell carcinoma related to IL-6
and T-cell immunosuppression. Oncotarget. 7:7913–7924. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kitamura H, Ohno Y, Toyoshima Y, Ohtake J,
Homma S, Kawamura H, Takahashi N and Taketomi A:
Interleukin-6/STAT3 signaling as a promising target to improve the
efficacy of cancer immunotherapy. Cancer Sci. 108:1947–1952. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang B, Liu J, Mo Y, Zhang K, Huang B and
Shang D: CD8+ T cell exhaustion and its regulatory mechanisms in
the tumor microenvironment: Key to the success of immunotherapy.
Front Immunol. 15:14769042024. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Tong Y, Yang L, Yu C, Zhu W, Zhou X, Xiong
Y, Wang W, Ji F, He D and Cao X: Tumor-secreted exosomal lncRNA
POU3F3 promotes cisplatin resistance in ESCC by inducing fibroblast
differentiation into CAFs. Mol Ther Oncolytics. 18:1–13. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Jiang J, Xu C, Han D, Lu Y, Yang F, Wang
J, Yan X, Mu X, Zhang J, Jia C, et al: Functional heterogeneity of
cancer-associated fibroblasts with distinct neoadjuvant
immunotherapy plus chemotherapy response in esophageal squamous
cell carcinoma. Biomark Res. 12:1132024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lu Y, Xin D, Guan L, Xu M, Yang Y, Chen Y,
Yang Y, Wang-Gillam A, Wang L, Zong S and Wang F: Metformin
downregulates PD-L1 expression in esophageal squamous cell
carcinoma by inhibiting IL-6 signaling pathway. Front Oncol.
11:7625232021. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Zhou YC, Zhu HL, Pang XZ, He Y, Shen Y and
Ma DY: The IL-6/STAT3 signaling pathway is involved in
Radiotherapy-mediated upregulation of PD-L1 in esophageal cancer.
Ann Clin Lab Sci. 55:28–38. 2025.PubMed/NCBI
|
|
119
|
Huseni MA, Wang L, Klementowicz JE, Yuen
K, Breart B, Orr C, Liu LF, Li Y, Gupta V, Li C, et al: CD8+ T
cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1
immunotherapy. Cell Rep Med. 4:1008782023. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Vilgelm AE: Illuminating the mechanism of
IL-6-mediated immunotherapy resistance. Cell Rep Med. 4:1009012023.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Huang P, Zhao M, Xia J, Li H, Sun J, Li X,
Yang C, Gao G, Zhou W, Zhong M and Yong H: IL-6 is a prognostic
biomarker in patients with advanced esophageal squamous cell
carcinoma received with PD-1 inhibitors. Front Immunol.
16:15690422025. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Ma H, Zhang S, Jiao P, Ding H, Wang F,
Zhao Y, Wu J and Guo Z: Serum IL-6 predicts immunotherapy-related
adverse and outcome in advanced gastric and esophageal cancer
patients with Anti-PD-1 treatment. Front Immunol. 16:15538822025.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Li CH, Sun XJ, Niu SS, Yang CY, Hao YP,
Kou JT, Li XZ and Wang XX: Overexpression of IQGAP1 promotes the
angiogenesis of esophageal squamous cell carcinoma through the AKT
and ERK-mediated VEGF-VEGFR2 signaling pathway. Oncol Rep.
40:1795–1802. 2018.PubMed/NCBI
|
|
124
|
Liu Y, Ge Q, Xu S, Li K and Liu Y:
Efficacy and safety of anlotinib plus programmed death-1 blockade
versus anlotinib monotherapy as second or further-line treatment in
advanced esophageal squamous cell carcinoma: A retrospective study.
Front Oncol. 12:9426782022. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tamura R, Tanaka T, Akasaki Y, Murayama Y,
Yoshida K and Sasaki H: The role of vascular endothelial growth
factor in the hypoxic and immunosuppressive tumor microenvironment:
Perspectives for therapeutic implications. Med Oncol. 37:22019.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Geindreau M, Ghiringhelli F and Bruchard
M: Vascular endothelial growth factor, a key modulator of the
Anti-tumor immune response. Int J Mol Sci. 22:48712021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Zhao Y and Adjei AA: Targeting
angiogenesis in cancer therapy: Moving beyond vascular endothelial
growth factor. Oncologist. 20:660–673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Yang YM, Hong P, Xu WW, He QY and Li B:
Advances in targeted therapy for esophageal cancer. Signal
Transduct Target Ther. 5:2292020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Zhang Y, Huang H, Coleman M, Ziemys A,
Gopal P, Kazmi SM and Brekken RA: VEGFR2 activity on myeloid cells
mediates immune suppression in the tumor microenvironment. JCI
Insight. 6:e1507352021. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Bourhis M, Palle J, Galy-Fauroux I and
Terme M: Direct and indirect modulation of T cells by VEGF-A
counteracted by Anti-angiogenic treatment. Front Immunol.
