1. Introduction
Endometriosis is one of the most common
gynecological diseases, with a rate of ~10% of women of
reproductive age and ~50% of those with infertility (1,2). It
is characterized by the presence of endometrial tissue outside the
uterine cavity (2). The main
symptoms are commonly associated with chronic pelvic pain and
infertility. Women affected suffer from dysmenorrhea (79%), pelvic
pain (69%), dyspareunia (45%), modified gut transit (constipation,
diarrhea in 36%), intestinal pain (29%), infertility (26%), ovarian
mass (20%), dysuria (10%) and other urinary disorders (6%)
(1,3,4).
The diagnosis of endometriosis is based on clinical
suspicion, a pelvic examination, ultrasound and magnetic resonance
imaging (MRI); however, laparoscopy, an invasive surgical
procedure, currently remains gold standard for diagnosis (5). The preferred diagnostic methods other
than laparoscopic inspection are transvaginal ultrasonography (TVS)
as the first line option. Endometriotic lesions were classified
into 3 phenotypes: Superficial peritoneal endometriosis, ovarian
endometrioma and deep infiltrating endometriosis (DIE). Superficial
endometriotic foci are difficult to diagnose not only by TVS, but
also by MRI and are usually identifiable only at laparoscopy.
Therefore, TVS is the first-line imaging technique for the
diagnosis of ovarian endometrioma and DIE (6). Several studies have demonstrated the
diagnostic accuracy and reliability of TVS for the detection of DIE
and pouch of Douglas obliteration (7). In clinical practice, physicians not
only diagnose the presence of endometriosis, but also evaluate the
spread of the disease and the severity of the symptoms. Usually,
the severity of the disease burden is determined based on the
location, diameter and depth of the lesions, the presence of deep
utero-sacral nodules and the density of adhesions, such as complete
obliteration of the cul-de sac. Endometriosis-related pain symptoms
may be associated with the severity of diseases, such as adhesions
and DIE. In addition, adhesion and fibrosis with anatomic
abnormalities will certainly lead to infertility, but even minimal
and mild endometriosis is often associated with infertility
(8). Sanchez et al found
that in vitro fertilization (IVF) outcomes in women with
endometriosis indicated that minimal/mild disease has a lower
fertilization rate than moderate/severe disease (9). It is often found that the severity of
the symptoms is not always associated with the spread or extent of
endometriosis. Furthermore, the lack of an association between
endometriosis-related pain and infertility makes it difficult to
determine the disease severity by imaging alone.
Although the etiology of endometriosis remains
unknown, the proposed hypothesis is based on 3 main theories:
Retrograde menstruation, coelomic metaplasia and Müllerian remnants
(2). Given the different contexts
in which endometriosis develops, a single etiological model is not
enough to explain its pathogenesis (10). Recent advances and controversies in
the etiology, pathogenesis, diagnosis, classification and treatment
of endometriosis have been previously discussed in detail (11). Genetics, epigenetics and the
related signaling pathways have been implicated in the pathogenesis
of endometriosis, and there has recently been interest in
inflammation, oxidative stress, iron metabolism, macrophages,
platelets, sensory nerve fiber and fibrosis (2,10).
It is now accepted that oxidative stress plays an important role in
the initiation and progression of endometriosis (12). Several basic and clinical studies
have been conducted on the effects of oxidative stress on the
endometriosis-related pain, male and female infertility, and the
development of fibrosis (2,10,13-15).
Currently, several endometriosis classification
systems are used in clinical practice. The classification systems
are mainly used to determine the extent of the anatomic
abnormality; however, the drawback is that they are not always
associated with the severity of the clinical symptoms. The present
review compares the strengths and weaknesses of the existing
classification systems and discusses their association with the
severity of the symptoms. The molecular mechanisms that trigger
endometriosis-related symptoms, such as pain, infertility and
fibrosis are also reviewed. The final aim of the present review was
to identify the biological mediators that contribute most to the
symptoms of endometriosis.
2. Literature search
A computerized literature search was conducted to
identify relevant studies reported in the English language. A
comprehensive literature search was conducted on the PubMed and
Embase databases between January, 2000 and December, 2019,
combining the keywords ‘endometriosis’, ‘classification’,
‘severity’, ‘pain’, ‘infertility’, ‘fibrosis’, ‘molecular’,
‘inflammation’, ‘oxidative stress’ and ‘iron’. A variety of
combinations of these terms were used, depending on which database
was searched (Table I).
Furthermore, the references of each article were searched to
identify potentially relevant studies. Publications of original
studies, review articles and some guidelines were included, while
those documenting opinions, points of view or anecdotes were
excluded.
 | Table INumber of articles hit by searching
for each keyword alone or in combination. |
Table I
Number of articles hit by searching
for each keyword alone or in combination.
| Key words | No. of refs. | No. of eligible
articles |
|---|
| Endometriosis |
|---|
| Classification | 778 | |
|
Pain | 281 | 29 |
|
Infertility | 202 | 19 |
|
Fibrosis | 41 | 11 |
| Severity | 2,175 | |
|
Pain | 1,021 | |
|
Molecular | 57 | 22 |
|
Infertility | 651 | |
|
Molecular | 58 | 19 |
|
Fibrosis | 113 | |
|
Molecular | 8 | 6 |
|
Inflammation | 124 | 55 |
|
Oxidative
stress | 28 | 18 |
|
Iron | 4 | 4 |
3. Characteristics of the relevant
studies
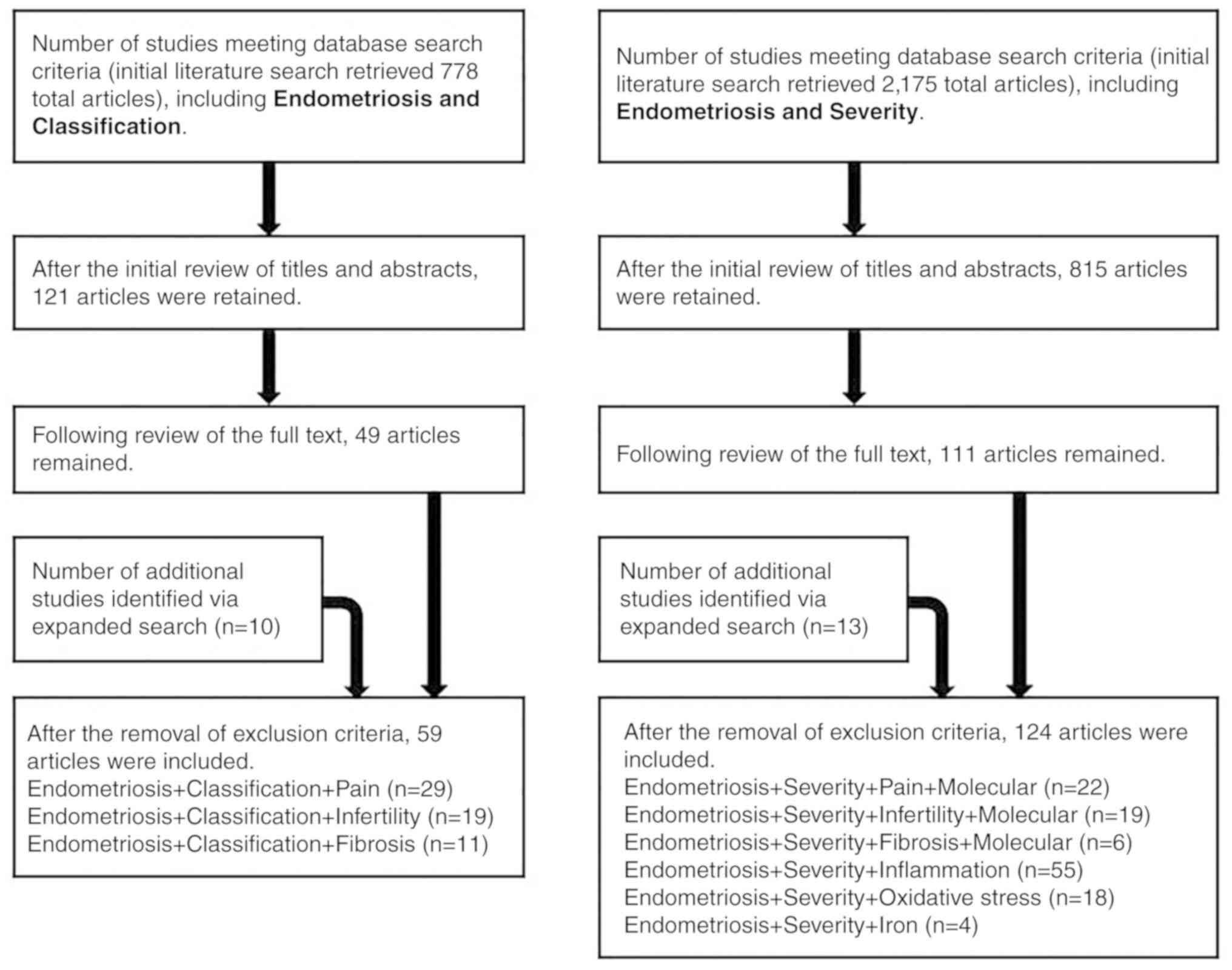
A selection flow chart for the search strategy is
presented in Fig. 1. Based on the
search strategy, a total of 778 and 2,175 articles were initially
identified on ‘endometriosis’ and ‘classification’ (Fig. 1, left panel) and ‘endometriosis’
and ‘severity’ (Fig. 1, right
panel), respectively. Following the initial review of titles and
abstracts, 121 and 815 articles were retained, respectively.
Following the review of the full text, 49 and 111 articles
remained, respectively, also following the removal of opinions,
points of view or anecdotes. The number of additional studies
identified by an expanded search was 10 and 13, respectively.
Finally, 59 and 124 articles were included, respectively.
4. Role of diagnostic imaging in evaluating
the severity of endometriosis
Laparoscopy remains the gold standard for the
diagnosis of endometriosis, although it is preferably diagnosed in
a non-invasive manner (16). It is
quite difficult to identify superficial diseases, peritoneal
lesions, or early/mild deep endometriosis by imaging modalities
(17,18), indicating that a negative diagnosis
cannot rule out endometriosis. On the other hand, TVS and MRI are
useful in detecting advanced stages of endometriosis. Endometriosis
with adhesions to surrounding organs, e.g., extensive inflammatory
adhesions between ovarian endometrioma and the rectum, is
considered severe. The items for determining adhesions in TVS using
the dynamic manipulation of the pelvic organs are the loss of
ovarian mobility and limited sliding between the posterior uterine
serosa and the bowels (17).
Holland et al revealed that the overall sensitivity and
specificity of TVS for the diagnosis of severe endometriosis were
85 and 98%, respectively (17).
Women with TVS features of ovarian endometriomas have greater
ovarian immobility than do women without these features, with the
sensitivity and specificity of 89 and 90%, respectively (19,20).
