|
1
|
Hardy J and Allsop D: Amyloid deposition
as the central event in the aetiology of Alzheimer's disease.
Trends Pharmacol Sci. 12:383–388. 1991.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Castellani RJ, Lee HG, Zhu X, Perry G and
Smith MA: Alzheimer disease pathology as a host response. J
Neuropathol Exp Neurol. 67:523–531. 2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Castellani RJ and Perry G: The
complexities of the pathology-pathogenesis relationship in
Alzheimer disease. Biochem Pharmacol. 88:671–676. 2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Soscia SJ, Kirby JE, Washicosky KJ, Tucker
SM, Ingelsson M, Hyman B, Burton MA, Goldstein LE, Duong S, Tanzi
RE and Moir RD: The Alzheimer's disease-associated amyloid
beta-protein is an antimicrobial peptide. PLoS One.
5(e9505)2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Moir RD, Lathe R and Tanzi RE: The
antimicrobial protection hypothesis of Alzheimer's disease.
Alzheimers Dement. 14:1602–1614. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mangold CA and Szpara ML: Persistent
infection with herpes simplex virus 1 and Alzheimer's disease-a
call to study how variability in both virus and host may impact
disease. Viruses. 11(966)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dubal DB, Zhu L, Sanchez PE, Worden K,
Broestl L, Johnson E, Ho K, Yu GQ, Kim D, Betourne A, et al: Life
extension factor klotho prevents mortality and enhances cognition
in hAPP transgenic mice. J Neurosci. 35:2358–2371. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Dubal DB and Yokoyama JS: Longevity gene
KLOTHO and Alzheimer disease-a better fate for individuals who
carry APOE ε4. JAMA Neurol. 77:798–800. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Belloy ME, Napolioni V, Han SS, Le Guen Y
and Greicius MD: Alzheimer's Disease Neuroimaging Initiative.
Association of klotho-vs heterozygosity with risk of Alzheimer
disease in individuals who carry APOE4. JAMA Neurol. 77:849–862.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lee S, Choi J, Mohanty J, Sousa LP, Tome
F, Pardon E, Steyaert J, Lemmon MA, Lax I and Schlessinger J:
Structures of β-klotho reveal a ‘zip code’-like mechanism for
endocrine FGF signalling. Nature. 553:501–505. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Crescenzi O, Tomaselli S, Guerrini R,
