Introduction
The cause of Alzheimer's disease (AD) is poorly
understood. The disease process is associated with β-amyloid (Aβ)
plaques, tau neurofibrillary tangles and neuroinflammation. In
1991, the amyloid hypothesis postulated that beta amyloid (Aβ)
accumulation is a key element (1).
Aβ was supposed to stimulate both the development of tau
neurofibrillary tangles and neuroinflammation. Aβ, tau and
inflammation each led to the destruction of neurons and synapses.
It follows that clearing the brain of Aβ would be beneficial, which
has not been the case. Therefore, Aβ is likely a result, not a
cause, of AD (2,3) and may be protective rather than
harmful (4).
Aβ has antimicrobial properties (4) and could represent a brain defense
against infection (5), in
particular against herpes simplex virus 1 (HSV-1). HSV-1 is found
in regions of the brain that are affected by AD in elderly
individuals. Additionally, neuronal infection with HSV-1 triggers
the accumulation of amyloid beta deposits and hyperphosphorylated
tau, and results in oxidative stress and synaptic dysfunction.
These factors are implicated in the development of AD (6).
The apolipoprotein E4 (apoE4) allele is the
strongest genetic risk factor for AD. Approximately 23% of the US
population carries an apoE4 allele. The apoE2 allele is less
common, 5% incidence, and is protective against AD.
Klotho (KL), encoded by the KL gene, may be another
AD-related protein. In mice, elevated KL levels extend lifespan,
enhance synaptic function and improve cognition during aging
(7). Cognitively normal older
