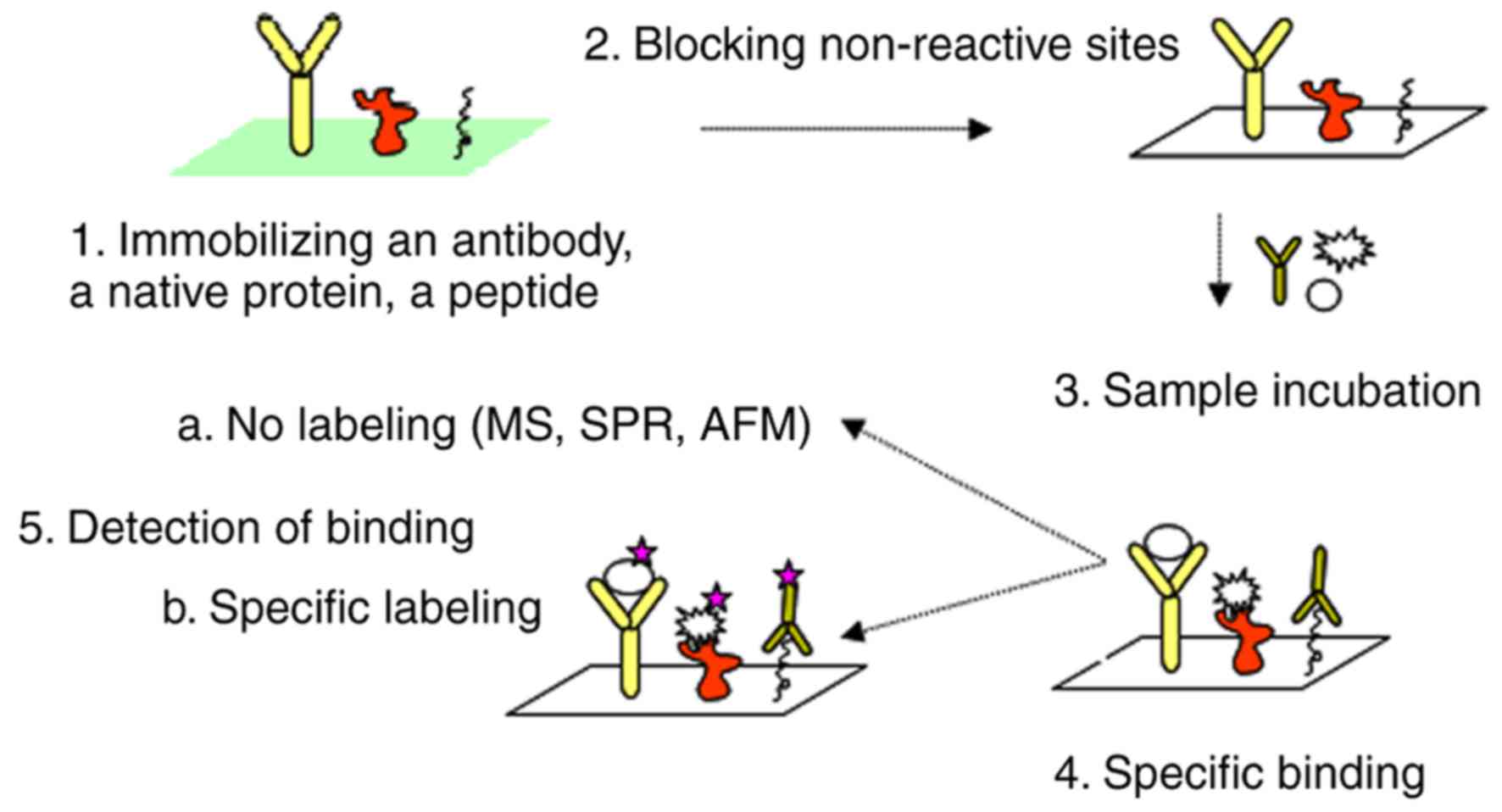
1. Introduction
History of array technologies
Array tools were developed more than 25 years ago
with the implementation of DNA microarray technology as a very
accurate platform intended to determine mRNA expression for the
concomitant determination of thousands of genes. One of the first
studies, if not actually the first one on this subject, was
published in 1995 when describing microarrays automatically printed
with complementary DNAs on glass so that corresponding genes could
be identified from samples. The use of low quantities of input
sample due to the high density of the array, the detection of rare
transcripts from only 2 µg total mRNA was reported. In that seminal
study, the expression levels of 45 Arabidopsis genes were measured
using two-color fluorescence hybridization (1). Relatively soon, the necessity of DNA
microarrays to evolve towards protein microarrays became obvious,
as mRNA profiles were not perfectly matching protein expression
(2).
Thus, although the number of human genes is in the
order of tens of thousands, the protein synthesis system can
express millions of protein types, these proteins being
structurally and functionally inter-connected for maintaining
tissues and cells homeostasis. With this finding, the scientific
stage was open to the development of protein microarrays. Thus,
protein microarrays were designed to cover the complex proteome
machine and to pursue in the identification of protein
functionality and interconnection. There are several types of
protein microarrays, although the first type that was developed was
based on specific antibody immobilization (3). Basically, it reproduced an
enzyme-linked immunosorbent assay (ELISA) setup, having the
advantage of highly specific recognition for the detection system.
When technology could spot, in small quantities, specific
antibodies without hindering their specificity and selectivity,
conventional immunoassays turned to spotted arrays that allowed for
multiple, specific and parallel detection of biomolecules from
small amount of biological samples (3). The overall scheme of a protein
microarray is presented in Fig.
1.
During its development and implementation in
clinical units, this technology gained sensitivity and overall
performance that led to its recognized validity. Further keeping
the similarity to ELISA types, another design of protein microarray
was developed. In this new version of protein microarray,
immobilized purified proteins and not antibodies were deposited on
the glass slides, and this new type was denominated as a functional
array (4,5). In this variant, various proteins can
be spotted from a certain cell type, a group of cells or a tissue
sample, or even an entire micro-organism can be placed on the slide
(4-6).
Functional microarrays can evaluate some key aspects in proteomics,
protein functions, interconnection in protein-protein binding,
metabolic/biochemical action, the association between a specific
ligand and its receptor, the interrelation between an enzyme and
its substrate, and can visualize immune-related protein(s)
triggered by an active response (7).
Another design of protein microarrays is the
reverse-phase type where total proteins from tissue/cell lysates or
specifically fractionated tissue/cell lysates are spotted on the
glass slides and their expression is hence quantified (8). Thus, in 2007, Speer et al
published for the first time, this new protein microarray type.
Their study demonstrated that in the need to depict functional
alterations within the proteome, reverse-phase protein microarray
can detect altered cellular protein molecular networks and cell
signaling pathways associated to human diseases, particularly
cancer. Tumorigenesis is based on protein molecular networks
alteration leading to disrupted cell signaling pathways,
uncontrolled proliferation, drug resistance, enhanced mobility and
the adaptation to a new microenvironment in the metastatic
processes (8).
From the very beginning, this new type of protein
microarray was intended to depict the pathology molecular profile,
as it can provide ‘functional read-out of cell signaling networks
or pathways for an individual patient’. The assertion of these
authors is of outmost importance in the clinical context of
personalized medicine where patient stratification for the most
efficient therapy is the ultimate goal (8).
In the biomedical research field, protein microarray
is increasing its utility in assisting treatment efficacy by
evaluating for example certain apoptotic markers (e.g., BCL-2,
BCL-X and BAD) upon the application of various therapies (9), or assessing transcriptional activity
in cells that were subjected to therapy (10). Thus, protein microarray has
developed into a proteomic tool that can deliver high-throughput
data for revealing novel therapeutic targets. There are currently
three main types of protein microarrays based on their reaction
principle and application: Analytical or antibody, reverse-phase
and functional protein microarrays (11).
Among all the developed variants, in oncology, the
antibody array type is the preferred one, where it applies
discovery and quantification to biomarkers. The antibody array
layout has high versatility and reproducibility (12). By contrast, the reverse-phase
array format has a lower standardization potential and multiplexing
possibilities and it is prone to cross-reactivity, as all the
proteins harbored by complex samples are investigated at once
(13).
These different protein microarray formats can
accommodate a variable number of spots, and can be used to
successfully aid personalized medicine. Protein arrays can
encompass >1,000 elements per array and these ones are termed
‘high-density arrays’ (14). Such
arrays are used for the identification of new proteins or novel
protein/protein interactions. Protein libraries or even unknown
elements can be spotted on the array and various biological samples
can be used. The detection is insured by antibodies that are
directly labeled with a fluorophore. Protein-protein binding events
can be also detected in these formats using specific antibodies
(15,16). Reverse-phase protein arrays use a
lower range of samples, up to hundreds, to identify a small number
of antigens. This format can use cell lysates, micro-dissected
tissues/cells, and biological fluids, such as plasma and serum. The
detection antibody labeled with a fluorophore would visualize the
reaction between capture antibody and analyte of interest from a
biological sample (17).
‘Low-density arrays’ with 9-100 elements per array
are antibody arrays that are used for the quantitative detection of
proteins in cells, tissues and biological fluids samples. The
density of the antibody array is constantly expanding due to the
generation of high-affinity antibodies (18). Thus, specific antibodies are
arrayed and they will capture antigens from samples. The identified
antigen will be directly labeled with a fluorophore or a secondary
antibody will be used for detection. This process is known from the
sandwich ELISA assay (19).
