Introduction
Cancer therapies that rely on the induction of
apoptosis have long faced significant challenges due to one of the
defining hallmarks of cancer cells; their inherent ability to evade
apoptotic cell death (1).
Traditionally, cell death has been considered to occur following
two main pathways: The physiological process of apoptosis and the
pathological process of necrosis. Previously, new forms of
programmed cell deaths have been discovered, one of which being
ferroptosis. In 2012, Dixon et al (2) coined the term ‘ferroptosis’ after
discovering a form of cell death induced by an increase in reactive
oxygen species (ROS) and lipid peroxidation (LPO) (2). The Nomenclature Committee on Cell
Death later classified ferroptosis as a form of non-apoptotic cell
death, based on its unique morphological and biochemical features
(3). This type of cell death
differs from apoptosis as it does not involve cell shrinkage, and
from necrosis as it does not result in cell swelling. Additionally,
it occurs independently of caspase activation (4).
The standard treatment for malignant tumors commonly
includes chemotherapy, often combined with other approaches such as
radiotherapy and surgical resection. Both chemotherapy and
radiotherapy are intended to target rapidly dividing cancer cells
but may also affect normal proliferating cells (5). In certain cancers, such as
hepatocellular carcinoma (HCC), resistance to chemotherapy can
develop secondary to the expression of resistance-associated genes,
thereby lowering the effectiveness of conventional therapeutics
(6).
The activation of ferroptosis has emerged as a
promising cancer therapeutic strategy. Inducing this non-apoptotic
form of regulated cell death has shown effectiveness across various
cancer types, offering new avenues for overcoming chemoresistance
and improving outcomes. In the present review, the molecular
mechanisms underlying ferroptosis were first explored, then the
findings specific to different cancer types were examined, and
finally the potential of ferroptosis-based therapeutic approaches
were assessed.
Mechanisms of ferroptosis
Understanding the intricacies of this cell death
pathway requires a closer look at the molecular regulators of iron
homeostasis, antioxidant defense and lipid metabolism.
Iron metabolism
Tumor cells often exhibit iron overload, making them
prone to ferroptotic cell death. Key iron metabolism-associated
proteins such as transferrin, transferrin receptor 1 (TFR1),
ferroportin, ferritin and divalent metal transporter 1 (DMT1) are
frequently dysregulated during carcinogenesis. The regulatory role
of iron metabolism in ferroptosis is illustrated in Fig. 1.
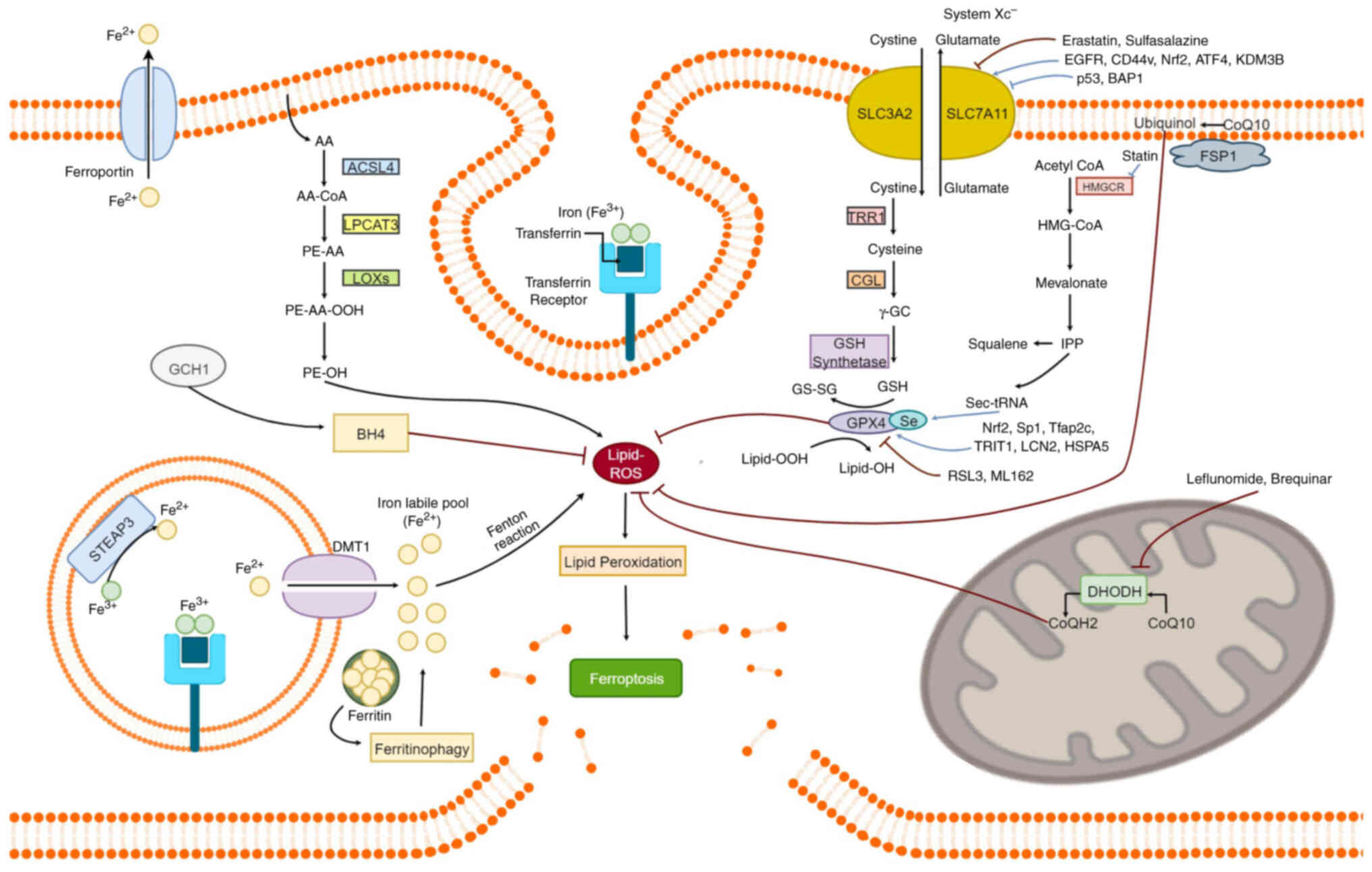 | Figure 1.Overview of key pathways and
molecular regulators involved in ferroptosis, an iron dependent
form of regulated cell death marked by the accumulation of ROS.
Iron metabolism increases the labile iron pool (Fe2+),
which drives LPO through Fenton reactions. The System
Xc− antiporter, composed of SLC7A11 and SLC3A2, imports
cystine in exchange for glutamate, enabling the synthesis of GSH, a
crucial antioxidant that supports GPX4 function. GPX4 protects
cells from ferroptosis by converting toxic lipid hydroperoxides
(Lipid-OOH) into non-toxic lipid-alcohols (Lipid-OH). Enzymes such
as ACSL4, LPCAT3 and LOXs facilitate phospholipid peroxidation,
enhancing ferroptosis. Ferritin degradation (ferritinophagy)
releases free iron, enhancing sensitivity to ferroptotic cell
death. In mitochondria, DHODH reduces CoQ to CoQH2, providing an
additional defense against lipid peroxidation, particularly when
GPX4 activity is impaired. In the cytosol, the GCH1/BH4 axis offers
a GPX4-independent antioxidant pathway, where BH4 synthesis helps
suppress lipid peroxidation and ferroptosis. Small molecules (for
example, erastin and RSL3) and regulatory proteins (for example,
FSP1, NRF2 and p53) modulate ferroptotic signaling through various
mechanisms, including GSH depletion and GPX4 inhibition. ROS,
reactive oxygen species; LPO, lipid peroxidation; GSH, reduced
glutathione; GPX4, glutathione peroxidase 4; LOX, lipoxygenase;
DHODH, dihydroorotate dehydrogenase; CoQ10, Coenzyme Q10; COX,
cyclooxygenase. |
Transferrin is a glycoprotein that transports iron
through the bloodstream in a controlled, non-toxic form (7). It plays an indirect yet significant
role in regulating ferroptosis by modulating the cellular iron
pool. Iron is delivered to cells via the interaction between
transferrin and TFR1 on the cell surface. TFR1 expression is
tightly linked to iron availability: It is upregulated under
conditions of low intracellular iron to enhance uptake, and
downregulated when iron is abundant to prevent overload (8). As such, TFR1 is a key regulator of
cellular iron homeostasis and plays a pivotal role in balancing a
cell's vulnerability or resistance to ferroptosis. Once
internalized, iron binds to ferritin, an intracellular iron-storage
protein. Accumulation of free Fe2+ in the cell,
resulting either from iron overload or from insufficient expression
or excessive degradation of ferritin, leads to the conversion of
hydrogen peroxide to hydroxyl radicals, through the Fenton
reaction, resulting in production of ROS. If cellular ROS
accumulates secondary to disruptions in the cell's antioxidant
system, it will lead to LPO, the hallmark of ferroptosis, and
subsequent cell death (9).
Ferritin is an intracellular iron-storage protein
that plays a central role in iron homeostasis by sequestering
excess iron and releasing it when needed (10). Structurally, ferritin consists of 24
subunits comprising heavy (H) and light (L) chains, which together
can store thousands of iron atoms (11). This prevents iron from participating
in Fenton reactions and minimizes oxidative stress (12). Similarly to TFR1, ferritin
expression is also linked to iron availability, as well as the
presence of certain hormonal regulators such as hepcidin (13). Elevated ferritin levels reduce the
pool of free iron and suppress ferroptosis. Conversely, low
ferritin expression increases labile iron levels, promoting Fenton
chemistry, LPO and ultimately, ferroptosis. In the context of
carcinogenesis, ferritin regulation is often disrupted. Tumor cells
frequently upregulate ferritin expression to buffer against
iron-induced oxidative stress, thereby enhancing resistance to
ferroptosis and promoting survival under iron-rich conditions
(14).
Ferroportin, a transmembrane protein that exports
iron from cells into the bloodstream to re-enter circulation,
recycles transferrin back into the bloodstream, regulating systemic
iron homeostasis. Hepcidin, a hormone synthesized by the liver,
tightly regulates ferroportin by inducing its degradation to
maintain iron homeostasis (15).
Elevated hepcidin levels lead to ferroportin internalization and
subsequent degradation resulting in reduced iron efflux and
ultimately increased ferroptosis. However, when hepcidin levels are
low, ferroportin is found more abundantly on the cell surface,
increasing iron efflux, and most importantly, decreasing the cell's
susceptibility to iron-induced stress and ferroptosis (16). Often, ferroportin is downregulated
in several types of cancer, leading to iron accumulation, and
rendering them more susceptible to ferroptosis (17). It has been reported that taking
advantage of this downregulation may provide an alternative
therapeutic strategy, leading to ferroptotic cell death in several
tumors. However, targeting ferroportin expression must be conducted
carefully as it could be a double-edged sword, potentially both
protecting and eliminating cancer cells (18).
DMT1, also known as natural resistance-associated
macrophage protein 2, is a transmembrane protein involved in the
uptake of dietary iron from the small intestine, the absorption of
iron by proliferating red blood cells, and the ejection of iron
from endosomes in numerous cell types (19). The expression of DMT1 is inversely
regulated by iron availability. Elevated iron levels lead to the
downregulation of DMT1, while low iron levels lead to its
upregulation (20). Cellular
sensitivity to ferroptosis increases when DMT1 is overexpressed.
DMT1 expression varies across tumor types with tumors having a high
demand for iron often showing DMT1 upregulation, whereas others
downregulate DMT1 as an anti-ferroptotic defense mechanism against
iron accumulation. It has been revealed that overactivation of DMT1
can promote ferroptosis in several cancers, highlighting its
potential as a therapeutic target (21).
It is now well established that ferroptosis occurs
in cells with dysregulated iron metabolism or impaired antioxidant
defense. Malfunction, mutation or dysregulation of any of the key
proteins involved in these processes can either reduce or enhance
cellular vulnerability to ferroptosis.
GSH/GPX4/System Xc−
axis
GSH and GPX4 as central antioxidant defenses
The GSH/GPX4/System Xc− axis serves as
the primary defense mechanism against ferroptosis by suppressing
the accumulation of lipid peroxides. At its core, the key
regulatory enzyme glutathione peroxidase 4 (GPX4) utilizes reduced
glutathione (GSH) to reduce lipid peroxides into non-toxic lipid
alcohols, thereby inhibiting ferroptosis (22). GSH is then regenerated from its
oxidized form (GSSG) by glutathione reductase. Therefore,
maintaining adequate intracellular GSH levels is vital for
sustaining GPX4 activity and blocking this cell death pathway
(23,24).
System Xc−
GSH biosynthesis is dependent on cystine uptake
mediated by the System Xc− amino acid antiporter, which
is expressed in various cell types, including hepatocytes,
fibroblasts and macrophages (25).
This transporter consists of two subunits, SLC3A2 and SLC7A11, and
exchanges intracellular L-glutamate for extracellular L-cystine.
Upon entry into the cell, the L-cystine molecule is reduced to
L-cysteine by thioredoxin reductase 1 (26). L-cysteine then combines with
glutamate to form γ-glutamyl cysteine, in a reaction catalyzed by
glutamate-cysteine-ligase (GCL). The subsequent addition of glycine
to the γ-glutamyl cysteine, catalyzed by the enzyme GSH synthetase,
generates the potent antioxidant GSH.
SLC7A11 regulation
The activity of the System Xc-antiporter is
primarily determined by the expression of its SLC7A11 subunit.
Elevated SLC7A11 expression promotes cystine uptake, facilitating
GSH synthesis promoting cell survival by suppressing ferroptosis.
Conversely, reduced SLC7A11 expression impairs this antioxidant
defense pathway, inducing ferroptotic cell death (27). Therefore, regulation of SLC7A11 is
critical in ferroptosis resistance (28).
Transcriptionally, transcription factors such as
activating factor 4 (ATF4) and nuclear factor erythroid 2-related
factor (NRF2) induce SLC7A11 expression under oxidative and
nutrient stress (cysteine deprivation) respectively, while in
cancer, ATF4 additionally supports tumor growth, proliferation and
angiogenesis (29–31).
Epigenetically, repressors including nuclear
deubiquitinase, anti-oncogene BRCA1-associated protein 1 and tumor
suppressor protein p53 have been shown to interact with the SCL7A11
gene promoter and repress its expression, reducing L-cysteine
uptake and increasing sensitivity to ferroptosis (32,33).
By contrast, the histone lysine demethylase 3B enzyme enhances
SLC7A11 expression by demethylating of histone 3 on lysine 9,
enhancing L-cystine uptake and resistance to ferroptosis (34).
Post-translationally, factors such as epidermal
growth factor receptor, adhesion molecule CD44v (expressed in
cancer stem cells) and deubiquitinases, stabilize SLC7A1,
protecting it from degradation and sustaining cystine uptake, thus
decreasing sensitivity to ferroptosis (35,36).
By contrast, small molecule inhibitors such as Erastin block
SLC7A11 function to induce this cell death pathway, highlighting
its potential as a therapeutic target (37).
