1. Introduction
Cancer is a complex, multifactorial disease that is
characterized by uncontrolled cellular proliferation, leading to
tumor development. It develops as a result of genetic and
epigenetic alterations in the genome caused by errors in the
replication of DNA or interactions with exogenous agents, such as
radiation and chemical carcinogens. The accumulation of such
tumor-promoting modulations in the DNA of cells endows them with
the ability to evade programmed cell death, a process known as
apoptosis, which is a crucial homeostatic mechanism that regulates
cell turnover and maintains cell populations in tissues. The types
of genes involved in the development of carcinogenesis include
tumor suppressor genes (e.g., p53 and Rb), oncogenes (e.g., myc and
Ras) and DNA repair genes (P53 and BRCA1) (1). Cancer is being extensively researched
as it is a leading cause of mortality worldwide. It accounted for
an estimated 9.6 million deaths only in 2018(2). Studies that have examined the risk
factors associated with the incidence of cancer have found that a
high body mass index (BMI, a sedentary lifestyle, smoking, alcohol
consumption and a low consumption of plant-based foods, are top
risk factors for cancer development, among others (2-5).
The disease is conventionally treated using surgery, radiation
therapy and chemotherapy (or a combination of these) depending on
the cancer type, its stage and its location. A great challenge in
the pursuit for effective cancer treatment is the genotypic and
phenotypic heterogeneity of tumor cells. Thus far, the cytotoxic
agents that have been used in chemotherapy act rapidly, dividing
abnormal cells by inducing cell cycle disruption or mitotic
division failure, effectively killing tumor cells, while also
killing normal cells with a high rate of turnover; this results in
adverse side-effects, which include depression of the bone marrow,
aplastic anemia, diarrhea, vomiting, alopecia etc. (6). To this effect, the greatest
limitations of chemotherapy are its non-selective cytotoxic
effects, its lack of efficiency and the associated financial cost.
Therefore, the need for the development of agents, not only with a
high efficacy and specificity, but also with a low toxicity is
crucial.
2. Chemoprevention
With an increase in cancer morbidity and mortality,
the advancement of chemopreventive agents is a key component to the
holistic approach in the prevention and treatment of cancer
accompanied by fewer adverse side-effects. ‘Chemoprevention refers
to the use of specific agents (natural or synthetic) to reverse,
suppress, or prevent the process of carcinogenesis’ (7). These agents target molecules involved
in the initiation of apoptosis, such as pro-apoptotic and
anti-apoptotic proteins (8).
Chemopreventive agents that have exhibited anticancer potential
include non-steroidal anti-inflammatory drugs (e.g., sulindac,
aspirin and celecoxib) (9), and
other FDA-approved drugs, such as Tamoxifen, Raloxifene and HPV
vaccines (Cervarix and Gardasil). However, Tamoxifen and Raloxifene
have shown adverse side effects in patients (10). Therefore, there is an urgent need
for the development of therapeutic agents using compounds that are
found naturally, such as phytochemicals in vegetables and fruits,
which are lower in toxicity and exhibit little to no adverse
side-effects as a result of their use in the prevention and
treatment of cancer (11).
According to recent studies, a diet rich in fruits and vegetables
has been inversely associated with the development of a number of
lifestyle-related diseases, such as diabetes and cancer (12-14).
However, Petrick et al and the Fukuoka colorectal cancer
study in Japan reported no association between the intake of
flavonoids and cancer (15,16).
Nevertheless, a plethora of studies mention that polyphenols and
isothiocyanates have exhibited effectiveness in the prevention of
and in reducing the risk of cancer (11-14).
3. Role of diet in the prevention of
carcinogenesis
Several dietary agents with potential for cancer
prevention have been identified and these include polyphenols,
isothiocyanates, selenium and allyl compounds (13). These compounds function by
controlling biological processes, such as cell proliferation, the
induction of programmed cell death, the cell cycle, DNA repair and
oxidative stress, affecting cancer pathways in various stages of
carcinogenesis (11). The
chemosensitivity of natural compounds favors their use as an
adjuvant therapy in conventional treatment; however, they can also
be utilized exclusively based on their different mechanisms of
action against tumor cells (14).
Phenolic compounds are secondary metabolites found in plants, that
have been extensively studied for their use as treatment and
preventive agents. Research highlights the potential of polyphenols
to interfere with multiple phases of carcinogenesis. Flavonoids
(polyphenolic compounds) are classified into 5 main subclasses as
follows: Flavones, flavanols, flavanonols, flavan-3-ols and
flavanones. In the present review, the anticarcinogenic properties
of flavones are discussed. Notably, in vitro and in
vivo studies on dietary flavones have demonstrated their
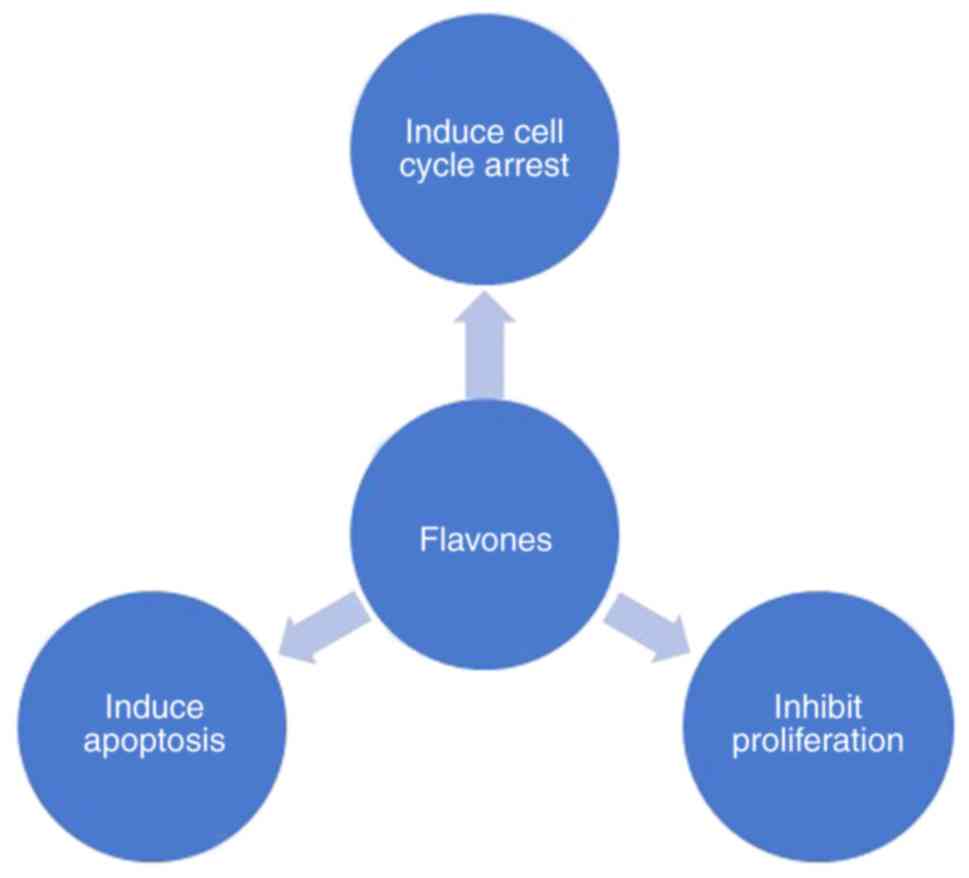
ability to modulate cellular processes, such as cell proliferation,
cell cycle arrest and apoptosis (Fig.
