Introduction
The medicinal properties of silver-based compounds
have been known for >2,000 years. Since the 19th century, these
properties have been associated with their antimicrobial activity.
More recently, advancements in the nanoscience and nanotechnology
fields have resulted in the development of several consumer
products, many of which are routinely used in daily life.
Inevitably, researchers are paying attention on metallic
nanoparticles due to their increasing microbial resistance against
metal ions, antibiotics and the development of resistant strains
(1). Among the metallic
nanoparticles, particular attention has been paid to silver
nanoparticles (AgNPs), which presently correspond to 24% of the
1,814 products listed in the Nanotechnology Consumer Products
Inventory (2). Efforts have been
made to explore their attractive properties, allowing their use as
antibacterial and anticancer drugs, in diagnostics and
optoelectronics, in water disinfection, cosmetics, and other
clinical/pharmaceutical applications. The majority of these
products are already available for purchase at grocery stores and
through the internet (3,4). Moreover, the silver antimicrobial
agents can be easily incorporated into several materials, such as
plastics and textiles, making them useful in a wide spectrum of
applications, maintaining their antimicrobial activity in
situ, in which traditional antimicrobial agents would be
unstable.
It is well known that nanoparticles can be
recognized by the immune system, which may result in the activation
of pro-inflammatory pathways (5).
The intentional or unintentional human exposure to AgNPs is
unavoidable and may also trigger innate immunity responses. The
innate immunity is the nonspecific and first line of the body's
defense system that plays an essential role in the early
recognition of non-self and foreign bodies and subsequent
pro-inflammatory response. Among the innate immune response cells,
leukocytes, neutrophils constitute the first cells to arrive to the
affected local of inflammation where they usually phagocyte and
neutralize the invader. This process involves the production of
reactive oxygen species (ROS) and reactive nitrogen species (RNS),
a process known as oxidative burst. When this production is
exacerbated and sustained, it may result in oxidative stress, a
condition involved in the development and worsening of several
diseases (6,7). It is currently accepted that several
chemical and physical properties of AgNPs, including their size,
shape and surface coatings, directly affect the nanoparticle
compatibility with the immune system. In this context, the coating
agents, essentially used to stabilize AgNPs, maintaining their
specific characteristics, are also responsible for the immunogenic
properties (8). Two commonly used
coating agents are sodium citrate and polyvinylpyrrolidone (PVP),
which impart a negative charge, giving AgNPs a wide appeal for
manufacturing and consumer use (9).
Previous studies have reported the pro-inflammatory
effects of AgNPs, in which it was demonstrated that such
nanoparticles are responsible for an increased number of
neutrophils in lungs/bronchoalveolar lavage fluid (9-12).
Nevertheless, the literature dealing with the direct interaction of
AgNPs with human neutrophils is still limited (13-15),
investigating only one particle type with one coating type and/or
one size, at a limited dose range. Therefore, the present study
examined the effect of 3 different sizes (5, 10 and 50 nm) of
citrate- and PVP-coated AgNPs on oxidative burst, calcium levels
and the viability of human neutrophils.
Materials and methods
Reagents
BioPure PVP and citrate coated-AgNPs (5, 10 and 50
nm) were obtained from nanoComposix. The manufacturer characterizes
each batch with: Transmission electron microscopy (TEM) to
determine size and shape distributions (all the AgNPs present a
spherical form) (Table I);
UV-visible spectroscopy to measure the optical properties; dynamic
light scattering to determine particle hydrodynamic diameter; and
zeta potential measurement to determine particle surface charge.
BioPure nanoparticles are extensively washed with the suspending
solvent to remove residual reactants from the manufacturing
process. Mass concentration is determined with inductively coupled
plasma mass spectroscopy (ICP-MS). The particles are sterile
filtered and tested for endotoxin contamination before delivery.
Throughout the study, nanomaterials were stored at
4˚C.
 | Table IDiameter measured by TEM and
coefficient of variation of the studied AgNPs, according the
manufacturer, nanoComposix. |
Table I
Diameter measured by TEM and
coefficient of variation of the studied AgNPs, according the
manufacturer, nanoComposix.
| | Diameter (nm) | Coefficient of
variation (%) |
|---|
| PVP-coated AgNPs
(nm) |
|
5 | 4.4±0.9 | 19.8 |
|
10 | 10.1±1.8 | 17.5 |
|
50 | 50±4 | 8.1 |
| Citrate-coated
AgNPs (nm) |
|
5 | 5.2±0.9 | 16.4 |
|
10 | 9.9±1.9 | 18.7 |
|
50 | 48±5 | 11.2 |
Trypan blue solution, histopaque 1077, histopaque
1119, dihydrorhodamine 123 (DHR), diphenyleneiodonium chloride
(DPI),
1-(5-chloronaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine
hydrochloride (ML-9),
3-[1-[3-(Dimethylamino)propyl]-5-methoxy-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione
(Gö6983), RPMI-1640 medium, fetal bovine serum, L-glutamine,
penicillin, streptomycin, Dulbecco's phosphate-buffered saline,
without calcium chloride and magnesium chloride (PBS) were obtained
from Sigma-Aldrich; Merck KGaA. The Annexin V-FLUOS Staining kit
was obtained from Roche Diagnostics GmbH. FLUO-4/AM was purchased
Life Technologies; Thermo Fisher Scientific, Inc. Vacuum tubes with
K3EDTA were purchased from Vacutainer Systems.
Equipment
Assays were performed in a microplate reader
(Synergy HT, BioTek Instruments, Inc.), using colorimetric and
fluorimetric detection, and a flow cytometer (Accuri™
C6, BD Biosciences).
Isolation of human neutrophils
All patient-related procedures and protocols were
performed in accordance with the Declaration of Helsinki and
approved by the Ethics Committee of Centro Hospitalar do Porto.
After written informed consent was obtained, venous
blood was collected by antecubital venipuncture into
K3EDTA vacuum tubes, in Centro Hospitalar do
Porto-Hospital de Santo António blood bank. The isolation of human
neutrophils was performed by the density gradient centrifugation
method, as previously reported by the authors' research group
(16). Briefly, 3 ml of histopaque
1077 were carefully layered on top of 3 ml of histopaque 1119 in a
15 ml polypropylene tube. Subsequently, 4.5 ml of the collected
blood were decanted on top of this discontinuous density gradient.