Beyond SLC7A11, GSH synthesis is regulated by the
enzyme GCL. Inhibition of GCL with sulfonamide or disruption of its
stability reduces GSH production and sensitizes cells to
ferroptosis (38–40). Moreover, pharmacological depletion
of cysteine, such as with engineered cyst(e)inase, selectively
induces cancer cell death by reducing GSH and elevating ROS
(41). Furthermore, salvage
pathways like trans-sulfuration can maintain cysteine and GSH pools
independently of System Xc−, representing an alternative
route to ferroptosis resistance (42,43).
GPX4 regulation
Finally, the selenoprotein GPX4 itself is a key
regulatory node in ferroptosis (44). Its expression is dependent on
selenium metabolism and the mevalonate pathway, which generates the
isopentenyl pyrophosphate required for the maturation of the
selenocysteine tRNA essential for the incorporation of Sec into its
catalytic site (45–47). Blocking the mevalonate pathway with
statins, cholesterol-lowering drugs, has been shown to impair GPX4
translation and maturation, thereby increasing cell sensitivity to
ferroptosis (47). At the
transcriptional level, GPX4 is regulated by the transcription
factors such as NRF2, Sp1 and Tfap2c and mTORC, LCN2 upregulates
both SLC7A11 and GPX4 to block ferroptosis (48–51).
Post-translationally, GPX4 and its stability is
primarily regulated by three major protein modifications:
Succination (fumarate binding), alkylation (RSL3, ML162, withaferin
A) and ubiquitination, which can target it for degradation
(52–56).
Epigenetically, chaperones such as heat shock
protein A5 (HSPA5) can directly bind to GPX4, shielding it from
proteasomal degradation and thereby stabilizing the protein
(57). Similarly, lipoxygenases
contribute to GPX4 inactivation by catalyzing the oxidation of its
Sec residue, inactivating its catalytic site (58).
Moreover, some aggressive and chemo-resistant
cancers, such as HCC, have been shown to exhibit increased
sensitivity to LPO. This supports the idea that combining
ferroptosis inducers that target the GSH/System Xc− and
GPX4 axis with conventional chemotherapeutic agents may offer a
novel and effective clinical strategy for cancer treatment
(59). The regulatory role of the
GSH/GPX4/System Xc− axis in ferroptosis is illustrated
in Fig. 1.
LPO and metabolism
Ferroptosis is triggered by the accumulation of
lipid peroxides. It has been previously demonstrated that
phosphatidylethanolamines containing polyunsaturated fatty acids
(PUFAs) such as Arachidonic acid (AA) or adrenac acid (AdA), which
are embedded in the cell membrane, are susceptible to LPO (60). Chemical modifications of these
acids, particularly acylation, can alter their properties and
significantly influence the regulation of ferroptosis. The
acylation of AA/AdA is performed by two enzymes: Acyl-CoA
synthetase long-chain family member 4 (ACSL4) and
lyso-phosphatidyl-choline acyltransferase 3 (LPCAT3) (61). Inhibition of either enzyme reduces
LPO and, thus, may suppress ferroptosis. Additionally, the
ferroptosis suppressor protein 1 (FSP1) plays a vital role in
protecting cells from ferroptosis due to decreased GPX4 levels.
FSP1 catalyzes the regeneration of coenzyme Q10 (CoQ10, also known
as ubiquinone) using NADPH. The reduced form of CoQ10, ubiquinol
traps lipid peroxyl radicals, effectively limiting LPO and
preventing ferroptotic cell death (62).
Among the numerous metabolic alterations observed in
cancer cells, compared with normal; cells, are changes in lipid
metabolism, which have significant implications for ferroptotic
cell death (63). Lipid metabolism
includes fatty acid synthesis and transport, mevalonate and
cholesterol synthesis pathways, as well as several other essential
signaling cascades (64). AA and
AdA are major substrates for LPO and require the activity of two
enzymes: Elongation of very long-chain fatty acid protein 5 and
fatty acid desaturase 1, both of which are highly expressed in
certain tumors such as gastric cancer cells (65). Upregulation of these enzymes has
been shown to increase resistance to ferroptosis in these cells. On
the contrary, epigenetic silencing of these enzymes through DNA
methylation of their respective promoter regions downregulates
their expression, restoring ferroptosis sensitivity (66).
During lipid synthesis, excess glucose is funneled
into the de novo lipogenesis pathway, which enables cells to
synthesize most of their necessary fatty acids, except for a few
essential PUFAs that depend on dietary intake, namely
alpha-linoleic acid and linoleic acid (67,68).
Compared with a healthy cell, cancer cells divert glucose for
anabolic processes instead of using it for oxidative energy
production (69). In fact, nearly
all fatty acids in tumor cells are derived from endogenous
synthesis (70). This metabolic
shift suggests that cancer cells may evade ferroptosis by
upregulating de novo lipogenesis, ensuring a continuous
supply of fatty acids.
Moreover, one of the products of this pathway,
triacylglycerol, contributes to the formation of lipid droplets
(LDs), which are energy storage units implicated in cancer cell
metabolism and proliferation (71).
Certain cyclooxygenase (COX) enzymes, necessary for LPO, have been
localized within LDs and shown to enhance angiogenesis, suggesting
that elevated LD levels may play a critical role in ferroptosis
resistance (72).
In case the de novo lipid synthesis pathway
is impaired, cancer cells compensate by increasing lipid uptake.
This is mediated by the increased expression of fatty acid
transporter (FATP), fatty acid translocase (FAT/Cluster of
differentiation 36 or CD36) and fatty acid-binding proteins, which
facilitate the import of extracellular fatty acids. These
transporters are not only highly expressed in cancer cells but are
also associated with increased tumor metastasis (73). Notably, studies have shown that the
inhibition of CD36 and FATP2 has been shown to suppress tumor
growth and promote ferroptosis, highlighting their potential as
therapeutic targets (74,75).
The mevalonate pathway, responsible for cholesterol
biosynthesis, is tightly linked to the regulation of ferroptosis
and the activity of the GPX4 selenoprotein (76). Within this pathway, the enzyme
3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR) catalyzes the
conversion of HMG-CoA to mevalonate. HMGCR is frequently
upregulated in cancer cells, where it promotes their proliferation
and migration. However, inhibiting this enzyme has been shown to
increase vulnerability to ferroptosis, largely due to its
disruptive effect on the overall pathway and the subsequent
impairment of GPX4 maturation and function (77). Moreover, the use of statins inhibits
HMGCR, leading to inactivation of GPX4 and the increase in
ferroptosis occurrence overall (59). The contribution of LPO to
ferroptosis is illustrated in Fig.
1.
GPX4-independent regulation of
ferroptosis
DHODH/CoqH2 axis
Recent studies have explored the role of
dihydroorotate dehydrogenase (DHODH) in the context of ferroptosis
regulation. DHODH is a mitochondrial inner-membrane enzyme capable
of reducing CoQ to regenerate CoqH2, thus mitigating LPO and
ultimately suppressing ferroptotic activity. This axis serves as
the main mitochondrial defense against LPO (78). In addition, loss of GPX4 function is
significantly compensated by enhanced production of DHODH-mediated
CoQH2, especially in cancer cells (79). DHODH inhibitors such as leflunomide
and Brequinar have shown promising results in cancers such as HCC
where GPX4 is lowly expressed, significantly enhancing cell
sensitivity to ferroptosis and boosting therapeutic approaches
(80,81).
GCH1/BH4 axis
While the DHODH/CoqH2 axis is localized in the
mitochondria, the GCH1/BH4 axis is primarily a cytosolic pathway,
recently identified as a GPX4-independent ferroptotic regulatory
system (82).
Guanosine-5′-triphoshpate cyclohydrolase-1 (GCH1) catalyzes the
biosynthesis of tetrahydrobiopterin (BH4), a prominent antioxidant
similar in function to CoQ10. A direct link between GCH1 expression
levels and cell resistance to ferroptosis has been reported.
Indeed, inhibition of GCH1 leads to low levels of BH4 and thus
drives cells towards accumulation of LPO and eventually,
ferroptotic death (83). By
contrast, upregulation of this axis protects cells from
ferroptosis, and emerging data highlights specific pathways, such
as the RAS pathway in oncogenic signaling, capable of enhancing
GCH1 transcription and suppressing ferroptosis (84).
Altogether, the DHODH/CoQH2 axis as well as the
GCH1/BH4 axis are both GPX4-independent regulatory systems of
ferroptosis, localized within the mitochondria and the cytosol,
respectively. Emerging research focuses on their vulnerability and
targetability in cancer therapy (85).
Ferroptosis across cancers
The molecular framework of ferroptosis manifests
differently across cancer types, with distinct patterns of iron
regulation, antioxidant capacity and lipid metabolism shaping each
tumor's response to ferroptotic stress. In the present review,
cancers where ferroptosis has been more extensively investigated
(for example, HCC, lung, breast, bladder and gastrointestinal
cancers) were prioritized, while noting that studies in ovarian,
cervical and thyroid cancers remain comparatively limited. To
provide a focused and comprehensive overview rather than an
exhaustive survey, HCC and bladder cancer were selected as
representative examples. HCC was chosen given the extensive body of
research available, whereas bladder cancer was included to
highlight a tumor type in which ferroptosis is less well explored.
Together, these examples capture both established and emerging
perspectives on ferroptosis-related vulnerabilities.
Bladder cancer
Mazdak et al (86) reported that patients with bladder
cancer had significantly lower serum iron levels compared with
healthy controls, suggesting that reduced free and serum iron may
play a role in promoting bladder cancer development. Indeed,
reduced levels of free iron have been shown to markedly promote the
growth of bladder cancer cells (87). However, treatment with baicalin, a
bioactive flavonoid compound, has been shown to elevate
intracellular chelated iron and generate ROS, promoting ferroptosis
in vitro and in vivo. To further elucidate the
underlying mechanisms, the expression of iron regulatory protein
and DNA damage-related proteins was assessed using western
blotting. Results showed increased transferrin, phosphorylated
histone H2AX, p53 and tumor suppressor p53 binding protein 1
levels, alongside a notable decrease in ferritin heavy chain 1
(FTH1) levels in baicalin-treated bladder cancer cell lines 5637
and KU-19-19. Interestingly, the use of ferroptosis inhibitors
reversed this effect, confirming that this is the predominant cell
death pathway (88).
Proteins that regulate ferroptosis, such as GPX4 and
SLC7A11, also play important roles in tumor-related signaling
pathways, particularly in bladder cancer development. SLC7A11
appears to be controlled by the deubiquinating enzyme OTUB1, which
is overexpressed in human bladder cancer and contributes to
ferroptosis resistance (36). In
vivo and in vitro experimental studies revealed that
deleting OTUB1 lowers SLC7A11 levels, decreases cystine uptake, and
induces ferroptosis, indicating that targeting SLC7A11 may
contribute to inhibition of bladder cancer progression (87,89).
Furthermore, ferroptosis in bladder cancer cells can
also occur due to a collapse of the antioxidant defense system and
ROS accumulation. Following treatment with erianin, elevated levels
of ROS and reduced GSH levels were reported. Additionally,
malondialdehyde (MDA) levels increased, and ferrous iron
accumulated in the bladder cancer cells, all of which drive
ferroptosis (90). Western blot
analysis further revealed that erianin significantly downregulated
several key ferroptosis-associated proteins, including NRF2, FTH1,
GPX4, heme oxygenase 1 (HO-1), glutaminase and xCT/SLC7A11 in
KU-19-19 and RT4 bladder cancer cells (91). Collectively, the downregulation of
these specific proteins impairs the cell's ability to maintain a
redox balance resulting in ferroptotic death.
Finally, recent research explores the crosstalk
between autophagy and ferroptosis in bladder cancer. FIN56, a
potent ferroptosis inducer through the degradation of GPX4 and
ferritin, was found to be enhanced when combined with Torin 2, an
autophagy activator. Interestingly, FIN56-induced ferroptosis was
suppressed by bafilomycin A1 and SAR405, both of which attenuated
LPO (92). These findings confirm
that ferroptosis may also occur dependent on autophagy,
specifically ferritin (ferritinophagy). A summary of
key-ferroptosis related vulnerabilities and therapeutic approaches
in this type of cancer is presented in Table I.
 | Table I.Cancer type-specific ferroptosis
vulnerabilities and therapeutic strategies. |
Table I.
Cancer type-specific ferroptosis
vulnerabilities and therapeutic strategies.
| Cancer type | Key
vulnerabilities | Ferroptosis
inducers | Major molecular
changes | Sensitization
strategies |
|---|
| Bladder | GSH/GPX4 depletion,
autophagy-dependence, iron/ROS imbalance | Baicalin, Erianin,
FIN56, RSL3 | ↓Serum iron;
↑ROS/Fe2+/MDA; ↑Tf/p53/p-H2AX/p53BP1;
↓FTH1/GPX4/SLC7A11/NRF2/HO-1/GLS; OTUB1-mediated ↑SLC7A11 | GSH/GPX4 depletion;
SLC7A11/OTUB1 suppression; autophagy-dependent ferritinophagy |
| HCC | GSTZ1-NRF2-GPX4
axis, PSAT1 regulation | Sorafenib,
Trigonelline, Punicallin | ↓GSTZ1,
↑SLC7A11/PSAT1 (context-dependent) | NRF2 targeting,
PSAT1/SLC7A11 inhibition, cysteine salvage blocking |
| NSCLC | High SLC7A11 and
GPX4 expression | Erastin, Sorafenib,
Cisplatin (combos) | ↑SLC7A11
(RBMS1-driven), GPX4 (mTORC1-mediated) | RBMS1 knockout,
mTORC1 inhibition, cisplatin combos |
| TNBC | Selenocysteine
biosynthesis (GPX4/SLC7A11) | RSL3,
Sulfasalazine | ↑GPX4 and SLC7A11
(selenocysteine-rich) | miR-5096
overexpression to target SLC7A11 |
|
Gastric/Colorectal | GPX4/SLC7A11
overexpression | Erastin | GPX4/SLC7A11
overexpression | CDO1 silencing to
sensitize cells |
| PDAC | Nrf2-mediated
GPX4/GSH, apoptosis resistance | Gemcitabine +
Erastin (with LONP1) | Gemcitabine
↑Nrf2/GPX4; LONP1 inhibits Nrf2/GPX4 | LONP1 + Erastin to
block GPX4 |
| Leukemia | Iron/ROS overload,
GSH/GPX4 and SLC7A11 dependence, lipid ROS stress | RSL3, FIN56, ML385,
BL-8040, Erastin (±BV6) | ↑Iron/ROS,
↑GSH/GPX4/SLC7A11/NRF2/FSP1; LPO; immune suppression | GSH/GPX4 and
SLC7A11 suppression; NRF2 inhibition; cysteine salvage disruption;
lipid ROS exploitation |
HCC
The primary therapeutic approach for inducing
ferroptosis in HCC cells involves targeting the system
Xc− antiporter and the GPX4 selenoprotein (93). Sorafenib, a multikinase inhibitor,
is commonly used as a first-line treatment for advanced HCC and has
been shown to stimulate ferroptosis (94). However, due to the chemorefractory
nature of HCC, the emergence of resistance-related genes limits its
efficacy. Upon treatment with sorafenib, dysregulation of several
transcription factors such as NRF2, retinoblastoma (Rb) protein,
hepatocyte nuclear factor 4α (HNF4α), HIC ZBTB Transcriptional
Repressor 1 (HIC1), as well as components of the Hippo pathway, can
be observed. A well-documented link exists between sorafenib and
the NRF2 signaling axis. By inhibiting system Xc−,
sorafenib depletes intracellular GSH, prompting compensatory
activation of the NRF2 pathway. Moreover, using trigonelline to
inhibit this pathway appears to enhance the antitumor effect of
sorafenib (95). Additionally, NRF2
upregulates the expression of several iron metabolism-related genes
such as HO-1, Metallothioenin-1G and Sigma-1 receptor, all of which
contribute to suppression of ferroptosis (96–98).
