1. Introduction
Adenomyosis is a benign uterine condition
characterized by the presence of ectopic endometrial glands and
stroma in the myometrium and muscular hypertrophy/hyperplasia
associated with increased vascularity and reactive fibrosis
(1). According to the most
convincing theory, adenomyosis was originally thought to originate
from the invagination of the basalis of the endometrium into the
inner myometrium (1,2). In addition, this disorder may
possibly result from metaplastic alterations of Müllerian duct
remnants. Adenomyosis is a clinical condition with a high
association of endometriosis and vice versa, with a marked
prevalence in women of reproductive age (3). Emerging evidence has indicated that
this disorder appears to consist of heterogeneous subtypes with
different causes and etiologies (4). Certain adenomyosis populations occur
in the uterine outer layer without affecting the inner myometrial
structures (4). A number of
various pathogenetic theories have described the mechanisms through
which adenomyosis develops; however, the origin of adenomyosis
remains unclear (1).
On the other hand, endometriosis is a common benign
disease caused by the presence of endometrial tissue outside the
uterine cavity or in ectopic locations, e.g., peritoneal implants,
ovarian cysts and deep infiltrating nodules (5). There are a number of hypotheses
regarding the complex etiology of endometriosis: Retrograde
menstruation, coelomic metaplasia and Müllerian duct remnants;
however, none have been postulated to completely explain the
etiology of endometriosis (5).
Several researchers have performed a large-scale survey for the
identification of differentially expressed genes (DEGs) between the
eutopic and ectopic endometrium of women with endometriosis
(6-11).
Some studies have examined the global transcriptome of isolated
ectopic and eutopic tissues from women with adenomyosis (12-14).
However, to date, to the best of our knowledge, there is no clear
evidence to indicate that fundamental differences exist in the
underlying mechanisms of disease development between adenomyosis
and endometriosis. Therefore, in this review, multiple independent
microarray datasets that are available through an open access,
comprehensive database, were utilized to identify reliable
adenomyosis-specific pathways and DEGs. The expression levels of
adenomyosis candidate genes were confirmed by the data published in
a peer-reviewed journal. The aim of this review was to summarize
the studies available to date on candidate key genes and functional
networks involved in the differentiation between adenomyosis and
endometriosis.
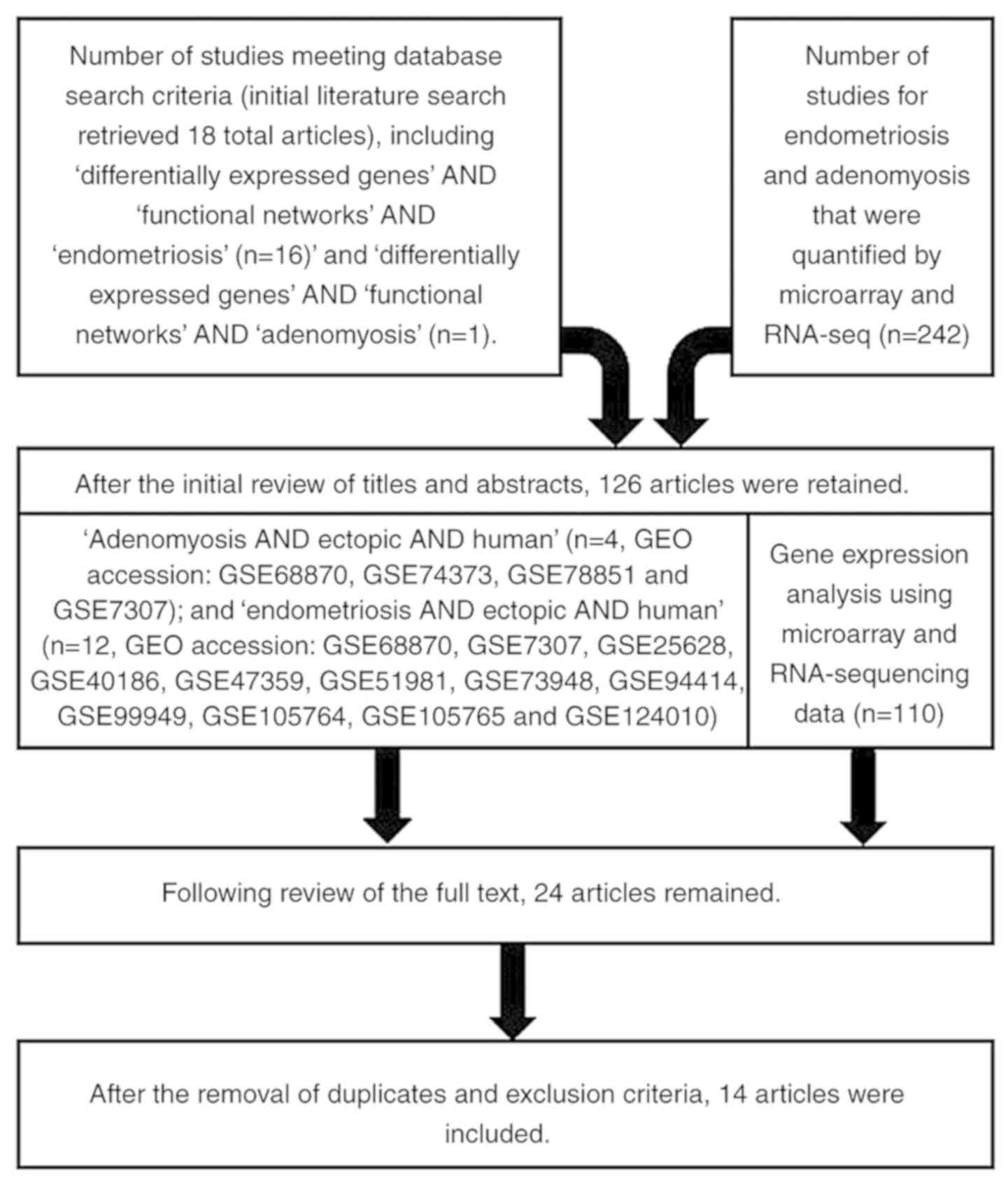
2. Search strategy, selection criteria and
data collection
A review of the literature was conducted in order to
screen candidate key genes and functional networks that
differentiate adenomyosis from endometriosis. A MEDLINE search was
performed using the key words ‘adenomyosis’, ‘endometriosis’,
‘differentially expressed genes’ and ‘functional networks’. English
language publications in PubMed and references from relevant
articles published between January, 2000 and December, 2018 were
analyzed. There were 17 articles available for ‘differentially
expressed genes’ AND ‘functional networks’ AND ‘endometriosis’, and
1 article available for ‘differentially expressed genes’ AND
‘functional networks’ AND ‘adenomyosis’ by search. References in
the studies identified were also searched. In addition, the
development of bioinformatics methods, including DNA microarray and
RNA-Seq technologies, could provide a better understanding of the
pathogenetic events of the development of adenomyosis and
endometriosis at the genome level. These data have been made
available through an open access, comprehensive PubMed database
that compiles up-to-date omics technologies regarding human
adenomyosis and endometriosis. Eligible archives for functional
genomics microarray datasets were collected from the National
Center for Biotechnology Information (NCBI)-Gene Expression Omnibus
(GEO) database, NCBI-GEO (http://www.ncbi.nlm.nih.gov/geo/). GEO dataset
searches up to December, 2018, using keywords ‘adenomyosis’ and
‘endometriosis’ and the accession number of meta-data tag. 47
articles were available in the GEO database for endometriosis and
adenomyosis by search. The inclusion criteria included the
following: i) Original genomic studies that screened for DEGs among
adenomyosis, endometriosis, or healthy individuals; ii) array
analysis studies of gene expression profiling; and iii) studies
comprised of raw files. The following data can be accessed via key
word search: ‘Adenomyosis AND ectopic AND human’ [n=4, GEO
accession nos. GSE68870(14),
GSE74373(12), GSE78851(13) and GSE7307]; and ‘endometriosis AND
ectopic AND human’ [n=12, GEO accession nos. GSE68870(14), GSE7307, GSE25628(6), GSE40186(7), GSE47359(11), GSE51981(8), GSE73948(9), GSE94414, GSE99949,
GSE105764(10),
GSE105765(10) and GSE124010]
(Table I). Fourteen publications
available in the GEO database were selected based on the final
selection taking into account the experiment type, overall design
and microarray platforms. The flow chart of the literature search
are presented in Fig. 1.
 | Table INCBI-GEO accession and datasets. |
Table I
NCBI-GEO accession and datasets.
| Diseased human
tissues | GEO accession
no. | Experiment
type | Overall design | Platforms | Summary | (Refs.) |
|---|
| Adenomyosis | GSE68870 | Expression
profiling by array | Transcriptionally
profiling of both normal and diseased human tissues representing
over 90 distinct tissue types, which includes endometriosis and
adenomyosis. | Affymetrix Human
Genome U133 Plus 2.0 Array | A total of 677
samples were processed and represent over 90 distinct tissuetypes,
including endometriosis and adenomyosis. | (14) |
| Adenomyosis | GSE74373 | Expression
profiling by array. Non-coding RNA profiling by array | A total of 3
samples were utilized to analyze the differential expression of
mRNAs and long noncoding RNAs between ectopic and eutopic
endometrium of adenomyosis. | Agilent-038314 CBC
Homo sapiens lncRNA + mRNA microarray V2.0 | Bioinformatics
analysis suggested a of metabolic abnormalities in adenomyosis,
which had many similarities with endometriosis. | (12) |
| Adenomyosis | GSE78851 | Expression
profiling by array | A total of 8
samples were used and analyzed by disease state. Endometrial
samples from hysterectomy specimens in proliferative phase of
menstrual cycle from symptomatic women with pathologically
confirmed diffuse adenomyosis were compared with endometrial
samples from normo-ovulatory healthy subjects with no endometrial
or uterine pathology. | Affymetrix Human
Gene 1.0 ST Array | Highly DEGs
included those involved in regulation of apoptosis, steroid hormone
responsiveness, and proteins involved in extracellular matrix
remodeling, as well as microRNAs of unknown significance. Affected
pathways included eukaryotic initiation factor 2 (eIF2α) signaling
(hypoxia-related pathway), oxidative phosphorylation, mitochondrial
dysfunction, estrogen receptor signaling, and mTOR signaling
(stress responsive pathway). Both eIF2α and mTOR play roles in the
cell-cycle arrest. | (13) |
| Endometriosis | GSE7307 | Expression
profiling by array | Transcriptionally
profiling of both normal and diseased human tissues representing
over 90 distinct tissue types, which includes endometriosis and
adenomyosis. | Affymetrix Human
Genome U133 Plus 2.0 Array | A total of 677
samples were processed and represent over 90 distinct tissue types,
including endometriosis and adenomyosis. | |
| Endometriosis | GSE25628 | Expression
profiling by array | Microarray studies
were performed using ectopic (8 samples) and eutopic endometrium (8
samples) from several affected woman in the proliferative phase. As
control we used endometrium from normal health donors in the same
phase (6 samples). | Affymetrix Human
Genome U133A 2.0 Array | BMP4 and GREM1 were
identified as DEGs for eutopic endometrium. They were involved in
the mesoderm-Müllerian duct differentiation. | (6) |
| Endometriosis | GSE40186 | Non-coding RNA
profiling by array | The primary culture
of endometriotic cyst stromal cells (ECSCs, n=8) and normal
endometrial stromal cells (NESCs, as controls, n=8) were utilized.