The tube was centrifuged at 890 x g for 30 min at 20˚C. Following
centrifugation, the neutrophil pellet was removed using a Pasteur
pipette and doubled in volume using PBS; the neutrophils were then
centrifuged at 870 x g for 5 min at 4˚C. The supernatant was
decanted and a mixture of 1.25 ml of PBS and 5.25 ml of sterile
distilled water was added to the neutrophil pellet to lyse any
remaining red blood cells. The tube was gently inverted for 2 min,
after which isotonicity was re-established by adding 2.2 ml of 3%
NaCl. This suspension was then submitted to a new centrifugation at
870 x g for 5 min at 4˚C after which the supernatant was decanted
and the neutrophil pellet resuspended in RPMI-1640 incubation
medium [(pH 7.4) supplemented with 10% fetal bovine serum, 2 mM
L-glutamine, 100 U/ml penicillin and 0.1 mg/ml streptomycin]. Cell
viability (>98%) and cell yield (number of cells/ml) were
determined by the trypan blue exclusion method using a Neubauer
chamber and an optic microscope. The suspension with the isolated
neutrophils was kept on ice until use.
Measurement of neutrophil oxidative
burst
The measurement of neutrophil oxidative burst was
performed by fluorescence, by monitoring the oxidation of DHR to
rhodamine 123 by neutrophil-generated ROS (15). Neutrophils (3x106
cells/ml) were incubated in a humidified incubator, at 37˚C, for 2
h with citrate or PVP-coated AgNPs (0-50 µg/ml), or PVP (15 µg/ml),
or citrate (0.3 µg/ml) or with Ag+ (AgNO3)
(12 µg/ml), followed by DHR (10 µM). At the end of this incubation
period, cells were centrifuged (400 x g for 5 min at 20˚C) and the
supernatant was discarded. The pellets were resuspended in 300 µl
of RPMI-1640 medium and the fluorescence was measured using a
microplate reader (excitation wavelength, 485 nm; and emission
wavelength, 520 nm). A control assay (without AgNPs) was always
performed in all the experiments.
Involvement of NADPH oxidase in the
AgNP-induced neutrophil oxidative burst
The measurement of neutrophil oxidative burst was
performed as described above with a few alterations. Neutrophils
(3x106 cells/ml) were incubated for 10 min with a NADPH
oxidase inhibitor, DPI (20 µM), followed by 2 h of incubation with
5 nm of citrate and PVP-coated AgNPs (50 µg/ml) and the probe DHR
(10 µM) at 37˚C. At the end of this incubation period, the protocol
used was the same as that described above.
Involvement of protein kinase C (PKC)
in the AgNP-induced neutrophil oxidative burst
The measurement of the neutrophil oxidative burst
was performed as described above with a few alterations.
Neutrophils (3x106 cells/ml) were incubated for 10 mins
with a PKC inhibitor, Gö6983 (5 µM), followed by 2 h of incubation
with 5 nm of citrate and PVP-coated-AgNPs (50 µg/ml) and the probe
DHR (10 µM) at 37˚C. At the end of this incubation period, the
protocol used was the same as that described above.
Measurement of intracellular free
calcium levels
The measurement of intracellular calcium levels was
performed as previously described by Ribeiro et al (17). Isolated neutrophils
(4x106 cells/ml) were pre-incubated with FLUO-4/AM (3
µM), during 30 min, in a humidified incubator, at 37˚C, followed by
the incubation with citrate and PVP-coated AgNPs (0-50 µg/ml) for 2
h. Cells were then centrifuged (870 x g for 5 min at 20˚C). The
pellet was resuspended in PBS and the cell number readjusted. The
monitoring of the intracellular free calcium flux was performed
using a microplate reader, at 37˚C, under continuous soft shaking.
The excitation and emission wavelengths used were 485 and 590 nm,
respectively.
The role of calcium flux in the
AgNP-induced neutrophil oxidative burst
To further elucidate the role of calcium flux in the
AgNP-induced neutrophil oxidative burst, the store-operated calcium
entry (SOCE) broadly investigated inhibitor, ML-9, was used as
follows: Neutrophils (3x106 cells/ml) were incubated
with ML-9 (100 µM) for 10 min and then incubated for 2 h with 5 nm
of citrate and PVP-coated AgNPs (50 µg/ml), followed by DHR (10 µM)
at 37˚C. At the end of this incubation period, the protocol used
was the same as that described above.
Evaluation of cell death
The evaluation of cell death was analyzed by flow
cytometry following simultaneous staining with Annexin V labeled
with fluorescein and propidium iodide, according to a previously
described method (18).
Neutrophils (1x106 cells/ml) were incubated in a
humidified incubator, at 37˚C, with citrate- or PVP-coated AgNPs
(0-50 µg/ml), or PVP (15 µg/ml), or citrate (0.3 µg/ml) or with
Ag+ (AgNO3) (12 µg/ml), for 2 h. The
commercial Annexin V-FLUOS Staining lit (Roche Diagnostics GmbH)
was used according to the manufacturer's instructions. Fluorescence
signals for each sample were collected using a flow cytometer. To
restrict the analysis to neutrophils only, a polygon gate was set
according to their light scattering properties (in a forward vs.
side scatter plot) excluding cell debris and other blood cells.
Fluorescence signals for at least 10,000 cells were collected in
logarithmic mode and the data were analyzed using C Flow (Accuri)
software. The green fluorescence due to Annexin V conjugated with
FITC was followed in channel 1 (FL1) and plotted as a histogram of
FL1 staining. Fluorescence due to the propidium iodide
incorporation was followed in channel 3 (FL3).
Statistical analysis
The GraphPad Prism 6 software was used to calculate
all the mean ± standard deviation of the mean (SEM), (from at least
6 individual experiments, performed in triplicate in each
experiment). Statistical comparison between groups was estimated
using one-way analysis of variance (ANOVA), followed by the
Bonferroni's post hoc test. In all cases, P-values <0.05 were
considered to indicate statistically significant differences. The
results were expressed according to the following equation:
where AU represents arbitrary units, AgNPs
represents cells treated with AgNPs and the Control represents
untreated cells (without AgNPs).