individuals who have higher serum KL levels exhibit enhanced
functional connectivity among brain regions that degenerate in AD
(8).
Approximately 20% of individuals carry a KL variant,
KL-VS. Heterozygosity (one copy) of KL-VS increases circulating
klotho, while reducing Aβ and lowering AD risk in apoE4 carriers
who are age 60 to 80 and cognitively normal (9). In the present study, the KL protein
structure was examined to determine whether it may interact with
Aβ.
Data collection methods
Protein data bank (pdb) entries for KL and Aβ were
searched on the RCSB Protein Data Bank. The following structures
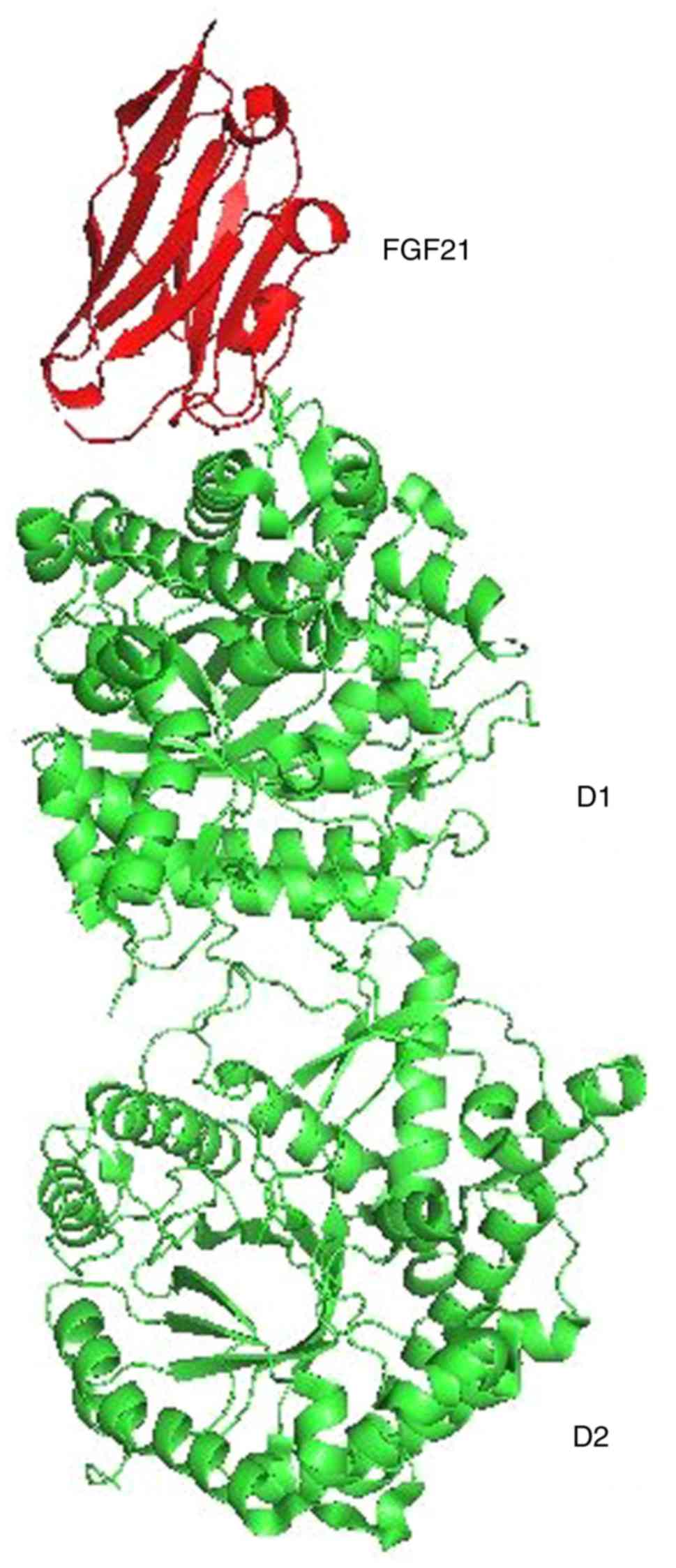
were identified: i) 5VAK (Fig. 1),
representing the crystal structure of β-KL in complex with FGF21CT
(C terminal tail). The method used was X-RAY diffraction. The
resolution was 2.61 Å, structure deposited on March 27, 2017 and
released on January 31, 2018(10).
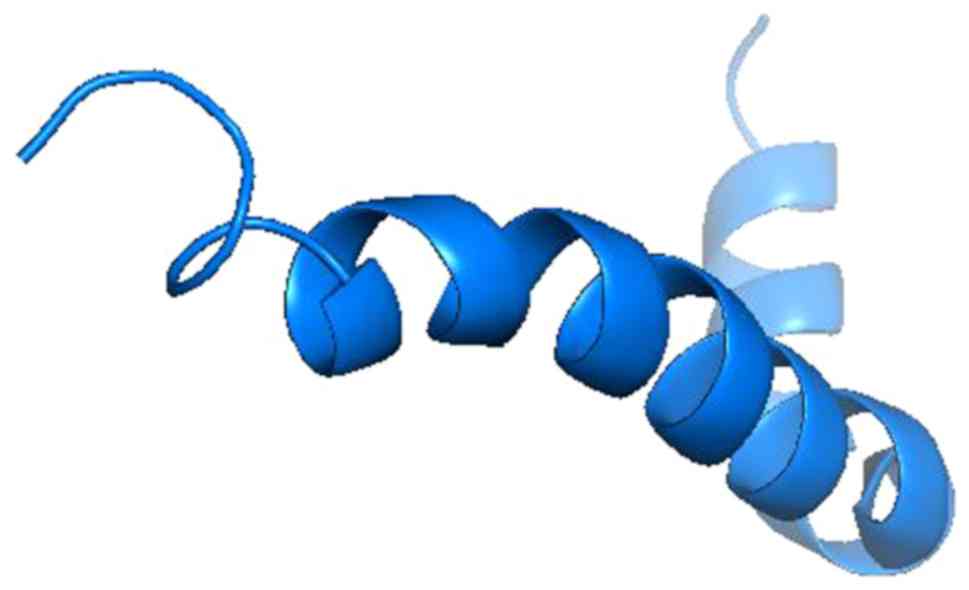
ii) 1IYT (Fig. 2), representing
the solution structure of the AD Aβ-peptide. The method used was
solution NMR, structure deposited on September 6, 2002 and released
on February 11, 2003(11).
The protein structures were superimposed and aligned
on PYMOL v 2.3.4 with the Super command, which super aligns
two protein selections. Super does a sequence-independent
structure-based dynamic programming alignment (unlike the
align command) followed by a series of refinement cycles
intended to improve the fit by eliminating pairing with high
relative variability. The Super command is more reliable
than align for proteins with low sequence similarity.