miRNA microarray analysis was performed. The miRNAs differentially
expressed between ECSCs and NESCs were identified, and aberrantly
expressed miRNAs in ECSCs were defined as the endometriosis related
miRNAs. | Agilent-021827
Human miRNA Microarray G4470C | miR-196b was
aberrantly expressed in endometriotic cyst stromal cells, whose
expression was repressed in endometriotic stromal cells. | (7) |
| Endometriosis | GSE47359 | Methylation
profiling by array | Bisulphite
converted DNA from cultured endometrial stromal cells (ESCs, n=3)
from eutopic endometria without endometriosis, ESCs with
endometriosis (n=3) and ESCs from chocolate cysts (n=3) were
hybridised to the Illumina infinium HumanMethylation27
BeadChip. | Illumina Human
Methylation27 BeadChip | DNA methylation
profiles were quite different between eutopic ESC and ectopic ESC,
whereas no clear difference were recognized between eutopic ESC
with and without endometriosis. Some of the differentially
methylated and/or expressed genes (NR5A1, STAR, STRA6 and HSD17B2)
are related with steroidogenesis in ESC. | (11) |
| Endometriosis | GSE51981 | Expression
profiling by array | The authors
analyzed endometrial samples from 148 women without or with
endometriosis and/or other uterine/pelvic pathologies, using whole
genome microarrays. | Affymetrix Human
Genome U133 Plus 2.0 Array | Differential gene
expression and pathway analyses revealed immune activation, altered
steroid and thyroid hormone signaling/metabolism and growth factor
signaling in endometrium of women with endometriosis. | (8) |
| Endometriosis | GSE73948 | Methylation
profiling by genome tiling array | Methylation arrays
were used to explore DNA methylation profiles of endometrial
tissues from various menstrual cycle phases from 24 patients with
endometriosis. | Illumina Human
Methylation450 BeadChip | Comparison of cycle
phase- and endometriosis-specific methylation profile changes
revealed that 13 out of 28 endometriosis-specific DMRs were present
in both datasets. | (9) |
| Endometriosis | GSE94414 | Expression
profiling by array | Samples of
endometriosis, ovary cysts and cervical endometrium were obtained
during surgical procedures. | CustomArray
Signaling Pathway platform version 2 | The authors
explored activation/deactivation of signaling pathways in
endometrium samples from patients with endometriosis. | |
| Endometriosis | GSE99949 | Expression
profiling by high throughput sequencing | AmpliSeq IonTorrent
sequencing of mRNA derived from ectopic lesions and matched eutopic
endometrium. There were 4 matched pairs overall; all donors were in
mid-secretory phase. | Ion Torrent
PGM | | |
| Endometriosis | GSE105764 | Non-coding RNA
profiling by high throughput sequencing | Eight paired
ectopic endometria (EC) and eutopic endometria (EU) from 8 patients
with advanced ovarian endometriosis obtained from Chinese were
selected and prepared for lncRNA sequencing. EC were obtained
during laparoscopy, and EU were obtained through curettage before
the laparoscopic procedure. Only patients in the secretary phase of
the menstrual cycle and without any hormonal treatment history were
included in the study. | Illumina HiSeq 2000
and 4000 platform. | Some key regulators
from the miR-449 and miR-34b/c cluster, miR-200 family,
miR-106a-363 cluster, miR-182/183, FOX family, GATA family, and E2F
family as well as CEBPA, SOX9 and HNF4A were suggested to play
vital regulatory roles in the pathogenesis of endometriosis. | (10) |
| Endometriosis | GSE105765 | Non-coding RNA
profiling by high throughput sequencing | Eight paired
ectopic endometria (EC) and eutopic endometria (EU) from 8 patients
with advanced ovarian endometriosis obtained from Chinese were
selected and prepared for lncRNA sequencing. EC were obtained
during laparoscopy, and EU were obtained through curettage before
the laparoscopic procedure. Only patients in the secretary phase of
the menstrual cycle and without any hormonal treatment history were
included in the study. | Illumina HiSeq 2000
and 4000 platform. | Some key regulators
from the miR-449 and miR-34b/c cluster, miR-200 family,
miR-106a-363 cluster, miR-182/183, FOX family, GATA family, and E2F
family as well as CEBPA, SOX9 and HNF4A were suggested to play
vital regulatory roles in the pathogenesis of endometriosis. | (10) |
| Endometriosis | GSE124010 | Non-coding RNA
profiling by array | Agilent miRNAs
microarray 21.0 for normal endometrium (n=3) and ectopic
endometrium (n=3) was established and analyzed by GeneSpringGX
software 11.0 (Agilent) | Agilent-070156
Human_miRNA_V21.0_Microarray046064 | The newly
identified miR-205-5p-ANGPT2-AKT/ERK axis illustrates the molecular
mechanism of endometriosis progression and may represent a novel
diagnostic biomarker and therapeutic target for disease
treatment. | |
3. Differentially expressed genes and their
functions between adenomyosis and endometriosis
We combined the data derived from GEO, analyzed the
similarities and differences between ectopic tissue samples from
patients with adenomyosis and those from patients with
endometriosis, and then focused on the unequivocal identification
of the adenomyosis related genes. The DEGs between adenomyosis
samples and endometriosis samples were screened using GEO2R
(http://www.ncbi.nlm.nih.gov/geo/geo2r). Probe sets
without corresponding gene symbols were removed. Following
pre-processing, DEGs with log2 fold change >2 or <1/2, a
false discovery rate <0.05 and P-value <0.01 were selected as
described previously (15).
Functional annotation and gene regulatory networks were performed
based on the Gene Ontology (GO) database (https://david.ncifcrf.gov/tools.jsp). The expression
of adenomyosis candidate genes were confirmed by the data published
in a peer-reviewed journal (https://www.ncbi.nlm.nih.gov/pubmed and https://www.ncbi.nlm.nih.gov/gene).
Screening of key genes with which to
differentiate adenomyosis from endometriosis
In this section, the gene expression features and
core regulatory genes which can be used to differentiate
adenomyosis from endometriosis by using high-throughput microarray
genomics datasets downloaded from the NCBI-GEO database are
discussed. The GEO database for adenomyosis [n=4, GEO accession
nos. GSE68870(14),
GSE74373(12), GSE78851(13) and GSE7307] and GEO database for
endometriosis [n=12, GEO accession nos. GSE68870(14), GSE7307, GSE25628(6), GSE40186(7), GSE47359(11), GSE51981(8), GSE73948(9), GSE94414, GSE99949,
GSE105764(10),
GSE105765(10) and GSE124010)]
enabled us to screen DEGs between the two conditions. Among these
databases, we selected the gene expression profiles of
GSE68870(14), GSE78851(13) and GSE7307 for adenomyosis and
GSE68870(14), GSE7307 and
GSE25628(6) for endometriosis,
particularly when taking their experiment type, overall design and
platforms into consideration (Table
I).
To reveal the differences in gene expression between
the adenomyosis and endometriosis tissues, the top ranked genes
with a log2 fold change >2 or log2 fold change <1/2 were
selected for analysis. Among a total of 3,119 genes collected from
the adenomyosis signatures, 2,845 genes (91.2%) overlapped in the
endometriosis signatures. This suggests that patients with
adenomyosis and endometriosis share a common genetic
pathophysiology. On the other hand, a total of 274 DEGs were
identified to be specific to ectopic lesions of patients with
adenomyosis. Among these adenomyosis candidate genes, we selected
50 genes (18.2%) whose expression profiles have been published in a
public repository, PubMed database (https://www.ncbi.nlm.nih.gov/pubmed/) (Table II). In comparison with the
genetic and proteomic data previously published for adenomyosis and
endometriosis, a set of DEGs, including insulin-like growth factor
1 (IGF1) (16-19),
osteopontin (OPN) (20-22),
KiSS-1 metastasis suppressor (KISS1) (23-27),
neural cell adhesion molecule 1 (NCAM1, also known as CD56)
(28,29), versican (VCAN) (13,30), L-selectin ligand (31) and Annexin A2 (ANXA2) (32-35),
were considered to be target genes for adenomyosis. These previous
studies revealed that, among these, 4 genes (IGF1, KISS1, NCAM1 and
ANXA2) were upregulated, and 3 genes (OPN, VCAN and L-selectin
ligand) were downregulated.
 | Table IIFunctional classification of
differentially expressed genes in adenomyosis and
endometriosis. |
Table II
Functional classification of
differentially expressed genes in adenomyosis and
endometriosis.
| | Differentially
expressed genes | |
|---|
| The major
pathways |
ADM>ENDa |
ADM<ENDb | ADM=END | (Refs.) |
|---|
| Survival and
apoptosis (n=13) | IGF1 | | | (16-19) |
| | | | BAX | (39) |
| | | | BCL2 | (38,55-57) |
| | | | IGF2 | (19) |
| | | | VEGF | (40-42) |
| | | | HGF | (43-45) |
| | | | NGF | (46-48) |
| | | | PDGF | (16,49) |
| | | | EGFR | (16,17,49,50) |
| | | | FGF2 | (49,51,52) |
| | | | mTOR | (53,54) |
| | | | BECN1 | (58,59) |
| | | | PDCD4 | (60,61) |
| Immune and
inflammatory response (n=12) | | OPN | | (20-22) |
| | | | KIR3DL1,
KIR2DL3 | (63,64) |
| | | | GM-CSF | (65,66) |
| | | | NF-κB | (67) |
| | | | TGF-β | (68-71) |
| | | | LIF | (72,73) |
| | | | TLR4 | (74,75) |
| | | | IL-1β | (46,76) |
| | | | IL-10 | (77,78) |
| | | | CXCL1 | (40,79,80) |
| | | | TrkB | (81,82) |
| | | | COX-2 | (83-86) |
| Invasion and
extracellular matrix remodeling (n=5) | KISS1 | | | (23-27) |
| | | | MMP-2, -9 | (83,87-89) |
| | | | MSN | (90,91) |
| | | | FAK | (92,93) |
| | | | LOX | (68,94) |
| Adhesion (n=5) | NCAM1 | | | (28,29) |
| | | VCAN | | (13,30) |
| | | L-selectin
ligand | | (31) |
| | | | SRGAP2 | (96,97) |
| | | | ADAM12 | (96,98,99) |
| Hormonal response
(n=5) | | | ESR1 | (37,67,102,103) |
| | | | ESR2 | (37,67,102,103) |
| | | | PR-B | (67,102,103) |
| | | | RXRα | (16,104) |
| | | | OXTR | (105-107) |
|
Epithelial-mesenchymal transition
(n=3) | ANXA2 | | | (32-35) |
| | | | ILK | (111-113) |
| | | | CTNNB1 | (45,114-117) |
| Cell cycle
regulation (n=3) | | | MSI1 | (119,120) |
| | | | SOX2 | (121) |
| | | | 4-Oct | (121) |
| Angiogenesis
(n=1) | | | HIF-1α | (41,123) |
| Oxidative damage
(n=1) | | | GPX | (13,125) |
| Mitochondrial
dysfunction (n=1) | | | SOD2 | (127,128) |
| Prostaglandin
biosynthesis (n=1) | | | PTGS2 | (83-86) |
Functional pathways of adenomyosis
candidate genes
GO term enrichment analysis suggested that DEGs were
significantly enriched in survival and apoptosis, immune and
inflammatory response, invasion and extracellular matrix
remodeling, adhesion, hormonal response, epithelial-mesenchymal
transition, cell cycle regulation, angiogenesis, oxidative damage,
mitochondrial dysfunction and prostaglandin biosynthesis (Table II). Taken together with the DEGs,
the enriched functions and pathway analysis revealed that the
adenomyosis candidate genes may be the core regulatory functions
and may play pivotal roles in the processes of invasion of the
endometrial basalis into the myometrium (KISS1), avoidance of the
immune attack (OPN), cell survival (IGF1), wound repair, healing
and scarring (NCAM1) and fibrosis (ANXA2). The biological functions
of adenomyosis candidate genes in implicating disease pathways were
comprehensively searched using the PubMed database.
Survival and apoptosis (13 genes).
Adenomyosis is involved in eutopic endometrial
invagination/infiltration into the inner myometrium by modulating
the proliferative, invasive and angiogenic responses, which is a
hallmark of adenomyosis (13).
Increased proliferation and decreased apoptosis may be critical for
the pathophysiology of adenomyosis and endometriosis; however, the
underlying regulatory mechanisms remain elusive (36). In adenomyosis, Ki-67 is expressed
in the glandular epithelium of the ectopic endometrium and
myometrial cells associated with hyperplasia and hypertrophy,
irrespective of the menstrual phases (36-38).