Previous studies have also elucidated the role of glutathione
S-transferase zeta 1 (GSTZ1), an enzyme involved in the
phenylalanine catabolism, which is markedly downregulated in HCC
cells (99). GSTZ1 does suppress
the NRF2 pathway, resulting in decreased GPX4 expression and
increased sensitivity of HCC cells to ferroptosis (100).
The tumor suppressor proteins Rb, HNF4α and HIC1 all
influence ferroptotic sensitivity via the regulation of the
phosphoserine aminotransferase 1 (PSAT1) gene, which plays a
critical role in GSH metabolism. Louandre et al (101) revealed that when Rb is inhibited,
cells are more susceptible to ferroptosis. Similarly, HNF4α and
HIC1 regulate PSAT1 in opposing ways, affecting redox homeostasis
and are therefore interesting targets for overcoming sorafenib
resistance (102). Additionally,
metabolic regulators such as c-Jun have also been associated with
higher levels of expression of PSAT1, thus conferring resistance to
sorafenib (103,104). Finally, dysregulation of the Hippo
pathway has been associated with the overexpression of SCL7A11
subunit of system Xc−, promoting the activity of ATF4
and further increasing resistance to ferroptosis (105).
Beyond these transcription factors, other mechanisms
present a clinical challenge to treatment of liver cancer. For
instance, the enzyme branched-chain amino acid transferase 2
(BCAT2) suppresses ferroptosis by maintaining intracellular GSH
levels (106). Similarly,
ATP-Binding Cassette Subfamily C Member 5 (ABCC5) is overexpressed
in HCC cells and may function analogously to BCAT2 by stabilizing
the expression of SLC7A11 (107).
On the other hand, inhibition of ABCC5 leads to GSH depletion and
marked reduction in sorafenib resistance (108). Additionally, RNA-binding proteins
such as DAZ-Associated protein 1 are upregulated in HCC cells and
contribute to ferroptosis resistance by enhancing SCL7A11 stability
at the transcriptional level. Besides that, micropinocytosis of
cysteine and trans-sulfuration pathways replenish intracellular
cysteine and contribute to GSH synthesis even in the presence of
system Xc− inhibitors, rendering cells resistant to
sorafenib-induced ferroptosis (109). A previous study has discussed the
protective role of the trans-sulfuration pathway that maintains
primary hepatocytes viability for several days, despite the absence
of cystine/cysteine in the medium (110).
Novel therapeutic approaches focus on targeting
proteins involved in these resistance pathways. Recently, a study
by Y. Chen et al (111)
elucidated the correlation of GPX4 with the phosphor-seryl-tRNA
kinase (PSTK) in HCC. Inhibition of PSTK was shown to reduce GSH
metabolism, thereby decreasing GPX4 activity and sensitizing HCC
cells to ferroptosis inducers such as erastin. Furthermore,
inhibition of the PSTK gene with punicalin, an agent used to
treat hepatitis B virus, a major risk factor for HCC development,
and Geraniin, a plant-derived therapeutic compound, resulted in
cytotoxic effects on HCC cells. Additionally, combination therapy
involving punicalin or geraniin with ferroptosis inducers like
sorafenib and erastin exhibited a synergistic effect, enhancing
cancer cell death without causing damage to surrounding organs such
as the kidney, intestine or lung in mouse models. These findings
underscore the therapeutic potential of concurrently targeting the
GPX4/GSH/System Xc− axis alongside conventional
chemotherapeutics as a strategy for overcoming drug-resistant HCC.
The major ferroptosis-related vulnerabilities mechanisms and
therapeutic strategies in this cancer are highlighted in Table I.
Other solid tumors
While ferroptosis offers a promising approach for
the treatment of liver and bladder cancer, it is also important to
highlight its potential in other malignancies such as lung,
gastrointestinal and breast cancers. In non-small cell lung cancer
(NSCLC), SLC7A11 subunit of the system Xc− antiporter is
highly expressed, promoting metastasis and reducing ROS levels by
facilitating cysteine uptake (112). This overexpression is attributed
to RNA-binding protein RBMS1 that directly enhances transcription
by stabilizing SLC7A11 mRNA. Knockout of this protein led to a
reduction in cysteine uptake and promoted ferroptosis (113). In parallel, the discovery of novel
microRNAs (miRNAs) has gained attention for therapeutic targeting.
These small RNAs bind to the 3′-untranslated region of mRNAs,
leading to its degradation. Notably, inhibition of miR-27a-3p
enhances the sensitivity of NSCLC cells to ferroptosis inducers
such as erastin (114). Alongside
SLC7A11, GPX4 also appears to be highly expressed due to enhanced
activation of the mTORC1 signaling pathway (115). Inhibition of this pathway leads to
downregulation of GPX4 as well as increased sensitivity to
lapatinib, a tyrosine kinase inhibitor that potentiates oxidative
stress and leads to ferroptosis (116). Although cisplatin is commonly used
as a first-line therapy, NSCLC cells quickly develop resistance.
Consequently, combination strategies involving low doses of
ciplastin with sorafenib or erastin have shown promise. These
combinations suppress the NRF2/SLC7A11 pathway, amplifying the
anticancer efficacy of cisplatin (117).
Similarly, in triple-negative breast cancer (TNBC),
both GPX4 and SLC7A11 are overexpressed, largely due to the
selenophilic nature of most breast cancer cells, which increases
Selenocysteine (Sec) biosynthesis and thus protects cells against
ferroptosis (118). Ferroptosis
resistance in TNBC is usually overcome by inhibiting GPX4 activity
with treatment of RSL3 or sulfasalazine (SAS) (119) or by overexpression miR-5096 which
specifically targets SLC7A11 transcription (120). Gastric and colorectal cancer also
exhibit similar levels of GPX4 and SLC7A11 overexpression (121,122) and thus high levels of ferroptosis
resistance. In gastric cancer, it has been shown that silencing of
the CD01 gene strengthens the adaptation of cancer cells to
oxidative stress, conferring resistance to erastin and promoting
proliferation (123). On the other
hand, poor pancreatic ductal adenocarcinoma prognosis is often
attributed to the lack of early diagnosis and resistance to
apoptosis-inducers, namely gemcitabine (124). Upon treatment with gemcitabine,
NRF2-mediated GPX expression increases and intracellular GSH levels
are elevated, which can subsequently be targeted by
ferroptosis-inducers for improved therapeutic outcomes (125). Previously, it has been reported
that mitochondrial protease Lon peptidase 1 is capable of
inhibiting NRF2-mediated GPX4 expression, thus promoting induced
ferroptosis when used in conjunction with erastin (126). In summary, the GSH/GPX4/System
Xc− axis plays a crucial role in ferroptosis across
numerous cancer types, presenting itself as a target for
ferroptosis-based therapies aimed at overcoming drug resistance in
cancer cells (127). Key
ferroptosis-associated vulnerabilities and sensitization strategies
across these cancer types are summarized in Table I.
Leukemia
Beyond solid tumors, leukemic cells display
dysregulated iron metabolism, leading to iron overload and
increased ROS production, which in turn disrupt normal
hematopoietic stem cell (HSC) functions like self-renewal,
differentiation and quiescence (128,129). Excess ROS drive further cell
apoptosis and reduce tumorigenic potential, while iron accumulation
reshapes the tumor microenvironment in ways that trigger
ferroptosis in cancer cells (130,131). However, leukemia cells take
advantage of iron overload to fuel their rapid proliferations by
relying on iron-dependent ribonucleotide reductase, an enzyme
required for DNA synthesis (132–134). In addition, iron buildup induced
apoptosis in nearby CD4+ T cells, CD8+ T
cells and natural killer cells, while expanding regulatory T cell
populations, thereby enabling leukemic cells to escape immune
surveillance (135,136). Moreover, unlike solid tumors,
patients with hematological malignancies often undergo repeated
blood transfusions due to chemotherapy and impaired erythropoiesis,
resulting in elevated iron and ROS levels. This, in turn, can
promote the tumorigenic transformation of HSCs by exhausting NADPH
oxidase and GSH (130). Together,
these findings highlight iron metabolism as a double-edged sword in
leukemia, as modulating iron homeostasis alters vulnerability of
leukemic cells to ferroptosis, while disease progression further
exacerbates iron overload in patients (137).
Furthermore, leukemia cells exhibit abnormally high
levels of GSH and GPX4, both of which are key suppressors of
ferroptosis (138). Moreover,
increased GPX4 expression has been associated with a poor prognosis
in patients with acute myeloid leukemia (AML) (139,140). Studies have shown that modulation
of GSH and GPX4 is critical for controlling cell death and
proliferation across leukemia subtypes (141). Indeed, GPX4 inhibitors (FIN56 or
RSL3) combined with the NRF2 inhibitor ML385 synergistically
trigger ferroptosis in AML cells (142). Similarly, blocking GSH synthesis
by BL-8040 has been shown to induce ferroptosis in T-cell acute
lymphoblastic leukemia (ALL) cells by promoting oxidative stress
(143). Conversely, supplementing
chronic lymphocytic leukemia cells with GSH or its precursor
N-acetylcysteine markedly improved cell viability, highlighting the
role of GSH in regulating leukemia cell proliferation through
ferroptosis (144). Collectively,
these findings underscore the role of GSH and GPX4 as key
regulators of ferroptosis in across leukemia subtypes, highlighting
the therapeutic potential of targeting the GSH/GPX4 axis to
resistance to conventional treatments (138).
SLC7A11 has been found to be frequently
overexpressed in leukemia and regulate cell proliferation by
regulating ferroptosis (138,145). Inhibiting SLC7A11, genetically or
chemically, leads to cysteine deprivation and reduces AML cell
viability and self-renewal by promoting ferroptosis, while
upregulation of SLC7A11 by the small molecule drug APR-246 enhances
cystine uptake and detoxifies lipid peroxides, protecting cells
from ferroptotic death (146,147). Nevertheless, leukemia cells can
bypass system Xc− inhibition via the trans-sulfuration
pathway to maintain cysteine and GSH levels, which highlights a key
metabolic resistance mechanism to ferroptosis (138).
In addition, dysregulated lipid metabolism and
oxidative stress in leukemic cells promotes lipid ROS accumulation
(148), which leads to LPO, a
hallmark of ferroptosis and a biomarker in patients with leukemia
(149). In ALL cells,
ferroptosis-mediated inhibition of leukemia growth by the combining
RSL3 and BV6 depends on lipid ROS and is preventable with the LPO
inhibitor Fer-1 (150). By
contrast, leukemic cells counteract this stress by activating
antioxidant defenses such as NRF2, SLC7A11 and FSP1, which
neutralize lipid ROS and shield cells from ferroptosis (138). Together, these findings underscore
ferroptosis as a critical vulnerability in leukemia, with iron
metabolism, antioxidant defenses and lipid ROS influencing disease
progression and presenting potential therapeutic targets.
Therapeutic strategies
Exploiting cancer-specific vulnerabilities to
ferroptosis has inspired the development of therapies that harness
this cell death pathway, from targeting metabolism to small
molecule inducers and combination treatments.
Monotherapy approaches
Targeting metabolic pathways
Iron metabolism modulation
Iron metabolism is frequently dysregulated in
various cancers, including breast cancer, pancreatic cancer,
lymphoma and HCC, making iron metabolism-related genes critical
targets for stimulating ferroptosis (151). The use of artesunate, an
antimalarial drug, has been shown to increase lysosomal activity
and iron concentration in cells (152). Additionally, artesunate has been
shown to influence the transcriptional regulation of iron-related
genes (153). One of the
upregulated genes is the nuclear receptor coactivator 4 (NCOA4)
gene, a selective cargo receptor that mediates degradation of
ferritin by autophagy, in a process termed ferritinophagy, and thus
increases cellular iron concentration and induces ferroptosis
(154). Cisplatin, another
chemotherapeutic agent, targets ferritin degradation in a similar
fashion by upregulating NCOA4 and contributing to GSH depletion,
leading to an increase in intracellular iron concentration and
reduced GPX4 activity, ultimately triggering ferroptosis (155). In addition to classical
chemotherapeutics, natural products such as Baicalin, derived from
the traditional Chinese medicinal herb Scutellaria
baicalensis has also been shown to influence iron metabolism
and promote ferroptosis. Baicalin facilitates iron buildup and LPO,
both critical for triggering ferroptosis. Treatment of osteosarcoma
cells with this natural compound has been shown to enhance iron
accumulation, ROS generation and MDA production, while decreasing
the ratio of GSH to GSSG (156).
However, different cancer types may respond
differently to modulators of iron metabolism. For example, some
tumors may develop resistance to iron overload caused by cisplatin
by upregulating ferroportin or activating NRF2 to strengthen
antioxidant defenses. Additionally, off-target ferroptosis in cells
with naturally high levels of iron may also occur, causing
undesirable systemic oxidative stress and posing a significant
clinical challenge. The role of iron modulation in
ferroptosis-based therapy is outlined in Fig. 2.