To evaluate conservation and alignment of the Aβ and
KL genomes across species, we used BLAT, the Blast-Like Alignment
Tool of the UCSC Genome Browser (12). BLAT can align a user sequence of 25
bases or more to the genome. As some level of mismatch is
tolerated, cross-species alignments may be performed provided the
species have not diverged too far from each other; this capability
allowed comparison of the Mouse Mammary Tumor Virus genome to the
human genome (13). BLAT
calculates a percent identity score to indicate differences between
sequences without a perfect match (i.e., without 100% identity).
The differences include mismatches and gaps (14).
Results
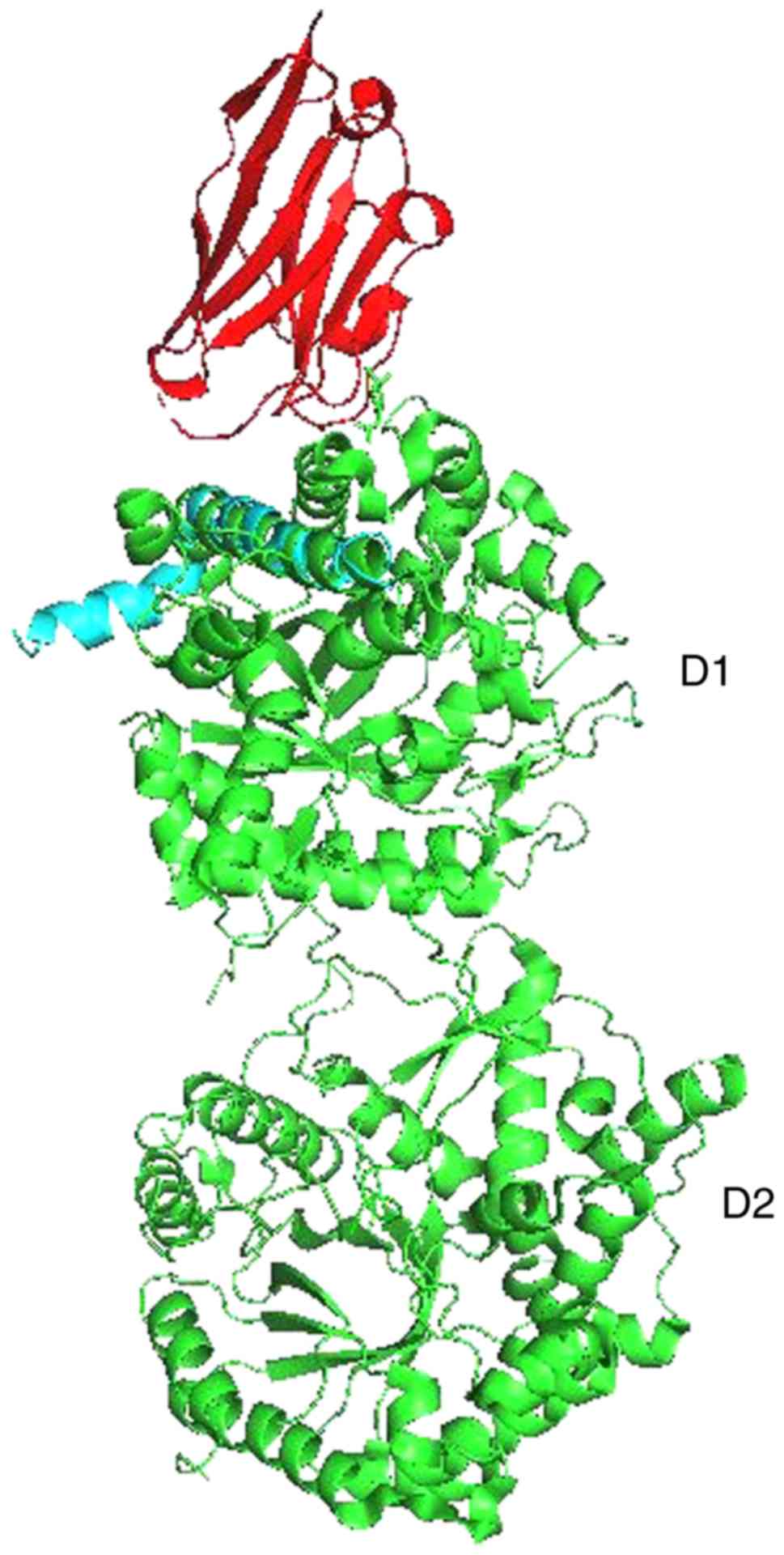
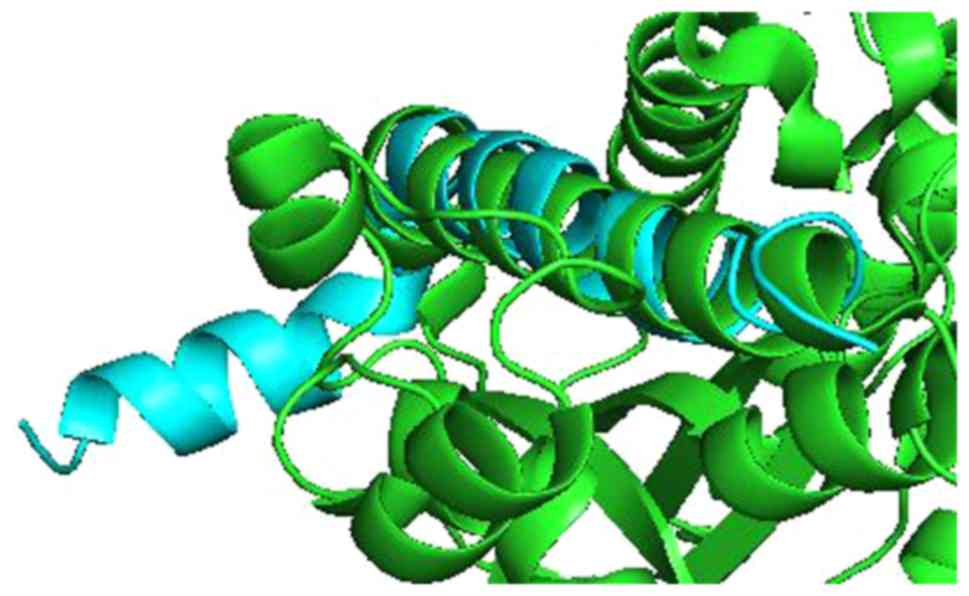
