Introduction
Cellular and genomic heterogeneity in tumors
involves the microenvironment, cancer stem cells and
epithelial-to-mesenchymal transition (EMT) (1,2). EMT
constitutes a complex and dynamic biological process during which
epithelial cells transdifferentiate towards a mesenchymal
phenotype. Unlike epithelial cells, which are characterized by
polarity and maintain firmly cell-to-cell adhesion contacts through
cellular adhesion molecules (CAMs), mesenchymal cells display an
increased mobility and loose organization within the extracellular
matrix. EMT plays an important role in physiological processes,
including organ formation during embryogenesis and tissue
regeneration; however, its involvement has also been confirmed in
tumor initiation, progression and metastasis. The transition can be
triggered through various stimuli, including different factors of
the tumor microenvironment (cytokines, growth factors, etc.), as
well as immune responses, hypoxia and antitumor drug treatment.
Notably, EMT is reversible, exhibiting plasticity, with mesenchymal
cells being capable of converting back to an epithelial phenotype
through a process known as mesenchymal-to-epithelial transition
(MET). The combination of EMT and MET can lead to a mixed, dynamic
population of cancer cells exhibiting both epithelial and
mesenchymal characteristics that also promote circulating tumor
cell (CTC) formation. This can result in the disruption of cellular
adhesion and increased migratory and invasive capabilities, which
can lead to metastasis (3,4).
The EMT phenotypic plasticity of tumor cells
contributes to molecular and cellular heterogeneity, that leads to
acquired drug resistance to cytotoxic or molecularly targeted
therapy in clinical practice. Moreover, the differential
pharmacological response limits the productivity and clinical
outcomes of innovative therapeutic approaches (5-7).
Moreover, the interplay of transcription (including the Snail,
Twist and Zeb families) and epigenetic factors (e.g., the miR-200
family, miR-205, miR-203, miR-34 and miR-29b) drive the regulatory
network program of EMT plasticity in cancer (6). It would be of interest if common
molecular drivers in these EMT and MET processes that are
deregulated in various types of tumors could be characterized;
following clinical validation, biomarkers could be developed which
may be used in cancer therapy.
Previously the authors characterized the expression
levels of several epithelial markers, namely desmoglein 3 (DSG3),
E-cadherin and β-/γ-catenins (β-/γ-catenins) in monolayer (ML) and
multicellular aggregates (MCAs) of the HSC-3 cell line (oral
squamous carcinoma) in vitro, as well as in clinical samples
of oral leukoplakia (OL) and oral squamous cell carcinoma (OSCC)
in vivo (8). Of note, the
downregulation of DSG3, E-cadherin and β-/γ-catenins was observed
to be significantly associated with the grade of OL-dysplasia and
OSCC samples (8). Furthermore, the
switch of expression and potent perinuclear aggregation of DSG3 and
γ-catenin were observed in both HSC-3 cells and OL/OSCC samples.
These observations support the involvement of DSG3 and γ-catenin in
the progression of oral epithelial cell malignancy. It was also
suggested that these genes may serve as potential predictive
biomarkers, along with E-cadherin and β-catenin, of the malignant
transformation risk of oral dysplasia and the biological behavior
(aggressiveness) of oral cancer, respectively (8).
In the present study, an in silico analysis
was performed using RNA-Seq data from The Cancer Genome Atlas
(TCGA) database (9), in order to
identify genes that are involved in the EMT and MET processes, and
that may serve as possible biomarkers and/or therapeutic targets in
clinical practice. For this purpose, differential gene and microRNA
(miRNA/miR) expression analyses were carried out between solid
tissue normal (STN) and primary solid tumor (PST) samples from 4
different types of cancer (head and neck, prostate and breast
cancer, and glioblastoma). The accurate separation of STN and PST
samples into distinct clusters was confirmed using principal
component analysis (PCA) based solely on the identified
differentially expressed (DE) genes/miRNAs (Fig. 1). Additionally, dbEMT 1.0, a
database containing EMT-related genes collected through extensive
literature search (10), was used
to further filter DE genes and miRNAs, and keep those that have
been found to be involved in EMT and/or MET. On the whole, the
present study identified and reported several DE and
EMT/MET-related genes and miRNAs in various types of malignancies,
whose potential in clinical utilization will be further evaluated
by characterizing their expression levels in cell line EMT models
and in clinical samples in the future.
Materials and methods
Cancer data
RNA-Seq and miRNA-Seq data from TCGA (9) were retrieved using TCGAbiolinks
(11). Specifically, from the
available pre-processed data types, gene and miRNA count data,
derived from HTSeq software (12),
were selected for 4 different types of cancer: i) Head-and-neck
squamous cell carcinoma (TCGA-HNSC); ii) breast cancer (TCGA-BRCA);
iii) prostate adenocarcinoma (TCGA-PRAD); and iv) glioblastoma
multiforme (TCGA-GBM).
Differential expression analysis
To ensure the power of statistical testing, only STN
and PST samples were selected to perform differential expression
analysis for genes and miRNAs using DESeq2(13).
Due to the lack of sufficient miRNA-Seq samples in
TCGA-GBM, miRNA differential expression analysis was performed only
for TCGA-BRCA, TCGA-HNSC and TCGA-PRAD.
A minimum threshold of 100 and 10 total number of
counts was set to filter out genes and miRNAs with very low counts,
respectively. Genes and miRNAs with an absolute log2 fold change
(LFC) >1 and a false discovery rate (FDR) (14) adjusted P-value <0.001 were
reported as statistically significant, DE targets.
Variance-stabilizing transformation (VST) was applied to DE gene or
miRNA expression values of all samples, followed by PCA. Both VST
and PCA were performed using DESeq2(13).
EMT-associated genes and miRNAs
Genes and miRNAs that have been associated with EMT
were retrieved from dbEMT 1.0, a database containing EMT-related
genes and miRNAs that were collected through extensive literature
search (10). Comparisons of EMT
targets with DE genes and miRNAs was performed with R statistical
programming language. Venn diagrams were created using limma
(15).
Survival analysis of DE genes and
miRNAs
Survival analysis was performed on DE genes and
miRNAs using clinical metadata from TCGA. Specifically, for each DE
gene or miRNA, PST samples were assigned into 2 separate groups,
depending on whether the target expression of each sample was
higher (high expression) or lower (low expression) than the median.
Kaplan-Meier analysis on the 2 groups was performed for each DE
gene or miRNA and selected, statistically significant targets are
reported in Kaplan-Meier survival curves. For the statistical
analysis of DE genes (P-value <0.001) and miRNAs (P-value
<0.1) the non-parametric log-rank test was used. An exception to
this was miR-16-1 in HNSC cancer samples, with a P-value of ~0.12.
Survival analysis was performed using a survival analysis package
(16,17) and survminer (18).
Cell cultures, RNA isolation and
RT-qPCR analysis
The established cell lines of human breast
epithelial carcinoma MCF-7 (Cellosaurus, CVCL-0031) and MDA-MB-231
(Cellosaurus, CVCL-0062), as well as the human tongue squamous
carcinoma HSC-3 (Cellosaurus, CVCL-1288) and keratinocyte HaCaT
(Cellosaurus, CVCL-0038) cancer cells that are routinely used in
the authors' laboratory were cultured as previously described
(19,20). Moreover, the RNA isolation, RT-cDNA
synthesis, as well as the RT-qPCR analysis were carried out as
previously described (19,20). In brief, total RNA was extracted
from cells using TRItidY G (Panreac, Applichem), quantified using a
Nanodrop ND-100 Spectrometer and reverse transcribed into cDNA by
applying the QuantiTect Reverse Transcription kit (Qiagen, Inc.).
qPCR was performed on a 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.) using KAPA
SYBR® FAST qPCR Master Mix (KAPA Biosystems) under
optimized conditions: 95˚C for 3 min followed by 40 cycles at 95˚C
for 3 sec and 60˚C for 20 sec. Primers designed and used during the
present study were as follows: (PPARG forward,
5'-TCG-AGG-ACA-CCG-GAG-AGG-3' and reverse,
5'-CAC-GGA-GCT-GAT-CCC-AAA-GT-3'; HMGA2 forward,
5'-GAA-AAA-CGG-CCA-AGA-GGC-AG-3' and reverse,
5'-AGA-GCT-ATC-CTG-GAC-TCC-TCC-3'; FOXM1 forward,
5'-ACC-GCT-ACT-TGA-CAT-TGG-AC-3' and reverse,
5'-GGG-AGT-TCG-GTT-TTG-ATG-GTC-3'; CAV-1 forward,
5'-CCC-AGG-GAA-ACC-TCC-TCA-CAG-3' and reverse,
5'-GGC-AGA-TAG-CAG-AAG-CGG-AC-3'; TGFB1-F forward,
5'-ACT-GCG-GAT-CTC-TGT-GTC-ATT-G-3' and reverse,
5'-ACA-GTA-GTG-TTC-CCC-ACT-GGT-C-3'; Vimentin forward,
5'-GGC-TCG-TCA-CCT-TCG-TGA-AT-3' and reverse,
5'-GAG-AAA-TCC-TGC-TCT-CCT-CGC-3'; β-actin forward,
5'-TTG-CTG-ACA-GGA-TGC-AGA-AG-3' and reverse,
5'-TGA-TCC-ACA-TCT-GCT-GGA-AG-3'). β-actin was used as an
endogenous control to normalize the gene expression levels.
