Introduction
Morphine continues to be used a reliable painkiller
for the alleviation of moderate to severe pain in acute and chronic
diseases. However, tolerance and dependence limit the clinical
application of morphine (1-3).
Opioid dependence to occurs within a relatively short period of
time following treatment initiation and is a major concern which
limits opioid administration (4).
Following morphine abstinence therapy, relapsing to morphine is one
of the most important issues that may occur even after a long
period of abstinence therapy (4,5).
It is a subjective feeling that can force the objective for drug
craving (1). Recently, several
investigations have been conducted with an aim of finding a
solution for this issue; however, these efforts have not been
successful and morphine relapse is continuing (5-7).
In this regard, chemicals with N-methyl-D-aspartate (NMDA)
antagonistic effects have been examined and promising results have
been achieved (8).
It has been shown that dopaminergic, GABAergic,
glutamatergic, serotonergic, adrenergic and orexinergic pathways
along with endogen opioid peptides are involved in the reward and
reinforcement pathway (4,5,9).
One of the most important systems that is involved in the addiction
and relapse to drugs of abuse is the dopaminergic system, which
begins from the ventral tegmental area (VTA) with projections to
nucleus accumbens (NAcc).
Resveratrol (RES; trans-3,4,5-trihydroxystilbene), a
natural polyphenol with the structure of phytoalexin is found in
human dietary compounds and in a large numbers of plants and
beverages, including peanuts, mulberries, grapes (particularly the
skin of black grapes) and red wines (10-13).
There is evidence to suggest that RES exerts a number of biological
effects, including antioxidant, anti-inflammatory, cardiovascular
protective, anti-cancer and neuroprotective and potentially
analgesic effects, without any known toxic effects (14-21).
RES has been shown to exert antinociceptive effects on acute and
inflammatory pain and RES pre-treatment has been shown to exert
antinociceptive effects in morphine-tolerant animals (22,23). RES and long-term morphine
co-administration has been shown to result in NMDAR upregulation
and RES can reduce the activation of NMDA receptors; this is
evidence of crosstalk between morphine tolerance and addiction
(23,24).
Furthermore, RES can improve learning and memory via
the microRNA-CREB pathway (20), prevent the increase in
acetylcholinesterase (AChE) activity and decrease memory impairment
(25), and improve the clearance
of amyloid beta peptides (26).
Moreover, it has been shown to exert cyclooxygenase and
lipoxygenase inhibitory effects that justify its analgesic effects
(27-29).
In a study conducted by Pérez-Severiano et al, RES
administration to the spinal cord was shown to reduce allodynia by
decreasing nitric oxide synthase (NOS) activity and neuronal NOS
(nNOS) expression (30).
Furthermore, other studies have demonstrated that RES inhibits the
expression of TNF-α, an important mediator of the induction of
inflammation (26,30).
Additionally, RES exerts inhibitory effects on the
release of glutamate from the cerebrocortical nerve terminals and
exerts NMDA antagonistic effects in cortical neurons. In fact,
drugs (e.g., haloperidol, clozapine, risperidone and SCH 23390)
that antagonize NMDA receptors and exert inhibitory effects on
glutamate receptors, have been shown to exert inhibitory effects on
morphine tendency in animal models. The authors have previously
demonstrated that Berberis vulgaris with NMDA antagonistic
effects reduces morphine relapsing and reinstatement (31). Moreover, the prefrontal cortex and
limbic area that project towards the VTA and NAcc and regulate the
release of dopamine via glutamate and N-methyl-D-aspartate (NMDA)
receptors, play important roles in reward processing. Memantine (an
NMDA receptor antagonist), promotes the acquisition of
morphine-induced CPP (5).
As RES antagonizes NMDA receptors, inhibits
glutamate release in the brain and exerts anti-inflammatory,
anti-neuropathic pain and neuroprotective effects, particularly in
neuroglia, it was hypothesized that RES can ameliorate morphine
tendency in animals using the CPP model.