Results
Neutrophil oxidative burst
The AgNP-induced neutrophil oxidative burst was
measured by DHR. This probe detects total intracellular ROS/RNS,
with certain specificity towards hydrogen peroxide
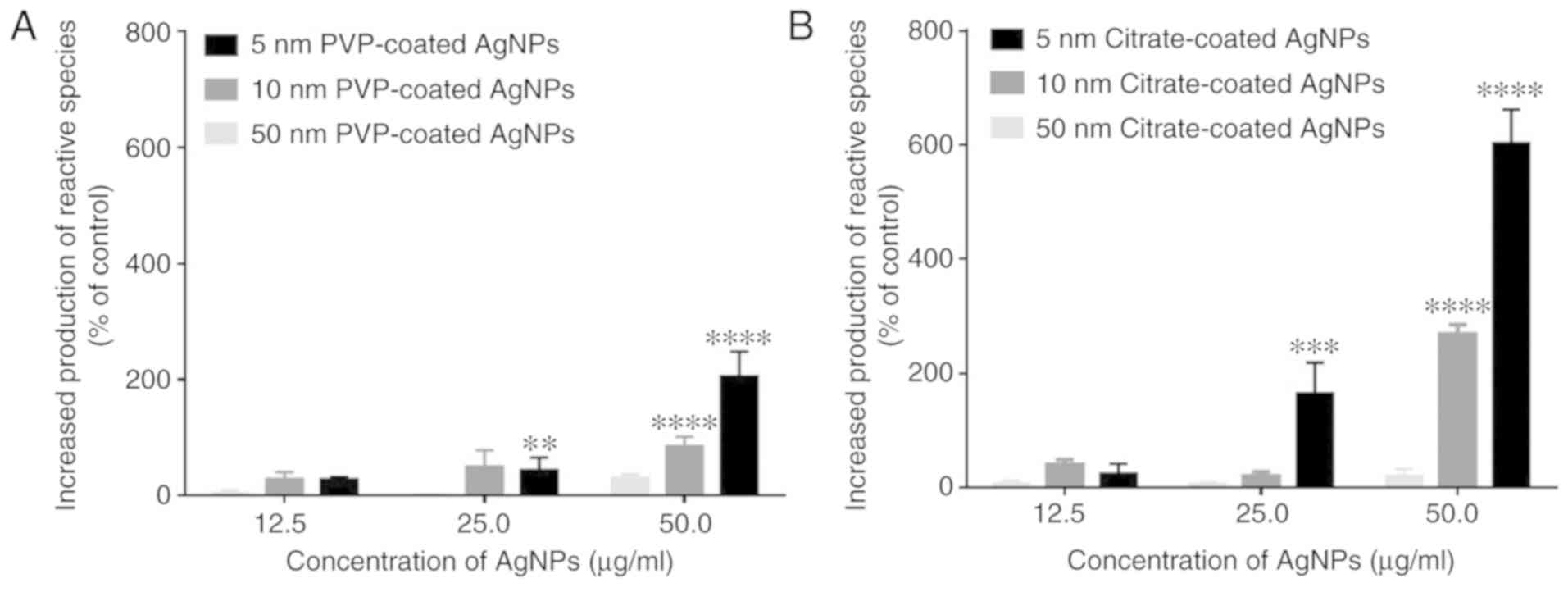
(H2O2) and hypochlorous acid (HOCl) (6). As shown in Fig. 1, both the PVP- and citrate-coated
AgNPs with sizes of 5 and 10 nm induced neutrophil oxidative burst
in a concentration-dependent manner, while the 50-nm-sized AgNPs
had no effect with both coatings. The results also evidenced a
higher reactivity for the 5-nm-sized AgNPs (significant effects
were observed beginning at 25 µg/ml) compared to the 10-nm-sized
AgNPs (significant effects were only observed at 50 µg/ml). It was
also clear that citrate-coated AgNPs induced greater reactive
species production than the PVP-coated AgNPs. None of the coating
agents, or Ag+ (in the form of AgNO3), per
se, induced the production of reactive species (data not
shown).
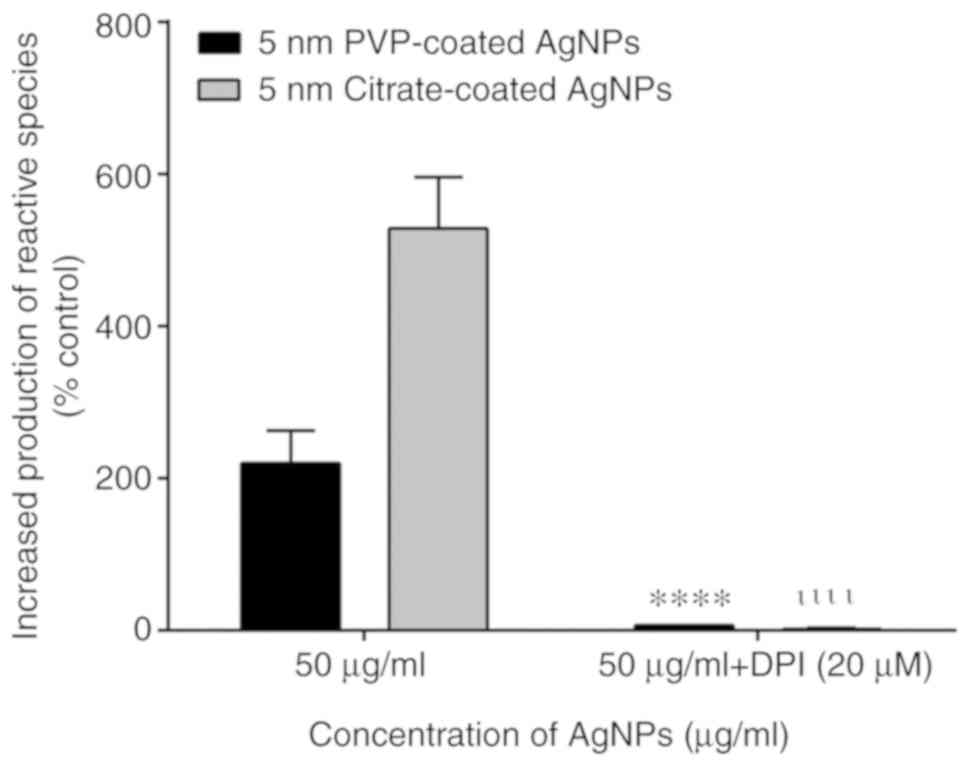
Contribution of NADPH oxidase to the
activation of neutrophil oxidative burst by AgNPs
NADPH oxidase is an enzymatic complex responsible
for initiating the production of reactive species by human
neutrophils. Thus, DPI, an inhibitor of NADPH oxidase, was used to
examine the involvement of this enzymatic complex in the
AgNP-induced neutrophil oxidative burst. The assays were performed
with the 5-nm-sized AgNPs (the nanoparticles that induced the most
pronounced effects in the neutrophil oxidative burst). The use of
DPI resulted in a decrease in both the PVP- and citrate-coated
AgNP-induced neutrophil oxidative burst. The fluorescent signal
decreased to values close to the basal levels, which indicated that
the AgNPs induced the production of reactive species via NADPH
oxidase activation (Fig. 2).
