Introduction
Since the first report of pneumonia due to infection
with the novel severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2) in Wuhan, China, namely infection with coronavirus
disease 2019 (COVID-19), the ongoing outbreak of the virus has
rapidly spread worldwide and has become a public health emergency
(1-3).
To date, >26.7 million individuals in >100 hundred countries
and regions were identified to have been infected with SARS-CoV-2,
according to the COVID-19 dashboard by Johns Hopkins University. Of
these, elderly patients are inclined to suffer from more severe
symptoms and a higher intensive care unit (ICU) admission rate than
other subpopulations (4,5). Generally, patients with COVID-19
infection exhibit clinical features, including fever and sputum,
paroxysmal cough, gastrorrhagia, pneumothorax, shortness of breath,
a low oxygen saturation, ground-glass opacity, pneumonia
infiltration and in particular, critically ill patients exhibit
what is known as the cytokine storm, the dysregulation of
immunocytes and multiple concomitant symptoms commonly resulting in
worse outcomes and a higher mortality rate (3,5,6).
State-of-the-art updates on clinical grade vaccines
and antiviral drugs for COVID-19 treatment are promising, but are
not yet forthcoming (7-9).
For instance, recent studies have revealed the involvement and
ultrastructure of angiotensin I converting enzyme 2 receptor (ACE2)
in mediating SARS-CoV-2 infection, which has been demonstrated as
the primary cell attachment molecule for SARS-CoV-2, and holds
promise for exploring the pathogenesis and drug development
(9-14).
Moreover, comprehensive treatments have been developed in clinical
trials to prevent and reverse the cytokine storm and pulmonary
injury caused by SARS-CoV-2, including spectrum antibiotics,
antiviral drugs, anti-inflammatory corticosteroids, Chinese
medicine, cytotherapy, immunotherapeutics and supportive
therapeutics (7,9,14-18).
However, as for elderly patients critically ill with COVID-19,
particularly those with multiple concomitant diseases, the efficacy
is far from satisfactory due to the hypoimmunity and multiple organ
failure, as well as the potentially controversial and severe
side-effects; thus, the development of safe and effective treatment
strategies if a matter of urgency (7,18,19).
Mesenchymal stem/stromal cells (MSCs) possess
advantaged immunomodulatory and hematopoietic-supporting
properties, together with a multi-lineage differentiation
potential; they have been identified from numerous tissues and hold
great promise for use in regenerative medicine (20-23).
Generally, the therapeutic effects of MSCs, include
immunoregulation and tissue repair, mainly through direct- or
trans-differentiation, in an autocrine and paracrine manner, as
well as by supplying the microenvironment and homing methods
(24-28).
For decades, low-immunogenic MSCs have been shown to be safe and
preferable for use in the management of recurrent and refractory
diseases, such as spinal injury, type 2 diabetes, graft-vs.-host
disease (GVHD), autoimmune diseases and hematological malignancies,
as well as for severe influenza H5N1 caused by acute respiratory
distress syndrome (ARDS) (20,24,29-31).
Recently, Leng et al and Wang et al reported the
exploration of adult tissue-derived MSCs upon 7 mild and severe
patients and revealed a favorable prognosis (14,32).