The expression of miRNAs was also carried out by
RT-qPCR using the miScript SYBR®-Green PCR kit (Qiagen,
Inc.). Total cellular RNA extraction and quantification was
performed as indicated above, whereas cDNA synthesis was executed
with the miScript II RT kit (Qiagen, Inc.). Hsa-miR-21-5p (miR-21,
5'-UAGCUUAUCAGACUGAUGUUGA-3') was designed and used during this
experiment and SNORD6 small nucleolar RNA, C/D box 6 (also known as
mgh28S-2412) (Qiagen, Inc.) was used as reference RNA gene. The
reaction conditions consisted of polymerase activation/denaturation
at 95˚C for 15 min, followed by 40 cycles at 94˚C for 15 sec and
55˚C for 30 sec.
In both cases, the relative mRNA/miRNA
concentrations were calculated using the 2-ΔΔCq method
(21) and the results obtained
were represented as fold changes in the diagrams.
Statistical analysis
The results from 2 independent biological
experiments (triplicate measurements) are shown and the data are
expressed as the means ± standard deviation (SE). Comparisons were
carried out using a Student's t test, whereas the statistical
analysis was performed using GraphPad Prism 6.0 (GraphPad Software,
Inc.). P<0.05 was considered to indicate a statistically
significant difference.
Results
DE genes and miRNAs
Accessing the GDC data portal through TCGAbiolinks
enabled the retrieval of RNA-Seq for 4 different types of
malignancies. For each project, STN and PST were identified as the
predominant categories containing the majority of samples.
Specifically, for TCGA-BRCA, TCGA-HNSC, TCGA-PRAD and TCGA-GBM, STN
+ PST samples numbering 113 + 1,102, 44 + 523, 52 + 498 and 5 +
156, respectively, were obtained, filtered for low count genes and
compared to identify DE genes. Based on strict criteria (please see
the ‘Materials and methods' section) a subset of all genes analyzed
was determined to be DE genes in each tumor (percentage of DE genes
