Introduction
The 19-24 kDa translationally controlled tumor
protein (TCTP), which is encoded by the TPT1 gene, is
evolutionary highly conserved and ubiquitously present in all
eukaryotic tissues and cell types (1-3).
It is also known as fortilin, p21, p23, Q23 or histamine-releasing
factor (HRF) (2). A wide range of
interactions with cellular proteins has been reported (1,4,5).
TCTP is involved in a number of biological processes, e.g. the cell
cycle (6-8),
cell growth (9,10) and cellular development (11,12),
protein synthesis (13),
cytoskeleton (6,14-16),
immune response (17-19)
and cell death (20,21). It has been associated with
carcinogenesis (22,23), but also with tumor reversion
(24,25). In tumor reversion, tumor cells lose
their malignant phenotype and begin reverting (26). In addition, TCTP has been shown to
be the most downregulated protein in revertant cells compared to
parental cancer cells (24,27).
Cancer is one of the foremost causes of mortality
worldwide (28). The most frequent
malignancies in total numbers are lung cancer, breast cancer and
prostate cancer (29). Modern
cancer treatment is based on surgery, chemotherapy, radiation,
immunotherapy, targeted therapy, hormone therapy, stem cell
transplantation and combinations of these. However, the majority of
these options still lack satisfactory efficiency and are associated
with severe side-effects, limiting both the patient's quality of
life and the therapeutic success. In particular, drug resistance is
challenging in medical oncology (30). The demand for novel drugs against
new targets continues to increase.
Targets for cancer therapy are defined as cellular
structures with different biological functions and/or expression
levels in cancer and normal cells. These changes contribute to
malignant transformation, and their pharmacological targeting may
lead to tumor suppression. Biological targets for cancer therapy
are also valuable prognostic factors for the course of cancer
(26).
TCTP overexpression has been reported in cancer
(31,32). Notably, Telerman and Amson
demonstrated a considerable downregulation of TCTP during the
process of tumor reversion (27).
Following malignant transformation, tumors retain their ability to
be reprogrammed, which initiates the re-differentiation of
de-dedifferentiated cells and to stop de-regulated tumor cell
growth. Therefore, it is imperative to better understand the
cellular and molecular mechanisms initiating and regulating tumor
reversion and to exploit this surprising phenomenon for therapeutic
purposes, in order to inhibit the malignant process and
proliferation. However, the relevance of TCTP as a novel target in
cancer therapy and particularly, in tumor reversion remains to be
clarified.
To date, TCTP has not been extensively investigated
as prognostic factor in cancer diseases (31,33).
The present study analyzed the association between TCTP and
clinical outcome at both the mRNA and protein level on a broad
basis. For this purpose, 12 datasets covering 5,651 patients and 8
cancer types were analyzed. In addition, immunohistochemical
analyses of 495 cases of 26 tumor types was performed and the
association between TCTP protein expression and clinical outcome
was also analyzed. This analysis was undertaken as a starting point
for the establishment of a novel tumor marker and target for cancer
therapy.
Materials and methods
Statistical evaluation of the Oncomine
database
TCTP mRNA expression data was attained from
the Oncomine database (https://www.oncomine.org). Only datasets including the
expression values of TPT1 (also known as TCTP) were
selected. Furthermore, only datasets were selected, where
comparative information was available for cancer tissue and their
matched normal tissues and where the survival status as clinical
outcome parameter was recorded. For reliable statistical
evaluation, only datasets with >151 samples each were included
into the evaluations (Fig. 1). A
total of 12 datasets with 8 cancer types from 5,651 samples
fulfilled these criteria. Among these were cancers of the bladder,
brain, breast (2 sets), colorectal, lung (3 sets), ovarian and
prostate, as well as lymphoma. Normalized TCTP expression
values were defined as ‘low’ and ‘high’ using median and mean as
cut-off values. The datasets for time-to-death distributions were
analyzed and estimated using Kaplan-Meier curves. The log-rank test
was used for significance assignment. IBM SPSS statistics V23
program (IBM, Inc.) was used for all statistical analyses.
Statistical differences with P-values <0.05 were regarded as
significant.
Tumor cases
A total of 495 formalin-fixed and paraffin-embedded
tumor cases covered 26 tumor types. The tissue microarrays (TMA)
T8235713 (BioCat GmbH) and BC000119 (Biomax Inc.) were commercially
available. Further TMAs were provided by the Tissue Bank of the
Institute of Pathology, University Medical Center of the
Johannes-Gutenberg-Universität, Mainz) with ethical approvals from
the Ethics Committee of the State Authorization Association for
Medical Issues (Landesärztekammer) Rheinland Pfalz to W.R.
(October 2, 2015; Ref. 837.031 9799) and to T.E. (March 22, 2018;
Ref. 2018-13179). The patients gave their approval for the
evaluation of tumor material and publication of data generated from
these investigations prior to participation.
Immunohistochemistry
The immunohistochemical staining protocol used in
the present study was as previously described (34). Paraffin was removed by 2 washing
steps with xylene (99%), 5 min each at room temperature.
Rehydration was conducted by graded washing steps with isopropanol
at various concentrations (100, 96 and 70%). Slides were washed
with PBS and heat-induced epitope-retrieval was performed for 20
min using hot vapor. The slides were incubated in 3%
H2O2 for 10 min at room temperature to avoid
chromogen binding and endogen peroxidase. Following 5 min of PBS
washing, UltraVision Protein Block and UltraVision Hydrogen
Peroxidase Block (Thermo Fisher Scientific, Inc.) were added for 20
min for the blockade of endogenous proteins and endogenous
peroxidase to reduce non-specific background staining. Primary TPT1
(TCTP) antibody (1:300 dilution; PA5-35332, Thermo Fisher
Scientific, Inc.) was added followed by incubation at 4˚C for 12 h.
After washing, horseradish-peroxidase-labeled polymers (1:100
dilution; TL-060-QPB, Thermo Fisher Scientific, Inc.) were added
for 1 h at room temperature according to the manufacturer's
protocol. Before counterstaining with hematoxylin for 3 min at room
temperature, diaminobenzidine (DAB Quanto Chromogen, 1:30 dilution;
TA-002-QHCX, Thermo Fisher Scientific, Inc.) was added for 5 min at
room temperature as a final staining step. The slides were
dehydrated by grading steps with ethanol at various concentrations
(70, 96 and 99%) and xylol followed by embedding in Entellan (Merck
KGaA). Negative control staining (cervix carcinoma) was performed
to assure the specificity of the staining process.
Immunohistochemical analysis of membrane-bound and nuclear
expression of TCTP was performed by 3D Histotech Digital Slide
Scanner (3DHISTECH Ltd.). For representative expression values, 6
different areas were selected from each probe. The expression of
TCTP was quantified using Pannoramic Viewer software version 1.15
(3DHISTECH Ltd., Budapest, Hungary) using the H-score. A total of 7
cases were excluded due to high necrotic damage and thus
insufficient interpretability.
Statistical analysis
One-way ANOVA was used to obtain the mean comparison
of TCTP H-score within different cancer types. Variations in the
distribution of the TCTP H-score were determined by the
Tukey-Kramer's test. TCTP was categorized into 4 degrees of
positive or negative groups according to intensity of stain and
thus expression. As for the TCTP H-scores, values >200 were
grouped as strongly positive, whereas H-scores ranging from 100 to
200 were grouped as moderately positive. H-scores ranging between
20 and 100 were grouped as weakly positive, and H-scores <20
were considered as negative. An ANOVA mean comparison test for the
nTCTP H-score, TNM stage and grade was first performed.
Subsequently, the independence of the H-score as a negative or
positive group with TNM stage and grade was then determined by the
Tukey-Kramer's test. IBM SPSS statistical V23 software (IBM, Inc.)
was used for all statistical analyses. Statistical differences with
P-values <0.05 were regarded as significant.