According to the ClinicalTrials.gov of NIH, a total number of 10
clinical trials focusing on MSC-based COVID-19 treatment from
China, France, Brazil and Jordan (https://clinicaltrials.gov/ct2/results?cond=Covid19&term=mesenchymal+stem+cells&cntry=&state=&city=&dist=)
have been newly registered among the 1,074 clinical trials upon
MSCs (https://clinicaltrials.gov/ct2/results?cond=&term=mesenchymal+stem+cells&cntry=&state=&city=&dist=),
which indicate the expectations of clinicians upon MSC-based
cytotherapy for ameliorating the outcomes of patients with
COVID-19; however, the unequivocal clinical efficacy remains to be
determined.
In the present study, an elderly patient critically
ill with COVID-19 with multiple concomitant diseases was enrolled,
and a systemic human umbilical cord-derived mesenchymal
stem/stromal cell (hUC-MSC) transplantation pilot study was
conducted following the informed consent of the patient and the
Ethics Committee of the Affiliated Hospital of Zunyi Medical
University. Following the administration of hUC-MSCs for 3 times,
the clinical symptoms and physical assessments were effectively
ameliorated and non-adverse effects were observed during
cytotherapy.
Case report
General information of the patient
with COVID-19
The enrolled 75-year-old male patient (body weight,
50 kg), with multiple comorbidities, including hypertension (for
>10 years), diabetes (for >1 year), and coronary heart
disease with auricular fibrillation (for >1 year), was first
diagnosed at the local county people's hospital on January 29, 2020
due to the feeling of limb weakness for 3 days (Table SI). As described, despite without
the history of offsite residence or exotic traveling, he had a
history of contact with individuals from the infective spot. On
January 31, 2020, the patient was in a critical condition and was
transferred to the Affiliated Hospital of Zunyi Medical University
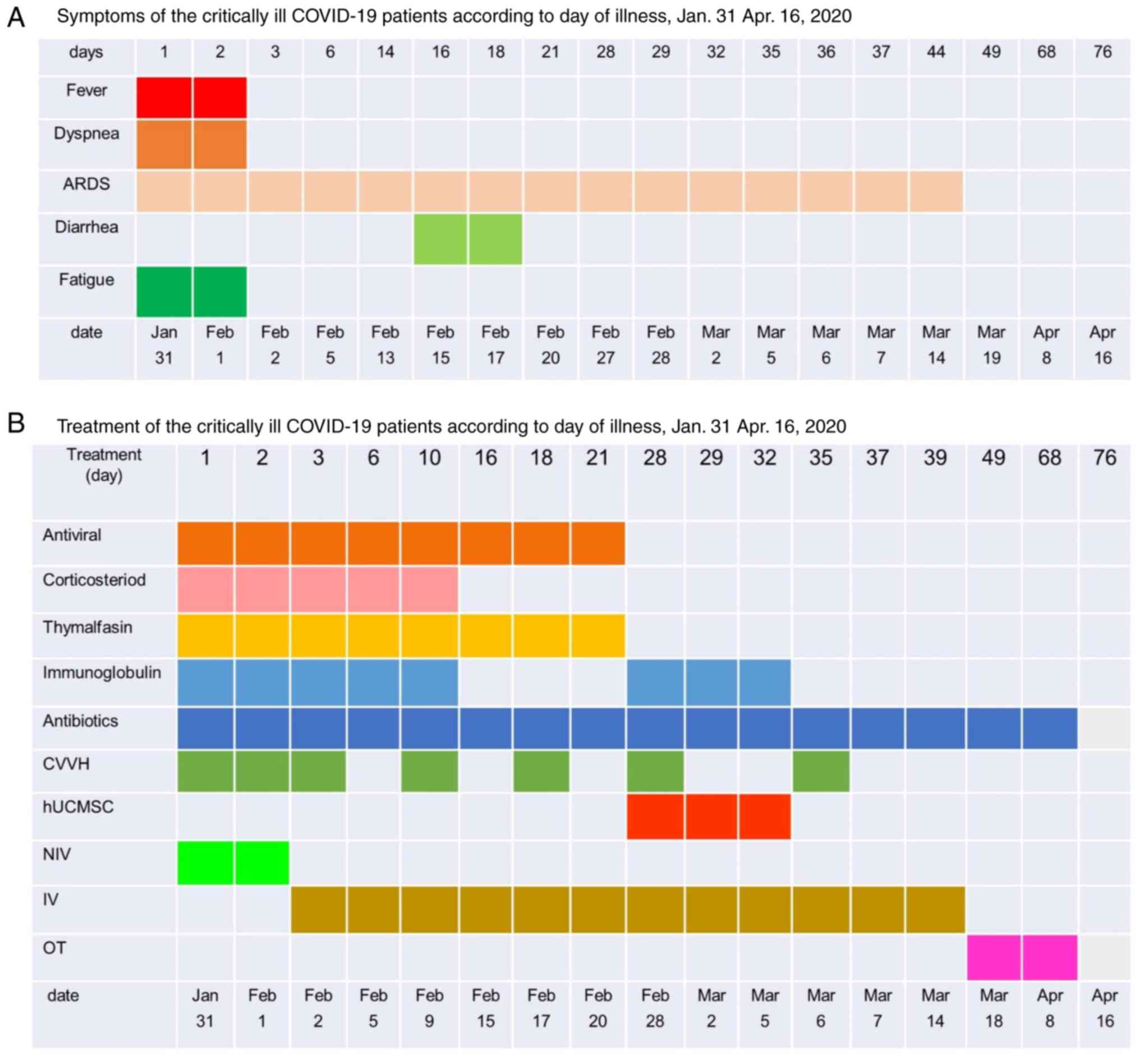
for further diagnosis and treatment (Fig. 1 and Table SI).
The patient was confirmed to have been infected with
COVID-19 based on the positive results of SARS-CoV-2 nucleic acid
analysis in the lixivium of nasopharyngeal swab, together with the
typical and multiple ground-glass opacity of exudative lesions in
both lungs (Fig. 2A and Data S1). According to the systemic
examination, the patient exhibited the following physical signs: A
low fever (37.6˚C); pulse (P), 54 beats per minute (bpm);
respiration (R), 45 bpm; blood pressure (BP), 136/79 mmHg; oxygen
saturation (SpO2), 95-98%; non-invasive
ventilator-assisted breathing (NIV); fraction of inspired
O2 (FiO2), 70-80%; oxygenation index (OI),
92.95 mmHg (Table SI). The
patient was conscious and listless, exhibited an acute appearance
and emaciated body, together with arrhythmia and atrial
fibrillation (Fig. 1A). Although
there were no abnormalities, such as cyanosis, tracheal deviation
and symmetrical thorax, thick breathing sounds, as well as moist
rales could be heard in both lungs. With the aid of a blood routine
detector for auxiliary examination, it was found that the patient
had a decreased content of white blood cells (WBCs;
3.76x109/l) and lymphocytes (LYMPH, 0.03%) (Table SI).
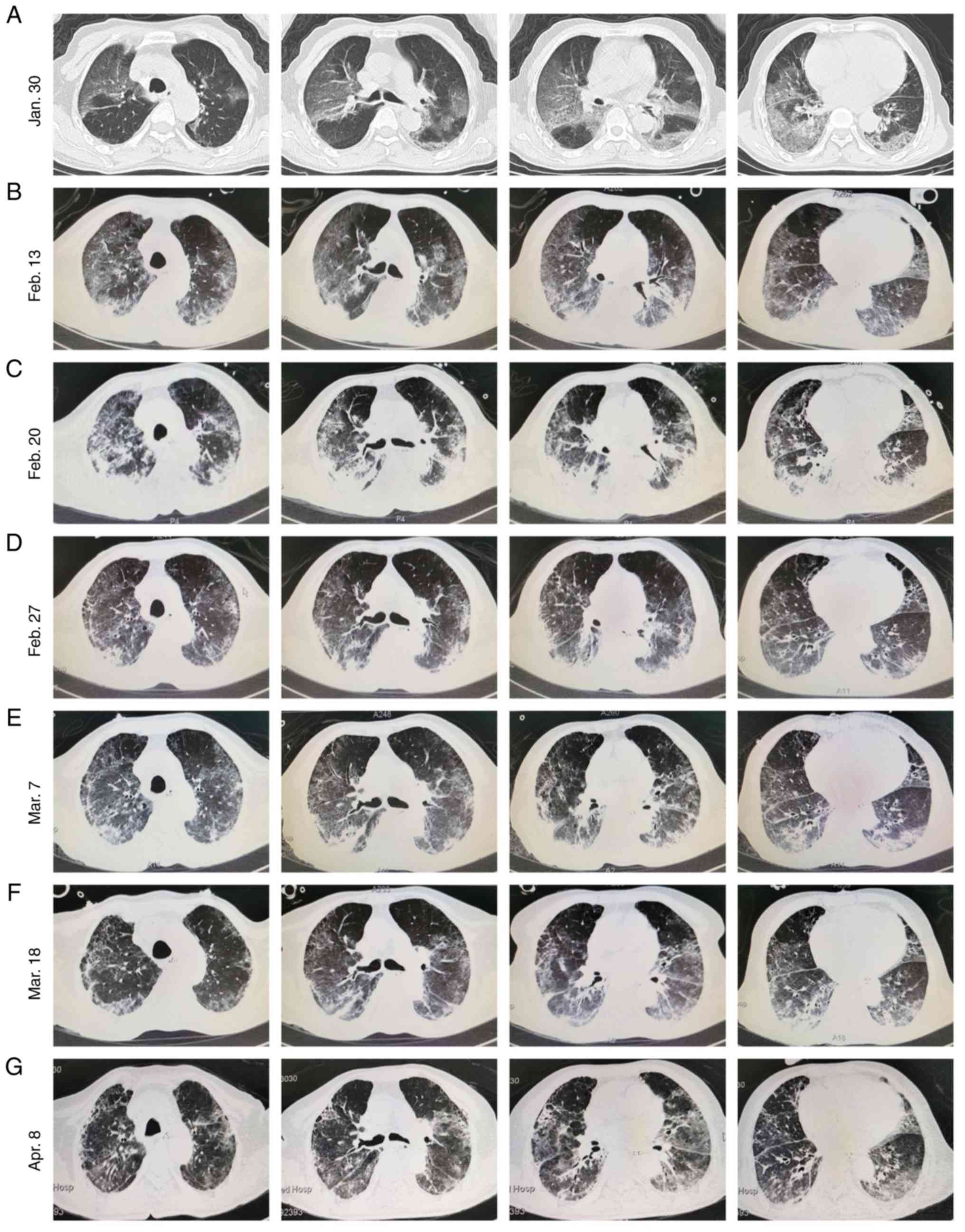 | Figure 2The dynamic chest radiographs during
traditionally comprehensive treatment and hUC-MSC administration.