As the ability of MRI to diagnose adhesions or complete
obliteration of the pouch of Douglas is similar to that of dynamic
TVS, there is no need to perform routine MRI following TVS
(7). Therefore, the diagnostic
accuracy of dynamic TVS is comparable with, and may be superior to,
routine MRI (17,18). However, MRI has the better
advantage of objectivity and reproducibility than TVS. Based on the
above, TVS and MRI are valuable tools for the diagnosis of the
severity of endometriosis, including adhesions; it may be possible
to develop a classification for endometriosis-related pain. On the
other hand, there is a major unsolved issue regarding
endometriosis-related infertility. Imaging diagnostics, which
primarily determine the presence of morphological abnormalities,
have limitations as disease progression is not associated with the
prevalence and severity of infertility (21). Several research groups have
developed a simplified classification to diagnose the severity of
endometriosis-related infertility (discussed below). The adhesion
scoring system created by Ichikawa et al can predict
post-operative adhesion and infertility (22). The scoring system also allows for
the selection of patients to undergo IVF following surgery
(22). Research on the association
between the progression of endometriosis (spreading and adhesion)
and the severity of symptoms (pain and infertility) is a topic for
the future.
5. Pros and cons of existing endometriosis
classification systems
The various classification schemes for endometriosis
staging developed by several professional organizations have been
proposed based on anatomic location and disease severity (3,23-26).
The classification system should at least be applied to various
endometriosis structures, lesion appearance, pelvic adhesions and
anatomic locations to determine the disease severity. First, the
advantages of the revised American Society for Reproductive
Medicine (r-ASRM) classification are its worldwide use, familiarity
and its easy integration into a number of other classification
systems (27). Second, the ENZIAN
classification is excellent for the assessment of DIE (3,24).
Third, the Endometriosis Fertility Index (EFI) classification can
be applied to the prediction of fertility (28). This chapter mainly outlines the
advantages and disadvantages of the r-ASRM classification, the
ENZIAN classification, the EFI classification and the American
Association of Gynecological Laparoscopists (AAGL)
classification.
The r-ASRM classification
In 1979, the American Fertility Society (AFS)
proposed a novel classification system that links the surgical
findings of endometriosis with fertility (29). This classification system was
revised in 1985 and became the current edition when the name of the
society was changed to the American Society for Reproductive
Medicine (r-ASRM) in 1996(29).
The r-ASRM score includes the following items: Peritoneal lesions,
ovarian lesions, posterior cul-de-sac obliteration and adnexal
adhesions (30). The total score
is calculated by adding points according to the size of
endometriotic lesions and the extent of adhesions, and the total
points are classified into stages I to IV (I, minimal; II, mild;
III, moderate; and IV, severe) (23,29,31).
Women with minimal or mild endometriosis have superficial implants
with very few adhesions, whereas moderate or severe endometriosis
is generally characterized by ovarian endometriomas with more
severe adhesions. The main color categories of peritoneal
endometriosis are classified into red (R), black (B) and white (W)
(29). The r-ASRM classification
system is standardized, widely used and accepted worldwide as a
global standard for almost all clinical studies (3,23,29).
This classification system has some limitations,
however (29). There is no
association between clinical symptoms and surgical findings due to
the lack of assessment of DIE and pain (29,31,32).
Even women presenting with minimal clinical symptoms may have
advanced disease. It has been pointed out that the advanced stage
is not related to the prognosis of infertility, as this
classification is less relevant for post-operative natural
pregnancy rates (29,31,32).
Thus, even women with very few endometrial lesions may have
infertility. However, as the advanced stages of endometriosis are
associated with a worse prognosis for IVF treatment compared to the
early stages or tubal factor infertility, the r-ASRM classification
is useful in predicting the IVF outcome (33). More specifically, the disadvantages
of this classification are the following: i) Staging is not fully
associated with morphological changes in organs; ii) a lack of
assessment of retroperitoneal changes and DIE; iii) poor prediction
of pregnancy success following treatment; and iv) limited
reproducibility (23). Moreover,
endometriosis-related pain and infertility are poorly associated
with the duration of the disease (23). Therefore, the clinical use of this
classification poses significant challenges, particularly in
predicting fertility and selecting therapeutic modalities (32). Several studies have searched for
candidate biomarkers (blood, ascites, cyst fluid, follicular fluid
and tissue that reflect the severity of the symptoms (please see
chapter 8 below entitled ‘Endometriosis-associated infertility: An
update on molecular aspects.’). In the future, a new classification
system consisting of anatomic abnormalities and these biomarkers
will need to be developed.
The ENZIAN classification
A working group meeting was held in Enzian, Austria
in 2005, mainly to create a new classification (the ENZIAN
classification) that includes retroperitoneal diseases and DIE
(3,24). In 2011, the ENZIAN classification
system was reviewed (3). Since DIE
can invade into surrounding structures, similar to cancer, this
classification was prototyped based on the TNM classification of
cervical cancer (25). The ENZIAN
score was classified into A (rectovaginal space and vagina), B
(sacrouterine ligaments, cardinal ligaments, pelvic sidewall and
external ureter compression) and C (rectum) compartments. The depth
of endometriosis invasion was classified as level 1 (<1 cm
depth), level 2 (1-3 cm) and level 3 (>3 cm) (25). The mode of invasion into other
organs was also subclassified as FA for adenomyosis, FB for bladder
involvement, FU for intrinsic ureter involvement, FI for intestinal
involvement and FO for involvement of other organs or structures,
such as the abdominal wall (3,24).
For patients with DIE, the ENZIAN classification system should be
used to completely describe the surgical findings, along with the
r-ASRM classification (34). The
ENZIAN scores can be viewed at www.endometriose-sef.de/dateien/ENZIAN_2013_web.pdf.
The historical background of the ENZIAN classification has been
previously described (23,35).
This classification has advantages and
disadvantages. Pain and dysmenorrhea are strongly associated with
the severity grade in this classification (35). The most affected compartment is the
posterior area (93.4%), mainly on the left side (67.8%), which
supports the clinical data that DIE is more likely to invade the
sigmoid colon (25). For the
evaluation of DIE, attempts have been made to incorporate the
ENZIAN classification into the r-ASRM classification (26,35).
Due to the poor association between the ENZIAN score and fertility,
this score cannot be used to predict the prognosis of
endometriosis-related infertility (34). However, it is currently used in
German-speaking countries, although it is not widely used
internationally (24).
The EFI
In 2010, the EFI was published (3,28).
This index has been validated to predict the pregnancy rate of
infertile patients after surgical diagnosis and treatment of
endometriosis (3,28). This index is useful for planning
post-operative assisted reproductive technology (ART) due to its
excellent evaluation of pregnancy prognosis (28). The EFI score includes historical
factors (age of the patient, duration of infertility and a history
of pregnancy) and surgical factors [least function (LF) score at
conclusion of surgery, AFS endometriosis score and AFS total score]
(28). This score is rated from 0
to 10, with 0 being the worst prognosis and 10 being the optimal
(28). When patients with
endometriosis are classified into 3 groups using the EFI score,
there is a significant difference in the cumulative pregnancy rate
between the groups. The EFI score allows for the prediction of
non-ART pregnancy in surgically-treated patients with endometriosis
(3,28). On the other hand, there is no
significant difference in the cumulative pregnancy rate when
divided into 4 groups by the r-ASMR classification (29,31,32),
indicating that the severity of the r-ASRM classification is not
associated with the pregnancy rate.
The AAGL classification
Currently, members of the American Association of
Gynecological Laparoscopists (AAGL) Society are developing a
classification system that focuses on endometriosis-related
infertility and pain (3,34). Based on the results of preliminary
studies demonstrating a better association between the AAGL
classification system and infertility, pain levels and surgical
difficulties, this classification may be used in place of the
r-ASRM classification system in the future (34). The document can be obtained from
the URL (http://www.aagl.org/wp-content/uploads/2013/03/NewsScope_Oct-Dec_2012.pdf).
Currently, the 4 classifications can be used in
various clinical settings. In summary, no single classification can
predict the severity of all pre- and post-operative symptoms of
endometriosis, particularly pain and fertility. The most important
factors are as follows: The classification needed should preferably
be simple, easy to use, reproducible, and taking into consideration
symptoms, such as pain and infertility, and predicting
prognosis.
6. An update on the endometriosis-associated
pain symptoms
Endometriosis is often found in women with
unexplained pelvic pain as it is associated with chronic cyclic
pain, dyspareunia, dysmenorrhea and sometimes dysuria. The presence
of DIE, vaginal lesions and adenomyosis is strongly associated with
deep dyspareunia, although the severity of the pain is not always
associated with the spread of the disease (36). Endometriosis is associated with
chronic inflammation, leading to tissue injury and repair, the
accumulation of excess extracellular matrix (ECM) components, scar
formation and ultimately, fibrosis (36). The cause of the pain symptoms
should not only take anatomic abnormalities, such as adhesions and
fibrosis into consideration, but also biological mechanisms,
including an imbalance of sensory and sympathetic innervation due
to the abnormal secretion of a variety of mediators. Estrogen
promotes the growth of nerve fibers that invade endometriotic
tissue and is involved in the hyperinnervation in close proximity
to various nerve plexuses or the activity of neurons throughout the
central nervous system (37).
Altered pelvic innervation or hyperinnervation can cause pelvic
pain in endometriosis via excessive neuroinflammation and
subsequent neurogenesis (36).
Cyclic hemorrhage from endometriosis causes local platelet
activation, producing nerve growth factor (NGF, a member of the
neurotrophin family) (38,39). Subsequently, the sensory nerves are
activated and induce neuroinflammation and excess fibrosis around
endometriotic foci (38,39). Furthermore, M2 macrophages
infiltrating endometrial lesions produce Th2 cytokines and mediate
the process of immunosuppression and neuroangiogenesis, causing the
abnormal distribution of nerve fibers, which is involved in the
generation of endometriosis-associated pain (36,40).
Since these nerve growth factors and Th2 cytokines may contribute
to sensory nerve hyperinnervation, it is necessary to measure these
factors in peripheral blood to objectively predict the severity of
pain symptoms (41). The signaling
interactions of pain mediators are described in chapter 9 below
entitled ‘Endometriosis-associated fibrosis: An update on molecular
aspects’.
7. Endometriosis-associated infertility: An
update on clinical aspects
The possible causes of infertility include the age
of the woman, oocyte quality, sperm quality, ovarian reserve, tubal
patency, uterine receptivity, anatomic abnormalities, inflammatory
changes in the uterus and pelvis, and the presence of endometriosis
(15,42-45).
Among these, endometriosis has a tremendous impact on fertility.
Similar to the mechanism of pain, infertility caused by
endometriosis includes anatomic and functional abnormalities.
The most likely anatomic features are pelvic
adhesions and fibrosis. Adnexal adhesions are considered to impair
gamete and embryo transport, resulting in reduced fertility.
However, infertility cannot be explained solely by anatomic
abnormalities (21).
Microenvironments, such as inflammation and oxidative stress
induced by endometriosis lead to ovarian dysfunction (21). Ovarian endometrioma does not
adversely affect the rate of spontaneous ovulation (46). However, Liu et al reported
that oocytes retrieved from women affected by endometriosis
exhibited low fertilization rates due to low rates of mature
oocytes (47). A meta-analysis of
27 observational studies, including 8,984 women, demonstrated that
even patients with early-stage endometriosis had reduced
fertilization rates compared to women without endometriosis
[relative risk (RR)=0.93, P=0.03)] (48). The presence of endometriosis can
cause inflammation, hormonal imbalance and oxidative stress in the
eutopic endometrium, resulting in impaired endometriotic
receptivity and implantation failure (49). In summary, endometriosis may affect
non-ART-induced pregnancies through anatomic abnormalities of the
fallopian tubes, the exposure of the endometrium to chronic
inflammation, and poor egg and sperm quality. Both the ASRM
committee opinion in 2012 and the European Society of Human
Reproduction and Embryology (ESHRE) guideline in 2014 announced
that both ovarian endometrioma per se or cystectomy for
endometriomas significantly reduced the ovarian reserve and that
surgical interventions for endometrioma prior to IVF treatment did
not increase pregnancy rates (1,50).
