Introduction
Head and neck cancer is the sixth most common type
of cancer worldwide (1), with
~500,000 new cases diagnosed annually. It constitutes 5% of all
cancer cases worldwide. In India, head and neck cancer accounts for
29.6% of all cancer cases among males (range, 24.3-34.3%) and
11.84% of all cancer cases among females (range, 10.5-15.5%) as per
different hospital-based registries (2,3).
Squamous cell carcinoma of the head and neck (SCCHN) constitutes
>90% of all head and neck cancers (1). In India, SCCHN arising from the oral
cavity is the most common type of cancer among males and the third
most common type among females (1,4).
Approximately, 16% of all cancer cases were registered as SCCHN at
the Regional Cancer Centre, Indira Gandhi Medical College, Shimla,
India, from 2001 to 2010, accounting for 10.6% of all cancer cases
among males and 5.4% of all cancer cases among females (unpublished
data). The geographic distribution reveals a very large variation
in the incidence of head and neck cancers in different countries,
with low incidences reported in Western Europe and high incidences
in South Asia, parts of Africa and South America (5).
The incidence of early-stage SCCHN (stage I or II)
is ~40%, whereas 60% of cases are reported with locally advanced
(stages III and IVA/B) and metastatic (stage IVC) disease (6). Therapeutic options for early-stage
SCCHN include both surgery and radiotherapy as a single treatment
modality, with a cure rate of ~80% (6,7).
Radiotherapy alone has long been the standard non-surgical therapy
for locally advanced disease (8,9). A
previous meta-analysis of individual patient data from >10,000
participants in 63 trials [Meta-Analysis of Chemotherapy on Head
and Neck Cancer (MACH-NC)] demonstrated that the addition of
chemotherapy to radiotherapy in both definitive and adjuvant
postoperative settings resulted in a 12% reduction in the risk of
mortality from head and neck cancer, corresponding to an absolute
improvement of 4% in the 5-year survival rates (10).
The use of induction chemotherapy followed by
radiotherapy has resulted in organ preservation without
compromising overall survival, when compared with radiotherapy
alone in the treatment of SCCHN (11). Previously, 2 phase III
TAX323(12) and TAX324(13) trials demonstrated the efficacy of
induction chemotherapy [TPF (docetaxel and cisplatin, day 1;
fluorouracil by continuous infusion, days 1 to 5)] plus
radiotherapy for the treatment of patients with locally advanced
(LA) unresectable SCCHN with overall response rates (ORRs) of 68%
and 72%, respectively. The aforementioned studies revealed that a
therapeutic gain may be achieved in patients with SCCHN when
concomitant chemoradiotherapy is preceded by induction
chemotherapy. Hence, the present study compared induction
chemotherapy followed by concomitant chemoradiotherapy (CRT) vs.
CRT alone in Indian patients with LA SCCHN.
Materials and methods
Study population
Patients of either sex, aged ≤70 years with a
histologically confirmed diagnosis of stage IVA/B SCCHN of the
oropharynx, hypopharynx and larynx, who were previously untreated
and had a Karnofsky performance status score of >70, were
included in the present study. The key exclusion criteria were the
following: A histology other than SCCHN, hemoglobin (Hb) levels ≤10
gm%, deranged liver and renal function tests and the presence of
distant metastasis.
Study design
The present study was a prospective, randomized
two-arm study conducted between July, 2014 and July, 2015. The
patients (n=52) were randomly divided by stratification into 2
treatment groups/arms: The induction chemotherapy (docetaxel,
cisplatin and 5-FU) followed by CRT arm (TPF + CRT arm; n=25) and
the conventional CRT alone arm (CRT arm; n=27). Randomization was
carried out by stratification, and the treatment assignment was
stratified according to the site of disease (hypopharynx, larynx or
oropharynx), N stage (node -ve or +ve) and T stage (T1 and T2 vs.
T3 and T4). Patients were randomly divided into the TPF + CRT arm
and CRT arm based on the treatment they received. Approximately
equal numbers of patients were assigned to each group.
Study treatments TPF + CRT arm. Patients
randomly divided into the TPF + CRT arm were administered induction
chemotherapy with docetaxel (75 mg/m2) on day 1, and
cisplatin (75 mg/m2) and 5-FU (750 mg) on days 1 and 2
in 3 weekly cycles for a total of 3 cycles. Granulocyte-colony
stimulating factor (G-CSF; Filgrastim, 300 µg) was administered
prophylactically on day 3 of each cycle. Dexamethasone (16 mg),
ranitidine (50 mg), chlorpheniramine maleate (5 mg) and ondansetron
(8 mg) were administered in each cycle. Dose modifications were
allowed as follows: i) The dose of docetaxel was reduced after any
episode of febrile neutropenia, grade 4 neutropenia (lasting >5
days), grade 4 thrombocytopenia, or >grade 3 asthenia; ii) The
dose of cisplatin was reduced to 75% of the original dose in
subsequent cycles if any of the following occurred: >grade 3
sensory neurotoxicity, ≥grade 2 nephrotoxicity, persistent grade 4
neutropenia or neutropenic fever following the dose reduction of
docetaxel; iii) The dose of 5-FU was reduced by 25% in any of the
following circumstances: For patients with grade 3 diarrhea lasting
for >7 days despite the administration of loperamide, mucositis
grade 3 lasting for >5 days, or grade 4 mucositis.