(A-C) The dynamic chest X-ray images of the patient on (A) January
30, (B) February 13 and (C) February 20, 2020. The streaky
ground-glass opacity of exudative lesions in both lungs was
steadily increased in density over time during traditionally
comprehensive treatment. (D-G) The dynamic chest X-ray images of
the patient on February 27 (D, the day before the first trial for
hUC-MSC transplantation), March 7 (E, 2 days after the third trial
for hUC-MSC transplantation), March 18 (F, 13 days after the third
trial for hUC-MSC transplantation), and April 8 (G, 34 days after
the third trial for hUC-MSC transplantation), 2020. The streaky
ground-glass opacity of exudative lesions in both lungs was
steadily decreased in density over time during hUC-MSC
administration with traditionally comprehensive treatment. hUC-MSC,
human umbilical cord-derived mesenchymal stem/stromal cell. |
Traditionally comprehensive treatment
not effective for the patient
On the basis of the abovementioned information, the
hospital specialist group came to the following conclusion: The
patient was a critically ill with COVID-19 with severe ARDS; the
patient also suffered from multiple organ dysfunction syndrome
(MODS) for respiratory, kidney and liver function; coronary
atherosclerotic heart disease with auricular fibrillation; and type
2 diabetes. Thereafter, the critically ill patient with COVID-19
with comorbidities was administered to the the intensive care unit
(ICU) for monitoring electrocardiogram (ECG), blood oxygen
saturation and dynamic blood pressure and NIV. Moreover, according
to the guidance of National Health Commission (NHC) of China,
comprehensive treatment was conducted on the patient, including
anti-viral and anti-infection, immune enhancement, hypoglycemic
management and nutrition therapy, as well as pharmacotherapy, such
as lopinavir/ritonavir, ribavirin, recombinant human interferon α2b
(hIL-α2b) and abidor (Fig. 1B). On
February 2, 2020, the patient was administered tracheal intubation
followed by tracheotomy (the following day) for invasive breathing
(IV) supportive therapy, and numerous pink frothy sputa were
visible. However, even though following a 30-day period of positive
support care and symptomatic therapy, the enrolled patient was not
able to be removed ventilator support due to the poor absorption of
infiltration shadow in the lungs (Figs. 1B, and 2B and C,
and Table SI).
hUC-MSC administration and safety
outcomes
Considering the persistent poor prognosis of
rehabilitative treatment and the shortfall of substantial
improvement of the patient, the hospital specialist group
unanimously gave the consultation for the application for MSC-based
cytotherapy, which was subsequently approved by the patient and by
the Ethics Committee of the Affiliated Hospital of Zunyi Medical
University (Figs. 1, 2C and D).
Having taken into sufficient consideration the
potential untoward effects and following the meticulous arrangement
of emergency measures, the patient was subjected to the intravenous
delivery of clinical-grade hUC-MSCs on February 28, March 2 and
March 5, respectively (Fig. 1B).
In total, 5x107 hUC-MSCs (Jiangxi Health-Biotech Stem
Cell Technology Co. Ltd, product lot no. 202002JF07, 202003JF02 and
202002JF05) in 100 ml saline were infused into the body of the
patient through a blood transfusion needle at a certain rate of 40
drops per min. Within 24 h after the transfusion process, no acute
untoward effects, such as infusion-associated and allergic
reactions (e.g., instantaneous low fever, polypnea or slight
trembling) were observed in the patient. Furthermore, no secondary
infection, delayed hypersensitivity or life-threatening events
occurred in the following 30 days of observation. Furthermore,
accompanied by the upregulation of immunoglobulin, the clinical
parameters, chest radiography and a SARS-CoV-2 nucleic acid test,
as well as the aforementioned comprehensive treatment, including
antibiotics, antiviral drugs and respiratory support by the
breathing machine, were recorded (Fig.
2E and F). The symptoms
observed prior to hUC-MSC administration, such as fatigue and
listlessness, low oxygen saturation and an acute appearance
disappeared, and arrhythmia and atrial fibrillation in the patient
were improved to a certain extent as well (Table SI).
hUC-MSC administration ameliorates the
outcomes of COVID-19-associated pneumonia
To systematically and meticulously dissect the
efficacy of systemic hUC-MSC transplantation upon the patient
critically ill with COVID-19 with multiple comorbidities, the chest
radiography was primarily used. Compared with the CT image of the
chest on February 27 (the day before MSC treatment), the first
application of hUC-MSCs (within 2 days since hUC-MSC
transplantation) did not reveal immediate and evidently
ameliorative effects upon the absorption of infiltration shadow in
the lungs, even though a series of the aforementioned vital signs
were significantly improved (Fig.
2D and E). However, 13 days
after the third trial of hUC-MSC transplantation, pneumonia
infiltration was suppressed, and in particular, spontaneous
remission in the ground-glass opacity was observed in both lungs on
April 8 (Fig. 2F and G).
hUC-MSC administration ameliorates the
clinical outcomes of the patient
From the view of the respiratory support curve, it
was found that both the SpO2 and OI were upregulated,
whereas the FiO2 was decreased, which was further
confirmed by the replacement of mechanical ventilation with
ordinary oxygen treatment (OT) (Figs.