Pal et al reported that the outcome of IVF-embryo transfer
(ET) did not depend on the severity of endometriosis, suggesting
that IVF-ET treatment overcomes the adverse effects on damaged
oocytes (51). However, a recent
meta-analysis of IVF treatment revealed that women with advanced
stages of endometriosis had lower implantation and clinical
pregnancy rates than patients without endometriosis (RR=0.79,
P=0.0008) (48). Further, De Wilde
et al reported that the presence of endometriosis, even in
mild cases, adversely affects pregnancy outcome following IVF-ET
treatment (21). Researchers have
discussed that changes in the endometriosis microenvironment due to
local inflammation and oxidative stress can lead to the reduced
quality of the oocyte, sperm and embryo, the impaired receptivity
of the endometrium and implantation failure, which has a negative
effect on pregnancy rates even following IVF treatment (15,42-45,49).
8. Endometriosis-associated infertility: An
update on molecular aspects
This chapter highlights the possible molecular
mechanisms underlying infertility associated with endometriosis.
Several elegant reviews have been published on the mechanisms of
endometriosis-related infertility (4,15).
The altered expression or abnormal activation of biochemical,
endocrine, immune, genetic and epigenetic factors in the
endometriosis microenvironment can lead to ovarian dysfunction and
subsequently, to infertility. In particular, the proinflammatory
mediators (cytokines, interleukins and immune dysfunction),
oxidative stress markers (hemoglobin, heme, free iron, ROS and
antioxidants), hormonal imbalance, proteolytic enzymes and soluble
adhesion molecules all may be potential markers for predicting
endometriosis-related infertility (15). The understanding of the mechanisms
through which endometriosis causes infertility may lead to the
discovery of markers that predict the association between the
severity of endometriosis and infertility. An overview of each of
the potential markers studied will be outlined.
Inflammatory cytokines
Endometriosis is considered to be a chronic
inflammatory disease (4,52). Miller et al performed a
comprehensive literature review of inflammatory and immune
dysfunction on endometriosis-associated infertility (49). In fact, the levels of
proinflammatory cytokines, including tumor necrosis factor-α
(TNF-α), interleukin (IL)-6 and IL-8, were increased in the
peritoneal fluid of women with endometriosis (49). These proinflammatory cytokines
produced by activated macrophages and natural killer (NK) cells may
be candidate markers for the diagnosis of infertility (53). Changes in inflammatory factors in
peritoneal fluid negatively affect the fallopian tube and
intrauterine environment, as the ampulla of the fallopian tube is
structurally exposed to peritoneal fluid (4). These cytokines in peritoneal fluids
enter the uterine cavity and increase prostaglandin production by
endometrial epithelial cells, which in turn stimulates the
overexpression of other inflammatory cytokines (54). Furthermore, TNF-α, IL-6 and IL-8
activate the inflammatory response, induce angiogenesis and are
also involved in tissue damage and repair. Physiological levels of
inflammatory cytokines are essential for implantation,
placentation, and pregnancy. The overexpression of TNF-α, IL-6 and
IL-8 can impair follicular steroidogenesis, growth and ovulation,
cause impaired fertility and implantation failure, and adversely
affect spontaneous pregnancy (4,54-56).
Several researchers have reported that elevated serum IL-6 and IL-8
levels are associated with the occurrence of infertility (4,54-56).
However, to date, to the best of our knowledge, no blood markers
have been reported to determine the severity of infertility. Even
other candidate markers overexpressed in the peritoneal cavity may
not be reflected in the peripheral blood possibly due to dilution.
Further studies are required to determine whether blood cytokine
levels are associated with the severity of endometriosis-related
infertility. Given the causes of infertility, cyclooxygenase
(COX)-2 inhibitors, cytokine modulators, or hormone-suppressing
therapies may hold promise for the treatment of infertility
(15). Since the pathogenesis of
endometriosis is the propagation of the inflammatory network by
macrophages, the targeted inhibition of an activation cascade that
will lead to an autoamplification of cytokine production, namely
the cytokine storm, may be novel promising therapeutic strategies
(57).
Iron and oxidative stress
Living things on earth have evolved to use iron, the
fourth most abundant element in the Earth's crust, to transport
oxygen. ROS are by-products produced by various cellular
compartments, including mitochondria, during the process of
consuming oxygen by aerobic organisms. The balance between ROS
production and ROS elimination/antioxidant production is crucial to
preventing ROS-induced adverse events. Physiological levels of ROS
play important regulatory roles in the processes of
folliculogenesis, oocyte maturation, endometrial cycle regulation,
luteolysis, implantation, embryogenesis and pregnancy through
various signaling pathways (58,59).
The excessive production of ROS causes detrimental effects on cells
through lipid peroxidation, protein oxidation and DNA damage, which
in turn negatively affects reproductive function (2,52).
Therefore, ROS act as important signaling molecules in
physiological processes, while they also play a role in
pathological processes involved in female reproductive function
(58). Based on the
above-mentioned facts, ROS act as a double-edged sword.
This chapter focuses on the iron-induced oxidative
stress in endometriosis. Retrograde menstruation contains
endometrial cells and red blood cells, and following hemolysis,
hemoglobin, heme and free iron (so-called hemoglobin species) are
released and accumulate in the pelvic cavity (14). In addition, within ovarian
endometrioma, hemorrhage from the ectopic endometrium occurs at
each menstruation and hemoglobin species accumulates in the cyst.
Ovarian endometrioma contains higher levels of hemoglobin species
compared to other types of ovarian cysts (60). Iron is a well-known inducer of
oxidative stress (14,52). The mechanisms through which free
iron induces ROS are as follows: Iron ions react with
H2O2 via the Fenton reaction to form hydroxyl
radicals (•OH). Hydroxyl radicals are highly reactive,
and therefore cause harmful oxidative damage to DNA, proteins and
membrane lipids (61). The
accumulation of iron in ovarian endometrioma may strongly promote
an imbalance in the redox status (61,62).
The emerging evidence to indicate that ROS-induced oxidative stress
is involved in the development and progression of endometriosis
[please see the chapter entitled ‘Iron-dependent progression of
endometriosis’ the previous study by Kobayashi et al
(62)]. The comprehensive
systematic review highlighted iron metabolism, oxidative stress
markers (serum, peritoneal fluid, follicular fluid, ovarian cortex,
and eutopic and ectopic endometrial tissue), genes involved in
oxidative stress, endometriosis-induced infertility and the
mechanism of carcinogenesis (2,62).
In addition to endometriosis, iron is also involved in cell damage,
apoptosis, carcinogenesis, fibrosis, and ultimately in the
dysfunction of various organs, including the heart, lungs, liver
and kidneys (63).
Iron levels have been investigated in each bodily
fluid of patients with endometriosis. Van Langendonckt et al
reported that iron levels in the peritoneal cavity of patients with
endometriosis were increased compared to the controls, although
serum iron levels were not (14).
However, Alizadeh et al found that serum iron levels in
patients with endometriosis were significantly higher compared with
the controls (64). Serum iron
allowed for the discrimination between patients with endometriosis
and controls with an area under the receiver operating
characteristic (ROC) curve (AUC) of 0.899 and a cut-off value of
1.73 mg/l (64). In addition, the
median ± SD total iron, heme and free iron levels in endometriotic
cyst fluids were previously shown to be 244.4±204.9, 303.9±324.4
and 13.5±16.2 mg/l, respectively (60). Thus, the iron levels in ovarian
endometrioma were 10-100-fold higher than those in serum, depending
on the hemoglobin species. High levels of intracystic iron can
oxidize a wide range of substrates and cause biological damage and
cell death to adjacent follicles (64). Following the degradation of
hemoglobin, iron is released from heme and accumulates in tissues
over a period of several days, which results in the deposition of
hemosiderin.
However, iron levels in bodily fluids of women with
endometriosis remain controversial. Montoya-Estrada et al
reported that the levels of peritoneal hemoglobin did not differ
between the endometriosis group and the non-endometriosis group
(65). Benaglia et al
reported that there was no significant difference in iron
concentrations in follicular fluid between ovaries affected with
endometriosis and those not affected (66). These data suggest that high levels
of iron in ovarian endometrioma do not affect oocyte quality as
they are less likely to spread through the cyst wall. Despite
conflicting data, a number of studies support the notion that
iron-induced oxidative stress impairs fertility in women with
endometriosis (2,52,56,59,63,64).
The iron concentration that adversely affects
fertility is unknown. In another study, basic research on
iron-related oxidative stress was performed on cerebral hemorrhage.
In animal models, recovery, injury, sequelae, or death from
intracerebral hemorrhage may depend on the degree of hemolysis,
iron release and oxidative stress that occurs following hemorrhage
(67). Therefore, iron chelators
may prevent neuronal death induced by oxidative stress. Experiments
using Medaka fish have demonstrated that oxidation products (e.g.,
Fe3+) at lower mg/l levels can induce reproductive
toxicity through oxidative stress (68). Although humans and fish cannot be
compared, taking the serum iron levels (>1.73 mg/l) of women
with endometriosis into consideration, the possibility of
reproductive toxicity due to direct gonadal damage cannot be ruled
out.
The oxidant-antioxidant imbalance
Herein, the oxidant-antioxidant balance in bodily
fluids in patients with endometriosis is reviewed. Several studies
have identified a variety of indicators of oxidative stress and
antioxidant capacity. Some indicators of oxidative stress, such as
ROS and iron, are elevated in the endometriosis group compared to
other infertile women without endometriosis or tubal infertility
(69). Extensive damage to DNA by
hemoglobin species is reflected as an increase in
8-hydroxy-2'-deoxyguanosine (8-OHdG) levels in endometriotic cyst
fluid (70) and cell nuclei
(71). There is evidence suggests
that women with stage I/II endometriosis have higher levels of
8-OHdG in follicular fluid compared to the controls (24.21±8.56 vs.
17.22±5.6 ng/ml) (72,73). Even in the early stages of
endometriosis, they are exposed to oxidative stress and are
associated with reduced oocyte quality (72). In addition, the levels of markers
of acute oxidative stress, such as malondialdehyde (MDA), the most
mutagenic product of lipid peroxidation (2), carbonyls and lipohydroperoxides
(LOOH) (65), myeloperoxidase
(MPO) (74), lipid peroxidation
(LPO) (69) and oxidized
low-density lipoprotein (oxLDL) (2) are elevated in the peripheral blood,
peritoneal fluids, or follicular microenvironments in patients with
endometriosis (73,75). The levels of oxidative stress,
represented by LOOH and MPO, are associated with the severity of
endometriosis, and are inversely associated with the percentage of
mature oocytes, the implantation rate and clinical pregnancy rate
(8,69,74-76).
These data indicate that oxidative stress exerts a major effect on
oocyte, sperm, and embryo quality and function.
Contrary to the level of oxidative stress, the
levels of several antioxidant molecules are significantly lower in
patients with endometriosis than in those without endometriosis.
Molecules associated with antioxidant capacity include a subunit of
the cystine/glutamate transporter xCT (SLC7A11), glutathione
peroxidase 4 (GPX4) and the glutamate-cysteine ligase (GCLC),
glutathione reductase (GR), catalase, superoxide dismutase (SOD),
iron metabolism genes transferrin receptor 1 (TfR1), ferroportin
(Fpn), heme oxygenase 1 (HO-1) and ferritin that are regulated by
the antioxidant response element of the transcription factor,
nuclear factor, erythroid 2 like 2 (NRF2) (52,69,77,78).