in each TCGA-project: TCGA-BRCA, 8.95%; TCGA-HNSC, 6.1%; TCGA-PRAD,
2.56%; TCGA-GBM, 6.52 %) (Table
I). Similarly, miRNA-Seq data were retrieved for 3 TCGA
projects and major sample categories STN and PST were used for DE
miRNA identification (TCGA-BRCA, TCGA-HNSC and TCGA-PRAD with STN +
PST samples numbering 104 + 1,096, 44 + 523, 52 + 498,
respectively). Following analysis, the percentage of DE miRNAs were
determined to be as follows: TCGA-BRCA, 19.1%; TCGA-HNSC, 18.33%;
TCGA-PRAD, 9.04% (Table I).
 | Table INumber of genes and miRNAs identified
as differentially expressed and EMT-associated in different
malignancies. |
Table I
Number of genes and miRNAs identified
as differentially expressed and EMT-associated in different
malignancies.
| TCGA project
ID | Type of
malignancy | Number of DE genes
(LFC >1 and FDR adjusted P-value <0.001) | Number of DE Genes
reported by dbEMT 1.0 | Number of DE miRNAs
(LFC >1 and FDR adjusted P-value <0.001) | Number of DE miRNAs
reported by dbEMT 1.0 |
|---|
| TCGA-HNSC | Head and neck
squamous carcinoma | 2,509 | 37 | 255 | 6 |
| TCGA-BRCA | Breast cancer | 4,227 | 73 | 271 | 10 |
| TCGA-PRAD | Prostate
cancer | 1,049 | 11 | 110 | 4 |
| TCGA-GBM | Glioblastoma
multiforme | 2,350 | 44 | | |
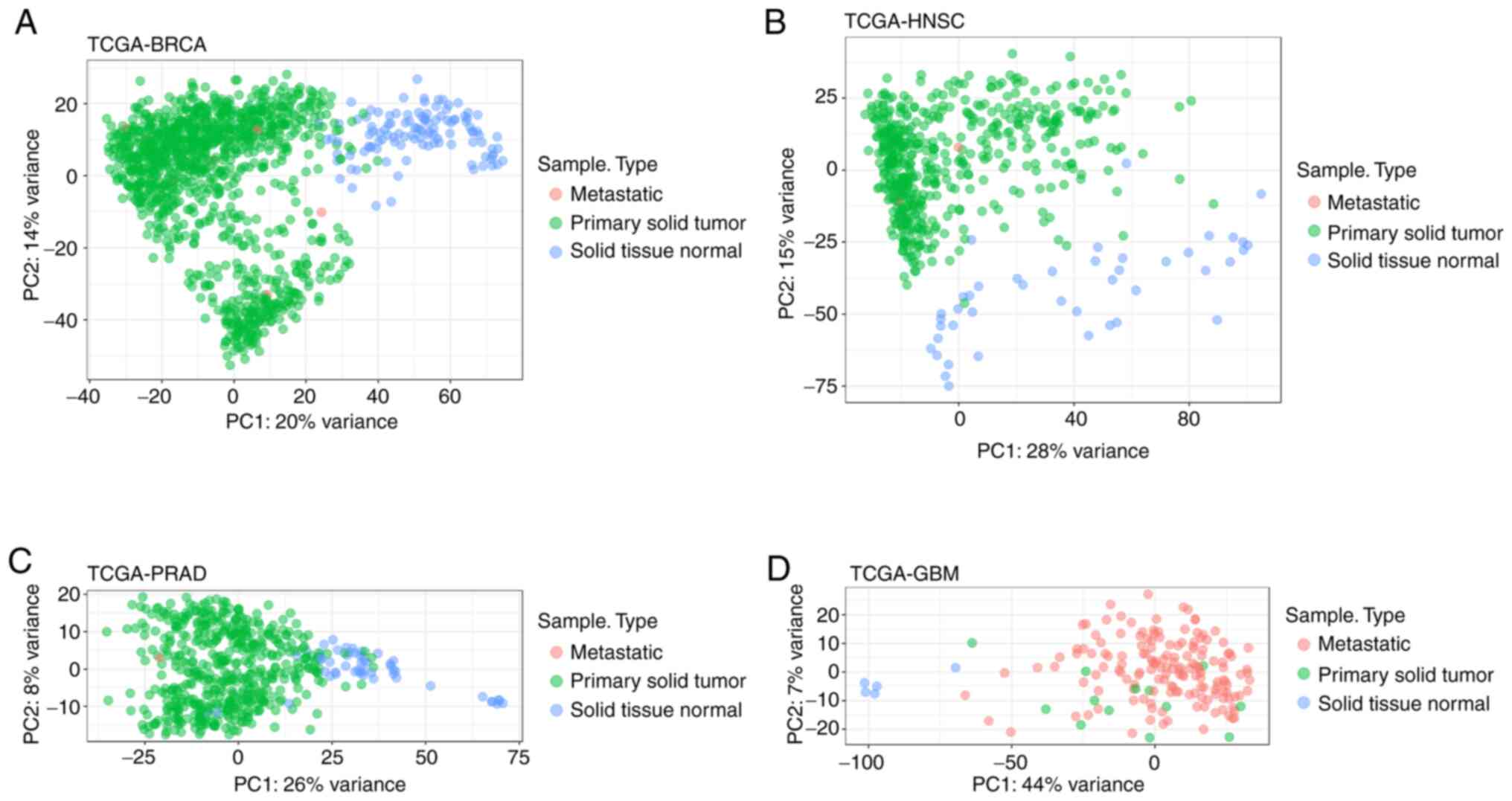
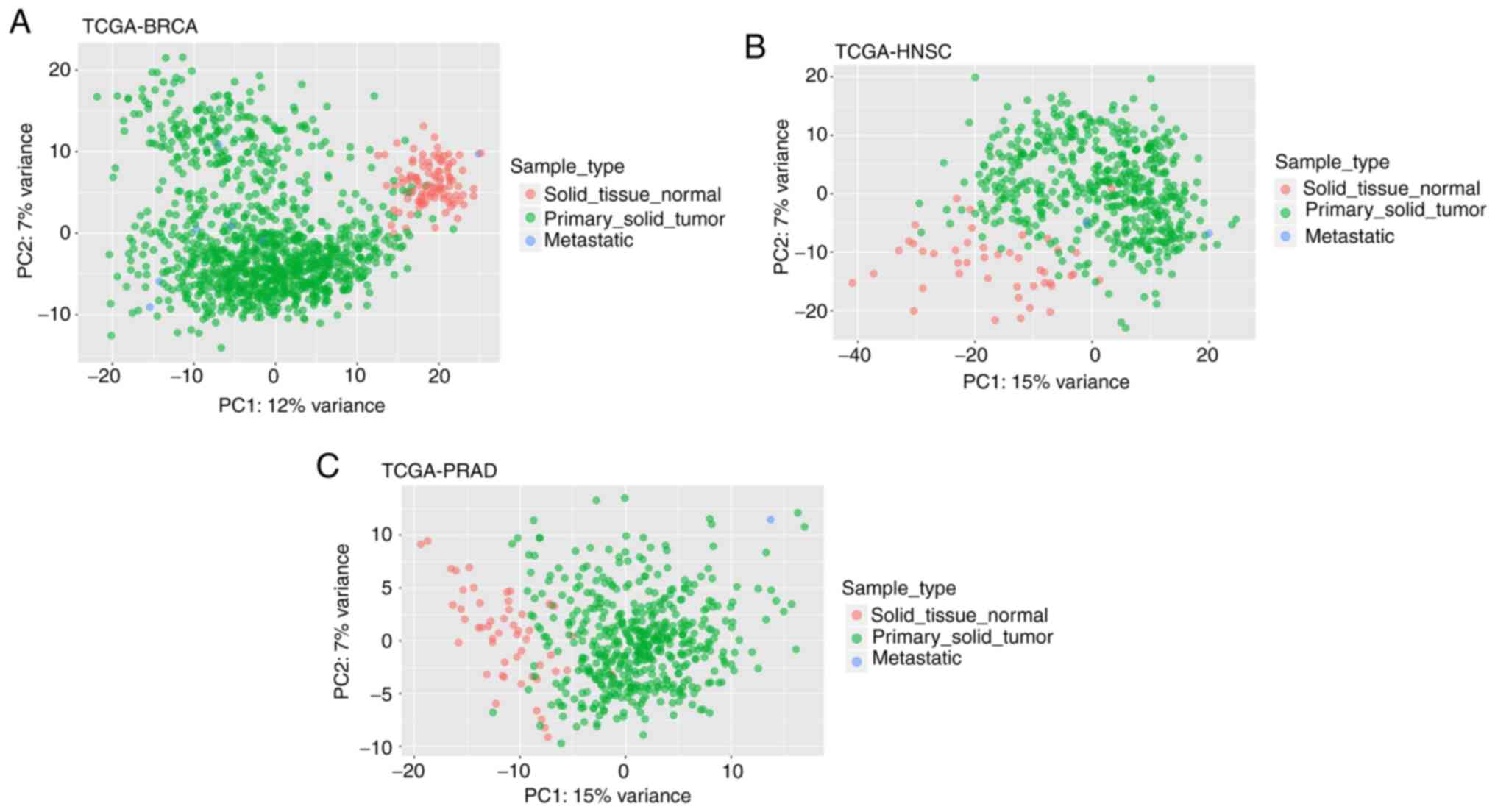
To further confirm these findings, PCA analysis was
performed using DE gene and miRNA expression values for all samples
of each TCGA-project. Following dimension reduction, loadings of
principal components (PC) 1 and 2 were plotted against each other
and displayed the formation of distinct groups between STN and PST,
as well as other sample types, such as metastatic, in each type of
cancer (Figs. 1 and 2).
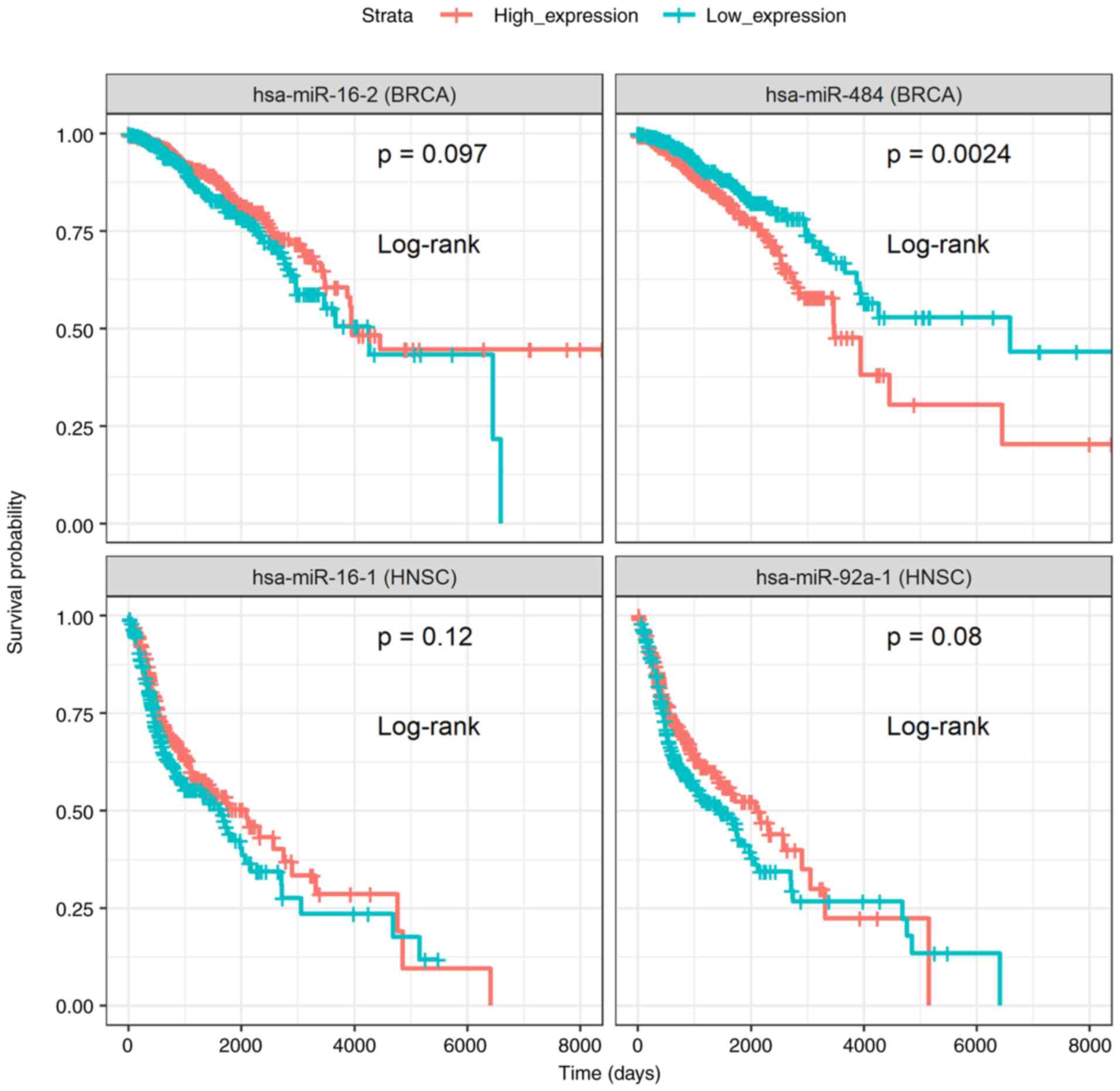
Moreover, survival analysis was performed on DE
genes and miRNAs (please see the ‘Materials and methods’ section).