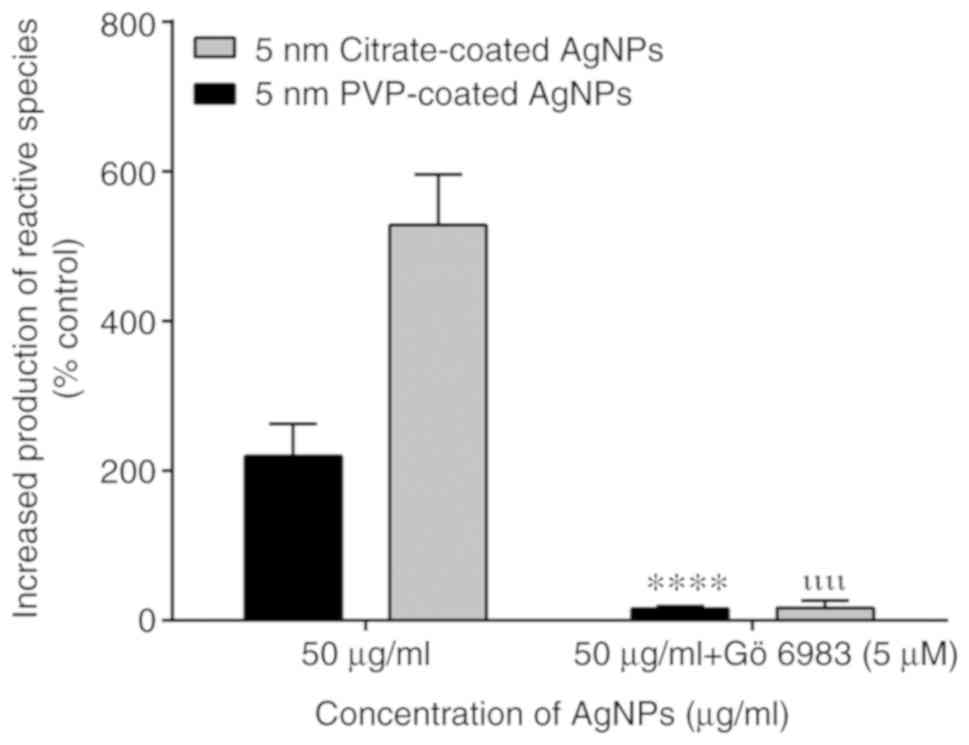
Contribution of PKC to the activation
of neutrophil oxidative burst by AgNPs
To analyze the involvement of PKC in the activation
of NADPH oxidase by AgNPs, a specific inhibitor of PKC, Gö6983, was
used. This inhibitor also prevented the activation of neutrophil
oxidative burst by the 5-nm-sized PVP- and citrate-coated AgNPs,
indicating that the observed effect was dependent on PKC activation
(Fig. 3).
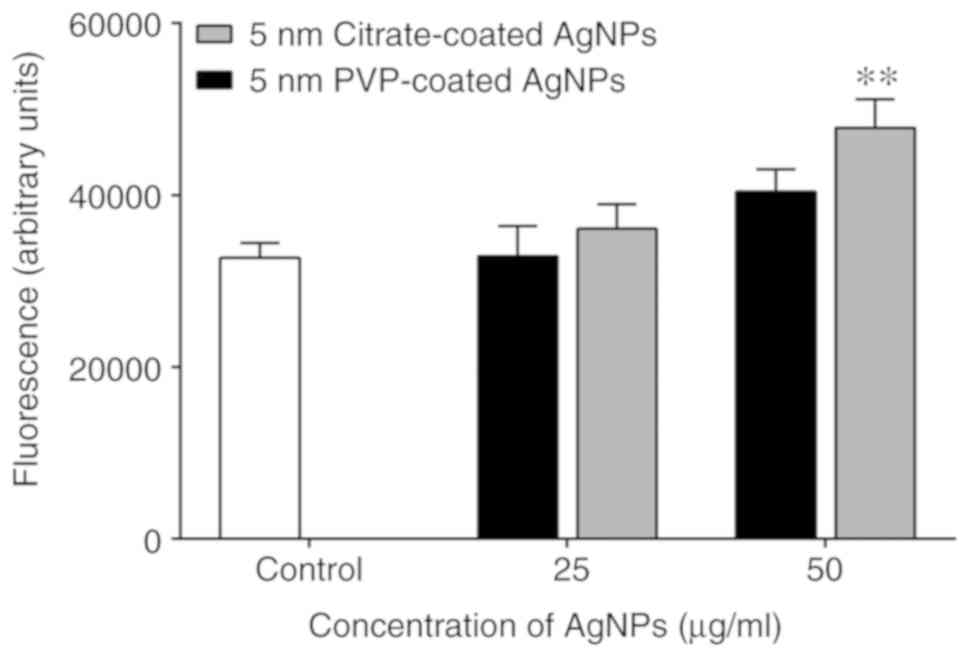
Effect of AgNPs on intracellular free
calcium levels
Calcium is a well-known intracellular second
messenger with proven involvement in a wide variety of biological
processes in human neutrophils. FLUO-4/AM, that belongs to a new
group of fluorescent indicators, was used to measure the free
cytosolic calcium. At the tested conditions, only the 5-nm-sized
citrate-coated AgNPs, at the concentration of 50 µg/ml were able to
induce an increase in the intracellular free calcium levels. The
PVP-coated AgNPs induced a slight increasing trend that did not
achieve statistical significance (Fig.
4).