For KL and Aβ, Pymol performed 6 cycles of
calculations on 165 aligned atoms, with a final root mean square
deviation of atomic positions (RMSD) of 1.792 Å for 148 atoms.
Amino acid residues phe76-val96 of KL aligned closely with residues
asp7-asn27 of Aβ (Figs. 3 and
4).
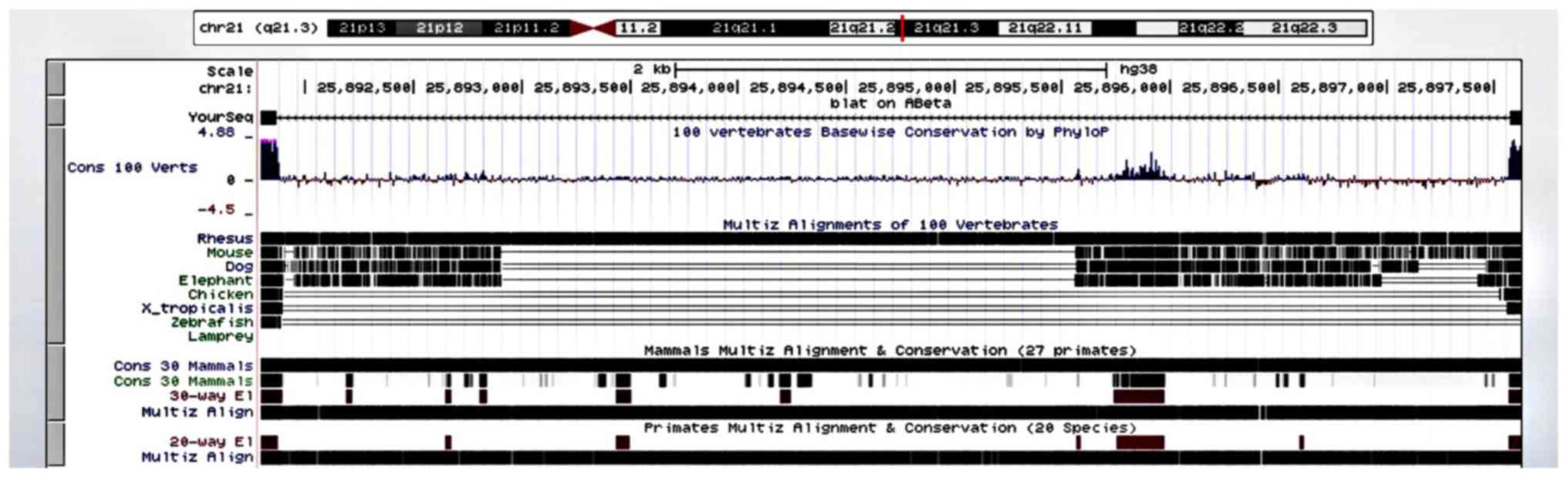
The results of the cross-species comparison of Aβ
revealed a high degree of alignment and conservation of human Aβ
(chr 21q21.3) in the rhesus monkey and 27 other primates. The
rhesus macaque diverged from ancestors of Homo sapiens
approximately 25 million years ago (15). There was much less alignment and
conservation in the mouse, dog, and elephant, even less in the
chicken, western clawed frog (Xenopus tropicalis), zebrafish
and lamprey (Fig. 5).