Results
Oncomine: Association of TCTP mRNA
expression and the survival of cancer patients
A total of 12 datasets of 8 cancer types with 5,651
samples from the Oncomine database was screened with the filter
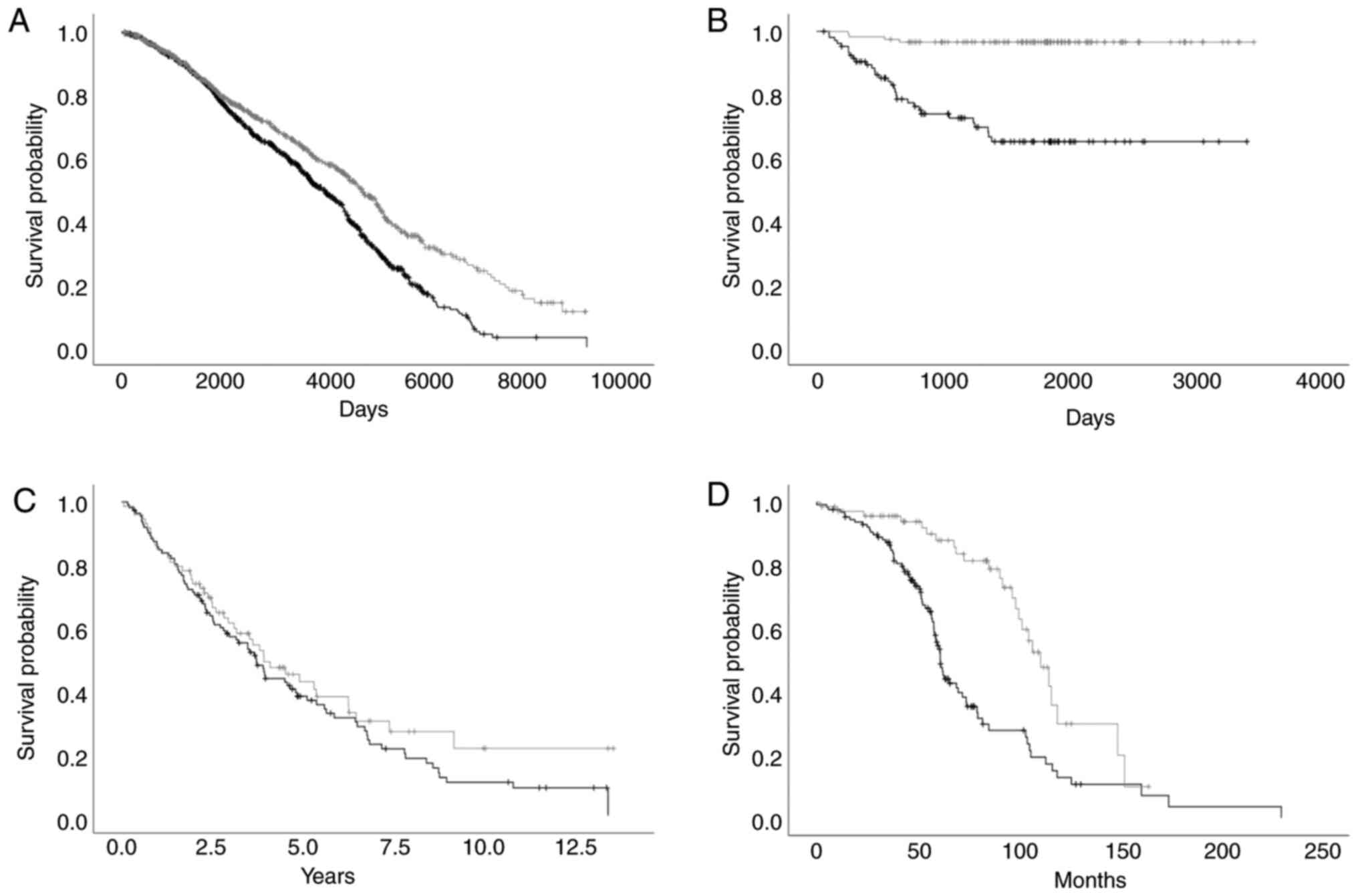
settings mentioned above. The workflow is displayed in Fig. 1. The mean cut-off value revealed a
significant (P<0.05) association between the TCTP mRNA
level and clinical outcome in 5 datasets out of the 12 examined
(41.7%). A significantly reduced overall survival time was
associated with high TCTP mRNA expression levels in the
datasets ‘Curtis Breast’ (-8.60%; P<0.001), ‘Okayama Lung’
(-27.08%; P<0.001), ‘Bonome Ovarian’ (-17.03%; P=0.042), ‘TCGA
Ovarian’ (-18.93%; P=0.019) and ‘Taylor Prostate’ (-30.10%;
P<0.001) (Table I and Fig. 2). The median cut-off value also
revealed a statistically significant association between the
TCTP mRNA level and clinical outcome in 5 out of 12 (41.7%)
datasets examined: ‘Curtis Breast’ (-17.84%; P<0.001), ‘Okayama
Lung’ (-25.13% P<0.001), ‘Rosenwald Lymphoma’ (-37.89%; P=0.016,
‘TCGA Ovarian’ (-22.16%; P=0.008) and ‘Taylor Prostate’ (-33.79%;
P<0.001) (Table I). A
significant association between high TCTP mRNA expression
levels and a poor overall survival was observed. Adverse effects
were not observed in the given datasets.
 | Table IAssociation between TCTP mRNA
expression and overall survival. |
Table I
Association between TCTP mRNA
expression and overall survival.
| | Overall survival
(mean) | Overall survival
(median) |
|---|
| | Mean survival time
(months) | | Mean survival time
(months) | |
|---|
| Cancer type | Dataset name | Low expression | High
expression | % Survival change
(low-high) | P-value | Low expression | High
expression | % Survival change
(low-high) | P-value |
|---|
| Brain | TCGA Brain | 19.17 | 21.22 | +10.69 | 0.275 | 19.17 | 21.22 | +10.69 | 0.275 |
| Breast | Curtis Breast | 143.55 | 131.20 | -8.60 |
<0.001a | 157.76 | 129.61 | -17.84 |
<0.001a |
| | TCGA Breast | 38.22 | 33.48 | -12.40 | 0.333 | 38.33 | 33.16 | -13.49 | 0.268 |
| Bladder | Lee Bladder | 83.42 | 81.87 | -1.86 | 0.847 | 80.63 | 84.40 | +4.68 | 0.769 |
| Colorectal | TCGA
Colorectal | 37.05 | 35.75 | -3.51 | 0.881 | 36.23 | 25.62 | -29.29 | 0.939 |
| Lung | Bhattacharjee
Lung | 53.77 | 49.15 | -8.59 | 0.905 | 52.85 | 52.56 | -0.01 | 0.898 |
| | Hou Lung | 58.89 | 61.57 | +4.55 | 0.890 | 57.70 | 62.53 | +8.37 | 0.751 |
| | Okayama Lung | 112.12 | 81.83 | -27.08 |
<0.001a | 111.94 | 83.81 | -25.13 |
<0.001a |
| Lymphoma | Rosenwald
Lymphoma | 63.93 | 101.56 | +58.86 | 0.063 | 106.89 | 66.39 | -37.89 | 0.016a |
| Ovarian | Bonome Ovarian | 68.99 | 57.24 | -17.03 | 0.042a | 67.22 | 57.46 | -14.52 | 0.051 |
| | TCGA Ovarian | 61.76 | 50.07 | -18.93 | 0.019a | 62.83 | 48.91 | -22.16 | 0.008a |
| Prostate | Taylor
Prostate | 110.03 | 76.90 | -30.10 |
<0.001a | 108.39 | 71.77 | -33.79 |
<0.001a |
The present study did not only wish to determine
whether TCTP mRNA expression was associated with the
survival times of the patients, but also whether TCTP protein
expression was of prognostic value.