1) in various types of cancer (17).
4. Flavones and apoptosis
Apoptosis is a crucial process resulting in the
removal of undesirable cells within physiological conditions. The
process is molecularly characterized by energy-reliant cascade
events and morphologically characterized by DNA fragmentation and
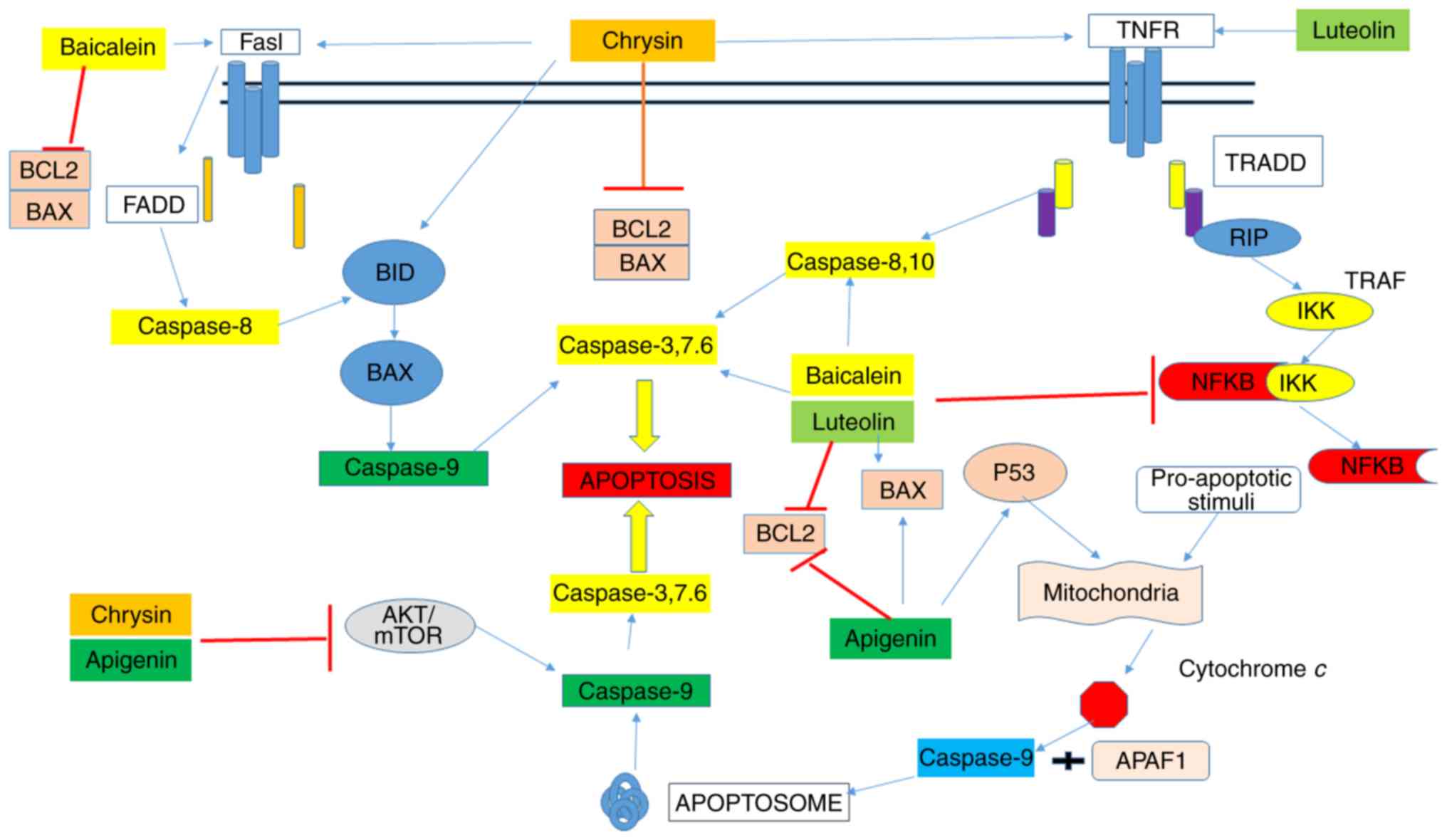
apoptotic body formation. Apoptosis occurs either via an intrinsic
pathway, where the process is activated from signals within the
cell, or through an extrinsic pathway where the process is
stimulated by death signals received from outside the cell, which
are then processed within the cell (18,19)
(Fig. 2). A critical
characteristic that distinguishes cancer cells from normal cells is
their ability to avoid apoptosis. The dysregulation of apoptotic
pathways plays a leading role in the onset of carcinogenesis.
Therefore, a popular strategy in alternative cancer treatment is
the utilization of dietary agents that trigger the apoptosis of
cancer cells by modulating apoptotic pathways (18).
