Introduction
Endometriosis is a complex benign, multifactorial,
estrogen-dependent gynecological condition characterized by the
growth of endometrial-like tissue outside the uterine cavity and is
associated with pelvic pain, dysmenorrhea, intestinal symptoms,
dyspareunia and infertility, although a low percentage of patients
with the condition may be asymptomatic (1-3).
Various genetic and environmental factors are known to influence
the susceptibility to endometriosis (4-6)
and heritability has been estimated at approximately 50% from twin
studies (7). Novel genes are
added to the list of endometriosis-associated genes given that
novel Genome Wide Association Studies (GWAS) and meta-analyses are
still in progress, while the role of epigenetic modifications in
the development of endometriosis is being investigated extensively
(8). Endometriosis can appear as
peritoneal lesions, ovarian endometriotic cysts and deeply
infiltrative endometriosis (9).
Whilst a high percentage (10-15%) of women of reproductive age
suffer from endometriosis (1),
the pathogenetic mechanisms leading to this disease remain unclear,
although several theories have been put forth thus far regarding
the development of endometriosis. However, there is strong evidence
to indicate that the development of endometriosis is highly
dependent on angiogenesis, due to its role in the ectopic
implantation of endometrial tissue, as well as its increased
activity into endometriotic lesions (10-12).
Angiogenesis deals with the fundamental process of the formation
and growth of new blood vessels from pre-existing ones and
represents a biological setting of high importance during
development and tissue growth, while it is involved in the
pathogenesis of a number of diseases (13,14). Moreover, angiogenesis is
considered to play a pivotal role in the pathogenesis of
endometriosis, given that it provides a substantial supply of
oxygen and essential nutrition required for the maintenance of
endometrial tissues (15).
Indeed, clinical studies have shown that dense vascularization
characterizes all endometriotic lesions (2,10).
There are numerous endogenous regulators of
angiogenesis. Among these, a main angiogenic mediator that has
attracted much attention is vascular endothelial growth factor
(VEGF), which has been suggested to play an important role in the
development of endometriosis in combination with its endothelial
selective receptor, VEGFR2(16),
also known as kinase insert domain receptor (KDR) (17). VEGF proteins play specific roles
in controlling the growth of new blood vessels, while VEGF receptor
signal transduction mediates endothelial cell proliferation,
migration, organization into functional vessels and the remodeling
of the vessel network (18,19). VEGF is produced by endothelial
cells, monocytes and fibroblasts in response to hypoxia, which is
the major physiological signal for angiogenesis through the
activation of its receptor, VEGFR2, which is a member of the
tyrosine kinase superfamily (20). A VEGFR2 homodimer is considered to
be the main conduit for VEGF signaling (21,22); however, heterodimers involving
other VEGF receptors may also transduce a signal (23,24).
Binding of growth factors, such as the VEGF dimer to
the extracellular domain of their trans-membrane receptors leads to
receptor dimerization, the activation of the intracellular tyrosine
kinase domain of the receptor (25) and the initiation of signaling
pathways (20). The extracellular
region of the receptor is composed of 7 Ig-like domains. Domain
deletion experiments have localized the VEGF binding site to
domains 2 and 3 (D2 and D3), with D2 seems to play a dominant role
(26,27). VEGFR2 D2 alone is necessary and
sufficient for high-affinity binding, although D3 enhances affinity
(28). By contrast, VEGFR2
constructs lacking domain D3 bind VEGF weakly or not at all;
however, domain D2 is also required (26,27). Importantly, both 4th-7th Ig-like
extracellular domains play a crucial role in signal transduction.