Involvement of calcium in the
activation of neutrophil oxidative burst by AgNPs
In order to determine the role of calcium in the
production of reactive species induced by AgNPs, the SOCE
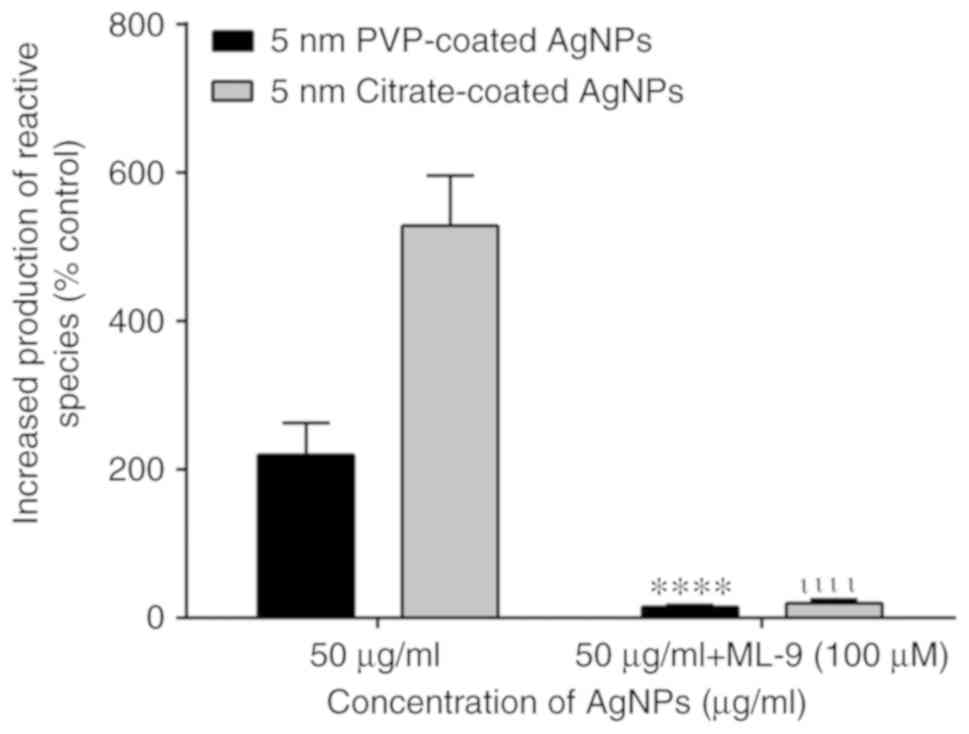
inhibitor, ML-9, was used. As shown in Fig. 5, the use of ML-9 decreased the
human neutrophil oxidative burst induced by 5-nm-sized citrate- and
PVP-coated AgNPs. These results clearly demonstrate the important
role of calcium in the effects of AgNPs.
Assessment of neutrophil apoptosis vs.
necrosis
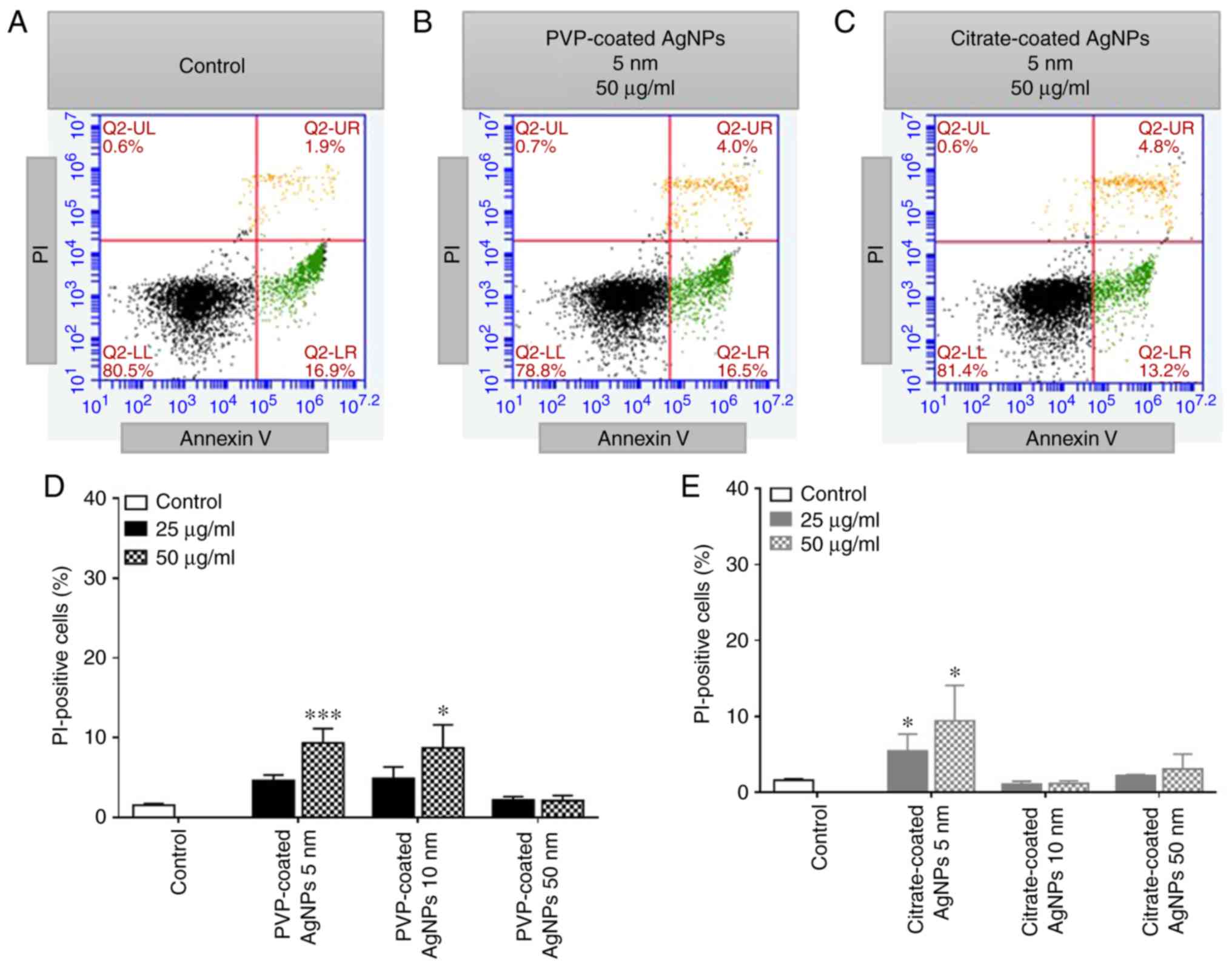
To investigate the ability of AgNPs to induce
apoptosis and/or necrosis, human neutrophils were exposed during 2
h to increasing concentrations of PVP- and citrate-coated AgNPs (5,
10 and 50 nm in size), as well as PVP (15 µg/ml), citrate (0.3
µg/ml) and Ag+ (AgNO3) (12 µg/ml). Under the
tested experimental conditions, the AgNPs did not induce neutrophil
apoptosis, since the number of Annexin V positive cells did not
increase significantly when compared with the control (without
AgNPs). By contrast, the number of PI-positive cells increased in
both types of coated AgNPs tested, as it can be seen in the
representative flow cytometry plots (Fig. 6A-C). The smallest-sized AgNPs of 5
nm, induced the most potent effect, with 25 µg/ml of the 5-nm-sized
of citrate-coated AgNPs being sufficient to induce an increase in
the number of necrotic cells (Fig.
6E). Under the tested experimental conditions, PVP, citrate and
Ag+, per se, did not influence neutrophil
viability (data not shown).