In total, 6 genes (Fig. 3) and 3
miRNAs (Fig. 4) were reported, for
which patients with high or low expression levels presented
considerable differences in survival. The results concerning the
association of the genes PGK1, PCMT1, FDG3,
HOXB9, NSUN5 and ZNF330 to patient survival
probability, are in agreement with those previously reported by the
Human Protein Atlas project (22),
with the exception of the unfavorable prognosis of HOXB9
overexpression in glioblastoma, which was identified during the
current analysis (Fig. 3). As
regards the miRNAs, miR-16, known for its tumor suppressive
functions (23-25),
miR-92a-1(26) and miR-484
(27-29)
exhibited an associated with a favorable and poor prognosis,
respectively, for patients with breast and head and neck cancer
(Fig. 4). These results support
the approach for the identification of DE targets.
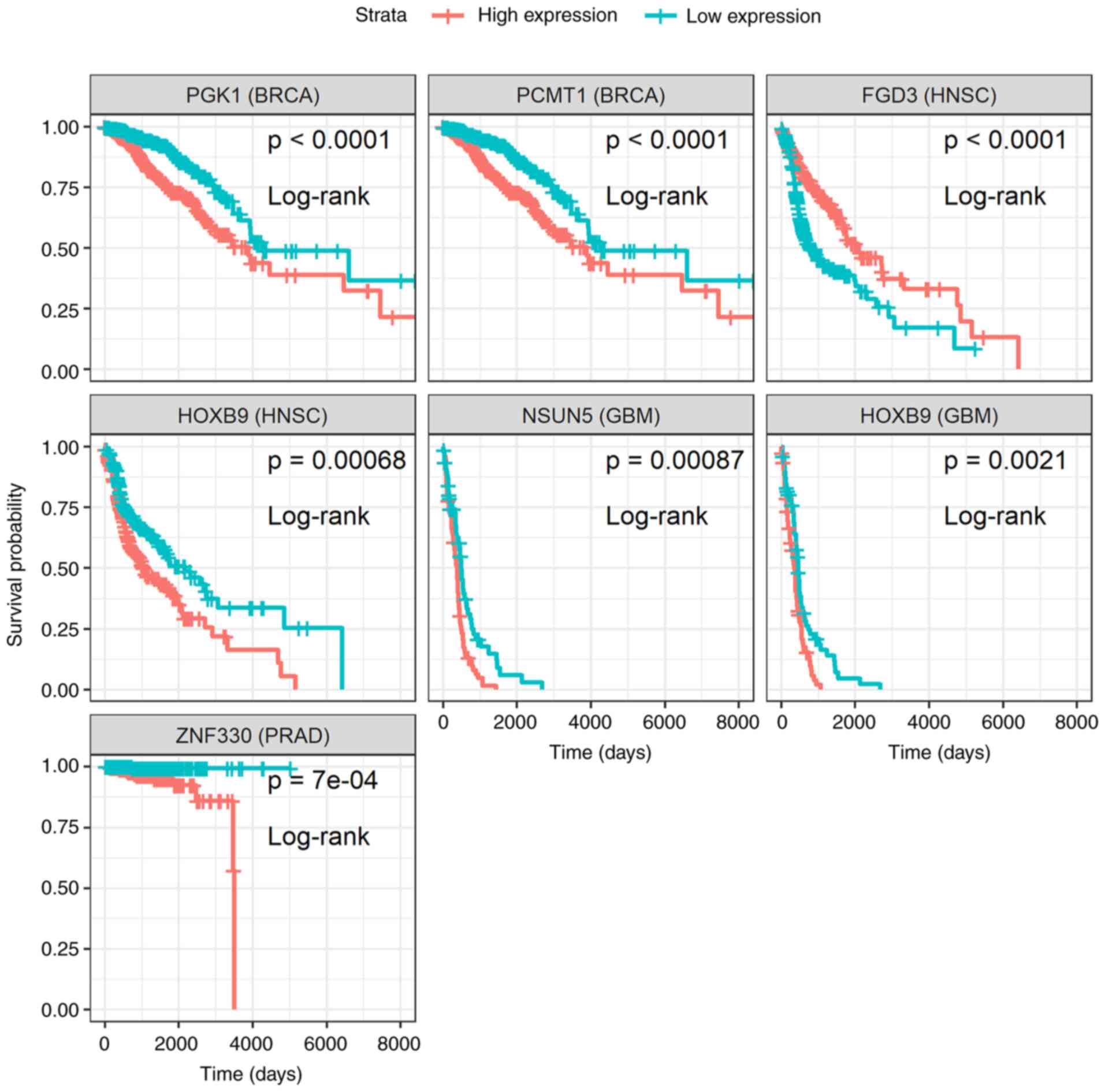 | Figure 3Kaplan-Meier survival curves of the
differentially expressed genes, PGK1, PCMT1,
FGD3, HOXB9, NSUN5 and ZNF330, whose
expression levels were significantly associated (P-value <0.001;
non-parametric log-rank test) with a favorable or poor survival
probability of patients with breast (BRCA), head and neck (HNSC),
glioblastoma (GBM) or prostate (PRAD) cancer. PST samples were
assigned into two separate groups depending on whether target
expression of each sample is higher (high expression) or lower (low
expression) than the median. Survival analysis was performed and
plots were created using survival methods (16,17)
and survminer (18). PST, primary
solid tumor; (Time: Is shown in days). |
EMT-associated genes and miRNAs
In total, 344 genes and 20 miRNAs that, following an
exhaustive literature search, constitute a collection of
well-characterized EMT-associated targets, were retrieved from
dbEMT 1.0(10). Direct comparisons
between the 2 gene collections revealed that only a small fraction
of DE genes has been identified as directly related to the EMT
process (percentage of DE genes that were EMT-related: TCGA-BRCA,
1.7%; TCGA-HNSC, 1.5%; TCGA-PRAD, 1%; TCGA-GBM, 1.9%) (Table I). A small number of miRNAs
reported in dbEMT 1.0 was also found in the present collection of
DE miRNAs (percentage of DE miRNAs that were EMT-related:
TCGA-BRCA, 3.7%; TCGA-HNSC, 2.4%; TCGA-PRAD, 3.6%) (Table I).
