1. Introduction
Influenza develops in approximately 20% of the
world's population each year. In the US, 30,000 to 100,000 deaths
occur annually due to influenza. The pandemic of 1918-1919 resulted
in 50 million to 100 million deaths.
Vaccination is the primary strategy for the
prevention of influenza; however, it is not always adequate. The
effectiveness of the seasonal influenza vaccine varies by season.
For example, during the period between November 23, 2018 to
February 2, 2019, the overall adjusted vaccine effectiveness
against all influenza virus infection associated with medically
attended acute respiratory illness was 47%. For children aged 6
months to 17 years, the overall vaccine effectiveness was 61%
(1). In addition, a growing body
of evidence indicates that the protective immune responses
triggered by flu vaccines wane in a matter of weeks (2). Antiviral drugs thus form an
important part of an effective approach to influenza and are
critical to planning for a pandemic (3).
Five drugs are currently available for the treatment
or prophylaxis of influenza infections: The adamantanes (amantadine
and rimantadine) and the neuraminidase inhibitors (zanamivir and
oseltamivir). In 2019, the FDA approved baloxavir marboxil (trade
name, Xofluza), a new class of drug which targets the endonuclease
function of the viral PA polymerase subunit and prevents the
transcription of viral mRNA (4).
Despite the success of baloxavir, certain strains of influenza A
(H3N2) exhibit a reduced susceptibility (5). Additional antiviral drugs are thus
required.
2. mTOR and influenza
The mammalian target of rapamycin (mTOR) signaling
pathway senses and responds to nutrient availability, energy
sufficiency, stress, hormones and mitogens to modulate protein
synthesis. The mTOR pathway is dysregulated in human diseases,
particularly in cancers. Rapamycin (sirolimus) is a bacterial
product that can inhibit mTOR via AMPK activation and the
inhibition of the PI3K/AKT/mTOR pathway (6).
mTOR signaling is necessary for the development of
influenza and modulates the antibody response to provide
cross-protective immunity to lethal infection with influenza virus.
In animal studies, rapamycin was shown to promote cross-strain
protection against lethal infection with influenza virus of various
subtypes when administered during immunization with influenza virus
subtype H3N2(7). Mitogenic
stimulation accelerates influenza-induced mortality in animals by
increasing susceptibility of alveolar type II cells to infection,
and pre-treatment with rapamycin reverses this effect (8).
In human studies, the treatment of severe H1N1
influenza-related pneumonia with rapamycin and steroids was shown
to improve the outcome (9,10). However, other researchers have
demonstrated that immune suppression caused by systemic steroids,
and possibly rapamycin as well, is associated with an increased
morbidity/mortality and a prolonged viral replication (11).
In order to avoid the systemic side-effects, some
investigators have postulated that the inhalation of rapamycin
would be desirable. Inhalable rapamycin preparations have been
formulated and tested on rats (12,13) but never in humans, and for good
reason: A side-effect of oral rapamycin is interstitial pneumonitis
(14). The inhalation of
rapamycin, with its well-documented lung toxicity, is
contraindicated.
3. Biguanides
Another class of drug, biguanides, can also inhibit
mTOR activation but has no lung toxicity. Biguanides are widely
used small molecule drugs prescribed as oral anti-diabetics. They
include the following: i) Metformin; ii) phenformin, withdrawn from
US market because of its propensity to cause lactic acidosis; iii)
buformin (1-butylbiguanide), an oral antidiabetic drug of the
biguanide class, chemically related to metformin and phenformin;
buformin was marketed by the German pharmaceutical company,
Grünenthal, as Silubin; and iv) benfosformin, etoformin,
tiformin
Metformin activates the 5' AMP-activated protein
kinase (AMPK) pathway through liver kinase B1 (LKB1), eventually
causing the inhibition of the mTOR pathway and thus, a reduction in
protein synthesis and cellular proliferation. Metformin also
appears to indirectly reduce AKT activation, through the
AMPK-mediated phosphorylation of insulin receptor substrate 1
(IRS-1), causing the inhibition of the mTOR pathway (15).
Biguanides have no known lung toxicity after decades
of use in millions of patients. Biguanides are cell proliferation
inhibitors, and their use in oncology holds considerable promise
(16,17).