The genes and pathways involved in the regulation of apoptosis have
been shown to be aberrantly expressed in the endometrium of women
with adenomyosis, compared to the controls (39). We found that the survival and
apoptosis-related genes were upregulated or downregulated in the
previous studies cited. Thus, the proliferation- and growth-related
genes, including IGF1 (16-19),
IGF2(19), vascular endothelial
growth factor (VEGF) (40-42),
hepatocyte growth factor (HGF) (43-45),
nerve growth factor (NGF) (46-48),
platelet-derived growth factor (PDGF) (16,49), epidermal growth factor receptor
(EGFR) (16,17,49,50), fibroblast growth factor 2 (FGF2)
(49,51,52) and mechanistic target of rapamycin
kinase (mTOR) (53,54), were upregulated in both the
ectopic and eutopic endometria from subjects with adenomyosis
compared with the eutopic endometria from women without
adenomyosis, while the anti-apoptotic or autophagy-related genes,
such as BCL2 apoptosis regulator (BCL2) (38,55-57),
beclin 1 (BECN1) (58,59) and programmed cell death 4 (PDCD4)
(60,61), were much higher in the ectopic and
eutopic endometria than in the control endometria. Furthermore, the
decreased expression of BCL2 associated X, apoptosis regulator
(BAX) has been observed in adenomyosis, suggesting that the
downregulation of apoptotic cell death machinery is a hallmark of
adenomyosis (39). Among these
genes, IGF1 was identified as one of the candidate key regulators
to differentiate adenomyosis from endometriosis.
i) IGF1. IGF1 may influence the growth and
survival of the endometrium by regulating its receptor IGF1R
signaling pathway. The immunohistochemical protein expression of
IGF1 and IGF1R has been shown to be upregulated in adenomyosis foci
and the myometrium in the proliferative phase than in the secretory
phase (16,17). It has been demonstrated that the
overexpression of IGF1R mRNA and protein is not altered throughout
the menstrual cycle in the eutopic endometrium of patients with
adenomyosis (17). On the other
hand, the protein expression of IGF1 and IGF1R has been shown to be
similar between ectopic and eutopic endometrial tissues of patients
with endometriosis throughout the cycle (18). The IGF1 levels in peritoneal fluid
have been shown to be higher in patients with endometriosis than in
control subjects, although the data remain controversial (19). Therefore, the IGF1 levels are
significantly higher in adenomyosis cases compared to endometriosis
cases. As previously demonstrated, the IGF2 levels did not differ
between the two diseases (19).
The expression levels of the ‘survival and
apoptosis’ related genes, including BAX, BCL2, IGF2, VEGF, HGF,
NGF, PDGF, EGFR, FGF2, MTOR, BECN1 and PDCD4, were similar between
the two diseases.
ii) BCL2. In general, ectopic cells of
adenomyosis and endometriosis can block apoptotic cell death, which
is regulated by the anti-apoptotic factor BCL2 family and caspase
family of proteins. BCL2 is upregulated in adenomyosis during the
proliferative phase, reaching a peak during ovulation to decrease
to values close to zero in the late luteal phase (57,62). BCL2 is also overexpressed in
endometrial stromal cells of patients with adenomyosis (57). Furthermore, the expression of BCL2
has been shown to be increased in endometriotic stromal cells
compared to the paired eutopic cells (38). BCL2 is persistently overexpressed
during both proliferative and luteal phases of the cycle in ovarian
endometriotic tissues (55).
iii) BAX. Bax protein forms a heterodimer
with Bcl-2, and functions as an apoptotic activator. The protein
expression of Bax has been shown to be decreased in the eutopic
endometrium from endometriosis compared with the control (39). No significant differences in BAX
expression have been between endometriosis and adenomyosis
(55).
iv) VEGF. Angiogenesis and neovascularization
are considered to be the major pathological features of adenomyosis
and endometriosis (42). VEGF
expression has been shown to be increased in the eutopic
endometrium in patients with adenomyosis compared to women without
adenomyosis (40,41). In adenomyosis, 17β-estradiol (E2)
has been shown to induce pro-angiogenic activity in vascular
endothelial cells through the snail family transcriptional
repressor 2 (SNAI2)-VEGF axis (42).
v) HGF. HGF may contribute to endometrial
cell invagination deep into the myometrium at the endo-myometrial
junction in women with adenomyosis through the
epithelial-mesenchymal transition (EMT) pathway (43). The upregulation of HGF, VEGFR2,
hypoxia inducible factor 1 subunit α (HIF-1α), PDGFB, neuropilin 1
(NRP1) and EPH receptor B4 (EPHB4) has been identified in the
ectopic and eutopic endometrium of women with endometriosis
compared to that of the controls (44,45).
vi) NGF. NGF mRNA expression has been shown
to be increased in the ectopic endometrium of patients with
adenomyosis (46) or
endometriosis (48) compared to
the eutopic endometrium or normal endometrium. E2 promotes NGF
production in adenomyosis endometrial stromal cells, but not in
control endometrial stromal cells (47).
vii) PDGF. The expression level of PDGF has
been shown to be decreased in adenomyosis (16) and endometriosis (49) compared with the control.
viii) EGFR. EGFR is downregulated in the
adenomyotic (16) and
endometriotic (50) tissues
compared to that in the normal endometrial tissues.
ix) FGF2. FGF2 has been shown to be
upregulated in adenomyosis (51)
and endometriosis (49) compared
to healthy controls, which may contribute to the proliferation of
ectopic cells through the FGF2/Extracellular signal-regulated
kinase (ERK)1/2 signaling pathway (52).
x) MTOR. Endometrial cells proliferate,
migrate and survive by modulating the phosphatidylinositol 3-kinase
(PI3K)/AKT/p-mTOR signaling pathway (53). The expression of p-mTOR has been
shown to be higher in the ectopic endometrium than in the eutopic
endometrium of patients with endometriosis or adenomyosis (53,54).
xi) BECN1. BECN1 is an autophagy-related gene
(58). The expression of Beclin 1
mRNA and protein has been shown to be decreased in both ectopic and
eutopic endometriotic tissues from women with adenomyosis (58) or endometriosis (59) compared with endometrium from
healthy women, suggesting that autophagy hisrelated to the
pathogenesis and progression of the two conditions.
xii) PDCD4. PDCD4 is involved in the
apoptotic pathway and is a tumor suppressor gene, which is
downregulated in adenomyosis (60) and endometriosis (61) possibly through the nuclear factor
(NF)-κB/mammalian target of rapamycin (MMP)2/MMP9 signaling
pathway.
Immune and inflammatory response (12 genes).
The alterations to the immune response and inflammation in the
surrounding microenvironment may play a role at different stages of
adenomyosis and endometriosis development, including initiation,
invasion, promotion, survival and progression. The pathway involved
in ‘Immune and inflammatory response’ includes OPN, killer cell
immunoglobulin-like receptors (KIRs; KIR3DL1 and KIR2DL3),
granulocyte-macrophage colony-stimulating factor (GM-CSF), NF-κB,
transforming growth factor-β (TGF-β), leukemia inhibitory factor
(LIF), Toll-like receptor (TLR), interleukin (IL)-1β, IL-10, C-X-C
motif chemokine ligand 1 (CXCL1), tropomycin receptor kinase B
(TrkB) and cyclooxygenase-2 (COX-2).
i) OPN. OPN is a multifunctional cytokine and
upregulates the expression of interferon-γ (IFN-γ) and IL-12.
IFN-γ, one of the immunostimulatory cytokines, activates natural
killer (NK) cell cytotoxicity. IL-12, a pleiotropic cytokine, also
plays an essential role in the activation of both innate (NK cells)
and adaptive (cytotoxic T lymphocytes) immunities. Higher OPN mRNA
and protein levels have been observed in both the ectopic and
eutopic endometrium from subjects with endometriosis compared with
the endometrium from women without endometriosis (22). Conversely, the expression levels
of OPN mRNA and protein have been shown to be lower in patients
with adenomyosis than in the controls (20,21). The altered expression of OPN
programs an immune environment that may be obligatory for
adenomyosis progression.
No significant difference in the expression levels
of the following genes was noted in adenomyosis and in
endometriosis: NK cell markers (KIR3DL1, KIR2DL3), GM-CSF, NF-κB,
TGF-β, LIF, TLR, IL-1β, IL-10, CXCL1, TrkB and COX-2.
ii) KIR. NK cells contribute to early defense
against infected cells and tumor cells. NK cells have a decreased
expression of inhibitory KIRs, including KIR3DL1 (also known as
NKB1) and KIR2DL3 (also known as GL183), on their surface and have
enhanced cytotoxic functions. In endometriosis, peripheral NK cells
and peritoneal NK cells exhibit an increased expression of KIRs and
have reduced levels of cytotoxicity (63). Endometriotic cells develop several
strategies to escape immune surveillance to avoid attack from the
immune system. Ectopic lesions of adenomyosis have an increased
expression of KIRs (64). By
contrast, the expression of inhibitory KIRs is decreased in the
eutopic endometrium, but not in the ectopic lesion, in women with
adenomyosis, demonstrating a greater cytotoxic activity (64).
iii) GM-CSF. GM-CSF is highly expressed in
the ectopic compared with the eutopic tissue in patients with
endometriosis (65), as well as
in patients with adenomyosis (66). GM-CSF-activated phagocytes cause
tissue damage during inflammation.
iv) NF-κB. NF-κB is a critical
pro-inflammatory regulator that has been suggested to play a
pivotal role in the expression of proinflammatory genes, including
cytokines, chemokines and adhesion molecules (67). NF-κB is considered to play a
central role in the pathogenesis of endometriosis and adenomyosis
(67).
v) TGF-β. The ectopic endometrium of women
with adenomyosis and endometriosis exhibits an increased expression
of TGF-β1 and phosphorylated SMAD family member 3 (Smad3), markers
of EMT, and increased fibrosis as compared with the normal
endometrium (68,69). TGF-β1/Smad3 and IL-6/Janus kinase
2 (JAK2)/signal transducer and activator of transcription 3 (STAT3)
signaling pathways are upregulated in adenomyosis (70). TGF-β is increased in the serum,
peritoneal fluid, ectopic endometrium and peritoneum of women with
endometriosis compared to women without endometriosis (71).
vi) LIF. LIF has biological actions in
maternal receptivity to blastocyst implantation, embryo
implantation and placental formation, possibly through the
activation of STAT3 and ERK signaling (72). Adenomyosis and endometriosis have
negative impacts on embryo implantation. Patients with adenomyosis
(72) or endometriosis (73) exhibit a decreased expression of
LIF and its receptor, LIFR, in the eutopic endometrium as compared
with healthy women.
vii) TLR4. The expression of TLRs (TLR1, 2,
4, 5 and 9) is higher in the ectopic endometrium and eutopic
endometrium in adenomyosis as compared with the normal endometrium,
and positively correlates with IL-6 and IL-8(74). Similar to adenomyosis, TLR4 is
overexpressed in endometriosis, indicating that both conditions are
a state of inflammatory pathology (75).
viii) IL-1β. The overexpression of IL-1β has
been identified in adenomyosis (46) and endometriosis (76), which is linked with an ability to
transform acute inflammation to the chronic inflammation.
ix) IL-10. The anti-inflammatory cytokine,
IL-10, may play critical roles in suppressing immunity against
embryo implantation (77). IL-10
protein is overexpressed in the ectopic and eutopic endometrium of
women with adenomyosis compared to the normal endometrium (78). The serum level of IL-10 in
patients with endometriosis is also higher than that in healthy
subjects (77).
x) CXCL1 (also known as GRO1 or GROα). The
CXCL1 chemokine is a secreted growth factor that signals through
the G-protein coupled receptor, CXC receptor 2, and acts as a
neutrophil-activating factor. CXCL1 is overexpressed in the
epithelium of the endometrium in adenomyosis (40) and endometriosis (79,80). VEGF contributes to the production
of CXCL1 in endometrial epithelial cells through the NF-κB
signaling pathway (40). IL-17A
also induces CXCL1 expression (80).
xi) TrkB. TrkB is a receptor for the main
neurotrophins (NGF and BDNF) and detected in endometriosis tissue.