1B, and 3A and B, and Table
SI). Together with the hemoglobin (Hb) and platelet (PLT)
values, a clinical laboratory examination revealed the ultimate
resumption of the content of WBCs and total lymphocyte count in the
peripheral blood of patient after 2 weeks from the hUC-MSC
transplantation (Figs. 3C and
D, and S1A and B, and Table SI). Furthermore, to assess the
potential effects on the cytokine storm, a sharp decrease was
observed in the levels of pivotal pro-inflammatory factors,
including IL-2, IL-4, IL-6, IL-10, TNF-α and TNF-γ in the patient
critically ill with COVID-19 (Figs.
3E-H, and S1C and D, and Table SI).
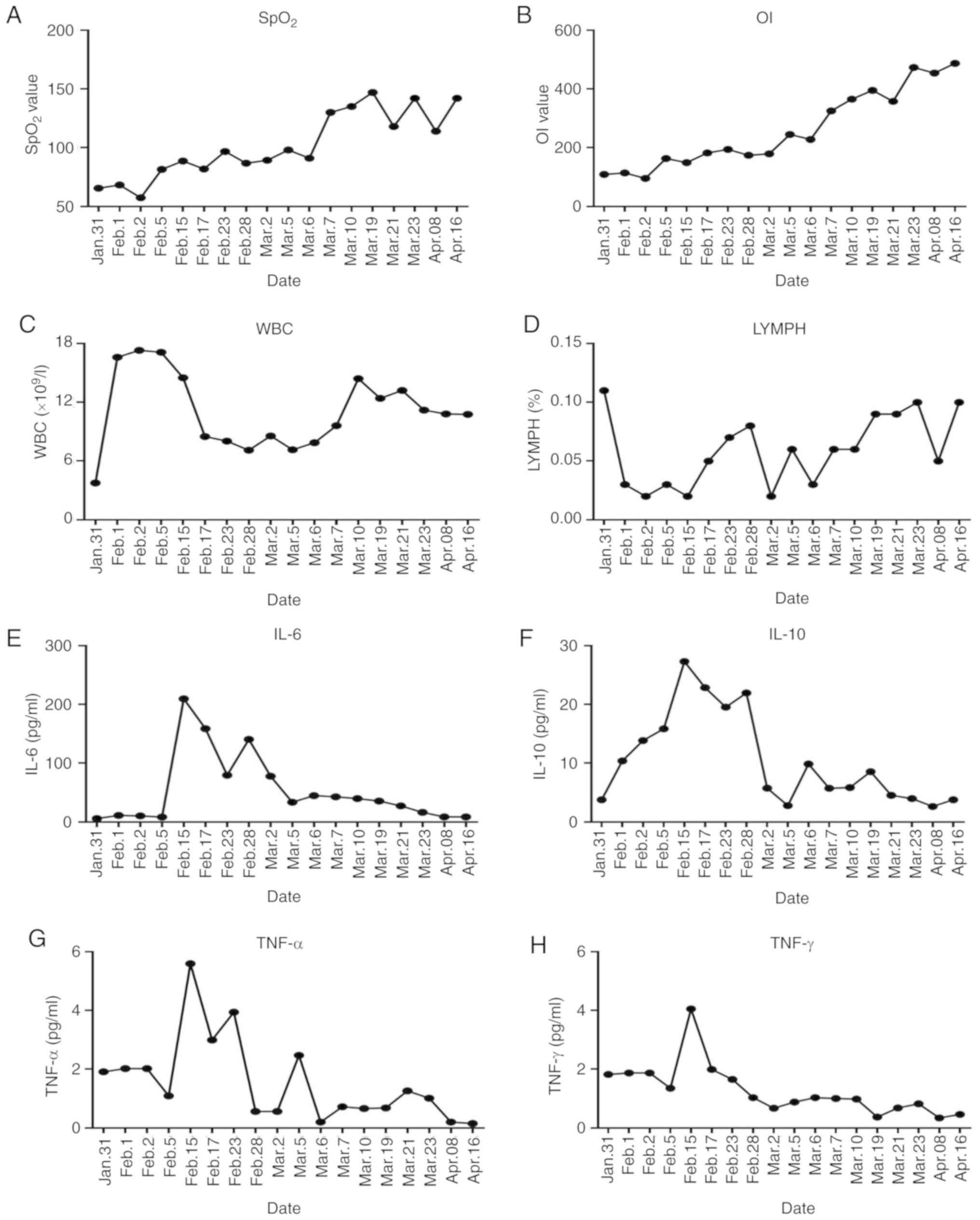 | Figure 3The dynamic variations of clinical
parameters during the patient treatment. (A and B) The dynamic
upregulation of (A) SpO2 value and (B) OI value in the
patient from Janruary 31 to April 16, 2020. (C and D) The dynamic
upregulation of WBCs (C, 109/l) and LYMPH (D, %) in the
peripheral blood of the patient from January 31 to April 16, 2020.
(E-H) The dynamic immunosuppression of IL-6 (E, pg/ml), IL-10 (F,
pg/ml), TNF-α (G, pg/ml), TNF-γ (H, pg/ml) in the peripheral blood
of the patient from January 31 to April 16th, 2020. SpO2, oxygen
saturation; OI, oxygenation index; WBCs, white blood cells; LYMPH,
lymphocytes. |
Simultaneously, the expression levels of
heart-associated [brain natriuretic peptide (BNP), Troponin T
(TNT)], kidney-associated [serum creatinine (SCR), blood urea
nitrogen (BUN)] and liver-associated [alanine transaminase (ALT),
aspartate aminotransferase (AST), albumin (ALB) and pre-serum
protein PA)] biomarkers were partially recovered following a
transient increase (Figs. 4A-D,
and S2A-D, and Table SI). Collectively, these results
indicated the ameliorative outcomes of MODS and multiple
comorbidities (e.g., coronary atherosclerotic heart disease with
auricular fibrillation).