Insufficient antioxidant capacity promotes harmful ROS formation
(52). In addition, recent studies
have focused on the antioxidants, HO-1(79) and CD44v9(71). HO-1 is constitutively present in M2
phenotype macrophages and exerts beneficial protective effects
against oxidative damage. In addition, CD44v9 expressed in
endometriotic cells stabilizes the glutamate-cystine transporter
xCT, thereby decreasing the intracellular levels of ROS (71). The CD44v9 system is involved in
survival, anti-apoptosis and anti-oxidative stress by maintaining
higher levels of antioxidants (80). In an oxidative stress environment,
iron-dependent lipid peroxidation leads to regulated non-apoptotic
cell death, also known as ferroptosis (78,81).
Endometriosis is characterized by resistance to ferroptosis
(82). In addition to
endometriosis, ferroptosis has been implicated in the pathological
cell death associated with degenerative diseases, stroke,
intracerebral hemorrhage, traumatic brain injury,
ischemia-reperfusion injury, kidney degeneration and carcinogenesis
(81). It is necessary to
elucidate the detailed mechanisms of avoiding ferroptosis in
endometriosis with reference to the molecular mechanisms of these
diseases.
It would also be of interest to elucidate the
mechanisms through which the oxidant-antioxidant imbalance
adversely affects fertility. There are considerable basic and
clinical studies supporting that ROS affects human fertility.
Notarstefano et al reported that the oxidative
stress-induced dysregulation of lipid and carbohydrate metabolism
occurs in the ovary affected by endometriotic lesions, as well as
in the contralateral healthy ovary (83). This cannot be explained by the
direct action of endometriosis alone, but by biological,
biochemical, genetic, or epigenetic modifications which may occur
in pelvic organs possibly through ROS-induced oxidative stress. It
has been demonstrated that the redox balance also affects the
clinical performance of ART. Follicular fluid 8-OHdG levels have
been shown to be inversely associated with the fertilization rate
following intra-cytoplasmic sperm injection (ICSI) (84). In addition, the levels of
follicular fluid and seminal plasma MDA are inversely associated
with the IVF results (85). On the
other hand, follicular fluid total GSH levels, total antioxidant
capacity (TAC) and total systemic antioxidant response (TAR) are
positively associated with the fertilization rate following ICSI
(86). Previously, a positive
association was observed between the increased expression of SOD1
in cumulus cells and the clinical pregnancy rate (87). Given that patients with
endometriosis have a lower follicular total GSH activity and that
IVF-ET/ICSI is successful in patients with endometriosis with a
high blood antioxidant capacity, it is likely that the
oxidant-antioxidant balance has a significant effect on fertility
(86). Since ROS in the culture
medium are known to adversely affect the post-fertilization
cleavage rate and blastocyst yield and quality (76), the supplementation of antioxidants
to the medium may increase the success rate of ART through the
reduction of oxidative stress (13). The degree of oxidative stress in
follicular fluid, peritoneal fluid and peripheral blood may be
involved in infertility by regulating the expression of genes
encoding cytokines and immunomodulators that are associated with
sustained inflammatory response and immune dysregulation (14). Taken together, these findings
strongly suggest that changes in the microenvironment due to
oxidative stress contribute to infertility.
Apart from endometriosis, the oxidant-antioxidant
imbalance has been described in several pathophysiological
conditions, including reproductive disorders, such as polycystic
ovary syndrome (PCOS) and infertility of unknown origin (13). In addition, being overweight,
smoking and alcohol consumption may affect fertility by promoting
excessive amounts of free radical production (13). Therefore, it is well known that a
healthy lifestyle can maintain good fertility.
Conversely, certain studies have demonstrated
opposite results, indicating that oxidative stress does not affect
reproductive function. The total oxidant status and antioxidant
capacity in the follicular fluid of patients with unilateral
ovarian endometrioma have been shown to not differ from those
without endometrioma (88).
Furthermore, oxidative stress in follicular fluid has been shown to
not impair oocyte fertility (89).
The association between oxidative stress and fertility remains
controversial; however, a number of studies have demonstrated
increased oxidative stress and reduced fertility in patients with
endometriosis (2,52,58,64).
There are at least two possible mechanisms for
endometriosis-related infertility: First, excessive oxidative
stress in follicular fluid leads to reduced antioxidant capacity,
and second, low antioxidant capacity results in high levels of
oxidative stress.
If oxidative stress is really involved in the
development of endometriosis, available antioxidants or iron
chelators can be selected as novel therapeutic targets. In
preliminary experiments using animals, antioxidants may be possible
therapeutic candidates for endometriosis-related fibrosis, which
are lipophilic antioxidants, including vitamin E, polyphenols,
ferrostatin-1 (Fer-1), or liproxstatin-1 (Lip-1) (90). Epigallocatechin-3-gallate (EGCG)
suppresses endometriosis-associated fibrosis by inhibiting the
transforming growth factor-1β (TGF-β1)-stimulated activation of
Smad and mitogen-activated protein kinase (MAPK) signaling pathways
(91). The injection of an iron
chelator, deferoxamine, into a mouse model of endometriosis
suppressed the growth of endometriotic lesions (92). Certain antioxidants, such as EGCG
and deferoxamine, are effective in the treatment of endometriosis
and fibrosis. There are only a few animal studies to identify
effective therapeutic candidates for endometriosis (91,92).
Showell et al reviewed whether oral antioxidant supplements
improve fertility in infertile women (93). Limited evidence suggests that
antioxidants, such as N-acetyl-cysteine, melatonin, L-arginine,
carnitine, selenium, vitamin E, vitamin B, vitamin C, vitamin D and
CoQ10, improve fertility (93). To
date, to the best of our knowledge, no clinical studies have been
conducted using available antioxidant supplements exclusively in
infertile women with endometriosis.
Iron-dependent progression of
endometriosis
It would be of interest to determine the reasons
why endometriosis requires toxic iron for its development and
progression. The authors have previously summarized the recent
advances in our understanding of the underlying epigenetic
mechanisms focusing on oxidative stress in endometriosis (94). The hypothesis is as follows: Iron
modulates the expression of genes involved in methylation, creating
an environment that contributes to impaired decidualization and
favors the growth of endometriotic cells (94). Iron-induced oxidative stress alters
the activity of enzymes responsible for the demethylation and
deacetylation of histones (95)
and regulates the expression of CpG demethylases, such as
ten-eleven translocation (TET) and jumonji (JMJ) (94). These demethylases recognize a wide
range of endogenous DNA methylation (94). The steroid hormone-mediated
decidual signaling pathway has been shown to be dysregulated in
endometriosis through the modification of DNA methylation. DNA
methylation is also closely involved in epithelial-mesenchymal
transition (EMT), such as the suppression of E-cadherin, and the
increased expression of vascular endothelial growth factor (VEGF)
(96), which may contribute to the
development and progression of endometriosis. There are at least 2
distinct phases of epigenetic modification in endometriosis: The
initial wave of iron-induced oxidative stress would be followed by
the second big wave of epigenetic modulation of endometriosis
susceptibility genes (94). Taken
together, endometrial and endometriotic foci with localized iron
overload are important to the subsequent pathophysiology of the
development of endometriosis. Oxidative stress is required for
endometriosis to survive and, as the lesion progresses, it causes
symptoms, such as pain and infertility.
9. Endometriosis-associated fibrosis: An
update on molecular aspects
It is generally considered that the greater the
number of anatomical distortions due to endometriosis adhesions and
fibrosis, the more often infertility occurs. Furthermore, soluble
factors such as inflammation, oxidative stress, endocrine
abnormalities and immunological disturbances are also important
causes of infertility in women with endometriosis (15,42-45).
Chronic wounds, such as not only endometriosis, but also diabetic
foot wounds and ulcers, usually deteriorate into non-healing
wounds, undergoing repeated tissue injury and repair (40,97,98).
Endometriosis-related fibrosis is triggered by an inflammatory
response, causing EMT, fibroblast-myofibroblast
transdifferentiation (FMT) and smooth muscle metaplasia (SMM), and
repeating this cycle (4,40,97,98).
Guo et al found that older endometriotic cysts had higher
concentrations of total bilirubin, ferritin, free iron and
collagen, exhibited higher density and viscosity, and also
exhibited stronger adhesions than younger cysts (97). Over time, ROS from accumulated iron
promote fibrosis, forming strong adhesions around endometriotic
lesions and eventually replacing the ovaries with fibrous tissue
(99). Fibrosis also physically
causes vascular damage, which can result in follicular loss,
reduced ovarian reserve and infertility (4,99,100).
The major cell types involved in the development of
endometriosis-associated fibrosis are platelets, various
inflammatory cells, such as macrophages, T lymphocytes, B
lymphocytes and NK cells, ectopic endometrial cells and sensory
nerve fibers (101,102). These cells produce key effectors.
such as fibrosis-related cytokines and chemokines (TGF-β, TNF-α,
IL-1 and L-6), a variety of neuropeptides, injury-related molecular
patterns, such as damage-associated molecular pattern (DAMP) and
receptor for advanced glycation end-products (RAGE) (101,102). This chapter describes the
molecular mechanisms involved in endometriosis fibrosis on a
cell-by-cell basis. The molecular mechanisms that regulate
endometriosis-associated pain, infertility and fibrosis are
illustrated in Fig. 2.