Materials and methods
Animals
Fifty-six male NMRI mice (purchased from Pasteur
Institute, Tehran, Iran; 23 days old weighing 25-30 g) were kept
under standard conditions (at 25˚C with 12 h/12 h light/dark
cycles) and had free access to food and water, ad libitum.
All tests were performed with respect to the guidelines for the
care and use of laboratory animals provided by Zabol University of
Medical Sciences, Zabol, Iran. This study was approved by Ethics
Committee of Zabol University of Medical Sciences, Zabol, Iran
(approval no. IR.ZBMU.REC.1398.157).
Chemicals
Morphine sulfate and RES were purchased from
Daropakhsh and Sigma, respectively.
Apparatus
The Plexiglas apparatus consisted of a box with
three compartments (30x30x35 cm dimensions) separated by removable
baffles. Two compartments were of the same size, but had different
colors (black and white), floor texture (the black compartment was
thicker than the white one) and odor (i.e., essence of banana in
the white compartment and acetic acid in the black compartment).
The third compartment, which was in gray, was in the middle of the
box and was connected to the two other compartments. Following each
experiment, all chambers were cleaned in order to remove any odor
interventions induced by animals' feces and/or urine.
Experimental procedure
This study comprised of 6 different stages namely,
pre-treatment, pre-conditioning, conditioning, post-conditioning,
extinction and the reinstatement test (Table I).
 | Table ITreatment schedule of the CPP
experiment. |
Table I
Treatment schedule of the CPP
experiment.
| Acquisition of place
preference | Extinction phase | Reinstatement
phase |
|---|
| Pre-treatment (2
days) | Pre-conditioning (1
day) | Conditioning (4
days) | Post-conditioning (1
day) | 7 days | 1 day |
|---|
| | | Normal saline +
normal saline, normal saline + morphine 40 mg/kg, morphine 40 mg/kg
+ Res 25, 50 and 75 mg/kg, normal saline + RES 25, 50 and 75
mg/kg | | | Normal saline +
morphine10 mg/kg morphine 10 mg/kg + RES 25, 50 and 75 mg/kg |
Pre-conditioning phase
This part of the study comprised 3 phases. During
the first 2 days, the animals were placed in the box without
guillotine doors (doors were opened) and were allowed to freely
move among the compartments. On day 3, the mice were placed in the
box only for 20 min and were allowed to move freely in all
compartments; the time each animal spent in each compartment
(black, white and gray) was measured to evaluate unconditioned
preference. Mice that stayed in each compartment for >600 sec
were excluded from the study. During the pre-conditioning phase,
there were no significant preferences of each animal for each
compartment.
Conditioning phase
This phase lasted for 4 days. The animals were
administered a single intra-peritoneal dose of normal saline (NS)
and placed in the black chamber of CPP for 1 h. After 4 h, they
received either morphine or RES, intraperitoneally. Following
treatment (with morphine or RES), they were placed for 1 h inside
the white CPP chamber. The animals were divided into 8 groups (n=7
per group) as follows: i) The normal saline + normal saline (SAL)
group; ii) the saline + morphine 40 mg/kg group; iii) the morphine
40 mg/kg + RES 25 mg/kg group; iv) the morphine 40 mg/kg + RES 50
mg/kg group; v) the morphine 40 mg/kg + RES 75 mg/kg group; vi) the
normal saline + RES 25 mg/kg group; vii) the normal saline + RES 50
mg/kg group; and viii) the normal saline + RES 75 mg/kg group.
Immediately following drug administration, the animals were placed
in the white compartment for 1 h.
Post-conditioning phase
The third part of this study was the
post-conditioning phase. Eight days after the experiment, the mice
were placed into the apparatus for 900 sec and allowed to move
freely among the compartments; the time that was spent in each
compartment (white, black and gray) was measured. The time that was
spent in the middle chamber was equally divid¬ed between the white
and black compartments. By abstracting, time spent during
pre-conditioning and post-conditioning can be measured for each
mouse. If the obtained result (time) is positive, it confirms that
the drug can induce a preference and vice versa (8,32).