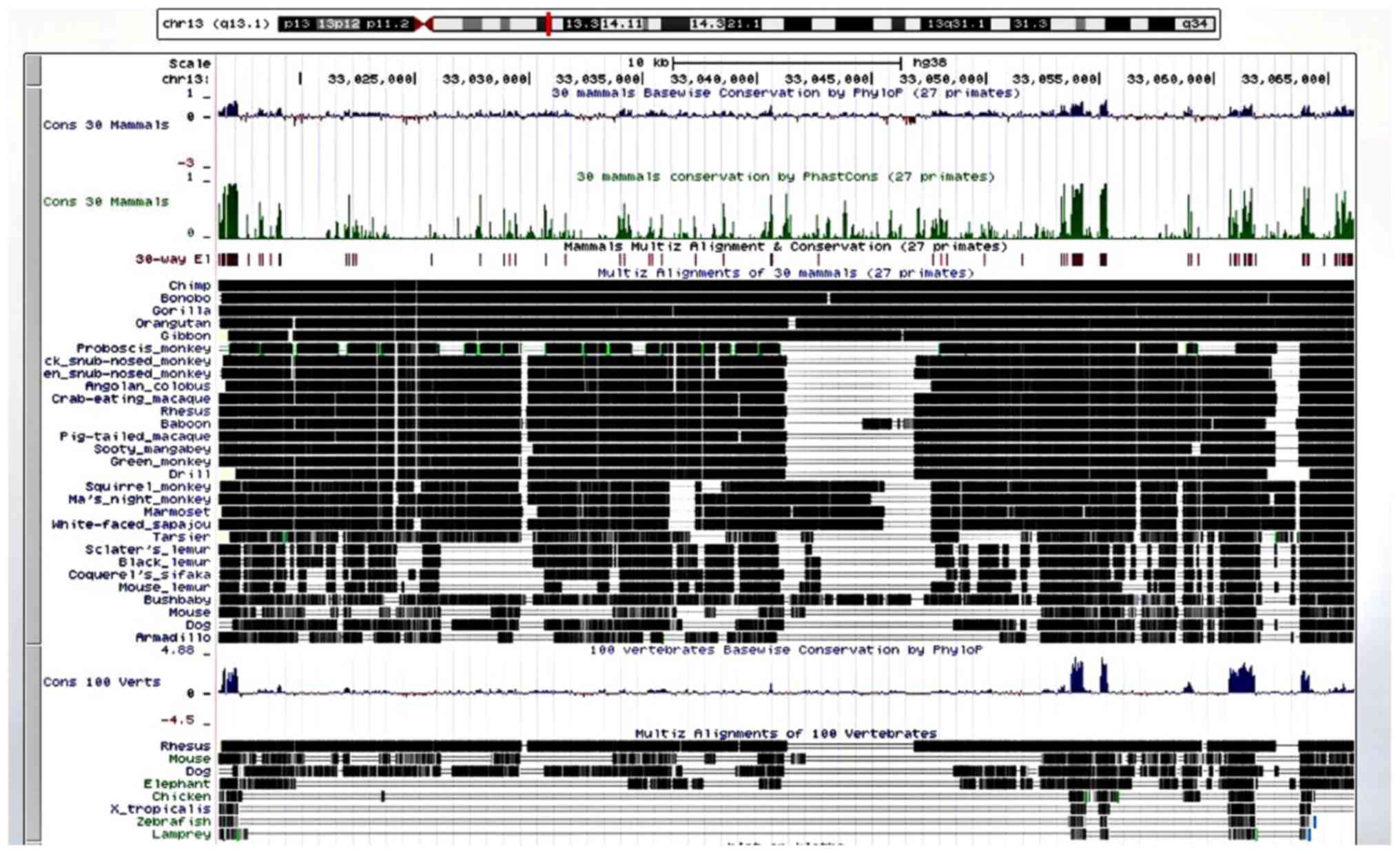
The results of the cross-species comparison of KL
revealed a high degree of alignment and conservation of KL (chr
13q13.1) in the chimp and 27 other primates, with less alignment
and conservation in the mouse, dog and elephant, even less in the
chicken, western clawed frog (Xenopus tropicalis), zebrafish
and lamprey (Fig. 6).