Following 3 cycles of induction chemotherapy,
concurrent CRT with cisplatin (30 mg/m2) on day 1 of
each week and conventional radiotherapy daily with 2 Gy fraction
for 5 days a week for a total of 6½ weeks (total, 66 Gy/6½
weeks/33#) were administered (Fig.
1A).
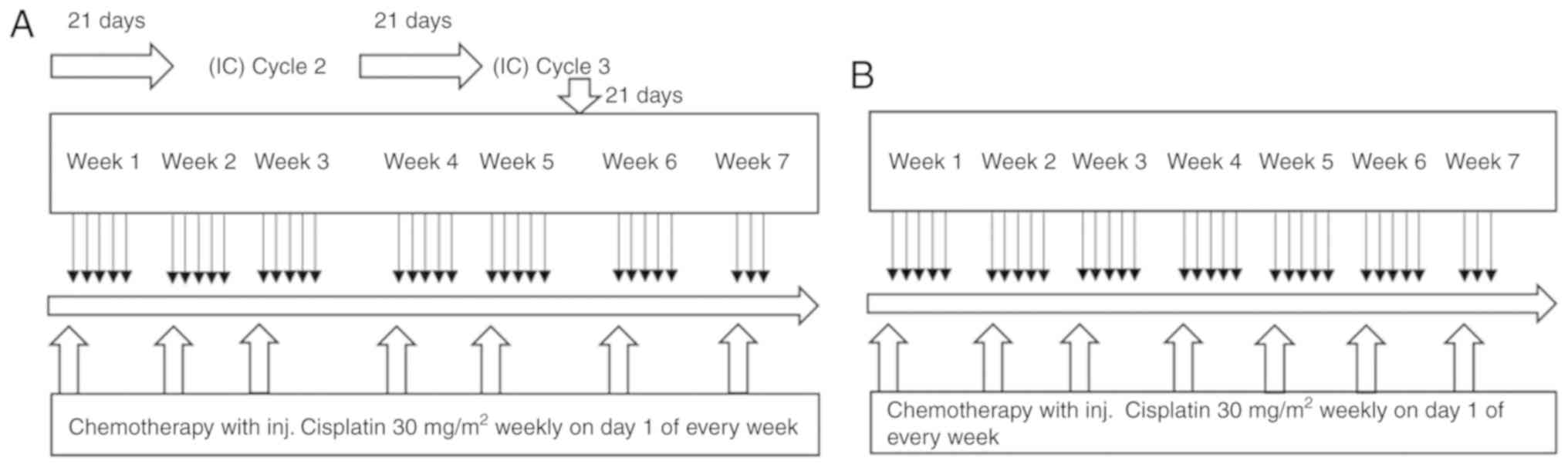 | Figure 1Study design. (A) TPF + CRT arm, (B)
CRT arm. In the TPF + CRT arm, following 3 cycles of induction
chemotherapy, concurrent chemoradiotherapy with cisplatin (30
mg/m2) on day 1 of each week and conventional
radiotherapy daily with 2 Gy fraction for 5 days a week for a total
of 6½ weeks (total, 66 Gy/6½ weeks/33#) were administered. In the
CRT arm, standard concomitant CRT with cisplatin (30
mg/m2) on day 1 of every week for 7 doses and
conventional radiotherapy daily with 2 Gy fraction for 5 days a
week for a total of 6½ weeks (total, 66 Gy/6½ weeks/33#) were
administered. TPF + CRT, induction chemotherapy [TPF (docetaxel and
cisplatin, day 1; fluorouracil by continuous infusion, days 1 to
5)] plus radiotherapy; CRT, concomitant chemoradiotherapy
alone. |
CRT arm (conventional CRT). Patients assigned
to the CRT arm received standard concomitant CRT with cisplatin (30
mg/m2) on day 1 of each week for 7 doses and
conventional radiotherapy daily with a 2 Gy fraction for 5 days a
week for a total of 6½ weeks (total, 66 Gy/6½ weeks/33#). G-CSF
(Filgrastim, 300 µg) was administered only if necessary, after
reviewing the investigations, not prophylactically (Fig. 1B).
Study assessments
The first follow-up was performed at 6 weeks
following treatment and subsequent follow-ups were performed every
2 months. The primary endpoint was the response rate (RECIST 1.1)
at 6 weeks as evaluated by the following criteria: i) Complete
response (CR): A complete regression of the lesion (primary, as
well as neck nodes); ii) Partial response (PR): A >50%
regression in the lesion in maximal diameter; iii) Stable disease:
If the lesion regressed <50% in maximal diameter; and iv)
Progressive disease: If the lesion increased by 25% or the
appearance of a new lesion or secondary metastatic disease were
noted.