Association of TCTP protein expression
and pathological parameters
Immunohistochemical investigations of 495 cases
derived from 26 tumor types were conducted for the evaluation of
the effects of TCTP protein expression on clinical parameters,
particularly regarding nuclear (nTCTP) or cytoplasmic expression
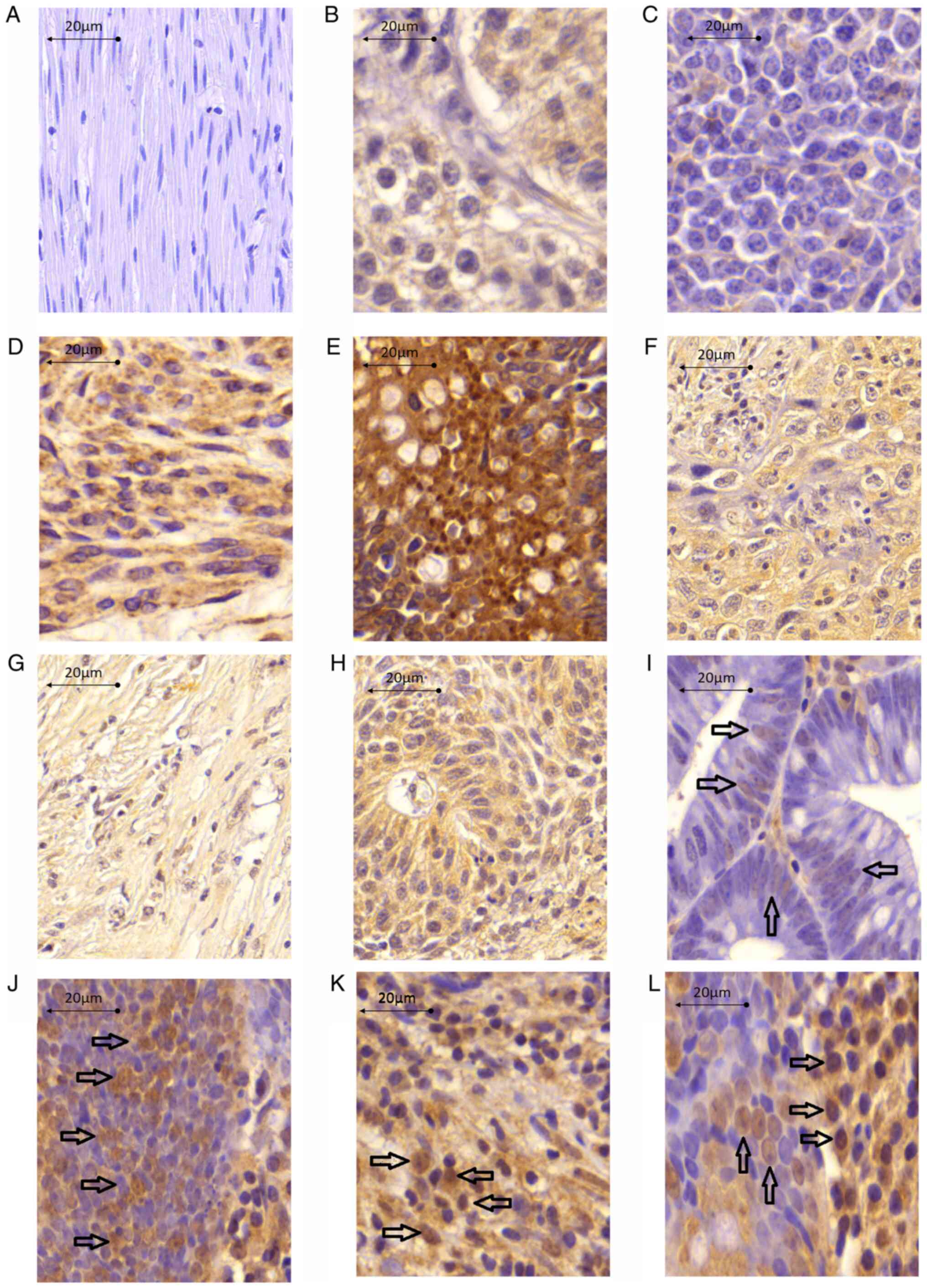
(cTCTP). The frequency of strong, moderate, weak and negative
staining was 12.35, 18.04, 4.34 and 28.05% for nTCTP, and 6.09,
14.68, 17.13 and 20.17% for cTCTP (data not shown). Typical
expression patterns are illustrated in Fig. 3. The distribution of nTCTP and
cTCTP expression was observed in different tumor types.
In all samples analyzed, the cTCTP levels were
elevated compared to corresponding normal tissue of the same
origin. The present study investigated adipose (liposarcoma),
adrenal (pheochromocytoma), bladder (transitional cell carcinoma),
brain (meningioma), colon (adenocarcinoma), duodenum
(adenocarcinoma), esophagus (squamous cell carcinoma), fallopian
tube (adenocarcinoma), gall bladder (adenocarcinoma), kidney
(granular cell carcinoma), liver (hepatocellular carcinoma), lung
(squamous cell carcinoma), ovary (adenocarcinoma), paratoid
(plasmacytoma), prostate (adenocarcinoma), rectum (adenocarcinoma),
skin (malignant melanoma), small intestine (Non-Hodgkin's
Lymphoma), soft tissue (rhabdomyosarcoma), stomach
(adenocarcinoma), testis (seminoma), throat (squamous cell
carcinoma), tonsil (squamous cell carcinoma) and uterus
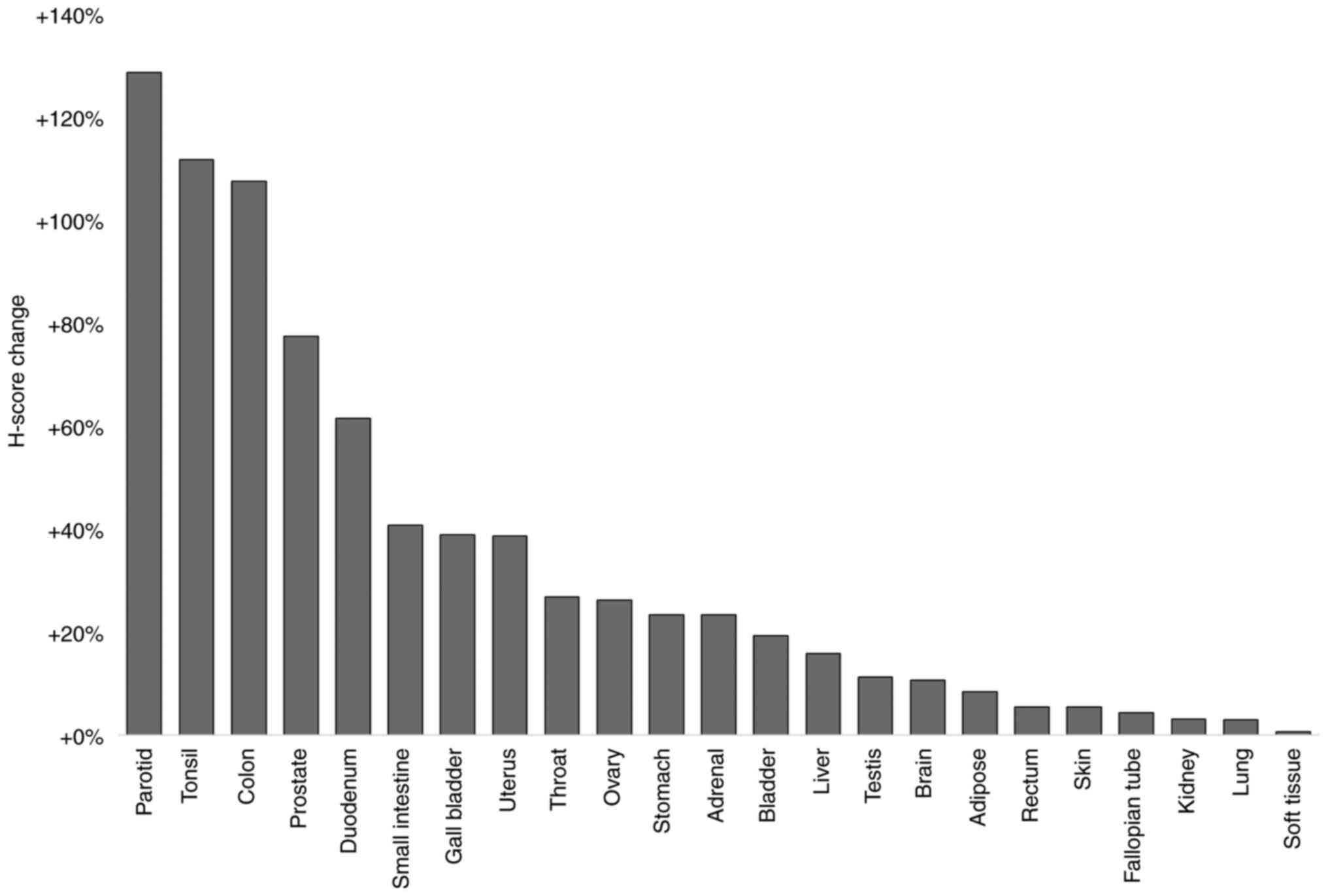
(adenocarcinoma) tumorous tissue (Fig.
4). A total of 18 out of 23 tumor types (79.2%) exhibited an
elevated cTCTP expression up to 50%. In duodenal cancer tissue,
cTCTP expression was elevated by 61.67%, and in prostate cancer
tissue, cTCTP was elevated by 77.65% compared to corresponding
normal tissue. In 3 tumor tissues, it was found that cTCTP
expression was more than doubled compared to normal tissue. These
were colon (107.84%), tonsil (112.03%) and paratoid (128.98%)
tumorous tissues (Fig. 4).