Table I presents an overall view
of the classification of major protein microarray formats.
 | Table IClassification of protein microarray
types (6). |
Table I
Classification of protein microarray
types (6).
| Classification
criteria | | Type | (Refs.) |
|---|
| Immobilized
structure | Direct | Standard:
Recombinant purified proteins are immobilized | (20,21) |
| | | Analytical:
Antibodies are immobilized | (5) |
| | Indirect | Reverse phase
protein microarray (total or fractionated cellular lysates) | (8) |
| Determined
parameter | Abundance | Capture | (22) |
| | | Indirect | (23) |
| | Functional | Protein in
situ arrays | (24,25) |
| | | In situ
puromycin arrays | (26) |
| | | Nucleic acids
programmable protein (NAPPA) array | (27,28) |
Protein microarrays: ‘Ongoing
future’
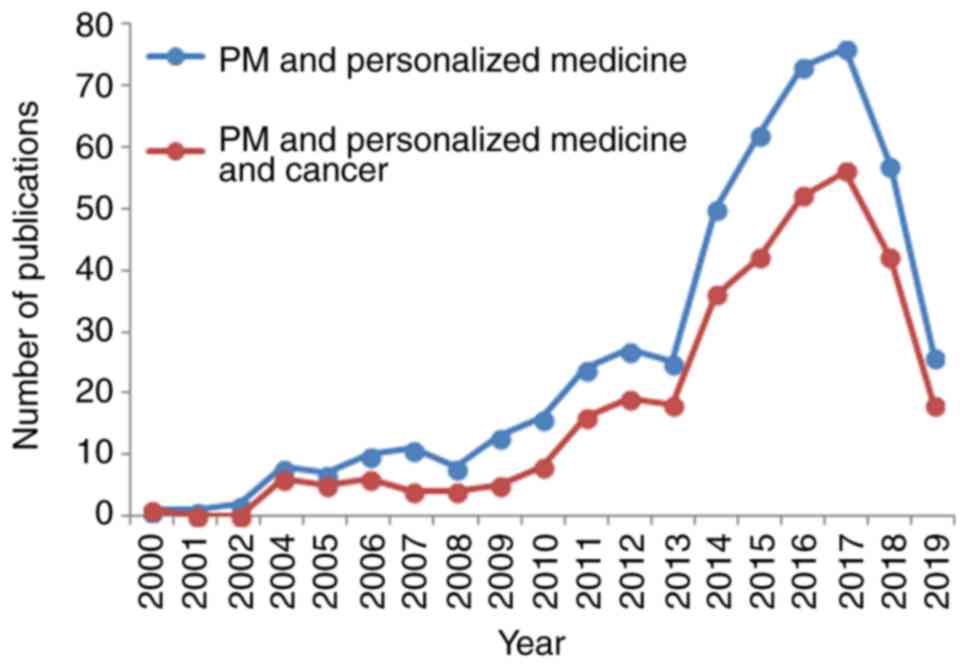
Following an investigation of the PubMed database
using key words, such as ‘Protein microarray’ AND ‘Personalized
Medicine’ and ‘Protein microarray’ AND ‘Personalized Medicine’ AND
‘Cancer’, we obtained some very interesting facts on this issue
(Fig. 2). First of all, an
increase in the number of publications can be observed, beginning
from the year 2012, and subsequently maintaining an accelerated
trend up to 2017, when the number of publications decreased.
Secondly, the publications for protein microarrays in personalized
medicine has an identical trend for its use in cancer, this trend
showing that the majority of publications in personalized medicine
are based on this type of human pathology.
Tumorigenesis is a multi-proteomic complex process
in which the transformed cells will crosstalk with immune and
stromal cells, favoring tumor development, dissemination,
neo-angiogenesis, enhance immune-escaping processes,
epithelial-to-mesenchymal transition, invasion and multi-drug
resistance (29). All these
processes rely on direct contact through protein-based receptors
and ligands or soluble protein molecules (e.g., growth factors,
cytokines and chemokines). Recently nucleic acids structures, such
as microRNAs encompassed in extracellular vesicles are also
reported as communication avenues (30).
Proteomics represents the large-scale study of
proteins, depicting structure, interactions and functions. Amidst
proteomics domain protein microarray stands in these
high-throughput approaches (31).
Protein microarray is involved in deciphering
cellular differentiation, transformation, angiogenesis,
tumorigenesis and metastasis. Due to its development, it can detect
alterations in protein expression levels, identify
post-translational modification, mRNA events, and identify
molecular networks triggered by therapeutic approaches, but also by
environmental factors. The future of protein microarray is
continuously expanding, so that profiling the cancer signaling
network, personalizing therapy and improving diagnosis and
prognosis would take a step forward (32).
The accelerated future of protein microarray relies
on several domains: Improving its technicalities approaches with
improved sensibility and specificity, expanding on areas, such as
cell-free microarrays and immunoproteomics and last, but not least,
develop the bioinformatics technologies that actively sustains this
type of technology (32).
Proteomics techniques would provide information on the proteins and
peptides, and the dynamics of their interconnections. Hence, a
number of methodological developments and innovations have been
reported over the past decade. Protein networks are best studied
using nucleic acid programmable protein array (NAPPA). Following
its design a decade ago (33), it
is evolving with high accuracy, increased reproducibility,
throughput and flexibility in diagnostic and therapeutic
applications. NAPPA is an essential tool that deciphers functional
proteomics along with protein-protein interaction (34). In NAPPA microarrays, the
extra-well fluorescence automated acquisition was reported.
Different approaches have been used to identify spots with
extra-well fluorescence (rings) in the microarray images and the
reported system, would identify in a significantly more rapid
manner, than any human would, this extra-fluorescence, while
maintaining high performance for microarray image analysis
(35).
Cell-free protein microarrays are an array variant
which display fresh proteins, avoiding storage and denaturation. In
basic and translational research, this is a format that is steadily
increasing in order to identify protein-protein interactions,
pathogen-host associations, post-translational modifications, and
antibody-type biomarkers (36).
Displaying actually naturally-folded proteins has increased the
risk of spot-to-spot crosstalk due to protein diffusion during
expression. Thus, recently, the multiplexed nucleic acid
programmable protein array (M-NAPPA) was reported. This improved
technology increases the number of displayed proteins through five
different gene plasmids in a single printed spot. Due to this
improved technology, M-NAPPA, an ultra-high density proteome
microarray could be done having >16,000 proteins per slide. This
multiplexing has improved features, and is a new protein microarray
for high-throughput translational research (37).
Immunoproteomics is actually a recent extension of
the proteomics domain study of immune-related proteins and peptides
(38) and encompasses the future
of protein microarrays. Immunoproteomics began over 30 years ago
for the identification of tumor antigens, and is one of the main
goals in oncology (39). Proteins
released by the tumor trigger an immune response activating
antigen-specific T and B cells. An efficient immunotherapy destroys
tumor cells, cellular proteins are released and T and B lymphocytes
are activated. As new antigens are spreading, auto-antibodies are
generated, and thus auto-antibodies against tumor antigens can be a
measure of efficient immunotherapy (40).
From the early 1980s, several immunoproteomics
methodologies were approached. Thus, serologic proteome analysis
(SERPA), accompanied by serological analysis of recombinant tumor
cDNA expression libraries (SEREX) aided by protein microarrays were
lately exploring tumour antigens using specific antibodies
(41). SERPA can screen antibody
profiles in patient serum using proteins appended to a membrane and
is a highly used immunoproteomics workflow. SEREX actually
discovered the first cancer testis antigen, NY-ESO-1(42). SERPA and SEREX have their
limitations, mainly due to the proteins on the SEREX membrane
expressed by the library expressed in bacteria, expression levels
that cannot cover human post-translational protein modification.
Furthermore, protein microarrays have additional characteristics,
such as thousands of pure proteins that are immobilized on a glass
surface. For example, ProtoArray® (functional array),
can analyze concomitantly thousands of proteins from a serum
sample. These types of protein microarray have developed a new
domain known as seromics (43).
Tumor-associated antigens and their generated antibodies are the
major proteins that can be detected using proteomics (44). The use of SEREX tumor antigens
that elicit a high IgG antibody titer in sera from patients
diagnosed with different types of cancer has been established
(45).
Cancer immunotherapies, entering clinical
management, have taken advantage from the research stage of protein
microarray technology. Auto-antibodies generated during therapy
were correlated with tumor progression in patients diagnosed with
prostate, lung, ovarian and breast cancer (46-48).
Sipuleucel-T therapy, actually the first approved adoptive cell
therapy, is based on an antigen spreading that leads to an immune
response and an improved overall survival (49). In prostate cancer, cytotoxic T
lymphocyte antigen 4 (CTLA-4) blockade generates a broad antibody
response in therapy responsive patients (49) and moreover, this response is
directed to proteins that were reported as mutated in prostate
cancers (50).
The advantages brought by protein microarray
technology include a reduction in biological sample volume, high
sensitivity/specificity and large data generation. The sensitivity
and specificity of a protein microarray with 329 proteins gives a
94% concordance with standard ELISAs (51). As in gene microarrays, there are
several standard methodologies that should be followed in order to
obtain accurate data, such as proper biological sample collection,
correct storage and standard laboratory procedures that avoid
inter- and intra-assay variation, thus reinforcing data
reproducibility (32).
In addition, bioinformatics specific for protein
microarray needs specific development in order to process large
amounts of data arising. Analyzing protein microarray data implies
the following steps: Data acquisition, data pre-processing,
visualization, differential analysis, computational annotation and
network analysis (52,53).