12:6168372021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Chi Y, Wang F, Zhang Y, Shan Z, Tao W,
Lian Y, Xin D, Fan Q and Sun Y: Apatinib inhibits tumour
progression and promotes antitumour efficacy of cytotoxic drugs in
oesophageal squamous cell carcinoma. J Cell Mol Med. 26:1905–1917.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Shaw P, Dwivedi SKD, Bhattacharya R,
Mukherjee P and Rao G: VEGF signaling: Role in angiogenesis and
beyond. Biochim Biophys Acta Rev Cancer. 1879:1890792024.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Rahma OE and Hodi FS: The intersection
between tumor angiogenesis and immune suppression. Clin Cancer Res.
25:5449–5457. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Mayoux M, Roller A, Pulko V, Sammicheli S,
Chen S, Sum E, Jost C, Fransen MF, Buser RB, Kowanetz M, et al:
Dendritic cells dictate responses to PD-L1 blockade cancer
immunotherapy. Sci Transl Med. 12:eaav74312020. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Kim CG, Jang M, Kim Y, Leem G, Kim KH, Lee
H, Kim TS, Choi SJ, Kim HD, Han JW, et al: VEGF-A drives
TOX-dependent T cell exhaustion in anti-PD-1-resistant
microsatellite stable colorectal cancers. Sci Immunol.
4:eaay05552019. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hou Z, Lai L, Wu H, Zou B, Xu N, Zhu D,
Wang X and Zhang H: Administering immunotherapy after anti-vascular
targeted therapy improves overall survival of patients with
metastatic clear cell renal cell carcinoma. J Cancer. 15:4527–4533.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Lochrin SE, Cugliari MK, Yeh R and
Shoushtari AN: Efficacy of axitinib in a US cohort of patients with
programmed cell death protein 1-resistant mucosal melanoma.
Melanoma Res. 34:450–456. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Gu L, Peng C, Liang Q, Huang Q, Lv D, Zhao
H, Zhang Q, Zhang Y, Zhang P, Li S, et al: Neoadjuvant toripalimab
plus axitinib for clear cell renal cell carcinoma with inferior
vena cava tumor thrombus: NEOTAX, a phase 2 study. Signal Transduct
Target Ther. 9:2642024. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Atkins MB, Plimack ER, Puzanov I, Fishman
MN, McDermott DF, Cho DC, Vaishampayan U, George S, Olencki TE,
Tarazi JC, et al: Axitinib in combination with pembrolizumab in
patients with advanced renal cell cancer: A non-randomised,
open-label, dose-finding, and dose-expansion phase 1b trial. Lancet
Oncol. 19:405–415. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yan Z, Yao ZH, Yao SN, Wang HY, Chu JF,
Song M, Zhao S and Liu YY: Camrelizumab plus apatinib successfully
treated a patient with advanced esophageal squamous cell carcinoma.
Immunotherapy. 12:1161–1166. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zhou Y, Liu Z, Yu A, Zhao G and Chen B:
Immune checkpoint inhibitor combined with antiangiogenic agent
synergistically improving the treatment efficacy for solid tumors.
Immunotargets Ther. 13:813–829. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Trüb M and Zippelius A: Tertiary lymphoid
structures as a predictive biomarker of response to cancer
immunotherapies. Front Immunol. 12:6745652021. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Deguchi S, Tanaka H, Suzuki S, Natsuki S,
Mori T, Miki Y, Yoshii M, Tamura T, Toyokawa T, Lee S, et al:
Clinical relevance of tertiary lymphoid structures in esophageal
squamous cell carcinoma. BMC Cancer. 22:6992022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Wang H, Li J, Wang Y, Chen Y, Zhang W, Pan
X, Su C, Li Z, Wang L and Gu J: IgG4-mediated M2 macrophage
polarization in tertiary lymphoid structures of esophageal cancer:
Implications for immunosuppression. Front Immunol. 15:14977832025.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Huang H, Zhao G, Wang T, You Y, Zhang T,
Chen X, Dong J, Gong L, Shang X, Cao F, et al: Survival benefit and
spatial properties of tertiary lymphoid structures in esophageal
squamous cell carcinoma with neoadjuvant therapies. Cancer Lett.