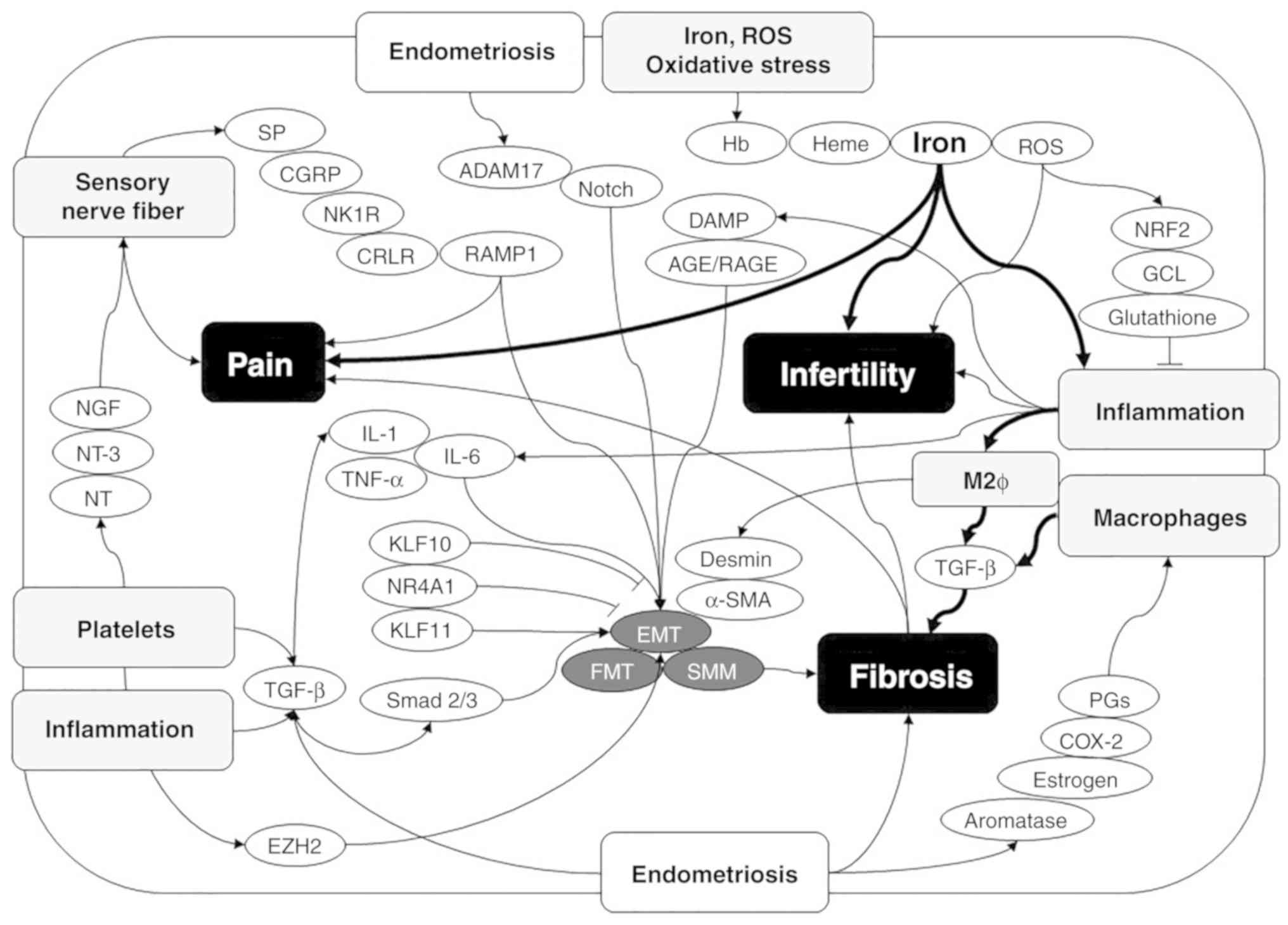 | Figure 2Molecular mechanisms underlying the
regulation of endometriosis-associated pain, infertility and
fibrosis. A variety of symptoms of endometriosis, such as pain,
infertility and fibrosis, involve interrelated signaling
mechanisms. First, endometriosis-associated pain symptoms are
caused by humoral factors from inflammation and activated
platelets, resulting in sensory nerve hyperinnervation. Second,
infertility may be affected by oxidative stress, inflammation and
finally fibrosis. Finally, fibrosis is formed by the interaction of
inflammation, M2 macrophages, activated platelets, sensory nerve
hyperinnervation, oxidative stress and iron. ADAM17, a disintegrin
and metalloproteinase-17; α-SMA, α-smooth muscle actin; CGRP,
calcitonin gene-related peptide; COX-2, cyclooxygenase-2; CRLR,
calcitonin receptor like receptor; EZH2, enhancer of zeste 2
polycomb repressive complex 2 subunit; GCL, glutamate-cysteine
ligase; Hb, hemoglobin; IL-1, interleukin-1; IL-6, interleukin-6;
KLF10, Kruppel like factor 10; KLF11, Kruppel like factor 11; NGF,
nerve growth factor; NK1R, neurokinin 1 receptor; NR4A1, nuclear
receptor subfamily 4 group A member 1; NRF2, nuclear factor,
erythroid 2 like 2; NT, neurotrophin; NT-3, neurotrophin-3; PGs,
prostaglandins; SP, substance P; RAMP1, receptor activity modifying
protein 1; ROS, reactive oxygen species; TGF-β, transforming growth
factor-β; and TNF-α, tumor necrosis factor-α. |
Platelets
Platelets are involved in endometriosis-associated
fibrosis, immune regulation and hyperinnervation. TGF-β1 produced
by activated platelets promotes fibrosis through the TGF-β/Smad
signaling pathway in endometriosis (103). Furthermore, endometrial stromal
cells, upon exposure to activated platelets, induce alpha-smooth
muscle actin (SMA) expression and promote fibrosis (103). These results indicate that
neurotrophic factors produced by activated platelets around the
lesions play an important role in endometriosis-related fibrosis
(38,103). Activated platelets are also
involved in organ fibrosis in a number of diseases other than
endometriosis. Platelet-derived TGF-β1 stimulates pathological
fibrosis in a variety of important organs such as the heart, liver,
lungs and kidneys (104).
Furthermore, platelet-derived TGF-β1 induces the reduced
cytotoxicity of NK cells in patients with endometriosis, suggesting
that platelets may directly participate in the progression of
endometriosis as immune-like cells (105). Finally, human platelets are rich
in neurotrophic factors, which consist of nerve growth factor
(NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin 3
(NT-3) and neurotrophin 4/5 (NT4/5) (39). The members of the neurotrophin
family are essential for the development, maturation, maintenance
and homeostasis of the nervous system, particularly by regulating
cell differentiation, survival, synaptic growth and death (39). Endometriotic lesions and
surrounding tissues can be hyperinnervated, possibly by NTs
secreted by platelets (38).
Furthermore, the neurotrophic factors induce the differentiation of
sensory neurons, which are involved in all major steps in the wound
healing and fibrosis of endometriotic lesions (38). Substance P (SP), also referred to
as neurokinin 1 (NK1), induces EMT, FMT, SMM and cell
contractility, produces collagen and matrix proteins, exacerbates
fibrosis and hyperalgesia, and increases lesion weight via
neurokinin 1 receptor (NK1R) activation (38,41).
Therefore, SP and its receptor, NK1R, may be candidate molecular
targets for the treatment of fibrosis.
Macrophages
In response to tissue damage, a variety of
inflammatory cells (macrophages and monocytes) and other cells
(hematopoietic, stromal and stem cells) are recruited to tissue
injury sites where they promote changes in the microenvironment
(98). Among the inflammatory cell
types, tissue macrophages and inflammatory monocytes are involved
in all processes of initiation, maintenance and the resolution of
inflammation (40,106). Macrophages adapt to environmental
changes to alter proinflammatory (M1) and anti-inflammatory (M2)
phenotypes and functions (106).
High ROS levels induced by iron overload promote macrophages to
polarize into M1-type macrophages (107). On the other hand, sustained
increases in iron levels lead to polarization toward the type 2
response (108). Macrophages
polarize over time toward the M2 subtype to reduce inflammation and
tissue destruction by M1 macrophages. Research using experimental
animal models has demonstrated that M2 macrophages induce EMT, FMT
and SMM to promote endometriosis-associated fibrosis (40). Iron establishes a vicious cycle
through the activation of macrophages, changes in polarity,
increased oxidative stress and enhanced fibrosis (59,109). Since fibrosis or scarring due to
persistent inflammation impairs ovarian function, M1/M2 macrophages
may represent a novel therapeutic target (98).
Sensory nerve fibers
Among the various phenotypes of endometriosis, DIE
in particular is known to be hyperinnervated due to its proximity
to the pelvic plexus (41).
Compared with ovarian endometrioma, several neuropeptide hormones
are overexpressed in DIE, resulting in higher nerve fiber density
(41). Neuropeptides such as SP,
NK1R, calcitonin receptor like receptor (CRLR), receptor activity
modifying protein 1(RAMP-1) and calcitonin gene-related peptide
(CGRP) are likely to play a role in the bidirectional communication
both within the nervous system and between neurons and
endometriotic cells (41). The
above-mentioned neuropeptide hormones induce the expression of
genes related to EMT and fibrosis, such as α-SMA, desmin, oxytocin
receptor and smooth muscle myosin heavy chain (41). The severity of DIE is associated
with the expression levels of neuropeptides, suggesting that
sensory nerves play a significant role in endometriosis-associated
pain and fibrosis (41).
Furthermore, neuropeptide hormones, such as NGF and NT-3 secreted
from peritoneal lesions promote the growth of nerve fibers in and
around peritoneal endometriotic lesions, resulting in adhesions and
pain (110).
Candidate markers for fibrotic
endometriosis
This chapter summaries individual markers strongly
associated with endometriosis-related fibrosis.
TGF-β1. Peritoneal mesothelial cells are
damaged in the inflammatory process, which activates
pro-inflammatory mediators, including peritoneal cytokines (TGF-α,
TGF-β, TNF-α, IL-1, IL-6 and prostaglandins), produces growth
factors (VEGF, epidermal growth factor and platelet-derived growth
factor) and promotes the fibrin/coagulation cascade [thrombin,
tissue factor, plasminogen activator inhibitor (PAI)-1/2], leading
to tissue healing and repair (111). Among these mediators, TGF-β1 is
predominantly expressed by macrophages, peritoneal mesothelial
cells and endometriotic cells, and promotes cell growth, migration,
extracellular matrix production, adhesions and fibrosis possibly
through the TGF-β1/Smad and TGF-β1/hypoxia-inducible factor-1
(HIF-1) signaling pathways (103,111). Furthermore, TGF-β1 strongly
induces peritoneal mesothelial-mesenchymal transition, adhesion and
ultimately fibrosis formation in the presence of inflammatory
cytokine such as IL-6 (103,111). Thus, TGF-β is considered to be
the most important factor in endometriosis-related fibrosis
(100).
Nuclear receptor subfamily 4 group A member 1
(NR4A1). Zeng et al found that the nuclear transcription
factor, NR4A1, reduced fibrosis through the suppression of the
TGF-β/Smad signaling pathway (100). In in vitro studies, as
well as in animal experiments, NR4A1 suppressed the progression of
fibrosis though the downregulation of collagen type I alpha 1 chain
(COL1A1) expression (100). NR4A1
may thus serve as the molecular target and therapy for the
management of fibrosis (100).
Kruppel like factor 11 (KLF11). The
transcription factor, KLF11, binds to the promoter regions of
collagen, matrix metalloproteinases and TGF-β family genes in
endometrial stromal cells and suppresses their protein expression,
resulting in inhibition of fibrosis formation (112). In an animal model of
endometriosis, Klf11-/- animals were shown to develop
multiple fibrous adhesions (112). On the other hand, Klf10, a
paralog of Klf11, exerts the opposite effect of Klf11 and promotes
fibrosis (113).
Aromatase. Endometrial cells that
overexpress aromatase protect themselves from destruction by
activated macrophages via estrogen production (114). Keskin et al found that the
aromatase inhibitor reduced postoperative adhesion formation in the
rat uterine horn experimental model (115). Maia et al demonstrated
that aromatase was overexpressed in endometriotic cells through an
epigenetic alteration that stimulated the demethylation of the
promoter region (114). On the
other hand, however, enhanced estrogen production by aromatase
overexpression promotes prostaglandin synthesis by stimulating
COX-2 activity (114). In
addition, prostaglandin is involved in tissue remodeling by
promoting angiogenesis and fibrosis (114). Although the molecular mechanisms
of adhesion and fibrosis may differ, the effect of aromatase on
fibrosis remains controversial.
Enhancer of zeste 2 polycomb repressive complex
2 subunit (EZH2). EZH2 is a subunit of the polycomb repressive
complex 2 (PRC2) catalyzing the trimethylation of histone H3 lysine
27 (H3K27) (116). Xiao et
al found that EZH2 induced angiogenesis, EMT, myofibroblast
transformation and tissue fibrosis through the TGFβ/Smad signaling
pathway and the upregulation of vimentin and transcription factors,
such as Snail and Slug, and the downregulation of E-cadherin
(116). Zhang et al found
that EZH2 was elevated not only in the ectopic endometrium of
endometriosis, but also in the eutopic endometrium compared to the
control endometrium (117). In
fact, an EZH2 inhibitor, 3-deazaneplanocin A (DZNep), inhibited
cell growth, EMT and fibrosis in a preclinical mouse model of
endometriosis (117). Activated
platelets also upregulate EZH2 expression, leading to endometrial
invasion and fibrosis (117).