Toxicity profiles were evaluated each week during
treatment and at the end of treatment. The toxicity was assessed
according to the Radiation Therapy Oncology Group toxicity criteria
(14). Treatment toxicities
occurring within 90 days of the commencement of radiotherapy were
considered acute and those occurring or persisting >90 days
after the commencement of radiotherapy were considered as late.
Statistical analysis
Quantitative data are presented as the means and
standard deviation, and qualitative data by frequency and
distribution. The Student's t-test and χ2 test were used
for the statistical comparisons of parametric data. Statistical
significance was considered as follows: P>0.05 as
non-significant, P=0.05-0.01 as significant and P<0.01 as highly
significant. SPSS (version 24) was used for statistical
analysis.
Results
Patient disposition and
demographics
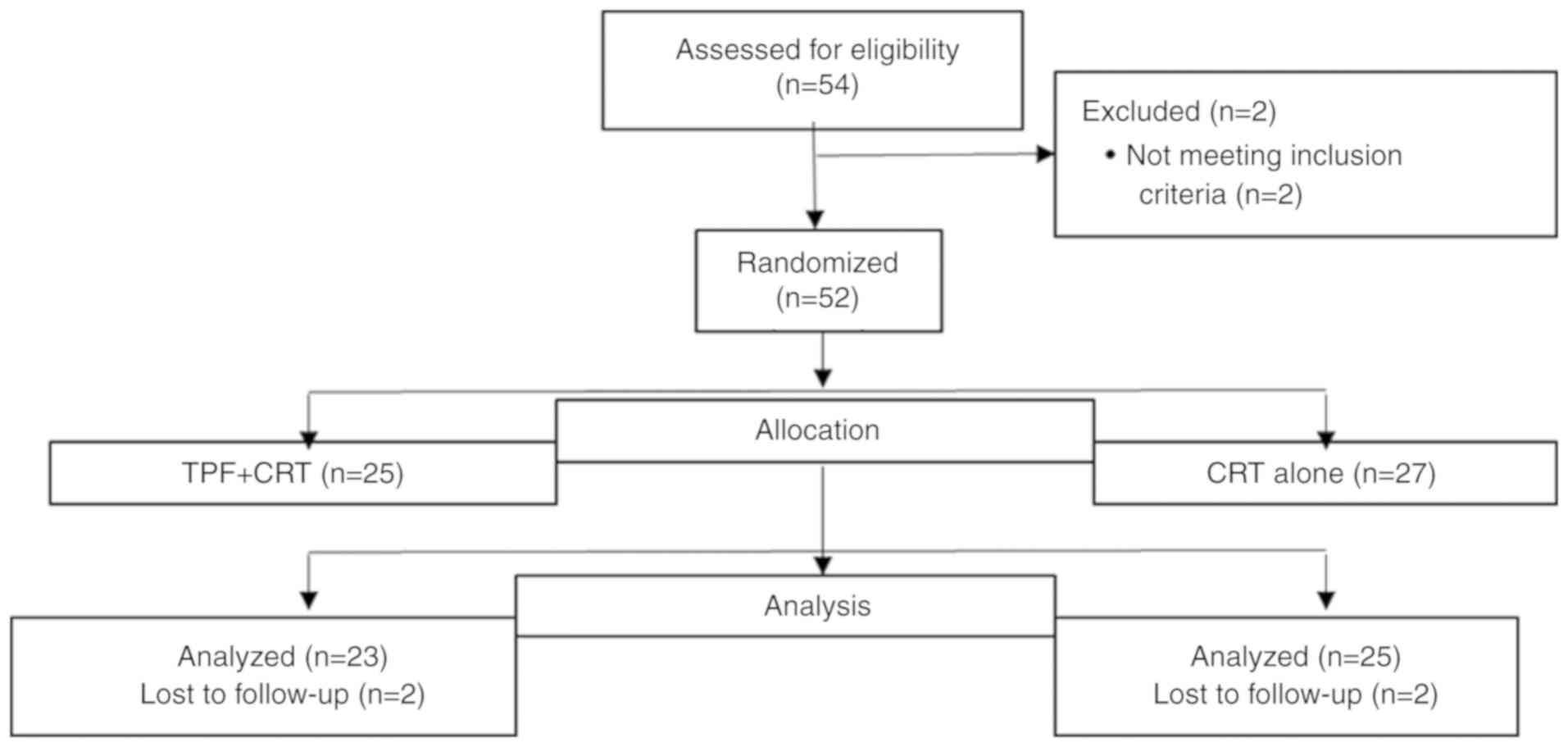
Of the 52 patients enrolled in the present study, 25
were randomized to the TPF + CRT arm and 27 to the CRT arm
(Fig. 2). The median age of the
patients was 56.2 years. The patients were followed-up for a median
duration of 4 months. The baseline characteristics (Table I) of the study participants did not
differ significantly between the 2 study arms. The present study
was conducted after due approval from the Institutional Ethics
Committee, Indira Gandhi Government Medical College (IGMC), Shimla,
Himachal Pradesh, India. All the patients provided written informed
consent for participation.