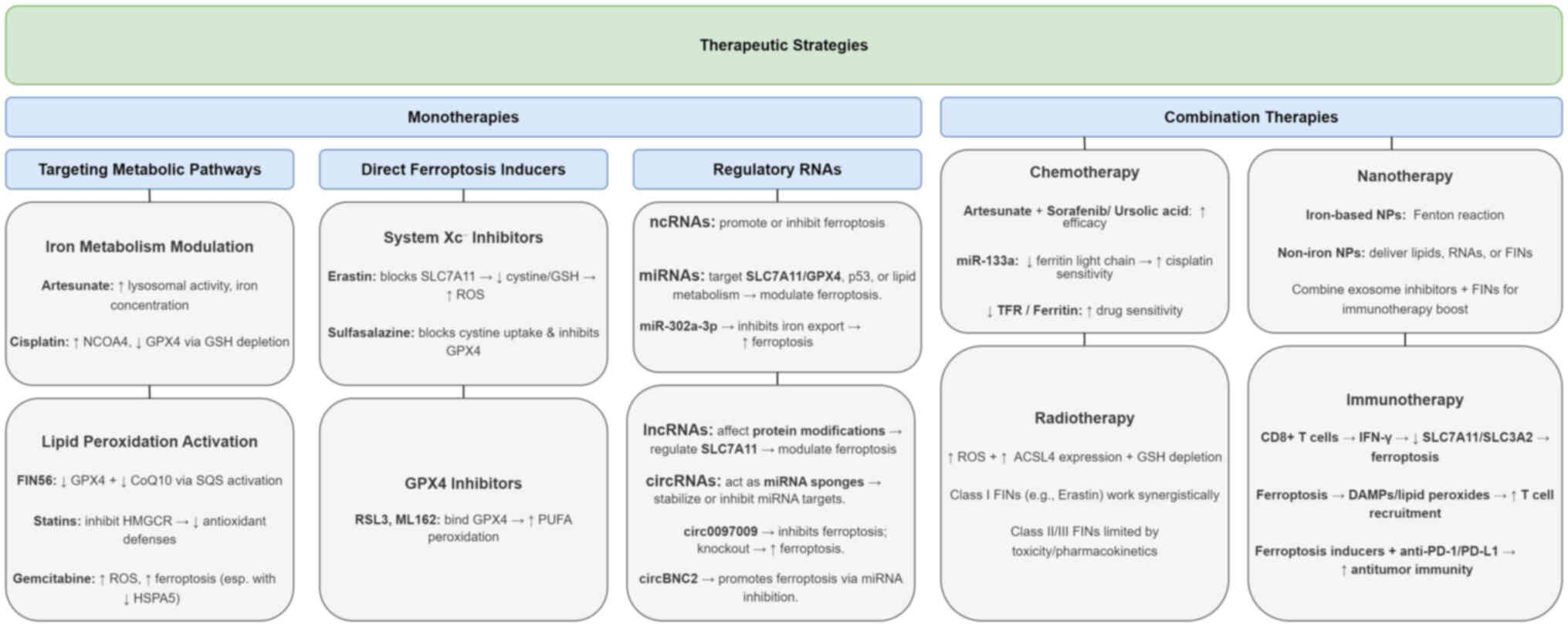 | Figure 2.Key therapeutic approaches to induce
ferroptosis in cancer cells can be classified into two main
categories: monotherapies and combination therapies. Monotherapies
can be further categorized as: i) targeting metabolic pathways,
including modulation of iron metabolism and activation of LPO; ii)
using direct ferroptosis inducers, such as inhibitors of System
Xc− or GPX4; and iii) employing regulatory RNAs, such as
miRNAs, lncRNAs and circRNAs. Combination therapies include
strategies that synergize with chemotherapy, radiotherapy,
immunotherapy, or nanotechnology-based approaches. LPO, lipid
peroxidation; GPX4, glutathione peroxidase 4; miRNAs or miRs,
microRNAs; lncRNAs, long non-coding RNAs; circRNAs, circular RNAs;
ROS, reactive oxygen species; GSH, reduced glutathione; NCOA4,
nuclear receptor coactivator 4; CoQ10, Coenzyme Q10; HMGCR,
3-hydroxy-3-methylglutaryl-CoA reductase; HSPA5, heat shock protein
family A (Hsp70) member 5. |
Activation of LPO
LPO may occur in two distinct pathways. The
non-enzymatic pathway involves the Fenton Reaction, where hydrogen
peroxide reacts with ferrous ions (Fe2+) to generate
hydroxyl radicals, contributing to oxidative damage and ferroptosis
(157). Alternatively, the
enzymatic pathway engages lipoxygenases (LOX), cytochrome p450
oxidoreductase (POR) and COX enzymes, all of which trigger
ferroptosis by facilitating ROS production (158). LOX enzymes catalyze the oxidation
of PUFAs in membrane phospholipids and may be transcriptionally
regulated by p53 to promote ferroptosis (159). Furthermore, POR donates electrons
from NADPH to downstream effectors such as cytochrome p450,
reducing them in the process and allowing the peroxidation of
membrane-bound PUFAs, which may eventually trigger ferroptotic cell
death (160).
Certain ferroptosis inducers, such as FIN56, act
through dual mechanisms: Promoting GPX4 degradation and activating
squalene synthase, which exhausts levels of CoQ10, a coenzyme
involved in the mevalonate pathway, thereby sensitizing cells to
ferroptosis (89). Alternatively,
pharmacological inhibition of this pathway through statins, which
block HMGCR, has also shown potential anticancer therapies by
impairing antioxidant defenses and enhancing ferroptotic
susceptibility (161).
Therapeutic strategies may also involve targeting
lipid metabolism in cancer cells. Specifically, modulating LPO
through enzymes such as COX, LOX and cytochrome POR presents a
promising avenue for inducing ferroptosis. Gemcitabine, an
antimetabolite that disrupts DNA replication and is commonly used
in treating pancreatic cancers, causes ROS accumulation and
stimulates ferroptosis (162).
Interestingly, the anticancer effect of gemcitabine is enhanced
when HSPA5 is unable to bind to and stabilize GPX4, leading to its
degradation and eventual ferroptosis (57).
Furthermore, erianin, a bioactive compound extracted
from Dendrobium chrysotoxum Lindl, has been reported to
trigger ferroptosis by iron-dependent LPO in multiple cancer cell
types. It has been shown to facilitate the buildup of harmful
lipid-based ROS and deplete GSH, a key cellular antioxidant,
thereby inducing oxidative stress and cell death, which are
hallmarks of ferroptosis (91,163–165). Moreover, it has been shown to
elevate intracellular Fe2+ concentrations, which
catalyze ROS generation via the Fenton reaction, further enhancing
LPO and ferroptotic cell death. It has also been reported to
suppress the expression of ferroptosis-protective proteins,
including GPX4 and SLC7A11. Since GPX4 plays a critical role in
detoxifying lipid peroxides, its downregulation results in the
accumulation of toxic lipid peroxides (163–165). The role of LPO activation in
ferroptosis-based therapy in cancer is summarized in Fig. 2.
Nonetheless, a major limitation of this approach
lies in the complexity and heterogeneity of lipid metabolism across
different tumor types. Some cancers may evade ferroptosis by
enriching their membranes with monounsaturated fatty acids instead
of PUFAs, reducing LPO susceptibility. Furthermore, the frequent
mutation of p53 in cancer cells can impair its ability to activate
pro-ferroptotic axis pathways, further complicating treatment
efforts.
Direct ferroptosis inducers
System Xc− inhibitors
Erastin is a small molecule compound capable of
inducing ferroptotic cell death by binding to and irreversibly
inhibiting the SLC7A11 subunit of System Xc− antiporter.
This inhibition reduces cystine uptake, resulting in diminished GSH
biosynthesis. Without GSH, GPX4 becomes non-functional, allowing
toxic lipid ROS to accumulate within the cell, causing oxidative
stress and ultimately, ferroptosis. Additionally, erastin has been
shown to interact with voltage-dependent anion channels on the
outer membrane of mitochondria, further contributing to ROS
production (166). In various
cancer types, including HCC, erastin sensitizes cells to
ferroptosis and shows promise as a potent anticancer therapeutic.
However, its clinical utility is limited to poor solubility,
metabolic instability and potential toxicity to healthy tissues,
particularly the kidneys (167).
To address these challenges, researchers are developing erastin
analogs and exploring advanced delivery systems, such as
nanotechnology and exosome-based encapsulation (168).
SAS, another ferroptosis inducer, similarly blocks
cystine uptake and inhibits GPX4. Nevertheless, its use in cancer
therapy is constrained by significant off-target effects, including
inhibition of the NF-KB signaling pathway and alterations in immune
cell function, which may raise concerns about safety and
specificity. These mechanisms of action are summarized in Fig. 2.
GPX4 inhibitors
When cancer cells develop resistance to System
Xc− inhibitors, targeting downstream effectors such as
GPX4 becomes a valuable alternative. RSL3 and ML162 are small
molecule inhibitors that directly bind to the Sec active site of
GPX4, blocking its lipid peroxide-reducing activity. As a result,
PUFAs undergo unchecked peroxidation, ultimately stimulating
ferroptosis (169). Although
structurally distinct, RSL3 and ML162 function in a similar fashion
and thus share similar limitations, including chemical instability
and broad off-target effects that may hinder regular cell function,
especially in neurons and kidneys. Much like the System
Xc− inhibitors, GPX4 inhibitors also suffer from low
solubility and rapid inactivation. Additionally, cancer cells may
develop resistance by altering membrane lipid composition to reduce
PUFA content and limit the effects of both RSL3 and ML162, or by
upregulating ferroptosis-suppressing proteins. One such protein is
FSP1, which is overexpressed in some cancers. FSP1 acts as an
oxidoreductase that generates reduced CoQ10, thereby preventing LPO
through a pathway parallel to the GSH/GPX4 axis. In a mouse model
using ferroptosis-resistant H460 lung cancer cell xenografts, FSP1
inhibition leads to significant tumor suppression in both GPX4
knockout and GPX4 KO/FSP1 groups (170). The aforementioned therapeutic
mechanisms are summarized in Fig.
2.
Regulatory RNAs: miRNAs, long
non-coding RNAs (lncRNAs) and circular RNAs (circRNAs)
Non-coding RNAs (ncRNA) are an increasingly
interesting topic for researchers as they have recently been shown
to regulate ferroptosis in cancer cells, having both stimulating
and inhibitory effects depending on the nature of the ncRNA. For
instance, miRNAs have been identified to target either SLC7A11 or
GPX4 and enhance or suppress ferroptosis (171). Other miRNAs may target the p53
signaling pathway or even regulate lipid metabolism and are capable
of greatly enhancing cell sensitivity to ferroptosis. It has been
recently reported that miR-302a-3p interacts with the
3′-untranslated region of FPN 1 and directly enhances ferroptosis
in NSCLC by inhibiting cellular iron export (172). Furthermore, lncRNAs also regulate
post-translational modifications of RNA-binding proteins through
numerous various mechanisms: Ubiquitination, acetylation and
phosphorylation. These modifications both directly and indirectly
modulate ferroptosis by affecting levels of specific protein
expression and degradation, notably the SLC7A11 protein (173). Finally, circRNAs may also play a
pivotal role in modulating ferroptotic sensitivity, as they often
act as sponges for miRNAs, stabilizing them and keeping them from
reaching their targets (174). In
a previous study, a specific circRNA, circ0097009, was found to be
overexpressed in HCC serving as a sponge for miR-1261, whose target
is the SLC7A11 subunit. Knockout of circ0097009 lead to decreased
expression of SLC7A11, inactivation of GPX4 and a decrease in
GSH/GSSG ratio, all of which result in increased sensitivity to
ferroptosis (175). Additionally,
some circRNA may sponge miRNA that would normally inhibit
ferroptosis. A recent study reported that exogenous delivery of
circBNC2 lead to increased LPO and ROS levels in prostate cancer,
greatly increasing cell sensitivity to ferroptosis by inhibiting
microRNA-4298 (176).
Combination therapy approaches
Synergy with chemotherapy
Currently, several effective strategies have been
identified to reverse tumor resistance, primarily by targeting iron
and lipid metabolism, two key regulators to ferroptosis (64). A major contributing factor to drug
resistance is the suppression of ferroptosis-related genes and
signaling pathways in tumor cells (177). For instance, clinical agents such
as artesunate have been shown to act synergistically with
sorafenib, enhancing its antitumor efficacy (178). Likewise, co-administration of
ursolic acid with sorafenib enhances its therapeutic effect
(179).
In the context of iron metabolism, a correlation
between tumor drug resistance and TFR expression has been recently
suggested, with evidence indicating that the downregulation of TFR
can help overcome resistance (180). In multiple myeloma cells, elevated
ferritin levels are considered to be associated to bortezomib
resistance, while iron supplementation promotes cell death. By
contrast, a reduction in ferritin has been shown to sensitize tumor
cells to bortezomib (181).
Additionally, miR-133a has been found to downregulate the ferritin
light chain subunit in cisplatin- and doxorubicin-resistant breast
cancer cells, increasing their sensitivity to the drugs (182). A summary of these mechanisms is
provided in Fig. 2.
In terms of lipid metabolism, cells with high GSH
expression levels and upregulation of the SCL7A11 light chain
subunit of System Xc− have been linked to resistance to
both radiation and chemotherapy (183). Moreover, numerous cancer cells
develop a dependency on the GPX4 enzyme, which contributes
massively to its acquired drug resistance. Loss of GPX4 function
renders cells more susceptible to ferroptosis and has been shown to
prevent tumor relapse in mouse models (184).
Synergy with radiation therapy
Radiation therapy may induce ferroptosis by
promoting oxidase activity via the production of highly reactive
hydroxyl radicals (185). It
triggers ferroptosis through three primary mechanisms: Producing
excess ROS that drive LPO, upregulating ACSL4 expression to enhance
polyunsaturated phospholipid biosynthesis, and depleting GSH,
thereby inhibits the protective effects of GPX4 against LPO
(61,186).
Tumor sensitivity to radiation therapy can be
increased by combining radiation therapy with ferroptosis inducers.
For example, class I FINs such as erastin and SAS inhibit cystine
uptake, while class II FINs like RSL3 and ML162 directly inhibit
GPX4. Class III FIN, such as FIN56, degrade GPX4 and deplete CoQ10
(64). Research indicates that the
combination of radiation therapy with ferroptosis inducers
significantly increases the radiosensitivity of NSCLC, yielding
improved outcomes than radiotherapy alone (187). Among the different classes of
FINs, class I FINs show the most promising synergistic effect with
radiotherapy, particularly through targeting SLC7A11. By contrast,
class II and III FINs face limitations due to poor pharmacokinetics
and higher cytotoxicity, which reduce their overall therapeutic
benefit in combination treatments (185). A summary is illustrated in
Fig. 2.
Synergy with immunotherapy
Emerging research suggests potential therapeutic
interplay between ferroptosis and immunotherapy. CD8+ T
cells secrete interferon-γ which in turn inhibits expression of
both the SLC7A11 and SLC3A2 subunits of the System Xc-, sensitizing
the cell to ferroptotic death (188). In turn, the lysed tumor cells
release damage-associated molecular patterns as well as LPO
products, enhancing recruitment of T-cells in a positive-feedback
manner to amplify antitumor immunity (189). Furthermore, with the rise of
immune checkpoint inhibitors as a viable therapeutic avenue,
combining both ferroptosis inducers with anti-PD-1/PD-L1 therapies
greatly enhance the antitumor response by reversing immune
suppression within the tumor microenvironment (190). In a recent study, mefloquine, an
antimalarial, was revealed to not only induce ferroptosis in
melanoma and lung cancer cells, but also sensitize them to both
ferroptosis and anti-PD-1 immunotherapy when combined with
T-cell-derived interferon-γ. Mefloquine was reported to
significantly upregulate LPCAT3, a key gene in LPO-associated
ferroptosis, highlighting how LPO can also boost the efficacy of
checkpoint blockade immunotherapy (191). The combined effective delivery of
both ferroptosis inducers as well as immune checkpoint inhibitors
remains a significant hurdle, as much of recent research focuses on
optimizing efficient delivery to maximize antitumor effects
(192,193).
Synergy with nanotherapy
Ferroptosis-based nanotherapies are currently under
development as a promising approach in cancer treatment.
Nanomaterials used in this context are generally categorized as
either iron-based or non-iron based (64). Iron-based nanoparticulate materials
can induce ferroptosis by releasing iron at the tumor site, where
it catalyzes the Fenton reaction to generate ROS (194). These nanoparticles can also be
engineered to carry ferroptosis inducers or chemotherapeutic
agents, such as erastin, to circumvent its poor solubility and
off-target effects (168,195).
Iron free or non-iron based nanotherapies operate
through alternative mechanisms, such as delivering lipids,
non-coding RNAs, or ferroptosis-inducing compounds (196). For instance, nanoparticle
formulations can supplement PUFAs, modulating LPO and promoting
ferroptosis in tumor cells (197).