To probe the functional importance of the residues involved in
D3-D4 domain interaction interfaces, and to assess their
contribution to VEGFR2 dimerization and VEGF binding, a series of
alanine mutants have been constructed (29). There is reason to believe that
domain 4 is involved in VEGFR2 dimerization, both from analogy with
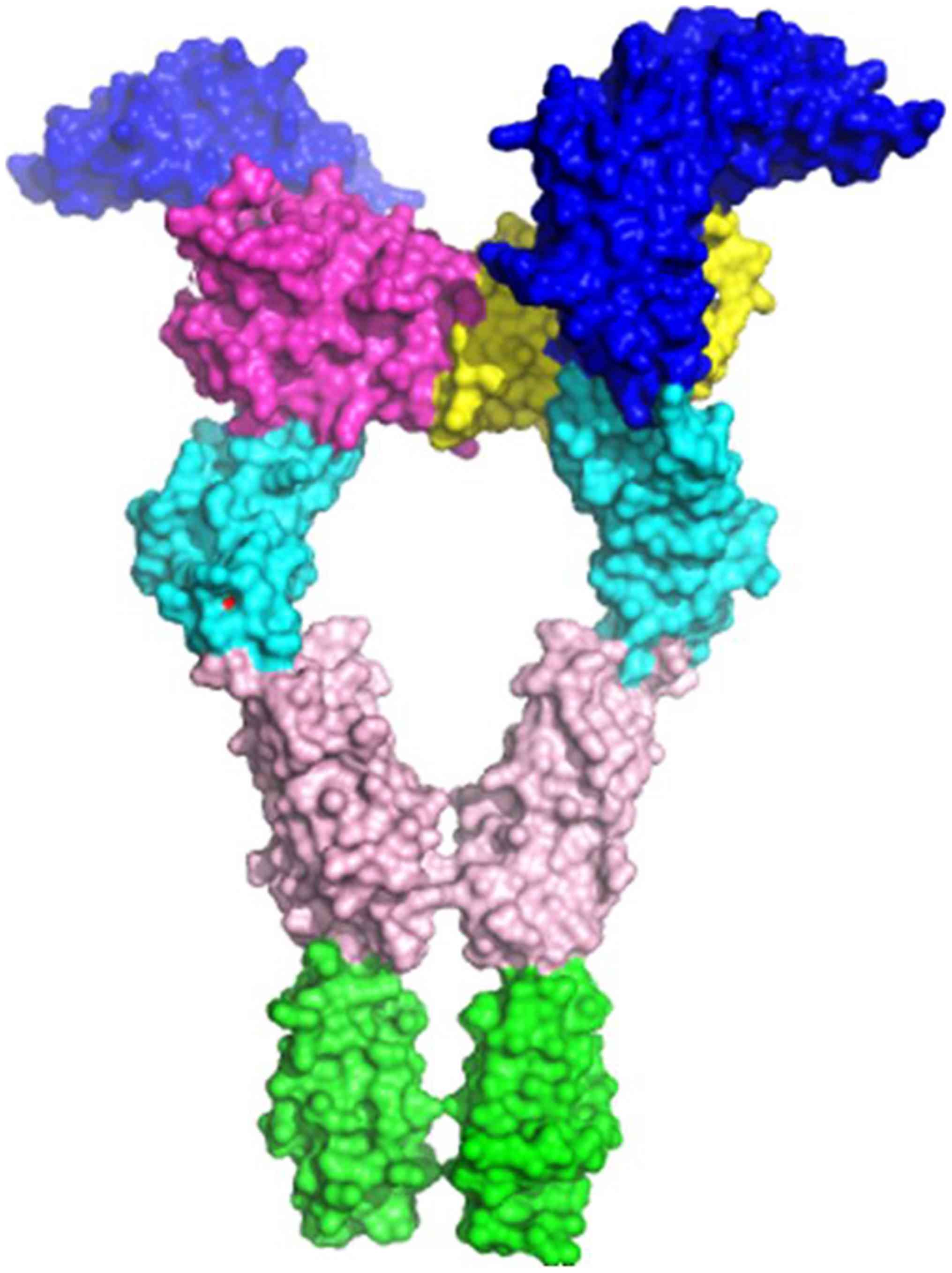
c-Kit (30) (Fig. 1) and from electron microscopy of
dimeric VEGFR2:VEGF complexes (31). Four contained mutations on the
β-sheet connecting loop, between amino acids 295 and 299 of the D3
domain, which also serve as part of the antibody binding interface,
affect the binding affinity of VEGF by a factor of 2(29).
Notably, it has been shown that angiogenesis differs
in benign endometrial polyps and in endometrial cancers and,
therefore, it has been proposed that the development of specific
angiogenic markers may be important for prognosis of these lesions
(32). However, it has been
proven that VEGFR2 cannot be used as a prognostic factor in routine
diagnostics for the selection of high-risk cases (32). Furthermore, taking into account
that angiogenesis is a critical component of normal implantation in
the early stages of pregnancy (33), VEGFR2 appears to mediate a
significant molecular signaling pathway for endometrial receptivity
(34).
Although angiogenesis is crucial for normal growth
and development and in protective responses, such as wound healing
and inflammation (35),
aberrancies in angiogenesis can be observed in various pathological
settings that lead to the pathogenesis of various diseases, such as
cancer, diabetic retinopathy, rheumatoid arthritis and systemic
sclerosis (14,36-38).
Considering that the pathogenesis of endometriosis is crucially
dependent on angiogenesis that is signaled via VEGF and its
receptor, VEGFR2, and taking into account various genetic
associations detected between the rs2305948 SNP of VEGFR2
and endometriosis in different ethnic/racial populations (39-41),
this study attempted to examine the potential genetic association
between the rs2305948 (V297I) SNP and endometriosis in a
homogeneous Greek population. This study also aimed to further
elucidate the functional significance of this polymorphism by using
a structural biological approach.
Patients and methods
Patient population and study
design
In this case control association study, 332 women
were enrolled (162 endometriosis patients and 170 controls) from
the Department of Obstetrics and Gynecology of Venizeleion Hospital
of Heraklion (Crete, Greece). The average age of the Greek
endometriosis and control cohorts was 32.25±7.1 and 29.49±6.7
years, respectively. All the women enrolled had undergone surgery
in the aforementioned tertiary care centre, while cases were
diagnosed surgically (laparotomy or laparoscopy) and biopsies were
used in order for the disease to be confirmed histologically. All
the members of the control group had given birth to 2-5 children
and had no previous medical record of chronic pelvic pain,
dysmenorrhea or dyspareunia. Both the cases and controls were
unrelated, living in the same urban environment and originated from
the same Greek population (Cretan). The stages of endometriosis
were defined by using the revised American Fertility Society
Classification (42).
Accordingly, 87 (53.70%) patients had stage I-II endometriosis and
75 (46.39%) patients had moderate to severe endometriosis (stage
III-IV). All the subjects were of self-reported Greek origin. The
study was performed in the Section of Molecular Pathology and Human
Genetics of the Medical School of Crete, after obtaining the
approval of the Research Committee of the Venizeleion General
Hospital of Heraklion (ECHR no. 47/773) and was carried out in
compliance with the declaration of Helsinki. Written informed
consent was obtained from all the patients and control subjects,
while the medical records were collected by the clinicians and
pathologists of Venizeleion General Hospital, including surgical
procedures and findings.