4. Buformin, phenformin and human
influenza
During the 1971 outbreak of influenza (1968
Hong-Kong H3N2 strain), 110 diabetic patients treated with
phenformin or buformin (group A) and 79 diabetic patients treated
with insulin or sulfonylurea derivatives (group B) were observed
(18). The incidence of
influenza was significantly lower in group A (6/110, 5.4%) than in
group B (19/79, 24%). This difference was statistically significant
(P=0.0003, Fisher's exact test) (18).
A smaller number of complications following
influenza in group A (1/110, 0.9%) as compared with group B (4/79,
5%) was not statistically significant (P=0.16, Fisher's exact test)
(18).
Biguanides act against other viruses, apart from
influenza. For example, polyhexamethylene biguanide exposure has
been shown to lead to the viral aggregation of MS2 bacteriophage
(19). Persistent interactions
between biguanide-based compound NB325 and CXCR4 result in
prolonged inhibition of human immunodeficiency virus type 1
infection (20).
5. Buformin and influenza in mice
Denys and Bocian examined the protective effect of
buformin (Silubin retard) against influenza in mice (21). They used the APR-8 influenza
virus strain. The source of the virus was the allantoic fluid of
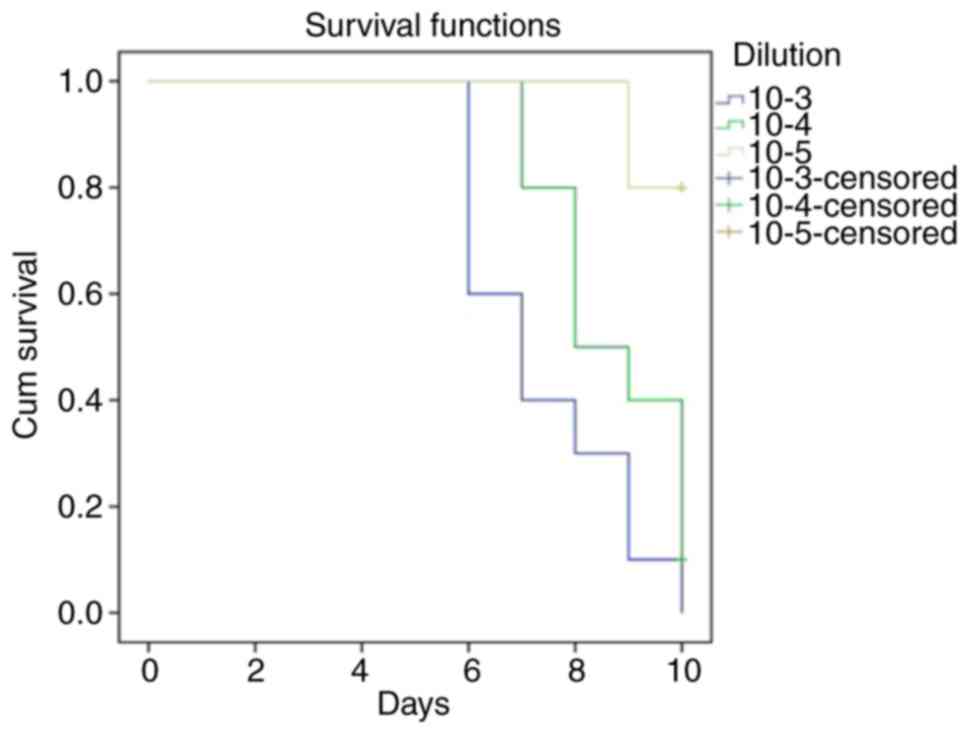
infected chick embryos. They determined, with the method of
surviving allantoic sections, that the minimal infectious dilution
of influenza virus causing hemagglutination was 10-5
(Table I). Allantoic sections
contain an inhibitor of hemagglutination, and this method is a
standard assay of viral infectivity (22).
 | Table IFive dilutions of influenza virus
tested on 8 allantoic sections. |
Table I
Five dilutions of influenza virus
tested on 8 allantoic sections.
| 10-2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10-3 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10-4 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10-5 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 10-6 | No | No | No | No | No | No | No | No |
The buformin preparation was dissolved in
phosphate-buffered 0.9% NaCl solution. A total of 110 white BALB/C
mice were used for the experiments, weighing 18-20 g. Mice were
infected with 0.05 ml influenza virus intranasally following mild
anesthesia. An LD50 infectious dilution assay for mice was carried
out and estimated to be 10-4 (Fig. 1). Half the animals treated died
within 10 days after being infected. This titer of APR-8 was used
in the buformin studies (21).