 | Table IBaseline characteristics of the study
participants. |
Table I
Baseline characteristics of the study
participants.
| Parameter | TPF + CRT (n=25) | CRT (n=27) |
|---|
| Age, mean, years | 54.9 | 60.7 |
|
Median | 56 | 60 |
|
Range | 37-70 | 45-70 |
|
31-40
years, n (%) | 1(4) | 0 |
|
41-50
years, n (%) | 6(24) | 6 (22.2) |
|
51-60
years, n (%) | 7(28) | 8 (29.6) |
|
61-70
years, n (%) | 11(44) | 13 (48.2) |
| Sex, n (%) | | |
|
Male | 23(92) | 26 (96.3) |
|
Female | 2(8) | 1 (3.7) |
| Smoker, n (%) | 22(88) | 26 (96.2) |
| Alcohol consumption n
(%) | | |
|
Chronic
alcohol consumption | 8(32) | 13 (48.1) |
|
Occasional
alcohol consumption | 9(36) | 10 (37.1) |
|
No alcohol
consumption | 8(32) | 4 (14.8) |
| Karnofsky performance
status, mean (range) | 87.6 (80-90) | 85.9 (80-90) |
| Hemoglobin levels
(g/dl), mean | 12.9 | 12.7 |
| Cancer sites, n
(%) | | |
|
Oropharynx | 15(60) | 14 (51.9) |
|
Larynx | 7(28) | 7 (25.9) |
|
Hypopharynx | 3(12) | 6 (22.2) |
| Cancer subsites, n
(%) | | |
|
Vallecula | 6(24) | 6 (22.2) |
|
Base of
tongue | 7(28) | 4 (14.8) |
|
Supraglottis | 5(20) | 5 (18.5) |
|
Tonsil | 2(8) | 4 (14.8) |
|
Pyriform
sinus | 2(8) | 4 (14.8) |
|
Glottis | 2(8) | 2 (7.4) |
|
Lateral
pharyngeal wall | 1(4) | 1 (3.7) |
|
Posterior
pharyngeal wall | 0 | 1 (3.7) |
| T stage, n (%) | | |
|
T1 | 2(8) | 2 (7.4) |
|
T2 | 10(40) | 8 (29.6) |
|
T3 | 6(24) | 8 (29.6) |
|
T4 | 7(28) | 9 (33.3) |
| N stage, n (%) | | |
|
N0 | 2 (8%) | 3 (11.1%) |
|
N1 | 3 (25%) | 4 (14.81%) |
|
N2 | 17 (68%) | 18 (66.7%) |
|
N3 | 3 (12%) | 2 (7.4%) |
| Cancer stage, n
(%) | | |
|
IVA | 20(80) | 22 (81.5) |
|
IVB | 5(20) | 5 (18.5) |
Treatment efficacy
In total, 2 patients in the TPF + CRT and CRT group
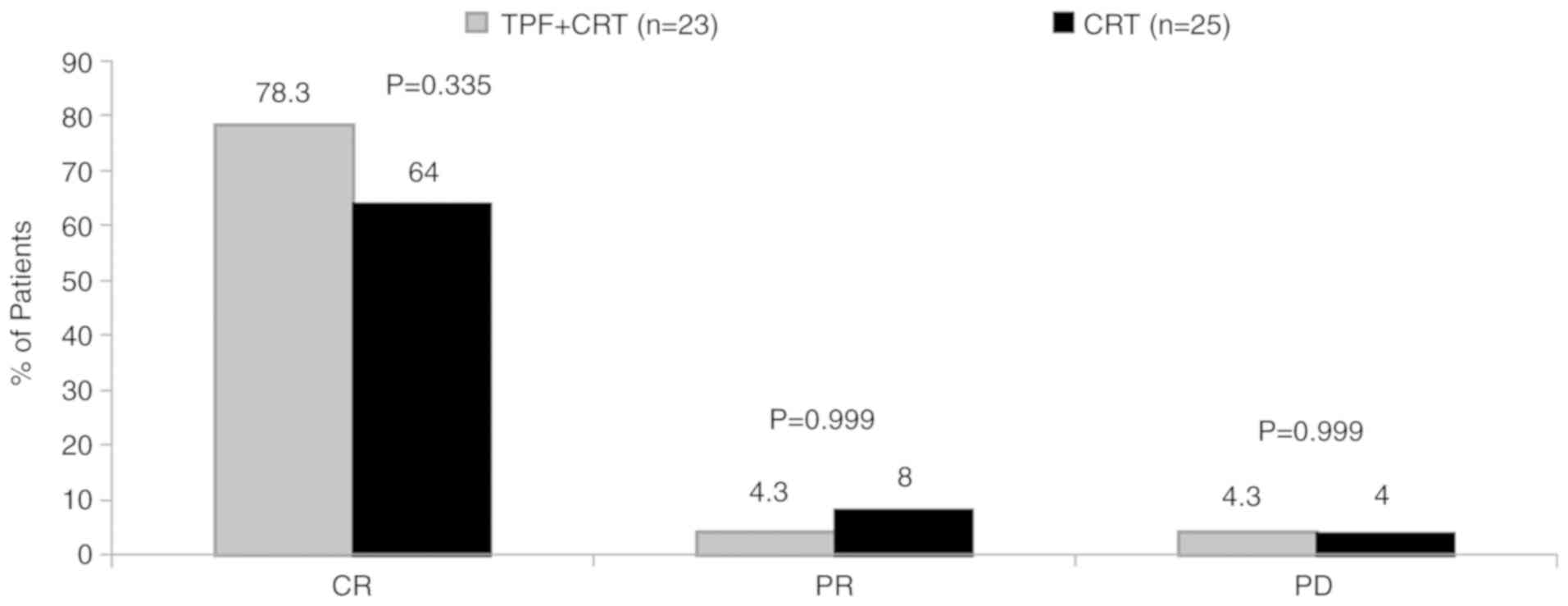
(in each group) were lost to follow-up. The ORR was 82.6% [CR,
78.3% (18/23); PR, 4.3% (1/23)] for the TPF + CRT arm and 72% [CR,
64% (16/25); PR, 8% (2/25)] for the CRT arm at the first follow-up
(Fig. 3). No significant
differences (P=0.999) were observed in disease response at the
primary site in both study arms. Disease progression was reported
in 1 patient in each arm. The response was not evaluated in 3 and 4
patients in the TPF + CRT and CRT arms, respectively.
For nodal response, the ORR was 82.6% [CR, 73.9%
(17/23); PR, 8.7% (2/23)] for the TPF + CRT arm vs. 76% [CR, 56%
(14/25); PR, 20% (5/25)] for the CRT arm. No statistically
significant differences were observed in nodal response between the
treatment arms at the first follow-up.