Genetic analysis of the V297I VEGFR2
polymorphism
Whole blood was collected pre-operatively in
ethylenediaminetetraacetic acid (EDTA)-containing tubes. Genomic
DNA was isolated from peripheral blood leukocytes using a
commercial kit (PureLink® Genomic DNA Mini kit;
Invitrogen; Thermo Fisher Scientific) according to the
manufacturer's instructions. The extracted DNA was stored at -20˚C
until analysis. The rs2305948 1192 C/T (V297I) SNP of the
VEGFR2 gene was genotyped via TaqMan 5'allelic
discrimination technology, using a predesigned SNP genotyping assay
provided by Applied Biosystems (TaqMan assay no. C_22271999_20) as
previously described in detail (43). Allelic discrimination plots were
all reviewed individually for quality and negative controls were
also run for this assay. The accuracy of the results was ensured
upon the amplification of a random 10% of the total samples. The
genotyping success rate was 98% (missingness: 2%).
Construction of VEGFR2 domains'
three-dimensional (3D) model
The three dimensional structure of the human VEGFR2
extracellular domain in complex with VEGF or antibodies (PDB codes
2X1W and 3S35) (29,44) as well as the crystal structure of
the extracellular domain of Kit (PDB code 2E9W) (30) were downloaded from the Protein
Data Bank (https://www.wwpdb.org/) and used to
analyze the consequences to structure and function of the mutation
p.Val297Ile located in the interface between the D3 and D4 Ig-like
domains. The mutant was constructed using molecular modeling with
the program Maestro (Schrodinger, LLC) which was also used to
analyze the conformational changes caused by the mutation.
Rotational flexibility on mutated side chains was tested due to the
restricted space in the mutation vicinity and the conformation with
the least bad contacts was adopted. All figures depicting 3D models
were created using the molecular graphics program PyMOL
V.2.2(45).
Statistical analysis
The cases and controls subjects used in the study