Additionally, tumor cells release exosomal PD-L1, which suppresses
T-cell activity, blocking immune checkpoint responses, and
contributing to therapy resistance (198). By combining exosome inhibitors
with ferroptosis inducers into a single nanotherapeutic unit,
researchers aim to harness both immune activation and ferroptotic
cell death. This strategy represents a novel and promising
direction in immunotherapy (187).
Despite their potential, ferroptosis-based nanotherapies remain
largely experimental. Further research, optimization and clinical
validation are needed to fully realize their therapeutic potential
in cancer treatment. A summary of these strategies is presented in
Fig. 2.
Discussion
Accumulation of ROS and LPO, resulting from an
imbalance in the cellular redox system, can trigger ferroptosis, a
form of programmed cell death. Over time, different tumor types
have become more aggressive and resistant to conventional therapies
such as surgical resection and chemotherapy, leading to poor
prognosis. Given the uncontrolled proliferation of cancer cells, it
is expected that, over the course of repeated treatment, some will
acquire mutations that confer resistance, mirroring the principles
of natural selection, where the fittest variants survive.
The emergence of drug-resistant tumors remains a
major challenge in oncology. However, since the initial discovery
of ferroptosis, mounting evidence has supported its potential as an
effective antitumor mechanism. Inhibition of key components of the
GPX4/GSH/System Xc− axis, along with related pathways
such as the mevalonate and trans-sulfuration pathways, has
demonstrated the ability to induce ferroptosis in various cancer
models.
Although clinical data on ferroptosis induction
remain limited, they are steadily increasing. For example, SAS,
which inhibits system Xc−, is currently being tested
against solid tumors and glioblastoma in clinical trials
(NCT04205357). Moreover, carbon nanoparticle-loaded iron
[CNSI-Fe(II)] is under clinical investigation as a
ferroptosis-inducing therapy for patients with advanced solid
tumors (NCT06048367).
Despite these promising findings, translating
preclinical results into clinical practice has been hindered by
challenges such as poor pharmacokinetics and significant side
effects observed with classical inducers. For instance, erastin
exhibits poor solubility and metabolic stability as observed in a
mouse liver microsome assay and, upon intraperitoneal
administration in mice, induces ferroptosis-associated alterations
in multiple organs, indicating potential toxicity to normal tissues
(167). Moreover, RSL3 at
tumoricidal doses is cytotoxic to normal cells such as neurons
(199), fibroblasts (200) and nephrocytes (201) and shows poor selectivity and
pharmacokinetics due to covalent binding to GPX4 via a reactive
alkyl chloride moiety (202).
Therefore, future studies should focus on enhancing
drug distribution, reducing systemic toxicity, and improving the
pharmacokinetics of currently used ferroptosis inducers. Moreover,
just as tumors evolve resistance to chemotherapy, they may also
adapt to evade ferroptosis. For example, prolonged treatment with
sorafenib has been associated with the overexpression of
resistance-related genes (203),
highlighting the need to understand the mechanisms underlying
acquired ferroptosis resistance and tumor heterogeneity.
To address these challenges, efforts are ongoing to
develop combination therapies, ranging from nanotechnology-based
approaches that improve drug solubility and reduce resistance
(204), to co-treatment with
traditional compounds such as artesunate alongside ferroptosis
inducers targeting the GPX4/GSH/System Xc− axis.
Additional strategies involve disrupting aberrant iron or lipid
metabolism in tumor cells to indirectly destabilize redox balance
and promote ferroptotic cell death.
Looking ahead, future research should prioritize
establishing rational combination regimens that optimize efficacy
and identifying predictive biomarkers to stratify patients most
likely to respond to ferroptosis-based therapies. Ultimately,
translating this promising strategy from bench to bedside will
require the development of next generation ferroptosis inducers
with enhanced selectivity and safety profiles, alongside
longitudinal clinical investigations to evaluate long-term
outcomes.
Recently, ferroptosis has been the focus of
numerous reviews, spanning both broad and disease-specific
perspectives. Broad overviews, such as those by Singh et al
(205), Ojo et al (206) and Ubellacker et al
(207) survey the underlying
mechanisms and general therapeutic potential of ferroptosis. By
contrast, disease-focused reviews include Hino et al
(208) on liver disease and HCC,
Meng et al (209) on
melanoma, Hsu et al (210)
on exosomal ncRNAs in lung cancer, and Chen et al (211) on digestive cancers and therapy
resistance. While collectively informative, these reviews are
either mechanistic surveys at a broad level or limited to single
cancer contexts, leaving a gap for integrative perspectives that
explicitly connect ferroptosis mechanisms to therapeutic resistance
across multiple malignancies.
By contrast, the present review employs a focused
yet comparative approach. In particular, HCC was selected, a cancer
well-characterized in ferroptosis research, and bladder cancer,
where ferroptosis is comparatively understudied, as representative
models. This dual focus enables us to merge well-established and
emerging findings, highlight research gaps and outline directions
for future research. In addition, rather than examining molecular
regulators in isolation as previous reviews have, our approach
associates iron metabolism, antioxidant defenses and LPO with drug
resistance, presenting a translational framework that bridges
molecular insights with clinical applications. Finally, the present
review evaluates therapeutic strategies, including monotherapies
such as ferroptosis inducers and regulatory RNAs, and synergistic
combinations with chemotherapy, radiotherapy, immunotherapy and
nanotherapy. Collectively, these points underscore the novel
insights and distinctive contribution of our manuscript relative to
prior reviews.
In conclusion, the present review highlights the
promising potential of combination therapies aimed at inducing
ferroptosis as a novel approach for cancer treatment, particularly
in drug-resistant tumors. Combining ferroptosis inducers with
existing treatments such as chemotherapy, radiotherapy and more
recently, nanotherapy, has shown encouraging results. Nonetheless,
further research is necessary given that most studies to date are
relatively recent and primarily conducted in xenograft models. In
summary, current findings suggest that targeting pathways involved
in iron metabolism, lipid metabolism and the GPX4/GSH/System
Xc− axis holds significant therapeutic promise. As
research in this field progresses, ferroptosis-based combination
strategies continue to emerge as a compelling new avenue for cancer
therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
NM drafted the initial manuscript. NEJ restructured
the manuscript's organization and ideas, created the figures and
table, and performed proofreading. RAH and MES wrote the final
draft and edited the manuscript. Data authentication is not
applicable. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, artificial
intelligence tools were used to improve the readability and
language of the manuscript, and subsequently, the authors revised
and edited the content produced by the artificial intelligence
tools as necessary, taking full responsibility for the ultimate
content of the present manuscript.
Glossary
Abbreviations
Abbreviations:
|
AA
|
arachidonic acid
|
|
ABCC5
|
ATP-binding cassette subfamily C
member 5
|
|
ACSL4
|
acyl-CoA synthetase long-chain family
member 4
|
|
AdA
|
adrenic acid
|
|
AML
|
acute myeloid leukemia
|
|
ATF4
|
activating transcription factor 4
|
|
BCAT2
|
enzyme branched-chain amino acid
transferase 2
|
|
BH4
|
tetrahydrobiopterin
|
|
CD36
|
cluster of differentiation 36 or
fatty acid translocase
|
|
circRNA
|
circular RNA
|
|
CoQ10
|
Coenzyme Q10
|
|
COX
|
cyclooxygenase
|
|
DHODH
|
dihydroorotate dehydrogenase
|
|
DMT1
|
divalent metal transporter 1
|
|
FATP
|
fatty acid transporter
|
|
FSP1
|
ferroptosis suppressor protein 1
|
|
FTH1
|
ferritin heavy chain 1
|
|
GCH1
|
guanosine-5′-triphosphate
cyclohydrolase-1
|
|
GCL
|
glutamate-cysteine ligase
|
|
GSH
|
reduced glutathione
|
|
GSSG
|
oxidized glutathione
|
|
GSTZ1
|
glutathione S-tranferase zeta 1
|
|
HCC
|
hepatocellular carcinoma
|
|
HIC1
|
HIC ZBTB transcriptional repressor
1
|
|
HMGCR
|
3-hydroxy-3-methylglutaryl-CoA
reductase
|
|
HNF4α
|
hepatocyte nuclear factor 4α
|
|
HO-1
|
heme oxygenase 1
|
|
HSC
|
hematopoietic stem cell
|
|
HSPA5
|
heat shock protein family A (Hsp70)
member 5
|
|
LD
|
lipid droplets
|
|
lncRNA
|
long-non-coding RNA
|
|
LOX
|
lipoxygenase
|
|
LPCAT3
|
lysophosphatidylcholine
acyl-transferase 3
|
|
LPO
|
lipid peroxidation
|
|
MDA
|
malondialdehyde
|
|
miRNA
|
microRNA
|
|
NCOA4
|
nuclear receptor coactivator 4
|
|
ncRNA
|
non-coding RNA
|
|
NRF2
|
nuclear factor erythroid 2-related
factor 2
|
|
NSCLC
|
non-small cell lung cancer
|
|
OTUB1
|
OTU deubiquitinase
|
|
POR
|
cytochrome P450 oxidoreductase
|
|
PSAT1
|
phosphoserine aminotransferase 1
|
|
PSTK
|
phosphor-seryl-tRNA kinase
|
|
PUFAs
|
polyunsaturated fatty acids
|
|
Rb
|
retinoblastoma
|
|
ROS
|
reactive oxygen species
|
|
SAS
|
sulfasalazine
|
|
Sec
|
selenocysteine
|
|
TFR1
|
transferrin receptor 1
|
|
TNBC
|
triple-negative breast cancer
|
References
|
1
|
Sharma A, Boise LH and Shanmugam M: Cancer
metabolism and the evasion of apoptotic cell death. Cancers
(Basel). 11:11442019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM and Yang
WS: Ferroptosis: An Iron-dependent form of nonapoptotic cell death.
Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galluzzi L, Vitale I, Aaronson SA, Abrams
JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews
DW, et al: Molecular mechanisms of cell death: Recommendations of
the Nomenclature Committee on Cell Death 2018. Cell Death Differ.
25:486–541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Čepelak I, Dodig S and Dodig DČ:
Ferroptosis: Regulated cell death. Arh Hig Rada Toksikol.
71:99–109. 2020.PubMed/NCBI
|
|
5
|
Debela DT, Muzazu SG, Heraro KD, Ndalama
MT, Mesele BW, Haile DC, Kitui SK and Manyazewal T: New approaches
and procedures for cancer treatment: Current perspectives. SAGE
Open Med. 9:205031212110343662021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kishi Y, Hasegawa K, Sugawara Y and Kokudo
N: Hepatocellular carcinoma: Current management and future
development-improved outcomes with surgical resection. Int J
Hepatol. 2011:7281032011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yiannikourides A and Latunde-Dada GO: A
short review of iron metabolism and pathophysiology of iron
disorders. Medicines (Basel). 6:852019.PubMed/NCBI
|
|
8
|
Camaschella C, Nai A and Silvestri L: Iron
metabolism and iron disorders revisited in the hepcidin era.
Haematologica. 105:260–272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhao Z: Iron and oxidizing species in
oxidative stress and Alzheimer's disease. Aging Med (Milton).
2:82–87. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kotla NK, Dutta P, Parimi S and Das NK:
The role of ferritin in health and disease: Recent advances and
understandings. Metabolites. 12:6092022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Knovich MA, Storey JA, Coffman LG, Torti
SV and Torti FM: Ferritin for the clinician. Blood Rev. 23:95–104.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abbaspour N, Hurrell R and Kelishadi R:
Review on iron and its importance for human health. Iran J Pediatr.
19:164–174. 2014.
|
|
13
|
Fillebeen C, Charlebois E, Wagner J,
Katsarou A, Mui J, Vali H, Garcia-Santos D, Ponka P, Presley J and
Pantopoulos K: Transferrin receptor 1 controls systemic iron
homeostasis by fine-tuning hepcidin expression to hepatocellular
iron load. Blood. 133:344–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Obeagu EI and Tanko MSM: Iron metabolism
in breast cancer: Mechanisms and therapeutic implications: A
narrative review. Ann Med Surg (Lond). 87:3403–3409. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh B, Arora S, Agrawal P and Gupta S:
Hepcidin: A novel peptide hormone regulating iron metabolism. Clin
Chim Acta. 412:823–830. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nemeth E and Ganz T: The role of hepcidin
in iron metabolism. Acta Haematol. 122:78–86. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan Z, Wei Z and Shaikh ZA: Suppression
of ferroportin expression by cadmium stimulates proliferation, EMT,
and migration in Triple-negative breast cancer cells. Toxicol Appl
Pharmacol. 356:36–43. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Belvin BR and Lewis JP: Ferroportin
depletes iron needed for cell cycle progression in head and neck
squamous cell carcinoma. Front Oncol. 12:10254342023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grillo AS, SantaMaria AM, Kafina MD,
Cioffi AG, Huston NC, Han M, Seo YA, Yien YY, Nardone C, Menon AV,
et al: Restored iron transport by a small molecule promotes
absorption and hemoglobinization in animals. Science. 356:608–616.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skjørringe T, Burkhart A, Johnsen K and
Moos T: Divalent metal transporter 1 (DMT1) in the brain:
Implications for a role in iron transport at the blood-brain
barrier, and neuronal and glial pathology. Front Mol Neurosci.
8:192015.PubMed/NCBI
|
|
21
|
Song Q, Peng S, Sun Z, Heng X and Zhu X:
Temozolomide drives ferroptosis via a DMT1-dependent pathway in
glioblastoma cells. Yonsei Med J. 62:8432021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ursini F and Maiorino M: Lipid
peroxidation and ferroptosis: The role of GSH and GPx4. Free Radic
Biol Med. 152:175–185. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aquilano K, Baldelli S and Ciriolo MR:
Glutathione: New roles in redox signaling for an old antioxidant.
Front Pharmacol. 5:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lu SC: Glutathione synthesis. Biochim
Biophys Acta. 1830:3143–3153. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu M, Zhu W and Pei D: System Xc-: A key
regulatory target of ferroptosis in cancer. Invest New Drugs.
39:1123–1131. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lewerenz J, Hewett SJ, Huang Y, Lambros
MP, Gout PW, Kalivas PW, Massie A, Smolders IJ, Methner A, Pergande
M, et al: The cystine/glutamate antiporter system x(c)(−) in health
and disease: From molecular mechanisms to novel therapeutic
opportunities. Antioxid Redox Signal. 18:522–555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Koppula P, Li Z and Gan B: Cystine
transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient
dependency, and cancer therapy. Protein Cell. 12:599–620. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee J and Roh J: SLC7A11 as a gateway of
metabolic perturbation and ferroptosis vulnerability in cancer.
Antioxidants (Basel). 11:24442022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma Q: Role of NRF2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kilberg MS, Shan J and Su N:
ATF4-dependent transcription mediates signaling of amino acid
limitation. Trends Endocrinol Metab. 20:436–443. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen D, Fan Z, Rauh M, Buchfelder M,
Eyupoglu IY and Savaskan NE: ATF4 promotes angiogenesis and
neuronal cell death and confers ferroptosis in a xCT-dependent
manner. Oncogene. 36:5593–5608. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang Y, Shi J, Liu X, Li F, Gong Z,
Koppula P, Sirohi K, Xu L, Wei Y, Lee H, et al: BAP1 links
metabolic regulation of ferroptosis to tumour suppression. Nat Cell
Biol. 20:1181–1192. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang Y, Lu Y, Zhang X, Cui W, Liu Y, Sun
Q, He Q, Zhao S, Zhang G, Wang Y, et al: Epigenetic regulation of
ferroptosis by H2B monoubiquitination and p53. EMBO Rep.