Extinction of place preference
At this stage, the animals were placed in the
apparatus and allowed to move freely between the compartments for
60 min/day for 7 days in order to reverse morphine dependency. The
time each mouse spent in the white compartment could not be
significant between the pre-conditioning and extinction phases. The
animals were then tested as described above. Following addiction,
the animals were placed into the CPP apparatus (for 900 sec on day
16) for morphine reinstatement, and the time spent in the different
compartments was measured (8,32).
At 30 min following drug administration, morphine 10 mg/kg + normal
saline (morphine group), or morphine 10 mg/kg + RES 25, 50 and 75
mg/kg were injected. The animals experienced a 15-min daily
extinction session, which consisted of the placement of the animals
in the apparatus (without guillotine doors separating the
compartments). On the 8th day, the time spent in the white
compartment for each group of animals became similar to that of
pre-conditioning sessions.
Reinstatement of place preference
On day 16, from step 1, four groups namely, the
morphine/saline, morphine/RES 25 mg/kg, morphine/RES 50 mg/kg and
morphine/RES 75 mg/kg were used. The animals were placed into the
CPP apparatus and allowed to move freely between the compartments
during which time they were recorded. A reminding dose of morphine
(10 mg/kg) was injected to each animal. After 30 min, RES 25, 50
and 75 mg/kg was intraperitoneally administered to each animal and
the time spent in the white compartment was recorded (8,32).
The time spent before and after the injection was calculated to
elucidate whether the animals exhibit reinstatement or not.
Statistical analysis
To compare differences among means, one-way ANOVA
with Tukey's post hoc tests were used. Results are presented as the
means ± SEM. A P<0.05 was considered to indicate a statistically
significant difference.
Results
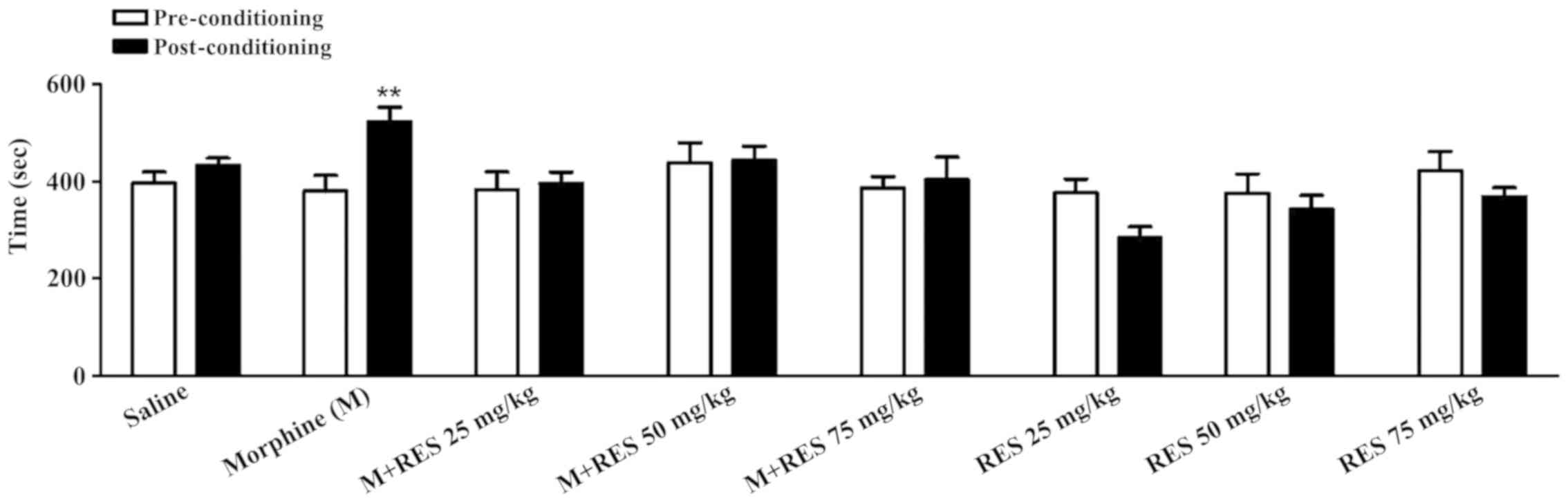
Effects of RES on morphine acquisition
of place preference test
The administration of RES (25, 50 and 75 mg/kg)
alone did not cause place preference and aversion. RES at 50 and 75
mg/kg inhibited CPP (Fig. 1). In
the conditioning phase, the animals were grouped as follows: SAL
(received normal saline plus normal saline); morphine (received
normal saline + morphine 40 mg/kg); M + RES 25 mg/kg (RES 25 mg/kg
of RES plus 40 mg/kg of morphine); M + RES 50 (received RES 50
mg/kg plus morphine 40 mg/kg); M + RES 75 (received RES 75 mg/kg +
morphine 40 mg/kg); RES 25 (received RES 25 mg/kg plus normal
saline); RES 50 (received RES 50 mg/kg plus normal saline); and RES
(received RES 75 mg/kg plus normal saline).