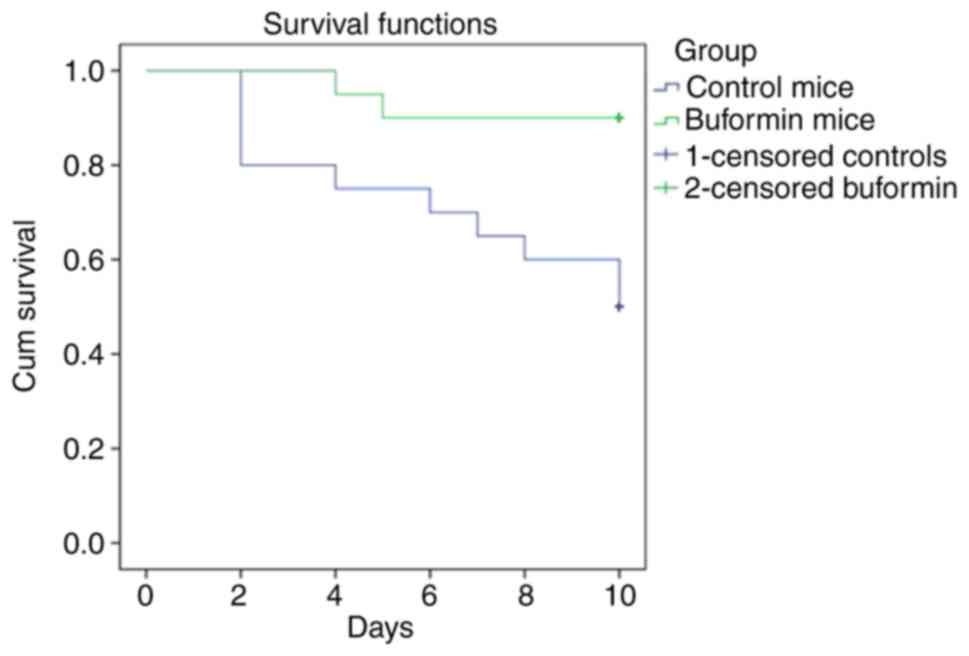
The buformin preparation was injected once daily
subcutaneously, at a dose of 20 mg/kg, beginning 24 h after 40
animals had been infected. Treatment was carried out over a period
of 4 days. Influenza-infected animals in the control group (40
animals) received 0.9% NaCl. The observations were carried out over
a period of 10 days (Fig. 2).
Buformin significantly improved survival (P<0.001). The
buformin=treated mice remained in a much better general condition,
compared to the control group that received no buformin. The lungs
of buformin-treated mice had less macroscopic inflammation. Further
studies indicated that phenformin also improved survival, though to
a lesser extent than buformin (23). The buformin results are
comparable to the effect of baloxavir marboxil combined with a
neuraminidase inhibitor (24).
6. Inhaled biguanides for influenza
Inhaling a biguanide for influenza would limit the
risk of systemic side-effects associated with biguanides due to the
low inhaled dose. Lactic acidosis is the main biguanide systemic
side-effect (25). Inhalation
would deliver a more predictable amount of biguanide to the lung
than oral dosing and is an established mode of delivery for a range
of therapeutic agents.
Precedence exists for inhaled drug use in influenza.
The neuraminidase inhibitor, zanamivir, is administered by
inhalation. Inhaled zanamivir requires 10 mg twice a day. The dose
of typical inhaled asthma medications is 10-100 µg day.
Oral metformin, then known as flumamine, was
examined as an anti-influenza and anti-malarial drug in the
Philippines during the late 1940s. Another biguanide anti-malarial
drug, biguanil, is still in use (26). A tendency for metformin to lower
blood glucose levels in some of the influenza patients was duly
noted (27,28).