were unrelated. Statistical analysis was performed using the
GraphPad Prism statistical program (GraphPad Software), by applying
the additive model. The Chi-squared (χ2) test, with one
or two degrees of freedom or the genetic variants under
investigation were evaluated for deviation from the Hardy-Weinberg
equilibrium (HWE) by comparing observed and expected genotype
frequencies by means of Chi-squared (χ2) test or
Fisher's exact test in the control groups (by using the program
named ‘Calculate’; Copyright TRG, SR, INMD, 2008). Power
calculations were performed by using the CaTS power calculator
(46). OR and 95% CI values were
calculated using the aforementioned GraphPad Prism statistical
program.
Results
Analysis of the structural
consequences of V297I VEGFR2 polymorphism
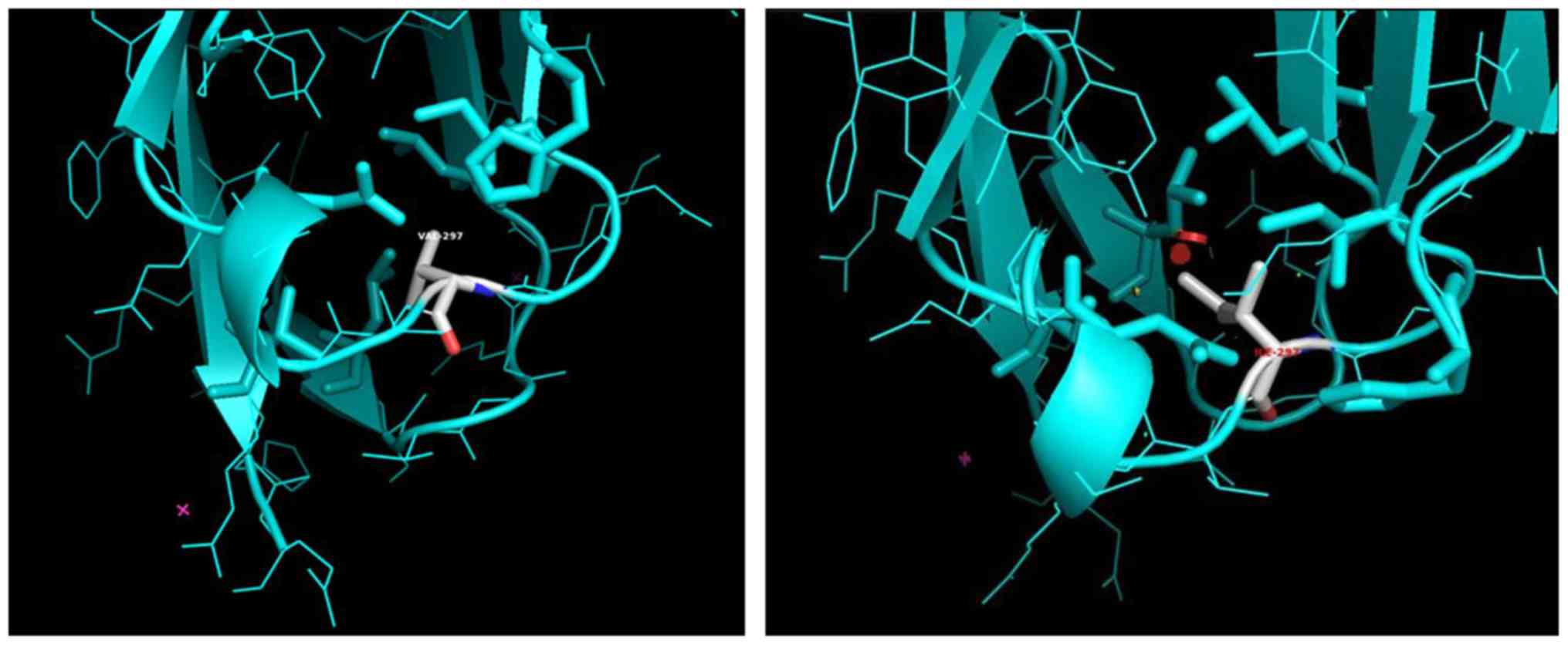
The rs2305948 SNP leads to the substitution of
valine (V), a Cβ branched amino acid residue, to a
larger Cγ branched hydrophobic residue, isoleucine (I)
at position 297 of the protein chain of the VEGFR2 monomer
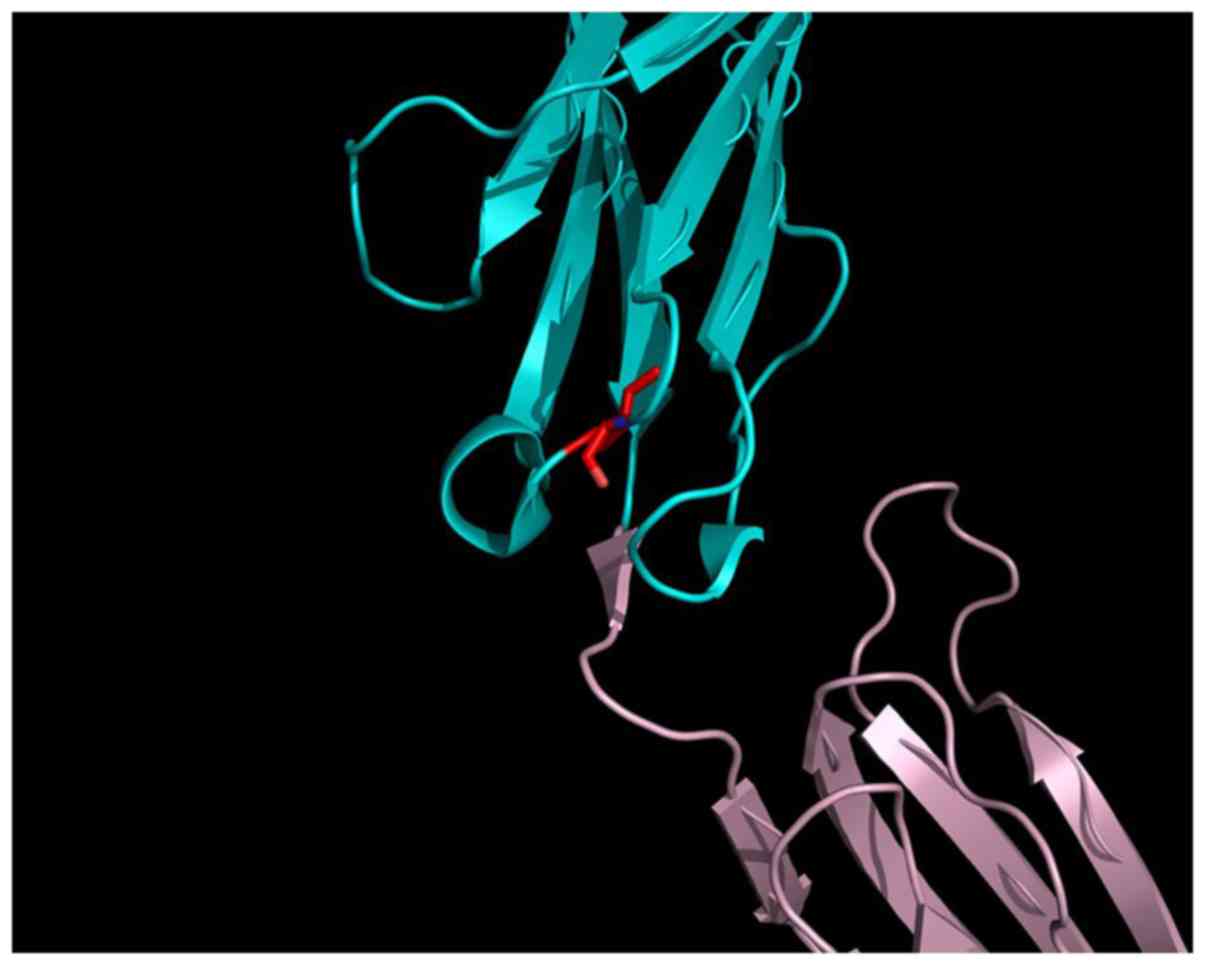
(Fig. 2A and B). Val297 is located close to the D4
interface, on a hairpin loop connecting two β-sheets, forming the
Ig-like β-barrel of the D3 Ig-like domain of the extracellular
VEGFR2 part (Fig. 3). The D4
domain is responsible for homotypic D4 contacts (47) upon ligand-mediated dimerization of
the VEGF receptors (25). This
mutation may alter the conformation of the β-sheet connecting loop
in the D3 Ig-like domain, by rearranging the domain 3:domain 4
interface of VEGFR2 in such a manner that the receptor can no
longer dimerize. As a consequence, the V297I mutation may affect
the efficiency of trans-autophosphorylation and cell signaling.
Sequence alignment between the D5 domain and the D7 domain of same
receptor and KIT D4, known to form homotypic receptor contacts in
VEGFR2 and KIT dimerization, respectively, indicate homology
between the domains on sequence motifs responsible for mediating
homotypic contacts.