The extinction and pre-conditioning phases did not
differ significantly after daily extinction sessions and the
conditioning disappeared. The injection of the priming dose of
morphine (10 mg/kg) reinstated CPP. RES at 50 and 75 mg/kg (but not
25 mg/kg) inhibited the reinstatement of place preference induced
by the first dose of morphine (Fig.
2). In total, as depicted in Fig.
2, RES at all doses reduced morphine post-conditioning and at
50 and 75 mg/kg, it inhibited morphine-induced reinstatement.
Discussion
The results of this study revealed that RES
decreased morphine-induced CPP, but did not cause morphine tendency
or repulsion. The results of reinstatement investigation revealed
that RES prevented morphine reinforcement (hedonism as well as
withdrawal discomfort may lead to continued consumption) induced by
the morphine (10 mg/kg) ‘reminding’ dose.
Similar to previous studies, repeated injections of
morphine (40 mg/kg) for 4 consecutive days produced morphine
dependency (27-29).
At 7 days following the initiation of the experiment, the animals
withdrew their morphine tendency when placed into CPP apparatus. On
the 8th day of the experiment, the effects of RES on morphine
tendency were examined. Following to aversion, morphine at a 10
mg/kg injection on day 16 can reinstate morphine CPP.
Opioidergic, dopaminergic and GABAergic neuronal
pathways along with NAcc, VTA, the amygdala and hippocampus
regulate morphine-induced CPP (30).
One of the most important systems involved in the
‘rewarding’ effects of morphine is the mesolimbic pathway.
Moreover, NMDA antagonists can prevent morphine tendency (5,16,32). Additionally, RES exerts inhibitory
effects on glutamate receptor especially NMDA receptors in cortical
neurons that are involved in the rewarding system (33). This effect of RES has been shown
to be mediated by the increment of glutamate release by reducing
the activity of voltage-dependent Ca2+ channels and MAP
kinase; moreover, RES inhibits glutamate release form
cerebrocortical nerve terminals by reducing the activity of N- and
P/Q-type Ca2+ channels (34). Furthermore, RES inhibits the
pre-synaptic release of glutamate, although post-synaptically, it
inhibits NMDA type glutamate receptor (33,34).
In acute opioid dependence, the rewarding effects
along with the development and expression of behavioral and opioid
neurochemical sensitization are related to glutamatergic
neurotransmission, and dopamine release is under the control of
glutamate and NMDA receptors (33). Opioids administration into the VTA
enhances dopamine release in the NAcc (29). The data of this study are
consistent with those of previous research indicating that NMDA
antagonists can diminish morphine tendency, as well as tolerance
and dependency (8).
Increased rates of dopamine release in the NAcc are
associated with the rewarding effects of morphine addiction
(31,33). The administration of MK 801, an
NMDA antagonist, has been shown to boost the morphine
antinociceptive effects via the suppression of calcium influx
(4,7).