EZH2 is an important epigenetic regulator as a marker of
endometriosis-associated fibrosis and may prove to be a potential
therapeutic candidate for the suppression of fibrosis.
NRF2. NRF2, a downstream target of the
Kelch-like ECH-associated protein (KEAP1), upregulates
anti-inflammatory gene expression and inhibits the progression of
inflammation via the antioxidant response element (ARE) signaling
pathway (118). Marcellin et
al found that NRF2 expression in ectopic endometrial tissue was
decreased compared to the eutopic endometrium in women with
endometriosis and without disease (119). The reduced NRF2 gene expression
may suppress the transcription of endogenous antioxidants, such as
glutathione and activate oxidative damage and inflammatory
response, thereby promoting the progression and fibrosis of
endometriosis (119).
Furthermore, a study using a mouse model of endometriosis revealed
that transplantation of NRF2-/- cells was more fibrotic
compared to wild-type cells (119). These data suggest that NRF2 may
be a potential candidate for the suppression of fibrotic
endometriosis.
A disintegrin and metalloproteinase-17
(ADAM17). Tissue damage upregulates the expression of ADAM17,
promotes macrophages and neutrophil infiltration, and eventually
causes further fibrosis (120).
González-Foruria et al found that oxidative stress in
endometriosis caused fibrosis via the ADAM17/Notch signaling
pathway (12). The Notch pathway
is involved in the regulation of myofibroblast differentiation in
chronic fibrosis including in the lung, kidney, liver, heart and
skin (121). Therefore, the
ADAM17/Notch signaling pathway is essential for fibrosis in diverse
organs and tissues (121).
Lysyl oxidase (LOX). LOX plays crucial roles
in tissue remodeling and fibrosis due to its crosslinking of
soluble collagen and elastin into insoluble, mature fibers
(122). Elevated LOX levels
catalyze collagen crosslinking to induce irreversible chronic
fibrosis in a variety of organs, including the kidneys. LOX is also
involved in the progression of endometriosis by promoting cell
invasion, EMT and fibrosis (122).
Others. In addition to the mediators
mentioned above, endometriosis-associated fibrosis also involves
damage-associated molecular patterns (DAMP), pattern recognition
receptors, advanced glycation end products (AGE), receptor for
advanced glycation end products (RAGE), extracellular lactate and
increased glycolysis (111). The
activation of pattern recognition receptor by DAMP stimulates cells
of the innate immune system to further activate toxic inflammatory
responses, promoting myofibroblast activation, fibrosis, or cell
death (123). High-mobility group
box-1 (HMGB1), a member of the DAMP family, increases in response
to tissue damage infection, inflammation, immune responses and
fibrosis by binding to specific cell-surface receptors (124). The expression of RAGE (125) and its ligand, HMGB1(124), is increased in endometriosis. Cao
et al reported that HMGB1 participated in the pathogenesis
of endometriosis-associated fibrosis though RAGE signaling
mechanisms (124).
10. Conclusion and future perspectives
Currently, the assessment of disease severity for
endometriosis is based on anatomical findings; however, no single
classification or scoring system has proven satisfactory. In order
to solve this issue and create a disease severity score tailored to
each symptom of endometriosis, not only anatomical findings, but
also biochemical parameters that objectively reflect the severity
of the disease are required. Oxidative stress and inflammation
serve as common biomarkers for the characteristic symptoms of
endometriosis, as illustrated by the bold curved arrows in Fig. 2. Among these biomarkers, the level
of iron in peritoneal fluid, ovarian endometrioma and peripheral
blood may reflect the severity of the disease. Future studies on
easily available biomarkers will provide useful information for the
development of novel classification systems for endometriosis. Both
hormonal therapy and surgical resection are currently the main
treatments for endometriosis; however, the elucidation of etiology
and pathophysiology will provide non-hormonal treatments to women
suffering from severe pain and women who wish to become pregnant
(126). The management of
endometriosis should be individualized, considering the patient's
background, risk factors and disease severity (16). The present review may prove to be
useful for exploring available biomarkers that are associated with
endometriosis-specific disease severity such as pain, infertility
and fibrosis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Japan
Society for the Promotion of Science (JSPS) Grants-in-Aid for
Scientific Research (KAKENHI) (grant nos. JP16K11150, 18K09269 and
18K09234 to HK).
Availability of data and materials
Not applicable.
Author's contributions
SI, SM, MK and MN performed the literature search
and collected data using web-based databases. SI and HK made
substantial contribution to conception of the study. SI contributed
to the study design and interpretation of the included research
studies. The final version of the manuscript has been read and
approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dunselman GA, Vermeulen N, Becker C,
Calhaz-Jorge C, D'Hooghe T, De Bie B, Heikinheimo O, Horne AW,
Kiesel L, Nap A, et al: ESHRE guideline: Management of women with
endometriosis. Hum Reprod. 29:400–412. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Scutiero G, Iannone P, Bernardi G,
Bonaccorsi G, Spadaro S, Volta CA, Greco P and Nappi L: Oxidative
stress and endometriosis: A systematic review of the literature.
Oxid Med Cell Longev. 2017(7265238)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Adamson GD: Endometriosis classification:
An update. Curr Opin Obstet Gynecol. 23:213–220. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Donnez J, Donnez O, Orellana R, Binda MM
and Dolmans MM: Endometriosis and infertility. Panminerva Med.
58:143–150. 2016.PubMed/NCBI
|
|
5
|
Spaczynski RZ and Duleba AJ: Diagnosis of
endometriosis. Semin Reprod Med. 21:193–208. 2003.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Guerriero S, Condous G, van den Bosch T,
Valentin L, Leone FP, Van Schoubroeck D, Exacoustos C, Installé AJ,
Martins WP, Abrao MS, et al: Systematic approach to sonographic
evaluation of the pelvis in women with suspected endometriosis,
including terms, definitions and measurements: A consensus opinion
from the international deep endometriosis analysis (IDEA) group.
Ultrasound Obstet Gynecol. 48:318–332. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Reid S and Condous G: Update on the
ultrasound diagnosis of deep pelvic endometriosis. Eur J Obstet
Gynecol Reprod Biol. 209:50–54. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Da Broi MG and Navarro PA: Oxidative
stress and oocyte quality: Ethiopathogenic mechanisms of
minimal/mild endometriosis-related infertility. Cell Tissue Res.
364:1–7. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sanchez AM, Vanni VS, Bartiromo L, Papaleo
E, Zilberberg E, Candiani M, Orvieto R and Viganò P: Is the oocyte
quality affected by endometriosis? A review of the literature. J
Ovarian Res. 10(43)2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Laganà AS, Garzon S, Götte M, Viganò P,
Franchi M, Ghezzi F and Martin DC: The pathogenesis of
endometriosis: Molecular and cell biology insights. Int J Mol Sci.
20: pii(E5615)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Rolla E: Endometriosis: Advances and
controversies in classification, pathogenesis, diagnosis, and
treatment. F1000Res. 8: pii(F1000): Faculty Rev-529.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
González-Foruria I, Santulli P, Chouzenoux
S, Carmona F, Chapron C and Batteux F: Dysregulation of the
ADAM17/Notch signalling pathways in endometriosis: From oxidative
stress to fibrosis. Mol Hum Reprod. 23:488–499. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Agarwal A, Aponte-Mellado A, Premkumar BJ,
Shaman A and Gupta S: The effects of oxidative stress on female
reproduction: A review. Reprod Biol Endocrinol.
10(49)2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Van Langendonckt A, Casanas-Roux F and
Donnez J: Oxidative stress and peritoneal endometriosis. Fertil
Steril. 77:861–870. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gupta S, Goldberg JM, Aziz N, Goldberg E,
Krajcir N and Agarwal A: Pathogenic mechanisms in
endometriosis-associated infertility. Fertil Steril. 90:247–257.
2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chapron C, Marcellin L, Borghese B and
Santulli P: Rethinking mechanisms, diagnosis and management of
endometriosis. Nat Rev Endocrinol. 15:666–682. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Holland TK, Yazbek J, Cutner A, Saridogan
E, Hoo WL and Jurkovic D: Value of transvaginal ultrasound in
assessing severity of pelvic endometriosis. Ultrasound Obstet
Gynecol. 36:241–248. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zanardi R, Del Frate C, Zuiani C and
Bazzocchi M: Staging of pelvic endometriosis based on MRI findings
versus laparoscopic classification according to the American
fertility society. Abdom Imaging. 28:733–742. 2003.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guerriero S, Ajossa S, Garau N, Alcazar
JL, Mais V and Melis GB: Diagnosis of pelvic adhesions in patients
with endometrioma: The role of transvaginal ultrasonography. Fertil
Steril. 94:742–746. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gerges B, Lu C, Reid S, Chou D, Chang T
and Condous G: Sonographic evaluation of immobility of normal and
endometriotic ovary in detection of deep endometriosis. Ultrasound
Obstet Gynecol. 49:793–798. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
De Wilde RL, Alvarez J, Brölmann H, Campo
R, Cheong Y, Lundorff P, Pawelczyk L, Roman H, di Spiezio Sardo A
and Wallwiener M: Adhesions and endometriosis: Challenges in
subfertility management: (An expert opinion of the ANGEL-the
anti-adhesions in gynaecology expert panel-group). Arch Gynecol
Obstet. 294:299–301. 2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ichikawa M, Akira S, Kaseki H, Watanabe K,
Ono S and Takeshita T: Accuracy and clinical value of an adhesion
scoring system: A preoperative diagnostic method using transvaginal
ultrasonography for endometriotic adhesion. J Obstet Gynaecol Res.
46:466–478. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Haas D, Shebl O, Shamiyeh A and Oppelt P:
The rASRM score and the Enzian classification for endometriosis:
Their strengths and weaknesses. Acta Obstet Gynecol Scand. 92:3–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tuttlies F, Keckstein J, Ulrich U,
Possover M, Schweppe KW, Wustlich M, Buchweitz O, Greb R, Kandolf
O, Mangold R, et al: ENZIAN-score, a classification of deep
infiltrating endometriosis. Zentralbl Gynakol. 127:275–281.
2005.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Morgan-Ortiz F, López-de la Torre MA,
López-Zepeda MA, Morgan-Ruiz FV, Ortiz-Bojórquez JC and
Bolívar-Rodríguez MA: Clinical characteristics and location of
lesions in patients with deep infiltrating endometriosis using the
revised Enzian classification. J Turk Ger Gynecol Assoc.
20:133–137. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haas D, Wurm P, Shamiyeh A, Shebl O,
Chvatal R and Oppelt P: Efficacy of the revised Enzian
classification: A retrospective analysis. Does the revised Enzian
classification solve the problem of duplicate classification in
rASRM and Enzian? Arch Gynecol Obstet. 287:941–945. 2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Endometriosis stages: Understanding the
different stages of endometriosis (https://www.endofound.org/endometriosis-stages).
|
|
28
|
Adamson GD and Pasta DJ: Endometriosis
fertility index: The new, validated endometriosis staging system.