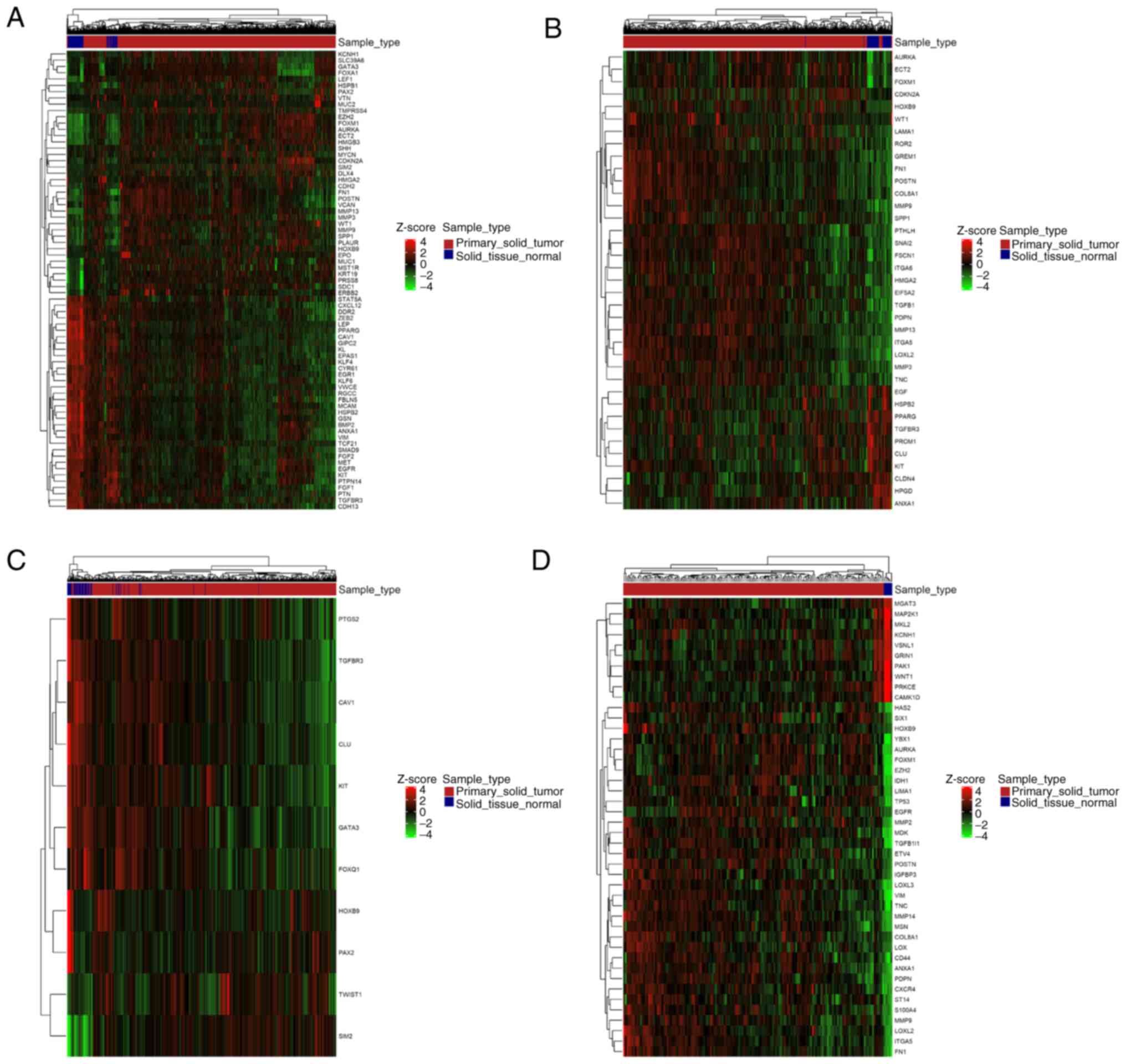
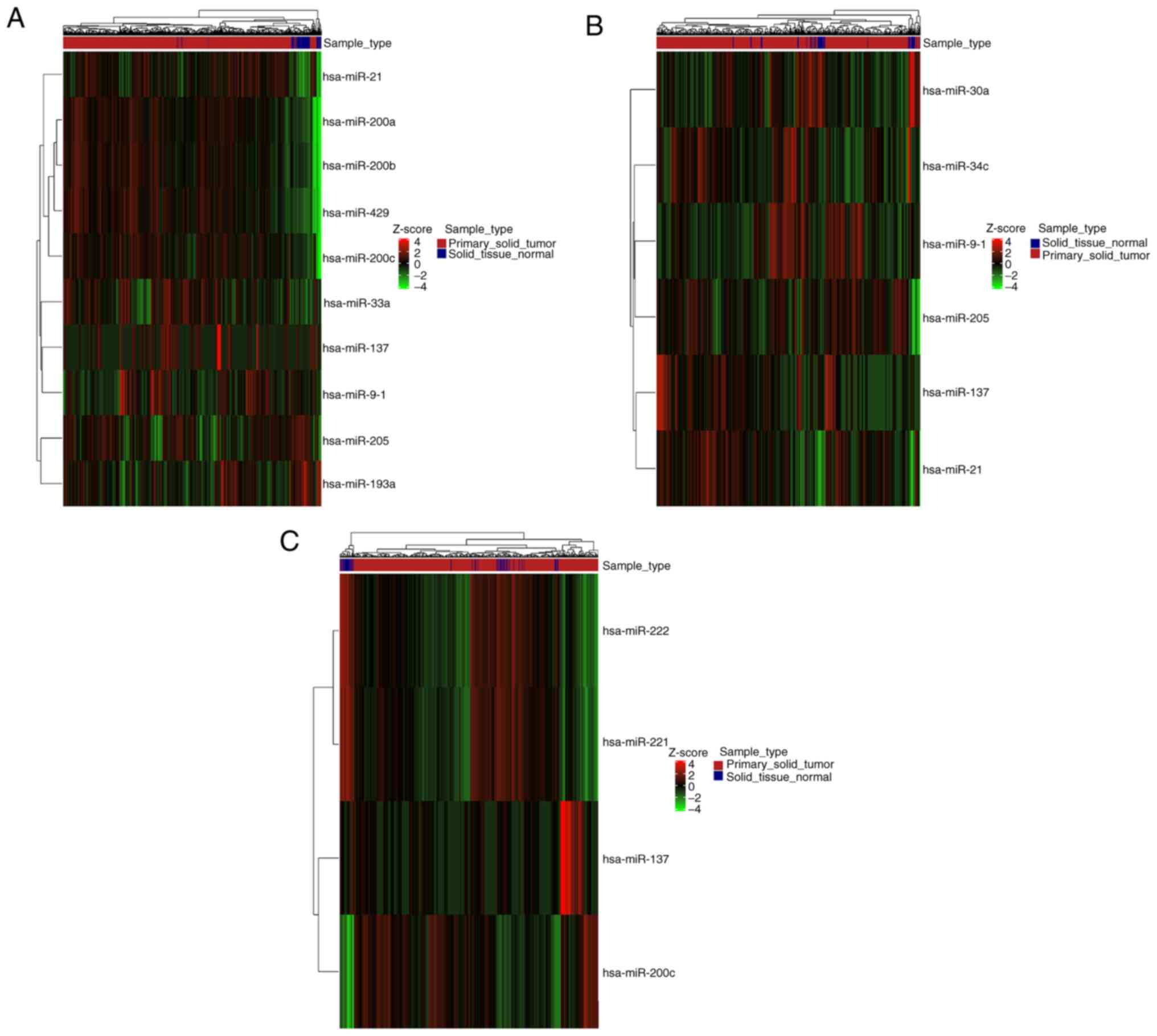
Furthermore, DE genes and miRNAs related to EMT were
found both up- and downregulated in each type of cancer (Figs. 5 and 6). Careful inspection of these results is
required to decipher the role of each gene and miRNA in EMT, at the
context of each malignancy.
EMT targets amongst different
malignancies
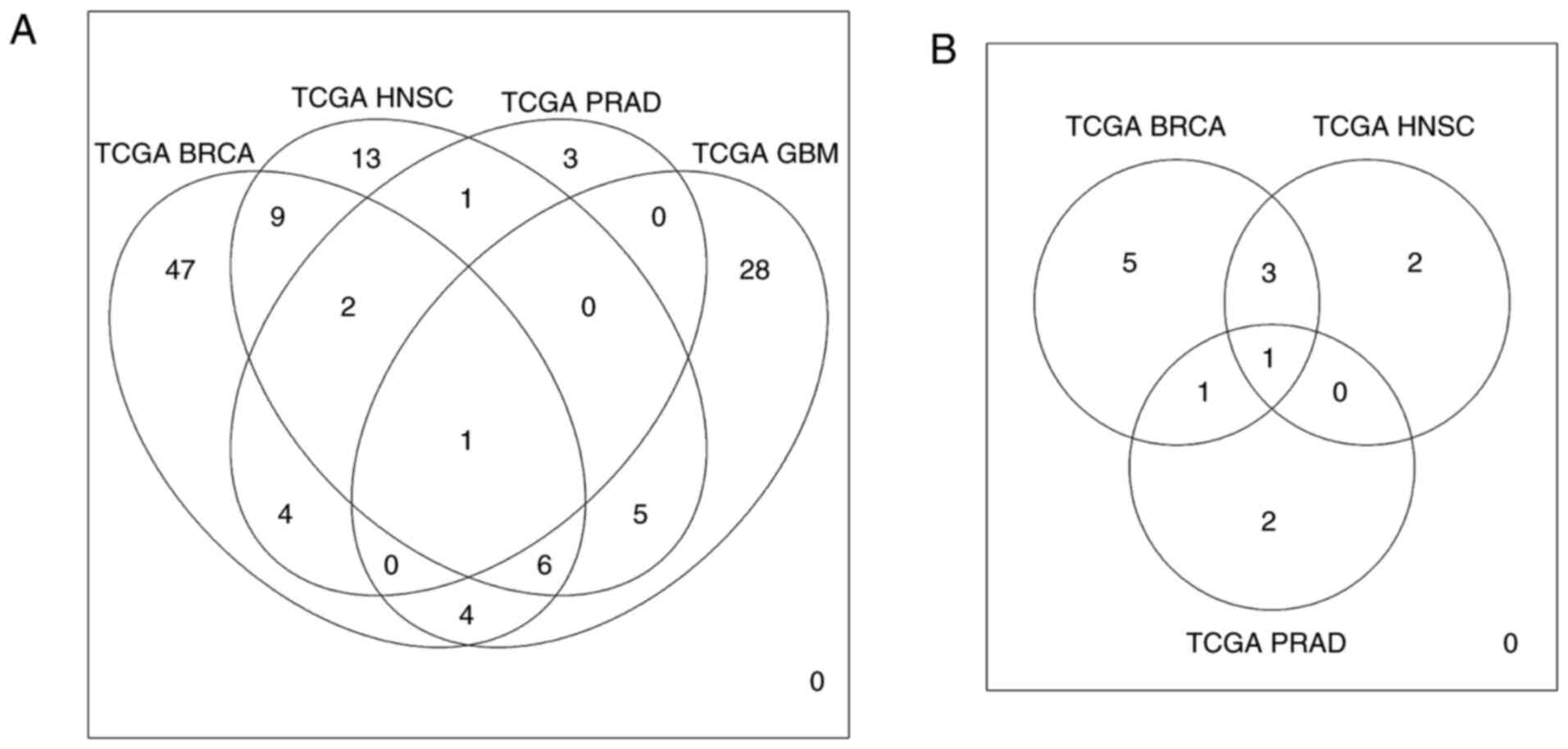
With an aim of identifying targets that are commonly
deregulated and EMT-associated between different types of cancer,
pairwise comparisons of DE genes and miRNAs associated with EMT
were performed. It was found that different types of malignancies
shared several deregulated molecules (Tables II and III), with HOXB9 and miR-137
being common for all types of malignancies that were examined
(Fig. 7).