The use of detection antibodies provides very good
sensitivity and specificity for protein identification, but it has
also some limitations. Antibodies are also proteins and thus, any
disturbances that will alter their structure (e.g., pH and
temperature) will affect the binding specificity. Antigen-antibody
interaction has complex kinetics, and again, any conditions that
would influence this interaction can hider both the specificity and
affinity of the bonding (54).
The selection of the ‘perfect antibody’ is another limitation of
the technology, as it should possess strong affinities and
specificity displayed for specific proteins, more so if intimate
modifications of the proteins are to be identified, e.g.,
phosphorylation, glycosylation or proteolysis-related compounds.
When the specific protein microarray has also a quantification
need, hundreds of supposed antibody-antigen complexes are
established having independent affinity parameters. Protein
concentrations in the samples, whether biological fluids,
cell/tissue lysates, can have hundreds of fold different scale.
Therefore, detection needs to cover concentrations over many orders
of magnitude (53,54). An overview of major advantages and
disadvantages for the most used protein microarrays variants
(55-57)
is presented in Table II.
 | Table IIAdvantages and disadvantages in using
the major protein microarray types in personalized medicine. |
Table II
Advantages and disadvantages in using
the major protein microarray types in personalized medicine.
| Main type of
protein array method |
Analytical/antibody/indirect labeling
(sandwich) |
Reverse-phase/direct labeling |
|---|
| |
- Assess
multiple analytes simultaneously |
| |
- Biomarker
discovery |
| |
- Low sample
consumption |
| Advantages | - Could be arrayed
as semi-quantitative or quantitative formats | - Compares patterns
of two analytes with different fluorophores |
| | - Improved
specificity by using two specific antibodies (for capture and
detection) | - Rapid screening
of many different samples |
| Disadvantages | - Requires two
specific antibodies for each target from sample of interest | - Specific
antibodies could give cross reactions and hence could provide false
results |
| | - Still a
challenging task to multiplex related to the feasibility of
designing arrays with thousands of analytes | - Rare analytes
from biological samples may not be caught |
| | | - Relatively high
production costs |
With the advent of the Human Genome Project
depicting just >20,000 genes, it became evident that a new
initiative should be covered at the proteome level. Hence, 2010 was
the year for launching the Human Proteome Project. The
technological strategy to cover this huge scientific endeavor
implied ‘three working pillars’: Mass spectrometry, arrays and
bioinformatics tools. If the first two pillars were based on the
advantages that quantitative mass spectrometry and protein capture
with antibodies brought a decade ago, the third one actually was
based on the global exchange of databases and the availability of
large primary data (58-59). This huge
on-going project has driven, apart from the enormous scientific
gain, a series of proteomics and bioinformatics methods. Hence,
shotgun and selected reaction monitoring (SRM)/multiple reaction
monitoring (MRM)-based proteomics were developed. The combination
of different omics technologies has led to the development of
intermingled domains, such as epitranscriptomics (60), proteogenomics, metabolomics and so
on (61). Moreover, the Human
Proteome Project has contributed to the clinical need for more
targeted/individualized therapies, laying the foundation for the
development of personalized medicine. Although fundamental research
purposes prevail in the development of array platforms, there is a
recent increasing trend in clinical research, diagnostics and even
industry applications.
2. Personalized medicine: A step forward in
improving disease management
Personalized medicine intends to unveil the
molecular mechanisms of disease onset and to integrate it with
individual genomics/proteomics/metabolomics profiles in order to
define the most suitable drugs and provide a prognosis of the
disease outcome (62). There are
several fields that must be accomplished when dealing with the
personalized medicine domain. First, a proper biomarker
characterization for predicting the outcome and correct diagnostic
markers must be identified, secondly it has to evaluate the
perfectly matched therapy regimen and last, but not least,
monitoring the disease and therapeutic efficacy come to complete a
personalized approach. All these items can be evaluated by omics
techniques (62).
‘Diagnostic biomarkers’ would be represented by
protein expression from signaling pathways accompanied by key
mutations. These markers will indicate the best drug(s) that will
offer the optimal response in therapy-related decisions.
‘Prognostic biomarkers’ would encompass somatic germline mutations,
epitranscriptomics modifications, miRNAs patterns and circulating
tumor cells, and would thus determine disease outcome (63).
In personalized medicine, there is a clear necessity
to identify the network comprising the
genome-transcriptome-proteome patient profile. In oncology, it was
estimated for 2018, that >1.5 million new cases of cancer would
be registered in the US, while there would be over half a million
cancer-related deaths. According to the Annual Report, the most
common types of cancer will be ‘breast cancer, lung and bronchus
cancer, prostate cancer, colon and rectum cancer, melanoma of the
skin, bladder cancer, non-Hodgkin lymphoma, kidney and renal pelvis
cancer, endometrial cancer, leukemia, pancreatic cancer, thyroid
cancer and liver cancer’. Moreover, cancer incidence could achieve
pandemic levels by the year 2025(64).
Therefore, a complex approach in health needs
high-throughput technologies that can sustain personalized
medicine. Developing the two-tiered health system (65) to two-tiered personalized medicines
is a demanding desiderate in oncology. The implementation of omics
facilities in clinical practice is warranted in order to offer
effective personalized medicine to the patient. However, in order
to for this to be accomplished, there are several draw-backs that
first need to be resolved, such as the high costs of
implementation, differences between data generation and the
capacity to analyze large amounts of data, the existence of
multidisciplinary teams and global economic relevance (66).
Protein microarrays paving the way for
personalized medicine
Personalized medicine intends to stratify patients
as per disease subtype, risk factors, evaluated prognosis and/or
treatment response. An interdisciplinary effort is needed between
several specialties, physicians, data scientists and health
insurance systems to provide un-biased advantage to clinical
practice (67).
As stated in the ‘Introduction’, DNA arrays can
analyze the whole transcriptome and can screen myriads of
single-nucleotide polymorphisms, allowing further correlations of
gene expression with disease progression. These analyses of
disease-specific mutations can lead to setting specific therapies
in accordance with their gene profiles. However, a more direct
correlation with disease development is established by protein
function, regulation and abundance. Driving the development of the
disease protein concentrations within an organ, tissue, or cell can
pinpoint an abnormality. Thus a patient's genotype can provide
information regarding a particular stratification of the disease;
however, the protein particularities, abundance, localization
within the tumor cell/tissue and so on, would follow and describe
the actual disease stage and would orient/personalize the therapy.
Thus, personalized medicine would cover: ‘Right patient/target,
right diagnosis, right treatment, right drug/target, and right
dose/time’ (68). This goal can
be achieved, however, only by combining genomic knowledge with
traditional clinical approaches, the patient's personal medical and
family history, and relevant clinical data, such as imaging and
in vitro diagnostics results (69). Profiling using protein
microarrays, can be efficiently applied in biomarker discovery,
validation and diagnosis, and can aid personalized medicine
(69). All the pathways that are
deregulated in tumorigenesis and that are the result of genetic
alterations accumulation can be, at least theoretically,
therapeutic targets in oncology. The real-life efficacy of such
molecular therapeutics is highly variable among individuals; thus,
minute details of such differences can be identified. Reverse-phase
protein array/microarray (RPPA/RPPM) can precisely map active
molecules in each patient. This identification is essential for the
optimization of therapy. RPPA is an antibody-based highly
quantitative proteomic technology, used for profiling the
expression and modification of signaling proteins, mainly in
low-abundance analytes cases. Clinical trials are using RPPA
technology molecular-targeted therapeutics (70); some of have not yet been completed
(https://clinicaltrials.gov/ct2/show/NCT01042379),
while others are already closed and waiting for the results of the
evaluation (https://clinicaltrials.gov/ct2/show/NCT01023477,
https://clinicaltrials.gov/ct2/show/NCT01074814). For
example, in breast cancer, which is a heterogeneous disease with
various histological and molecular variants, personalized medicine
has become a major goal for patient management over the past years.
In this type of cancer, molecular profiling and genomic analysis
based on array technologies have led to the discovery of targeted
drug therapies (71).
Using a designed RPPA, personalized therapy was
intended to search for the most effective drug. Thus, drug
sensitivity can be predicted in this system based on the
quantitative profiles of protein expression (72,73). Signaling transduction pathways
that trigger oncogenesis can also be depicted by proteomics
profiling and these particularities can lead the option for
personalized therapy (74,75).
To increase the quantization sensitivity of RPPA
(76,77) labeling was reported to be far more
accurate when using quantum dots (Qdots). Briefly, sample lysates
are used for serial dilution and then immobilized on the array.
Primary and secondary antibodies detect the immobilized proteins,
and the reaction is further detected by Qdot assay. Qdot is
actually a fluorophore with a nanometal structure that develops a
clear linear signal, photo-bleaching from the organic fluorophores
(78-80).
In this form, RPPA would detect post-translational modifications,
such as phosphorylation, modifications that are seminal for
depicting intracellular events related to drug sensitivity
(72).
Another recent version of protein arrays was
published, bringing new information to personalized medicine. Thus,
antibody co-localization microarray (ACM), avoids some draw-backs
from classical protein microarrays (e.g., reagent-induced
cross-reactivity), as detection antibodies are printed atop of
their individual capture antibodies. Several parameters are
improved, and therefore low volumes of sample and hence, low
quantities of reagents are used. In this manner, up to 108
different protein-targets can be measured in hundreds of samples,
displaying high specificity and sensitivity (81).