Metformin is taken orally twice daily by diabetic
patients, with a maximum total dose of 2.5 g/day. Reducing the
inhaled dose of an oral drug by a factor of 10-20 typically results
in the same local concentration in the airways as by oral
administration. Thus, just to equal what the oral dose of metformin
would deliver to the airways, a subject would need to inhale
125-250 mg metformin per day; or, if broken into 3 doses/day, 40-80
mg/dose. Delivering this amount of metformin powder to the lungs is
at the upper limit of acceptability, and would result in reduced
compliance, bronchospasm and cough (29).
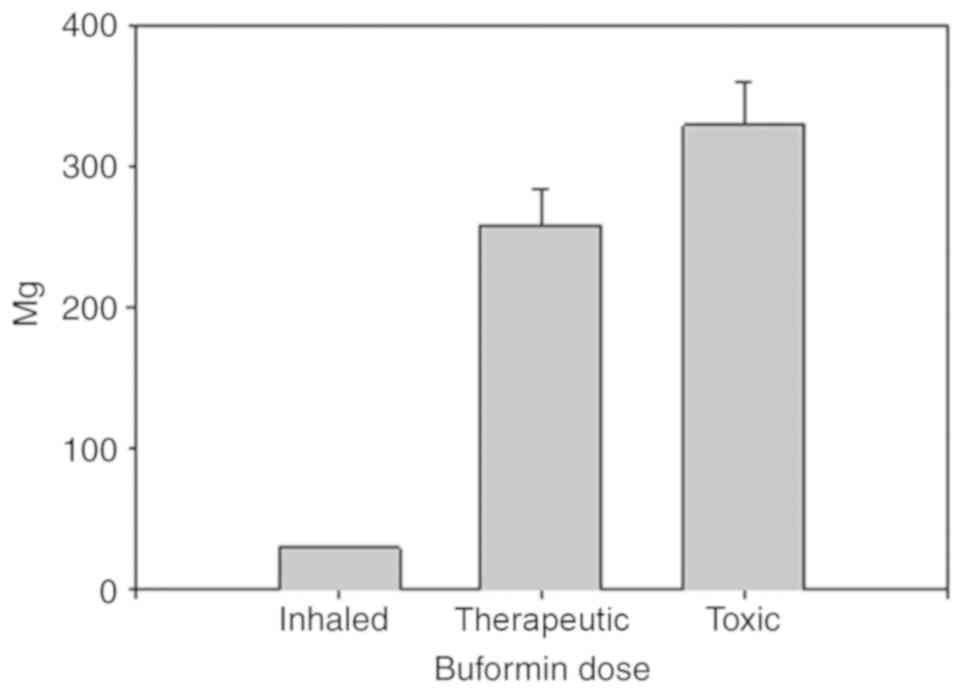
Buformin has eight times the potency of metformin.
The inhalation of buformin as opposed to metformin, could reduce
the dose by a factor of eight. The usual maximum oral dose of
buformin is 300 mg/day. Decreasing the inhaled dose by a factor of
10 to 20, 3 doses per day inhaled buformin could be administered at
5 to 10 mg per dose, much less than metformin. This dose of
buformin, 15 to 30 mg per day, would be highly unlikely to produce
lactic acidosis, the main biguanide complication. In a previous
study, the toxic oral buformin dose was 329±30 mg/day in 24
patients who developed lactic acidosis while using buformin.
Another group of 24 patients administered 258±25 mg/day buformin
did not develop lactic acidosis (25). In other words, the inhaled
buformin dose can be increased 10-fold above what would be needed
to treat influenza and would still be well below the systemic toxic
dose (Fig. 3). This is a key
strength of buformin.
Inhaled buformin has a relatively long lung
residence time. Buformin has an octanol/water partition coefficient
(log P) of -1.2 and is hydrophilic. Hydrophilic small molecules
with a log P-value <0 have a mean lung half-life (t½) of
approximately 1 h (29).
Hydrophobic small molecules have a mean lung half-life of
approximately 1 min and are less suitable as an influenza treatment
because they pass through the lung so rapidly.
A final advantage of buformin over phenformin is
that it improves survival of influenza-infected mice with higher
efficiency than phenformin (23).