Furthermore, the present study investigated the
distribution of nTCTP and cTCTP among breast, colon, kidney, lung,
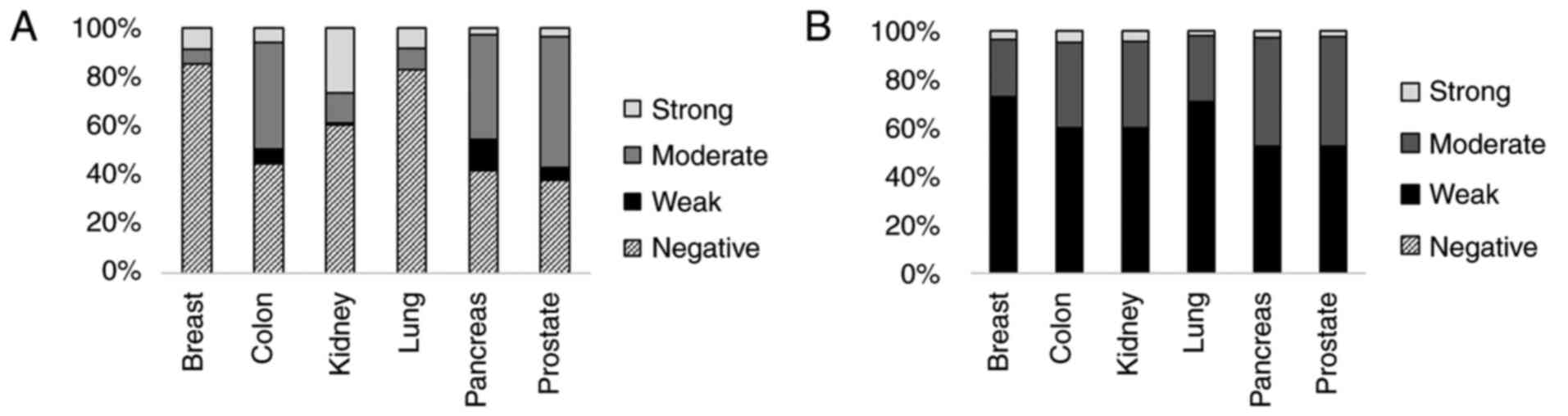
pancreas and prostate cancer. As shown in Fig. 5, TCTP expression was not detected
in >40% of the nuclei of all tissues analyzed. Breast cancer
revealed the highest rate of TCTP-negative nuclei (85.32%), and
prostate cancer exhibited the lowest rate of TCTP-negative nuclei
(37.83%). The percentage of nuclei with a strong TCTP expression
ranged from 2.86% (pancreas tumor) to 26.94% (kidney tumor).
Cytoplasmic TCTP was expressed more in all tumor types mentioned
above. Of all samples, >99.08% exhibited at least a weak cTCTP
expression. The ratio of weak cTCTP expression was >50% in all
samples, and weak and moderate cTCTP expression was present in
>95% of all the cancer types analyzed. The ratio of strong cTCTP
expression ranged from 1.95% (lung tumor) to 4.87% (colon
tumor).
Data on tumor stage and tumor grade were available
for breast, colon, kidney, lung, pancreas and prostate cancers. The
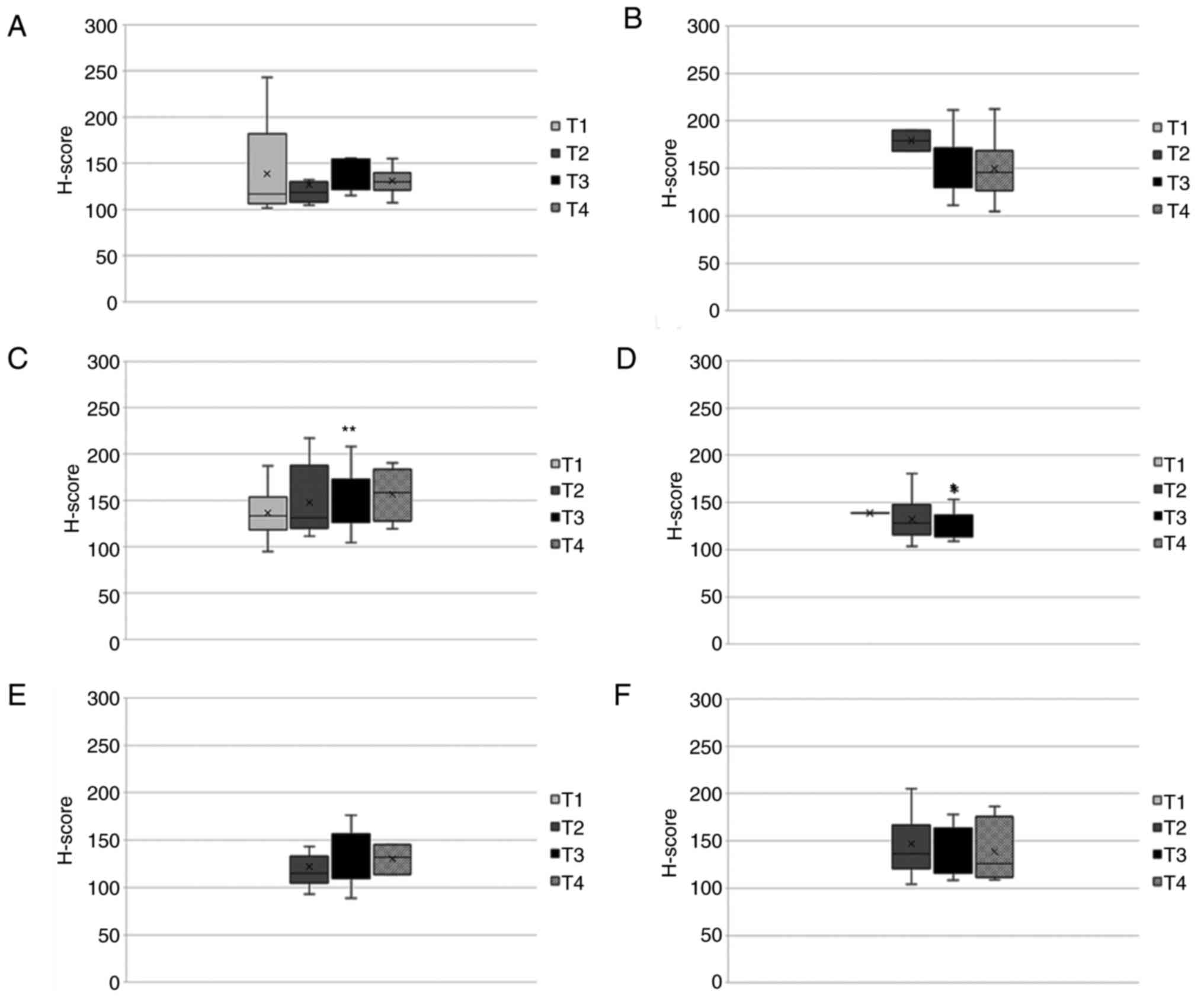
present study evaluated nTCTP expression in those tumor types and
their association with the T stage (Fig. 6). Herein, an ANOVA mean comparison
test for the nTCTP H-score, TNM stage and grade was first
performed. Subsequently, the independence of the H-score as a
negative or positive group with TNM stage and grade was then
determined by Tukey-Kramer's test.