20:e475632019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Y, Zhao Y, Wang H, Zhang C, Wang M,
Yang Y, Xu X and Hu Z: Histone demethylase KDM3B protects against
ferroptosis by upregulating SLC7A11. FEBS Open Bio. 10:637–643.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsuchihashi K, Okazaki S, Ohmura M,
Ishikawa M, Sampetrean O, Onishi N, Wakimoto H, Yoshikawa M,
Seishima R, Iwasaki Y, et al: The EGF receptor promotes the
malignant potential of glioma by regulating amino acid transport
system xc(−). Cancer Res. 76:2954–2963. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu T, Jiang L, Tavana O and Gu W: The
deubiquitylase OTUB1 mediates ferroptosis via stabilization of
SLC7A11. Cancer Res. 79:1913–1924. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dixon SJ, Patel DN, Welsch M, Skouta R,
Lee ED, Hayano M, Thomas AG, Gleason CE, Tatonetti NP, Slusher BS
and Stockwell BR: Pharmacological inhibition of cystine-glutamate
exchange induces endoplasmic reticulum stress and ferroptosis.
Elife. 3:e025232014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gao M, Monian P, Quadri N, Ramasamy R and
Jiang X: Glutaminolysis and transferrin regulate ferroptosis. Mol
Cell. 59:298–308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang WS, Sriramaratnam R, Welsch M,
Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji
AF, Clish CB, et al: Regulation of ferroptotic cancer cell death by
GPX4. Cell. 156:317–331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yang Y, Lin J, Guo S, Xue X, Wang Y, Qiu
S, Cui J, Ma L, Zhang X and Wang J: RRM2 protects against
ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell
Int. 20:162020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cramer SL, Saha A, Liu J, Tadi S, Tiziani
S, Yan W, Triplett K, Lamb C, Alters SE, Rowlinson SW, et al:
Systemic depletion of L-cyst(e)ine with cyst(e)inase increases
reactive oxygen species and suppresses tumor growth. Nat Med.
23:120–127. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sbodio JI, Snyder SH and Paul BD:
Regulators of the transsulfuration pathway. Br J Pharmacol.
176:583–593. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hayano M, Yang W, Corn CK, Pagano NC and
Stockwell BR: Loss of cysteinyl-tRNA synthetase (CARS) induces the
transsulfuration pathway and inhibits ferroptosis induced by
cystine deprivation. Cell Death Differ. 23:270–278. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weaver K and Skouta R: The selenoprotein
glutathione peroxidase 4: From molecular mechanisms to novel
therapeutic opportunities. Biomedicines. 10:8912022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tuo Q, Masaldan S, Southon A, Mawal C,
Ayton S, Bush AI, Lei P and Belaidi AA: Characterization of
selenium compounds for anti-ferroptotic activity in neuronal cells
and after cerebral ischemia-reperfusion injury. Neurotherapeutics.
18:2682–2691. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hao S, Liang B, Huang Q, Dong S, Wu Z, He
W and Shi M: Metabolic networks in ferroptosis (Review). Oncol
Lett. 16:5279–5287. 2018.
|
|
47
|
Zheng J and Conrad M: The metabolic
underpinnings of ferroptosis. Cell Metab. 32:920–937. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shin D, Kim EH, Lee J and Roh J: Nrf2
inhibition reverses resistance to GPX4 inhibitor-induced
ferroptosis in head and neck cancer. Free Radic Biol Med.
129:454–462. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Alim I, Caulfield JT, Chen Y, Swarup V,
Geschwind DH, Ivanova E, Seravalli J, Ai Y, Sansing LH, SteMarie
EJ, et al: Selenium drives a transcriptional adaptive program to
block ferroptosis and treat stroke. Cell. 177:1262–1279.e25. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang Y, Swanda RV, Nie L, Liu X, Wang C,
Lee H, Lei G, Mao C, Koppula P, Cheng W, et al: mTORC1 couples
cyst(e)ine availability with GPX4 protein synthesis and ferroptosis
regulation. Nat Commun. 12:15892021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chaudhary N, Choudhary BS, Shah S, Khapare
N, Dwivedi N, Gaikwad A, Joshi N, Raichanna J, Basu S, Gurjar M, et
al: Lipocalin 2 expression promotes tumor progression and therapy
resistance by inhibiting ferroptosis in colorectal cancer. Int J
Cancer. 149:1495–1511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li F, Long H, Zhou Z, Luo H, Xu S and Gao
L: System Xc-/GSH/GPX4 axis: An important antioxidant system for
the ferroptosis in drug-resistant solid tumor therapy. Front
Pharmacol. 13:9102922022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kerins M, Milligan JF, Wohlschlegel JA and
Ooi A: Fumarate hydratase inactivation in hereditary leiomyomatosis
and renal cell cancer is synthetic lethal with ferroptosis
induction. Cancer Sci. 109:2757–2766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cui C, Yang F and Li Q: Post-translational
modification of GPX4 is a promising target for treating
ferroptosis-related diseases. Front Mol Biosci. 9:9015652022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Grossman E, Ward CC, Spradlin JN, Bateman
LA, Huffman TR, Miyamoto DK, Kleinman JI and Nomura DK: Covalent
ligand discovery against druggable hotspots targeted by anticancer
natural products. Cell Chem Biol. 24:1368–1376.e4. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Yang L, Chen X, Yang Q, Chen J, Huang Q,
Yao L, Ding Y, Wu J, Zhang P, Tang D, et al: Broad spectrum
deubiquitinase inhibition induces both apoptosis and ferroptosis in
cancer cells. Front Oncol. 10:9492020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhu S, Zhang Q, Sun X, Zeh HJ, Lotze MT,
Kang R and Tang D: HSPA5 regulates ferroptotic cell death in cancer
cells. Cancer Res. 77:2064–2077. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang WS, Kim KJ, Gaschler MM, Patel MM,
Shchepinov MS and Stockwell BR: Peroxidation of polyunsaturated
fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci
USA. 113:E4966–E4975. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Viswanathan VS, Ryan M, Dhruv HD, Gill S,
Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada
K, Aguirre AJ, et al: Dependency of a therapy-resistant state of
cancer cells on a lipid peroxidase pathway. Nature. 547:453–457.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kagan VE, Mao G, Qu F, Angeli JPF, Doll S,
Croix C, Dar HH, Liu B, Tyurin VA, Ritov VB, et al: Oxidized
arachidonic and adrenic PEs navigate cells to ferroptosis. Nat Chem
Biol. 13:81–90. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dixon SJ, Winter GE, Musavi L, Lee ED,
Snijder B, Rebsamen M, Superti-Furga G and Stockwell BR: Human
haploid cell genetics reveals roles for lipid metabolism genes in
nonapoptotic cell death. ACS Chem Biol. 10:1604–1609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Doll S, Freitas FP, Shah R, Aldrovandi M,
Da Silva MC, Ingold I, Grocin AG, Da Silva TNX, Panzilius E, Scheel
C, et al: FSP1 is a glutathione-independent ferroptosis suppressor.
Nature. 575:693–698. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Snaebjornsson MT, Janaki-Raman S and
Schulze A: Greasing the wheels of the cancer machine: The role of
lipid metabolism in cancer. Cell Metab. 31:62–76. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sun Y, Xue Z, Huang T, Che X and Wu G:
Lipid metabolism in ferroptosis and Ferroptosis-based cancer
therapy. Front Oncol. 12:9416182022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee JY, Nam M, Son H, Hyun K, Jang SY, Kim
JW, Kim MW, Jung Y, Jang E, Yoon S, et al: Polyunsaturated fatty
acid biosynthesis pathway determines ferroptosis sensitivity in
gastric cancer. Proc Natl Acad Sci USA. 117:32433–32442. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim JW, Lee J, Oh M and Lee EW: An
integrated view of lipid metabolism in ferroptosis revisited via
lipidomic analysis. Exp Mol Med. 55:1620–1631. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Lee J, Kim WK, Bae K, Lee SC and Lee EW:
Lipid metabolism and ferroptosis. Biology (Basel).
10:1842021.PubMed/NCBI
|
|
68
|
Song Z, Xiaoli AM and Yang F: Regulation
and metabolic significance of de novo lipogenesis in adipose
tissues. Nutrients. 10:13832018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Carracedo A, Cantley LC and Pandolfi PP:
Cancer metabolism: Fatty acid oxidation in the limelight. Nat Rev
Cancer. 13:227–232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Roehrig F and Schulze A: The multifaceted
roles of fatty acid synthesis in cancer. Nat Rev Cancer.
16:732–749. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Olzmann JA and Carvalho P: Dynamics and
functions of lipid droplets. Nat Rev Mol Cell Biol. 20:137–155.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Cruz ALS, Barreto EA, Fazolini NPB, Viola
JPB and Bozza PT: Lipid droplets: Platforms with multiple functions
in cancer hallmarks. Cell Death Dis. 6:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Guerrero-Rodriguez S, Mata-Cruz C,
Pérez-Tapia SM and Velasco-Velázquez MA: Role of CD36 in cancer
progression, stemness, and targeting. Front Cell Dev Biol.
10:10790762022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Veglia F, Tyurin VA, Di Blasi M, De Leo A,
Kossenkov AV, Donthireddy L, To TKJ, Schug ZT, Basu S, Wang F, et
al: Fatty acid transport protein 2 reprograms neutrophils in
cancer. Nature. 569:73–78. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zielinski ZAM and Pratt DA: Cholesterol
autoxidation revisited: Debunking the dogma associated with the
most vilified of lipids. J Am Chem Soc. 138:6932–6935. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chushi L, Wu W, Xie K, Feng Y, Xie N and
Chen X: HMGCR is up-regulated in gastric cancer and promotes the
growth and migration of the cancer cells. Gene. 587:42–47. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deng R, Fu L, Liang H, Ai X, Liu F, Li N,
Wu L, Li S, Yang X, Lin Y, et al: Inhibition of mitochondrial
complex I induces mitochondrial ferroptosis by regulating CoQH2
levels in cancer. Cell Death Dis. 16:2542025. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mao C, Liu X, Zhang Y, Lei G, Yan Y, Lee
H, Koppula P, Wu S, Zhuang L, Fang B, et al: DHODH-mediated
ferroptosis defence is a targetable vulnerability in cancer.
Nature. 593:586–590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu J, Wang Y, Jiang R, Xue R, Yin X, Wu M
and Meng Q: Ferroptosis in liver disease: New insights into disease
mechanisms. Cell Death Discov. 7:2762021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhan M, Ding Y, Huang S, Liu Y, Xiao J, Yu
H, Lu L and Wang X: Lysyl oxidase-like 3 restrains mitochondrial
ferroptosis to promote liver cancer chemoresistance by stabilizing
dihydroorotate dehydrogenase. Nat Commun. 14:31232023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kraft VAN, Bezjian CT, Pfeiffer S,
Ringelstetter L, Müller C, Zandkarimi F, Merl-Pham J, Bao X,
Anastasov N, Kössl J, et al: GTP cyclohydrolase
1/tetrahydrobiopterin counteract ferroptosis through lipid
remodeling. ACS Cent Sci. 6:41–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hu Q, Wei W, Wu D, Huang F, Li M, Li W,
Yin J, Peng Y, Lu Y, Zhao Q, et al: Blockade of GCH1/BH4 axis
activates ferritinophagy to mitigate the resistance of colorectal
cancer to Erastin-induced ferroptosis. Front Cell Dev Biol.
10:8103272022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lim JKM, Stölting F, Levy T, Thewes L,
Picard D, Tishina S, Zhang H, Lewandowska O, Reiff T, Remke M, et
al: Oncogenic RAS signaling suppresses ferroptosis via
transcriptional upregulation of GCH1. bioRxiv. Jan
29–2024.doi.org/10.1101/2024.01.27.577524.
|
|
85
|
Cao J, Chen X, Chen L, Lu Y, Wu Y, Deng A,
Pan F, Huang H, Liu Y, Li Y, et al: DHODH-mediated mitochondrial
redox homeostasis: A novel ferroptosis regulator and promising
therapeutic target. Redox Biol. 85:1037882025. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Mazdak H, Yazdekhasti F, Movahedian A,
Mirkheshti N and Shafieian M: The comparative study of serum iron,
copper, and zinc levels between bladder cancer patients and a
control group. Int Urol Nephrol. 42:89–93. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
An WX, Gupta R, Zhai K, Wang YR, Xu WH and
Cui Y: Current and potential roles of ferroptosis in bladder
cancer. Curr Med Sci. 44:51–63. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Kong N, Chen X, Feng J, Duan T, Liu S, Sun
X, Chen P, Pan T, Yan L, Jin T, et al: Baicalin induces ferroptosis
in bladder cancer cells by downregulating FTH1. Acta Pharm Sin B.
11:4045–4054. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Shimada K, Skouta R, Kaplan A, Yang WS,
Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ and
Stockwell BR: Global survey of cell death mechanisms reveals
metabolic regulation of ferroptosis. Nat Chem Biol. 12:497–503.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Stepanic V and Kucerova-Chlupacova M:
Review and chemoinformatic analysis of ferroptosis modulators with
a focus on natural plant products. Molecules. 28:4752023.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xiang Y, Chen X, Wang W, Zhai L, Sun X,
Feng J, Duan T, Zhang M, Pan T, Yan L, et al: Natural product
erianin inhibits bladder cancer cell growth by inducing ferroptosis
via NRF2 inactivation. Front Pharmacol. 12:7755062021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Sun Y, Berleth N, Wu W, Schlutermann D,
Deitersen J, Stuhldreier F, Berning L, Friedrich A, Akgun S,
Mendiburo MJ, et al: Fin56-induced ferroptosis is supported by
autophagy-mediated GPX4 degradation and functions synergistically
with mTOR inhibition to kill bladder cancer cells. Cell Death Dis.
12:10282021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Zhang X, Sui S, Wang L, Li H, Zhang L, Xu
S and Zheng X: Inhibition of tumor propellant glutathione
peroxidase 4 induces ferroptosis in cancer cells and enhances
anticancer effect of cisplatin. J Cell Physiol. 235:3425–3437.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Lachaier E, Louandre C, Godin C, Saidak Z,
Baert M, Diouf M, Chauffert B and Galmiche A: Sorafenib induces
ferroptosis in human cancer cell lines originating from different
solid tumors. Int J Oncol. 34:6417–6422. 2014.
|
|
95
|
Sun X, Ou Z, Chen R, Niu X, Chen D, Kang R
and Tang D: Activation of the p62-Keap1-NRF2 pathway protects
against ferroptosis in hepatocellular carcinoma cells. Hepatology.
63:173–184. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chiang S, Chen S and Chang L: A dual role
of HEME oxygenase-1 in cancer cells. Int J Mol Sci. 20:392019.