Discussion
Neutrophils are considered one of the first and
primary cell types that process circulating nanoparticles,
mediating the host inflammatory and immunological response, which
involve the production of reactive species. Although neutrophils
play an important role in early stages of inflammation, damage can
occur in affected tissues if the stimulus persists (19). The information available in the
literature concerning the interaction of AgNPs with neutrophils is
controversial, probably due to the differences found in the
experimental conditions, as the cellular model used, the
concentrations used, etc. Moreover, given that the Ag+
release rate is variable and depends on multiple factors
(e.g., AgNPs size and surface area and ambient conditions),
it is expected that the citrate- and PVP-coated AgNPs induce
different responses. To the best of our knowledge, this is the
first study in which PVP- and citrate-coated AgNPs were tested
together against human neutrophil main activities. Silva et
al (20) instilled
Sprague-Dawley rats with AgNPs and concluded that both citrate- and
PVP-coated AgNPs induced inflammation, possibly through the
increase in neutrophils levels. However, that effect was
independent of the coating agent used. By contrast, Seiffert et
al (9)
intratracheally-administered PVP- and citrate-coated AgNPs to
Brown-Norway rats and reported that there was a persistent
neutrophilic inflammation more pronounced when citrate-coated AgNPs
were used. Herein, the effects of various concentrations of AgNPs
(5, 10 and 50 nm), coated with citrate and PVP on human neutrophils
were investigated. The concentrations used in the present study can
be attained in individuals following years of exposure, or
following acute accidental exposure to AgNPs. As a good example,
using exposure data from a AgNPs manufacturing facility, 10 µg/ml
of AgNPs would approximately correspond to the total cellular
deposition following 74 working weeks (8 h per day, 5 days per
week) (21).
The production of reactive species, characteristic
of the neutrophil oxidative burst, is initiated by the generation
of superoxide anion radical (O2•-)
through the activation of a multicomponent enzyme system, NADPH
oxidase. This enzymatic complex is constituted by several subunits:
Cytochrome b558, that is a heterodimer composed by two
transmembrane proteins, gp91phox (phox: Phagocyte oxidase) and
p22phox; and other four proteins, p47phox, p67phox, p40phox and
Rac2, dispersed in cytosol. In neutrophils, NADPH oxidase occurs in
a resting state, a primed state, a fully activated state and in a
hyperactive state. In circulating neutrophils, NADPH oxidase occurs
in a resting state, with the subunits distributed in the membrane
and cytosol, ensuring a dormant inactivated state of the enzyme.
The primed NADPH oxidase is a ‘ready-to-go’ state, which allows the
enzyme to assemble all the subunits, gp91phox being able to use the
cytosolic NADPH as the electron donor to reduce oxygen and produce
O2•-. The production of the first
reactive specie indicate that the enzyme is fully activated
(22,23). Subsequently, other reactive species
are produced, in a cascade form, as H2O2,
which is concomitantly used, together with chlorine ions, by
myeloperoxidase (MPO), a heme protein present in azurophil granules
of neutrophils, to form hypochlorous acid (HOCl) (24). The information available in the
literature about the effects of AgNPs on the human neutrophil
oxidative burst is limited. For example, Poirier et al
(14) described that
citrate-coated AgNPs of 20 or 70 nm in size did not induce ROS
production following exposure for 1 h. In turn, the authors have
previously described that 10 nm of PVP-coated AgNPs induced
neutrophil oxidative burst, an effect not achieved by 50 nm AgNPs
(15). In another study involving
neutrophils, it was reported that 20 nm AgNPs were more
pro-inflammatory than 110 nm AgNPs in terms of neutrophil influx to
the lungs, but not in terms of eosinophilic influx (9). These results suggest that the
smallest-sized AgNPs induce a greater effect on human neutrophils.
To investigate this hypothesis, the present study tested 3 sizes of
AgNPs, 5, 10 and 50 nM, coated with PVP or citrate. For this
purpose, DHR was used as a fluorescent probe. The results presented
herein are in line with those previously described. The
smallest-sized AgNPs induced a greater production of reactive
species, irrespective of the coating agent used. It was previously
demonstrated by the authors, through TEM images, that 10 nm of
PVP-coated AgNPs were internalized in human neutrophils, and were
located inside the cell, throughout the cytosol and also more
intrinsically in phagosomes. AgNPs sized 50 nm were not able to
enter cells, remaining outside the cells, close to the cellular
membrane (15). In addition,
Poirier et al (14) failed
to detect the presence of citrate-coated AgNPs sized 70 nm inside
human neutrophils. This difference in cellular localization could
explain the size-dependent effect on human neutrophil activities.
The so-called ‘Trojan-horse’ mechanism, in which nanoparticles are
internalized within cells and then release high levels of toxic
ions, has been proposed for AgNPs (25). In that sense, the present study
examines the effect of the ion Ag+, through the analysis
of AgNO3, and it was concluded that, under these
experimental conditions, Ag+, per se, did not
induce the neutrophil oxidative burst. This suggests that
Ag+ may not be the main factor contributing for the
AgNP-induced activation of human neutrophils.
It is important to note that AgNPs coated with
citrate triggered the most intensive response by neutrophils. PVP
and citrate are frequently used coatings, essentially due to their
low toxicity. PVP protects AgNPs from agglomerating by stabilizing
the Ag+ and H+ in the suspension. The
coordination of Ag+ on the particle surface, with the N
or O atoms of PVP, leads to the formation of a surface layer that
inhibits agglomeration due to steric hindrance (26). By contrast, citrate is used in the
form of citrate anions, which serve as a reducing agent, as well as
to provide electrostatic repulsion, stabilizing the particles
suspension (27,28). To exclude any effect of the coating
agent, PVP and citrate were tested per se, in the maximum
proportion predictably existing in the AgNPs and no effect was
detected on the neutrophil oxidative burst. As such, the effect
seems to be due to the complex AgNPs coated with the capping
agent.
It is currently accepted that PKC is implicated in
the neutrophil oxidative burst, through the phosphorylation of
p47phox, resulting in the assembly and activation of
phagocytic NADPH oxidase (29).
Neutrophils contain 5 of the 11 known isoforms of PKC. These
comprise 3 conventional isoforms designated α‚ βI and βII, which
are dependent on phosphatidylserine (PS), diacylglycerol (DAG) and
calcium. There is also one novel isoform designated PKCd, which
also depends on phosphatidylserine and DAG, but is
calcium-independent; and one atypical isoform designated PKCz which
is DAG and calcium-independent but can be activated by
phosphatidylserine, phosphatidic acid, or phosphatidylinositol. The
expression of other isoforms, namely PKCz, PKCi/l, and PKCq,
remains a matter of debate (30).