Nuclear TCTP was similarly expressed in all tumors
throughout all stages. For kidney cancer, a slight non-significant
increase in nTCTP expression from T1-T4 was observed. Clear
conclusions could not be drawn from the results for the other tumor
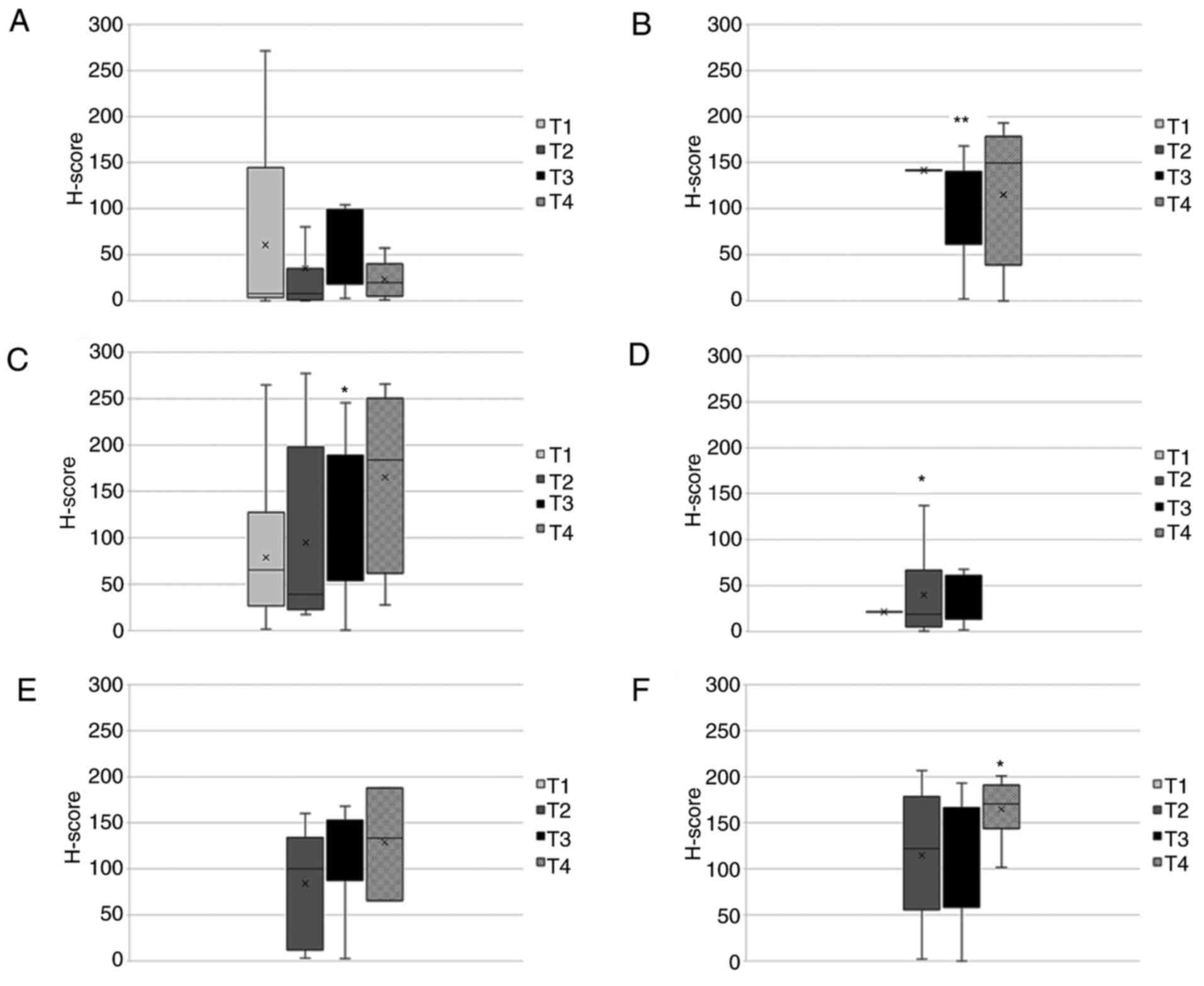
types. As shown in Fig. 7, cTCTP
was associated with T stage. The expression patterns were more
heterogeneous compared to those in shown in Fig. 6. For kidney cancer and pancreatic
cancer, a slight non-significant increase in cTCTP expression
associated with T1-T2 was observed. The results from the other
tumor types were not conclusive.
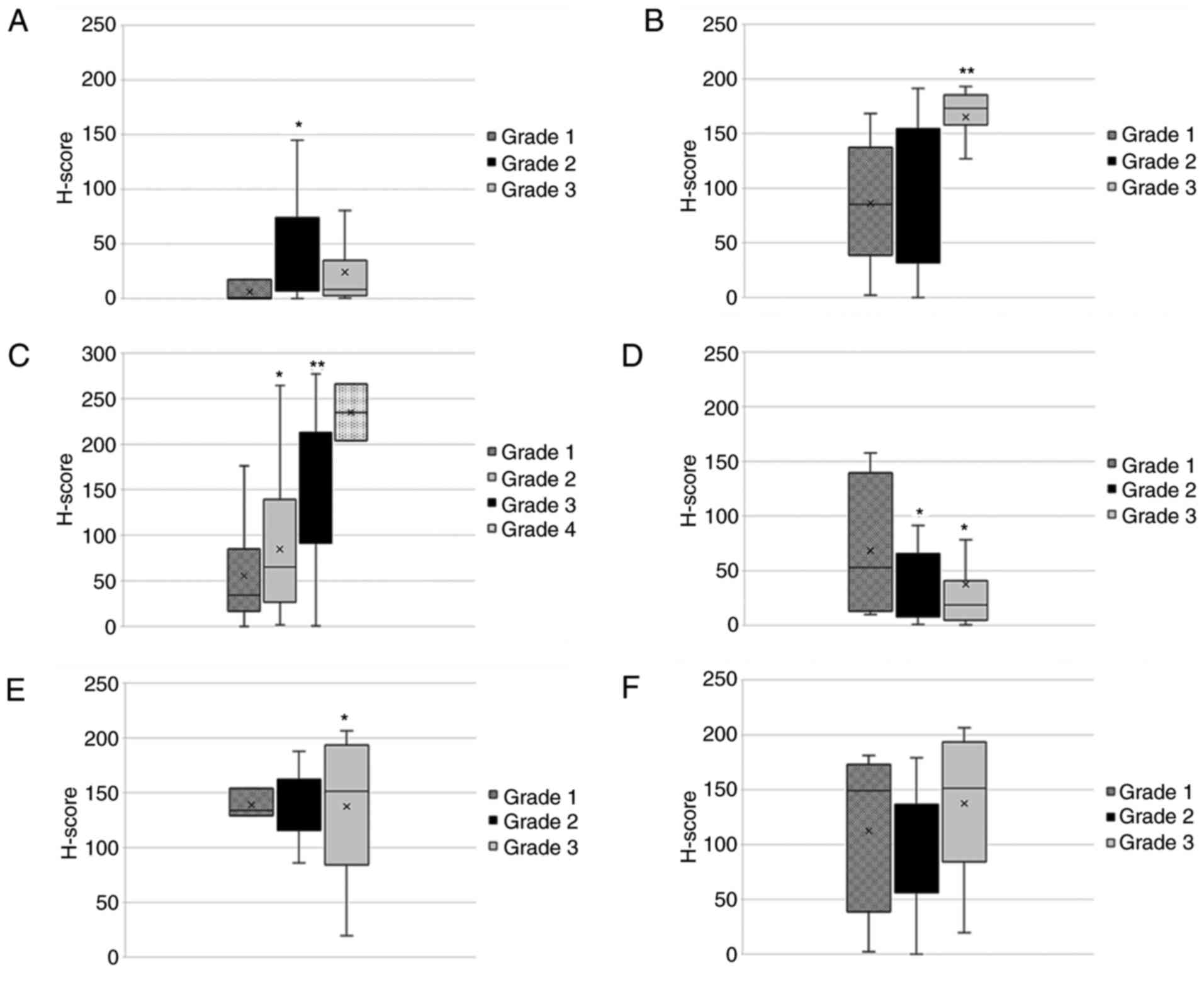
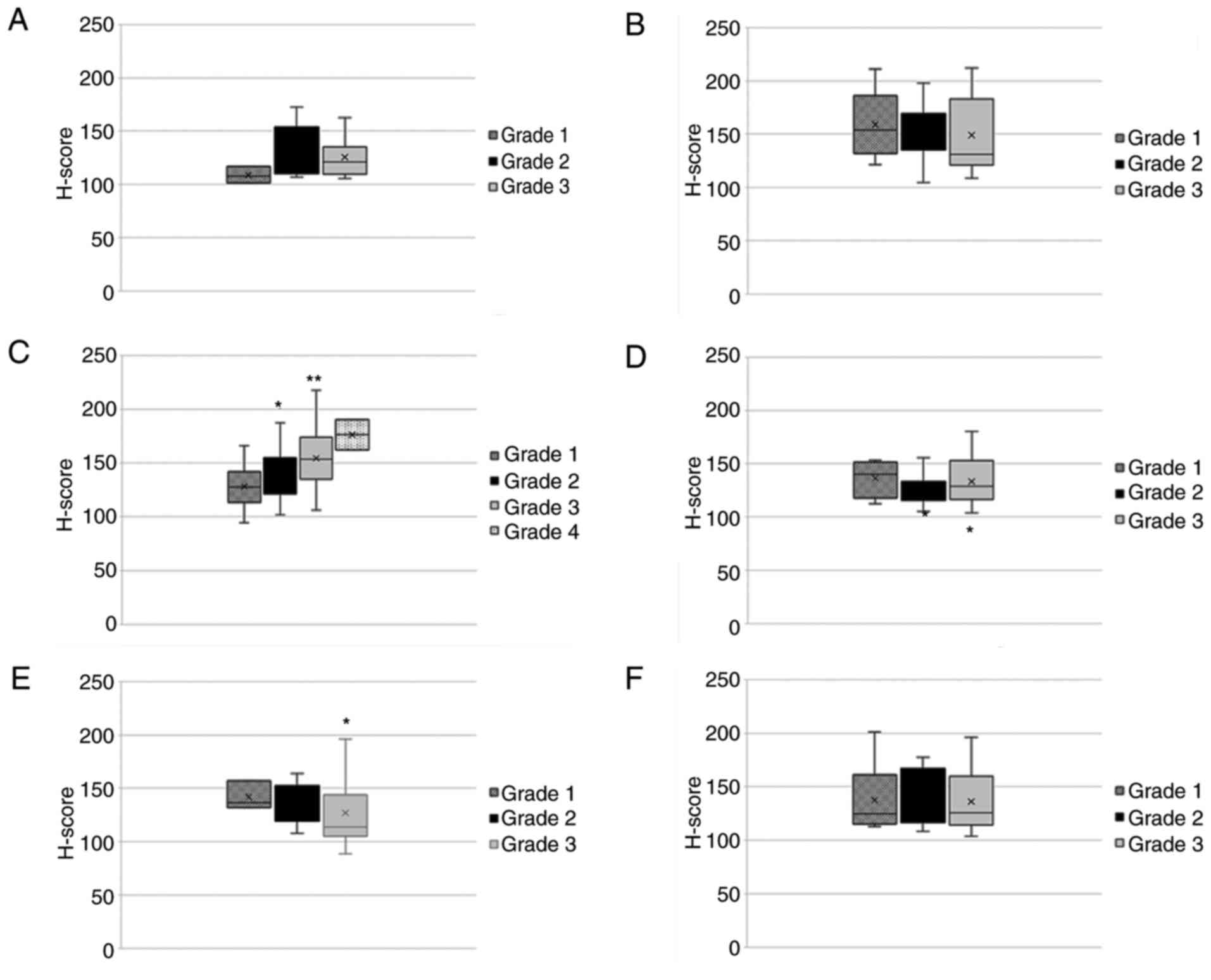
The association of nTCTP with tumor grade (Fig. 8) revealed more heterogeneous
expression patterns compared to those shown in Fig. 6. For kidney cancer, a significant
increase in nTCTP expression associated with the increasing tumor
grade (grade 1-4) was observed. For colon cancer, nTCTP expression
significantly increased for grade 3 tumors compared to grade 1 or 2
tumors. However, no difference between grade 1 and 2 tumors was
found. nTCTP expression in lung cancer significantly decreased with
the higher tumor grade. For breast, pancreas and prostate cancer,
no significant change in nTCTP expression was observed for the
association with tumor grade. The investigation of cTCTP and tumor
grade revealed a significant increase in cTCTP expression
associated with tumor grade 1-3 for kidney cancer (Fig. 9). No significant changes were
observed for the other tumor types analyzed.