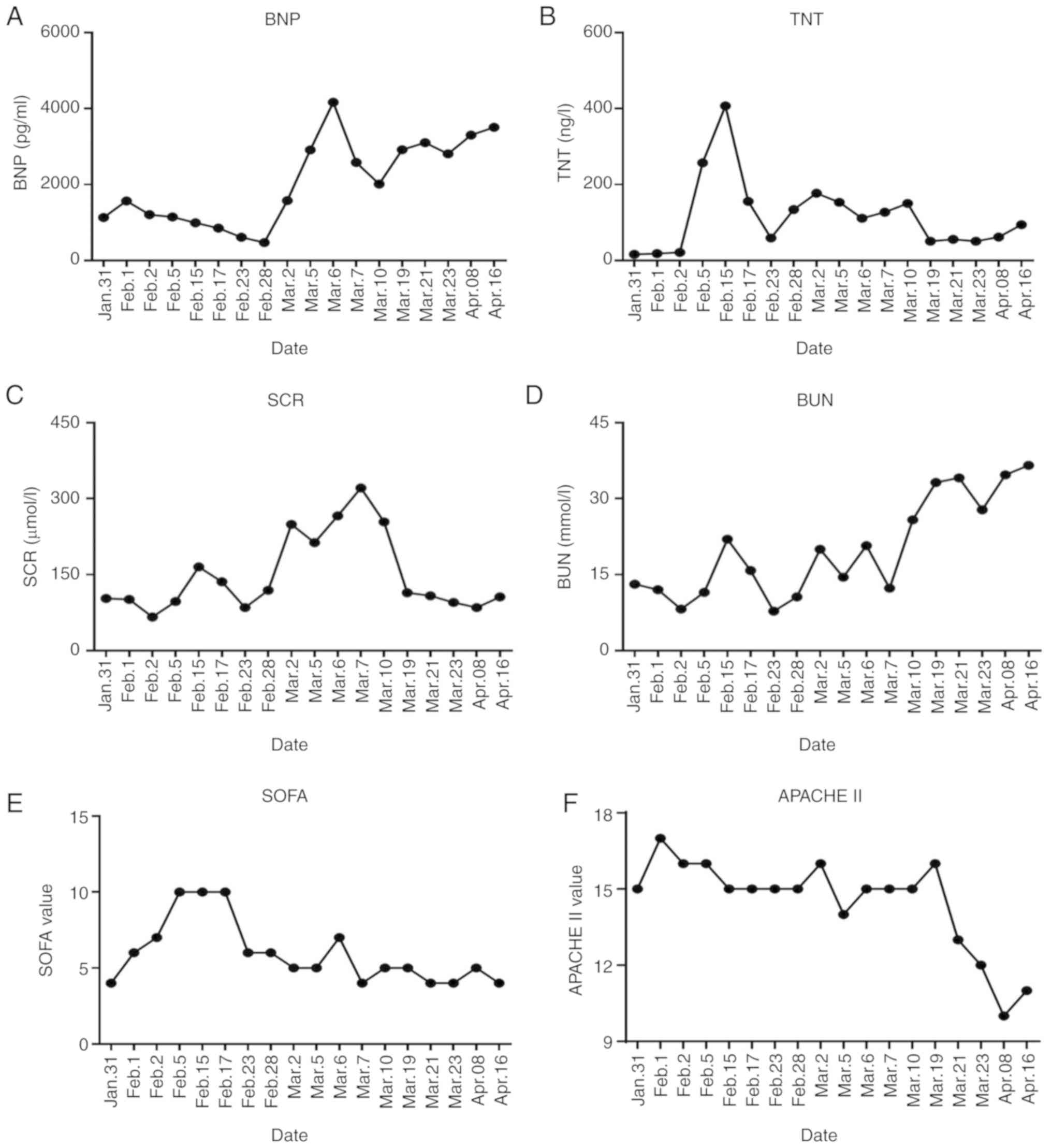 | Figure 4The dynamic variations of multiple
comorbidities-associated parameters and the severity of illness
during the patient treatment. (A and B) The dynamic variation of
heart-related indicators including the upregulation of BNP (A,
pg/ml) and downregulation of TNT (B, ng/l) in the patient from
January 31 to March 23th, 2020. (C and D) The dynamic variation of
kidney-related indicators including the instantaneous upregulation
of SCR (C, µmol/l) and delayed upregulation of BUN (D, mmol/l) in
the patient from January 31 to April 16, 2020. (E and F) The
dynamic variation of severity of illness-associated indicators
including the standard (E) SOFA value, and (F) APACHE II value in
the patient from January 31 to April 16, 2020. BNP, brain
natriuretic peptide; TNT, troponin T; BUN, blood urea nitrogen;
APACHE II, acute physiology and chronic health evaluation II; SOFA,
sequential organ failure assessment. |
For the purpose of comprehensively evaluating the
efficacy of hUC-MSCs on COVID-19 infection, the multifaceted
disease scores were conducted according to the standard indexes.
From the dynamic variations of the curves, it was found tht both
the sequential organ failure assessment (SOFA) and pneumonia
severity index (PSI) scores were immediately decreased after the
third trial of hUC-MSC transplantation, while the Acute physiology
and chronic health evaluation II (PACHE II) exhibited a delayed
decrease for 2 weeks (Figs. 4E and
F, and S2E). Above all, with the aid of hUC-MSC
administration and traditionally comprehensive treatment, the
SARS-CoV-2 nucleic acid test result of the patient finally yielded
a negative result as well.
Discussion
The ongoing outbreak of COVID-19 pneumonia caused by
SARS-CoV-2 has become a worldwide public hygiene event and has
caused the death of >877,500 individuals due to a severe
inflammation response and cytokine storm, particularly for
critically ill elderly patients with pneumonia-induced ARDS and
multiple comorbidities (3-6,33).
Due to the deficiency of targeted therapeutics, as well as
specialized antiviral vaccines and drugs, there is an urgency for
the development of MSC-based cytotherapy to reverse the
immunodysregulation and high mortality, and to facilitate
functional reconstruction (8,9,18).
The present study is a pilot case report study on the systemic
infusion of hUC-MSCs for patients critically ill with COVID-19. On
the basis of traditionally comprehensive treatment, hUC-MSC
administration did not trigger primary or secondary untoward
effects, but effectively ameliorated the outcomes of the patient,
such as vital signs, deteriorated clinical parameters and pneumonia
associated with SARS-CoV-2 infection, and related ARDS and
MODS.
In general,SARS-CoV-2 belongs to the genus and
exhibits great phylogenetic similarity to the coronavirus from
bats, which is distinguished from the 2003 SARS-CoV and the Middle
East respiratory syndrome coronavirus (MERS-CoV) at the molecular
level (7,8,32,34-37).
Despite the early outbreak of COVID-19 in Wuhan of China, yet
increasingly evidences were inclined to dismiss the possibility
from the classification of SARS-CoV-2 (33,34,37,38).
Although patient zero and the spectrum of intermediaries are still
unknowable, yet the multifaceted characterization of latent period
and asymptomatic carriers together with the main routes of
human-to-human transmission and comprehensive diagnosis have made
the prevention and control of COVID-19 outbreaks in the early
period of exponential growth more available (3,7,37,39).