Discussion
Aβ is an ancient neuropeptide expressed in
vertebrates. Many primate species share the human Aβ sequence,
which has been highly conserved over millions of years (16). The conservation of KL is similar.
The high degree of conservation suggests that both sequences play
an important role in survival.
KL is a membrane protein that is related to
β-glucuronidases, enzymes that break down carbohydrates. KL has
tandem glucosidase domains, D1 and D2 (Fig. 1). Three subtypes of KL have been
identified: α-KL, β-KL and γ-KL. Low levels of KL are present in
patients with chronic renal failure. KL may be one element involved
in degenerative processes, such as arteriosclerosis, osteoporosis
and skin atrophy often observed in renal failure. Mutations in the
KL protein have been associated with aging, bone loss and alcohol
consumption (17,18).
In vivo, Aβ and KL may function like
ubiquitin and the substrate proteins to which it binds. Ubiquitin
is a small (8.6 kDa) regulatory protein present in most tissues of
eukaryotic organisms, that is, it occurs ubiquitously (19). The conjugation and binding of
ubiquitin to a substrate protein is called ubiquitination.
Ubiquitination affects proteins in many ways. Ubiquitin can alter
protein cellular location, affect protein activity, and promote
protein interactions. Similarly, after Aβ conjugates and binds to
klotho, Aβ and KL could enhance the marking of brain regions for
delivery of fibroblast growth factor 21 (FGF21) (10).
FGF21 is neuroprotective and may delay the onset of
AD. FGF21 is a circulating endocrine hormone, mainly secreted by
the liver, mostly during fasting. FGF21 acts by binding to its
receptor FGFR1 and co-receptor β-KL. FGF21 regulates energy
consumption by influencing glucose and lipid metabolism. Deranged
FGF21 signaling might account for some forms of neurodegeneration,
and FGF21 could be therapeutic in AD (20). Structurally, FGF21 is a 181 amino
acid peptide (~22.3 kDa molecular mass) derived from a 209 amino
acid mature protein encoded by the FGF21 gene located on chromosome
19.
ApoE4 is an independent risk factor for AD. KL can
act on ApoE4 to prevent pathological β-amyloid production or
deposition, enhance synaptic functions, and increased brain
connectivity. The KL-VS status could thus mitigate ApoE4 risks for
AD and could be used to further stratify individuals who carry
APOE4 in clinical trials for the disease. KL itself could represent
a therapeutic for the prevention or treatment of AD in individuals
who carry ApoE4(7). It would be
vital in future molecular studies to factor in the relationship of
ApoE4, klotho, and the occurrence of AD.
The present demonstration of amino acid residues
phe76-val96 of KL aligning closely with residues asp7-asn27 of Aβ
suggests that Aβ could enhance the ability of KL to draw FGF21 to
regions of incipient neurodegeneration in AD. The problem arises
with age. Older people do not heal or repair tissue damage as well
as younger individuals. As neurodegeneration advances in an older
individual, perhaps caused by neuroinflammation related to
HSV-1(21), increasing amounts of
amyloid are produced, forming an adhesive web, as the brain tries
to hold the pathologic process in check. Meanwhile, damage
increases and spreads. Progressive neurodegeneration and cognitive
decline are the outcome.