Flavones are a subclass of flavonoids which are
polyphenolic compounds present in various fruits and vegetables,
are known to have antiviral, antioxidant and anticancer activities.
Main members of flavones include tangeretin, apigenin, chrysin,
nobiletin luteolin and baicalein (13,14,17).
Flavones have been broadly studied as anticancer agents and are
known to inhibit tumor development in cancer cells by inducing
apoptosis. Scientific reports have demonstrated that flavones
activate the apoptotic pathway in cancer cells through different
mechanisms (14,17,18).
The flavones, apigenin, luteolin, baicalein and chrysin, have
specifically exhibited shown proteasome-inhibitory effects
characterized by decrease in activity the induction of the
apoptosis of various cancer cell (17-22).
Moreover, flavones have a high safety profile, with no adverse
side-effects (19). Studies have
elucidated certain biochemical mechanisms through which flavones
exert their anticancer effects; these include the following: i)
Apoptosis induction (18,23,24);
ii) cell cycle arrest at the G1 or G2/M phase
(17,23,24);
iii) inhibition of enzymes involved in metabolism [namely,
cytochromes P450 (CYPs)] (18,26);
iv) inhibition of reactive oxygen species (ROS) formation by
activating phase II metabolizing enzymes (24,25,27);
and v) inhibition of vascular endothelial growth factor (VEGF) and
basic fibroblast growth factor (bFGF)-mediated angiogenesis
(28,29).
In the present review, the anticancer potential of 4
prevalent flavones, namely chrysin, luteolin, baicalein and
apigenin, as well as their molecular mechanisms of action that
entail their anticancer potential are discussed.
Chrysin
Chrysin, a naturally occurring flavone, is found in
honey, Passiflora caerulea (blue passion flower),
Oroxylum indicum and bee propolis (30,31).
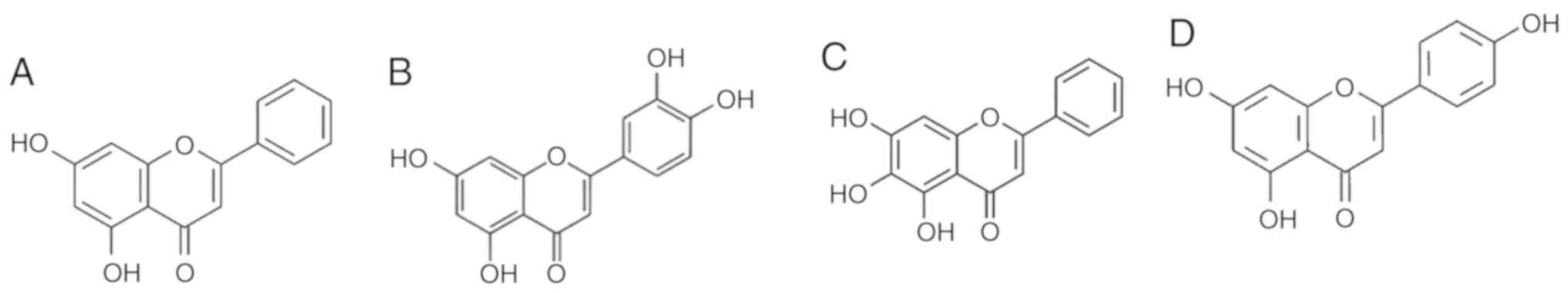
It is specifically a dihydroxyflavone in which the two hydroxy
groups are located at positions 5 and 7 (Fig. 3A) and has been investigated for its
anti-allergic, anti-inflammatory, antibacterial, antihypertensive,
antioxidant and antitumor properties (13,30,31).
Chrysin has been evaluated as a potent anticancer
agent and has exhibited tremendous potential against various cancer
cell lines, including, breast, bladder, cervical and colorectal
cancer. Chrysin has been shown to induce G2/M arrest in SW480
colorectal cancer cells, the inhibition of cyclooxygenase (COX-2)
via nuclear factor (NF) interleukin (IL)-6 (NF-IL-6), and the
fragmentation and apoptosis of CaCo2 cells (17).
Chrysin induces cytotoxicity, induces apoptosis and
inhibits the migration of various cell lines (30,31).
The cytotoxic effects of chrysin and the pathways associated with
these effects have been elucidated. Certain studies have reported
that chrysin induces apoptosis via the intrinsic pathway
specifically (30-32),
while others have reported that chrysin induces apoptosis via both
the intrinsic pathway and the extrinsic pathway (13,32,33).
A study on human melanoma cells found that chrysin was able to
reduce the viability of the cells in a dose-dependent manner with
an IC50 value of 35.8 and 28.3 µM in (human uveal melanoma cells)
M17 and SP6.5 cell lines, respectively. Further molecular
investigation revealed that mitochondrial permeability and
cytochrome c levels in the cytosol had increased, and the
expression of caspase -3 and -9 was upregulated by chrysin
treatment, while the expression of caspase-8 remained unaltered
(30). Concurrently, treatment
with chrysin has been shown to activate p53/caspase-9 in
hepatocellular carcinoma (HCC); the anti-apoptotic [B-cell lymphoma
2 (Bcl-2)] proteins, including B-cell lymphoma-extra large (Bcl-xL)
and Bcl-2 exhibited a decreased expression, while pro-apoptotic
proteins exhibited an increased expression; this suggested that
chrysin induced apoptosis via th e mitochondrial pathway that was
associated with the upregulation of caspase-3, BID and
Bcl-2-associated X protein (BAX), and the downregulation of
Bcl-2(34). However, chrysin has
been shown to induce the TNF-mediated apoptosis of colorectal
cancer, and both the TNF mediated and mitochondria-mediated
apoptosis of colon cancer cells (13,32,33).
Similarly, another study reported that there was no upregulation of
caspase-8, while there was an upregulation of caspase-9 and -3, as
a result of treatment of bladder cancer cells with chrysin
(31). Several other studies have
also shown that chrysin treatment induces the apoptosis of human
colorectal and HCC cells (13,33,35).
These studies have confirmed that chrysin induces the apoptosis of
various cell lines by modulating different pathways.