At the median follow-up of 3.5 months, 17 (73.9%)
patients in the TPF + CRT arm and 15 (60%) patients in the CRT arm
achieved CR (P=0.307). Similarly, complete nodal response at the
median follow-up of 3.5 months was achieved in 16 (69.6%) and 13
(52%) of the patients in the TPF + CRT and CRT arms, respectively
(P=0.367).
No statistically significant differences were found
in CR in both the treatment arms when analyzed for different
subgroups: Sex, smoking status, alcohol status, smoking and
alcoholic status, diet, cancer site and cancer stage. A trend for a
better response with TPF + CRT was observed across cancer sites
(oropharynx, larynx and hypopharynx) and cancer stages (IVA and
IVB), although the difference was not statistically significant
(Table II).
 | Table IISubgroup evaluation for both
groups. |
Table II
Subgroup evaluation for both
groups.
| | TPF + CRT
(n=23) | CRT (n=25) | |
|---|
| Parameter | CR (%) | No CR (%) | CR (%) | No CR (%) | P-value |
|---|
| Sex | | | | | |
|
Male | 18 (85.7) | 3 (14.3) | 16 (66.7) | 8 (33.3) | 0.177 |
|
Female | 1(50) | 1(50) | 0 | 1(100) | 0.999 |
| Smoker | 15(75) | 5(25) | 16(64) | 9(36) | 0.059 |
| Alcohol
consumption, n (%) | 13 (81.25) | 3 (18.8) | 15 (68.1) | 7 (31.8) | 0.469 |
| No alcohol
consumption, n (%) | 5 (71.4) | 2 (28.6) | 2 (66.7) | 1 (33.3) | 0.999 |
| Smoking and alcohol
consumption, n (%) | 12(80) | 3(20) | 15 (68.2) | 7 (31.8) | 0.481 |
| Non-vegetarian, n
(%) | 18(90) | 2(10) | 14 (63.6) | 8 (36.4) | 0.071 |
| Vegetarian, n
(%) | 2 (66.7) | 1 (33.3) | 2 (66.7) | 1 (33.3) | 0.999 |
| Site | | | | | |
|
Oropharynx | 11 (78.6) | 3 (21.4) | 8 (61.5) | 5 (38.5) | 0.420 |
|
Larynx | 4(80) | 1(20) | 3(50) | 3(50) | 0.546 |
|
Hypopharynx | 2 (66.7) | 1 (33.3) | 3(50) | 3 (33.3) | 0.999 |
| Cancer stage | | | | | |
|
IVA | 13 (72.2) | 5 (27.8) | 13(65) | 7(35) | 0.633 |
|
IVB | 4(80) | 1(20) | 3(60) | 2(40) | 0.999 |
Safety
During treatment, majority of the patients suffered
from grade 3 skin toxicity, which was higher with TPF + CRT as
compared to CRT (65.2 vs. 48%; P=0.262). Grade 3 mucositis was
significantly (P=0.016) higher in the patients in the TPF + CRT arm
(52.2%) compared to those in the CRT arm (16%). Grade 2 laryngeal
toxicities were observed in the majority of patients, including
hoarseness or whispered speech, throat pain and cough. Grade 2 and
3 pharyngeal toxicities combined were higher in the CRT (grade 2,
64%; grade 3, 24%) vs. the TPF + CRT arm (grade 2, 60.7%; grade 3,
17.4%), although no significant differences were observed.