7. Inhaled buformin or phenformin for
coronavirus
Coronavirus disease 2019 (COVID-19) is an infectious
disease caused by SARS-CoV-2, a virus closely related to the SARS
virus. The disease is the cause of the 2019-2020 coronavirus
outbreak. It is primarily spread between individuals by small
droplets emitted from infected individuals when they breathe or
cough. The PI3K/AKT/mTOR signaling responses play important roles
in MERS-CoV infection and may represent a novel drug target for
therapeutic intervention strategies (30). The inhalation of buformin or
phenformin for coronavirus may be an effective novel treatment that
would limit the risk of systemic side-effects associated with
biguanides due to a low inhaled dose.
8. Conclusions and future perspectives
The repurposing of old drugs as antivirals holds
considerable promise. Statins are a prime example. A randomized
placebo-controlled phase II clinical trial (NCT02056340) aimed at
evaluating the potential effect of atorvastatin to reduce the
severity of illness in influenza-infected patients is currently
underway (31). The inhalation
of buformin may represent a novel route of administration for an
old drug that may also be used a novel treatment strategy for
influenza, coronavirus and other viral infections.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SL as the sole author of the present review article
was responsible for the conception and design of this article, as
well as for the literature search, writing and manuscript
preparation and revisions. The author has read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Doyle JD, Chung JR, Kim SS, Gaglani M,
Raiyani C, Zimmerman RK, Nowalk MP, Jackson ML, Jackson LA, Monto
AS, et al: Interim estimates of 2018-19 seasonal influenza vaccine
effectiveness-United States, February 2019. MMWR Morb Mortal Wkly
Rep. 68:135–139. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cohen J: Waning immunity. Science.
364:224–227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Moscona A: Neuraminidase inhibitors for
influenza. N Engl J Med. 353:1363–1373. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
O'Hanlon R and Shaw ML: Baloxavir
marboxil: The new influenza drug on the market. Curr Opin Virol.
35:14–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takashita E, Ichikawa M, Morita H, Ogawa
R, Fujisaki S, Shirakura M, Miura H, Nakamura K, Kishida N,
Kuwahara T, et al: Human-to-human transmission of influenza A(H3N2)
virus with reduced susceptibility to baloxavir, Japan, February
2019. Emerg Infect Dis. 25:2108–2111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ma XM and Blenis J: Molecular mechanisms
of mTOR-mediated translational control. Nat Rev Mol Cell Biol.
10:307–318. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Keating R, Hertz T, Wehenkel M, Harris TL,
Edwards BA, McClaren JL, Brown SA, Surman S, Wilson ZS, Bradley P,
et al: The kinase mTOR modulates the antibody response to provide
cross-protective immunity to lethal infection with influenza virus.
Nat Immunol. 14:1266–1276. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Nikolaidis NM, Noel JG, Pitstick LB,
Gardner JC, Uehara Y, Wu H, Saito A, Lewnard KE, Liu H, White MR,
et al: Mitogenic stimulation accelerates influenza-induced
mortality by increasing susceptibility of alveolar type II cells to
infection. Proc Natl Acad Sci USA. 114:E6613–E6622. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang CH, Chung FT, Lin SM, Huang SY, Chou
CL, Lee KY, Lin TY and Kuo HP: Adjuvant treatment with a mammalian
target of rapamycin inhibitor, sirolimus, and steroids improves
outcomes in patients with severe H1N1 pneumonia and acute
respiratory failure. Crit Care Med. 42:313–321. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chuang YC, Ruan SY and Huang CT:
Compelling results of adjuvant therapy with sirolimus for severe
H1N1 pneumonia. Crit Care Med. 42:e687–e688. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ison MG: Adjuvant immunosuppression in the
management of severe influenza: Friend or foe? Crit Care Med.
42:457–459. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gupta A, Pant G, Mitra K, Madan J,
Chourasia MK and Misra A: Inhalable particles containing rapamycin
for induction of autophagy in macrophages infected with
Mycobacterium tuberculosis. Mol Pharm. 11:1201–1207.