Moreover, it has been reported by a number of
studies that the cytotoxic effects of chrysin are mediated via the
generation of ROS (24,31). A previous study demonstrated the
inhibition of the proliferation of ovarian cancer cells and the
induction of cell death by an increase in the concentration of
cytoplasmic Ca2+ and ROS levels, as well the loss of
mitochondrial membrane potential induced by chrysin treatment
(24). Chrysin treatment resulted
in the inhibition of AKT/mTOR pathway in triple negative breast
cancer and also led to the activation of the tumor necrosis factor
(TNF) pathway in multiple cell lines, including lung (A549), rectal
(SW837) and colorectal (DLD1 and HCT116) cancer cells by the
activation of TNFα and TNFβ gene expression levels. In HCT116
cells, chrysin induced apoptosis via the mitochondrial pathway;
also in A549 human lung adenocarcinoma and HeLa human cancer cell
lines chrysin led to tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis (13,32,33).
A number of studies have also investigated the
anticancer potential of Chrysin in vivo using tumor growth
assays/tumor xenografts. As previously reported, the
immunohistochemical analysis of tumor tissue exhibited a decreased
expression of hexokinase-2 (HK-2) (overexpressed in several types
of cancer) following treatment with chrysin, which demonstrated the
effect of chrysin on HK-2 in vivo; the reduction of HK-2 in
tumor tissue resulted in glycolysis suppression and thus, the
supply of energy to maintain tumor growth was blocked, and the
tumor cell proliferative ability was weakened (35). A similar study using (human primary
glioblastoma cell) U87 xenografts found that 3 weeks of chrysin
treatment led to a reduction of tumor weight in mice compared to
that of mice delivered treated with refined olive oil as a control;
western blot analysis of the tumors revealed that treatment with
chrysin blocked tumor xenograft growth via the downregulation of
the extracellular signal-regulated kinase (ERK)/nuclear factor
E2-related factor 2 (Nrf2) signaling pathway (36). Another study found that chrysin
activated Notch1 signaling in an (anaplastic thyroid cancer) ATC
xenograft model. The chrysin-treated samples exhibited a moderate
to strong expression of Notch1 intracellular domain (NICD),
indicating that the apoptosis induced by chrysin was associated
with Notch1 activation (37).
Luteolin
Luteolin (3,4,5,7-tetrahydroxy flavone) (Fig. 3B), is a phytochemical ubiquitously
found in dietary sources, such as green peppers, olive oil, celery,
chamomile tea, peppermint, oregano and broccoli (38,39).
Luteolin has been shown to exhibit anti-inflammatory, anti-oxidant
and potent cytotoxic activity against various cancer cell lines. It
has also been shown to lead to G2 cell cycle arrest and the
apoptosis of A549 lung cancer cells via the mitochondrial pathway,
whereas it causes either G0/G1 arrest in SH-SY5Y neuroblastoma
tumor cells with the loss of mitochondrial potential or G1/S arrest
with an increase in the ratio of BAX/Bcl-2 in SMMC-7721 and
BEL-7402 (human hepatoma) cells (13,38).
Luteolin has been shown to induce apoptosis by
modulating key molecules in both the intrinsic and extrinsic
pathways in numerous cancer cell lines, including HT-29, COLO-320
DM, HCT-15, SW480, CaCo-2, HeLa, EC1, KYSE450 and CAOV3/DDP cells
(17,39-41).
Studies have revealed that luteolin exerts its effects by
upregulating the expression of caspase-3/-7/-8, death receptors,
TRAIL and Fas/FasL. It also causes inequity in the Bax/Bcl-xL ratio
via the inhibition of E6/E7 oncoproteins (17,40,41).
Luteolin induces apoptosis by reducing Bcl-2 expression (39) and increasing caspase-3 and
caspase-8 expression (42). It
also inhibits NF-κB and activates TNF-α-induced apoptosis. Another
elucidated mechanism of action is that luteolin promotes the
generation of ROS, leading to TNF-α-induced apoptosis. ROS
generated as a consequence of luteolin treatment have also been
shown to activate AMP-activated protein kinase (AMPK), a regulator
of NF-κB, which may initiate the cytotoxic effect of luteolin.
Furthermore, luteolin induces cytotoxicity to human non-small-cell
lung cancer (A549 cells) via the phosphorylation of JNK, and
induces apoptosis through the intrinsic pathway, while
simultaneously inhibiting NF-κB (42). Another mechanism of apoptosis
induction by luteolin is the accumulation and stabilization of p53;
luteolin has been shown to enhance p53 expression in two human
colon carcinoma cell lines, CO115 and HCT15(38).
In addition to this, in vivo studies have
found that luteolin reduces tumor weight and volume in mice.
Furthermore, Notch1, β-catenin and Ki-67 expression have been shown
to be reduced in tumors from luteolin-treated mice. These results
suggest the possibility that luteolin can suppress the progression
of gastric cancer via the inhibition of Notch1 expression (43). Another study using human non-small
cell lung carcinoma (NCI-H1975) xenograft tumors demonstrated a
significant tumor growth inhibition upon the administration of
luteolin to mice. Concomitantly, there was no decrease in body
weight and no signs of toxicity were manifested following luteolin
administration. Immunohistochemical detection displayed that
luteolin treatment caused a progressive and significant decrease in
the expression of proliferating cell nuclear antigen (PCNA; a cell
proliferation marker), CD34 (a microvessel density marker) and
epidermal growth factor (EGF) receptors (44).