TrkB is expressed in te eutopic endometrium of proliferative phase
of women with endometriosis (81), while its expression has been
detected in the secretory endometrium of women with adenomyosis
(82). TrkB has not been found in
the endometrium of the proliferative or secretory phase in the
control group (81).
xii) COX-2 [also known as
prostaglandin-endoperoxide synthase 2 (PTGS2)]. COX-2 is
responsible for the prostaglandin biosynthesis involved in
inflammation. Several studies have demonstrated the overexpression
of COX-2 in adenomyosis (83,84) and endometriosis, particularly in
the endometriotic ovarian cyst wall (85,86). IL-1β induces the expression of
COX-2 and VEGF through the MAPK/ERK signaling pathway (86).
Invasion and extracellular matrix remodeling (5
genes). The eutopic endometrium of women with adenomyosis
exhibits multiple pathways that predispose to the endometrial
infiltration and invasion (13).
ECM degradation and remodeling may be the cause of eutopic and
ectopic endometrial invasion in adenomyosis and endometriosis.
Below, key DEGs that promote adenomyotic and endometriotic cell
migration and invasion are discussed.
i) KISS1. KISS1 was initially found to
function as a metastasis suppressor and also involved in endocrine
functions, glucose homeostasis and insulin secretion (26). KISS1 suppresses the metastases of
melanomas, colon cancer and breast cancer through inhibiting
cell-matrix adhesion, cytoskeletal reorganization, chemotaxis and
invasion (24). KISS1 prevents
the growth and colonization of metastatic cells in distant sites
and delays the metastatic cascade. KISS1 exerts multiple effects on
cancer cell biology through a G-protein-coupled receptor, GPR54.
The expression of KISS1 is upregulated in adenomyosis (23), but not in endometriosis (25). Thus, the question may be raised as
to whether and how KISS1 is involved in the pathogenesis of
adenomyosis. The existing data are controversial. Fratangelo et
al reported that KISS1 plays a role in the early steps of
breast cancer development (27).
Although MMP-2, MMP-9, moesin (MSN), focal adhesion
kinase (FAK) and lysyl oxidase (LOX) are overexpressed in the
ectopic endometrium of women with adenomyosis and endometriosis,
the expression levels of these genes have been found to be similar
between the two conditions.
ii) MMP. The increased expression of MMP-2
and -9 in the eutopic endometrium may play a role in the
development of adenomyosis (83,87) and endometriosis (88). The MMP-7-181A/G polymorphism may
be associated with the susceptibility to adenomyosis and
endometriosis (89).
iii) MSN. MSN is a member of the ezrin,
radixin and moesin (ERM) protein family that participates in the
key events of the carcinogenesis processes, such as cellular
morphology, adhesion, migration and tumor invasion through a
cross-linking between membrane proteins and the actin-based
cytoskeleton. MSN mRNA and protein expression levels have been
shown to be upregulated in adenomyosis (90). In addition, MSN is overexpressed
and produced in ovarian endometrioma (91).
iv) FAK. FAK is a cytoplasmic protein
tyrosine kinase that is overexpressed and activated in several
types of cancer (92). FAK
promotes cell motility, survival and proliferation through the EMT
process and the PI3K/AKT signaling pathway (92). FAK expression is increased in
adenomyosis (92) and
endometriosis (93). FAK may play
a role in the pathogenesis of the two conditions.
v) LOX. LOX is an enzyme involved in collagen
deposition, extracellular membrane remodeling and invasive
potential. LOX exressionis increased in adenomyosis (68) and endometriosis (94). The aberrant expression of LOX
destabilizes the ECM, resulting in ECM degradation and
remodeling.
Adhesion (5 genes). The adhesion and
migration of endometrial cells are required to establish
adenomyotic lesions within the myometrium. The levels of
adhesion-related genes, neural cell adhesion molecule 1 (NCAM1),
versican (VCAN), L-selectin ligand, SLIT-ROBO Rho GTPase activating
protein 2 (SRGAP2) and disintegrin and metalloproteinase
domain-containing protein 12 (ADAM12), are altered in both the
ectopic and eutopic endometrium from patients with adenomyosis
compared with the eutopic endometrium from women without
adenomyosis. Among these, NCAM1 is upregulated and VCAN and
L-selectin ligand are downregulated in the ectopic endometrium in
adenomyosis than in the corresponding eutopic endometrium and
control groups. A adenomyotic cell-induced switch of attachment
into detachment may, in turn, trigger a range of cell invasive
signals accompanied by destruction of ECM, thus creating an optimal
niche for adenomyotic cells to grow.
i) NCAM1 (also known as CD56). NCAM1, also
known as CD56, is a NK cell marker and is involved in cell-to-cell
interactions, as well as cell-matrix interactions. NCAM1 expression
has been shown to be increased in the ectopic endometrium of
patients with adenomyosis (28),
while a decreased NCAM1 expression has been observed in the ectopic
endometrium of patients with endometriosis (29). Muscle repair and scarring are
driven by migratory CD56+ NK cells, macrophages and
fibroblasts that infiltrate injury sites and secrete growth factors
(95). This suggests that
adenomyosis-related wound healing and scarring may be associated
with an increased CD56+ cell infiltrate. Therefore, an
increased NCAM1 expression may be associated with the severity of
dysmenorrhea (28).
ii) VCAN, versican. VCAN, a major component
of the ECM, is a large chondroitin sulfate proteoglycan. This gene
plays a central role in tissue morphogenesis and maintenance
through cell adhesion, migration, proliferation and angiogenesis.
VCAN is one of the molecules upregulated in endometriosis (30), while this protein is downregulated
in adenomyosis (13).
iii) L-selectin ligand. The selectins mediate
inflammatory leukocyte trafficking on vascular endothelial cell
surfaces, which transduces the intracellular signal transduction
processes through selectin ligands. L-selectins induce the integrin
β2-dependent tyrosine phosphorylation of multiple proteins,
resulting in the enhancement of target cell adhesion, slow rolling
and recruitment. The expression of L-selectin ligands is increased
in endometriosis, but decreased in adenomyosis (31). Aberrant adhesion represents an
acquired advantage of tumor cells directly responsible for an
invasive phenotype, which is considered to be a hallmark of cancer
progression. A reduced expression of adhesion molecules may
contribute to an enhanced invasive phenotype of adenomyotic cells.
However, constitutive defects in L-selectin ligand expression may
be attributed to poor uterine receptivity and cause implantation
failure (31). Future studies are
required to focus on the mechanisms through which L-selectin
ligands affect adenomyotic cell adhesion, migration and
invasion.
iv) SRGAP2. SLITs stimulates the GTPase
activity of RAC1 through the transmembrane receptor roundabout
(ROBO). SLIT/ROBO signaling has been linked to roles in neuronal
migration, leukocyte chemotaxis, angiogenesis and cancer
progression (96). The
microvascular density is associated with SLIT expression (96). SRGAP2 increases cell adhesion
spreading and decreases cell migration. SLIT/ROBO expression is
higher in the ectopic endometrium from women with adenomyosis
(97) and endometriosis (96) compared with the normal
endometrium. SLIT/ROBO expression is higher in recurrent
endometriosis cases than in non-recurrent cases (96).
v) ADAM12. ADAM12 has been shown to be
involved in the regulation of a variety of biological processes,
including fertilization, muscle development, neurogenesis and tumor
angiogenesis. ADAM12 facilitates tumor angiogenesis through the
activation of EGFR/STAT3/AKT-dependent pathways (98). Similar to adenomyosis, ADAM12
levels are upregulated in endometriosis that has undergone a switch
to the angiogenic phenotype (96). ADAM12 is involved in heparin
binding EGF-like growth factor (HB-EGF) shedding in endometriosis
(99).
Hormonal response (5 genes). Estrogen
receptor (ER) may be an important regulator in controlling gene
expression in endometriosis and may contribute to disease
formation, establishment, maintenance and progression (100). Functional downstream target
genes of ER may contribute to the epigenetic susceptibility to
endometriosis (100). An
enhanced ERβ (ESR2) activity stimulates the progression of
endometriosis by inhibiting tumor necrosis factor-α (TNF-α)-induced
apoptosis and increasing IL-1β-dependent cellular adhesion and
proliferation (101). The
steroid hormone-mediated decidualization signaling pathway is
considered to be dysregulated in endometriosis (100). Progesterone resistance is
associated with endometriosis and contributes to impaired
decidualization. The expression levels of ESR2, progesterone
receptor isoform B (PR-B), retinoid X receptor α (RXRα) and
oxytocin receptor (OXTR) genes and/or proteins are dysregulated in
the ectopic endometrial tissues of women with adenomyosis and
endometriosis. We could not find any key genes that can
differentiate adenomyosis from endometriosis.
i) Estrogen receptor 1 (ESR1; ERα); ii) ESR2
(ERβ); and iii) progesterone receptor isoform B (PR-B). The
ectopic endometrium in adenomyosis is rarely influenced by hormonal
changes and has potent proliferative properties (37). In adenomyosis and endometriosis,
the promoters of ERβ and PR-B are hypomethylated and
hypermethylated, respectively (67,102,103). ER-β overexpression and the lack
of PR-B expression may explain progesterone resistance (103). A similar expression pattern of
ER-β and PR-B was found in adenomyosis and endometriosis.
iv) RXRA (RXRα). RXRA is a member of the
steroid and thyroid hormone receptor superfamily of transcriptional
regulators. A decreased RXRA expression has been found in
endometriotic stromal cells of patients with adenomyosis (16) and endometriosis (104).
v) OXTR. OXTR is upregulated in smooth muscle
cells and epithelial cells in adenomyosis (105) and peritoneal endometriotic
lesions and ovarian endometriotic cysts (106). OXTR overexpression may be
responsible for increased uterine contractility and dysmenorrhea
(107).
EMT (3 genes). EMT contributes pathologically
to both adenomyosis and endometriosis, and this transition may be
the fundamental event, such as increased cellular invasiveness and
fibrogenesis (108). We explored
key genes, including ANXA2, integrin linked kinase (ILK) and
catenin beta1 (CTNNB1). ANXA2 may be a susceptibility gene that
controls this transition.
i) ANXA2. ANXA2 acts as a tumor-associated
protein and promotes cancer proliferation, invasion, EMT and
metastasis (35). ANXA2
expression has been found to be upregulated (32) and downregulated (33,34) in adenomyosis and endometriosis,
respectively. E2 stimulates the expression of ANXA2 in adenomyotic
endometrial cells and induces EMT and angiogenesis via the
β-catenin/T-cell factor (Tcf) and HIF-1α/VEGF-A signaling pathways
(32). In addition, ANXA2
contributes to lung (109) and
liver (110) injury and
fibrosis, suggesting that ANXA2 may be involved in fibrotic process
in adenomyosis occurring at the inner or the outer myometrium
ii) ILK. αVβ3-integrins promote tumor growth
and metastasis by activating ILK via the Akt/mTOR and glycogen
synthase kinase 3 beta (GSK-3β)/β-catenin signaling pathways
(111). ILK plays a role in
intercellular adhesion and triggers the process of EMT, which plays
a significant role in the pathogenesis of adenomyosis and
endometriosis (112).
ILK-induced EMT promotes the invasive phenotype in adenomyosis
(112) and endometriosis
(113).
iii) CTNNB1. The aberrant activation of the
Wnt/β-catenin pathway may be involved in the pathophysiology of
adenomyosis (114,115) and endometriosis (116,117). E2 drives Wnt/β-catenin triggered
up-regulation of MMP-9 and VEGF (114,117).
Cell cycle regulation (3 genes).
p27kip1 and p21CIP are key cell cycle
regulators of endometriosis (95,118). The dysregulation of the cell
cycle control is one of the hallmarks of adenomyosis and
endometriosis. Musashi RNA binding protein 1 (MSI1) and
pluripotency markers [SRY-box transcription factor 2 (SOX2)/POU
class 5 homeobox 1 (OCT4)] are overexpressed in adenomyosis;
however, they are not able to distinguish adenomyosis from
endometriosis.
i) MSI1 (also known as Musashi-1). MSI1, a
stem cell marker, was identified as a regulator of cancer
progression. MSI1 reduces the expression of p21CIP and
promotes cell proliferation and cell division. MSI1 expression has
been shown to be increased in adenomyosis (119) and endometriosis (120).
ii) SOX2; and iii) OCT4 (also known as
POU5F1). The transcription pluripotency factors, SOX2 and OCT4,
have been shown to be overexpressed in adenomyosis and
endometriosis (121).