As protein microarrays are furnishing a plethora of
records, several systems for data analysis have been reported.
Ingenuity Pathway Analysis (IPA) is the most commonly used software
for protein microarray data exploration (54). This software links to published
database and finds function(s) and pathway(s) for microarray
analysis. It can be used to integrate complex data from gene
expression, microRNAs, single nucleotide polymorphisms (SNPs) and
protein microarray (82). IPA
ranks the genes and the encoded proteins as plausible candidate
biomarker(s), identifies if a particular gene/protein can be
detected in various tissues and/or other body fluids, it selects
the relevant parameters for a specific biomarker discovery and
shows possible links to a specific disease/biological process;
moreover, it generates a list of unique markers to one treatment or
disease, or reveals markers common for different
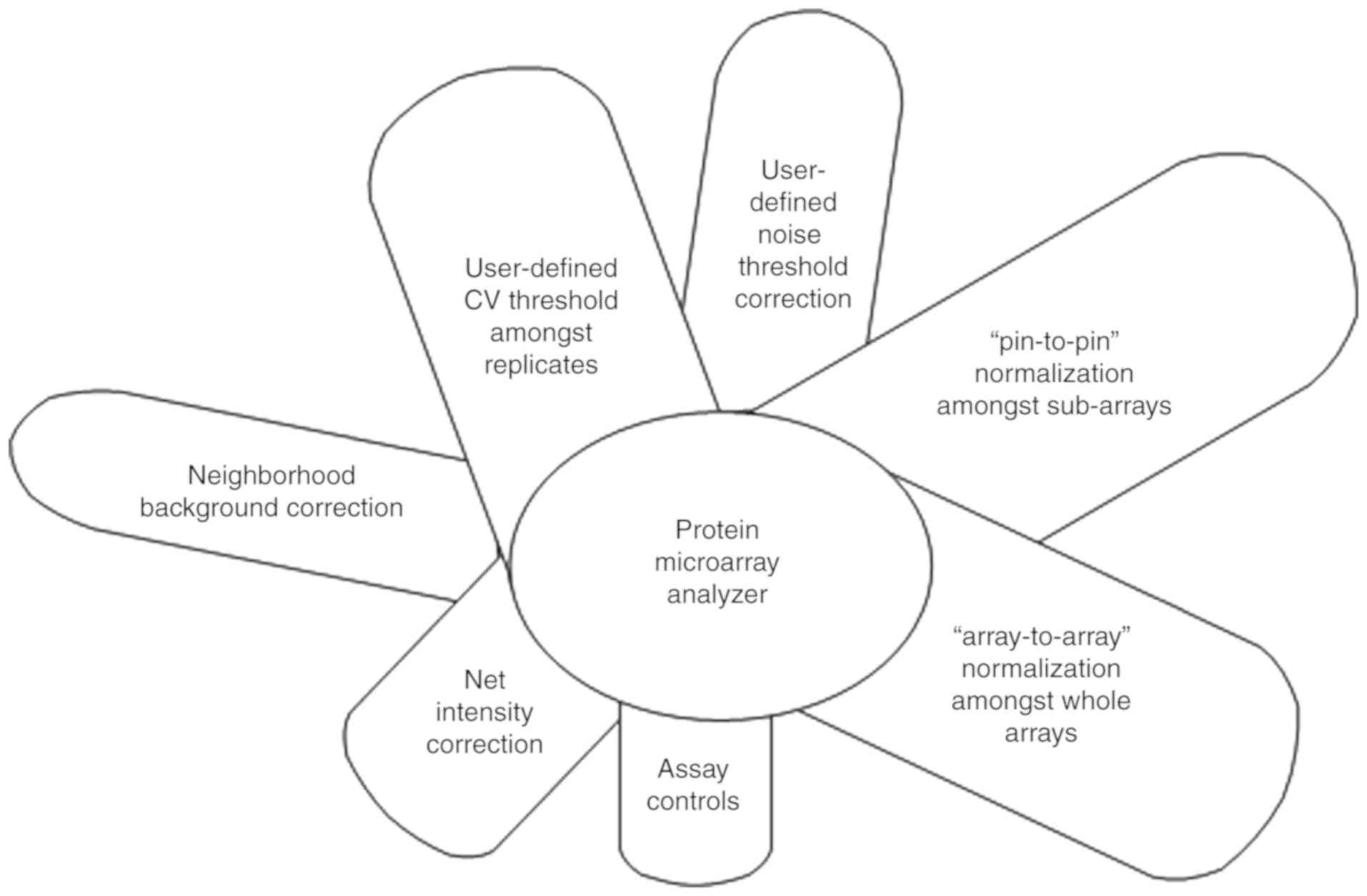
diseases/therapies. Practice has shown that improvements in data
processing systems are warranted; thus, recently reported Protein
Microarray Analyzer software has several improved tools, as shown
in Fig. 3.
To identify tumor-associated antigens (TAAs),
antibody response and new antigen discovery other software were
specifically developed for protein microarrays used in seromics,
namely Prospector, LIMMA package, PAA package and Spotfire package
(49,50,53,83).
The newest intervention of protein microarray
technology was reported for the revolutionary immunotherapies that
were recently approved. New combinations of therapies are tested in
pre-clinical settings. In mutant Kirsten rat sarcoma (KRAS) and
tumor protein 53 (TP53) (KP) mouse models of non-small cell lung
cancer (NSCLC), combinations of anti-programmed cell death protein
1 ligand (PD-L1), anti-CTLA-4 with mitogen-activated protein kinase
kinase (MEK) inhibitors decreased tumor growth and metastasis. RPPA
analysis and flow cytometric analysis of the tumors revealed an
enhanced expression of Tregs and CTLA-4. Combining anti-CTLA-4 and
anti PD-L1 with MEK inhibitor in a mouse model with good disease
evolution has driven a phase I/II clinical protocol in humans that
is now undergoing regulatory revision and enrollment began in
2019(84).
3. Protein microarray in oncology
One of the main target domain of personalized
medicine is the field of oncology, where the number of pre-clinical
models and clinical trials has increased over the past years
(85); thus, new proteomics
technologies have been put to use in acknowledging
proteomic/genomic/transcriptomic/metabolic individualities
(71). In this section, we will
comment on the most frequent human pathologies that entail protein
microarray technology in order to come one step closer to
personalized therapy.
Personalized medicine through protein
microarray in pulmonary disease
Pulmonary diseases infer a large range of biological
samples, from sputum, to pulmonary epithelial lining fluid,
bronchoalveolar and nasal lavage fluids, to exhaled breath
condensate, and finally, blood plasma/serum (86). Due to this extended array of
samples, the proteins that can be identified in these diseases
comprise a huge span of molecules in terms of types and
concentrations. Various proteomics technologies have been used;
thus, PRoteomics IDEntifications (PRIDE) of the European
Bioinformatics Institute has issued, over the past years, a
database, containing >70,000 assays (87). In a previous study, the analysis
of plasma from >100 children diagnosed with asthma evaluated for
proteome patterns demonstrated that in comparison to the controls,
there were 3 overexpressed proteins [chemokine (C-C motif) ligand 5
(CCL5), hematopoietic prostaglandin D2 synthase (HPGDS) and
neuropeptide S receptor (member of the G protein-coupled receptor 1
family) (NPSR)] with potential to be used as biomarkers (88).
Sputum proteomes from lung diseases [e.g., asthma
and chronic obstructive pulmonary disease (COPD)] have been proven
to exhibit enhanced concentrations of various other proteins, such
as calgranulin A and B (89).
Lung tissue from non-smokers compared to smokers and COPD, has been
shown to have a different proteome, in which cathepsin D (CTSD),
dihydropyrimidinase like 2 (DPYSL2), transglutaminase 2 (TGM2) and
tripeptidyl peptidase 1 (TPP1) are differently expressed (90,91).
In acute respiratory distress (ARDS), drug
development and biomarkers to prognosticate the disease are crucial
(92). Deregulated cellular
pathways leading to inflammation and epithelial injury have been
revealed in ARDS. In lung tissue, as well as in biological fluids,
several proteins have been found to be overexpressed, such as
apolipoprotein A1, hemoglobin α and hemoglobin β (93), osteopontin, matrix
metalloproteinase (MMP)7, CXCL7, chemokine (C-X-C motif) ligand 7
(CXCL7), chemokine (C-C motif) ligand 18 (CCL18) and eosinophil-
and neutrophil-derived proteins (94). However, there are still no
validated markers available for subclassifying patients (92,95,96).
Therefore, subgroups of patients displaying particular molecular
and clinical parameters could be identified using integrative omics
data that will be required to accelerate personalized medicine
upcoming in pulmonary diseases (97).
Lung cancer is the primary cause of cancer-related
mortality in the United States, with a low 5-year survival rate of
18%; therefore, this type of disease is an important subject in the
light of personalized medicine. In a recent study, apart from the
genomic profiling of lung cancer cell-surface markers, protein
microarray was used to validate the high expression of the six
selected markers that were identified in all the analyses [carbonic
anhydrase 9 (CA9), carbonic anhydrase 12 (CA12), cancer/testis
antigen 83 (CT83; also known as CXorf61), G protein-coupled
receptor 87 (GPR87), LY6/PLAUR domain containing 3 (LYPD3) and
solute carrier family 7 member 11 (SLC7A11)], these factors being
associated with a worse survival. Hence, these markers could be
further used for personalized care in patients with lung cancer
(98). In lung cancer therapy,
the identification of immune-checkpoints before therapy commences
is a recent goal of personalized medicine. Tissue arrays and
multiplex immunofluorescence have been used to evaluate 25
different types of immune-checkpoints and neoantigens. A recent
study demonstrated that in lung therapy, protein-protein
interaction and thorough intracellular signaling pathway mapping
can reveal immune-checkpoint nodes that are associated with
positive outcomes of the administered therapy (99).