View Article : Google Scholar
|
|
97
|
Sun X, Niu X, Chen R, He W, Chen D, Kang R
and Tang D: Metallothionein-1G facilitates sorafenib resistance
through inhibition of ferroptosis. Hepatology. 64:488–500. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Bai T, Lei P, Zhou H, Liang R, Zhu R, Wang
W, Zhou L and Sun Y: Sigma-1 receptor protects against ferroptosis
in hepatocellular carcinoma cells. J Cell Mol Med. 23:7349–7359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Yang F, Li J, Deng H, Wang Y, Lei C, Wang
Q, Jin X, Liang L, Xia J, Pan X, et al: GSTZ1-1 deficiency
activates NRF2/IGF1R axis in HCC via accumulation of oncometabolite
succinylacetone. EMBO J. 38:e1019642019. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wang Q, Cheng B, Qin X, Gao Q, Huang A,
Wang K and Tang N: GSTZ1 sensitizes hepatocellular carcinoma cells
to sorafenib-induced ferroptosis via inhibition of NRF2/GPX4 axis.
Cell Death Dis. 12:4262021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Louandre C, Marcq I, Bouhlal H, Lachaier
E, Godin C, Saidak Z, François C, Chatelain D, Debuysscher V,
Barbare J, et al: The retinoblastoma (Rb) protein regulates
ferroptosis induced by sorafenib in human hepatocellular carcinoma
cells. Cancer Lett. 356:971–977. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhang Y, Li J, Dong X, Meng D, Zhi X, Liu
Y and Yao L: PSAT1 regulated oxidation-reduction balance affects
the growth and prognosis of epithelial ovarian cancer. Onco Targets
Ther. 13:5443–5453. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Haga Y, Kanda T, Nakamura M, Nakamoto S,
Sasaki R, Takahashi K, Wu S and Yokosuka O: Overexpression of c-Jun
contributes to sorafenib resistance in human hepatoma cell lines.
PLoS One. 12:e01741532017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chen Y, Zhu G, Liu Y, Wu Q, Zhang X, Bian
Z, Zhang Y, Pan Q and Sun F: O-GlcNAcylated c-Jun antagonizes
ferroptosis via inhibiting GSH synthesis in liver cancer. Cell
Signal. 63:1093842019. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Gao R, Kalathur RKR, Coto-Llerena M, Ercan
C, Buechel D, Song S, Piscuoglio S, Dill MT, Camargo FD,
Christofori G, et al: YAP/TAZ and ATF4 drive resistance to
sorafenib in hepatocellular carcinoma by preventing ferroptosis.
EMBO Mol Med. 13:e143512021. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang K, Zhang Z, Tsai H, Liu Y, Gao J,
Wang M, Liu S, Cao X, Xu Z, Chen H, et al: Branched-chain amino
acid aminotransferase 2 regulates ferroptotic cell death in cancer
cells. Cell Death Differ. 28:1222–1236. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Qiu Y, Li H, Xie J, Qiao X and Wu J:
Identification of ABCC5 among ATP-binding cassette transporter
family as a new biomarker for hepatocellular carcinoma based on
bioinformatics analysis. Int J Gen Med. 14:7235–7246. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Huang W, Chen K, Lu Y, Zhang D, Cheng Y,
Li L, Huang W, He G, Liao H, Cai L, et al: ABCC5 facilitates the
acquired resistance of sorafenib through the inhibition of
SLC7A11-induced ferroptosis in hepatocellular carcinoma. Neoplasia.
23:1227–1239. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Byun J, Lee S, Kang G, Lee YR, Park SY,
Song I, Yun JW, Lee J, Choi Y and Park K: Macropinocytosis is an
alternative pathway of cysteine acquisition and mitigates
sorafenib-induced ferroptosis in hepatocellular carcinoma. J Exp
Clin Cancer Res. 41:22962022. View Article : Google Scholar
|
|
110
|
Lee JY, Kang ES, Kobayashi S, Homma T,
Sato H, Seo HG and Fujii J: The viability of primary hepatocytes is
maintained under a low cysteine-glutathione redox state with a
marked elevation in ophthalmic acid production. Exp Cell Res.
361:178–191. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Chen Y, Li L, Lan J, Yang C, Rao X, Zhao
J, Xing T, Ju G, Song G and Lou J: CRISPR screens uncover
protective effect of PSTK as a regulator of chemotherapy-induced
ferroptosis in hepatocellular carcinoma. Mol Cancer. 21:112022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Liu Y, Fan X, Zhao Z and Shan XH: LncRNA
SLC7A11-AS1 contributes to lung cancer progression through
facilitating TRAIP expression by inhibiting miR-4775. Onco Targets
Ther. 13:6295–6302. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Zhang W, Sun Y, Bai L, Zhi L, Yang Y, Zhao
Q, Chen C, Qi Y, Gao W, He W, et al: RBMS1 regulates lung cancer
ferroptosis through translational control of SLC7A11. J Clin
Invest. 131:e1520672021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lu X, Kang N, Ling X, Pan M, Du W and Gao
S: MIR-27A-3P promotes non-small cell lung cancer through
SLC7A11-mediated ferroptosis. Front Oncol. 11:7593462021.
View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Gargalionis AN, Papavassiliou KA and
Papavassiliou AG: Implication of MTOR signaling in NSCLC:
Mechanisms and therapeutic perspectives. Cells. 12:20142023.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ni J, Chen K, Zhang J and Zhang X:
Inhibition of GPX4 or mTOR overcomes resistance to lapatinib via
promoting ferroptosis in NSCLC cells. Biochem Biophys Res Commun.
567:154–160. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Li Y, Yan H, Xu X, Liu H, Wu C and Zhao L:
Erastin/sorafenib induces cisplatin-resistant non-small cell lung
cancer cell ferroptosis through inhibition of the Nrf2/xCT pathway.
Oncol Lett. 19:323–333. 2020.PubMed/NCBI
|
|
118
|
Carlisle AE, Lee N, Matthew-Onabanjo AN,
Spears ME, Park SJ, Youkana D, Doshi MB, Peppers A, Li R, Joseph
AB, et al: Selenium detoxification is required for cancer-cell
survival. Nat Metab. 2:603–611. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Yu H, Yang C, Lei J, Guo S, Chen R, Li K,
Qu F, Tao K, Fu Y, Liu F, et al: Sulfasalazine-induced ferroptosis
in breast cancer cells is reduced by the inhibitory effect of
estrogen receptor on the transferrin receptor. Oncol Rep.
42:2650–2660. 2019.
|
|
120
|
Yadav P, Sharma P, Sundaram S, Venkatraman
G, Bera AK and Karunagaran D: SLC7A11/xCT is a target of miR-5096
and its restoration partially rescues miR-5096-mediated ferroptosis
and antitumor effects in human breast cancer cells. Cancer Lett.
522:211–224. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sugezawa K, Morimoto M, Yamamoto M,
Matsumi Y, Nakayama Y, Hara K, Uejima C, Kihara K, Matsunaga T,
Tokuyasu N, et al: GPX4 regulates tumor cell proliferation via
suppressing ferroptosis and exhibits prognostic significance in
gastric cancer. Anticancer Res. 42:5719–5729. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Zeuner A, Todaro M, Stassi G and De Maria
R: Colorectal cancer stem cells: From the crypt to the clinic. Cell
Stem Cell. 15:692–705. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hao S, Yu J, He W, Huang Q, Zhao Y, Liang
B, Zhang S, Wen Z, Dong S, Rao J, et al: Cysteine dioxygenase 1
mediates Erastin-induced ferroptosis in human gastric cancer cells.
Neoplasia. 19:1022–1032. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Ye G, Guan W, Cao Z, Guo W, Xiong G, Zhao
F, Feng M, Qiu J, Liu Y, Zhang MQ, et al: Integrative genomic
analysis of gemcitabine resistance in pancreatic cancer by
patient-derived xenograft models. Clin Cancer Res. 27:3383–3396.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Thummuri D, Khan S, Underwood PW, Zheng P,
Wiegand J, Zhang X, Budamagunta V, Sobh A, Tagmount A, Loguinov A,
et al: Overcoming gemcitabine resistance in pancreatic cancer using
the BCL-XL-Specific degrader DT2216. Mol Cancer Ther. 21:184–192.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang H, Liu C, Zhao Y, Zhang W, Xu K, Li
D, Zhou Y, Li H, Xiao G, Lu B and Gao G: Inhibition of LONP1
protects against erastin-induced ferroptosis in pancreatic ductal
adenocarcinoma PANC1 cells. Biochem Biophys Res Commun.
522:1063–1068. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Wang Y, Wu X, Zhao R, Yu-Lin L, Zou W,
Chen J and Wang H: Overcoming cancer chemotherapy resistance by the
induction of ferroptosis. Drug Resist Updat. 66:1009162023.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ludin A, Gur-Cohen S, Golan K, Kaufmann
KB, Itkin T, Medaglia C, Lu XJ, Ledergor G, Kollet O and Lapidot T:
Reactive oxygen species regulate hematopoietic stem cell
self-renewal, migration and development, as well as their bone
marrow microenvironment. Antioxid Redox Signal. 21:1605–1619. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Fischbacher A, von Sonntag C and Schmidt
TC: Hydroxyl radical yields in the Fenton process under various pH,
ligand concentrations and hydrogen peroxide/Fe(II) ratios.
Chemosphere. 182:738–744. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Rassool FV, Gaymes TJ, Omidvar N, Brady N,
Beurlet S, Pla M, Reboul M, Lea N, Chomienne C, Thomas NS, et al:
Reactive oxygen species, DNA damage, and error-prone repair: A
model for genomic instability with progression in myeloid leukemia?
Cancer Res. 67:8762–8771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Hole PS, Zabkiewicz J, Munje C, Newton Z,
Pearn L, White P, Marquez N, Hills RK, Burnett AK, Tonks A and
Darley RL: Overproduction of NOX-derived ROS in AML promotes
proliferation and is associated with defective oxidative stress
signaling. Blood. 122:3322–3330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Fonseca-Nunes A, Jakszyn P and Agudo A:
Iron and cancer risk-a systematic review and meta-analysis of the
epidemiological evidence. Cancer Epidemiol Biomarkers Prev.
23:12–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Manz DH, Blanchette NL, Paul BT, Torti FM
and Torti SV: Iron and cancer: Recent insights. Ann N Y Acad Sci.
1368:149–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Long F, Lin Z, Long Q, Lu Z, Zhu K, Zhao M
and Yang M: CircZBTB46 protects acute myeloid leukemia cells from
ferroptotic cell death by upregulating SCD. Cancers (Basel).
15:4592023. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Aurelius J, Thorén FB, Akhiani AA, Brune
M, Palmqvist L, Hansson M, Hellstrand K and Martner A: Monocytic
AML cells inactivate antileukemic lymphocytes: Role of NADPH
oxidase/gp91(phox) expression and the PARP-1/PAR pathway of
apoptosis. Blood. 119:5832–5837. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Chen J, Lu WY, Zhao MF, Cao XL, Jiang YY,
Jin X, Xu P, Yuan TT, Zhang YC, Chai X, et al: Reactive oxygen
species mediated T lymphocyte abnormalities in an iron-overloaded
mouse model and iron-overloaded patients with myelodysplastic
syndromes. Ann Hematol. 96:1085–1095. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Liu Y, Du Z, Huang J, Li T, Zhang J, Li Y,
Yi W and Chen C: Ferroptosis in hematological malignant tumors.
Front Oncol. 13:11275262023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Bai Y, Luo Y, Yuan Y, Li X, Jin J, Ping R,
Guo J, Jin L, Yu Y and Xiong Y: Ferroptosis: A novel therapeutic
warrior in the battle against leukemia. Apoptosis. 30:1776–1795.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Wei J, Xie Q, Liu X, Wan C, Wu W, Fang K,
Yao Y, Cheng P, Deng D and Liu Z: Identification the prognostic
value of glutathione peroxidases expression levels in acute myeloid
leukemia. Ann Transl Med. 8:6782020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ma Z, Ye W, Huang X, Li X, Li F, Lin X, Hu
C, Wang J, Jin J, Zhu B and Huang J: The ferroptosis landscape in
acute myeloid leukemia. Aging (Albany NY). 15:13486–13503. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Yu Y, Yan Y, Niu F, Wang Y, Chen X, Su G,
Liu Y, Zhao X, Qian L, Liu P and Xiong Y: Ferroptosis: A cell death
connecting oxidative stress, inflammation and cardiovascular
diseases. Cell Death Discov. 7:1932021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Liu X, Zhong S, Qiu K, Chen X, Wu W, Zheng
J, Liu Y, Wu H, Fan S, Nie D, et al: Targeting NRF2 uncovered an
intrinsic susceptibility of acute myeloid leukemia cells to
ferroptosis. Exp Hematol Oncol. 12:472023. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Xia J, Sun S, Jotte MR, Uy GL, Sorani E,
Abi Vainstein L, Shemesh Davish L, Caldwell KE, Hawkins WG and Link
DC: Combined inhibition of CXCR4 signaling and System
xc-transporter activity induces synthetic lethality in T-ALL cells
by suppressing GSH and inducing ferroptosis. Blood. 136 (Suppl
1):S372020. View Article : Google Scholar
|
|
144
|
Zhang W, Trachootham D, Liu J, Chen G,
Pelicano H, Garcia-Prieto C, Lu W, Burger JA, Croce CM, Plunkett W,
et al: Stromal control of cystine metabolism promotes cancer cell
survival in chronic lymphocytic leukaemia. Nat Cell Biol.
14:276–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Liu J, Xia X and Huang P: xCT: A critical
molecule that links cancer metabolism to redox signaling. Mol Ther.
28:2358–2366. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Birsen R, Larrue C, Decroocq J, Johnson N,
Guiraud N, Gotanegre M, Cantero-Aguilar L, Grignano E, Huynh T,
Fontenay M, et al: APR-246 induces early cell death by ferroptosis
in acute myeloid leukemia. Haematologica. 107:403–416. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Pardieu B, Pasanisi J, Ling F, Dal Bello
R, Penneroux J, Su A, Joudinaud R, Chat L, Wu HC, Duchmann M, et
al: Cystine uptake inhibition potentiates front-line therapies in
acute myeloid leukemia. Leukemia. 36:1585–1595. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Rashkovan M and Ferrando A: Metabolic
dependencies and vulnerabilities in leukemia. Genes Dev.
33:1460–1474. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Zheng X, Jin X, Ye F, Liu X, Yu B, Li Z,
Zhao T, Chen W, Liu X, Di C and Li Q: Ferroptosis: A novel
regulated cell death participating in cellular stress response,
radiotherapy, and immunotherapy. Exp Hematol Oncol. 12:652023.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Dächert J, Schoeneberger H, Rohde K and
Fulda S: RSL3 and erastin differentially regulate redox signaling
to promote Smac mimetic-induced cell death. Oncotarget.
7:63779–63792. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Brown RAM, Richardson KL, Kabir TD,
Trinder D, Ganss R and Leedman PJ: Altered iron metabolism and
impact in cancer biology, metastasis, and immunology. Front Oncol.