Baicalein
Baicalein (5,6,7-trihydroxyflavone) (Fig. 3C) is a flavone that was isolated
originally from the roots of Scutellaria lateriflora and
Scutellaria baicalensis. Baicalein can also be found in
thyme and Oroxylum indicum and it has demonstrated to
exhibit antioxidant, anti-viral, anti-inflammatory and anticancer
properties (45). It has also been
shown to exert anticancer effects on HCT116 human colon cancer
cells by inhibiting inflammation and inducing apoptosis via the
extrinsic pathway and the inactivation of the AKT/PI3K pathway; in
HT29 cells, it has been shown to induce cell cycle arrest in the G1
phase, increase the ratio of BAX/Bcl-2 and to inactivated the
AKT/PI3K pathway (17). Its
anticancer properties have been attributed to its potent inhibition
of several cyclins or cyclin-dependent kinases (CDKs) to regulate
the cell cycle, and the downregulation of AKT/mammalian target of
rapamycin (mTOR) and MAPK pathways. Both in vitro and in
vivo studies have demonstrated that baicalein induces the
apoptosis in HT29 colon cancer via the activation of BAX and the
downregulation of Bcl-2, and the depolarization of mitochondria; it
also inhibits migration via the inhibition of matrix
metalloproteinase (MMP)2 and MMP9. The induction of apoptosis and
the inhibition of metastasis are mediated via the AKT pathway
(17,45). A number of studies have reported
the potential of baicalein to induce the apoptosis of various
cancer cells, such as HCC (HCCj5), cervical cancer (HeLa) and
non-small lung cancer cells; although underlying mechanisms are
different between cell lines, overall, apoptosis occurs via the
upregulation of the expression of pro-apoptotic proteins, such as
cytochrome c and Bax, and the downregulation of the
expression of anti-apoptotic proteins, such as Bcl-2. This
increases the Bax/Bcl-2 ratio and triggers the intrinsic apoptotic
pathway through the mitochondria, leading to executioner caspase
(caspase-3/-9) activation and the cleavage of poly(ADP-ribose)
polymerase (PARP) (17,44-46).
Baicalein induces apoptosis by the activation of
caspase-9/-3 in breast cancer cells (MDA-MB-231), cervical cancer
cells (HeLa and U14) and bladder cancer cells (T24); baicalein
induces cell death by upregulating the expression of Bax, caspases
and FasL, and downregulating the expression of Bcl-2 (47-49).
Similar results were found in HT29colon cancer cells and SGC-7901
gastric cells. In addition, DNA fragmentation was reported in these
cell lines. In vivo studies on HT29 colon tumor xenografts
verified similar results, while in HCT116 cells, the activation of
caspases was observed (45).
Another study on MDA-MB-231 cells stated that baicalein
considerably diminished the expression of special AT-rich
sequence-binding protein-1 (SATB1), SNAIL and vimentin, Wnt1 and
β-catenin proteins, while it augmented the expression of E-cadherin
(50). In pancreatic cancer (PaCa)
cells, baicalein has been shown to augment the Bax/Bcl-2 ratio,
stimulate the release of mitochondrial cytochrome c, and
upregulate caspase-3, - 7 and -9 expression. Baicalein and its
inhibitory effect on human colon cancer were investigated in
vivo and in vitro and the results convey that baicalein
exerts a compelling inhibitory effect on HCT-116 cells (17,50).
Similarly, by altering NF-κB activity, baicalein inhibits cervical
cancer cell proliferation as well, and promotes cell apoptosis and
can also induce apoptosis by inactivating the PI3K/AKT pathway
(17,46,51).
A number of in vivo studies have validated
that baicalein exhibits significant anticancer potential in several
types of cancer. Upon the gene expression analysis of
baicalein-treated H-460 (large cell lung cancer xenograft tumor
model) xenografts, it was found that certain genes were
differentially regulated in the baicalein-treated group, compared
to the control group. The most significant changes were exhibited
by genes involved in the DNA damage repair pathway and cell cycle
control. The genes ITGB3 (+6.96) and TNFRSF25 (+3.4),
which play a role in the induction of apoptosis, were the most
significantly upregulated (52).
Another study found that baicalein was able to inhibit the in
vivo tumor growth of a breast cancer xenogeneic mouse model by
modulating the expression of DNA-damage-inducible transcript 4
(DDIT4), which is responsible for the inhibition of mTOR (53). It has also been demonstrated that
baicalein induces the apoptosis of cervical cancer cells by
increasing the expression of Bax and decreasing the expression of
Bcl-2, which inhibits tumor growth in an in vivo tumor model
(49,51). It has also been reported that
baicalein downregulates myeloid cell leukemia (Mcl)-1 protein
expression, thereby promoting the apoptosis of PaCa (prostate
cancer) cells (45).
Apigenin
Apigenin, a 4',5,7-trihydroxyfavone (Fig. 3D), is abundantly found in fruits
and vegetables such as grapes, parsley and apples, and even in
beverages such as red wine and chamomile tea (17,18).
It aids multiple physiological functions that are beneficial, as it
has been shown to exhibit antioxidant, antiviral, anti-inflammatory
and antibacterial properties (18). Additionally, there has been ample
research conducted on apigenin and its anticancer properties.
Several studies have stated that apigenin suppresses the
proliferation of various cancer cell lines, including melanoma,
hepatic cancer, prostate cancer, lung cancer, colorectal cancer and
breast cancer in vitro and in vivo (11-14,17,18),
by modulating several biological pathways involved in induction of
apoptosis, the inhibition of the cell cycle and the suppression of
cell migration and cell invasion. What makes apigenin an
effective agent is its ability to trigger apoptosis via both the
intrinsic and extrinsic pathways. It achieves this by upregulating
the expression of pro-apoptotic proteins, while downregulating that
of anti-apoptotic proteins. Research has revealed that it functions
by modulating a p53-dependent pathway, which is a pathway related
to the induction of apoptosis and cell cycle arrest (28).