 | Table IIDifferentially expressed and
EMT-associated genes in different malignancies. |
Table II
Differentially expressed and
EMT-associated genes in different malignancies.
| Gene symbol | Ensemble gene
ID | TCGA_BRCA | TCGA_HNSC | TCGA_PRAD | TCGA_GBM |
|---|
| ZEB2 |
ENSG00000169554 | TRUE | FALSE | FALSE | FALSE |
| EGFR |
ENSG00000146648 | TRUE | FALSE | FALSE | TRUE |
| EPAS1 |
ENSG00000116016 | TRUE | FALSE | FALSE | FALSE |
| ERBB2 |
ENSG00000141736 | TRUE | FALSE | FALSE | FALSE |
| MET |
ENSG00000105976 | TRUE | FALSE | FALSE | FALSE |
| CDH2 |
ENSG00000170558 | TRUE | FALSE | FALSE | FALSE |
| KLF4 |
ENSG00000136826 | TRUE | FALSE | FALSE | FALSE |
| KLF6 |
ENSG00000067082 | TRUE | FALSE | FALSE | FALSE |
| WT1 |
ENSG00000184937 | TRUE | TRUE | FALSE | FALSE |
| LEF1 |
ENSG00000138795 | TRUE | FALSE | FALSE | FALSE |
| SIM2 |
ENSG00000159263 | TRUE | FALSE | TRUE | FALSE |
| FN1 |
ENSG00000115414 | TRUE | TRUE | FALSE | TRUE |
| EGR1 |
ENSG00000120738 | TRUE | FALSE | FALSE | FALSE |
| KIT |
ENSG00000157404 | TRUE | TRUE | TRUE | FALSE |
| BMP2 |
ENSG00000125845 | TRUE | FALSE | FALSE | FALSE |
| CAV1 |
ENSG00000105974 | TRUE | FALSE | TRUE | FALSE |
| PPARG |
ENSG00000132170 | TRUE | TRUE | FALSE | FALSE |
| TGFBR3 |
ENSG00000069702 | TRUE | TRUE | TRUE | FALSE |
| FOXA1 |
ENSG00000129514 | TRUE | FALSE | FALSE | FALSE |
| STAT5A |
ENSG00000126561 | TRUE | FALSE | FALSE | FALSE |
| GATA3 |
ENSG00000107485 | TRUE | FALSE | TRUE | FALSE |
| ANXA1 |
ENSG00000135046 | TRUE | TRUE | FALSE | TRUE |
| DDR2 |
ENSG00000162733 | TRUE | FALSE | FALSE | FALSE |
| FOXM1 |
ENSG00000111206 | TRUE | TRUE | FALSE | TRUE |
| HSPB1 |
ENSG00000106211 | TRUE | FALSE | FALSE | FALSE |
| VIM |
ENSG00000026025 | TRUE | FALSE | FALSE | TRUE |
| SMAD9 |
ENSG00000120693 | TRUE | FALSE | FALSE | FALSE |
| GSN |
ENSG00000148180 | TRUE | FALSE | FALSE | FALSE |
| CYR61 |
ENSG00000142871 | TRUE | FALSE | FALSE | FALSE |
| MST1R |
ENSG00000164078 | TRUE | FALSE | FALSE | FALSE |
| VCAN |
ENSG00000038427 | TRUE | FALSE | FALSE | FALSE |
| MYCN |
ENSG00000134323 | TRUE | FALSE | FALSE | FALSE |
| TCF21 |
ENSG00000118526 | TRUE | FALSE | FALSE | FALSE |
| HMGA2 |
ENSG00000149948 | TRUE | TRUE | FALSE | FALSE |
| CDKN2A |
ENSG00000147889 | TRUE | TRUE | FALSE | FALSE |
| MUC1 |
ENSG00000185499 | TRUE | FALSE | FALSE | FALSE |
| AURKA |
ENSG00000087586 | TRUE | TRUE | FALSE | TRUE |
| FGF2 |
ENSG00000138685 | TRUE | FALSE | FALSE | FALSE |
| MCAM |
ENSG00000076706 | TRUE | FALSE | FALSE | FALSE |
| PAX2 |
ENSG00000075891 | TRUE | FALSE | TRUE | FALSE |
| PTPN14 |
ENSG00000152104 | TRUE | FALSE | FALSE | FALSE |
| SHH |
ENSG00000164690 | TRUE | FALSE | FALSE | FALSE |
| SPP1 |
ENSG00000118785 | TRUE | TRUE | FALSE | FALSE |
| CDH13 |
ENSG00000140945 | TRUE | FALSE | FALSE | FALSE |
| VTN |
ENSG00000109072 | TRUE | FALSE | FALSE | FALSE |
| FBLN5 |
ENSG00000140092 | TRUE | FALSE | FALSE | FALSE |
| KRT19 |
ENSG00000171345 | TRUE | FALSE | FALSE | FALSE |
| EZH2 |
ENSG00000106462 | TRUE | FALSE | FALSE | TRUE |
| ECT2 |
ENSG00000114346 | TRUE | TRUE | FALSE | FALSE |
| PLAUR |
ENSG00000011422 | TRUE | FALSE | FALSE | FALSE |
| MMP9 |
ENSG00000100985 | TRUE | TRUE | FALSE | TRUE |
| PTN |
ENSG00000105894 | TRUE | FALSE | FALSE | FALSE |
| POSTN |
ENSG00000133110 | TRUE | TRUE | FALSE | TRUE |
| CXCL12 |
ENSG00000107562 | TRUE | FALSE | FALSE | FALSE |
| EPO |
ENSG00000130427 | TRUE | FALSE | FALSE | FALSE |
| FGF1 |
ENSG00000113578 | TRUE | FALSE | FALSE | FALSE |
| DLX4 |
ENSG00000108813 | TRUE | FALSE | FALSE | FALSE |
| MMP3 |
ENSG00000149968 | TRUE | TRUE | FALSE | FALSE |
| LEP |
ENSG00000174697 | TRUE | FALSE | FALSE | FALSE |
| PRSS8 |
ENSG00000052344 | TRUE | FALSE | FALSE | FALSE |
| MMP13 |
ENSG00000137745 | TRUE | TRUE | FALSE | FALSE |
| KL |
ENSG00000133116 | TRUE | FALSE | FALSE | FALSE |
| HOXB9 |
ENSG00000170689 | TRUE | TRUE | TRUE | TRUE |
| SLC39A6 |
ENSG00000141424 | TRUE | FALSE | FALSE | FALSE |
| SDC1 |
ENSG00000115884 | TRUE | FALSE | FALSE | FALSE |
| GIPC2 |
ENSG00000137960 | TRUE | FALSE | FALSE | FALSE |
| HMGB3 |
ENSG00000029993 | TRUE | FALSE | FALSE | FALSE |
| TMPRSS4 |
ENSG00000137648 | TRUE | FALSE | FALSE | FALSE |
| RGCC |
ENSG00000102760 | TRUE | FALSE | FALSE | FALSE |
| VWCE |
ENSG00000167992 | TRUE | FALSE | FALSE | FALSE |
| MUC2 |
ENSG00000198788 | TRUE | FALSE | FALSE | FALSE |
| KCNH1 |
ENSG00000143473 | TRUE | FALSE | FALSE | TRUE |
| HSPB2 |