VEGFR2 rs2305948 SNP is not associated
with endometriosis in a Greek population
In the case of the rs2305948 SNP of the
VEGFR2 gene, no statistically significant difference was
found in the frequency of the T allele between the cases and
controls (P=0.89, OR=1.073, 95% CI, 0.61-1.88) (Table I). Similarly, no statistically
significant difference was observed in the frequencies of the TT
and CT genotypes between the cases vs. the controls (P=1, OR=0.960,
95% CI, 0.06-15.5) and P=0.87, OR=1.091, 95% CI, 0.59-2.00,
respectively) (Table I).
 | Table IGenotypes and allele frequencies of
the VEGFR2 rs2305948 SNP analyzed in 162 women with
endometriosis and 170 healthy controls. |
Table I
Genotypes and allele frequencies of
the VEGFR2 rs2305948 SNP analyzed in 162 women with
endometriosis and 170 healthy controls.
|
Genotypes/Alleles | Endometriosis | Controls | P-value | OR (95% CI) |
|---|
| Genotypes | n=162 | n=170 | | |
|
CC | 138 (85.18%) | 143 (84.12%) | | |
|
CT | 23 (14.20%) | 26 (15.29%) | 0.87 | 1.091
(0.59-2.00) |
|
TT | 1 (0.62%) | 1 (0.59%) | 1 | 0.960
(0.06-15.5) |
| Alleles | n=324 | n=340 | | |
|
C | 299 (92.28%) | 312 (91.76%) | | |
|
T | 25 (7.72%) | 28 (8.24%) | 0.89 | 1.073
(0.61-1.88) |
Notably, in an analysis conducted for endometriosis,
no significant association was detected as regards the TT and CT
genotypes of this SNP in patients with stage I/II of the disease
and the controls (P=1, OR=0.520, 95% CI, 0.03-8.40 and P=0.85,
OR=1.120, 95% CI, 0.53-2.35, respectively), as depicted in Table II. Furthermore, no evidence for
the association with endometriosis cases stratified to stages I and
II was found for the T allele (P=1, OR=1.026, 95% CI, 0.52-2.00)
(Table II).
 | Table IIGenotype and allele frequency of the
VEGFR2 rs2305948 SNP analyzed in 87 women with endometriosis
(stage I and II) and 170 healthy controls. |
Table II
Genotype and allele frequency of the
VEGFR2 rs2305948 SNP analyzed in 87 women with endometriosis
(stage I and II) and 170 healthy controls.
|
Genotypes/Alleles | Endometriosis | Controls | P-value | OR (95% CI) |
|---|
| Genotypes | n=87 | n=170 | | |
|
CC | 74 (34.48%) | 143 (84.12%) | | |
|
CT | 12 (42.53%) | 26 (15.29%) | 0.85 | 1.120
(0.53-2.35) |
|
TT | 1 (22.99%) | 1 (0.59%) | 1 | 0.520
(0.03-8.40) |
| Alleles | n=174 | n=340 | | |
|
C | 160 (91.95%) | 312 (91.76%) | | |
|
T | 14 (8.06%) | 28 (8.24%) | 1 | 1.026
(0.52-2.00) |
Similarly, when the patients were stratified to
stages III and IV and analyzed, no significant association with
endometriosis was detected either at the genotype or allele
frequencies of rs2305948. Thus, when genotype CT+TT or allele ‘T’
frequencies of patients with stage III/IV endometriosis were
compared with the controls, no statistically significant difference
was observed (P=1, OR 1.10, 95% CI, 0.51-2.35 and P=0.86, OR 1.13,
95% CI, 0.55-2.34, respectively), as depicted in Table III. The SNP under investigation
did not deviate significantly from the expected Hardy-Weinberg
proportion either for cases or for controls (P-value for deviation
was 0.20).
 | Table IIIGenotype and allele frequency of the
VEGFR2 rs2305948 SNP analyzed in 75 women with endometriosis
(stage III and IV) and 170 healthy controls. |
Table III
Genotype and allele frequency of the
VEGFR2 rs2305948 SNP analyzed in 75 women with endometriosis
(stage III and IV) and 170 healthy controls.
|
Genotypes/Alleles | Endometriosis | Controls | P-value | OR (95% CI) |
|---|
| Genotypes | n=75 | n=170 | | |
|
CC | 64 (85.33%) | 143 (84.12%) | | |
|
CT + TT | 11 (14.67%) | 27 (15,88%) | 1 | 1.10
(0.51-2.35) |
| Alleles | n=150 | n=340 | | |
|
C | 139 (92.67%) | 312 (91.76%) | | |
|
T | 11 (7.33%) | 28 (8.24%) | 0.86 | 1.13
(0.55-2.34) |
Discussion
Aiming for a better understanding of the putative
role of VEGFR2 in endometriosis, the authors conducted the present
structural biological and genetic study. It was hypothesized that
variations of the VEGFR2 gene may alter the biological
function of the encoded protein and, as a consequence, this genetic
factor may further influence the endothelial function in women that
develop endometriosis. The structural data presented in the current
study suggest that the rs2305948 (V297I) polymorphism may be
causative for the development of endometriosis due to its effect on
the impairment in cell signaling. The structural analysis provided
insights into receptor/ligand and dimer formation interactions,
which are essential for the understanding of receptor/ligand
specificity, and may explain previously published data (25,29,47). The Val297Ile polymorphism may play
an important role in D3-D4 Ig-domain interaction, thus affecting
the efficiency of dimerization, essential for kinase activation and
the cell signaling mechanism.
As previously suggested, the rs2305948 SNP results
in a significant increase in the VEGF binding efficiency to VEGFR2
and, as a consequence, an impairment in VEGFR2 function may be
associated with vascular dysfunction, abnormal vascular repair and
vascular diseases (48). In this
study, the authors performed a case-control association analysis in
an attempt to reveal, for the first time, an association of the
rs2305948 (V297I) VEGFR2 SNP with an increased
susceptibility to endometriosis in a Greek population. It is worth
noting that the available literature reports concerning the role of
this polymorphism in endometriosis have yielded controversial
results. However, this polymorphism was not found to be associated
with endometriosis, although the genotype frequencies of rs2305948
from the International HapMap data (49) were very similar to those of this
study. Of note, similar frequencies of the rs2305948 SNP have been
demonstrated in a previous study conducted in the Cretan population
(50), aiming to detect a
putative association between this SNP and systemic lupus
erythematosus (SLE). Importantly, the VEGFR2 gene locus has
not been identified in GWAS for endometriosis susceptibility thus
far (6), a finding that is in
accordance with the results of the present case-control study.