Angiogenesis (1 gene). Ectopic endometriotic
lesions produce multiple pro-angiogenic factors, including VEGF,
angiopoietin (Ang)-1 and Ang-2, PDGF, IL-1β, IL-6 and IL-8, TNF-α,
and TGF-β, Notch and HIF-1α that were also upregulated in eutopic
endometrium from patients with endometriosis in comparison with
endometrium from healthy women (122). HIF-1α represents a key regulator
of angiogenesis. HIF-1α expression has been shown to be increased
in adenomyosis (41,123) and endometriosis (120).
i) HIF-1α. The ectopic endometrium of women
with adenomyosis (41) and
endometriosis (123) may
contribute to an increased HIF-1α expression and the stimulation of
angiogenesis.
Oxidative damage (1 gene). Hemoglobin and
iron originating from cyclic menstrual hemorrhage generate a wide
range of reactive oxygen species (ROS) and hydroxy radicals, which
also induce redox signaling (124). Oxidative stress and redox
signaling may be implicated in the pathophysiology of adenomyosis
and endometriosis.
i) GPX, glutathione peroxidase. The
expression of the antioxidant protein, GPX, in adenomyosis has been
shown t obe upregulated throughout the menstrual cycle (13,125). The aberrant expression of GPX in
the ectopic and eutopic endometrium also suggests a
pathophysiological role in endometriosis (125). Thus, the expression level of GPX
may be similar between two conditions.
Mitochondrial dysfunction (1 gene)
i) Superoxide dismutase 2 (SOD2; also known as
Mn-SOD). Since mitochondrial DNA is sensitive to oxidative
stress, ROS induces mitochondrial dysfunction. Adenomyosis and
endometriosis may be associated with mitochondrial dysfunction, as
reflected by enhanced inflammatory reaction and decreased ATP
production (13,126,127). ROS may induce mitochondrial
antioxidant enzyme, superoxide dismutase (Mn-SOD) in ectopic
endometrium of adenomyosis (128) and endometriosis (127).
Prostaglandin biosynthesis (1 gene)
i) Prostaglandin-endoperoxide synthase 2
(PTGS2). The expression of (PTGS2 (also known as prostaglandin
H synthase) is high in the ectopic endometrium of patients with
adenomyosis and endometriosis compared with eutopic endometrium,
suggesting that ectopic lesions are capable of prostaglandin
synthesis (84,129).
4. Conclusions and future
considerations
Despite the advent of numerous genetic techniques
and molecular assessment, direct comparisons between adenomyosis
and endometriosis have not been performed to date, at least to the
best of our knowledge. In this review, we discuss the gene
expression features and core regulatory genes with which to
differentiate adenomyosis from endometriosis. Bioinformatics
approaches were applied to analyze the high-throughput microarray
datasets that were downloaded from NCBI-GEO. Patients with
adenomyosis displayed both coincident (91.2%) and distinct (8.8%)
gene expression profiles compared to patients with endometriosis.
Through the comparison of the two conditions, we identified 274
(8.8%) adenomyosis candidate genes that have little overlap with
endometriosis. Among these, we selected 50 genes whose expression
profiles have been published in a public repository (https://www.ncbi.nlm.nih.gov/pubmed/).
Compared to endometriosis, adenomyosis exhibits an upregulation of
IGF1, KISS1, NCAM1 and ANXA2, and a downregulation of OPN, VCAN and
L-selectin ligand. The candidate genes specifically altered in
adenomyosis may be related to the processes of invasion of the
endometrial basalis into the myometrium (KISS1), cell survival and
apoptosis (IGF1 and OPN), wound healing and scarring (NCAM1) and
fibrosis (ANXA2).
First, several researchers have explored the
proposed mechanisms of the pathogenesis of endometriosis. Recent
biological studies have used large-scale datasets combined with
bioinformatics to investigate global patterns of gene expression in
a group of clinically heterogeneous disorders. The differential
gene expression profiles were compared between ectopic and eutopic
endometrial tissues of endometriosis via global genomics,
transcriptomics and proteomics, coupled with bioinformatics and
biostatistics (6-11,130).
Endometriosis is involved in pathways, such as hormonal regulation,
proliferation, anti-apoptosis, cell cycle regulation, cytokines,
chemokines, (pro)inflammatory and immune response, stress response
and detoxification, metabolism, cell adhesion, motility, invasion
and survival of endometriotic cells. These data have paid special
attention to the role of oxidative stress, which may be implicated
in the pathophysiology of endometriosis, causing a general
inflammatory response (131,132). The retrograde flow of menstrual
blood followed by severe hemolysis occurs during the development of
endometriosis, which results in high levels of free heme and iron
(131,132). These compounds lead to DNA
damage, oxidatively modify lipids and proteins, and subsequently
promote cell death, fibrosis and carcinogenesis (131). Iron may also alter genome
stability through oxidative DNA damage and may have an impact on
gene expression (132). The
highly expressed genes in endometriosis mainly take part in the
process of stress response, detoxification and anti-apoptosis
(131,132). Both the ectopic endometrium and
eutopic endometrium in endometriosis exhibit anti-apoptotic
activity (133). The aberrant
expression of anti-apoptotic molecules, such as BCL2 may be
involved in viability of ectopic endometrial cells by increasing
cell proliferation and decreasing cell apoptosis (38,55). Given that endometriotic cells live
in harsh environmental conditions, an altered gene expression may
result in enhanced anti-apoptosis in order to cope with oxidative
stress.
Second, both adenomyosis and endometriosis are
identical to their pathological and clinical features, although
there are some differences with regard to the proposed
pathogenesis. Adenomyosis results from the invagination of the
endometrial basalis into the myometrium, while endometriosis
originates from retrograde menstruation. The retrograde
menstruation theory does not explain adenomyosis (134). Emerging evidence indicates that
adenomyosis is a heterogeneous gynecologic condition and is
subcategorized into at least 3 subtypes: Subtype I adenomyosis
results from the invagination of the endometrial basalis into the
myometrium; subtype II occurs in the uterine outer layer without
affecting the inner structures; and subtype III adenomyosis results
from metaplasia (2,4). The identification of DEGs of each
subtype will provide a basis for the understanding of the complex
pathophysiology and etiology of adenomyosis and these genes may be
used as biomarkers of the early development of adenomyosis. In
addition, to explore the proposed mechanisms of the pathogenesis of
adenomyosis, the differential gene expression profiles were
compared between ectopic and eutopic endometrial tissues of
adenomyosis. Compared with the eutopic endometrium of women with
adenomyosis, the major pathways in terms of highly significant
functional networks in ectopic lesions of adenomyosis included ER
signaling (steroid hormone responsiveness); cell death and survival
signaling (proliferation, angiogenesis and apoptosis); cell-to-cell
signaling (cell adhesion, interaction, movement and invasion);
signaling involved in extracellular matrix remodeling; EMT
signaling; would healing, scarring and fibrosis; oxidative damage
signaling (oxidative phosphorylation); inflammatory and immune
response; mitochondrial dysfunction; prostaglandin biosynthesis;
and altered lncRNA and miRNA expression profiles (12-14,36,37,41,67,70,84,125,131,135-137).
Herndon et al reported that comparative transcriptomic
analysis identified 1,024 DEGs, 140 upregulated and 884
downregulated genes, in the endometrium of women with and without
adenomyosis (13). The eutopic
endometrium in patients with adenomyosis has undergone extensive
gene expression changes (13).
Not only the ectopic endometrium, but also the eutopic endometrium
in patients with adenomyosis have fundamental abnormalities that
may predispose to deep myometrial invasion, inflammation, would
healing, scarring and then fibrosis, suggesting that these
abnormalities may play an important role in the pathogenesis of
adenomyosis.
Third, we discuss for the first time, to the best
of our knowledge, the differential gene expression profiles of
ectopic tissues from women with adenomyosis and those from women
with endometriosis to explore core regulatory genes to
differentiate adenomyosis from endometriosis. The present review
provided a list of tissue-specific pathways that may be most suited
for adenomyosis, which were enriched in the terms related to
survival and apoptosis, immune and inflammatory response, invasion
and extracellular matrix remodeling, adhesion and EMT (Table II). It was demonstrated that
patients with adenomyosis displayed both coincident (91.2%) and
distinct (8.8%) gene expression profiles compared to patients with
endometriosis. One may raise the question whether the two
conditions represent a continuum or separated clinical entities.
The answer to the question we posed is that the patterns of gene
expression profiling inferred from the two conditions are
genetically similar. However, particularly noteworthy was that this
study has revealed distinct molecular signatures specific for
adenomyosis features. We found that 8.8% of the genes are
considered to be adenomyosis candidate genes, leading to an
improved knowledge of adenomyosis etiology. Vannuccini et al
reported that adenomyosis and endometriosis share common genetic
mutations, although recent studies offer a comprehensive landscape
of different pathogenetic mechanisms, including sex steroid hormone
receptors, inflammatory molecules, extracellular matrix degradation
enzymes, growth factors and neuroangiogenic factors (138). A notable characteristic is that
adenomyosis may be more invasive and survival benefit (increased
expression of IGF1 and KISS1) than endometriosis; adenomyosis has
an array of defensive mechanisms that can be adapted to cope with
immune attack (decreased expression of OPN); and adenomyotic
lesions show increased accumulation of ECM, leading ultimately to
fibrosis, possibly through increased expression of ANXA2. Several
genes and pathways that are specific to adenomyosis may provide
potential therapeutic targets for adenomyosis in the future
(135).
Finally, the results of this review are derived
from high-throughput microarray genomic datasets submitted by the
research community, which renders it difficult to draw firm
conclusions with regard to the etiology of adenomyosis from
existing evidence. Much of the published research datasets can be
influenced by the diagnostic approach, age, menstrual cycle phase,
the severity of the disease, intake of oral contraceptives, number
of given births, tissue heterogeneity in lesions, the concomitant
presence of endometriosis, or the difference in epigenetic
modifications. Since both lesions consist of the different cell
types (e.g., epithelial glandular cells, stromal cells,
fibroblasts, myofibroblasts, mesenchymal cells, inflammatory cells,
or immune cells), it is unclear which cells contribute to gene
expression variability. Among the 274 adenomyosis candidate genes,
we reviewed only 50 genes whose expression profiles are being
published in a public repository. To date, the function of most of
the candidate genes remain largely unknown. Notwithstanding these
limitations, this review will help to elucidate the molecular
mechanisms underlying the pathogenesis of adenomyosis.
In conclusion, herein, we present a comprehensive
gene expression analysis of adenomyosis and endometriosis by using
NCBI-GEO database to identify adenomyosis candidate genes and
clarify the complex pathogenetic mechanisms.
5. Summary
Through eligible microarray data sets collected
from NCBI-GEO, in this review, we present a large-scale survey for
the identification of candidate functional networks and several key
regulators to differentiate adenomyosis from endometriosis. The
results of bioinformatics analysis revealed that adenomyosis
patients displayed both coincident (91.2%) and distinct (8.8%) gene
expression profiles compared to endometriosis patients. Both
conditions share common pathways, but typical candidate genes are
uniquely regulated in adenomyosis. According to the functional
annotation of the DEG modules, the processes of invasion, immune
response, cell survival, wound repair and fibrosis pathways are
strongly associated with biological process terms in
adenomyosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
SI and HN collected the datasets regarding the
NCBI-GEO database and analyzed the databases to identify
adenomyosis candidate genes. HK contributed to the conception,
design and interpretation of this study. HK wrote the first draft.