Salvadori S, D'Ursi AM, Temussi PA and Picone D: Solution structure
of the Alzheimer amyloid beta-peptide (1-42) in an apolar
microenvironment. Similarity with a virus fusion domain. Eur J
Biochem. 269:5642–5648. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
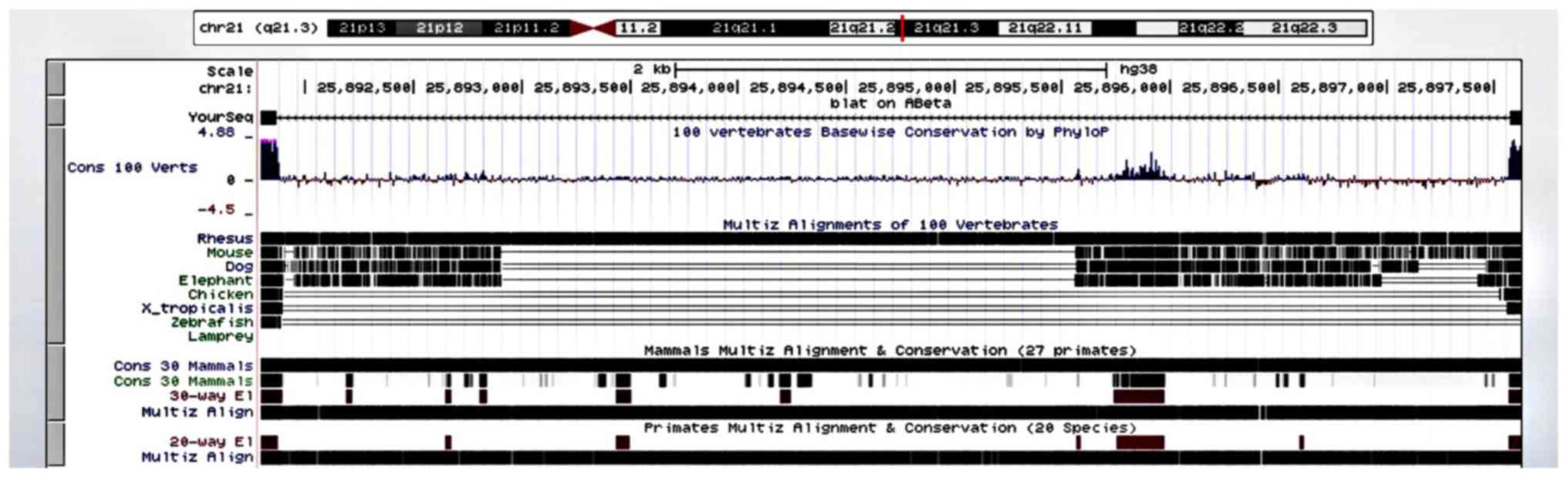
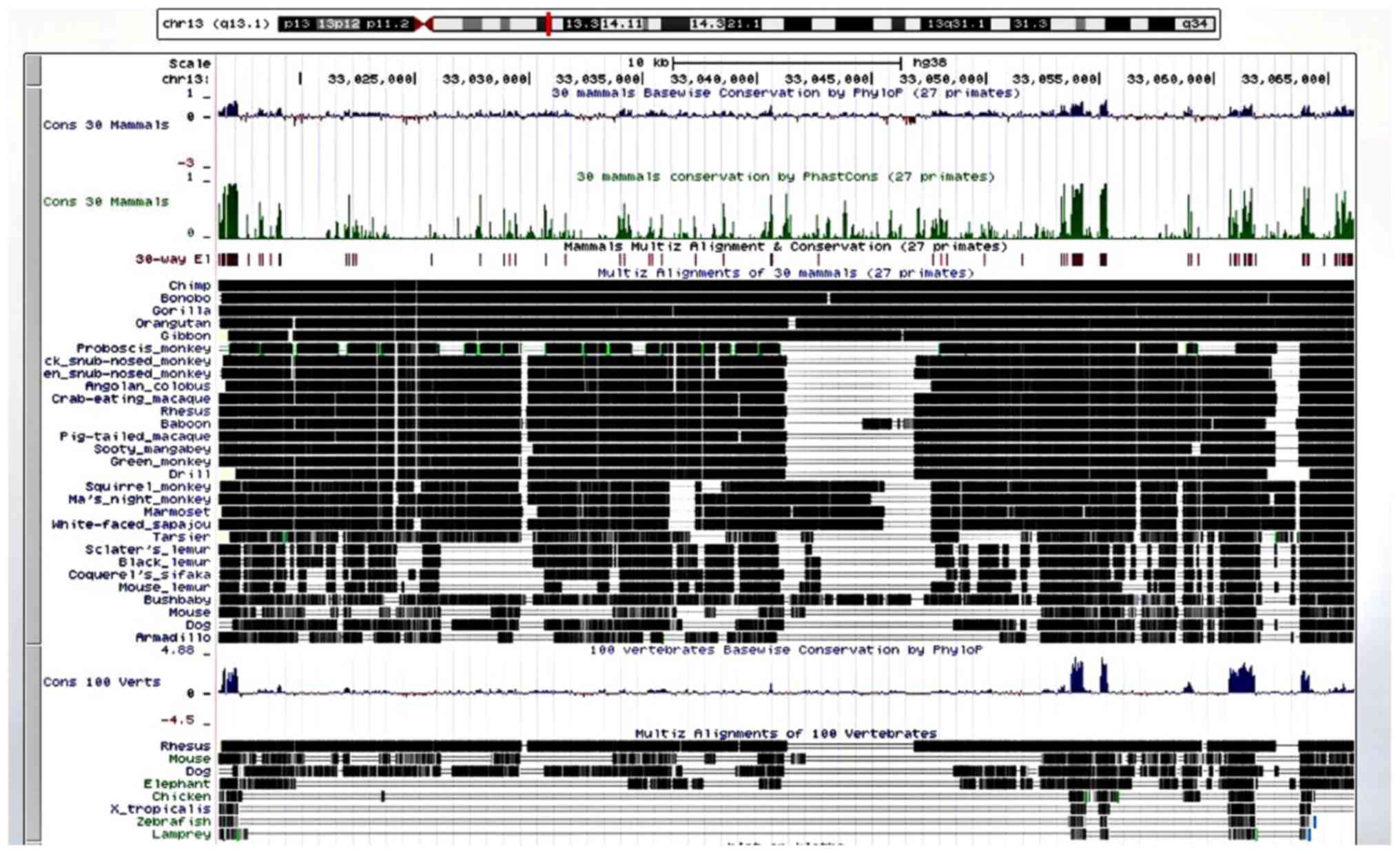
Kuhn RM, Haussler D and Kent WJ: The UCSC
genome browser and associated tools. Brief Bioinform. 14:144–161.
2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lehrer S and Rheinstein PH: Mouse mammary
tumor viral env sequences are not present in the human genome but
are present in breast tumors and normal breast tissues. Virus Res.
266:43–47. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bhagwat M, Young L and Robison RR: Using
BLAT to find sequence similarity in closely related genomes. Curr
Protoc Bioinformatics. 10(Unit10.8)2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Rhesus Macaque Genome Sequencing and
Analysis Consortium. Gibbs R, Rogers J, Katze MG, Bumgarner R,
Weinstock GM, Mardis ER, Remington KA, Strausberg RL, Venter JC,
Wilson RK, et alEvolutionary and biomedical insights from the
rhesus macaque genome. Science. 316:222–234. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Moir RD and Tanzi RE: Low evolutionary
selection pressure in senescence does not explain the persistence
of Aβ in the vertebrate genome. Front Aging Neurosci.
11(70)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuro-O M: Klotho. Pflugers Arch.
459:333–343. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kuro-O M: The Klotho proteins in health
and disease. Nat Rev Nephrol. 15:27–44. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pickart CM and Eddins MJ: Ubiquitin:
Structures, functions, mechanisms. Biochim Biophys Acta.
1695:55–72. 2004.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Taliyan R, Chandran SK and Kakoty V:
Therapeutic approaches to Alzheimer's type of dementia: A focus on
FGF21 mediated neuroprotection. Curr Pharm Des. 25:2555–2568.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Komaroff AL: Can infections cause
Alzheimer disease? JAMA. 324:239–240. 2020.PubMed/NCBI View Article : Google Scholar
|