Previously, it has been reported that a functional
polymorphism in the VEGF gene may be associated with the risk of
developing endometriosis in women in Northern China, through
changes in the transcriptional activity of VEGF (51). Based on the same population, Kang
et al (39) presented data
suggesting that the 1192C/T polymorphism of rs2305948 of the
VEGFR-2 gene may be associated with endometriosis in women
in Northern China Han ethnicity, by conferring protection to women
carrying the minor allele ‘T’. However, these data have to be
examined and evaluated with a critical eye, considering that the
control group was recruited based on an ultrasound-based diagnosis
only, negative for endometriosis and, as known, this method cannot
exclude the occurrence of pelvic endometriosis. Moreover, Cardoso
et al (41) conducted a
study based on a Brazilian population and found that rs2305948 was
also protective against the development of endometriosis, with this
SNP also reducing cyclical urinary symptoms. VEGFR-2 is encoded by
the KDR gene, located on chromosome 4q11-q12 and consists of
30 exons (27). The SNP under
investigation is located in exon 7, within the immunoglobulin-like
domain 3, and previous studies have illustrated that it
substantially decreases the efficiency of VEGF binding to VEGFR-2
(48,52). Therefore, it is reasonable to
speculate that the decreased binding capacity of VEGF appearing as
a result of the 1192C/T polymorphism may lead to a decrease the
activity of angiogenesis, thus reducing the risk of endometriosis
(39). However, a study performed
in a population from Belgium (40) presented data demonstrating an
association of 1192C/T polymorphism with an increased risk of
developing endometriosis. Furthermore, previous studies have
demonstrated an association of this SNP with the risk of developing
diseases, such as diffuse large B cell lymphoma, colorectal cancer,
coronary heart disease and ischemic stroke (53,54). Thus, accumulative data seem to
suggest that common genetic variants in the VEGF and
VEGFR-2 genes, resulting in alterations regarding the
VEGF-VEGFR signaling, may play a role in the molecular pathogenesis
of endometriosis.
It is worth noting a limitation of this study.
Although this study sample size was sufficient, the statistical
power of this study was relatively low due to the very low
frequency (globally observed) of the minor allele ‘T’ of the
rs2305948 SNP. However, the authors avoided the inclusion of
additional individuals from mainland Greece or immigrants that
could reduce the genetic homogeneity of the sample.
To the best of our knowledge, this study is the
first to investigate the association of the rs2305948 SNP of the
VEGFR2 gene with endometriosis in the Greek population.
Apart from the important role of this SNP in the VEGF/VEGFR2
binding efficiency, its involvement in the development of various
diseases and its association with an increased risk of developing
endometriosis in different cohorts to date have been well
established. However, this study did not succeed in confirming that
this polymorphism contributes significantly either to an increased
susceptibility for endometriosis or to the severity of this
condition. The failure to confirm previous findings may be
attributed either to inter-population differences or to
interactions between genetic and non-genetic factors. Thus, the
results of the present study demonstrate that it is difficult to
identify generalizable risk alleles in endometriosis and highlight
the importance of conducting comparative studies in different
populations in order to determine true risk alleles for this
condition.
Acknowledgements
The authors would like to thank all the clinicians
and the pathologists for providing the data and pathological
reports for this study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EE, CM, MM and GNG designed the study and drafted
the manuscript; EE, GNG, CM, IM, MM, IK, DAS and MIZ searched the
literature; EE, MM, IK, GNG and MIZ analyzed and interpreted the
data; MIZ, IM and DAS critically revised the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee for Human Research of
Venizeleio Hospital approved the respective protocol (ECHR no.
47/773). Informed consent was obtained from all the
participants.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Managing Editor of the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
All the other authors declare that they have no competing
interests.
References
|
1
|
Cramer DW and Missmer SA: The epidemiology
of endometriosis. Ann N Y Acad Sci. 955:11–22, discussion 34-36,
396-406. 2002.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Giudice LC: Clinical practice.
Endometriosis. N Engl J Med. 362:2389–2398. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fauconnier A, Staraci S, Huchon C, Roman
H, Panel P and Descamps P: Comparison of patient- and
physician-based descriptions of symptoms of endometriosis: A
qualitative study. Hum Reprod. 28:2686–2694. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Simpson JL, Elias S, Malinak LR and
Buttram VC Jr: Heritable aspects of endometriosis. I. Genetic
studies. Am J Obstet Gynecol. 137:327–331. 1980.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Stefansson H, Geirsson RT,
Steinthorsdottir V, Jonsson H, Manolescu A, Kong A, Ingadottir G,
Gulcher J and Stefansson K: Genetic factors contribute to the risk
of developing endometriosis. Hum Reprod. 17:555–559.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Sapkota Y, Steinthorsdottir V, Morris AP,
Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards
TL, Jones S, et al: iPSYCH-SSI-Broad Group: Meta-analysis
identifies five novel loci associated with endometriosis
highlighting key genes involved in hormone metabolism. Nat Commun.
8(15539)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Saha R, Pettersson HJ, Svedberg P,
Olovsson M, Bergqvist A, Marions L, Tornvall P and Kuja-Halkola R:
Heritability of endometriosis. Fertil Steril. 104:947–952.