More specifically, it has been found that the
inhibition of cancer cells by apigenin, specifically colon cancer
cells (HT-29, SW480, HCT-116 and CaCo-2) occurs through the
downregulation of Cdc-2, cyclin B1 and Cdc-25, and the upregulation
of p21 and p53. Further investigation revealed that cells treated
with apigenin exhibited an accumulation in the G2/M phase of the
cell cycle, whereas in HT29 cells exhibiting mutant p53 exhibited
the induction of p38 and ERK, and the downregulation of mTOR and
cyclin D1(17). A similar study
using pancreatic cells (with p53 gene mutations) indicated that
apigenin induced cell cycle arrest followed by apoptotic cell death
through p53-related pathways (54). In colon adenocarcinoma (SW480)
cells, apigenin was shown to increase the expression of caspases
and Bax, and inhibit Bcl-2 expression (17). The treatment of bladder carcinoma
cells with apigenin activated the intrinsic mitochondrial pathway
marked by the release of cytochrome c, the stimulation of
Bax, PARP and caspases, and the inhibition of Bcl-2(53). It has also been reported that
apigenin amplifies the production of ROS, leading to cytotoxicity
in all cervical cancer cell lines (SiHa, HeLa, C33A and CaSki
(27). In a previous study,
apigenin induced WAF1/p21 (cell cycle inhibitor), p53 protein,
caspase-3 activation. Furthermore, apoptosis was indicated by DNA
fragmentation in these cells (55). Another study using colon cancer
cells demonstrated the induction of apoptosis by apigenin via the
inhibition of the phosphorylation of signal transducer and
activator of transcription 3 (STAT3) and the downregulation Bcl-xL
and Mcl-1, which are anti-apoptotic (56). Another similar study demonstrated
that in malignant mesothelioma cells, apigenin induced an increase
in the Bax/Bcl-2 ratio, p53 expression and the subsequent
stimulation of caspases-9 and -8 led to apigenin-induced apoptosis,
which was marked by the cleavage of PARP (57). It was also demonstrated by another
study that apoptosis was induced by apigenin in cholangiocarcinoma
via a caspase-dependent pathway (58). Normal cells have not exhibited any
significant cytogenotoxic effects upon apigenin treatment; this
specificity is associated with its low toxicity indicate its
potential as natural chemopreventive agent (59).
The anticancer potential of apigenin has also been
explored in several in vivo models. Studies have revealed
that apigenin inhibits colorectal cancer induced by azoxymethane
(AOM) in Sprague-Dawley (SD) rats and that apigenin decreases tumor
volume in human prostate cancer (PC-3). Treatment of tumors with
apigenin was also shown to result in an increase in the apoptotic
proportion of cells in the tumor. More importantly, apigenin intake
did not seem to reduce body weight and appetite in the animals
(17,60). Another similar in vivo study
reported that apigenin modulated the PI3K/AKT/FoxO-signaling
pathway, restricting the process of tumorigenesis in transgenic
adenocarcinoma of mouse prostate (TRAMP) mice (61). Furthermore, apigenin intake also
led to tumor growth inhibition in U937 (human leukemia cells)
xenografts. This was accompanied by the inhibition of AKT and
induction of JNK (57).
Although flavones are potential anticancer agents,
some investigators have identified the low bioavailability and
instability (enzymes, acid, interference by other nutrients) of
these compounds inside the gut during the processing of food in the
digestive system. Thus, the best method recommended thus far is
delivery of these agents via nanoparticles (14,20).
5. Conclusion and future prospects
Cancer is a multifactorial and multi-step process
characterized by a variety of hallmarks, with uncontrolled
proliferation and resistance to apoptosis being the most important.
Cancer positions among the most domineering medical issues
affecting the human population, and chemopreventive approaches
signify a hopeful strategy with which to prevent occurrence and
death. Flavones, as natural compounds, may elicit great variability
in their therapeutic results. Although a number of in vitro
studies have been conducted, clinical trials using specific
concentrations of these agents are underway. Furthermore,
experimental and clinical studies focused on flavones need to be
performed in order to clarify the value of these molecules in
cancer treatment. Although ample data have been collected, further
investigations are warranted to investigate the use of flavones as
a treatment option, taking into consideration the proper delivery
system of these agents for clinical settings.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
AH conceptualized the study and designed the study
content and figures, and was also involved in the editing of the
manuscript. RR was involved in the writing of the manuscript, in
the literature search and in the processing of the figures/images.
RS was involved in data collection. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gelbart WM, Lewontin RC, Wessler SR,
Suzuki DT, Miller JH and Griffiths AJF: An Introduction to Genetic
Analysis, 8th edition. W.H.Freeman & Co Ltd. 2004.
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Arem H and Loftfield E: Cancer
epidemiology: A survey of modifiable risk factors for prevention
and survivorship. Am J Lifestyle Med. 12:200–210. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Theodoratou E, Timofeeva M, Li X, Meng X
and Ioannidis JPA: Nature, nurture, and cancer risks: Genetic and
nutritional contributions to cancer. Annu Rev Nutr. 37:293–320.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu S, Zhu W, Thompson P and Hannun YA:
Evaluating intrinsic and non-intrinsic cancer risk factors. Nat
Commun. 9(3490)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Tripathi KD: Essentials of Medical
Pharmacology, 7th edition. Jaypee Brothers Medical Publishers (P)
Ltd, 2013.
|
|
7
|
Steward WP and Brown K: Cancer
chemoprevention: A rapidly evolving field. Br J Cancer.
109(17)2013.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Aggarwal BB, Takada Y and Oommen OV: From
chemoprevention to chemotherapy: Common targets and common goals.
Expert Opin Investig Drugs. 13:1327–1338. 2004.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Patterson SL, Colbert Maresso K and Hawk
E: Cancer chemoprevention: Successes and failures. Clin Chem.
59:94–101. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sauter ER: Breast cancer prevention:
current approaches and future directions. Eur J Breast Health.
14:64–71. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Melo FHM, Oliveira JS, Sartorelli VOB
and Montor WR: Cancer chemoprevention: Classic and epigenetic
mechanisms inhibiting tumorigenesis. What have we learned so far?
Front Oncol. 8(644)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Heller MC, Keoleian GA and Willett WC:
Toward a life cycle-based, diet-level framework for food
environmental impact and nutritional quality assessment: A critical
review. Environ Sci Technol. 47:12632–12647. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhou Y, Zheng J, Li Y, Xu DP, Li S, Chen
YM and Li HB: Natural polyphenols for prevention and treatment of
cancer. Nutrients. 8(E515)2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Costea T, Hudiță A, Ciolac OA, Gălățeanu
B, Ginghină O, Costache M, Ganea C and Mocanu MM: Chemoprevention
of colorectal cancer by dietary compounds. Int J Mol Sci.
19(E3787)2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Petrick JL, Steck SE, Bradshaw PT, Trivers
KF, Abrahamson PE, Engel LS, He K, Chow WH, Mayne ST, Risch HA, et
al: Dietary intake of flavonoids and oesophageal and gastric
cancer: Incidence and survival in the United States of America
(USA). Br J Cancer. 112:1291–1300. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang ZJ, Ohnaka K, Morita M, Toyomura K,
Kono S, Ueki T, Tanaka M, Kakeji Y, Maehara Y, Okamura T, et al:
Dietary polyphenols and colorectal cancer risk: The Fukuoka
colorectal cancer study. World J Gastroenterol. 19:2683–2690.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Koosha S, Alshawsh MA, Looi CY, Seyedan A
and Mohamed Z: An association map on the effect of flavonoids on
the signaling pathways in colorectal cancer. Int J Med Sci.