2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Carvalho SR, Watts AB, Peters JI, Liu S,
Hengsawas S, Escotet-Espinoza MS and Williams RO III:
Characterization and pharmacokinetic analysis of crystalline versus
amorphous rapamycin dry powder via pulmonary administration in
rats. Eur J Pharm Biopharm. 88:136–147. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Weiner SM, Sellin L, Vonend O, Schenker P,
Buchner NJ, Flecken M, Viebahn R and Rump LC: Pneumonitis
associated with sirolimus: Clinical characteristics, risk factors
and outcome-a single-centre experience and review of the
literature. Nephrol Dial Transplant. 22:3631–3637. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Clements A, Gao B, Yeap SH, Wong MK, Ali
SS and Gurney H: Metformin in prostate cancer: Two for the price of
one. Ann Oncol. 22:2556–2560. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Pollak M: Potential applications for
biguanides in oncology. J Clin Invest. 123:3693–3700.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Lehrer S, Rheinstein P and Mulshine J:
Inhaled buformin for lymphangioleiomyomatosis and early (airway
confined) lung cancer. American Association for Cancer Research
Annual Meeting Abstracts: 14.A.638.AACR, 2014.
|
|
18
|
Babinski S and Giermaziak H: Influenza
epidemic in 1971 in diabetics treated with 1-butyl-biguanidine
hydrochloride (Silubin retard) and 1-phenylethyl-biguanidine
hydrochloride (Phenformin). Pol Tyg Lek. 28:1815–1817. 1973.(In
Polish). PubMed/NCBI
|
|
19
|
Pinto F, Maillard JY, Denyer SP and
McGeechan P: Polyhexamethylene biguanide exposure leads to viral
aggregation. J Appl Microbiol. 108:1880–1888. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Thakkar N, Pirrone V, Passic S, Keogan S,
Zhu W, Kholodovych V, Welsh W, Rando R, Labib M, Wigdahl B and
Krebs FC: Persistent interactions between biguanide-based compound
NB325 and CXCR4 result in prolonged inhibition of human
immunodeficiency virus type 1 infection. Antimicrob Agents
Chemother. 54:1965–1972. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Denys A and Bocian J: Effect of
Silubin-retard (1-butyl-biguanide hydrochloride) on the course of
influenza-virus infection in mice. Pol Tyg Lek. 25:332–334.
1970.(In Polish). PubMed/NCBI
|
|
22
|
Orthel FW: Influenza virus titrations and
the inhibitor of hemagglutination in normal allantoic fluid. Arch
Gesamte Virusforsch. 38:347–356. 1972.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bocian J, Denys A and Czernek Z: Effect of
biguanidine derivatives phenformin and buformin on course of
infection with influenza virus in mouse. Dissertationes
Pharmaceuticae et Pharmacologicae. 23(581)1971.
|
|
24
|
Fukao K, Noshi T, Yamamoto A, Kitano M,
Ando Y, Noda T, Baba K, Matsumoto K, Higuchi N, Ikeda M, et al:
Combination treatment with the cap-dependent endonuclease inhibitor
baloxavir marboxil and a neuraminidase inhibitor in a mouse model
of influenza A virus infection. J Antimicrob Chemother. 74:654–662.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Luft D, Schmülling RM and Eggstein M:
Lactic acidosis in biguanide-treated diabetics: A review of 330
cases. Diabetologia. 14:75–87. 1978.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Garcia Rubino ME, Carrillo E, Ruiz Alcala
G, Dominguez-Martin A, A Marchal J and Boulaiz H: Phenformin as an
anticancer agent: Challenges and prospects. Int J Mol Sci.
20(E3316)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Garcia EY: Flumamine, a new synthetic
analgesic and anti-flu drug. J Philipp Med Assoc. 26:287–293.
1950.PubMed/NCBI
|
|
28
|
Bailey CJ: Metformin: Historical overview.
Diabetologia. 60:1566–1576. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Patton JS, Fishburn CS and Weers JG: The
lungs as a portal of entry for systemic drug delivery. Proc Am
Thorac Soc. 1:338–344. 2004.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kindrachuk J, Ork B, Hart BJ, Mazur S,
Holbrook MR, Frieman MB, Traynor D, Johnson RF, Dyall J, Kuhn JH,
et al: Antiviral potential of ERK/MAPK and PI3K/AKT/mTOR signaling
modulation for Middle East respiratory syndrome coronavirus
infection as identified by temporal kinome analysis. Antimicrob
Agents Chemother. 59:1088–1099. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pizzorno A, Padey B, Terrier O and
Rosa-Calatrava M: Drug repurposing approaches for the treatment of
influenza viral infection: Reviving old drugs to fight against a
long-lived enemy. Front Immunol. 10(531)2019.PubMed/NCBI View Article : Google Scholar
|