Similar to the results of this study, a previous
study on the effect of dextromethorphan, an NMDA antagonist, on
morphine demonstrated that dextromethorphan reduced morphine
tolerance and dependency (29).
Dextromethorphan can increase morphine antinociceptive effects and
decrease tolerance and dependence towards it (29).
Excitatory neurotransmitters, including NMDA play
crucial roles in hyperalgesia and morphine tolerance. It has been
shown that memantine, an NMDA antagonist, decreases morphine
tendency and CPP (16,33) and attenuates morphine rewarding
potential, as evaluated by the method of morphine
self-administration in mice (32). Other NMDA antagonists have been
shown to exert inhibitory effects on morphine rewarding activity in
a CPP model. Mechanistically, the activation of the morphine
rewarding system requires the stimulation of NMDA receptors in the
NAcc and VTA. Previously, the authors demonstrated that Berberis
vulgaris aqueous extract reduces morphine tendency and
reinstatement presumably via NMDA antagonistic effects (31).
Moreover, previous studies have shown that RES
exerts antinociceptive effects on acute pain and chronic
inflammation and increases the antinociceptive effects of morphine
in morphine-tolerant animals (22,23). Since RES exerts antinociceptive
effects, potentiates antinociceptive effects in morphine-tolerant
animals and reduces morphine relapse and reinstatement, it may
prove to a valuable natural product for abstinence therapy.
In conclusion, this study demonstrated that RES
markedly suppressed morphine-induced CPP and improved extinction.
The findings of this study demonstrate that RES can potentiate the
antinociceptive effects of morphine and reduces morphine tendency
and reinstatement.
Acknowledgements
Not applicable.
Funding
This study was partly supported by a grant from the
Vice Chancellor of Research, Zabol University of Medical Sciences,
Zabol, Iran.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
MH and RR were involved in the study design, and in
the drafting and editing of the manuscript; SJ, SE and MAA
performed the experiments; AT, DAS and CN were involved in the
study design and in data interpretation. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by Ethics Committee of Zabol
University of Medical Sciences, Zabol, Iran (approval no.
IR.ZBMU.REC.1398.157).
Patient consent for publication
Not applicable.
Competing interests
DAS is the Managing Editor of the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
The other authors declare that they have no competing
interests.
References
|
1
|
Kest B, McLemore G, Kao B and Inturrisi
CE: The competitive
α-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor
antagonist LY293558 attenuates and reverses analgesic tolerance to
morphine but not to delta or kappa opioids. J Pharmacol Exp Ther.
283:1249–1255. 1997.PubMed/NCBI
|
|
2
|
Mayer DJ, Mao J, Holt J and Price DD:
Cellular mechanisms of neuropathic pain, morphine tolerance, and
their interactions. Proc Natl Acad Sci USA. 96:7731–7736.
1999.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tsatsakis A, Docea AO, Calina D, Tsarouhas
K, Zamfira LM, Mitrut R, Sharifi-Rad J, Kovatsi L, Siokas V,
Dardiotis E, et al: A Mechanistic and Pathophysiological Approach
for Stroke Associated with Drugs of Abuse. J Clin Med.
8(1295)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kratzer U and Schmidt WJ: The anti-craving
drug acamprosate inhibits the conditioned place aversion induced by
naloxone-precipitated morphine withdrawal in rats. Neurosci Lett.
252:53–56. 1998.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Theberge FR, Li X, Kambhampati S, Pickens
CL, St Laurent R, Bossert JM, Baumann MH, Hutchinson MR, Rice KC,
Watkins LR, et al: Effect of chronic delivery of the Toll-like
receptor 4 antagonist (+)-naltrexone on incubation of heroin
craving. Biol Psychiatry. 73:729–737. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dhawan K, Kumar S and Sharma A: Reversal
of Morphine Tolerance and Dependence by Passiflora incarnata - A
Traditional Medicine to Combat Morphine Addiction. Pharm Biol.