13:374–385. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yan X, Qi M, Li P, Zhan Y and Shao H:
Apigenin in cancer therapy: Anti-cancer effects and mechanisms of
action. Cell Biosci. 7(50)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Lin CH, Chang CY, Lee KR, Lin HJ, Chen TH
and Wan L: Flavones inhibit breast cancer proliferation through the
Akt/FOXO3a signaling pathway. BMC Cancer. 15(958)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Amawi H, Ashby CR Jr and Tiwari AK: Cancer
chemoprevention through dietary flavonoids : What's limiting? Chin
J Cancer. 36(50)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Moga MA, Dimienescu OG, Arvatescu CA,
Mironescu A, Dracea L and Ples L: The role of natural polyphenols
in the prevention and treatment of cervical cancer-an overview.
Molecules. 21(E1055)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Landis-Piwowar KR, Milacic V and Dou QP:
Relationship between the methylation status of dietary flavonoids
and their growth-inhibitory and apoptosis-inducing activities in
human cancer cells. J Cell Biochem. 105:514–523. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Ryu S, Lim W, Bazer FW and Song G: Chrysin
induces death of prostate cancer cells by inducing ROS and ER
stress. J Cell Physiol. 232:3786–3797. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lim W, Ryu S, Bazer FW, Kim SM and Song G:
Chrysin attenuates progression of ovarian cancer cells by
regulating signaling cascades and mitochondrial dysfunction. J Cell
Physiol. 233:3129–3140. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Granato M, Gilardini Montani MS,
Santarelli R, D'Orazi G, Faggioni A and Cirone M: Apigenin, by
activating p53 and inhibiting STAT3, modulates the balance between
pro-apoptotic and pro-survival pathways to induce PEL cell death. J
Exp Clin Cancer Res. 36(167)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Romagnolo DF, Donovan MG, Papoutsis AJ,
Doetschman TC and Selmin OI: Genistein prevents BRCA1 CpG
methylation and proliferation in human breast cancer cells with
activated aromatic hydrocarbon receptor. Curr Dev Nutr.
1(e000562)2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Souza RP, Bonfim-Mendonça PS, Gimenes F,
Ratti BA, Kaplum V, Bruschi ML, Nakamura CV, Silva SO, Maria-Engler
SS and Consolaro ME: Oxidative stress triggered by apigenin induces
apoptosis in a comprehensive panel of human cervical cancer-derived
cell lines. Oxid Med Cell Longev. 2017(1512745)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sung B, Chung HY and Kim ND: Role of
apigenin in cancer prevention via the induction of apoptosis and
autophagy. J Cancer Prev. 21:216–226. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ci Y, Qiao J and Han M: Molecular
mechanisms and metabolomics of natural polyphenols interfering with
breast cancer metastasis. Molecules. 21(E1634)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Xue C, Chen Y, Hu DN, Iacob C, Lu C and
Huang Z: Chrysin induces cell apoptosis in human uveal melanoma
cells via intrinsic apoptosis. Oncol Lett. 12:4813–4820.
2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Xu Y, Tong Y, Ying J, Lei Z, Wan L, Zhu X,
Ye F, Mao P, Wu X, Pan R, et al: Chrysin induces cell growth
arrest, apoptosis, and ER stress and inhibits the activation of
STAT3 through the generation of ROS in bladder cancer cells. Oncol
Lett. 15:9117–9125. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chylińska-Wrzos P, Lis-Sochocka M and
Jodlowska-Jedrych B: Chrysin and its potential antineoplastic
effect. Eur J Biol Res. 7:245–254. 2017.
|
|
33
|
Ronnekleiv-Kelly SM, Nukaya M, Díaz-Díaz
CJ, Megna BW, Carney PR, Geiger PG and Kennedy GD: Aryl hydrocarbon
receptor-dependent apoptotic cell death induced by the flavonoid
chrysin in human colorectal cancer cells. Cancer Lett. 370:91–99.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zhang Q, Ma S, Liu B, Liu J, Zhu R and Li
M: Chrysin induces cell apoptosis via activation of the
p53/Bcl-2/caspase-9 pathway in hepatocellular carcinoma cells. Exp
Ther Med. 12:469–474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xu D, Jin J, Yu H, Zhao Z, Ma D, Zhang C
and Jiang H: Chrysin inhibited tumor glycolysis and induced
apoptosis in hepatocellular carcinoma by targeting hexokinase-2. J
Exp Clin Cancer Res. 36(44)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wang J, Wang H, Sun K, Wang X, Pan H, Zhu
J, Ji X and Li X: Chrysin suppresses proliferation, migration, and
invasion in glioblastoma cell lines via mediating the ERK/Nrf2
signaling pathway. Drug Des Devel Ther. 12:721–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Yu XM, Phan T, Patel PN, Jaskula-Sztul R
and Chen H: Chrysin activates Notch1 signaling and suppresses tumor
growth of anaplastic thyroid carcinoma in vitro and in vivo.
Cancer. 119:774–781. 2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Tuorkey MJ: Molecular targets of luteolin
in cancer. Eur J Cancer Prev. 25:65–76. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ham S, Kim KH, Kwon TH, Bak Y, Lee DH,
Song YS, Park SH, Park YS, Kim MS, Kang JW, et al: Luteolin induces
intrinsic apoptosis via inhibition of E6/E7 oncogenes and
activation of extrinsic and intrinsic signaling pathways in
HPV-18-associated cells. Oncol Rep. 31:2683–2691. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chen P, Zhang JY, Sha BB, Ma YE, Hu T, Ma
YC, Sun H, Shi JX, Dong ZM and Li P: Luteolin inhibits cell
proliferation and induces cell apoptosis via down-regulation of
mitochondrial membrane potential in esophageal carcinoma cells EC1
and KYSE450. Oncotarget. 8:27471–27480. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wang H, Luo Y, Qiao T, Wu Z and Huang Z:
Luteolin sensitizes the antitumor effect of cisplatin in
drug-resistant ovarian cancer via induction of apoptosis and
inhibition of cell migration and invasion. J Ovarian Res.