For instance, the suspected SARS-CoV-2-infected patients in China
are diagnosed and quarantined on the basis of evidence, including
epidemiological, demographic, clinical, laboratory and radiological
characteristics according to guidance for the diagnosis and
treatment of 2019 novel coronavirus infected pneumonia from China
CDC and WHO recommendations (4-6,15).
For instance, numerous strategies have been developed for
SARS-CoV-2 detection, including SARS-CoV-2 RNA and protein, and
even the early ELISA- or collaurum-based antibody response (IgG,
IgM and IgA) against the virus (9). Although traditionally comprehensive
treatment, including supportive symptomatic treatment (e.g.,
steroids, oxygen therapy and fluid management) and therapeutic
intervention (e.g., immunomodulatory adjuvants and substances)
plays a critical role in the treatment of COVID-19-infected
patients, the efficacy of suppressing transmission and ameliorating
disease is far from satisfactory, due to the time-consuming
development for unequivocal clinical-grade targeted vaccines and
antiviral drugs (9,19,40).
For example, scientific or clinical data upon broad-spectrum
antiviral drug repurposing, high-throughput drug screening, late
humoral response and persistence of alternative neutralizing
antibodies during SARS-CoV-2 infection is desperately scarce
(9). Worse still, the
controversial outcome and the side-effects of therapeutic drugs,
such as gastrorrhagia and osteoporosis are suspected to be caused
by glucocorticoids (18,19). Moreover, elderly patients are apt
to suffer from worse symptoms (e.g., ARDS), and are associated with
a higher ICU admission rate and higher mortality rate when compared
with other demographics (6,33).
To date, basic research and clinical trials have indicated the
pivotal role of higher concentrations of pro-inflammatory factors
(e.g., IL-6, IL-10, G-CSF, MCP-1 and TNF-α) and the resultant
severe cytokine storm, which further accelerate the ground-glass
opacity and pneumonia infiltration, as well as multiple organ
destruction and dysfunction in the critically ill COVID-19 patient
(3,5). Above all, despite the large flow of
scientific or clinical explorations on mechanistic characteristics
and possible drug repositioning rationale, the eventual mitigation
and containment of this COVID-19 epidemic diseases still depends on
the current exponentially increasing knowledge of the SARS-CoV-2
pathology, virology and cross-species transmission (9).
Since the development of new drugs is a
time-consuming and costly process, drug repurposing, such as MSCs-
and the gene therapy-based approach is a promising strategy for
identifying therapeutic solutions during the time-critical pandemic
(9,41). Since the 1960s, MSCs were initially
isolated from bone marrow followed by various adult tissues (e.g.,
adipose tissue and dental pulp) and perinatal tissues (e.g.,
umbilical cord and placenta), as well as human pluripotent stem
cells (e.g., hESCs, iPSCs) (20,42-44).
Of the aforementioned MSCs, hBM-MSCs and hUC-MSCs are associated
with the most applications and long-term in vitro
proliferation capacity, respectively (20,24).
For decades, the authors of the present study, as well as others,
have reported the clinical and preclinical applicability of MSCs in
refractory and recurrent diseases, such as Crohn's disease,
aplastic anemia, acute myocardial infarction, GVHD and even
H5N1-infected pneumonia which cause acute lung injury and acute
respiratory distress syndrome (ALI/ARDS), which are similar to the
symptoms of SARS-CoV-2 infection (20,24,25,42,43,45).
Very recently, Leng et al and Wang et al reported the
clinical remission of a 65-year-old female critically ill patient
with COVID-19 with hUC-MSC transplantation for 3 times as well,
whereas the lung damage was relatively mild, which was confirmed by
another studies (14,32). However, the safety and
effectiveness assessment on elderly patients critically ill with
COVID-19 with severe pneumonia-induced ARDS and MODS-associated
multiple comorbidities remains largely unknown. Herein, prior to
potentially large-scale clinical investigations and applications,
the feasibility and dependability of hUC-MSC administration on a
critically ill elderly patient with COVID were preliminarily
confirmed, including immunodysfunction, cytokine storm, severe
pneumonia-induced ARDS, MODS and the concomitant comorbidities as
well, which add new references to MSC-based COVID-19 treatment,
particularly for patients without a therapeutic response to
traditional medications, such as immunoglobulins, hormones,
antibiotics and anti-viral vaccines (14,46).
Furthermore, it was also realized that the case report alone is far
from sufficient for the full interpretation of MSC-based therapy
for COVID-19-infected patients. Therefore, the replicability of the
results obtained with this representative patient are not
sufficient. Additionally, the potentially bidirectional
immunoregulation effects and equilibrium upon cytokine storm
inhibition and SARS-CoV-2 replication should not be neglected, and
the safety and effectiveness assessment of MSC-based cytotherapy on
COVID-19-infected patients should be further examined in clinical
trials with a large number of patients.
Supplementary Material
Supplementary procedures
The dynamic variations of hemogram and
pro-inflammatory cytokines during treatment. (A and B) The dynamic
variations of Hb (A, g/l) and PLT (B, 109/l) in the
peripheral blood of the patient from January 31 to April 16, 2020.
(C and D The dynamic immunosuppression of IL-2 (C, pg/ml), and IL-4
(D, pg/ml) in the peripheral blood of the patient from January 31
to April 16, 2020. Hb, haemoglobin; PLT, platelet.
The dynamic variations of
liver-associated parameters and the severity of illness during the
patient treatment. (A-C) The dynamic variation of liver-related
indicators including ALT (A, U/l), AST (B, U/l), ALB (C, g/l) and
PA (D, μg/dl) in the patient from January 31 to April 16,
2020. (E) The dynamic variation of severity of illness-associated
indicator (PSI value) in the patient from January 31 to April 16,
2020. ALT, alanine transaminase; AST, aspartate aminotransferase;
ALB, albumin; PA, pre-serum protein.
Characteristics of the 75-year-old
patient with COVID-19.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81960024, 81700119
and 81900126), the Major Program of the National Natural Science
Foundation of China (grant no. 81330015), the National Science and
Technology Major Projects of China for ‘Major New Drugs Innovation
and Development’ (grant no. 2014ZX09508002-003), the Natural
Science Foundation of Tianjin (grant no. 19JCQNJC12500), the
Project funded by China Postdoctoral Science Foundation (grant no.
2019M661033), the Natural Science Foundation of Hebei (grant no.