Personalized medicine in breast and
ovarian cancers using protein microarray.
As breast cancer is the second leading cause of
cancer-related mortality among women worldwide, research has
advanced at an accelerated pace. Over the past ten years,
significant assessments, such as cytogenetics, SNPs and gene
expression arrays, copy number variation and DNA methylation, have
aimed to divide breast cancer types on a genetic basis. Apart from
the genomic profiles, proteomics has begun to ‘take the
battlefield’. Thus, ten years ago, a comparison of proteomics
profiling of invasive ductal carcinoma and normal tissues
demonstrated that out of the 160 proteins/phospho-proteins tested,
56 were differentially expressed [e.g., Twist family BHLH
transcription factor (Twist), Fas cell surface death receptor
(Fas), proliferating cell nuclear antigen (PCNA), phosphatase and
tensin homolog (PTEN) and cyclin B1]. These proteomics profiles
distinguished tumor tissue from normal tissue with a 96% accuracy
(54).
Recent proteomics studies have focused on
drug-induced signaling events that would trigger a process that is
of seminal importance to clinical application, namely acquired drug
resistance. Following treatment with agents targeting human
epidermal growth factor receptor 2 (HER2), phosphoproteome of the
tumor cells was quantified using tandem mass tag liquid
chromatography/tandem mass spectrometry (TMT LC-MS/MS). The
activation of kinases families (e.g., serine/threonine and tyrosine
kinases) following treatment was identified by peptide Chip array.
Using these proteomics approaches, a common adaptive kinase
response process was reported, involving the activation of the
focal adhesion kinase 1 (FAK1), protein kinase C-δ (PRKCD) and
ephrin (EPH) family receptors. These approaches bring into the
light individual networks that can be activated when acquiring
resistance to HER2-targeted therapies (100).
In the same area, an experimental model was recently
reported, in which HR+/HER2+ patient-derived
xenograft (PDX) were established in order to individualize
HER2+ breast cancer therapies. Transcriptomic and
proteomic profiling were used (RNA sequencing and RPPA) for
establishing the molecular particularities of the PDX models. The
reported results revealed that apart from standard trastuzumab
therapies, the combination with the dual mammalian target of
rapamycin (mTOR) complex inhibitor impeded tumor growth. Thus, this
study opens the door for personalized medicine clinical trials
(101).
From lysates obtained from samples of breast cancer
tissues, a personalized medicine protocol was recently reported
using RPPA. This protocol could be used for the pharmacodynamic
effects of standard therapies in various molecular subtypes
(102).
Drug resistance in breast cancer is a therapeutic
domain where protein microarray can bring new information.
Chemotherapy-resistant breast cancer stem cells (CSCs) were
analyzed with protein arrays and the paclitaxel-resistant phenotype
was associated with the overexpression of several proteins, such as
growth factors, MMP proteins, Frizzled proteins and interleukin
(IL)-23(103).
Ovarian cancer also has a large array of subtypes,
serous, clear cell, endometrioid and mucinous epithelial ovarian
carcinoma, all these having various chemotherapeutic sensitivities.
Clear cell carcinoma (CCC) has high rates of recurrence associated
with low chemosensitivity. Using RPPA, possible protein biomarkers
have been investigated in patients with CCC and in high-grade
serous carcinoma (HGSC). Thus, HER2 and PD-L1 expression levels
were higher in patients with CCC, and aurora kinase A (AURKA) and
PD-L1 were associated with CCC chemoresistance. The reported
differences would lead to new candidate target drugs (104).
In CCC, various activating pathways have been
reported, yet again, possible personalized drug targets (105). Proteins appending to various
networks (Table III) (106,107) were recently identified using
RPPA. For example, 11 out of 117 proteins identified from CCC
samples were appending to different signaling networks in
comparisons to other ovarian cancer samples, giving ground to the
further development of personalized therapy in this particular type
of ovarian cancer which is difficult to treat (108).
 | Table IIISignaling pathways that are enhanced
in ovarian clear cell carcinoma in comparison to other sub-types
(108). |
Table III
Signaling pathways that are enhanced
in ovarian clear cell carcinoma in comparison to other sub-types
(108).
| Pathway | Proteins |
|---|
| mTOR | PI3K/AKT |
| VEGF | HIF-1α/VEGF |
| HNF-1β | HNF-1β |
| IL-6 | IL-6/STAT3 |
| MET | Ligand hepatocyte
growth factor |
Other types of diseases taking
advantage of protein microarray technology in personalized
medicine
In clear cell renal carcinoma (ccRCC), which is the
most frequent renal cancer type, it is assumed that one third of
patients would progress after surgery. Therefore, establishing
molecular patterns that would stratify patients would significantly
improve survival. In vitro cultures established from patient
specimens have been used to develop orthotopic xenograft tumors in
animal models. RPPA was used to evaluate the proteome in tumor
cells and it was shown that tumor-propagating cells had clear
altered kinase cascades, alterations that were associated with
stage, the angiogenesis level and mTOR pathways. Testing in
vitro and in vivo pharmacological action on ccRCC tumor
cells can bring a personalized screening for therapies in patients,
hence personalizing the therapy. Accordinly, only a high-throughput
profiling, such as the one provided by RPPA could cover all the
triggered pathways (109).
Custom RPPA was used to establish the protein
profiling in pediatric acute myeloid leukemia (AML) bone marrow
samples in comparison to normal samples. Protein functional groups
and protein clusters identification has shown that there are 12
protein clusters that can stratify AML patients into 8 protein
signatures. The identification of particular protein signatures
creates the premises for specific combinations of therapies with
increased therapeutic efficacy (110). Thus, in a phase II clinical
trial, the efficacy and safety of a combination of the pan-AKT
inhibitor (GSK2141795) and the MEK inhibitor, trametinib, in
RAS-mutated AML were examined. Using RPPA the phospho-flow analysis
assessment of the MEK and AKT pathways was performed in patients
receiving this combination of drugs (111).
In colorectal cancer the immunoproteomics endeavor
was reported for discovering auto-antibodies as possible cancer
markers. Tissue proteins were extracted from primary tumors,
metastastic and benign tissues, and autoantigens were identified.
Olfactomedin 4 (OLFM4), CD11b, integrin α2 (ITGA2), periostin and
thrombospondin-2 were the main proteins found to be overexpressed
in tumors in comparison to benign samples using a tissue
microarray. These autoantigens can have prognostic significance in
colorectal cancer that has a tendency to induce liver metastases.
Autoantibodies can be found in the sera of patients diagnosed with
colorectal cancer; thus, finding the tissue antigens that are
specific for the neoplastic tissue is of outmost importance in
personalizing therapy (112).
When studying the association of angiogenesis-related proteins with
anti-angiogenic therapy in colorectal cancer protein, arrays were
used. The proteome profiler array identified in dynamics the
proteins before, after treatment and after tumor progression. The
antibody arrays revealed that during treatment, alterations in the
levels of protein, such as MMP8, tissue inhibitor of matrix
metalloproteinase (TIMP)4 and epidermal growth factor (EGF) were
observed. IL-8, Activin A and IGFBP-2, had a low association with
chemotherapy induction and disease progression (113).
In organ transplantation, immunological rejection is
the main clinical drawback; thus, the optimal proteomics
characterization would ensure the best match between donor and
recipient. Recently, a screening tool was developed using peptide
array from the donor's human leukocyte antigen (HLA) to assess
post-transplant sera from the recipient and evaluate the risk of
immune-mediated rejection. In this pilot study, up to 600
individual peptides were customized. On these arrays pre- and
post-transplant sera of recipient were investigated and, with great
accuracy the immune epitopes that were involved in the immune
response were detected. These personalized arrays could pin-point
the donor-specific HLA epitopes and further allow the therapeutic
approach to be personalized in organ transplantation (114).
In acknowledging the enhanced role of protein
microarrays in research and clinical application we foresee an
increased use of this technology in biomarker discovery and
validation and their increased involvement in personalized
medicine.
4. Conclusions and future perspectives
Particular proteomic-individualities of patients
have recently driven the initiation of personalized medicine and
these particularities can be thoroughly done only with advanced
technologies (115). The Human
Genome Project, has shown that there are multiple players in human
disease development where the transcriptome, proteome and
metabolome entered this field. However, the Human Proteome Project
would never have been accomplished without the aid of several
high-through-put proteomic technologies, one of these being
antibody-based protein microarrays (21). New formats were constantly
evolving to tackle the personalized proteome analysis. With these
new formats of microarrays, parallel analyses can be carried out,
investigating variations in protein structure and moreover protein
interaction particular to each biological sample (116).
As cancer has a complex multifactorial trait and it
is the result of acquired dysregulation at various levels (genomic,
epigenomic, proteomic and metabolomics) complex multi-molecular
signatures obtained from these domains would bring new information
on cancer diagnosis, prognosis and personalized treatment.
Platforms combining protein microarrays with bioinformatics, are
bringing new tools for the further development of personalized
medicine to medical and scientific communities. Protein microarrays
are involved in oncology for identifying and validating new
biomarkers, depicting molecules for early detection, and can
monitor disease and select optimal therapeutic strategies. These
platforms can intervene in all fields of oncology, as this
proteomics technology can screen a multitude of parameters and
encumbers tremendous future potential for applications in
diagnostic and personalized medicine.