11(93)2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Liao Y, Xu Y, Cao M, Huan Y, Zhu L, Jiang
Y, Shen W and Zhu G: Luteolin induces apoptosis and autophagy in
mouse macrophage ANA-1 cells via the Bcl-2 pathway. J Immunol Res.
2018(4623919)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Zang M, Hu L, Fan ZY, Wang HX, Zhu ZL, Cao
S, Wu XY, Li JF, Su LP, Li C, et al: Luteolin suppresses gastric
cancer progression by reversing epithelial-mesenchymal transition
via suppression of the Notch signaling pathway. J Transl Med.
15(52)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Hong Z, Cao X, Li N, Zhang Y, Lan L, Zhou
Y, Pan X, Shen L, Yin Z and Luo L: Luteolin is effective in the
non-small cell lung cancer model with L858R/T790M EGF receptor
mutation and erlotinib resistance. Br J Pharmacol. 171:2842–2853.
2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu H, Dong Y, Gao Y, Du Z, Wang Y, Cheng
P, Chen A and Huang H: The fascinating effects of baicalein on
cancer: A review. Int J Mol Sci. 17(E1681)2016.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Y, Xia J, Tang X, Tang L, Mao X,
Zhang Y and Yu X: Baicalein promotes the apoptosis of HeLa cells by
inhibiting ERK1/2 expression. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi
J. 32:1507–1512. 2016.(In Chinese). PubMed/NCBI
|
|
47
|
Choi EO, Park C, Hwang HJ, Hong SH, Kim
GY, Cho EJ, Kim WJ and Choi YH: Baicalein induces apoptosis via
ROS-dependent activation of caspases in human bladder cancer 5637
cells. Int J Oncol. 49:1009–1018. 2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yan W, Ma X, Zhao X and Zhang S: Baicalein
induces apoptosis and autophagy of breast cancer cells via
inhibiting PI3K/AKT pathway in vivo and vitro. Drug Des Devel Ther.
12:3961–3972. 2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Peng Y, Guo C, Yang Y, Li F, Zhang Y,
Jiang B and Li Q: Baicalein induces apoptosis of human cervical
cancer HeLa cells in vitro. Mol Med Rep. 11:2129–2134.
2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ma X, Yan W, Dai Z, Gao X, Ma Y, Xu Q,
Jiang J and Zhang S: Baicalein suppresses metastasis of breast
cancer cells by inhibiting EMT via downregulation of SATB1 and
Wnt/β-catenin pathway. Drug Des Devel Ther. 10:1419–1441.
2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yu X, Liu Y, Wang Y, Mao X, Zhang Y and
Xia J: Baicalein induces cervical cancer apoptosis through the
NF-κB signaling pathway. Mol Med Rep. 17:5088–5094. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Cathcart MC, Useckaite Z, Drakeford C,
Semik V, Lysaght J, Gately K, O'Byrne KJ and Pidgeon GP:
Anti-cancer effects of baicalein in non-small cell lung cancer
in-vitro and in-vivo. BMC Cancer. 16(707)2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang Y, Han E, Xing Q, Yan J, Arrington A,
Wang C, Tully D, Kowolik CM, Lu DM, Frankel PH, et al: Baicalein
upregulates DDIT4 expression which mediates mTOR inhibition and
growth inhibition in cancer cells. Cancer Lett. 358:170–179.
2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
King JC, Lu QY, Li G, Moro A, Takahashi H,
Chen M, Go VL, Reber HA, Eibl G and Hines OJ: Evidence for
activation of mutated p53 by apigenin in human pancreatic cancer.
Biochim Biophys Acta. 1823:593–604. 2012.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Shi MD, Shiao CK, Lee YC and Shih YW:
Apigenin, a dietary flavonoid, inhibits proliferation of human
bladder cancer T-24 cells via blocking cell cycle progression and
inducing apoptosis. Cancer Cell Int. 15(33)2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Maeda Y, Takahashi H, Nakai N, Yanagita T,
Ando N, Okubo T, Saito K, Shiga K, Hirokawa T, Hara M, et al:
Apigenin induces apoptosis by suppressing Bcl-xl and Mcl-1
simultaneously via signal transducer and activator of transcription
3 signaling in colon cancer. Int J Oncol. 52:1661–1673.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Masuelli L, Benvenuto M, Mattera R, Di
Stefano E, Zago E, Taffera G, Tresoldi I, Giganti MG, Frajese GV,
Berardi G, et al: In vitro and in vivo anti-tumoral effects of the
flavonoid apigenin in malignant mesothelioma. Front Pharmacol.
8(373)2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Subhasitanont P, Chokchaichamnankit D,
Chiablaem K, Keeratichamroen S, Ngiwsara L, Paricharttanakul NM,
Lirdprapamongkol K, Weeraphan C, Svasti J and Srisomsap C: Apigenin
inhibits growth and induces apoptosis in human cholangiocarcinoma
cells. Oncol Lett. 14:4361–4371. 2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Vrhovac Madunic I, Madunić J, Antunović M,
Paradžik M, Garaj-Vrhovac V, Breljak D, Marijanović I and Gajski G:
Apigenin, a dietary flavonoid, induces apoptosis, DNA damage, and
oxidative stress in human breast cancer MCF-7 and MDA MB-231 cells.
Naunyn Schmiedebergs Arch Pharmacol. 391:537–550. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Shukla S, Kanwal R, Shankar E, Datt M,
Chance MR, Fu P, MacLennan GT and Gupta S: Apigenin blocks IKKα
activation and suppresses prostate cancer progression. Oncotarget.
6:31216–31232. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Shukla S, Bhaskaran N, Babcook MA, Fu P,
Maclennan GT and Gupta S: Apigenin inhibits prostate cancer
progression in TRAMP mice via targeting PI3K/Akt/FoxO pathway.
Carcinogenesis. 35:452–460. 2014.PubMed/NCBI View Article : Google Scholar
|