2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang Z, Zang C, Rosenfeld JA, Schones DE,
Barski A, Cuddapah S, Cui K, Roh TY, Peng W, Zhang MQ, et al:
Combinatorial patterns of histone acetylations and methylations in
the human genome. Nat Genet. 40:897–903. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Viganò P, Parazzini F, Somigliana E and
Vercellini P: Endometriosis: Epidemiology and aetiological factors.
Best Pract Res Clin Obstet Gynaecol. 18:177–200. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
McLaren J: Vascular endothelial growth
factor and endometriotic angiogenesis. Hum Reprod Update. 6:45–55.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gupta S, Agarwal A, Sekhon L, Krajcir N,
Cocuzza M and Falcone T: Serum and peritoneal abnormalities in
endometriosis: Potential use as diagnostic markers. Minerva
Ginecol. 58:527–551. 2006.PubMed/NCBI
|
|
12
|
Hey-Cunningham AJ, Peters KM, Zevallos HB,
Berbic M, Markham R and Fraser IS: Angiogenesis, lymphangiogenesis
and neurogenesis in endometriosis. Front Biosci (Elite Ed).
5:1033–1056. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Folkman J: Antiangiogenesis in cancer
therapy--endostatin and its mechanisms of action. Exp Cell Res.
312:594–607. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Maruotti N, Cantatore FP, Crivellato E,
Vacca A and Ribatti D: Angiogenesis in rheumatoid arthritis. Histol
Histopathol. 21:557–566. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Smith SK: Angiogenesis, vascular
endothelial growth factor and the endometrium. Hum Reprod Update.
4:509–519. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Rocha AL, Reis FM and Taylor RN:
Angiogenesis and endometriosis. Obstet Gynecol Int.
2013(859619)2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Terman BI, Carrion ME, Kovacs E, Rasmussen
BA, Eddy RL and Shows TB: Identification of a new endothelial cell
growth factor receptor tyrosine kinase. Oncogene. 6:1677–1683.
1991.PubMed/NCBI
|
|
18
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sekiguchi K, Ito Y, Hattori K, Inoue T,
Hosono K, Honda M, Numao A, Amano H, Shibuya M, Unno N, et al: VEGF
Receptor 1-Expressing Macrophages Recruited from Bone Marrow
Enhances Angiogenesis in Endometrial Tissues. Sci Rep.
9(7037)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Schlessinger J: Cell signaling by receptor
tyrosine kinases. Cell. 103:211–225. 2000.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ferrara N: Vascular endothelial growth
factor: Basic science and clinical progress. Endocr Rev.
25:581–611. 2004.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Holmes K, Roberts OL, Thomas AM and Cross
MJ: Vascular endothelial growth factor receptor-2: Structure,
function, intracellular signalling and therapeutic inhibition. Cell
Signal. 19:2003–2012. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang K, Andersson C, Roomans GM, Ito N
and Claesson-Welsh L: Signaling properties of VEGF receptor-1 and
-2 homo- and heterodimers. Int J Biochem Cell Biol. 33:315–324.
2001.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Neagoe PE, Lemieux C and Sirois MG:
Vascular endothelial growth factor (VEGF)-A165-induced prostacyclin
synthesis requires the activation of VEGF receptor-1 and -2
heterodimer. J Biol Chem. 280:9904–9912. 2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Stuttfeld E and Ballmer-Hofer K: Structure
and function of VEGF receptors. IUBMB Life. 61:915–922.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Fuh G, Li B, Crowley C, Cunningham B and
Wells JA: Requirements for binding and signaling of the kinase
domain receptor for vascular endothelial growth factor. J Biol
Chem. 273:11197–11204. 1998.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lu D, Kussie P, Pytowski B, Persaud K,
Bohlen P, Witte L and Zhu Z: Identification of the residues in the
extracellular region of KDR important for interaction with vascular
endothelial growth factor and neutralizing anti-KDR antibodies. J
Biol Chem. 275:14321–14330. 2000.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wiesmann C, Fuh G, Christinger HW,
Eigenbrot C, Wells JA and de Vos AM: Crystal structure at 1.7 A
resolution of VEGF in complex with domain 2 of the Flt-1 receptor.
Cell. 91:695–704. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Franklin MC, Navarro EC, Wang Y, Patel S,
Singh P, Zhang Y, Persaud K, Bari A, Griffith H, Shen L, et al: The
structural basis for the function of two anti-VEGF receptor 2
antibodies. Structure. 19:1097–1107. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yuzawa S, Opatowsky Y, Zhang Z, Mandiyan
V, Lax I and Schlessinger J: Structural basis for activation of the
receptor tyrosine kinase KIT by stem cell factor. Cell.
130:323–334. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ruch C, Skiniotis G, Steinmetz MO, Walz T
and Ballmer-Hofer K: Structure of a VEGF-VEGF receptor complex
determined by electron microscopy. Nat Struct Mol Biol. 14:249–250.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Hvingel B, Lieng M, Roald B and Ørbo A:
Vascular markers CD31, CD34, actin, VEGFB, and VEGFR2, are
prognostic markers for malignant development in benign endometrial
polyps. Open J Obstet Gynecol. 2:18–26. 2012. View Article : Google Scholar
|
|
33
|
Silva LA, Klein C, Ealy AD and Sharp DC:
Conceptus-mediated endometrial vascular changes during early
pregnancy in mares: An anatomic, histomorphometric, and vascular
endothelial growth factor receptor system immunolocalization and
gene expression study. Reproduction. 142:593–603. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Douglas NC, Tang H, Gomez R, Pytowski B,
Hicklin DJ, Sauer CM, Kitajewski J, Sauer MV and Zimmermann RC:
Vascular endothelial growth factor receptor 2 (VEGFR-2) functions
to promote uterine decidual angiogenesis during early pregnancy in
the mouse. Endocrinology. 150:3845–3854. 2009.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hall K and Ran S: Regulation of tumor
angiogenesis by the local environment. Front Biosci. 15:195–212.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
36
|
Wilkinson-Berka JL: Vasoactive factors and
diabetic retinopathy: Vascular endothelial growth factor,
cycoloxygenase-2 and nitric oxide. Curr Pharm Des. 10:3331–3348.