40:576–580. 2002.
|
|
7
|
Tabatabai SM, Dashti S, Doosti F and
Hosseinzadeh H: Phytotherapy of opioid dependence and withdrawal
syndrome: A review. Phytother Res. 28:811–830. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Ribeiro Do Couto B, Aguilar MA, Manzanedo
C, Rodríguez-Arias M and Miñarro J: Effects of NMDA receptor
antagonists (MK-801 and memantine) on the acquisition of
morphine-induced conditioned place preference in mice. Prog
Neuropsychopharmacol Biol Psychiatry. 28:1035–1043. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Guo SJ, Cui Y, Huang ZZ, Liu H, Zhang XQ,
Jiang JX and Xin WJ: Orexin A-mediated AKT signaling in the dentate
gyrus contributes to the acquisition, expression and reinstatement
of morphine-induced conditioned place preference. Addict Biol.
21:547–559. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Al-Harthi SE, Alarabi OM, Ramadan WS,
Alaama MN, Al-Kreathy HM, Damanhouri ZA, Khan LM and Osman AM:
Amelioration of doxorubicin induced cardiotoxicity by resveratrol.
Mol Med Rep. 10:1455–1460. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hamza RZ and El-Shenawy NS:
Anti-inflammatory and antioxidant role of resveratrol on
nicotine-induced lung changes in male rats. Toxicol Rep. 4:399–407.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Theodotou M, Fokianos K, Moniatis D,
Kadlenic R, Chrysikou A, Aristotelous A, Mouzouridou A, Diakides J
and Stavrou E: Effect of resveratrol on non-alcoholic fatty liver
disease. Exp Ther Med. 18:559–565. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Goutzourelas N, Stagos D, Spanidis Y,
Liosi M, Apostolou A, Priftis A, Haroutounian S, Spandidos DA,
Tsatsakis AM and Kouretas D: Polyphenolic composition of grape stem
extracts affects antioxidant activity in endothelial and muscle
cells. Mol Med Rep. 12:5846–5856. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hashemzaei M, Barani AK, Iranshahi M,
Rezaee R, Tsarouhas K, Tsatsakis AM, Wilks MF and Tabrizian K:
Effects of resveratrol on carbon monoxide-induced cardiotoxicity in
rats. Environ Toxicol Pharmacol. 46:110–115. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hashemzaei M, Karami SP, Delaramifar A,
Sheidary A, Tabrizian K, Rezaee R, Shahsavand S, Arsene AL,
Tsatsakis AM and Taghdisi SM: Anticancer effects of
co-administration of daunorubicin and resveratrol in MOLT-4, U266
B1 and RAJI cell lines. Farmacia. 64:36–42. 2016.
|
|
16
|
Labinskyy N, Csiszar A, Veress G, Stef G,
Pacher P, Oroszi G, Wu J and Ungvari Z: Vascular dysfunction in
aging: Potential effects of resveratrol, an anti-inflammatory
phytoestrogen. Curr Med Chem. 13:989–996. 2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Russo GL: Ins and outs of dietary
phytochemicals in cancer chemoprevention. Biochem Pharmacol.
74:533–544. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Tabrizian K, Musavi SS, Rigi M,
Hosseindadi F, Kordi S, Shamshirgaran F, Bazi A, Shahraki J, Rezaee
R and Hashemzaei M: Behavioral and molecular effects of
intrahippocampal infusion of auraptene, resveratrol, and curcumin
on H-89-induced deficits on spatial memory acquisition and
retention in Morris water maze. Hum Exp Toxicol. 38:775–784.
2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Tabrizian K, Shahraki J, Bazzi M, Rezaee
R, Jahantigh H and Hashemzaei M: Neuro-Protective Effects of
Resveratrol on Carbon Monoxide-Induced Toxicity in Male Rats.