Acknowledgements
Not applicable.
Funding
This study was supported by the following grants:
PN-III-P1-1.2-PCCDI-2017-0341 (PATHDERM),
PN-III-P1-1.2-PCCDI-2017-0782 (REGMED) and PN 19.29.01.01.
Availability of data and materials
Not applicable.
Authors' contributions
MN, MB and CC were involved in the conception and
design of the manuscript, and in the writing, drafting, revising
and editing of the manuscript, and in the processing of the figures
and tables. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Schena M, Shalon D, Davis RW and Brown PO:
Quantitative monitoring of gene expression patterns with a
complementary DNA microarray. Science. 270:467–470. 1995.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gygi SP, Rochon Y, Franza BR and Aebersold
R: Correlation between protein and mRNA abundance in yeast. Mol
Cell Biol. 19:1720–1730. 1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Haab BB: Antibody arrays in cancer
research. Mol Cell Proteomics. 4:377–383. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen CS and Zhu H: Protein microarrays.
Biotechniques. 40(423, 425, 427)2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kopf E and Zharhary D: Antibody arrays-An
emerging tool in cancer proteomics. Int J Biochem Cell Biol.
39:1305–1317. 2007.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Constantin C, Surcel M, Munteanu A and
Neagu M: Protein microarray technology for antibody detection
associated to human pathology. Roman Arch Microbiol Immunol.
77:236–244. 2018.
|
|
7
|
Poetz O, Schwenk JM, Kramer S, Stoll D,
Templin MF and Joos TO: Protein microarrays: Catching the proteome.
Mech Ageing Dev. 126:161–170. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Speer R, Wulfkuhle J, Espina V, Aurajo R,
Edmiston KH, Liotta LA and Petricoin EF III: Development of reverse
phase protein microarrays for clinical applications and
patient-tailored therapy. Cancer Genomics Proteomics. 4:157–164.
2007.PubMed/NCBI
|
|
9
|
Matei C, Tampa M, Ion RM, Georgescu SR,
Dumitrascu GR, Constantin C and Neagu M: Protein microarray for
complex apoptosis monitoring of dysplastic oral keratinocytes in
experimental photodynamic therapy. Biol Res. 47(33)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Demyanenko SV, Uzdensky AB, Sharifulina
SA, Lapteva TO and Polyakova LP: PDT-induced epigenetic changes in
the mouse cerebral cortex: A protein microarray study. Biochim
Biophys Acta. 1840:262–270. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sutandy FX, Qian J, Chen CS and Zhu H:
Overview of protein microarrays. Curr Protoc Protein Sci. 27:Unit
27.1. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wilson JJ, Burgess R, Mao YQ, Luo S, Tang
H, Jones VS, Weisheng B, Huang RY, Chen X and Huang RP: Antibody
arrays in biomarker discovery. Adv Clin Chem. 69:255–324.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Díez P, Dasilva N, González-González M,
Matarraz S, Casado-Vela J, Orfao A and Fuentes M: Data analysis
strategies for protein microarrays. Microarrays (Basel). 1:64–83.
2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Angenendt P, Kreutzberger J, Glökler J and
Hoheisel JD: Generation of high density protein microarrays by
cell-free in situ expression of unpurified PCR products. Mol Cell
Proteomics. 5:1658–1666. 2006.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Schweitzer B, Predki P and Snyder M:
Microarrays to characterize protein interactions on a
whole-proteome scale. Proteomics. 3:2190–2199. 2003.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alhamdani MS, Schröder C and Hoheisel JD:
Oncoproteomic profiling with antibody microarrays. Genome Med.
1(68)2009.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Wulfkuhle JD, Aquino JA, Calvert VS,
Fishman DA, Coukos G, Liotta LA and Petricoin EF III: Signal
pathway profiling of ovarian cancer from human tissue specimens
using reverse-phase protein microarrays. Proteomics. 3:2085–2090.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuk CS, Lee HK, Kim HT, Choi YK, Lee BC,
Chun BH and Chung N: Development and evaluation of a protein
microarray chip for diagnosis of hepatitis C virus. Biotechnol
Lett. 26:1563–1568. 2004.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gowan SM, Hardcastle A, Hallsworth AE,
Valenti MR, Hunter LJ, de Haven Brandon AK, Garrett MD, Raynaud F,
Workman P, Aherne W and Eccles SA: Application of meso scale
technology for the measurement of phosphoproteins in human tumour
xenografts. Assay Drug Dev Technol. 5:391–401. 2007.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sigalotti L, Covre A, Fratta E, Parisi G,
Colizzi F, Rizzo A, Danielli R, Nicolay HJ, Coral S and Maio M:
Epigenetics of human cutaneous melanoma: Setting the stage for new
therapeutic strategies. J Transl Med. 8(56)2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Honda K, Ono M, Shitashige M, Masuda M,
Kamita M, Miura N and Yamada T: Proteomic approaches to the
discovery of cancer biomarkers for early detection and personalized
medicine. Jpn J Clin Oncol. 43:103–109. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yang L, Guo S, Li Y, Zhou S and Tao S:
Protein microarrays for systems biology. Acta Biochim Biophys Sin
(Shanghai). 43:161–71. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
LaBaer J and Ramachandran N: Protein
microarrays as tools for functional proteomics. Curr Opin Chem
Biol. 9:14–19. 2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hu S, Xie Z, Qian J, Blackshaw S and Zhu
H: Functional protein microarray technology. Wiley Interdiscip Rev
Syst Biol Med. 3:255–268. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
He M, Stoevesandt O and Taussig MJ: In
situ synthesis of protein arrays. Curr Opin Biotechnol. 19:4–9.
2008.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tao SC and Zhu H: Protein chip fabrication
by capture of nascent polypeptides. Nat Biotechnol. 24:1253–1254.
2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Miersch S and LaBaer J: Nucleic acid
programmable protein arrays: Versatile tools for array-based
functional protein studies. Curr Protoc Protein Sci Chapter.
27:Unit27.2. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wright C, Sibani S, Trudgian D, Fischer R,
Kessler B, LaBaer J and Bowness P: Detection of multiple
autoantibodies in patients with ankylosing spondylitis using
nucleic acid programmable protein arrays. Mol Cell Proteomics.
11:M9 00384. 2012.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Neagu M and Constantin C: Immunogenicity
of Stem Cell in Tumorigenesis Versus Regeneration. In: Stem Cells
between Regeneration and Tumorigenesis. Tanase C and Neagu M (eds).
Bentham Pbl. House. pp202–234. 2016. View Article : Google Scholar
|
|
30
|
Chiodoni C, Di Martino MT, Zazzeroni F,
Caraglia M, Donadelli M, Meschini S, Leonetti C and Scotlandi K:
Cell communication and signalling: How to turn bad language into
positive one. J Exp Clin Cancer Res. 38(128)2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
James P: Protein identification in the
post-genome era: The rapid rise of proteomics. Q Rev Biophys.
30:279–331. 1997.PubMed/NCBI
|
|
32
|
Joos T and Bachmann J: Protein
microarrays: Potentials and limitations. Front Biosci (Landmark
Ed). 14:4376–4385. 2009.PubMed/NCBI
|
|
33
|
Ramachandran N, Raphael JV, Hainsworth E,
Demirkan G, Fuentes MG, Rolfs A, Hu Y and LaBaer J: Next-generation
high-density self-assembling functional protein arrays. Nat
Methods. 5:535–538. 2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Manzano-Romána R and Fuentes M: A decade
of nucleic acid programmable protein arrays (NAPPA) availability:
News, actors, progress, prospects and access. J Proteomics.
198:27–35. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rivera R, Wang J, Yu X, Demirkan G, Hopper
M, Bian X, Tahsin T, Magee DM, Qiu J, LaBaer J and Wallstrom G:
Automatic identification and quantification of extra-well
fluorescence in microarray images. J Proteome Res. 16:3969–3977.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yu X, Petritis B, Duan H, Xu D and LaBaer
J: Advances in cell-free protein array methods. Expert Rev
Proteomics. 15:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu X, Song L, Petritis B, Bian X, Wang H,
Viloria J, Park J, Bui H, Li H, Wang J, et al: Multiplexed nucleic
acid programmable protein arrays. Theranostics. 7:4057–4070.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Bulman A, Neagu M and Constantin C:
Immunomics in skin cancer-improvement in diagnosis, prognosis and
therapy monitoring. Curr Proteomics. 10:202–217. 2013.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Neagu M and Constantin C: New Insights in
Cutaneous Melanoma Immune-Therapy-Tackling Immune-Suppression and
Specific Anti-Tumoral Response. In: Melanoma. Murph M (ed).
IntechOpen. pp225–246. 2015. View
Article : Google Scholar
|
|
40
|
Butterfield LH, Ribas A, Dissette VB,
Amarnani SN, Vu HT, Oseguera D, Wang HJ, Elashoff RM, McBride WH,
Mukherji B, et al: Determinant spreading associated with clinical
response in dendritic cell-based immunotherapy for malignant
melanoma. Clin Cancer Res. 9:998–1008. 2003.PubMed/NCBI
|
|
41
|
Yuan J, Wang E and Fox BA: Immune
monitoring technology primer: Protein microarray (‘seromics’). J
Immunother Cancer. 4(2)2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen YT, Scanlan MJ, Sahin U, Türeci O,
Gure AO, Tsang S, Williamson B, Stockert E, Pfreundschuh M and Old
LJ: A testicular antigen aberrantly expressed in human cancers
detected by autologous antibody screening. Proc Natl Acad Sci USA.