Further studies are required to explore these
findings in depth. It would be worthwhile to examine the function
of klotho and FGF21 in animal experiments.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
SL and PHR contributed equally to the study. Both
authors were involved in the conception and design of the study, in
data collection and analysis, as well as in the revisions of the
manuscript. Both authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hardy J and Allsop D: Amyloid deposition
as the central event in the aetiology of Alzheimer's disease.
Trends Pharmacol Sci. 12:383–388. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castellani RJ, Lee HG, Zhu X, Perry G and
Smith MA: Alzheimer disease pathology as a host response. J
Neuropathol Exp Neurol. 67:523–531. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Castellani RJ and Perry G: The
complexities of the pathology-pathogenesis relationship in
Alzheimer disease. Biochem Pharmacol. 88:671–676. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Soscia SJ, Kirby JE, Washicosky KJ, Tucker
SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi
RE and Moir RD: The Alzheimer's disease-associated amyloid
beta-protein is an antimicrobial peptide. PLoS One.
5(e9505)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moir RD, Lathe R and Tanzi RE: The
antimicrobial protection hypothesis of Alzheimer's disease.
Alzheimers Dement. 14:1602–1614. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mangold CA and Szpara ML: Persistent
infection with herpes simplex virus 1 and Alzheimer's disease-a
call to study how variability in both virus and host may impact
disease. Viruses. 11(966)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dubal DB, Zhu L, Sanchez PE, Worden K,
Broestl L, Johnson E, Ho K, Yu GQ, Kim D, Betourne A, et al: Life
extension factor klotho prevents mortality and enhances cognition
in hAPP transgenic mice. J Neurosci. 35:2358–2371. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dubal DB and Yokoyama JS: Longevity gene
KLOTHO and Alzheimer disease-a better fate for individuals who
carry APOE ε4. JAMA Neurol. 77:798–800. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Belloy ME, Napolioni V, Han SS, Le Guen Y
and Greicius MD: Alzheimer's Disease Neuroimaging Initiative.
Association of klotho-vs heterozygosity with risk of Alzheimer
disease in individuals who carry APOE4. JAMA Neurol. 77:849–862.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee S, Choi J, Mohanty J, Sousa LP, Tome
F, Pardon E, Steyaert J, Lemmon MA, Lax I and Schlessinger J:
Structures of β-klotho reveal a ‘zip code’-like mechanism for
endocrine FGF signalling. Nature. 553:501–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crescenzi O, Tomaselli S, Guerrini R,
Salvadori S, D'Ursi AM, Temussi PA and Picone D: Solution structure
of the Alzheimer amyloid beta-peptide (1-42) in an apolar
microenvironment. Similarity with a virus fusion domain. Eur J
Biochem. 269:5642–5648. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kuhn RM, Haussler D and Kent WJ: The UCSC
genome browser and associated tools. Brief Bioinform. 14:144–161.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lehrer S and Rheinstein PH: Mouse mammary
tumor viral env sequences are not present in the human genome but
are present in breast tumors and normal breast tissues. Virus Res.
266:43–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bhagwat M, Young L and Robison RR: Using
BLAT to find sequence similarity in closely related genomes. Curr
Protoc Bioinformatics. 10(Unit10.8)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rhesus Macaque Genome Sequencing and
Analysis Consortium. Gibbs R, Rogers J, Katze MG, Bumgarner R,
Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC,
Wilson RK, et alEvolutionary and biomedical insights from the
rhesus macaque genome. Science. 316:222–234. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moir RD and Tanzi RE: Low evolutionary
selection pressure in senescence does not explain the persistence
of Aβ in the vertebrate genome. Front Aging Neurosci.
11(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuro-O M: Klotho. Pflugers Arch.
459:333–343. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kuro-O M: The Klotho proteins in health
and disease. Nat Rev Nephrol. 15:27–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pickart CM and Eddins MJ: Ubiquitin:
Structures, functions, mechanisms. Biochim Biophys Acta.
1695:55–72. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Taliyan R, Chandran SK and Kakoty V:
Therapeutic approaches to Alzheimer's type of dementia: A focus on
FGF21 mediated neuroprotection. Curr Pharm Des. 25:2555–2568.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Komaroff AL: Can infections cause
Alzheimer disease? JAMA. 324:239–240. 2020.PubMed/NCBI View Article : Google Scholar
|