Fertil Steril. 94:1609–1615. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Andres MP, Borrelli GM and Abrão MS:
Endometriosis classification according to pain symptoms: Can the
ASRM classification be improved? Best Pract Res Clin Obstet
Gynaecol. 51:111–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Revised American society for reproductive
medicine classification of endometriosis: 1996. Fertil Steril 67:
817-821, 1997.
|
|
31
|
Sapkota Y, Attia J, Gordon SD, Henders AK,
Holliday EG, Rahmioglu N, MacGregor S, Martin NG, McEvoy M, Morris
AP, et al: Genetic burden associated with varying degrees of
disease severity in endometriosis. Mol Hum Reprod. 21:594–602.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Stovall DW, Bowser LM, Archer DF and
Guzick DS: Endometriosis-associated pelvic pain: Evidence for an
association between the stage of disease and a history of chronic
pelvic pain. Fertil Steril. 68:13–18. 1997.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pop-Trajkovic S, Popović J, Antić V,
Radović D, Stefanović M and Vukomanović P: Stages of endometriosis:
Does it affect in vitro fertilization outcome. Taiwan J Obstet
Gynecol. 53:224–226. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Johnson NP, Hummelshoj L, Adamson GD,
Keckstein J, Taylor HS, Abrao MS, Bush D, Kiesel L, Tamimi R,
Sharpe-Timms K, et al: World Endometriosis Society consensus on the
classification of endometriosis. Hum Reprod. 32:315–324.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Haas D, Oppelt P, Shebl O, Shamiyeh A,
Schimetta W and Mayer R: Enzian classification: Does it correlate
with clinical symptoms and the rASRM score? Acta Obstet Gynecol
Scand. 92:562–566. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Wu J, Xie H, Yao S and Liang Y: Macrophage
and nerve interaction in endometriosis. J Neuroinflammation.
14(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Berkley KJ, Rapkin AJ and Papka RE: The
pains of endometriosis. Science. 308:1587–1589. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu X, Yan D and Guo SW: Sensory
nerve-derived neuropeptides accelerate the development and
fibrogenesis of endometriosis. Hum Reprod. 34:452–468.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Rocco ML, Soligo M, Manni L and Aloe L:
Nerve growth factor: Early studies and recent clinical trials. Curr
Neuropharmacol. 16:1455–1465. 2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Duan J, Liu X, Wang H and Guo SW: The M2a
macrophage subset may be critically involved in the fibrogenesis of
endometriosis in mice. Reprod Biomed Online. 37:254–268.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yan D, Liu X and Guo SW: Neuropeptides
substance P and calcitonin gene related peptide accelerate the
development and fibrogenesis of endometriosis. Sci Rep.
9(2698)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Azem F, Lessing JB, Geva E, Shahar A,
Lerner-Geva L, Yovel I and Amit A: Patients with stages III and IV
endometriosis have a poorer outcome of in vitro
fertilization-embryo transfer than patients with tubal infertility.
Fertil Steril. 72:1107–1109. 1999.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Garrido N, Navarro J, García-Velasco J,
Remoh J, Pellice A and Simón C: The endometrium versus embryonic
quality in endometriosis-related infertility. Hum Reprod Update.
8:95–103. 2002.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Augoulea A, Mastorakos G, Lambrinoudaki I,
Christodoulakos G and Creatsas G: The role of the oxidative-stress
in the endometriosis-related infertility. Gynecol Endocrinol.
25:75–81. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Jackson LW, Schisterman EF, Dey-Rao R,
Browne R and Armstrong D: Oxidative stress and endometriosis. Hum
Reprod. 20:2014–2020. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Leone Roberti Maggiore U, Scala C,
Venturini PL, Remorgida V and Ferrero S: Endometriotic ovarian
cysts do not negatively affect the rate of spontaneous ovulation.
Hum Reprod. 30:299–307. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu F, He L, Liu Y, Shi Y and Du H: The
expression and role of oxidative stress markers in the serum and
follicular fluid of patients with endometriosis. Clin Exp Obstet
Gynecol. 40:372–376. 2013.PubMed/NCBI
|
|
48
|
Harb HM, Gallos ID, Chu J, Harb M and
Coomarasamy A: The effect of endometriosis on in vitro
fertilisation outcome: A systematic review and meta-analysis. BJOG.
120:1308–1320. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Miller JE, Ahn SH, Monsanto SP, Khalaj K,
Koti M and Tayade C: Implications of immune dysfunction on
endometriosis associated infertility. Oncotarget. 8:7138–7147.
2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Practice Committee of the American Society
for Reproductive Medicine. Endometriosis and infertility: A
committee opinion. Fertil Steril. 98:591–598. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Pal L, Shifren JL, Isaacson KB, Chang Y,
Leykin L and Toth TL: Impact of varying stages of endometriosis on
the outcome of in vitro fertilization-embryo transfer. J Assist
Reprod Genet. 15:27–31. 1998.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Donnez J, Binda MM, Donnez O and Dolmans
MM: Oxidative stress in the pelvic cavity and its role in the
pathogenesis of endometriosis. Fertil Steril. 106:1011–1017.
2016.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Wang XM, Ma ZY and Song N: Inflammatory
cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid flora were
associated with infertility in patients with endometriosis. Eur Rev
Med Pharmacol Sci. 22:2513–2518. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yamauchi N, Harada T, Taniguchi F, Yoshida
S, Iwabe T and Terakawa N: Tumor necrosis factor-alpha induced the
release of interleukin-6 from endometriotic stromal cells by the
nuclear factor-kappaB and mitogen-activated protein kinase
pathways. Fertil Steril. 82 (Suppl 3):S1023–S1028. 2004.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Falconer H, Sundqvist J,
Gemzell-Danielsson K, von Schoultz B, D'Hooghe TM and Fried G: IVF
outcome in women with endometriosis in relation to tumour necrosis
factor and anti-Müllerian hormone. Reprod Biomed Online.
18:582–588. 2009.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Malvezzi H, Hernandes C, Piccinato CA and
Podgaec S: Interleukin in endometriosis-associated
infertility-pelvic pain: Systematic review and meta-analysis.
Reproduction. 158:1–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Beste MT, Pfäffle-Doyle N, Prentice EA,
Morris SN, Lauffenburger DA, Isaacson KB and Griffith LG: Molecular
network analysis of endometriosis reveals a role for
c-Jun-regulated macrophage activation. Sci Transl Med.
6(222ra16)2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Agarwal A, Gupta S and Sharma RK: Role of
oxidative stress in female reproduction. Reprod Biol Endocrinol.
3(28)2005.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Agarwal A, Gupta S, Sekhon L and Shah R:
Redox considerations in female reproductive function and assisted
reproduction: From molecular mechanisms to health implications.
Antioxid Redox Signal. 10:1375–1403. 2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yoshimoto C, Iwabuchi T, Shigetomi H and
Kobayashi H: Cyst fluid iron-related compounds as useful markers to
distinguish malignant transformation from benign endometriotic
cysts. Cancer Biomark. 15:493–499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Yamaguchi K, Mandai M, Toyokuni S,
Hamanishi J, Higuchi T, Takakura K and Fujii S: Contents of
endometriotic cysts, especially the high concentration of free
iron, are a possible cause of carcinogenesis in the cysts through
the iron-induced persistent oxidative stress. Clin Cancer Res.
14:32–40. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kobayashi H, Yamada Y, Kanayama S,
Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T and Oi
H: The role of iron in the pathogenesis of endometriosis. Gynecol
Endocrinol. 25:39–52. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Gammella E, Recalcati S, Rybinska I,
Buratti P and Cairo G: Iron-induced damage in cardiomyopathy:
Oxidative-dependent and independent mechanisms. Oxid Med Cell
Longev. 2015(230182)2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Alizadeh M, Mahjoub S, Esmaelzadeh S,
Hajian K, Basirat Z and Ghasemi M: Evaluation of oxidative stress
in endometriosis: A case-control study. Caspian J Intern Med.
6:25–29. 2015.PubMed/NCBI
|
|
65
|
Montoya-Estrada A, Coria-García CF,
Cruz-Orozco OP, Aguayo-González P, Torres-Ramos YD, Flores-Herrera
H, Hicks JJ, Medina-Navarro R and Guzmán-Grenfell AM: Increased
systemic and peritoneal oxidative stress biomarkers in
endometriosis are not related to retrograde menstruation. Redox
Rep. 24:51–55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Benaglia L, Paffoni A, Mangiarini A,
Restelli L, Bettinardi N, Somigliana E, Vercellini P and Fedele L:
Intrafollicular iron and ferritin in women with ovarian
endometriomas. Acta Obstet Gynecol Scand. 94:646–653.
2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Karuppagounder SS, Alim I, Khim SJ,
Bourassa MW, Sleiman SF, John R, Thinnes CC, Yeh TL, Demetriades M,
Neitemeier S, et al: Therapeutic targeting of oxygen-sensing prolyl
hydroxylases abrogates ATF4-dependent neuronal death and improves
outcomes after brain hemorrhage in several rodent models. Sci
Transl Med. 8(328ra29)2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Yang CH, Kung TA and Chen PJ: Differential
alteration in reproductive toxicity of medaka fish on exposure to
nanoscale zerovalent iron and its oxidation products. Environ
Pollut. 252:1920–1932. 2019.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Singh AK, Chattopadhyay R, Chakravarty B
and Chaudhury K: Markers of oxidative stress in follicular fluid of
women with endometriosis and tubal infertility undergoing IVF.
Reprod Toxicol. 42:116–124. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Fujimoto Y, Imanaka S, Yamada Y, Ogawa K,
Ito F, Kawahara N, Yoshimoto C and Kobayashi H: Comparison of redox
parameters in ovarian endometrioma and its malignant
transformation. Oncol Lett. 16:5257–5264. 2018.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Niiro E, Kawahara N, Yamada Y, Yoshimoto
C, Shimada K, Sudo T and Kobayashi H: Immunohistochemical
expression of CD44v9 and 8-OHdG in ovarian endometrioma and the
benign endometriotic lesions adjacent to clear cell carcinoma. J
Obstet Gynaecol Res. 45:2260–2266. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Da Broi MG, Jordão AA Jr, Ferriani RA and
Navarro PA: Oocyte oxidative DNA damage may be involved in
minimal/mild endometriosis-related infertility. Mol Reprod Dev.
85:128–136. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Da Broi MG, de Albuquerque FO, de Andrade
AZ, Cardoso RL, Jordão Junior AA and Navarro PA: Increased
concentration of 8-hydroxy-2'-deoxyguanosine in follicular fluid of
infertile women with endometriosis. Cell Tissue Res. 366:231–342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Santanam N, Zoneraich N and Parthasarathy
S: Myeloperoxidase as a potential target in women with
endometriosis undergoing IVF. Reprod Sci. 24:619–626.
2017.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Seino T, Saito H, Kaneko T, Takahashi T,
Kawachiya S and Kurachi H: Eight-hydroxy-2'-deoxyguanosine in
granulosa cells is correlated with the quality of oocytes and
embryos in an in vitro fertilization-embryo transfer program.
Fertil Steril. 77:1184–1190. 2002.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Agarwal A, Gupta S and Sharma R: Oxidative
stress and its implications in female infertility-a clinician's
perspective. Reprod Biomed Online. 11:641–650. 2005.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Choi YS, Cho S, Seo SK, Park JH, Kim SH
and Lee BS: Alteration in the intrafollicular thiol-redox system in
infertile women with endometriosis. Reproduction. 149:155–162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Conrad M, Kagan VE, Bayir H, Pagnussat GC,
Head B, Traber MG and Stockwell BR: Regulation of lipid
peroxidation and ferroptosis in diverse species. Genes Dev.