601:2171782024. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Mori T, Tanaka H, Suzuki S, Deguchi S,
Yamakoshi Y, Yoshii M, Miki Y, Tamura T, Toyokawa T, Lee S, et al:
Tertiary lymphoid structures show infiltration of effective
tumor-resident T cells in gastric cancer. Cancer Sci.
112:1746–1757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Hu C, You W, Kong D, Huang Y, Lu J, Zhao
M, Jin Y, Peng R, Hua D, Kuang DM and Chen Y: Tertiary lymphoid
Structure-associated B cells enhance CXCL13+CD103+CD8+
Tissue-resident memory T-Cell response to programmed cell death
protein 1 blockade in cancer immunotherapy. Gastroenterology.
166:1069–1084. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zhang D, Jiang D, Jiang L, Ma J, Wang X,
Xu X, Chen Z, Jiang M, Ye W, Wang J, et al: HLA-A+ tertiary
lymphoid structures with reactivated tumor infiltrating lymphocytes
are associated with a positive immunotherapy response in esophageal
squamous cell carcinoma. Br J Cancer. 131:184–195. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Yarchoan M, Hopkins A and Jaffee EM: Tumor
mutational burden and response rate to PD-1 inhibition. N Engl J
Med. 377:2500–2501. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhang W, Wang P and Pang Q: Immune
checkpoint inhibitors for esophageal squamous cell carcinoma: A
narrative review. Ann Transl Med. 8:11932020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhou X, Bao W, Zhu X, Wang D, Zeng P, Xia
G, Xing M, Zhan Y, Yan J, Yuan M and Zhao Q: Molecular
characteristics and multivariate survival analysis of 43 patients
with locally advanced or metastatic esophageal squamous cell
carcinoma. J Thorac Dis. 16:1843–1853. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Indini A, Massi D, Pirro M, Roila F,
Grossi F, Sahebkar A, Glodde N, Bald T and Mandalà M: Targeting
inflamed and non-inflamed melanomas: Biological background and
clinical challenges. Semin Cancer Biol. 86:477–490. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Zheng M: Tumor mutation burden for
predicting immune checkpoint blockade response: The more, the
better. J Immunother Cancer. 10:e0030872022. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
McGrail DJ, Pilié PG, Rashid NU, Voorwerk
L, Slagter M, Kok M, Jonasch E, Khasraw M, Heimberger AB, Lim B, et
al: High tumor mutation burden fails to predict immune checkpoint
blockade response across all cancer types. Ann Oncol. 32:661–672.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Yan C, Huang H, Zheng Z, Ma X, Zhao G,
Zhang T, Chen X, Cao F, Wei H, Dong J, et al: Spatial distribution
of tumor-infiltrating T cells indicated immune response status
under chemoradiotherapy plus PD-1 blockade in esophageal cancer.
Front Immunol. 14:11380542023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Ti W, Wei T, Wang J and Cheng Y:
Comparative analysis of mutation status and immune landscape for
squamous cell carcinomas at different anatomical sites. Front
Immunol. 13:9477122022. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Chan TA, Yarchoan M, Jaffee E, Swanton C,
Quezada SA, Stenzinger A and Peters S: Development of tumor
mutation burden as an immunotherapy biomarker: Utility for the
oncology clinic. Ann Oncol. 30:44–56. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wang L, Jia YM, Zuo J, Wang YD, Fan ZS,
Feng L, Zhang X, Han J, Lyu WJ and Ni ZY: Gene mutations of
esophageal squamous cell carcinoma based on next-generation
sequencing. Chin Med J (Engl). 134:708–715. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zhang N, Shi J, Shi X, Chen W and Liu J:
Mutational characterization and potential prognostic biomarkers of
chinese patients with esophageal squamous cell carcinoma. Onco
Targets Ther. 13:12797–12809. 2020. View Article : Google Scholar : PubMed/NCBI
|