10:4762020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Yang N, Tan S, Ng SC, Shi Y, Zhou J, Tan
KSW, Wong WF and Shen H: Artesunate induces cell death in human
cancer cells via enhancing lysosomal function and lysosomal
degradation of ferritin. J Biol Chem. 289:33425–33441. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ooko E, Saeed ME, Kadioglu O, Sarvi S,
Colak M, Elmasaoudi K, Janah R, Greten HJ and Efferth T:
Artemisinin derivatives induce iron-dependent cell death
(ferroptosis) in tumor cells. Phytomedicine. 22:1045–1054. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Santana-Codina N and Mancias JD: The role
of NCOA4-mediated ferritinophagy in health and disease.
Pharmaceuticals (Basel). 11:1142018. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel antitumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Wen RJ, Dong X, Zhuang HW, Pang FX, Ding
SC, Li N, Mai YX, Zhou ST, Wang JY and Zhang JF: Baicalin induces
ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4
regulatory axis. Phytomedicine. 116:1548812023. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Tang Z, Huang Z, Huang Y, Chen Y, Huang M,
Liu H, Ye Q, Zhao J and Jia B: Ferroptosis: The silver lining of
cancer therapy. Front Cell Dev Biol. 9:7658592021. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Shintoku R, Takigawa Y, Yamada K, Kubota
C, Yoshimoto Y, Takeuchi T, Koshiishi I and Torii S:
Lipoxygenase-mediated generation of lipid peroxides enhances
ferroptosis induced by erastin and RSL3. Cancer Sci. 108:2187–2194.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Chu B, Kon N, Chen D, Li T, Liu T, Jiang
L, Song S, Tavana O and Gu W: ALOX12 is required for p53-mediated
tumour suppression through a distinct ferroptosis pathway. Nat Cell
Biol. 21:579–591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Zou Y, Li H, Graham E, Deik A, Eaton JK,
Wang W, Sandoval-Gomez G, Clish CB, Doench JG and Schreiber SL:
Cytochrome P450 oxidoreductase contributes to phospholipid
peroxidation in ferroptosis. Nat Chem Biol. 16:302–309. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhou Y, Tashiro J, Kamatani S, Irie N,
Suzuki A, Ishikawa T, Warita K, Oltvai ZN and Warita T: HMG-CoA
reductase degrader, SR-12813, counteracted statin-induced
upregulation of HMG-CoA reductase and augmented the anticancer
effect of atorvastatin. Biochem Biophys Res Commun. 677:13–19.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Yang J, Xu J, Zhang B, Tan Z, Meng Q, Hua
J, Liu J, Wang W, Shi S, Yu X and Liang C: Ferroptosis: At the
crossroad of gemcitabine resistance and tumorigenesis in pancreatic
cancer. Int J Mol Sci. 22:109442021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Chen L, Sun R and Fang K: Erianin inhibits
tumor growth by promoting ferroptosis and inhibiting invasion in
hepatocellular carcinoma through the JAK2/STAT3/SLC7A11 pathway.
Pathol Int. 74:119–128. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Zheng Y, Zheng Y, Chen H, Tan X, Zhang G,
Kong M, Jiang R, Yu H, Shan K, Liu J, et al: Erianin triggers
ferroptosis in colorectal cancer cells by facilitating the
ubiquitination and degradation of GPX4. Phytomedicine.
139:1564652025. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Tian XY, Han R, Huang QY, Zhou MY, Luo B,
Chen XR and Xu JC: Erianin inhibits oral cancer cell growth,
migration, and invasion via the Nrf2/HO-1/GPX4 pathway. Asian Pac J
Trop Biomed. 12:4372022. View Article : Google Scholar
|
|
166
|
Yagoda N, Von Rechenberg M, Zaganjor E,
Bauer AJ, Yang WS, Fridman DJ, Wolpaw AJ, Smukste I, Peltier JM,
Boniface JJ, et al: RAS-RAF-MEK-dependent oxidative cell death
involving voltage-dependent anion channels. Nature. 447:865–869.
2007. View Article : Google Scholar
|
|
167
|
Zhao J, Xu B, Xiong Q, Feng Y and Du H:
Erastin-induced ferroptosis causes physiological and pathological
changes in healthy tissues of mice. Mol Med Rep. 24:7132021.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Yu M, Gai C, Li Z, Ding D, Zheng J, Zhang
W, Lv S and Li W: Targeted exosome-encapsulated erastin induced
ferroptosis in triple negative breast cancer cells. Cancer Sci.
110:3173–3182. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Sun S, Shen J, Jiang J, Wang F and Min J:
Targeting ferroptosis opens new avenues for the development of
novel therapeutics. Signal Transduct Target Ther. 8:3722023.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Bersuker K, Hendricks JM, Li Z, Magtanong
L, Ford B, Tang PH, Roberts MA, Tong B, Maimone TJ, Zoncu R, et al:
The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit
ferroptosis. Nature. 575:688–692. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Zhao Y, Liu J, Wang S and Li X: Role of
non-coding RNA-regulated ferroptosis in colorectal cancer. Cell
Death Discov. 11:3152025. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ghosal J, Sinchana VK and Chakrabarty S:
Ferroptosis meets microRNAs: A new frontier in anticancer therapy.
Free Radic Biol Med. 226:266–278. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Ju Y, Lv Y, Liu X, Lu J, Shi Y, Guo H, Xu
S, Tian J, Yang J and Zhong J: Role of long non-coding RNAs in the
regulation of ferroptosis in tumors. Front Immunol. 16:15685672025.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Yang R, Ma L, Wan J, Li Z, Yang Z, Zhao Z
and Ming L: Ferroptosis-associated circular RNAs: Opportunities and
challenges in the diagnosis and treatment of cancer. Front Cell Dev
Biol. 11:11603812023. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Lyu N, Zeng Y, Kong Y, Chen Q, Deng H, Ou
S, Bai Y, Tang H, Wang X and Zhao M: Ferroptosis is involved in the
progression of hepatocellular carcinoma through the
circ0097009/miR-1261/SLC7A11 axis. Ann Transl Med. 9:6752021.
View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Pan X, Chen K, Gao W, Xu M, Meng F, Wu M,
Wang ZQ, Li YQ, Xu W, Zhang M and Luo Y: Circular RNA circBNC2
inhibits tumorigenesis by modulating ferroptosis and acts as a
nanotherapeutic target in prostate cancer. Mol Cancer. 24:292025.
View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Sleire L, Skeie BS, Netland IA, Førde HE,
Dodoo E, Selheim F, Leiss L, Heggdal JI, Wang J and Enger PØ: Drug
repurposing: Sulfasalazine sensitizes gliomas to gamma knife
radiosurgery by blocking cystine uptake through system Xc-, leading
to glutathione depletion. Oncogene. 34:5951–5959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Li Z, Dai HQ, Huang XW, Feng J, Deng JH,
Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al: Artesunate
synergizes with sorafenib to induce ferroptosis in hepatocellular
carcinoma. Acta Pharm Sin B. 42:301–310. 2021. View Article : Google Scholar
|
|
179
|
Li H, Yu Y, Liu Y, Luo Z, Law BYK, Zheng
Y, Huang X and Li W: Ursolic acid enhances the antitumor effects of
sorafenib associated with Mcl-1-related apoptosis and
SLC7A11-dependent ferroptosis in human cancer. Pharmacol Res.
182:1063062022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Wang H, Zhang Z, Ruan S, Qiu Y, Chen Y,
Cui J, Wang X, Huang S and Hou B: Regulation of iron metabolism and
ferroptosis in cancer stem cells. Front Oncol. 13:12515612023.
View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Campanella A, Santambrogio P, Fontana F,
Frenquelli M, Cenci S, Marcatti M, Sitia R, Tonon G and Camaschella
C: Iron increases the susceptibility of multiple myeloma cells to
bortezomib. Haematologica. 98:971–979. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chekhun VF, Lukyanova NY, Burlaka AP,
Bezdenezhnykh NA, Shpyleva SI, Tryndyak VP, Beland FA and Pogribny
IP: Iron metabolism disturbances in the MCF-7 human breast cancer
cells with acquired resistance to doxorubicin and cisplatin. Int J
Oncol. 43:1481–1486. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Habib E, Linher-Melville K, Lin H and
Singh G: Expression of xCT and activity of system xc(−) are
regulated by NRF2 in human breast cancer cells in response to
oxidative stress. Redox Biol. 5:33–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Hangauer MJ, Viswanathan VS, Ryan MJ, Bole
D, Eaton JK, Matov A, Galeas J, Dhruv HD, Berens ME, Schreiber SL,
et al: Drug-tolerant persister cancer cells are vulnerable to GPX4
inhibition. Nature. 551:247–250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Lei G, Mao C, Yan Y, Li Z and Gan B:
Ferroptosis, radiotherapy, and combination therapeutic strategies.
Protein Cell. 12:836–857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Ye LF, Chaudhary K, Zandkarimi F, Harken
A, Kinslow CJ, Upadhyayula PS, Dovas A, Higgins D, Tan H, Zhang Y,
et al: Radiation-induced lipid peroxidation triggers ferroptosis
and synergizes with ferroptosis inducers. ACS Chem Biol.
15:469–484. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Lei G, Zhang Y, Koppula P, Liu X, Zhang J,
Lin SH, Ajani JA, Xiao Q, Liao Z, Wang H and Gan B: The role of
ferroptosis in ionizing radiation-induced cell death and tumor
suppression. Cell Res. 30:146–162. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
188
|
Lin Z, Zou S and Wen K: The crosstalk of
CD8+ T cells and ferroptosis in cancer. Front Immunol.
14:12554432024. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Zheng Y, Sun L, Guo J and Ma J: The
crosstalk between ferroptosis and anti-tumor immunity in the tumor
microenvironment: Molecular mechanisms and therapeutic controversy.
Cancer Commun (Lond). 43:1071–1096. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Xia W, Lv Y, Zou Y, Kang Z, Li Z, Tian J,
Zhou H, Su W and Zhong J: The role of ferroptosis in colorectal
cancer and its potential synergy with immunotherapy. Front Immunol.
15:15267492025. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Tao Q, Liu N, Wu J, Chen J, Chen X and
Peng C: Mefloquine enhances the efficacy of anti-PD-1 immunotherapy
via IFN-γ-STAT1-IRF1-LPCAT3-induced ferroptosis in tumors. J
Immunother Cancer. 12:e0085542024. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Wang T, Li Z, Lei J, Zhang Y, Tong Y, Guan
X and Wang S: RGD peptide-functionalized micelles loaded with
crocetin ameliorate doxorubicin-induced cardiotoxicity. Int J Pharm
X. 9:1003262025.PubMed/NCBI
|
|
193
|
Zhang T, Gu F, Lin W, Shao H, Jiang A and
Guan X: Boosting cancer immunotherapy: Drug delivery systems
leveraging ferroptosis and immune checkpoint blockade. Front
Immunol. 16:16112992025. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Manivasagan P, Joe A, Han H, Thambi T,
Selvaraj M, Chidambaram K, Kim J and Jang E: Recent advances in
multifunctional nanomaterials for photothermal-enhanced
Fenton-based chemodynamic tumor therapy. Mater Today Bio.
13:1001972022. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Ma P, Xiao H, Chang Y, Liu J, Cheng Z,
Song H, Zhang X, Li C, Wang J, Gu Z and Lin J: Enhanced cisplatin
chemotherapy by iron oxide nanocarrier-mediated generation of
highly toxic reactive oxygen species. Nano Lett. 17:928–937. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Luo L, Wang H, Tian W, Li X, Zhu Z, Huang
R and Luo H: Targeting ferroptosis-based cancer therapy using
nanomaterials: Strategies and applications. Theranostics.
11:9937–9952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Yang J, Gong Y, Sontag DP, Corbin IR and
Minuk GY: Effects of Low-density lipoprotein docosahexaenoic acid
nanoparticles on cancer stem cells isolated from human hepatoma
cell lines. Mol Biol Rep. 45:1023–1036. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Poggio M, Tao H, Pai CC, Chu B, Belair CD,
Chang A, Montabana E, Lang UE, Fu Q, Fong L and Blelloch R:
Suppression of exosomal PD-L1 induces systemic antitumor immunity
and memory. Cell. 177:414–427.e13. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Jelinek A, Heyder L, Daude M, Plessner M,
Krippner S, Grosse R, Diederich WE and Culmsee C: Mitochondrial
rescue prevents glutathione peroxidase-dependent ferroptosis. Free
Radic Biol Med. 117:45–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Wu J, Feng Z, Chen L, Li Y, Bian H, Geng
J, Zheng ZH, Fu X, Pei Z, Qin Y, et al: TNF antagonist sensitizes
synovial fibroblasts to ferroptotic cell death in Collagen-induced
arthritis mouse models. Nat Commun. 13:6762022. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Qin LY, Guan P, Wang JX, Chen Y, Zhao YS,
Yang SC, Guo YJ, Wang N and Ji ES: Therapeutic potential of
Astragaloside IV against Adriamycin-induced renal damage in rats
via ferroptosis. Front Pharmacol. 13:8125942022. View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Eaton JK, Ruberto RA, Kramm A, Viswanathan
VS and Schreiber SL: Diacylfuroxans are masked nitrile oxides that
inhibit GPX4 covalently. J Am Chem Soc. 141:20407–20415. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Firtina Karagonlar Z, Koc D, Iscan E,
Erdal E and Atabey N: Elevated hepatocyte growth factor expression
as an autocrine c-Met activation mechanism in acquired resistance
to sorafenib in hepatocellular carcinoma cells. Cancer Sci.
107:407–416. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Liu K, Huang L, Qi S, Liu S, Xie W, Du L,
Cui J, Zhang X, Zhang B, Liu L, et al: Ferroptosis: The
entanglement between traditional drugs and nanodrugs in tumor
therapy. Adv Healthc Mater. 12:e22030852023. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Singh M, Arora HL, Naik R, Joshi S,
Sonawane K, Sharma NK and Sinha BK: Ferroptosis in cancer:
Mechanism and therapeutic potential. Int J Mol Sci. 26:38522025.
View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Ojo OA, Grant S, Nwafor-Ezeh PI,
Maduakolam-Aniobi TC, Akinborode TI, Ezenabor EH and Ojo AB:
Ferroptosis as the new approach to cancer therapy. Cancer Treat Res
Commun. 43:1009132025.PubMed/NCBI
|
|
207
|
Ubellacker JM and Dixon SJ: Prospects for
ferroptosis therapies in cancer. Nat Cancer. 6:13262025. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Hino K, Nishina S and Yanatori I:
Ferroptosis: Biology and role in liver disease. J Gastroenterol.
Sep 18–2025.doi: 10.1007/s00535-025-02300-5 (Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Meng Y, Zhou Q, Dian Y, Zeng F, Deng G and
Chen X: Ferroptosis: A targetable vulnerability for melanoma
treatment. J Invest Dermatol. 145:1323–1344. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Hsu CY, Al-Hasnaawei S, Kumar A, Ballal S,
Mahmood AA, Kalia R, Joshi KK, Ray S and Pramanik A: Exosomal
non-coding RNA as a key mediator of ferroptosis in lung cancer.
Cell Signal. 136:1121352025. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Chen W, Han L, Wang J and Song L:
Ferroptosis: The dawn of reversing drug resistance in digestive
cancers. Genes Dis. 10:1018732025. View Article : Google Scholar
|
