Furthermore, the association between nTCTP and cTCTP
expression, and metastatic status in kidney cancer was
investigated. As shown in Fig.
10, the presence of metastases in distant organs led to
significantly higher expression levels of both, nTCTP and cTCTP.
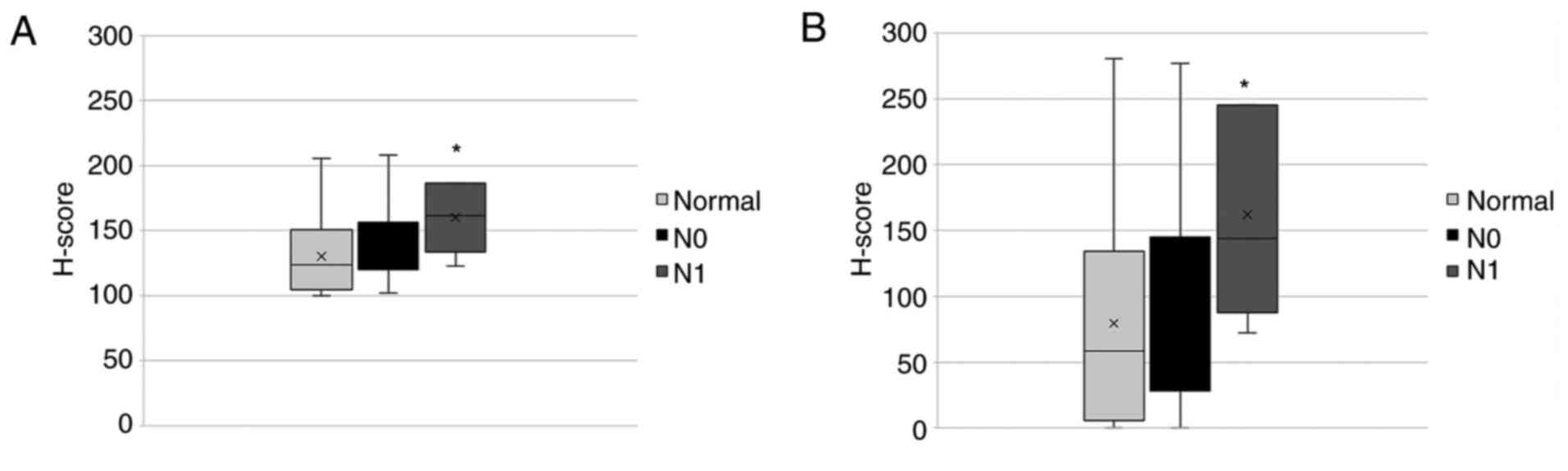
Similar observations were found regarding the nodal status
(Fig. 11). The expression of
nTCTP was significantly elevated in tumors with a nodal status
above N1. This was also found for cTCTP. However, nTCTP and cTCTP
were not differentially expressed in tumors of status N0 or N1.
Discussion
TCTP is an evolutionary highly conserved
multifunctional protein with diverse roles in key regulatory
processes such as cell growth, protein synthesis, cell cycle
progression, apoptosis, immune response and cancer. It can be found
in all Eukaryotic species (35)
and interacts with a wide range of different proteins (3). Previous results have revealed its
role in tumor reversion (26) and
the induction of pluripotent stem cells (36). TCTP downregulation leads to
reprogramming cells either into apoptosis or tumor reversion
(37). The protein is highly
expressed in human cancer (38).
Tumor reversion is clinically attractive as target for cancer
treatment (39). Advances in
oncology have led to higher life expectancies, resulting in
increased overall cancer incidences in both developed and
developing countries (40). High
numbers of different malignancies cause enormous costs and economic
damages throughout the world (41-43).
Cancer research is continuously aiming to identify novel targets
for new active compounds. However, classical chemotherapy remains a
mainstay in tumor therapy according to the cancer treatment
guidelines of leading health authorities. A frequent problem is the
non-sufficient selectivity between tumor and normal cells, which
causes severe side-effects, such as hair loss, vomiting, anemia and
bone marrow suppression (44). In
addition, drug resistance limits the therapeutic success and
quality of life of patients (45).
Therefore, there is an urgent necessity to identify new targets for
more effective tumor therapies. The present study was conducted to
evaluate both the role of TCTP as a novel target and prognostic
factor in cancer therapy. To date, there are only limited studies
available on TCTP as a prognostic factor, at least to the best of
our knowledge (31,33,46,47).
However, a comprising overview of the relevance of TCTP and
clinical prognosis, such as the one presented herein was
lacking.
For breast cancer, the present study observed a
significantly shorter overall survival time (-12.35 months,
P<0.001), if TCTP was highly expressed compared to a low
TCTP expression in one dataset. The smaller dataset ‘TCGA
Breast’ exhibited the same tendency (overall survival time
reduction of 4.74 months, P=0.333). However, this association was
not significant. TCTP has been previously reported as a
prognostic factor (25), which was
validated by the present study. In lung cancer, the high expression
of TCTP was accompanied by a decrease of 30.29 overall
survival months (P<0.001). For two smaller studies, the change
in survival time was not significant. The role of TCTP as a
prognostic factor has been discussed (48); however, no scientific study has
been reported as yet, at least to the best of our knowledge. In
ovarian cancer, a high TCTP expression was associated with
decreased overall survival times. Patients with a high TCTP
status lived 11.75 (P=0.042) resp. 11.69 (P=0.019) months shorter
compared to patients with a low TCTP expression status. An
immunohistological study on 119 ovarian cancer cases stressed the
role of TCTP as promotor of tumor progression (33). The present study complemented that
study by investigating 798 tumor cases. A high TCTP
expression was accompanied by a survival time that was 33.13 months
shorter (P<0.001) in prostate cancer. By now, no comparable
study has been conducted for prostate cancer. In lymphoma, a
reverse effect was observed. A high TCTP expression was
significantly associated (P=0.016) with a prolonged survival
(+37.63 months), if the median was selected as the expression
cut-off. As large B- cell lymphoma (49) was the only non-solid malignancy we
analyzed; a different clinical outcome was to be expected.
Furthermore, the present study examined TCTP protein
expression in tissues of 24 tumor types (adipose, adrenal, bladder,
brain, colon, duodenum, esophagus, fallopian tube, gall bladder,
kidney, liver, lung, ovarian, paratoid, prostate, rectum, skin,
small intestine, soft tissue, stomach, testis, throat, tonsil and
uterine cancer) and observed elevated cytoplasmic TCTP expression
in all of these (Fig. 4).