Significantly higher grade 3 hematological toxicities were observed
in the TPF + CRT vs. the CRT arm (60.9 vs. 24%, P=0.012).
Gastrointestinal toxicity was slightly higher in the TPF + CRT
(28%) vs. the CRT (25.9%) arm. Febrile neutropenia was observed in
1 (4%) patient receiving TPF + CRT. Treatment interruptions (TPF +
CRT, 40%; CRT, 37%) were observed as follows: TPF + CRT arm: Due to
grade 3 and 4 skin toxicity, grade 3 and 4 mucositis and
hematological toxicity; CRT arm: Due to the withdrawal of consent
and 1 patient leaving the treatment in between.
Toxicity at first follow-up
In the TPF + CRT arm, 65.2% of the patients had
grade 1 salivary gland toxicity compared with 36% patients in the
CRT arm. Skin reactions occurring during the treatment were healed
at first follow-up. Mucositis was not healed in 1 patient (4.34%)
in the TPF + CRT arm and 2 patients (8%) in the CRT arm. The
majority of the patients had grade 1 salivary gland toxicity
(Table III).
 | Table IIIToxicity profile at first
follow-up. |
Table III
Toxicity profile at first
follow-up.
| Parameter | TPF + CRT (n=23)
(%) | CRT (n=25) (%) | P-value |
|---|
| Skin
toxicities | | | |
|
No
toxicity | 20(87) | 20(80) | 0.612 |
| Mucositis | | | |
|
No
toxicity | 19 (82.6) | 18 (78.3) | 0.458 |
|
Grade 1 | 1 (4.34) | 2(8) | 0.999 |
| Salivary Gland
Toxicity | | | |
|
No
toxicity | 4 (17.4) | 11(44) | 0.069 |
|
Grade 1 | 15 (65.2) | 9(36) | 0.054 |
|
Grade 2 | 1 (4.3) | 0 | 0.999 |
| Late Toxicity:
Depigmentation | 16 (69.6) | 14(56) | 0.376 |
| Late Toxicity:
Subcutaneous Fibrosis | 3 (13.0) | 3(12) | 0.999 |
| Late Toxicity:
Salivary gland | | | |
|
No
toxicity | 3 (13.0) | 3(12) | 0.999 |
|
Grade 1 | 19 (82.6) | 21(84) | 0.999 |
|
Grade 2 | 1 (4.34) | 1(4) | 0.999 |
Late skin toxicity,
depigmentation
Depigmentation was present in 16 (69.6%) of the
patients in the TPF + CRT arm compared with 14 (56%) of patients in
the CRT arm. Subcutaneous fibrosis was present in 13% of the
patients in the TPF + CRT arm compared with 12% in the CRT arm
(Table III).
Discussion
Concomitant chemoradiation has demonstrated an 8%
absolute survival advantage in the meta-analysis conducted by
Pignon et al, and has now become the standard of care in LA
SCCHN cancers (10). The
combination of induction chemotherapy with concomitant CRT for the
treatment of SCCHN has been examined in several clinical trials.
Browman et al reported a pooled analysis of 18 randomized
controlled trials (RCTs) in 3,192 patients, in which concomitant
chemotherapy-radiation therapy was compared to radiation therapy
alone. Overall, the chemotherapy-radiation therapy arm was superior
for the reduction in mortality compared with radiation therapy
alone (P<0.0001). The results further demonstrated that
platinum-based concomitant CRT is superior to conventional
radiotherapy alone in improving survival in locally advanced SCCHN
(15).
The current randomized prospective study compared
the combination of induction chemotherapy with the TPF regimen
followed by concurrent CRT vs. CRT alone in 52 patients with
advanced SCCHN at the Regional Cancer Centre in India. The study
demonstrated a higher local control at primary and nodal sites with
TPF + CRT as compared with CRT alone, though the difference was not
statistically significant. The ORR was reported in 82.6% patients
with TPF + CRT vs. 72% with CRT.