In the present study, a broad-spectrum PKC inhibitor was used and a
decrease in the neutrophil oxidative burst, induced by the citrate-
and PVP-coated AgNPs was observed. Despite the possible role of
other kinases in the neutrophil activation, the obtained results
indicate that the AgNPs induced the production of reactive species
through the activation of PKC.
It has been proposed that calcium and DAG, both
downstream products of phospholipase C (PLC), activate PKC, which
in turn, completes a negative feedback loop by inhibiting PLC.
Moreover, the classical PKC are distinguished from all other PKC as
they require elevated calcium levels for maximal activity (31). In that sense, it was the authors'
intention to determine whether AgNPs alter the intracellular
calcium levels in human neutrophils. The results demonstrated that
the citrate-coated AgNPs induced a significant increase in the
intracellular calcium levels, which could explain the greater
production of reactive species by these nanoparticles. By contrast,
the PVP-coated AgNPs induced only a slight increase which was not
statistically significant. The calcium movements in neutrophils can
occur through a rapid emptying of the calcium stores, as
endoplasmic reticulum, and then some intermediate mechanism
translates this information to plasma membrane channels that enable
calcium to entry and refill the depleted stores, in a process
generally known as SOCE (17).
Therefore, to determine the involvement of calcium in
citrate-coated AgNP-induced neutrophil oxidative burst, ML-9, a
SOCE broadly investigated inhibitor, was used and the production of
reactive species was detected with DHR. The fluorescent signal
decreased when ML-9 was used, revealing an important role of
calcium in neutrophil activation by citrate-coated AgNPs.
The next step was to determine whether all the
above-mentioned events triggered by AgNPs and mostly by the
citrate-coated AgNPs, interfere with neutrophil viability, focusing
on apoptosis vs. necrosis. Apoptosis was detected by measuring the
externalization of phosphatidylserine on the plasma membrane using
fluorescent-tagged Annexin V. Under the tested experimental
conditions, the number of Annexin V positive cells of treated or
untreated cells did not differ significantly, suggesting that AgNPs
did not induce neutrophil apoptosis.
PI, a popular red-fluorescent nuclear and chromosome
counterstain was also used to analyze the effect of AgNPs on
neutrophil viability. The necrotic effect was confirmed and the
citrate-coated AgNPs induced higher levels of necrosis, since the
smallest-sized AgNPs (5 nm) induced necrosis at lower
concentrations, than the PVP-coated AgNPs. Flow cytometry provides
information about the size and granularity of the cells, even
without any specific marker. It was also observed that the
citrate-coated AgNPs altered the morphology of the cells (data not
shown) to a greater extent than the PVP-coated AgNPs. The reported
cytotoxicity of AgNPs also varies among authors. Nguyen et
al (32) examined the
cytotoxic effects of uncoated, PVP- and citrate-coated AgNPs (10,
50 and 75 nm) on J774A.1 macrophages and HT29 epithelial cells.
They concluded that the uncoated AgNPs were more cytotoxic than the
coated AgNPs. Moreover, the PVP-coated AgNPs resulted in a greater
loss of cell viability compared to the citrate-coated AgNPs,
indicating a surface coating-dependent toxicity. By contrast, Wang
et al (28) reported that
PVP and citrate differentially affected the cytotoxicity of AgNPs
on BEAS-2B cells, as demonstrated by the finding that PVP-coated
AgNPs induced lower cytotoxicity, as was also found in the present
study. The authors proposed that the formation of N-Ag+
or O-Ag+ complexes by PVP results in reduced
Ag+ bioavailability. In addition, the authors of that
study also stated that citrate lacks potent coordinating effects,
and cannot provide protection against the cytotoxic effects of
Ag+ (28). This could
explain the higher rates of cytotoxicity of citrate-coated
AgNPs.
In conclusion, though the widespread use of AgNPs
may indicate a good safety record, special attention is required to
the extended exposure to these nanoparticles, considering its
pro-inflammatory potential, which seems to increase as
nanoparticles size decrease, specifically when citrate coating is
used, as shown in the present study. It was demonstrated that AgNPs
induced an increase in intracellular calcium levels, which are
involved in the activation of PKC, resulting in the assembly of
NADPH oxidase subunits with the subsequent production of reactive
species. This excessive production of reactive species most
probably contributes to the AgNP-induced decrease in neutrophil
viability. The findings of the present study provide new insight
into the interaction of AgNPs with human neutrophils, which is
essential for the safer use of these nanoparticles.
Acknowledgements
The authors gratefully acknowledge Dr Margarida Amil
and the nursing staff of the Centro Hospitalar do Porto-Hospital de
Santo António blood bank for the collaboration in the recruitment
of blood donors to participate in the study.
Funding
The present study was supported by
UID/QUI/50006/2020 with funding from FCT/MCTES through national
funds, and ‘Programa Operacional Competitividade e
Internacionalização’ (COMPETE) (POCI-01-0145-FEDER-029248). DR and
MF acknowledge the financial support from the European Union [FEDER
funds through the Operational Competitiveness Program (COMPETE)]
(POCI-01-0145-FEDER-029253 and POCI-01-0145-FEDER-029248,
respectively).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
MF, FC and EF were involved in the conception and
design of the study, and in the drafting of the manuscript. ML, AS,
TS and DR performed all the experimental procedures, and
contributed to the writing of the manuscript. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
All patient-related procedures and protocols were
performed in accordance with the Declaration of Helsinki and
approved by the Ethics Committee of Centro Hospitalar do Porto.
Written informed consent was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Beyene HD, Werkneh AA, Bezabh HK and
Ambaye TG: Synthesis paradigm and applications of silver
nanoparticles (AgNPs), a review. Sustain Mater Technol. 13:18–23.
2017.
|
|
2
|
Vance ME, Kuiken T, Vejerano EP, McGinnis
SP, Hochella MF Jr, Rejeski D and Hull MS: Nanotechnology in the
real world: Redeveloping the nanomaterial consumer products
inventory. Beilstein J Nanotechnol. 6:1769–1780. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Williams KM, Gokulan K, Cerniglia CE and
Khare S: Size and dose dependent effects of silver nanoparticle
exposure on intestinal permeability in an in vitro model of the
human gut epithelium. J Nanobiotechnol. 14(62)2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bouwmeester H, Dekkers S, Noordam MY,
Hagens WI, Bulder AS, de Heer C, ten Voorde SE, Wijnhoven SW,
Marvin HJ and Sips AJ: Review of health safety aspects of
nanotechnologies in food production. Regul Toxicol Pharmacol.