2004.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Dobrzycka B, Kinalski M, Piechocka D and
Terlikowski SJ: The role of estrogens in angiogenesis in the female
reproductive system. Endokrynol Pol. 60:210–214. 2009.(In Polish).
PubMed/NCBI
|
|
38
|
Riccieri V, Stefanantoni K, Vasile M,
Macrì V, Sciarra I, Iannace N, Alessandri C and Valesini G:
Abnormal plasma levels of different angiogenic molecules are
associated with different clinical manifestations in patients with
systemic sclerosis. Clin Exp Rheumatol. 29 (Suppl 65):S46–S52.
2011.PubMed/NCBI
|
|
39
|
Kang S, Shi YY, Li Y, Wang N, Lu YC, Zhou
RM and Zhao XW: Association between genetic variants of the VEGFR-2
gene and the risk of developing endometriosis in Northern Chinese
Women. Gynecol Obstet Invest. 76:32–37. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vodolazkaia A, Yesilyurt BT, Kyama CM,
Bokor A, Schols D, Huskens D, Meuleman C, Peeraer K, Tomassetti C,
Bossuyt X, et al: Vascular endothelial growth factor pathway in
endometriosis: Genetic variants and plasma biomarkers. Fertil
Steril. 105:988–996. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Cardoso JV, Abrão MS, Vianna-Jorge R,
Ferrari R, Berardo PT, Machado DE and Perini JA: Combined effect of
vascular endothelial growth factor and its receptor polymorphisms
in endometriosis: A case-control study. Eur J Obstet Gynecol Reprod
Biol. 209:25–33. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
The American Fertility Society: Revised
American Fertility Society classification of endometriosis: 1985.
Fertil Steril 43: 351-352, 1985.
|
|
43
|
Matalliotakis M, Zervou MI, Matalliotaki
C, Rahmioglu N, Koumantakis G, Kalogiannidis I, Prapas I, Zondervan
K, Spandidos DA, Matalliotakis I, et al: The role of gene
polymorphisms in endometriosis. Mol Med Rep. 16:5881–5886.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Leppänen VM, Prota AE, Jeltsch M, Anisimov
A, Kalkkinen N, Strandin T, Lankinen H, Goldman A, Ballmer-Hofer K
and Alitalo K: Structural determinants of growth factor binding and
specificity by VEGF receptor 2. Proc Natl Acad Sci USA.
107:2425–2430. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Schrödinger LLC: The PyMOL Molecular
Graphics System 2016 version 2.2. uripymol.org/2/support.htmlsimplepymol.org/2/support.html.
Accessed March 5, 2019.
|
|
46
|
Skol AD, Scott LJ, Abecasis GR and Boehnke
M: Joint analysis is more efficient than replication-based analysis
for two-stage genome-wide association studies. Nat Genet.
38:209–213. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
47
|
Yang Y, Xie P, Opatowsky Y and
Schlessinger J: Direct contacts between extracellular
membrane-proximal domains are required for VEGF receptor activation
and cell signaling. Proc Natl Acad Sci USA. 107:1906–1911.
2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang Y, Zheng Y, Zhang W, Yu H, Lou K,
Zhang Y, Qin Q, Zhao B, Yang Y and Hui R: Polymorphisms of KDR gene
are associated with coronary heart disease. J Am Coll Cardiol.
50:760–767. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
International HapMap Consortium: The
International HapMap Project. Nature 426: 789-796, 2003.
|
|
50
|
Vazgiourakis V, Zervou MI, Eliopoulos E,
Sharma S, Sidiropoulos P, Franek BS, Myrthianou E, Melissourgaki M,
Niewold T, Boumpas DT, et al: Implication of VEGFR2 in Systemic
Lupus Erythematosus: A structural biological and genetic approach.
Clin Exp Rheumatol. 31:97–102. 2012.PubMed/NCBI
|
|
51
|
Liu Q, Li Y, Zhao J, Sun DL, Duan YN, Wang
N, Zhou RM and Kang S: Association of polymorphisms -1154G/A and
-2578C/A in the vascular endothelial growth factor gene with
decreased risk of endometriosis in Chinese women. Hum Reprod.
24:2660–2666. 2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang WL, Sun K, Wang Y, Hu FB and Hui RT:
Abstract 2377: Interaction of the Ile297 variant of vascular
endothelial growth factor receptor-2 gene and homocysteine on the
risk of stroke recurrence. Circulation. 116 (Suppl)(521)2007.
|
|
53
|
Hansen TF, Sørensen FB, Spindler KL, Olsen
DA, Andersen RF, Lindebjerg J, Brandslund I and Jakobsen A:
Microvessel density and the association with single nucleotide
polymorphisms of the vascular endothelial growth factor receptor 2
in patients with colorectal cancer. Virchows Arch. 456:251–260.
2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Oh SH, Min KT, Jeon YJ, Kim MH, Moon JS,
Kim HS, Kim WC, Kim OJ, Park EK and Kim NK: Association between
kinase insert domain-containing receptor gene polymorphism and
haplotypes and ischemic stroke. J Neurol Sci. 308:62–66.
2011.PubMed/NCBI View Article : Google Scholar
|