ENSG00000170276 | TRUE | TRUE | FALSE | FALSE |
| TGFB1 |
ENSG00000105329 | FALSE | TRUE | FALSE | FALSE |
| SNAI2 |
ENSG00000019549 | FALSE | TRUE | FALSE | FALSE |
| EGF |
ENSG00000138798 | FALSE | TRUE | FALSE | FALSE |
| TNC |
ENSG00000041982 | FALSE | TRUE | FALSE | TRUE |
| ITGA6 |
ENSG00000091409 | FALSE | TRUE | FALSE | FALSE |
| PTHLH |
ENSG00000087494 | FALSE | TRUE | FALSE | FALSE |
| ITGA5 |
ENSG00000161638 | FALSE | TRUE | FALSE | TRUE |
| ROR2 |
ENSG00000169071 | FALSE | TRUE | FALSE | FALSE |
| CLDN4 |
ENSG00000189143 | FALSE | TRUE | FALSE | FALSE |
| HPGD |
ENSG00000164120 | FALSE | TRUE | FALSE | FALSE |
| GREM1 |
ENSG00000166923 | FALSE | TRUE | FALSE | FALSE |
| CLU |
ENSG00000120885 | FALSE | TRUE | TRUE | FALSE |
| LAMA1 |
ENSG00000101680 | FALSE | TRUE | FALSE | FALSE |
| PROM1 |
ENSG00000007062 | FALSE | TRUE | FALSE | FALSE |
| LOXL2 |
ENSG00000134013 | FALSE | TRUE | FALSE | TRUE |
| FSCN1 |
ENSG00000075618 | FALSE | TRUE | FALSE | FALSE |
| COL8A1 |
ENSG00000144810 | FALSE | TRUE | FALSE | TRUE |
| EIF5A2 |
ENSG00000163577 | FALSE | TRUE | FALSE | FALSE |
| PDPN |
ENSG00000162493 | FALSE | TRUE | FALSE | TRUE |
| TWIST1 |
ENSG00000122691 | FALSE | FALSE | TRUE | FALSE |
| PTGS2 |
ENSG00000073756 | FALSE | FALSE | TRUE | FALSE |
| FOXQ1 |
ENSG00000164379 | FALSE | FALSE | TRUE | FALSE |
| TP53 |
ENSG00000141510 | FALSE | FALSE | FALSE | TRUE |
| MAP2K1 |
ENSG00000169032 | FALSE | FALSE | FALSE | TRUE |
| PRKCE |
ENSG00000171132 | FALSE | FALSE | FALSE | TRUE |
| CD44 |
ENSG00000026508 | FALSE | FALSE | FALSE | TRUE |
| MMP2 |
ENSG00000087245 | FALSE | FALSE | FALSE | TRUE |
| YBX1 |
ENSG00000065978 | FALSE | FALSE | FALSE | TRUE |
| TGFB1I1 |
ENSG00000140682 | FALSE | FALSE | FALSE | TRUE |
| CXCR4 |
ENSG00000121966 | FALSE | FALSE | FALSE | TRUE |
| MDK |
ENSG00000110492 | FALSE | FALSE | FALSE | TRUE |
| MSN |
ENSG00000147065 | FALSE | FALSE | FALSE | TRUE |
| SIX1 |
ENSG00000126778 | FALSE | FALSE | FALSE | TRUE |
| S100A4 |
ENSG00000196154 | FALSE | FALSE | FALSE | TRUE |
| PAK1 |
ENSG00000149269 | FALSE | FALSE | FALSE | TRUE |
| IGFBP3 |
ENSG00000146674 | FALSE | FALSE | FALSE | TRUE |
| MMP14 |
ENSG00000157227 | FALSE | FALSE | FALSE | TRUE |
| ST14 |
ENSG00000149418 | FALSE | FALSE | FALSE | TRUE |
| MKL2 |
ENSG00000186260 | FALSE | FALSE | FALSE | TRUE |
| ETV4 |
ENSG00000175832 | FALSE | FALSE | FALSE | TRUE |
| WNT1 |
ENSG00000125084 | FALSE | FALSE | FALSE | TRUE |
| LOX |
ENSG00000113083 | FALSE | FALSE | FALSE | TRUE |
| LIMA1 |
ENSG00000050405 | FALSE | FALSE | FALSE | TRUE |
| GRIN1 |
ENSG00000176884 | FALSE | FALSE | FALSE | TRUE |
| LOXL3 |
ENSG00000115318 | FALSE | FALSE | FALSE | TRUE |
| VSNL1 |
ENSG00000163032 | FALSE | FALSE | FALSE | TRUE |
| IDH1 |
ENSG00000138413 | FALSE | FALSE | FALSE | TRUE |
| CAMK1D |
ENSG00000183049 | FALSE | FALSE | FALSE | TRUE |
| MGAT3 |
ENSG00000128268 | FALSE | FALSE | FALSE | TRUE |
| HAS2 |
ENSG00000170961 | FALSE | FALSE | FALSE | TRUE |
 | Table IIIDifferentially expressed and
EMT-associated miRNAs in different malignancies. |
Table III
Differentially expressed and
EMT-associated miRNAs in different malignancies.
| miRNA | TCGA_BRCA | TCGA_HNSC | TCGA_PRAD |
|---|
| hsa-mir-137 | TRUE | TRUE | TRUE |
| hsa-mir-193a | TRUE | FALSE | FALSE |
| hsa-mir-200a | TRUE | FALSE | FALSE |
| hsa-mir-200b | TRUE | FALSE | FALSE |
| hsa-mir-200c | TRUE | FALSE | TRUE |
| hsa-mir-205 | TRUE | TRUE | FALSE |
| hsa-mir-21 | TRUE | TRUE | FALSE |
| hsa-mir-33a | TRUE | FALSE | FALSE |
| hsa-mir-9-1 | TRUE | TRUE | FALSE |
| hsa-mir-429 | TRUE | FALSE | FALSE |
| hsa-mir-30a | FALSE | TRUE | FALSE |
| hsa-mir-34c | FALSE | TRUE | FALSE |
| hsa-mir-221 | FALSE | FALSE | TRUE |
| hsa-mir-222 | FALSE | FALSE | TRUE |
Assessment of DE genes and miRNAs
related to EMT in breast, and head and neck cell carcinoma
lines
Based on the data obtained, the present study wished
to assess the expression level in a number of DE genes in
well-characterized human breast, and head and neck cancer cell
lines. To this end, the selection was made for caveolin-1, FOXM1
and Vimentin for the human breast epithelial carcinoma cell lines,
MCF-7 and MDA-MB-231. As for the head and neck cancer cell lines,
the human oral HSC-3 and keratinocyte HaCaT cancer cells were used
to assess the gene expression levels of HMGA2, TGFB1, FOXM1 and
PPARG. Moreover, from the DE miRNAs, the expression of miR-21 was
selected and was assessed in all these 4 cell lines.