32:602–619. 2018.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Yamada Y, Uchiyama T, Ito F, Kawahara N,
Ogawa K, Obayashi C and Kobayashi H: Clinical significance of M2
macrophages expressing heme oxygenase-1 in malignant transformation
of ovarian endometrioma. Pathol Res Pract. 215:639–643.
2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Akasaka J, Uekuri C, Shigetomi H, Koike M
and Kobayashi H: Hepatocyte nuclear factor (HNF)-1β and its
physiological importance in endometriosis. Biomed Rep. 1:13–17.
2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Stockwell BR, Friedmann Angeli JP, Bayir
H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK,
Kagan VE, et al: A regulated cell death nexus linking metabolism,
redox biology, and disease. Cell. 171:273–285. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Ng SW, Norwitz SG, Taylor HS and Norwitz
ER: Endometriosis: The role of iron overload and ferroptosis.
Reprod Sci, Feb 19, 2020 (Epub ahead of print).
|
|
83
|
Notarstefano V, Gioacchini G, Byrne HJ,
Zacà C, Sereni E, Vaccari L, Borini A, Carnevali O and Giorgini E:
Vibrational characterization of granulosa cells from patients
affected by unilateral ovarian endometriosis: New insights from
infrared and Raman microspectroscopy. Spectrochim Acta A Mol Biomol
Spectrosc. 212:206–214. 2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Nishihara T, Matsumoto K, Hosoi Y and
Morimoto Y: Evaluation of antioxidant status and oxidative stress
markers in follicular fluid for human in vitro fertilization
outcome. Reprod Med Bio. 17:481–486. 2018.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Kumar S, Mishra V, Thaker R, Gor M,
Perumal S, Joshi P, Sheth H, Shaikh I, Gautam AK and Verma Y: Role
of environmental factors & oxidative stress with respect to in
vitro fertilization outcome. Indian J Med Res. 148
(Suppl):S125–S133. 2018.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Velthut A, Zilmer M, Zilmer K, Kaart T,
Karro H and Salumets A: Elevated blood plasma antioxidant status is
favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online.
26:345–352. 2013.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Donabela FC, Meola J, Padovan CC, de Paz
CC and Navarro PA: Higher SOD1 gene expression in cumulus cells
from infertile women with moderate and severe endometriosis. Reprod
Sci. 22:1452–1460. 2015.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Nakagawa K, Hisano M, Sugiyama R and
Yamaguchi K: Measurement of oxidative stress in the follicular
fluid of infertility patients with an endometrioma. Arch Gynecol
Obstet. 293:197–202. 2016.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Jozwik M, Wolczynski S, Jozwik M and
Szamatowicz M: Oxidative stress markers in preovulatory follicular
fluid in humans. Mol Hum Reprod. 5:409–413. 1999.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zilka O, Shah R, Li B, Friedmann Angeli
JP, Griesser M, Conrad M and Pratt DA: On the mechanism of
cytoprotection by ferrostatin-1 and liproxstatin-1 and the role of
lipid peroxidation in ferroptotic cell death. ACS Cent Sci.
3:232–243. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Matsuzaki S and Darcha C: Antifibrotic
properties of epigallocatechin-3-gallate in endometriosis. Hum
Reprod. 29:1677–1687. 2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Defrère S, Van Langendonckt A, Vaesen S,
Jouret M, González Ramos R, Gonzalez D and Donnez J: Iron overload
enhances epithelial cell proliferation in endometriotic lesions
induced in a murine model. Hum Reprod. 21:2810–2816.
2006.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Showell MG, Mackenzie-Proctor R, Jordan V
and Hart RJ: Antioxidants for female subfertility. Cochrane
Database Syst Rev. 7(CD007807)2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Ito F, Yamada Y, Shigemitsu A, Akinishi M,
Kaniwa H, Miyake R, Yamanaka S and Kobayashi H: Role of oxidative
stress in epigenetic modification in endometriosis. Reprod Sci.
24:1493–1502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Niu Y, DesMarais TL, Tong Z, Yao Y and
Costa M: Oxidative stress alters global histone modification and
DNA methylation. Free Radic Biol Med. 82:22–28. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Sun Q, Ding D, Liu X and Guo SW:
Tranylcypromine, a lysine-specific demethylase 1 (LSD1) inhibitor,
suppresses lesion growth and improves generalized hyperalgesia in
mouse with induced endometriosis. Reprod Biol Endocrinol.
14(17)2016.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Guo SW, Ding D, Shen M and Liu X: Dating
endometriotic ovarian cysts based on the content of cyst fluid and
its potential clinical implications. Reprod Sci. 22:873–883.
2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Capobianco A, Cottone L, Monno A, Manfredi
AA and Rovere-Querini P: The peritoneum: Healing, immunity, and
diseases. J Pathol. 243:137–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Sanchez AM, Viganò P, Somigliana E,
Panina-Bordignon P, Vercellini P and Candiani M: The distinguishing
cellular and molecular features of the endometriotic ovarian cyst:
From pathophysiology to the potential endometrioma-mediated damage
to the ovary. Hum Reprod Update. 20:217–230. 2014.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Zeng X, Yue Z, Gao Y, Jiang G, Zeng F,
Shao Y, Huang J, Yin M and Li Y: NR4A1 is Involved in fibrogenesis
in ovarian endometriosis. Cell Physiol Biochem. 46:1078–1090.
2018.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Viganò P, Ottolina J, Bartiromo L,
Bonavina G, Schimberni M, Villanacci R and Candiani M: Cellular
components contributing to fibrosis in endometriosis: A literature
review. J Minim Invasive Gynecol. 27:287–295. 2020.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Seki E and Schwabe RF: Hepatic
inflammation and fibrosis: Functional links and key pathways.
Hepatology. 61:1066–1079. 2015.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Zhang Q, Duan J, Liu X and Guo SW:
Platelets drive smooth muscle metaplasia and fibrogenesis in
endometriosis through epithelial-mesenchymal transition and
fibroblast-to-myofibroblast transdifferentiation. Mol Cell
Endocrinol. 428:1–16. 2016.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Guo SW, Du Y and Liu X: Platelet-derived
TGF-β1 mediates the down-modulation of NKG2D expression and may be
responsible for impaired natural killer (NK) cytotoxicity in women
with endometriosis. Hum Reprod. 31:1462–1474. 2016.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Wynn TA and Vannella KM: Macrophages in
tissue repair, regeneration, and fibrosis. Immunity. 44:450–462.
2016.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Zhou Y, Que KT, Zhang Z, Yi ZJ, Zhao PX,
You Y, Gong JP and Liu ZJ: Iron overloaded polarizes macrophage to
proinflammation phenotype through ROS/acetyl-p53 pathway. Cancer
Med. 7:4012–4022. 2018.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Agoro R, Taleb M, Quesniaux VFJ and Mura
C: Cell iron status influences macrophage polarization. PLoS One.
13(e0196921)2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Ngô C, Chéreau C, Nicco C, Weill B,
Chapron C and Batteux F: Reactive oxygen species controls
endometriosis progression. Am J Pathol. 175:225–234.
2009.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Barcena de Arellano ML, Arnold J, Lang H,
Vercellino GF, Chiantera V, Schneider A and Mechsner S: Evidence of
neurotrophic events due to peritoneal endometriotic lesions.
Cytokine. 62:253–261. 2013.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Wilson RB: Hypoxia, cytokines and stromal
recruitment: Parallels between pathophysiology of encapsulating
peritoneal sclerosis, endometriosis and peritoneal metastasis.
Pleura Peritoneum. 3(20180103)2018.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Daftary GS, Zheng Y, Tabbaa ZM,
Schoolmeester JK, Gada RP, Grzenda AL, Mathison AJ, Keeney GL,
Lomberk GA and Urrutia R: A novel role of the Sp/KLF transcription
factor KLF11 in arresting progression of endometriosis. PLoS One.
8(e60165)2013.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Delaney AA, Khan Z, Zheng Y, Correa LF,
Zanfagnin V, Shenoy CC, Schoolmeester JK, Saadalla AM, El-Nashar S,
Famuyide AO, et al: KLF10 mediated epigenetic dysregulation of
epithelial CD40/CD154 promotes endometriosis. Biol Reprod.
95(62)2016.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Maia H Jr, Haddad C, Coelho G and Casoy J:
Role of inflammation and aromatase expression in the eutopic
endometrium and its relationship with the development of
endometriosis. Womens Health (Lond). 8:647–658. 2012.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Keskin HL, Sirin YS, Keles H, Turgut O,
Ide T and Avsar AF: The aromatase inhibitor letrozole reduces
adhesion formation after intraperitoneal surgery in a rat uterine
horn model. Eur J Obstet Gynecol Reprod Biol. 167:199–204.
2013.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Xiao X, Senavirathna LK, Gou X, Huang C,
Liang Y and Liu L: EZH2 enhances the differentiation of fibroblasts
into myofibroblasts in idiopathic pulmonary fibrosis. Physiol Rep.
4: pii(e12915)2016.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Zhang Q, Dong P, Liu X, Sakuragi N and Guo
SW: Enhancer of Zeste homolog 2 (EZH2) induces
epithelial-mesenchymal transition in endometriosis. Sci Rep.
7(6804)2017.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Ahmed SM, Luo L, Namani A, Wang XJ and
Tang X: Nrf2 signaling pathway: Pivotal roles in inflammation.
Biochim Biophys Acta Mol Basis Dis. 1863:585–597. 2017.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Marcellin L, Santulli P, Chouzenoux S,
Cerles O, Nicco C, Dousset B, Pallardy M, Kerdine-Römer S, Just PA,
Chapron C and Batteux F: Alteration of Nrf2 and glutamate cysteine
ligase expression contribute to lesions growth and fibrogenesis in
ectopic endometriosis. Free Radic Biol Med. 110:1–10.
2017.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Kefaloyianni E, Muthu ML, Kaeppler J, Sun
X, Sabbisetti V, Chalaris A, Rose-John S, Wong E, Sagi I, Waikar
SS, et al: ADAM17 substrate release in proximal tubule drives
kidney fibrosis. JCI Insight. 1: pii(E87023)2016.PubMed/NCBI View Article : Google Scholar
|
|
121
|
Hu B and Phan SH: Notch in fibrosis and as
a target of anti-fibrotic therapy. Pharmacol Res. 108:57–64.
2016.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Ruiz LA, Báez-Vega PM, Ruiz A, Peterse DP,
Monteiro JB, Bracero N, Beauchamp P, Fazleabas AT and Flores I:
Dysregulation of lysyl oxidase expression in lesions and
endometrium of women with endometriosis. Reprod Sci. 22:1496–1508.
2015.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Kubes P and Mehal WZ: Sterile inflammation
in the liver. Gastroenterology. 143:1158–1172. 2012.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Cao Y, Liu X and Guo SW: Plasma high
mobility group Box 1 (HMGB1), osteopontin (OPN), and hyaluronic
acid (HA) as admissible biomarkers for endometriosis. Sci Rep.
9(9272)2019.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Sharma I, Dhawan V, Saha SC, Rashmi B and
Dhaliwal LK: Implication of the RAGE-EN-RAGE axis in endometriosis.
Int J Gynaecol Obstet. 110:199–202. 2010.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Zondervan KT, Becker CM, Koga K, Missmer
SA, Taylor RN and Viganò P: Endometriosis. Nat Rev Dis Primers.
4(9)2018.PubMed/NCBI View Article : Google Scholar
|