Differential analysis of nTCTP and cTCTP revealed mostly a
cytoplasmic TCTP localization in breast, colon, kidney, lung,
pancreas and prostate cancer (Fig.
5). The analysis of nTCTP and TNM stage did not reveal any
significant results. However, a tendency towards a high TCTP
expression accompanied by a high TNM stage was observed in breast,
kidney and pancreatic cancer (Fig.
6). As regards cTCTP, the same tendency was observed in kidney
and pancreatic cancer (Fig. 7).
The tumor grades of the cancer types mentioned above were examined
and a significant (P=0.033) increase in nTCTP expression was
observed with the increasing tumor grade in kidney cancer. This
result was even more significant for cTCTP (P=0.028). In colon
cancer, nTCTP expression was significantly higher (P=0.009) in
samples from patients with grade 3 compared to those with grade 1
and 2 disease. The other tissues did not exhibit any significant
associations.
The present study further analyzed the metastatic
status and nodal status of kidney cancer tissue samples. Tissues of
patients with distant metastases exhibited a significantly higher
expression of both nTCTP (P=0.043) and cTCTP (P=0.042) compared to
those without metastases. Previously, a high TCTP expression has
only been only linked by other authors to the metastases of
gallbladder carcinoma (50)
colorectal carcinoma (51),
melanoma (52), cholangiocarcinoma
(38) and glioma (53).
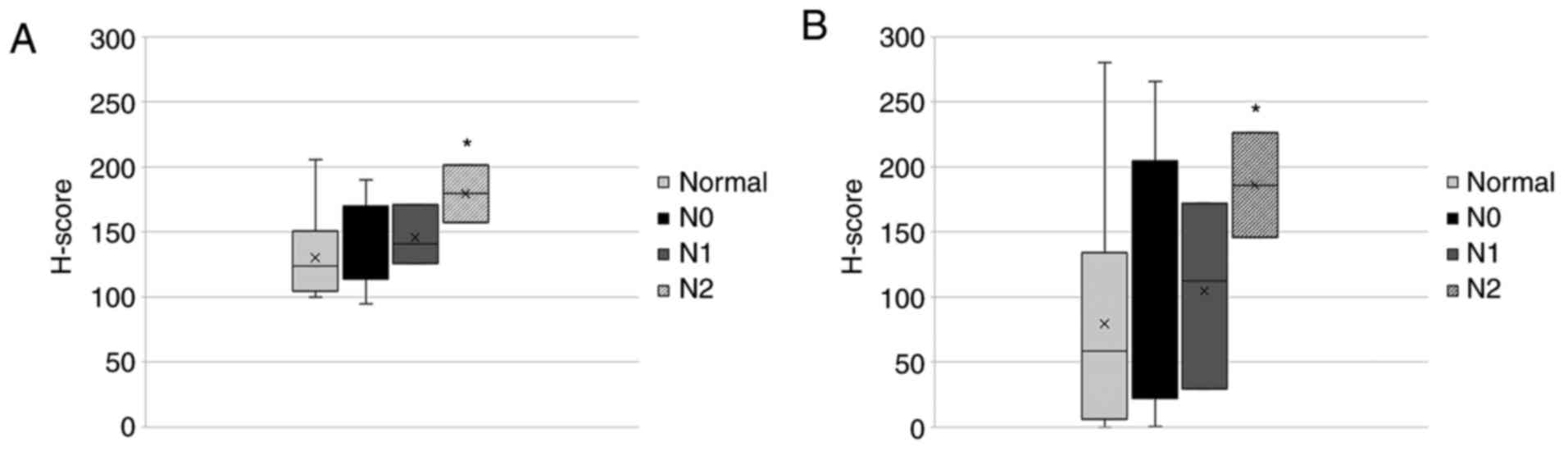
As regards the nodal status, nTCTP was significantly
more expressed (P=0.028) in patients with N2 status compared to N0
or N1 status. Differences in nTCTP expression were not observed for
tissues from patients with N0 or N1 status. The same result was
observed for cTCTP expression. cTCTP was significantly (P=0.036)
more expressed in kidney tumor tissue from patients with N2 status
compared to N0 or N1 status. Differences between samples from
patients with N0 or N1 status were not detected. To our knowledge,
an association between TCTP expression and the nodal status has not
been previously described.
In conclusion, the present study demonstrates that
TCTP expression was higher in malignant tissue compared to normal
tissue in 24 cancer types. Both mRNA and protein expression
reflected the prognostic value of TCTP for cancer patients. In
breast, lung ovarian and prostate cancer, elevated TCTP mRNA levels
were associated with shorter survival times. A high TCTP
expression, particularly in the nucleus, corresponded with a poor
prognosis, represented not only by tumor stage and grade, but also
by the presence of metastases and cancer in nearby lymph nodes in
kidney cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NF performed the experiments, and conducted the
statistical analyses, and wrote the draft of the manuscript NF and
TE set up the concept and designed the study. TE supervised the
project and corrected the manuscript. MEMS was involved in the
conception and conduction of the immunohistological experiments. WR
and EL delivered tissue arrays and corrected the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Tissue arrays were provided by the Tissue Bank of
the Institute of Pathology, University Medical Center of the
Johannes-Gutenberg-Universität, Mainz) with ethical approval from
the Ethics Committee of the State Authorization Association for
Medical Issues (Landesärztekammer) Rheinland Pfalz to WR
(October 2, 2015; Ref. 837.031 9799) and to TE (March 22, 2018;
Ref. 2018-13179). The patients gave written informed consent for
evaluation of tumor material and publication of data generated from
these investigations prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Acunzo J, Baylot V, So A and Rocchi P:
TCTP as therapeutic target in cancers. Cancer Treat Rev.
40:760–769. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bommer UA and Thiele BJ: The
translationally controlled tumour protein (TCTP). Int J Biochem
Cell Biol. 36:379–385. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Li S and Ge F: Current understanding of
the TCTP interactome. Results Probl Cell Differ. 64:127–136.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Assrir N, Malard F and Lescop E:
Structural Insights into TCTP and its interactions with ligands and
proteins. Results Probl Cell Differ. 64:9–46. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Seo EJ, Fischer N and Efferth T: Role of
TCTP for cellular differentiation and cancer therapy. Results Probl
Cell Differ. 64:263–281. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kubiak JZ and Kloc M: Elusive role of TCTP
protein and mRNA in cell cycle and cytoskeleton regulation. Results
Probl Cell Differ. 64:217–225. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Betsch L, Boltz V, Brioudes F, Pontier G,
Girard V, Savarin J, Wipperman B, Chambrier P, Tissot N, Benhamed
M, et al: TCTP and CSN4 control cell cycle progression and
development by regulating CULLIN1 neddylation in plants and
animals. PLoS Genet. 15(e1007899)2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jojic B, Amodeo S and Ochsenreiter T: The
translationally controlled tumor protein TCTP is involved in cell
cycle progression and heat stress response in the bloodstream form
of Trypanosoma brucei. Microb Cell. 5:460–468. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koziol MJ and Gurdon JB: TCTP in
development and cancer. Biochem Res Int.
2012(105203)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Chen SH, Wu PS, Chou CH, Yan YT, Liu H,
Weng SY and Yang-Yen HF: A knockout mouse approach reveals that
TCTP functions as an essential factor for cell proliferation and
survival in a tissue- or cell type-specific manner. Mol Biol Cell.
18:2525–2532. 2007.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kubiak JZ, Bazile F, Pascal A,
Richard-Parpaillon L, Polanski Z, Ciemerych MA and Chesnel F:
Temporal regulation of embryonic M-phases. Folia Histochem
Cytobiol. 46:5–9. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hsu YC, Chern JJ, Cai Y, Liu M and Choi
KW: Drosophila TCTP is essential for growth and proliferation
through regulation of dRheb GTPase. Nature. 445:785–788.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cans C, Passer BJ, Shalak V,
Nancy-Portebois V, Crible V, Amzallag N, Allanic D, Tufino R,
Argentini M, Moras D, et al: Translationally controlled tumor
protein acts as a guanine nucleotide dissociation inhibitor on the
translation elongation factor eEF1A. Proc Natl Acad Sci USA.
100:13892–13897. 2003.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bazile F, Pascal A, Arnal I, Le Clainche
C, Chesnel F and Kubiak JZ: Complex relationship between TCTP,
microtubules and actin microfilaments regulates cell shape in
normal and cancer cells. Carcinogenesis. 30:555–565.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tsarova K, Yarmola EG and Bubb MR:
Identification of a cofilin-like actin-binding site on
translationally controlled tumor protein (TCTP). FEBS Lett.