The final version of the manuscript has been read and approved by
all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Harada T, Khine YM, Kaponis A, Nikellis T,
Decavalas G and Taniguchi F: The impact of adenomyosis on Women's
fertility. Obstet Gynecol Surv. 71:557–568. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
García-Solares J, Donnez J, Donnez O and
Dolmans MM: Pathogenesis of uterine adenomyosis: Invagination or
metaplasia? Fertil Steril. 109:371–379. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Puente JM, Fabris A, Patel J, Patel A,
Cerrillo M, Requena A and Garcia-Velasco JA: Adenomyosis in
infertile women: Prevalence and the role of 3D ultrasound as a
marker of severity of the disease. Reprod Biol Endocrinol.
14(60)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kishi Y, Suginami H, Kuramori R, Yabuta M,
Suginami R and Taniguchi F: Four subtypes of adenomyosis assessed
by magnetic resonance imaging and their specification. Am J Obstet
Gynecol. 207:114.e1–e7. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Vercellini P, Viganò P, Somigliana E and
Fedele L: Endometriosis: Pathogenesis and treatment. Nat Rev
Endocrinol. 10:261–275. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Crispi S, Piccolo MT, D'Avino A, Donizetti
A, Viceconte R, Spyrou M, Calogero RA, Baldi A and Signorile PG:
Transcriptional profiling of endometriosis tissues identifies genes
related to organogenesis defects. J Cell Physiol. 228:1927–1934.
2013.PubMed/NCBIPubMed/NCBI View Article : Google Scholar
|
|
7
|
Abe W, Nasu K, Nakada C, Kawano Y,
Moriyama M and Narahara H: miR-196b targets c-myc and Bcl-2
expression, inhibits proliferation and induces apoptosis in
endometriotic stromal cells. Hum Reprod. 28:750–761.
2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tamaresis JS, Irwin JC, Goldfien GA,
Rabban JT, Burney RO, Nezhat C, DePaolo LV and Giudice LC:
Molecular classification of endometriosis and disease stage using
high-dimensional genomic data. Endocrinology. 155:4986–4999.
2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Saare M, Modhukur V, Suhorutshenko M,
Rajashekar B, Rekker K, Sõritsa D, Karro H, Soplepmann P, Sõritsa
A, Lindgren CM, et al: The influence of menstrual cycle and
endometriosis on endometrial methylome. Clin Epigenetics.
8(2)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhao L, Gu C, Ye M, Zhang Z, Li L, Fan W
and Meng Y: Integration analysis of microRNA and mRNA paired
expression profiling identifies deregulated microRNA-transcription
factor-gene regulatory networks in ovarian endometriosis. Reprod
Biol Endocrinol. 16(4)2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yamagata Y, Nishino K, Takaki E, Sato S,
Maekawa R, Nakai A and Sugino N: Genome-wide DNA methylation
profiling in cultured eutopic and ectopic endometrial stromal
cells. PLoS One. 9(e83612)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou C, Zhang T, Liu F, Zhou J, Ni X, Huo
R and Shi Z: The differential expression of mRNAs and long
noncoding RNAs between ectopic and eutopic endometria provides new
insights into adenomyosis. Mol Biosyst. 12:362–370. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Herndon CN, Aghajanova L, Balayan S,
Erikson D, Barragan F, Goldfien G, Vo KC, Hawkins S and Giudice LC:
Global transcriptome abnormalities of the eutopic endometrium from
women with adenomyosis. Reprod Sci. 23:1289–1303. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jiang JF, Sun AJ, Xue W, Deng Y and Wang
YF: Aberrantly expressed long noncoding RNAs in the eutopic
endometria of patients with uterine adenomyosis. Eur J Obstet
Gynecol Reprod Biol. 199:32–37. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tang Q, Wang Q, Zhang Q, Lin SY, Zhu Y,
Yang X and Guo AY: Gene expression, regulation of DEN and HBx
induced HCC mice models and comparisons of tumor, para-tumor and
normal tissues. BMC Cancer. 17(862)2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Levy M, Mittal K, Chiriboga L, Zhang X,
Yee H and Wei JJ: Differential expression of selected gene products
in uterine leiomyomata and adenomyosis. Fertil Steril. 88:220–223.
2007. View Article : Google Scholar
|
|
17
|
Konopka B, Skasko E, Kluska A, Goluda M,
Janiec-Jankowska A, Paszko Z and Ujec M: Changes in the
concentrations of receptors of insulin-like growth factor-I,
epithelial growth factor, oestrogens and progestagens in
adenomyosis foci, endometrium and myometrium of women during
menstrual cycle. Eur J Gynaecol Oncol. 19:93–97. 1998.PubMed/NCBI
|
|
18
|
Chang SY and Ho YS: Immunohistochemical
analysis of insulin-like growth factor I, insulin-like growth
factor I receptor and insulin-like growth factor II in
endometriotic tissue and endometrium. Acta Obstet Gynecol Scand.
76:112–117. 1997.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kim JG, Suh CS, Kim SH, Choi YM, Moon SY
and Lee JY: Insulin-like growth factors (IGFs), IGF-binding
proteins (IGFBPs), and IGFBP-3 protease activity in the peritoneal
fluid of patients with and without endometriosis. Fertil Steril.
73:996–1000. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Streuli I, Santulli P, Chouzenoux S,
Chapron C and Batteux F: Serum osteopontin levels are decreased in
focal adenomyosis. Reprod Sci. 24:773–782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xiao Y, Li T, Xia E, Yang X, Sun X and
Zhou Y: Expression of integrin β3 and osteopontin in the eutopic
endometrium of adenomyosis during the implantation window. Eur J
Obstet Gynecol Reprod Biol. 170:419–422. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Cho S, Ahn YS, Choi YS, Seo SK, Nam A, Kim
HY, Kim JH, Park KH, Cho DJ and Lee BS: Endometrial osteopontin
mRNA expression and plasma osteopontin levels are increased in
patients with endometriosis. Am J Reprod Immunol. 61:286–293.
2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kolioulis I, Zafrakas M, Grimbizis G,
Miliaras D, Timologou A, Bontis JN and Tarlatzis BC:
Immunohistochemical expression pattern of metastasis suppressor
KISS-1 protein in adenomyosis lesions and normal endometrium. Eur J
Obstet Gynecol Reprod Biol. 210:64–68. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Ji K, Ye L, Mason MD and Jiang WG: The
Kiss-1/Kiss-1R complex as a negative regulator of cell motility and
cancer metastasis (Review). Int J Mol Med. 32:747–754.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Timologou A, Zafrakas M, Grimbizis G,
Miliaras D, Kotronis K, Stamatopoulos P and Tarlatzis BC:
Immunohistochemical expression pattern of metastasis suppressors
KAI1 and KISS1 in endometriosis and normal endometrium. Eur J
Obstet Gynecol Reprod Biol. 199:110–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hussain MA, Song WJ and Wolfe A: There is
Kisspeptin- and then there is kisspeptin. Trends Endocrinol Metab.
26:564–572. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fratangelo F, Carriero MV and Motti ML:
Controversial role of Kisspeptins/KiSS-1R signaling system in tumor
development. Front Endocrinol (Lausanne). 9(192)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wang F, Shi X, Qin X, Wen Z, Zhao X and Li
C: Expression of CD56 in patients with adenomyosis and its
correlation with dysmenorrhea. Eur J Obstet Gynecol Reprod Biol.
194:101–105. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Oosterlynck DJ, Meuleman C, Lacquet FA,
Waer M and Koninckx PR: Flow cytometry analysis of lymphocyte
subpopulations in peritoneal fluid of women with endometriosis. Am
J Reprod Immunol. 31:25–31. 1994.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tani H, Sato Y, Ueda M, Miyazaki Y,
Suginami K, Horie A, Konishi I and Shinomura T: Role of Versican in
the pathogenesis of peritoneal endometriosis. J Clin Endocrinol
Metab. 101:4349–4356. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lai TH, Chang FW, Lin JJ and Ling QD:
Endometrial L-selectin ligand is downregulated in the mid-secretory
phase during the menstrual cycle in women with adenomyosis. Taiwan
J Obstet Gynecol. 57:507–516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhou S, Yi T, Liu R, Bian C, Qi X, He X,
Wang K, Li J, Zhao X, Huang C and Wei Y: Proteomics identification
of annexin A2 as a key mediator in the metastasis and
proangiogenesis of endometrial cells in human adenomyosis. Mol Cell
Proteomics. 11(M112.017988)2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wu MH, Chuang PC, Lin YJ and Tsai SJ:
Suppression of annexin A2 by prostaglandin E2 impairs
phagocytic ability of peritoneal macrophages in women with
endometriosis. Hum Reprod. 28:1045–1053. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fowler PA, Tattum J, Bhattacharya S,
Klonisch T, Hombach-Klonisch S, Gazvani R, Lea RG, Miller I,
Simpson WG and Cash P: An investigation of the effects of
endometriosis on the proteome of human eutopic endometrium: A
heterogeneous tissue with a complex disease. Proteomics. 7:130–142.
2007.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen CY, Lin YS, Chen CH and Chen YJ:
Annexin A2-mediated cancer progression and therapeutic resistance
in nasopharyngeal carcinoma. J Biomed Sci. 25(30)2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yang JH, Wu MY, Chen CD, Chen MJ, Yang YS
and Ho HN: Altered apoptosis and proliferation in endometrial
stromal cells of women with adenomyosis. Hum Reprod. 22:945–952.
2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Matsumoto Y, Iwasaka T, Yamasaki F and
Sugimori H: Apoptosis and Ki-67 expression in adenomyotic lesions
and in the corresponding eutopic endometrium. Obstet Gynecol.
94:71–77. 1999.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jones RK, Searle RF and Bulmer JN:
Apoptosis and bcl-2 expression in normal human endometrium,
endometriosis and adenomyosis. Hum Reprod. 13:3496–3502.
1998.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hu H, Li H and He Y: MicroRNA-17
downregulates expression of the PTEN gene to promote the occurrence
and development of adenomyosis. Exp Ther Med. 14:3805–3811.
2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lai TH, Wu PH and Wu WB: Involvement of
NADPH oxidase and NF-κB activation in CXCL1 induction by vascular
endothelial growth factor in human endometrial epithelial cells of
patients with adenomyosis. J Reprod Immunol. 118:61–69.
2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Goteri G, Lucarini G, Montik N, Zizzi A,
Stramazzotti D, Fabris G, Tranquilli AL and Ciavattini A:
Expression of vascular endothelial growth factor (VEGF), hypoxia
inducible factor-1alpha (HIF-1alpha), and microvessel density in
endometrial tissue in women with adenomyosis. Int J Gynecol Pathol.
28:157–163. 2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Huang TS, Chen YJ, Chou TY, Chen CY, Li
HY, Huang BS, Tsai HW, Lan HY, Chang CH, Twu NF, et al:
Oestrogen-induced angiogenesis promotes adenomyosis by activating
the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med.
18:1358–1371. 2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Khan KN, Kitajima M, Hiraki K, Fujishita
A, Nakashima M and Masuzaki H: Involvement of hepatocyte growth
factor-induced epithelial-mesenchymal transition in human
adenomyosis. Biol Reprod. 92(35)2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Yerlikaya G, Balendran S, Pröstling K,
Reischer T, Birner P, Wenzl R, Kuessel L, Streubel B and Husslein
H: Comprehensive study of angiogenic factors in women with
endometriosis compared to women without endometriosis. Eur J Obstet
Gynecol Reprod Biol. 204:88–98. 2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Fukaya T, Sugawara J, Yoshida H, Murakami
T and Yajima A: Intercellular adhesion molecule-1 and hepatocyte
growth factor in human endometriosis: Original investigation and a
review of literature. Gynecol Obstet Invest. 47 (Suppl 1):S11–S17.
1999.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Carrarelli P, Yen CF, Funghi L, Arcuri F,
Tosti C, Bifulco G, Luddi A, Lee CL and Petraglia F: Expression of
inflammatory and neurogenic mediators in adenomyosis. Reprod Sci.
24:369–375. 2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li Y, Zou S, Xia X and Zhang S: Human
adenomyosis endometrium stromal cells secreting more nerve growth
factor: Impact and effect. Reprod Sci. 22:1073–1082.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Kajitani T, Maruyama T, Asada H, Uchida H,
Oda H, Uchida S, Miyazaki K, Arase T, Ono M and Yoshimura Y:
Possible involvement of nerve growth factor in dysmenorrhea and
dyspareunia associated with endometriosis. Endocr J. 60:1155–1164.