Phytother Res. 31:1310–1315. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Zhao YN, Li WF, Li F, Zhang Z, Dai YD, Xu
AL, Qi C, Gao JM and Gao J: Resveratrol improves learning and
memory in normally aged mice through microRNA-CREB pathway. Biochem
Biophys Res Commun. 435:597–602. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ghazanfarpour M, Najafi MN, Roozbeh N,
Mashhadi ME, Keramat-Roudi A, Mégarbane B, Tsatsakis A, Moghaddam
MMM and Rezaee R: Therapeutic approaches for neonatal abstinence
syndrome: A systematic review of randomized clinical trials. Daru.
27:423–431. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gentilli M, Mazoit JX, Bouaziz H, Fletcher
D, Casper RF, Benhamou D and Savouret JF: Resveratrol decreases
hyperalgesia induced by carrageenan in the rat hind paw. Life Sci.
68:1317–1321. 2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Tsai RY, Chou KY, Shen CH, Chien CC, Tsai
WY, Huang YN, Tao PL, Lin YS and Wong CS: Resveratrol regulates
N-methyl-D-aspartate receptor expression and suppresses
neuroinflammation in morphine-tolerant rats. Anesth Analg.
115:944–952. 2012.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shen CH, Tsai RY, Tai YH, Lin SL, Chien CC
and Wong CS: Intrathecal etanercept partially restores morphine's
antinociception in morphine-tolerant rats via attenuation of the
glutamatergic transmission. Anesth Analg. 113:184–190.
2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schmatz R, Mazzanti CM, Spanevello R,
Stefanello N, Gutierres J, Corrêa M, da Rosa MM, Rubin MA,
Chitolina Schetinger MR and Morsch VM: Resveratrol prevents memory
deficits and the increase in acetylcholinesterase activity in
streptozotocin-induced diabetic rats. Eur J Pharmacol. 610:42–48.
2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Marambaud P, Zhao H and Davies P:
Resveratrol promotes clearance of Alzheimer's disease amyloid-β
peptides. J Biol Chem. 280:37377–37382. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
MacCarrone M, Lorenzon T, Guerrieri P and
Agrò AF: Resveratrol prevents apoptosis in K562 cells by inhibiting
lipoxygenase and cyclooxygenase activity. Eur J Biochem. 265:27–34.
1999.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Subbaramaiah K, Chung WJ, Michaluart P,
Telang N, Tanabe T, Inoue H, Jang M, Pezzuto JM and Dannenberg AJ:
Resveratrol inhibits cyclooxygenase-2 transcription and activity in
phorbol ester-treated human mammary epithelial cells. J Biol Chem.
273:21875–21882. 1998.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Subbaramaiah K, Michaluart P, Chung WJ,
Tanabe T, Telang N and Dannenberg AJ: Resveratrol inhibits
cyclooxygenase-2 transcription in human mammary epithelial cells.
Ann N Y Acad Sci. 889:214–223. 1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pérez-Severiano F, Bermúdez-Ocaña DY,
López-Sánchez P, Ríos C and Granados-Soto V: Spinal nerve ligation
reduces nitric oxide synthase activity and expression: Effect of
resveratrol. Pharmacol Biochem Behav. 90:742–747. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Imenshahidi M, Qaredashi R, Hashemzaei M
and Hosseinzadeh H: Inhibitory Effect of Berberis vulgaris
Aqueous Extract on Acquisition and Reinstatement Effects of
Morphine in Conditioned Place Preferences (CPP) in Mice.
Jundishapur J Nat Pharm Prod. 9(e16145)2014.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qaredashi R, Hosseinzadeh H and Hashemzaei
M: Inhibitory effect of Berberis vulgaris aqueous extract on
acquisition and reinstatement effects of morphine in conditioned
place preferences (CPP) in mice. Res Pharm Sci. 7(S839)2012.
|
|
33
|
Gao ZB, Chen XQ and Hu GY: Inhibition of
excitatory synaptic transmission by trans-resveratrol in rat
hippocampus. Brain Res. 1111:41–47. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chang Y and Wang SJ: Inhibitory effect of
glutamate release from rat cerebrocortical nerve terminals by
resveratrol. Neurochem Int. 54:135–141. 2009.PubMed/NCBI View Article : Google Scholar
|