The TAX323(12)
trial in patients with advanced SCCHN (stages III and IV) with an
unresectable disease demonstrated an ORR of 68% with TPF + CRT
regimen. The TAX 324(13) trial
reported loco-regional control in 72% patients with both resectable
and unresectable advanced SCCHN (stage III or IV) who were treated
with 3-cycles of induction chemotherapy with TPF regimen
(docetaxel, cisplatin and 5-FU) followed by concomitant
radiotherapy. The phase III EORTC trial of TPF followed by
radiotherapy in unresectable LA SCCHN (n=177) patients showed a
response rate of 67.8% (16). The
GSTTC trial that compared the induction TPF followed by concomitant
treatment (n=206) vs. concomitant treatment alone (208) in patients
with LA SCCHN revealed an ORR of 76% with induction regimen; the
CRs were significantly higher in the induction chemotherapy arm
(42.5% vs. 28%, P=0.0028) (17).
The efficacy results of these studies are comparable to those
reported in the present study.
The efficacy of TPF + CRT (n=50) vs. CRT alone
(n=51) in patients with LA SCCHN was evaluated by Paccagnella et
al in a European population (18). CR was reported in 50% patients in
the TPF + CRT arm vs. 21.2% in the CRT arm, compared to 78.3 and
64%, respectively as observed in the present study. On subset
analysis, a trend for a better response was observed with TPF + CRT
vs. CRT across the larynx, oropharynx and hypopharynx in the
present study, although the results were not statistically
significant.
In the present study, confluent fibrinous mucositis
with pain (grade 3 acute mucositis) and grade 4 mucositis was
observed more often in the TPF + CRT arm (52%) as compared with the
CRT arm (18.5%) during radiation treatment (P=0.016). Febrile
neutropenia was reported in 1 patient with TPF + CRT. In the DeCIDE
trial, the most common grade 3/4 toxicities during induction
chemotherapy (TPF + CRT arm) were febrile neutropenia (11%) and
mucositis (9%) (19). In the
present study, a higher trend for skin, laryngeal and GI toxicities
was observed with TPF + CRT compared with CRT. Furthermore, a
higher trend for acute toxicities was observed with the TPF + CRT
regimen with significant differences observed for mucositis and
hematological toxicities. Grade 3/4 hematological toxicity was
markedly higher with TPF + CRT. The acute toxicity results in the
present study were comparable to those observed in the DeCIDE,
PRADIGM and GSTCC trials (17,19,20).
None of the patients in the present study exhibited any skin
reactions at the first follow-up and all previous skin reactions
were healed completely. However, 1 patient in the TPF + CRT arm and
2 patients in the CRT arm had mucositis and were still healing even
after 6 weeks of completion of radiation therapy at the first
follow-up; however, at the second follow-up up, all acute
toxicities in patients of both the arms were completely healed. A
similar trend for higher late toxicities in the form of
subcutaneous fibrosis was observed in the TPF + CRT arm as compared
with the CRT arm, although the difference in late toxicities was
not statistically significant.
Some limitations of the present study should be
mentioned. These include the small sample size and the
unavailability of the progression-free survival (PFS) and overall
survival (OS) data.
In conclusion, local and nodal response at first
follow-up was higher with TPF + CRT regimen compared with CRT,
although the difference was not statistically significant. The
toxicities were higher in the TPF + CRT arm with significant
differences for mucositis and hematological toxicities. However, at
the first follow-up, mucositis was present in only 1 patient in the
TPF + CRT arm vs. 2 patients in the CRT arm. Although in the
present study, no statistically significant differences were
observed in late toxicities, a longer follow-up time is required to
draw any meaningful conclusion. The present study demonstrates the
feasibility of sequential therapy in the management of locally
advanced head and neck cancer in the Indian population. Triple
drug-based sequential therapy was tolerable in the Indian
population context. Further large scale studies with longer
follow-up times are warranted to confirm these results in Indian
patients and in other populations.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AR and PR performed the research, were involved in
the acquisition of data, critically revised the manuscript for
important intellectual content, and approved the final manuscript.
AR, PR, ManojG, RS and ManishG designed the study, were involved in
the data interpretation, critically revised the manuscript for
important intellectual content, and approved the final manuscript.
All authors made substantial contributions to the present study,
read and approved the final manuscript and agree to be accountable
for all aspects of the work.
Ethics approval and consent to
participate
The present study was conducted after due approval
from the Institutional Ethics Committee, Indira Gandhi Government
Medical College (IGMC), Shimla, Himachal Pradesh, India. All the
patients provided written informed consent for participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vigneswaran N and Williams MD:
Epidemiologic trends in head and neck cancer and aids in diagnosis.
Oral Maxillofac Surg Clin North Am. 26:123–141. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Indian Council of Medical Research.
National Cancer Registry Programme: Ten year consolidated report of
the hospital based cancer registries 1984-1993. New Delhi.
https://ncdirindia.org/NCRP/Old_Reports/HBCR_TEN/inside_pages.pdf.