H2020206403), the Emergency Project funded by Department of Science
and Technology of Jiangxi Province (2020, to ZHa), the Key project
funded by Department of Science and Technology of Shangrao City
(2020, to ZHa) and the Science and Technology Project of Tianjin
(grant no. 17ZXSCSY00030).
Availability of data and materials
The data used to support the findings of the study
are included in the present article. Additional data related to
this study are also available from the corresponding author. Trial
registration: The safety and effectiveness of human umbilical cord
mesenchymal stem cells in the treatment of acute respiratory
distress syndrome of severe novel coronavirus pneumonia (COVID-19),
ChiCTR2000030116, was registered on February 23, 2020 (http://www.chictr.org.cn/showproj.aspx?proj=49901;
prospectively registered). The analysis of clinical characteristics
and therapeutic effect of 9 cases of novel coronavirus pneumonia
(COVID-19), ChiCTR2000031930 was registered on April 15, 2020
(http://www.chictr.org.cn/showproj.aspx?proj=51602;
retrospective registration).
Authors' contributions
HC, LZ and ZHe were involved in the collection and
assembly of data and manuscript writing. DW, LL, WZ and TC were
involved in the collection and assembly of data. ZD was involved in
the preparation of the hUC-MSCs. LZ and ZHa were involved in data
interpretation, manuscript writing and revision. LZ, MC and ZHa
were involved in the conception and design of the study, and in
data analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The procedures performed on the patient followed the
internationally recognized guidelines. The ethical approval of the
research was signed by the Ethics Committee of the Affiliated
Hospital of Zunyi Medical University in China (approval no.
KLL-2020-013).
Patient consent for publication
The patient signed an informed consent to the
publication of his case report.
Competing interests
The authors declare no that they have no competing
interests.
References
|
1
|
Holshue ML, DeBolt C, Lindquist S, Lofy
KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural
A, et al: First case of 2019 novel coronavirus in the United
States. N Engl J Med. 382:929–936. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Li JY, You Z, Wang Q, Zhou ZJ, Qiu Y, Luo
R and Ge XY: The epidemic of 2019-novel-coronavirus (2019-nCoV)
pneumonia and insights for emerging infectious diseases in the
future. Microbes Infect. 22:80–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li Q, Guan X, Wu P, Wang X, Zhou L, Tong
Y, Ren R, Leung KSM, Lau EHY, Wong JY, et al: Early transmission
dynamics in wuhan, China, of novel coronavirus-infected pneumonia.
N Engl J Med. 382:1199–1207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chen N, Zhou M, Dong X, Qu J, Gong F, Han
Y, Qiu Y, Wang J, Liu Y, Wei Y, et al: Epidemiological and Clinical
characteristics of 99 cases of 2019 novel coronavirus pneumonia in
Wuhan, China: A descriptive study. Lancet. 395:507–513.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J,
Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics
of 138 hospitalized patients with 2019 novel Coronavirus-infected
pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jiang S, Du L and Shi Z: An emerging
coronavirus causing pneumonia outbreak in Wuhan, China: Calling for
developing therapeutic and prophylactic strategies. Emerg Microbes
Infect. 9:275–277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou Y, Hou Y, Shen J, Huang Y, Martin W
and Cheng F: Network-based drug repurposing for novel coronavirus
2019-nCoV/SARS-CoV-2. Cell Discov. 6(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nitulescu GM, Paunescu H, Moschos SA,
Petrakis D, Nitulescu G, Ion GND, Spandidos DA, Nikolouzakis TK,
Drakoulis N and Tsatsakis A: Comprehensive analysis of drugs to
treat SARSCoV2 infection: Mechanistic insights into current COVID19
therapies (Review). Int J Mol Med. 46:467–488. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wan Y, Shang J, Graham R, Baric RS and Li
F: Receptor recognition by the novel coronavirus from Wuhan: An
analysis Based on Decade-long structural studies of SARS
coronavirus. J Virol. 94:e00127–20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shang J, Ye G, Shi K, Wan Y, Luo C, Aihara
H, Geng Q, Auerbach A and Li F: Structural basis of receptor
recognition by SARS-CoV-2. Nature. 581:221–224. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lan J, Ge J, Yu J, Shan S, Zhou H, Fan S,
Zhang Q, Shi X, Wang Q, Zhang L and Wang X: Structure of the
SARS-CoV-2 spike Receptor-binding domain bound to the ACE2
receptor. Nature. 581:215–220. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kuba K, Imai Y, Rao S, Gao H, Guo F, Guan
B, Huan Y, Yang P, Zhang Y, Deng W, et al: A crucial role of
angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced
lung injury. Nat Med. 11:875–879. 2005.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han
Q, Shan G, Meng F, Du D, Wang S, et al: Transplantation of ACE2(-)
mesenchymal stem cells improves the outcome of patients with
COVID-19 pneumonia. Aging Dis. 11:216–228. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yu F, Du L, Ojcius DM, Pan C and Jiang S:
Measures for diagnosing and treating infections by a novel
coronavirus responsible for a pneumonia outbreak originating in
Wuhan, China. Microbes Infect. 22:74–79. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou G and Zhao Q: Perspectives on
therapeutic neutralizing antibodies against the Novel Coronavirus
SARS-CoV-2. Int J Biol Sci. 16:1718–1723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dhama K, Sharun K, Tiwari R, Dadar M,
Malik YS, Singh KP and Chaicumpa W: COVID-19, an emerging
coronavirus infection: Advances and prospects in designing and
developing vaccines, immunotherapeutics, and therapeutics. Hum
Vaccin Immunother. 16:1232–1238. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Shang L, Zhao J, Hu Y, Du R and Cao B: On
the use of corticosteroids for 2019-nCoV pneumonia. Lancet.
395:683–684. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Russell CD, Millar JE and Baillie JK:
Clinical evidence does not support corticosteroid treatment for
2019-nCoV lung injury. Lancet. 395:473–475. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhao Q, Zhang L, Wei Y, Yu H, Zou L, Huo
J, Yang H, Song B, Wei T, Wu D, et al: Systematic comparison of
hUC-MSCs at various passages reveals the variations of signatures
and therapeutic effect on acute Graft-versus-host disease. Stem
Cell Res Ther. 10(354)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yao J, Chen N, Wang X, Zhang L, Huo J, Chi
Y, Li Z and Han Z: Human supernumerary Teeth-derived apical
papillary stem cells possess preferable characteristics and
efficacy on hepatic fibrosis in mice. Stem Cells Int.