2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Lee SR, Kim SH, Lee YJ, Hong SH, Chae HD,
Kim CH, Kang BM and Choi YM: Expression of epidermal growth factor,
fibroblast growth factor-2, and platelet-derived growth factor-A in
the eutopic endometrium of women with endometriosis. J Obstet
Gynaecol Res. 33:242–247. 2007.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Yang RQ, Teng H, Xu XH, Liu SY, Wang YH,
Guo FJ and Liu XJ: Microarray analysis of microRNA deregulation and
angiogenesis-related proteins in endometriosis. Genet Mol Res. Jun
3, 2016 (Epub ahead of print). doi: 10.4238/gmr.15027826.
View Article : Google Scholar
|
|
51
|
Propst AM, Quade BJ, Gargiulo AR, Nowak RA
and Stewart EA: Adenomyosis demonstrates increased expression of
the basic fibroblast growth factor receptor/ligand system compared
with autologous endometrium. Menopause. 8:368–371. 2001.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Guo Q, Zhang H, Zhao X, Fu Y, Zhang J and
Li M: Loss of expressions of Dusp6, Sprouty4, and Sef, negative
regulators of FGF2/ERK1/2 signaling, in the endometrium of women
with adenomyosis. Int J Gynecol Pathol. 33:288–297. 2014.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Streuli I, Santulli P, Chouzenoux S,
Chapron C and Batteux F: Activation of the MAPK/ERK cell-signaling
pathway in uterine smooth muscle cells of women with Adenomyosis.
Reprod Sci. 22:1549–1560. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Guo J, Gao J, Yu X, Luo H, Xiong X and
Huang O: Expression of DJ-1 and mTOR in eutopic and ectopic
endometria of patients with endometriosis and adenomyosis. Gynecol
Obstet Invest. 79:195–200. 2015.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Goumenou A, Panayiotides I, Matalliotakis
I, Vlachonikolis I, Tzardi M and Koumantakis E: Bcl-2 and Bax
expression in human endometriotic and adenomyotic tissues. Eur J
Obstet Gynecol Reprod Biol. 99:256–260. 2001.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Preya UH, Woo JH, Choi YS and Choi JH:
HNF1β protects endometriotic cells against apoptotic cell death by
upregulating the expression of anti-apoptotic genes. Biol Reprod.
Jun 18, 2019 (Epub ahead of print). doi: 10.1093/biolre/ioz127.
PubMed/NCBI View Article : Google Scholar
|
|
57
|
Li J, Yanyan M, Mu L, Chen X and Zheng W:
The expression of Bcl-2 in adenomyosis and its effect on
proliferation, migration, and apoptosis of endometrial stromal
cells. Pathol Res Pract. 215(152477)2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ren Y, Mu L, Ding X and Zheng W: Decreased
expression of Beclin 1 in eutopic endometrium of women with
adenomyosis. Arch Gynecol Obstet. 282:401–406. 2010.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhang L, Liu Y, Xu Y, Wu H, Wei Z and Cao
Y: The expression of the autophagy gene beclin-1 mRNA and protein
in ectopic and eutopic endometrium of patients with endometriosis.
Int J Fertil Steril. 8:429–436. 2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Liu Y, Tan X, Wang Z, Li Y, Gao M, Li Y,
Fang Z, Sun Y, Zhang L, Wang X and Wei Z: Down-regulation of tumor
suppressor PDCD4 expression in endometrium of adenomyosis patients.
Curr Res Transl Med. 64:123–128. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Li Y, Wang X, Wang X, Wan L, Liu Y, Shi Y,
Zhang L, Fang Z and Wei Z: PDCD4 suppresses proliferation,
migration, and invasion of endometrial cells by inhibiting
autophagy and NF-κB/MMP2/MMP9 signal pathway. Biol Reprod.
99:360–372. 2018.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Maia H Jr, Maltez A, Studart E, Athayde C
and Coutinho EM: Effect of menstrual cycle and hormonal treatment
on ki-67 and bcl-2 expression and adenomyosis. Gynecol Endocrinol.
20:127–131. 2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Thiruchelvam U, Wingfield M and O'Farrelly
C: Natural killer cells: Key players in endometriosis. Am J Reprod
Immunol. 74:291–301. 2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yang JH, Chen MJ, Chen HF, Lee TH, Ho HN
and Yang YS: Decreased expression of killer cell inhibitory
receptors on natural killer cells in eutopic endometrium in women
with adenomyosis. Hum Reprod. 19:1974–1978. 2004.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Monsanto SP, Edwards AK, Zhou J,
Nagarkatti P, Nagarkatti M, Young SL, Lessey BA and Tayade C:
Surgical removal of endometriotic lesions alters local and systemic
proinflammatory cytokines in endometriosis patients. Fertil Steril.
105:968–977.e5. 2016.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Propst AM, Quade BJ, Nowak RA and Stewart
EA: Granulocyte macrophage colony-stimulating factor in adenomyosis
and autologous endometrium. J Soc Gynecol Investig. 9:93–97.
2002.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Nie J, Lu Y, Liu X and Guo SW:
Immunoreactivity of progesterone receptor isoform B, nuclear factor
kappaB, and IkappaBalpha in adenomyosis. Fertil Steril. 92:886–889.
2009.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Liu X, Shen M, Qi Q, Zhang H and Guo SW:
Corroborating evidence for platelet-induced epithelial-mesenchymal
transition and fibroblast-to-myofibroblast transdifferentiation in
the development of adenomyosis. Hum Reprod. 31:734–749.
2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Shen M, Liu X, Zhang H and Guo SW:
Transforming growth factor β1 signaling coincides with
epithelial-mesenchymal transition and fibroblast-to-myofibroblast
transdifferentiation in the development of adenomyosis in mice. Hum
Reprod. 31:355–369. 2016.PubMed/NCBI View Article : Google Scholar
|
|
70
|
An M, Li D, Yuan M, Li Q, Zhang L and Wang
G: Interaction of macrophages and endometrial cells induces
epithelial-mesenchymal transition-like processes in adenomyosis.
Biol Reprod. 96:46–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Young VJ, Ahmad SF, Duncan WC and Horne
AW: The role of TGF-β in the pathophysiology of peritoneal
endometriosis. Hum Reprod Update. 23:548–559. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yen CF, Liao SK, Huang SJ, Tabak S, Arcuri
F, Lee CL, Arici A, Petraglia F, Wang HS and Kayisli UA: Decreased
endometrial expression of leukemia inhibitory factor receptor
disrupts the STAT3 signaling in adenomyosis during the implantation
window. Reprod Sci. 24:1176–1186. 2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Dimitriadis E, Stoikos C, Stafford-Bell M,
Clark I, Paiva P, Kovacs G and Salamonsen LA: Interleukin-11, IL-11
receptoralpha and leukemia inhibitory factor are dysregulated in
endometrium of infertile women with endometriosis during the
implantation window. J Reprod Immunol. 69:53–64. 2006.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Jiang C, Liu C, Guo J, Chen L, Luo N, Qu
X, Yang W, Ren Q and Cheng Z: The Expression of Toll-like receptors
in eutopic and ectopic endometrium and its implication in the
inflammatory pathogenesis of adenomyosis. Sci Rep.
7(7365)2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Khan KN, Kitajima M, Fujishita A,
Nakashima M and Masuzaki H: Toll-like receptor system and
endometriosis. J Obstet Gynaecol Res. 39:1281–1292. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Sikora J, Mielczarek-Palacz A and
Kondera-Anasz Z: Association of the precursor of interleukin-1β and
peritoneal inflammation-role in pathogenesis of endometriosis. J
Clin Lab Anal. 30:831–837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang J, Huang C, Jiang R, Du Y, Zhou J,
Jiang Y, Yan Q, Xing J, Hou X, Zhou J, et al: Decreased endometrial
IL-10 impairs endometrial receptivity by downregulating HOXA10
expression in women with adenomyosis. Biomed Res Int.
2018(2549789)2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wang F, Li H, Yang Z, Du X, Cui M and Wen
Z: Expression of interleukin-10 in patients with adenomyosis.
Fertil Steril. 91:1681–1685. 2009.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Szamatowicz J, Laudański P, Tomaszewska I
and Szamatowicz M: Chemokine growth-regulated-alpha: A possible
role in the pathogenesis of endometriosis. Gynecol Endocrinol.
16:137–141. 2002.PubMed/NCBI
|
|
80
|
Takamura M, Osuga Y, Izumi G, Yoshino O,
Koga K, Saito A, Hirata T, Hirota Y, Harada M, Hasegawa A and
Taketani Y: Interleukin-17A is present in neutrophils in
endometrioma and stimulates the secretion of growth-regulated
oncogene-α (Gro-α) from endometrioma stromal cells. Fertil Steril.
98:1218–1224.e1-e2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Yu X, Ren H, Liu T, Yong M and Zhong H:
Expression and significance of ERβ and TrkB in endometriosis. Clin
Exp Obstet Gynecol. 43:75–81. 2016.PubMed/NCBI
|
|
82
|
Huang Y, Zheng W, Mu L, Ren Y, Chen X and
Liu F: Expression of tyrosine kinase receptor B in eutopic
endometrium of women with adenomyosis. Arch Gynecol Obstet.
283:775–780. 2011.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Tokyol C, Aktepe F, Dilek FH, Sahin O and
Arioz DT: Expression of cyclooxygenase-2 and matrix
metalloproteinase-2 in adenomyosis and endometrial polyps and its
correlation with angiogenesis. Int J Gynecol Pathol. 28:148–156.
2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Chen YJ, Li HY, Chang YL, Yuan CC, Tai LK,
Lu KH, Chang CM and Chiou SH: Suppression of migratory/invasive
ability and induction of apoptosis in adenomyosis-derived
mesenchymal stem cells by cyclooxygenase-2 inhibitors. Fertil
Steril. 94:1972–1979.e1-e4. 2010.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Fagotti A, Ferrandina G, Fanfani F, Legge
F, Lauriola L, Gessi M, Castelli P, Barbieri F, Minelli L and
Scambia G: Analysis of cyclooxygenase-2 (COX-2) expression in
different sites of endometriosis and correlation with
clinico-pathological parameters. Hum Reprod. 19:393–397.
2004.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Huang F, Cao J, Liu Q, Zou Y, Li H and Yin
T: MAPK/ERK signal pathway involved expression of COX-2 and VEGF by
IL-1β induced in human endometriosis stromal cells in vitro. Int J
Clin Exp Pathol. 6:2129–2136. 2013.PubMed/NCBI
|
|
87
|
Li T, Li YG and Pu DM: Matrix
metalloproteinase-2 and -9 expression correlated with angiogenesis
in human adenomyosis. Gynecol Obstet Invest. 62:229–235. 2006.
|
|
88
|
Szymanowski K, Mikołajczyk M, Wirstlein P
and Dera-Szymanowska A: Matrix metalloproteinase-2 (MMP-2), MMP-9,
tissue inhibitor of matrix metalloproteinases (TIMP-1) and
transforming growth factor-β2 (TGF-β2) expression in eutopic
endometrium of women with peritoneal endometriosis. Ann Agric
Environ Med. 23:649–653. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Shan K, Lian-Fu Z, Hui D, Wei G, Na W, Xia
J and Yan L: Polymorphisms in the promoter regions of the matrix
metalloproteinases-7, -9 and the risk of endometriosis and
adenomyosis in China. Mol Hum Reprod. 12:35–39. 2006.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Ohara R, Michikami H, Nakamura Y, Sakata
A, Sakashita S, Satomi K, Shiba-Ishii A, Kano J, Yoshikawa H and
Noguchi M: Moesin overexpression is a unique biomarker of
adenomyosis. Pathol Int. 64:115–122. 2014.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Ametzazurra A, Matorras R, García-Velasco
JA, Prieto B, Simón L, Martínez A and Nagore D: Endometrial fluid
is a specific and non-invasive biological sample for protein
biomarker identification in endometriosis. Hum Reprod. 24:954–965.