53:52–62. 2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Luo YH, Chang LW and Lin P: Metal-based
nanoparticles and the immune system: Activation, inflammation, and
potential applications. Biomed Res Int. 2015(143720)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Freitas M, Lima JL and Fernandes E:
Optical probes for detection and quantification of neutrophils'
oxidative burst. A review. Anal Chim Acta. 649:8–23.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ribeiro D, Freitas M, Lima JL and
Fernandes E: Proinflammatory pathways: The modulation by
flavonoids. Med Chem Rev. 35:877–936. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Recordati C, De Maglie M, Bianchessi S,
Argentiere S, Cella C, Mattiello S, Cubadda F, Aureli F, D'Amato M,
Raggi A, et al: Tissue distribution and acute toxicity of silver
after single intravenous administration in mice: Nano-specific and
size-dependent effects. Part Fibre Toxicol. 13(12)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Seiffert J, Hussain F, Wiegman C, Li F,
Bey L, Baker W, Porter A, Ryan MP, Chang Y, Gow A, et al: Pulmonary
toxicity of instilled silver nanoparticles: Influence of size,
coating and rat strain. PLoS One. 10(e0119726)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Park EJ, Bae E, Yi J, Kim Y, Choi K, Lee
SH, Yoon J, Lee BC and Park K: Repeated-dose toxicity and
inflammatory responses in mice by oral administration of silver
nanoparticles. Environ Toxicol Pharmacol. 30:162–168.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Park EJ, Choi K and Park K: Induction of
inflammatory responses and gene expression by intratracheal
instillation of silver nanoparticles in mice. Arch Pharm Res.
34:299–307. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Alessandrini F, Vennemann A, Gschwendtner
S, Neumann AU, Rothballer M, Seher T, Wimmer M, Kublik S,
Traidl-Hoffmann C, Schloter M, et al: Pro-inflammatory versus
immunomodulatory effects of silver nanoparticles in the lung: The
critical role of dose, size and surface modification. Nanomaterials
(Basel). 7(pii: E300)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Poirier M, Simard JC, Antoine F and Girard
D: Interaction between silver nanoparticles of 20 nm (AgNP20) and
human neutrophils: Induction of apoptosis and inhibition of de novo
protein synthesis by AgNP20 aggregates. J Appl Toxicol. 34:404–412.
2014.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Poirier M, Simard JC and Girard D: Silver
nanoparticles of 70 nm and 20 nm affect differently the biology of
human neutrophils. J Immunotoxicol. 13:375–385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Soares T, Ribeiro D, Proenca C, Chisté RC,
Fernandes E and Freitas M: Size-dependent cytotoxicity of silver
nanoparticles in human neutrophils assessed by multiple analytical
approaches. Life Sci. 145:247–254. 2016.
|
|
16
|
Freitas M, Porto G, Lima JL and Fernandes
E: Isolation and activation of human neutrophils in vitro. The
importance of the anticoagulant used during blood collection. Clin
Biochem. 41:570–575. 2008.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ribeiro D, Freitas M, Rocha S, Lima J,
Carvalho F and Fernandes E: Calcium pathways in human
neutrophils-the extended effects of thapsigargin and ML-9. Cells.
7(pii: E204)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Freitas M, Costa VM, Ribeiro D, Couto D,
Porto G, Carvalho F and Fernandes E: Acetaminophen prevents
oxidative burst and delays apoptosis in human neutrophils. Toxicol
Lett. 219:170–177. 2013.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ng LG, Ostuni R and Hidalgo A:
Heterogeneity of neutrophils. Nat Rev Immunol. 19:255–265.
2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Silva RM, Anderson DS, Franzi LM, Peake
JL, Edwards PC, Van Winkle LS and Pinkerton KE: Pulmonary effects
of silver nanoparticle size, coating, and dose over time upon
intratracheal instillation. Toxicol Sci. 144:151–162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gliga AR, Skoglund S, Wallinder IO, Fadeel
B and Karlsson HL: Size-dependent cytotoxicity of silver
nanoparticles in human lung cells: The role of cellular uptake,
agglomeration and Ag release. Part Fibre Toxicol.
11(11)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Belambri SA, Rolas L, Raad H,
Hurtado-Nedelec M, Dang PM and El-Benna J: NADPH oxidase activation
in neutrophils: Role of the phosphorylation of its subunits. Eur J
Clin Invest. 48 (Suppl 2)(e12951)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Babior BM: The leukocyte NADPH oxidase.
Isr Med Assoc J. 4:1023–1024. 2002.PubMed/NCBI
|
|
24
|
Manda-Handzlik A and Demkow U:
Neutrophils: The role of oxidative and nitrosative stress in health
and disease. Adv Exp Med Biol. 857:51–60. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hsiao IL, Hsieh YK, Wang CF, Chen IC and
Huang YJ: Trojan-horse mechanism in the cellular uptake of silver
nanoparticles verified by direct intra- and extracellular silver
speciation analysis. Environ Sci Technol. 49:3813–3821.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang H, Qiao X, Chen J, Wang X and Ding S:
Mechanisms of PVP in the preparation of silver nanoparticles. Mater
Chem Phys. 94:449–453. 2005.
|
|
27
|
Henglein A and Giersig M: Formation of
colloidal silver nanoparticles: Capping action of citrate. J Phys
Chem B. 103:9533–9539. 1999.
|
|
28
|
Wang X, Ji Z, Chang CH, Zhang H, Wang M,
Liao YP, Lin S, Meng H, Li R, Sun B, et al: Use of coated silver
nanoparticles to understand the relationship of particle
dissolution and bioavailability to cell and lung toxicological
potential. Small. 10:385–398. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Cosentino-Gomes D, Rocco-Machado N and
Meyer-Fernandes JR: Cell signaling through protein kinase C
oxidation and activation. Int J Mol Sci. 13:10697–10721.
2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bertram A and Ley K: Protein kinase C
isoforms in neutrophil adhesion and activation. Arch Immunol Ther
Exp (Warsz). 59:79–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tintinger GR, Theron AJ, Steel HC,
Cockeran R, Pretorius L and Anderson R: Protein kinase C promotes
restoration of calcium homeostasis to platelet activating
factor-stimulated human neutrophils by inhibition of phospholipase.
C. J Inflamm (Lond). 6(29)2009.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nguyen KC, Seligy VL, Massarsky A, Moon
TW, Rippstein P, Tan J and Tayabali AF: Comparison of toxicity of
uncoated and coated silver nanoparticles. J Phys Conf Ser.
429(012025)2013.
|