Accessed July 10, 2014.
|
|
3
|
Dhull AK, Atri R, Dhankhar R, Chauhan AK
and Kaushal V: Major risk factors in head and neck cancer: A
retrospective analysis of 12-year experiences. World J Oncol.
9:80–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Warnakulasuriya S: Global epidemiology of
oral and oropharyngeal cancer. Oral Oncol. 45:309–316.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gupta N, Gupta R, Acharya AK, Patthi B,
Goud V, Reddy S, Garg A and Singla A: Changing trends in oral
cancer-a global scenario. Nepal J Epidemiol. 6:613–619.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Szturz P and Vermorken JB: Treatment of
elderly patients with squamous cell carcinoma of the head and neck.
Front Oncol. 6(199)2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Marur S and Forastiere AA: Head and neck
squamous cell carcinoma: Update on epidemiology, diagnosis, and
treatment. Mayo Clin Proc. 91:386–396. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Forastiere A, Koch W, Trotti A and
Sidransky D: Head and neck cancer. N Engl J Med. 345:1890–1900.
2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Posner MR, Haddad RI, Wirth L, Norris CM,
Goguen LA, Mahadevan A, Sullivan C and Tishler RB: Induction
chemotherapy in locally advanced squamous cell cancer of the head
and neck: Evolution of the sequential treatment approach. Semin
Oncol. 31:778–785. 2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Pignon JP, Bourhis J, Domenge C and
Designe L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: Three meta-analyses of updated
individual data. MACH-NC Collaborative Group. meta-analysis of
chemotherapy on head and neck cancer. Lancet. 355:949–955.
2000.PubMed/NCBI
|
|
11
|
Dietz A, Wiegand S, Kuhnt T and Wichmann
G: Laryngeal preservation approaches: Considerations for new
selection criteria based on the DeLOS-II trial. Front Oncol.
9(625)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Vermorken JB, Remenar E, van Herpen C,
Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss
JH, et al: Cisplatin, fluorouracil, and docetaxel in unresectable
head and neck cancer. N Engl J Med. 357:1695–1704. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, et al: Cisplatin and fluorouracil alone or with
docetaxel in head and neck cancer. N Engl J Med. 357:1705–1715.
2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cox JD, Stetz J and Pajak TF: Toxicity
criteria of the Radiation therapy oncology group (RTOG) and the
European organization for research and treatment of cancer (EORTC).
Int J Radiat Oncol Biol Phys. 31:1341–1346. 1995.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Browman GP, Hodson DI, Mackenzie RJ,
Bestic N and Zuraw L: Cancer Care Ontario Practice Guideline
Initiative Head and Neck Cancer Disease Site Group. Choosing a
concomitant chemotherapy and radiotherapy regimen for squamous cell
head and neck cancer: A systematic review of the published
literature with subgroup analysis. Head Neck. 23:579–589.
2001.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Vermorken JB, Remenar E, ven Herpen C,
Lluch JG, Stewart S, Gorlia T, Degardin TG, Schollen K and Bernier
J: Standard cisplatin/infusional 5-fluorouracil (PF) vs. docetaxel
(T) plus PF (TPF) as neoadjuvant chemotherapy for nonresectable
locally advanced squamous cell carcinoma of the head andneck
(LA-SCCHN): A phase III trial of the EORTC head and neck cancer
group (EORTC #24971). J Clin Oncol. 22 (14 Suppl)(S5508)2004.
|
|
17
|
Ghi MG, Paccagnella A, Ferrari D, Foa P,
Alterio D, Codeca C, Nole F, Verri E, Orecchia R, Morelli F, et al:
Induction TPF followed by concomitant treatment versus concomitant
treatment alone in locally advanced head and neck cancer. A phase
II-III trial. Ann Oncol. 28:2206–2212. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Paccagnella A, Ghi MG, Loreggian L,
Buffoli A, Koussis H, Mione CA, Bonetti A, Campostrini F, Gardani
G, Ardizzoia A, et al: Concomitant chemoradiotherapy versus
induction docetaxel, cisplatin and 5 fluorouracil (TPF) followed by
concomitant chemoradiotherapy in locally advanced head and neck
cancer: A phase II randomized study. Ann Oncol. 21:1515–1522.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cohen EE, Karrison TG, Kocherginsky M,
Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB,
Yunus F, et al: Phase III randomized trial of induction
chemotherapy in patients with N2 or N3 locally advanced head and
neck cancer. J Clin Oncol. 32:2735–2743. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Haddad R, O'Neill A, Rabinowits G, Tishler
R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler JJ, et
al: Induction chemotherapy followed by concurrent chemoradiotherapy
(sequential chemoradiotherapy) versus concurrent chemoradiotherapy
alone in locally advanced head and neck cancer (PARADIGM): A
randomised phase 3 trial. Lancet Oncol. 14:257–264. 2013.PubMed/NCBI View Article : Google Scholar
|