2009.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Zheng D, Duan H, Wang S, Xu Q, Gan L, Li J
and Dong Q: FAK regulates epithelial-mesenchymal transition in
adenomyosis. Mol Med Rep. 18:5461–5472. 2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Mu L, Zheng W, Wang L, Chen XJ, Zhang X
and Yang JH: Alteration of focal adhesion kinase expression in
eutopic endometrium of women with endometriosis. Fertil Steril.
89:529–537. 2008.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ruiz LA, Báez-Vega PM, Ruiz A, Peterse DP,
Monteiro JB, Bracero N, Beauchamp P, Fazleabas AT and Flores I:
Dysregulation of Lysyl oxidase expression in lesions and
endometrium of women with endometriosis. Reprod Sci. 22:1496–1508.
2015.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Zhang D, Li Y, Tian J, Zhang H and Wang S:
MiR-202 promotes endometriosis by regulating SOX6 expression. Int J
Clin Exp Med. 8:17757–1764. 2015.PubMed/NCBI
|
|
96
|
Shen F, Liu X, Geng JG and Guo SW:
Increased immunoreactivity to SLIT/ROBO1 in ovarian endometriomas:
A likely constituent biomarker for recurrence. Am J Pathol.
175:479–488. 2009.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Nie J, Liu X, Zheng Y, Geng JG and Guo SW:
Increased immunoreactivity to SLIT/ROBO1 and its correlation with
severity of dysmenorrhea in adenomyosis. Fertil Steril.
95:1164–1167. 2011. View Article : Google Scholar
|
|
98
|
Roy R, Dagher A, Butterfield C and Moses
MA: ADAM12 is a novel regulator of tumor angiogenesis via STAT3
signaling. Mol Cancer Res. 15:1608–1622. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Miller MA, Moss ML, Powell G, Petrovich R,
Edwards L, Meyer AS, Griffith LG and Lauffenburger DA: Targeting
autocrine HB-EGF signaling with specific ADAM12 inhibition using
recombinant ADAM12 prodomain. Sci Rep. 5(15150)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Koike N, Higashiura Y, Akasaka J, Uekuri
C, Ito F and Kobayashi H: Epigenetic dysregulation of endometriosis
susceptibility genes (Review). Mol Med Rep. 12:1611–1616.
2015.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Han SJ, Jung SY, Wu SP, Hawkins SM, Park
MJ, Kyo S, Qin J, Lydon JP, Tsai SY, Tsai MJ, et al: Estrogen
receptor β modulates apoptosis complexes and the inflammasome to
drive the pathogenesis of endometriosis. Cell. 163:960–974.
2015.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Jichan N, Xishi L and Guo SW: Promoter
hypermethylation of progesterone receptor isoform B (PR-B) in
adenomyosis and its rectification by a histone deacetylase
inhibitor and a demethylation agent. Reprod Sci. 17:995–1005.
2010.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Bulun SE, Cheng YH, Pavone ME, Xue Q,
Attar E, Trukhacheva E, Tokunaga H, Utsunomiya H, Yin P, Luo X, et
al: Estrogen receptor-beta, estrogen receptor-alpha, and
progesterone resistance in endometriosis. Semin Reprod Med.
28:36–43. 2010.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Pavone ME, Dyson M, Reirstad S, Pearson E,
Ishikawa H, Cheng YH and Bulun SE: Endometriosis expresses a
molecular pattern consistent with decreased retinoid uptake,
metabolism and action. Hum Reprod. 26:2157–2164. 2011.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Zhang Y, Yu P, Sun F, Li TC, Cheng JM and
Duan H: Expression of oxytocin receptors in the uterine junctional
zone in women with adenomyosis. Acta Obstet Gynecol Scand.
94:412–418. 2015.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Mechsner S, Bartley J, Loddenkemper C,
Salomon DS, Starzinski-Powitz A and Ebert AD: Oxytocin receptor
expression in smooth muscle cells of peritoneal endometriotic
lesions and ovarian endometriotic cysts. Fertil Steril. 83 (Suppl
1):S1220–S1231. 2005.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Guo SW, Mao X, Ma Q and Liu X:
Dysmenorrhea and its severity are associated with increased uterine
contractility and overexpression of oxytocin receptor (OTR) in
women with symptomatic adenomyosis. Fertil Steril. 99:231–240.
2013.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Matsuzaki S and Darcha C: Involvement of
the Wnt/β-catenin signaling pathway in the cellular and molecular
mechanisms of fibrosis in endometriosis. PLoS One.
8(e76808)2013.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Schuliga M, Jaffar J, Berhan A, Langenbach
S, Harris T, Waters D, Lee PVS, Grainge C, Westall G, Knight D and
Stewart AG: Annexin A2 contributes to lung injury and fibrosis by
augmenting factor Xa fibrogenic activity. Am J Physiol Lung Cell
Mol Physiol. 312:L772–L782. 2017.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Kolgelier S, Demir NA, Inkaya AC, Sumer S,
Ozcimen S, Demir LS, Pehlivan FS, Arslan M and Arpaci A: Serum
levels of Annexin A2 as a candidate biomarker for hepatic fibrosis
in patients with chronic hepatitis B. Hepat Mon.
15(e30655)2015.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: A cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Zhou W, Peng Z, Zhang C, Liu S and Zhang
Y: ILK-induced epithelial-mesenchymal transition promotes the
invasive phenotype in adenomyosis. Biochem Biophys Res Commun.
497:950–956. 2018.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Zheng QM, Chen XY, Bao QF, Yu J and Chen
LH: ILK enhances migration and invasion abilities of human
endometrial stromal cells by facilitating the
epithelial-mesenchymal transition. Gynecol Endocrinol.
34:1091–1096. 2018.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY,
Ahn JY, Broaddus RR, Taketo MM, Lydon JP, Leach RE, et al:
β-Catenin activation contributes to the pathogenesis of adenomyosis
through epithelial-mesenchymal transition. J Pathol. 231:210–222.
2013.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Chen YJ, Li HY, Huang CH, Twu NF, Yen MS,
Wang PH, Chou TY, Liu YN, Chao KC and Yang MH: Oestrogen-induced
epithelial-mesenchymal transition of endometrial epithelial cells
contributes to the development of adenomyosis. J Pathol.
222:261–270. 2010.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Yang YM and Yang WX:
Epithelial-to-mesenchymal transition in the development of
endometriosis. Oncotarget. 8:41679–41689. 2017.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Matsuzaki S, Botchorishvili R, Pouly JL
and Canis M: Targeting the Wnt/β-catenin pathway in endometriosis:
A potentially effective approach for treatment and prevention. Mol
Cell Ther. 2(36)2014.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Gonçalves GA: p27kip1 as a key
regulator of endometriosis. Eur J Obstet Gynecol Reprod Biol.
221:1–4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kozachenko IF, Dzhamalutdinova KM,
Faizullina NM and Shchegolev AI: Immunohistochemical parameters of
Musashi-1 in nodular and diffuse adenomyosis. Bull Exp Biol Med.
163:506–509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Götte M, Wolf M, Staebler A, Buchweitz O,
Kelsch R, Schüring AN and Kiesel L: Increased expression of the
adult stem cell marker Musashi-1 in endometriosis and endometrial
carcinoma. J Pathol. 215:317–329. 2008.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Song Y, Xiao L, Fu J, Huang W, Wang Q,
Zhang X and Yang S: Increased expression of the pluripotency
markers sex-determining region Y-box 2 and Nanog homeobox in
ovarian endometriosis. Reprod Biol Endocrinol.
12(42)2014.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Djokovic D and Calhaz-Jorge C:
Angiogenesis as a therapeutic target in endometriosis. Acta Med
Port. 27:489–497. 2014.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y,
Li N, He H, Du Y and Liu Y: Hypoxia-inducible factor-1α promotes
endometrial stromal cells migration and invasion by upregulating
autophagy in endometriosis. Reproduction. 153:809–820.
2017.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Scutiero G, Iannone P, Bernardi G,
Bonaccorsi G, Spadaro S, Volta CA, Greco P and Nappi L: Oxidative
stress and endometriosis: A systematic review of the literature.
Oxid Med Cell Longev. 2017(7265238)2017.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Ota H, Igarashi S, Kato N and Tanaka T:
Aberrant expression of glutathione peroxidase in eutopic and
ectopic endometrium in endometriosis and adenomyosis. Fertil
Steril. 74:313–318. 2000.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Hsu AL, Townsend PM, Oehninger S and
Castora FJ: Endometriosis may be associated with mitochondrial
dysfunction in cumulus cells from subjects undergoing in vitro
fertilization-intracytoplasmic sperm injection, as reflected by
decreased adenosine triphosphate production. Fertil Steril.
103:347–352.e1. 2015.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Chen C, Zhou Y, Hu C, Wang Y, Yan Z, Li Z
and Wu R: Mitochondria and oxidative stress in ovarian
endometriosis. Free Radic Biol Med. 136:22–34. 2019.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Ishikawa M, Nakata T, Yaginuma Y,
Nishiwaki K, Goishi K and Saitoh S: Expression of superoxide
dismutase (SOD) in adenomyosis. Am J Obstet Gynecol. 169:730–734.
1993. View Article : Google Scholar
|
|
129
|
Van Voorhis BJ, Huettner PC, Clark MR and
Hill JA: Immunohistochemical localization of prostaglandin H
synthase in the female reproductive tract and endometriosis. Am J
Obstet Gynecol. 163:57–62. 1990.PubMed/NCBI View Article : Google Scholar
|
|
130
|
Kobayashi H, Uekuri C and Shigetomi H:
Towards an understanding of the molecular mechanism of
endometriosis: Unbalancing epithelial-stromal genetic conflict.
Gynecol Endocrinol. 30:7–15. 2014.PubMed/NCBI View Article : Google Scholar
|
|
131
|
Kobayashi H, Yamada Y, Kanayama S,
Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T and Oi
H: The role of iron in the pathogenesis of endometriosis. Gynecol
Endocrinol. 25:39–52. 2009.PubMed/NCBI View Article : Google Scholar
|
|
132
|
Akasaka J, Uekuri C, Shigetomi H, Koike M
and Kobayashi H: Hepatocyte nuclear factor (HNF)-1β and its
physiological importance in endometriosis. Biomed Rep. 1:13–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
133
|
Meresman GF, Vighi S, Buquet RA,
Contreras-Ortiz O, Tesone M and Rumi LS: Apoptosis and expression
of Bcl-2 and Bax in eutopic endometrium from women with
endometriosis. Fertil Steril. 74:760–766. 2000.PubMed/NCBI View Article : Google Scholar
|
|
134
|
Benagiano G, Brosens I and Habiba M:
Structural and molecular features of the endomyometrium in
endometriosis and adenomyosis. Hum Reprod Update. 20:386–402.
2014.PubMed/NCBI View Article : Google Scholar
|
|
135
|
Xiaoyu L, Weiyuan Z, Ping J, Anxia W and
Liane Z: Comparative serum proteomic analysis of adenomyosis using
the isobaric tags for relative and absolute quantitation technique.
Fertil Steril. 100:505–510. 2013.PubMed/NCBI View Article : Google Scholar
|
|
136
|
Hever A, Roth RB, Hevezi PA, Lee J,
Willhite D, White EC, Marin EM, Herrera R, Acosta HM, Acosta AJ and
Zlotnik A: Molecular characterization of human adenomyosis. Mol Hum
Reprod. 12:737–748. 2006.PubMed/NCBI View Article : Google Scholar
|
|
137
|
Kobayashi H, Iwai K, Niiro E, Morioka S
and Yamada Y: Fetal programming theory: Implication for the
understanding of endometriosis. Hum Immunol. 75:208–217.
2014.PubMed/NCBI View Article : Google Scholar
|
|
138
|
Vannuccini S, Tosti C, Carmona F, Huang
SJ, Chapron C, Guo SW and Petraglia F: Pathogenesis of adenomyosis:
An update on molecular mechanisms. Reprod Biomed Online.
35:592–601. 2017.PubMed/NCBI View Article : Google Scholar
|