94:1914–1918. 1997.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fulton KM and Twine SM: Immunoproteomics:
Current technology and applications. Methods Mol Biol. 1061:21–57.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sahin U, Tureci O, Schmitt H, Cochlovius
B, Johannes T, Schmits R, Stenner F, Luo G, Schobert I and
Pfreundschuh M: Human neoplasms elicit multiple specific immune
responses in the autologous host. Proc Natl Acad Sci USA.
92:11810–11813. 1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ladd JJ, Chao T, Johnson MM, Qiu J, Chin
A, Israel R, Pitteri SJ, Mao J, Wu M, Amon LM, et al: Autoantibody
signatures involving glycolysis and splicesome proteins precede a
diagnosis of breast cancer among postmenopausal women. Cancer Res.
73:1502–1513. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Madoz-Gurpide J, Kuick R, Wang H, Misek DE
and Hanash SM: Integral protein microarrays for the identification
of lung cancer antigens in sera that induce a humoral immune
response. Mol Cell Proteomics. 7:268–281. 2008.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bouwman K, Qiu J, Zhou H, Schotanus M,
Mangold LA, Vogt R, Erlandson E, Trenkle J, Partin AW, Misek D, et
al: Microarrays of tumour cell derived proteins uncover a distinct
pattern of prostate cancer serum immunoreactivity. Proteomics.
3:2200–2207. 2003. View Article : Google Scholar
|
|
48
|
GuhaThakurta D, Sheikh NA, Fan LQ, Kandadi
H, Meagher T, Hall SJ, Kantoff PW, Higano CS, Small EJ, Gardner TA,
et al: Humoral immune response against nontargeted tumour antigens
after treatment with sipuleucel-T and its association with improved
clinical outcome. Clin Cancer Res. 21:3619–3630. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kwek SS, Dao V, Roy R, Hou Y, Alajajian D,
Simko JP, Small EJ and Fong L: Diversity of antigen-specific
responses induced in vivo with CTLA-4 blockade in prostate cancer
patients. J Immunol. 189:3759–3766. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Graff JN, Puri S, Bifulco CB, Fox BA and
Beer TM: Sustained complete response to CTLA-4 blockade in a
patient with metastatic, castration-resistant prostate cancer.
Cancer Immunol Res. 2:399–403. 2014.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Gnjatic S, Ritter E, Büchler MW, Giese NA,
Brors B, Frei C, Murray A, Halama N, Zörnig I, Chen YT, et al:
Seromic profiling of ovarian and pancreatic cancer. Proc Natl Acad
Sci USA. 107:5088–5093. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Abel L, Kutschki S, Turewicz M, Eisenacher
M, Stoutjesdijk J, Meyer HE, Woitalla D and May C: Autoimmune
profiling with protein microarrays in clinical applications. Biomed
Biochim Acta. 1844:977–987. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Turewicz M, May C, Ahrens M, Woitalla D,
Gold R, Casjens S, Pesch B, BrüningT Meyer HE, Nordhoff E, et al:
Improving the default data analysis workflow for large autoimmune
biomarker discovery studies with ProtoArrays. Proteomics.
13:2083–2087. 2013.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Zhang DY, Ye F, Gao L, Liu X, Zhao X, Che
Y, Wang H, Wang L, Wu J, Song D, et al: Proteomics, pathway array
and signaling network-based medicine in cancer. Cell Div.
4(20)2009.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schumacher S, Muekusch S and Seitz H:
Up-to-date applications of microarrays and their way to
commercialization. Microarrays (Basel). 4:196–213. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Ayoglu B, Schwenk JM and Nilsson P:
Antigen arrays for profiling autoantibody repertoires. Bioanalysis.
8:1105–1126. 2016.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Stoevesandt O, Taussig MJ and He M:
Protein microarrays: High-throughput tools for proteomics. Expert
Rev Proteomics. 6:145–157. 2009.PubMed/NCBI View Article : Google Scholar
|
|
58
|
No authors listed. The call of the human
proteome. Nat Methods. 7(661)2010.PubMed/NCBI
|
|
59
|
Legrain P, Aebersold R, Archakov A,
Bairoch A, Bala K, Beretta L, Bergeron J, Borchers CH, Corthals GL,
Costello CE, et al: The human proteome project: Current state and
future direction. Mol Cell Proteomics. 10:M111.009993.
2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Jantsch MF, Quattrone A, O'Connell M, Helm
M, Frye M, Macias-Gonzales M, Ohman M, Ameres S, Willems L, Fuks F,
et al: Positioning Europe for the EPITRANSCRIPTOMICS challenge. RNA
Biol. 15:829–831. 2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
González-Gomariz J, Guruceaga E,
López-Sánchez M and Segura V: Proteogenomics in the context of the
human proteome project (HPP). Expert Rev Proteomics. 16:267–275.
2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Tanase C, Albulescu R and Neagu M: Updates
in immune-based multiplex assays. J Immunoassay Immunochem. 40:1–2.
2019.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Popa ML, Albulescu R, Neagu M, Eugen
Hinescu ME and Tanase C: Multiplex assay for multiomics advances in
personalized-precision medicine. J Immunoassay Immunochem. 40:3–25.
2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Cronin KA, Lake AJ, Scott S, Sherman RL,
Noone AM, Howlader N, Henley SJ, Anderson RN, Firth AU, Ma J, et
al: Annual report to the nation on the status of cancer, part I:
National cancer statistics. Cancer. 124:2785–2800. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Smith ER: A two-tiered health care system:
Is there anything new? Can J Cardiol. 23:915–916. 2007.(In English;
French). PubMed/NCBI
|
|
66
|
Alyass A, Turcotte M and Meyre D: From big
data analysis to personalized medicine for all: Challenges and
opportunities. BMC Med Genomics. 8(33)2015. View Article : Google Scholar
|
|
67
|
Fröhlich H, Balling R, Beerenwinkel N,
Kohlbacher O, Kumar S, Lengauer T, Maathuis MH, Moreau Y, Murphy
SA, Przytycka TM, et al: From hype to reality: Data science
enabling personalized medicine. BMC Med. 16(150)2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Wong SH: Pharmacogenomics and personalized
medicine. In: Handbook of drug monitoring methods: Therapeutics and
drugs of abuse. Dasgupta A (ed). Humana Press, New York, NY.
pp211–223. 2007.
|
|
69
|
Yu X, Schneiderhan-Marra N and Joos TO:
Protein microarrays for personalized medicine. Clin Chem.
56:376–387. 2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Masuda M and Yamada T: Signalling pathway
profiling by reverse-phase protein array for personalized cancer
medicine. Biochim Biophys Acta. 1854:651–657. 2015.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Rosa M: Advances in the molecular analysis
of breast cancer: Pathway toward personalized medicine. Cancer
Control. 22:211–219. 2015.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Kim DC, Wang X, Yang CR and Gao JX: A
framework for personalized medicine: Prediction of drug sensitivity
in cancer by proteomic profiling. Proteome Sci. 10 (Suppl
1)(S13)2012.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wistuba II, Gelovani JG, Jacoby JJ, Davis
SE and Herbst RS: Methodological and practical challenges for
personalized cancer therapies. Nat Rev Clin Oncol. 8:135–141.
2011.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Mueller C, Liotta L and Espina V: Reverse
phase protein microarrays advance to use in clinical trials. Mol
Oncol. 4:461–481. 2010.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kornblau SM, Tibes R, Qiu YH, Chen W,
Kantarjian HM, Andreeff M, Coombes KR and Mills GB: Functional
proteomic profiling of AML predicts response and survival. Blood.
113:154–164. 2009.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Cain JW, Hauptschein RS, Stewart JK, Bagci
T, Sahagian GG and Jay DG: Identification of CD44 as a surface
biomarker for drug resistance by surface proteome signature
technology. Mol Cancer Res. 9:637–647. 2011.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Liotta LA, Espina V, Mehta AI, Calvert V,
Rosenblatt K, Geho D, Munson PJ, Young L, Wulfkuhle J and Petricoin
EF III: Protein microarrays: Meeting analytical challenges for
clinical applications. Cancer Cell. 3:317–325. 2003.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Spurrier B, Ramalingam S and Nishizuka S:
Reverse-phase protein lysate microarrays for cell signaling
analysis. Nat Protoc. 3:1796–1808. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Kim YB, Yang CR and Gao J: Functional
proteomic pattern identification under low dose ionizing radiation.
Artif Intell Med. 49:177–185. 2010.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Wang X, Dong Y, Jiwani AJ, Zou Y, Pastor
J, Kuro OM, Habib AA, Ruan M, Boothman DA and Yang CR: Improved
protein arrays for quantitative systems analysis of the dynamics of
signaling pathway interactions. Proteome Sci. 9(53)2011.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Laforte V, Lo PS, Li H and Juncker D:
Antibody colocalization microarray for cross-reactivity-free
multiplexed protein analysis. Methods Mol Biol. 1619:239–261.