2020(6489396)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lu S, Ge M, Zheng Y, Li J, Feng X, Feng S,
Huang J, Feng Y, Yang D, Shi J, et al: CD106 is a novel mediator of
bone marrow mesenchymal stem cells via NF-κB in the bone marrow
failure of acquired aplastic anemia. Stem Cell Res Ther.
8(178)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Du W, Li X, Chi Y, Ma F, Li Z, Yang S,
Song B, Cui J, Ma T, Li J, et al: VCAM-1+ placenta chorionic
villi-derived mesenchymal stem cells display potent Pro-angiogenic
activity. Stem Cell Res Ther. 7(49)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Huo J, Zhang L, Ren X, Li C, Li X, Dong P,
Zheng X, Huang J, Shao Y, Ge M, et al: Multifaceted
characterization of the signatures and efficacy of mesenchymal
stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther.
11(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Samsonraj RM, Raghunath M, Nurcombe V, Hui
JH, van Wijnen AJ and Cool SM: Concise review: Multifaceted
characterization of human mesenchymal stem cells for use in
regenerative medicine. Stem Cells Transl Med. 6:2173–2185.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pourgholaminejad A, Aghdami N, Baharvand H
and Moazzeni SM: The effect of pro-inflammatory cytokines on
immunophenotype, differentiation capacity and immunomodulatory
functions of human mesenchymal stem cells. Cytokine. 85:51–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fan XL, Zhang Y, Li X and Fu QL:
Mechanisms underlying the protective effects of mesenchymal stem
cell-based therapy. Cell Mol Life Sci. 77:2771–2794.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kfoury Y and Scadden DT: Mesenchymal cell
contributions to the stem cell niche. Cell Stem Cell. 16:239–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Darwish I, Mubareka S and Liles WC:
Immunomodulatory therapy for severe influenza. Expert Rev Anti
Infect Ther. 9:807–822. 2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang X, Yang Y, Zhang L, Lu Y, Zhang Q,
Fan D, Zhang Y, Zhang Y, Ye Z and Xiong D: Mesenchymal stromal
cells as vehicles of tetravalent bispecific tandab (CD3/CD19) for
the treatment of B cell lymphoma combined with IDO pathway
inhibitor D-1-methyl-tryptophan. J Hematol Oncol.
10(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song
B, Li C and Han Z, Zhang L and Han Z: High-efficient generation of
VCAM-1(+) mesenchymal stem cells with multidimensional
superiorities in signatures and efficacy on aplastic anaemia mice.
Cell Prolif. 53(e12862)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang L, Liu T, Liang R, Wang G, Liu Y, Zou
J, Liu N, Zhang B, Liu Y, Ding X, et al: Mesenchymal stem cells
ameliorate β cell dysfunction of human type 2 diabetic islets by
reversing β cell dedifferentiation. EBioMedicine.
51(102615)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu Z, Shi L, Wang Y, Zhang J, Huang L,
Zhang C, Liu S, Zhao P, Liu H, Zhu L, et al: Pathological findings
of COVID-19 associated with acute respiratory distress syndrome.
Lancet Respir Med. 8:420–422. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H,
Wang W, Song H, Huang B, Zhu N, et al: Genomic characterisation and
epidemiology of 2019 novel coronavirus: implications for virus
origins and receptor binding. Lancet. 395:565–574. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Guarner J: Three emerging coronaviruses in
two decades. Am J Clin Pathol. 153:420–421. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Meo SA, Alhowikan AM, Al-Khlaiwi T, Meo
IM, Halepoto DM, Iqbal M, Usmani AM, Hajjar W and Ahmed N: Novel
coronavirus 2019-nCoV: Prevalence, biological and clinical
characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med
Pharmacol Sci. 24:2012–2019. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Forster P, Forster L, Renfrew C and
Forster M: Phylogenetic network analysis of SARS-CoV-2 genomes.
Proc Natl Acad Sci USA. 117:9241–9243. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Li X, Zai J, Zhao Q, Nie Q, Li Y, Foley BT
and Chaillon A: Evolutionary history, potential intermediate animal
host, and cross-species analyses of SARS-CoV-2. J Med Virol.
92:602–611. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Riou J and Althaus CL: Pattern of early
human-to-human transmission of Wuhan 2019 novel coronavirus
(2019-nCoV), December 2019 to January 2020. Euro Surveill.
25(2000058)2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Colson P, Rolain JM and Raoult D:
Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int J
Antimicrob Agents. 55(105923)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Nazeam J, Mohammed EZ, Raafat M, Houssein
M, Elkafoury A, Hamdy D and Jamil L: Based on principles and
insights of COVID-19 epidemiology, genome sequencing, and
pathogenesis: Retrospective analysis of sinigrin and Prolixin(RX)
(Fluphenazine) provides Off-label drug candidates. SLAS Discov: Aug
17, 2020 (Epub ahead of print). doi: 10.1177/2472555220950236.
|
|
42
|
Zhang L, Wang H, Liu C, Wu Q, Su P, Wu D,
Guo J, Zhou W, Xu Y, Shi L and Zhou J: MSX2 initiates and
accelerates mesenchymal stem/stromal cell specification of hPSCs by
regulating TWIST1 and PRAME. Stem Cell Reports. 11:497–513.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Wei Y, Hou H, Zhang L, Zhao N, Li C, Huo
J, Liu Y, Zhang W, Li Z, Liu D, et al: JNKi- and DAC-programmed
mesenchymal stem/stromal cells from hESCs facilitate hematopoiesis
and alleviate hind limb ischemia. Stem Cell Res Ther.
10(186)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Soontararak S, Chow L, Johnson V, Coy J,
Wheat W, Regan D and Dow S: Mesenchymal stem cells (MSC) derived
from induced pluripotent stem cells (iPSC) equivalent to
Adipose-derived MSC in promoting intestinal healing and microbiome
normalization in mouse inflammatory bowel disease model. Stem Cells
Transl Med. 7:456–467. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liang X, Ding Y, Zhang Y, Tse HF and Lian
Q: Paracrine mechanisms of mesenchymal stem cell-based therapy:
Current status and perspectives. Cell Transplant. 23:1045–1059.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ye J and Gimble JM: Regulation of stem
cell differentiation in adipose tissue by chronic inflammation.
Clin Exp Pharmacol Physiol. 38:872–878. 2011.PubMed/NCBI View Article : Google Scholar
|