584:4756–4760. 2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mishra DK, Srivastava P, Sharma A, Prasad
R, Bhuyan SK, Malage R, Kumar P and Yadava PK: Translationally
controlled tumor protein (TCTP) is required for TGF-β1 induced
epithelial to mesenchymal transition and influences cytoskeletal
reorganization. Biochim Biophys Acta Mol Cell Res. 1865:67–75.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wu W, Wu B, Ye T, Huang H, Dai C, Yuan J
and Wang W: TCTP is a critical factor in shrimp immune response to
virus infection. PLoS One. 8(e74460)2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Taylor KJ, Van TT, MacDonald SM, Meshnick
SR, Fernley RT, Macreadie IG and Smooker PM: Immunization of mice
with plasmodium TCTP delays establishment of plasmodium infection.
Parasite Immunol. 37:23–31. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
MacDonald SM, Rafnar T, Langdon J and
Lichtenstein LM: Molecular identification of an IgE-dependent
histamine-releasing factor. Science. 269:688–690. 1995.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li F, Zhang D and Fujise K:
Characterization of fortilin, a novel antiapoptotic protein. J Biol
Chem. 276:47542–47549. 2001.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yang Y, Yang F, Xiong Z, Yan Y, Wang X,
Nishino M, Mirkovic D, Nguyen J, Wang H and Yang XF: An N-terminal
region of translationally controlled tumor protein is required for
its antiapoptotic activity. Oncogene. 24:4778–4788. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bommer UA, Vine KL, Puri P, Engel M,
Belfiore L, Fildes K, Batterham M, Lochhead A and Aghmesheh M:
Translationally controlled tumour protein TCTP is induced early in
human colorectal tumours and contributes to the resistance of
HCT116 colon cancer cells to 5-FU and oxaliplatin. Cell Commun
Signal. 15(9)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Li S, Chen M, Xiong Q, Zhang J, Cui Z and
Ge F: Characterization of the translationally controlled tumor
protein (TCTP) interactome reveals novel binding partners in human
cancer cells. J Proteome Res. 15:3741–3751. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Tuynder M, Susini L, Prieur S, Besse S,
Fiucci G, Amson R and Telerman A: Biological models and genes of
tumor reversion: Cellular reprogramming through tpt1/TCTP and
SIAH-1. Proc Natl Acad Sci USA. 99:14976–14981. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Amson R, Karp JE and Telerman A: Lessons
from tumor reversion for cancer treatment. Curr Opin Oncol.
25:59–65. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Tuynder M, Fiucci G, Prieur S, Lespagnol
A, Géant A, Beaucourt S, Duflaut D, Besse S, Susini L, Cavarelli J,
et al: Translationally controlled tumor protein is a target of
tumor reversion. Proc Natl Acad Sci USA. 101:15364–15369.
2004.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Telerman A and Amson R: The molecular
programme of tumour reversion: The steps beyond malignant
transformation. Nat Rev Cancer. 9:206–216. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Miao X, Chen YB, Xu SL, Zhao T, Liu JY, Li
YR, Wang J, Zhang J and Guo GZ: TCTP overexpression is associated
with the development and progression of glioma. Tumour Biol.
34:3357–3361. 2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Amson R, Pece S, Marine JC, Di Fiore PP
and Telerman A: TPT1/ TCTP-regulated pathways in phenotypic
reprogramming. Trends Cell Biol. 23:37–46. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chen C, Deng Y, Hua M, Xi Q, Liu R, Yang
S, Liu J, Zhong J, Tang M, Lu S, et al: Expression and clinical
role of TCTP in epithelial ovarian cancer. J Mol Histol.
46:145–156. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Michaelsen FW, Saeed ME, Schwarzkopf J and
Efferth T: Activity of Artemisia annua and artemisinin derivatives,
in prostate carcinoma. Phytomedicine. 22:1223–1231. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Koo N, Shin AY, Oh S, Kim H, Hong S, Park
SJ, Sim YM, Byeon I, Kim KY, Lim YP, et al: Comprehensive analysis
of translationally controlled tumor protein (TCTP) provides
insights for lineage-specific evolution and functional divergence.
PLoS One. 15(e0232029)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Brioudes F, Thierry AM, Chambrier P,
Mollereau B and Bendahmane M: Translationally controlled tumor
protein is a conserved mitotic growth integrator in animals and
plants. Proc Natl Acad Sci USA. 107:16384–16389. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Proietti S, Cucina A, Pensotti A, Biava
PM, Minini M, Monti N, Catizone A, Ricci G, Leonetti E, Harrath AH,
et al: Active fraction from embryo fish extracts induces reversion
of the malignant invasive phenotype in breast cancer through
down-regulation of TCTP and modulation of E-cadherin/β-catenin
pathway. Int J Mol Sci. 20(2151)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Phanthaphol N, Techasen A, Loilome W,
Thongchot S, Thanan R, Sungkhamanon S, Khuntikeo N, Yongvanit P and
Namwat N: Upregulation of TCTP is associated with
cholangiocarcinoma progression and metastasis. Oncol Lett.
14:5973–5979. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Powers S and Pollack RE: Inducing stable
reversion to achieve cancer control. Nat Rev Cancer. 16:266–270.
2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gu X, Zheng R, Xia C, Zeng H, Zhang S, Zou
X, Yang Z, Li H and Chen W: Interactions between life expectancy
and the incidence and mortality rates of cancer in China: A
population-based cluster analysis. Cancer Commun (Lond).
38(44)2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Luengo-Fernandez R, Leal J, Gray A and
Sullivan R: Economic burden of cancer across the European Union: A
population-based cost analysis. Lancet Oncol. 14:1165–1174.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Mariotto AB, Yabroff KR, Shao Y, Feuer EJ
and Brown ML: Projections of the cost of cancer care in the United
States: 2010-2020. J Natl Cancer Inst. 103:117–128. 2011.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Sankaranarayanan R, Ramadas K and Qiao Yl:
Managing the changing burden of cancer in Asia. BMC Med.
12(3)2014.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Weber GF: DNA Damaging Drugs. In:
Molecular Therapies of Cancer. Weber GF (ed). Springer, Cham,
International Publishing, pp9-112, 2015.
|
|
45
|
Hwang SY, Chang SJ and Park BW: Does
chemotherapy really affect the quality of life of women with breast
cancer? J Breast Cancer. 16:229–235. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Lucibello M, Adanti S, Antelmi E, Dezi D,
Ciafrè S, Carcangiu ML, Zonfrillo M, Nicotera G, Sica L, De Braud F
and Pierimarchi P: Phospho-TCTP as a therapeutic target of
Dihydroartemisinin for aggressive breast cancer cells. Oncotarget.
6:5275–5291. 2015.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Ramani P, Nash R, Sowa-Avugrah E and
Rogers C: High levels of polo-like kinase 1 and phosphorylated
translationally controlled tumor protein indicate poor prognosis in
neuroblastomas. J Neurooncol. 125:103–111. 2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Sun R, Lu X, Gong L and Jin F: TCTP
promotes epithelial-mesenchymal transition in lung adenocarcinoma.
Onco Targets Ther. 12:1641–1653. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Rosenwald A, Wright G, Chan WC, Connors
JM, Campo E, Fisher RI, Gascoyne RD, Muller-Hermelink HK, Smeland
EB, Giltnane JM, et al: The use of molecular profiling to predict
survival after chemotherapy for diffuse large-B-cell lymphoma. N
Engl J Med. 346:1937–1947. 2002.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Zhang F, Ma Q, Xu Z, Liang H, Li H, Ye Y,
Xiang S, Zhang Y, Jiang L, Hu Y, et al: Dihydroartemisinin inhibits
TCTP-dependent metastasis in gallbladder cancer. J Exp Clin Cancer
Res. 36(68)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Xiao B, Chen D, Luo S, Hao W, Jing F, Liu
T, Wang S, Geng Y, Li L, Xu W, et al: Extracellular translationally
controlled tumor protein promotes colorectal cancer invasion and
metastasis through Cdc42/JNK/MMP9 signaling. Oncotarget.
7:50057–50073. 2016.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bae SY, Kim HJ, Lee KJ and Lee K:
Translationally controlled tumor protein induces epithelial to
mesenchymal transition and promotes cell migration, invasion and
metastasis. Sci Rep. 5(8061)2015.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Jin H, Zhang X, Su J, Teng Y, Ren H and
Yang L: RNA interference-mediated knockdown of translationally
controlled tumor protein induces apoptosis, and inhibits growth and
invasion in glioma cells. Mol Med Rep. 12:6617–6625.
2015.PubMed/NCBI View Article : Google Scholar
|