2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Lim MS, Carlson ML, Crockett DK, Fillmore
GC, Abbott DR, Elenitoba-Johnson OF, Tripp SR, Rassidakis GZ,
Medeiros LJ, Szankasi P and Elenitoba-Johnson KS: The proteomic
signature of NPM/ALK reveals deregulation of multiple cellular
pathways. Blood. 114:1585–1595. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Gnjatic S, Wheeler C, Ebner M, Ritter E,
Murray A, Altorki NK, Ferrara CA, Hepburne-Scott H, Joyce S,
Koopman J, et al: Seromic analysis of antibody responses in
non-small cell lung cancer patients and healthy donors using
conformational protein arrays. J Immunol Methods. 341:50–58.
2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Gaudreau PO, Peng D, Rodriguez BL,
Fradette J, Gibson L, Kundu S, Chen L, et al: Co-clinical trials of
MEK inhibitor, anti PD-L1 and anti CTLA-4 combination treatment in
non-small cell lung cancer. J ImmunoTherapy Cancer. 6 (Suppl
1)(S114)2018.
|
|
85
|
Vilgelm AE, Johnson DB and Richmond A:
Combinatorial approach to cancer immunotherapy: Strength in
numbers. J Leukoc Biol. 100:275–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Terracciano R, Pelaia G, Preianò M and
Savino R: Asthma and COPD proteomics: Current approaches and future
directions. Proteomics Clin Appl. 9:203–220. 2015. View Article : Google Scholar
|
|
87
|
Vizcaino JA, Csordas A, Del-Toro N, Dianes
JA, Griss J, Lavidas I, Mayer G, Perez-Riverol Y, Reisinger F,
Ternent T, et al: 2016 update of the PRIDE database and its related
tools. Nucleic Acids Res. 44(11033)2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Hamsten C, Häggmark A, Grundström J, Mikus
M, Lindskog C, Konradsen JR, Eklund A, Pershagen G, Wickman M,
Grunewald J, et al: Protein profiles of CCL5, HPGDS, and NPSR1 in
plasma reveal association with childhood asthma. Allergy.
71:1357–1361. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Gharib SA, Nguyen EV, Lai Y, Plampin JD,
Goodlett DR and Hallstrand TS: Induced sputum proteome in healthy
subjects and asthmatic patients. J Allergy Clin Immunol.
128:1176–1184, e1176. 2011.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ohlmeier S, Nieminen P, Gao J, Kanerva T,
Rönty M, Toljamo T, Bergmann U, Mazur W and Pulkkinen V: Lung
tissue proteomics identifies elevated transglutaminase 2 levels in
stable chronic obstructive pulmonary disease. Am J Physiol Lung
Cell Mol Physiol. 310:L1155–L1165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Barnes PJ: Corticosteroid resistance in
patients with asthma and chronic obstructive pulmonary disease. J
Allergy Clin Immunol. 131:636–645. 2013.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Levitt JE and Rogers AJ: Proteomic study
of acute respiratory distress syndrome: Current knowledge and
implications for drug development. Expert Rev Proteomics.
13:457–469. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Priyadharshini VS and Teran LM:
Personalized medicine in respiratory disease: Role of proteomics.
Adv Protein Chem Struct Biol. 102:115–146. 2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Foster MW, Morrison LD, Todd JL, Snyder
LD, Thompson JW, Soderblom EJ, Plonk K, Weinhold KJ, Townsend R,
Minnich A and Moseley MA: Quantitative proteomics of
bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. J
Proteome Res. 14:1238–1249. 2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Lavoie JR, Ormiston ML, Perez-Iratxeta C,
Courtman DW, Jiang B, Ferrer E, Caruso P, Southwood M, Foster WS,
Morrell NW and Stewart DJ: Proteomic analysis implicates
translationally controlled tumour protein as a novel mediator of
occlusive vascular remodeling in pulmonary arterial hypertension.
Circulation. 129:2125–2135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Abdul-Salam VB, Wharton J, Cupitt J,
Berryman M, Edwards RJ and Wilkins MR: Proteomic analysis of lung
tissues from patients with pulmonary arterial hypertension.
Circulation. 122:2058–2067. 2010.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Kan M, Shumyatcher M and Himes BE: Using
omics approaches to understand pulmonary diseases. Respir Res.
18(149)2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Cohen AS, Khalil FK, Welsh EA, Schabath
MB, Enkemann SA, Davis A, Zhou JM, Boulware DC, Kim J, Haura EB and
Morse DL: Cell-surface marker discovery for lung cancer.
Oncotarget. 8:113373–113402. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Monette A, Bergeron D, Ben Amor A, Meunier
L, Caron C, Mes-Masson AM, Kchir N, Hamzaoui K, Jurisica I and
Lapointe R: Immune-enrichment of non-small cell lung cancer
baseline biopsies for multiplex profiling define prognostic immune
checkpoint combinations for patient stratification. J Immunother
Cancer. 7(86)2019.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Schwill M, Tamaskovic R, Gajadhar AS, Kast
F, White FM and Plückthun A: Systemic analysis of tyrosine kinase
signaling reveals a common adaptive response program in a
HER2-positive breast cancer. Sci Signal. 12:eaau2875.
2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Hsu PY, Wu VS, Kanaya N, Petrossian K, Hsu
HK, Nguyen D, Schmolze D, Kai M, Liu CY, Lu H, et al: Dual mTOR
kinase inhibitor MLN0128 sensitizes HR+/HER2+
breast cancer patient-derived xenografts to trastuzumab or
fulvestrant. Clin Cancer Res. 24:395–406. 2018.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Lee J, Geiss GK, Demirkan G, Vellano CP,
Filanoski B, Lu Y, Ju Z, Yu S, Guo H, Bogatzki LY, et al:
Implementation of a multiplex and quantitative proteomics platform
for assessing protein lysates using DNA-barcoded antibodies. Mol
Cell Proteomics. 17:1245–1258. 2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Kars MD and Yıldırım G: Determination of
the target proteins in chemotherapy resistant breast cancer stem
cell-like cells by protein array. Eur J Pharmacol. 848:23–29.
2019.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Li M, Li H, Liu F, Bi R, Tu X, Chen L, Ye
S and Cheng X: Characterization of ovarian clear cell carcinoma
using target drug-based molecular biomarkers: Implications for
personalized cancer therapy. J Ovarian Res. 10(9)2017.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Mabuchi S, Sugiyama T and Kimura T: Clear
cell carcinoma of the ovary: Molecular insights and future
therapeutic perspectives. J Gynecol Oncol. 27(e31)2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Yanaihara N, Hirata Y, Yamaguchi N,
Noguchi Y, Saito M, Nagata C, Takakura S, Yamada K and Okamoto A:
Antitumor effects of interleukin-6 (IL-6)/interleukin-6 receptor
(IL-6R) signalling pathway inhibition in clear cell carcinoma of
the ovary. Mol Carcinog. 55:832–841. 2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Yamashita Y: Ovarian cancer: New
developments in clear cell carcinoma and hopes for targeted
therapy. Jpn J Clin Oncol. 45:405–407. 2015.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Sereni MI, Baldelli E, Gambara G, Deng J,
Zanotti L, Bandiera E, Bignotti E, Ragnoli M, Tognon G, Ravaggi A,
et al: Functional characterization of epithelial ovarian cancer
histotypes by drug target based protein signaling activation
mapping: Implications for personalized cancer therapy. Proteomics.
15:365–373. 2015.PubMed/NCBI View Article : Google Scholar
|
|
109
|
di Martino S, De Luca G, Grassi L,
Federici G, Alfonsi R, Signore M, Addario A, De Salvo L,
Francescangeli F, Sanchez M, et al: Renal cancer: New models and
approach for personalizing therapy. J Exp Clin Cancer Res.
37(217)2018.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Hoff FW, Hu CW, Qiu Y, Ligeralde A, Yoo
SY, Mahmud H, de Bont ESJM, Qutub AA, Horton TM and Kornblau SM:
Recognition of recurrent protein expression patterns in pediatric
acute myeloid leukemia identified new therapeutic targets. Mol
Cancer Res. 16:1275–1286. 2018.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Ragon BK, Odenike O, Baer MR, Stock W,
Borthakur G, Patel K, Han L, Chen H, Ma H, Joseph L, et al: Oral
MEK 1/2 inhibitor trametinib in combination with AKT inhibitor
GSK2141795 in patients with acute myeloid leukemia with RAS
mutations: A phase II study. Clin Lymphoma Myeloma Leuk.
S2152-2650:31542–31548. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Yang Q, Bavi P, Wang JY and Roehrl MH:
Immuno-proteomic discovery of tumour tissue autoantigens identifies
olfactomedin 4, CD11b, and integrin alpha-2 as markers of
colorectal cancer with liver metastases. J Proteomics. 168:53–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Hagman H, Bendahl PO, Lidfeldt J, Belting
M and Johnsson A: Protein array profiling of circulating
angiogenesis-related factors during bevacizumab containing
treatment in metastatic colorectal cancer. PLoS One.
13(e0209838)2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Liu P, Souma T, Wei AZ, Xie X, Luo X and
Jin J: Personalized peptide arrays for detection of HLA
alloantibodies in organ transplantation. J Vis Exp. 2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Soni A, Gowthamarajan K and Radhakrishnan
A: Personalized medicine and customized drug delivery systems: The
new trend of drug delivery and disease management. Int J Pharm
Compd. 22:108–121. 2018.PubMed/NCBI
|
|
116
|
Syafrizayanti Lueong SS, Di C, Schaefer
JV, Plückthun A and Hoheisel JD: Personalised proteome analysis by
means of protein microarrays made from individual patient samples.
Sci Rep. 7(39756)2017.PubMed/NCBI View Article : Google Scholar
|