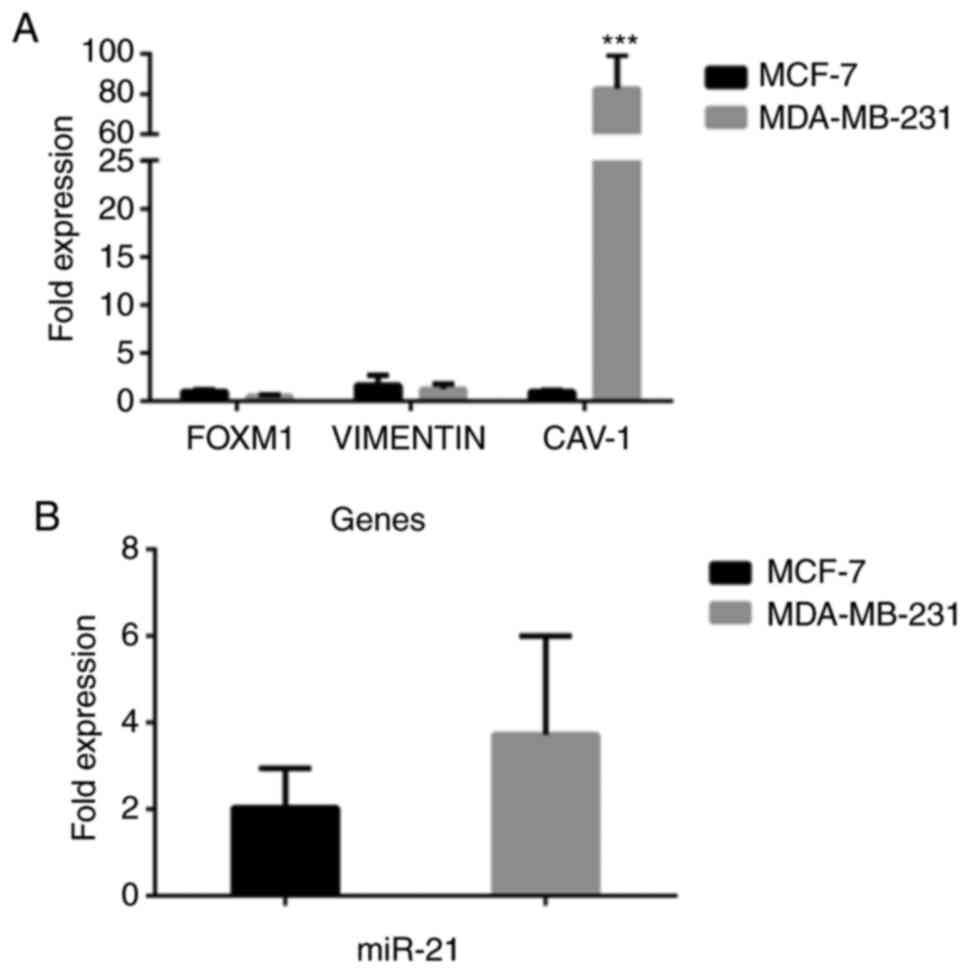
As shown in Fig. 8,
for the breast epithelial carcinoma cell lines, the expression of
caveolin-1 (Fig. 8A) was markedly
higher in the MDA-MB-231 cells than in the MCF-7 cells
(P<0.001). As regards the expression of miR-21 (Fig. 8B), it was higher again in the
MDA-MB-231 cells compared to the MCF-7 cells, although the
difference was not statistically significant. Such gene and miR-21
differential expression profiles may contribute to the observed
metabolic and phenotypic behavior of these 2 cell lines. Indeed,
although they are both invasive ductal breast carcinoma cells, they
have a number of phenotypic and genotypic differences. MCF-7 are
estrogen receptor-positive cells, whereas MDA-MB-231 cells are
estrogen and progesterone receptor-negative; in addition, MCF-7
cells express the epithelial phenotype in contrast to the
MDA-MB-231 cells that are more mesenchymal (30-32).
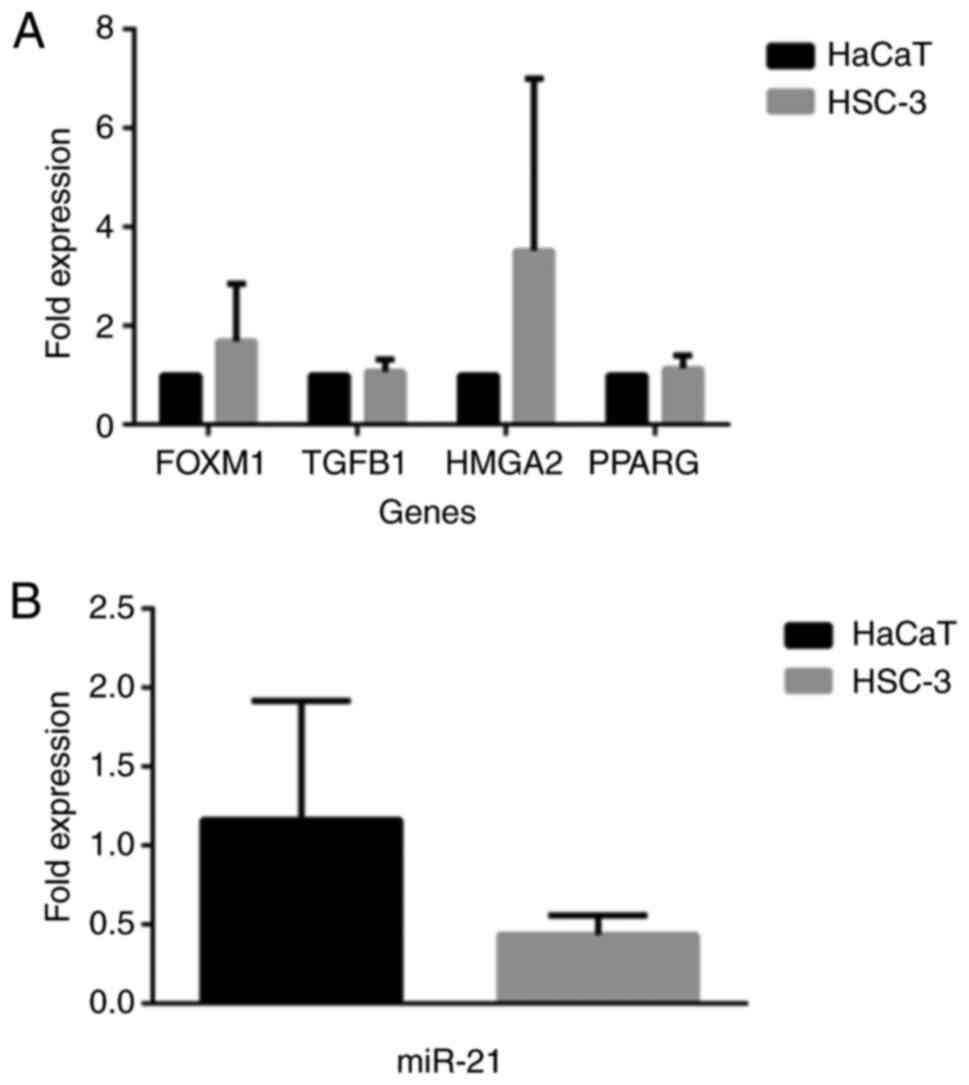
Similarly, as shown in Fig. 9, the expression profiles of the
FOXM1 and HMGA2 genes exhibited higher levels in the HSC-3 as
compared to the HaCaT cells (Fig.
9A), although this difference was not statistically
significant. On the contrary, miR-21 was found to have an increased
expression in HaCaT compared to HSC-3 cells, a result that was
again, not significant. It is interesting that the pattern of
expression of these EMT-related genes and that of miR-21 varies in
these 2 cell lines; however, it is still not known to what extent
such behavior contributes to any metabolic and phenotypic property
seen in these 2 cell lines.
Discussion
Through preliminary in silico analysis, the
present study identified a list of genes and miRNAs that were DE
and associated with EMT and/or MET in different types of cancer,
such as head and neck, breast and prostate cancer, and
glioblastoma. Moreover, RT-qPCR analysis revealed differential
expression profiles of selected EMT-related genes and miR-21 in a
number of human breast, and head and neck carcinoma cell lines.
TCGA-BRCA and -HNSC cancer samples shared the most DE and dbEMT 1.0
reported genes and miRNAs, in accordance with their common
epithelial tissue origin. The present study, by analyzing the
expression profiles of EMT-related genes and miRNAs in patient
cancer samples, suggests that HOXB9 and miR-137 present the same
deregulated patterns, independent of tumor type. HOXB9 belongs to
HOX gene family that in human plays crucial role in physiology and
pathophysiology, by modulating cell development, differentiation
and growth. It is noteworthy that the aberrant expression of HOX
genes has been shown to contribute to cancer progression and
development (33,34). Indeed, it has been observed that
HOX genes exhibit a dysregulated expression in leukemia, ovarian
and lung cancer (35-38).
The function of HOXB9 novel tumor suppressor in the regulation of
colon adenocarcinoma progression has also been identified (39). Moreover, it has been shown that
HOXB9 is associated with the emergence of radioresistance, as well
as the development of resistance in anti-VEGF therapy in colorectal
cancer (40,41). Importantly, a recent study
identified HOXB9 as one key gene in a 5-gene molecular prognostic
signature in patients with laryngeal cancer (42). In addition, the implication of HOX9
in prostate cancer cell progression has been recently proposed
(43). Furthermore, the present
study reports HOXB9, along with PGK1 (44,45),
PCMT1 (46,47), NSUN5 (48,49)
and ZNF330(50), as unfavorable
markers for the survival of patients with the types of cancer
examined herein. Of note, the present study demonstrated showed
that HOXB9 overexpression was associated with a poor prognosis for
patients with both head and neck cancer, previously reported in
Human Protein Atlas (22), and
those with glioblastoma. An exception was FGD3 (51,52),
with its overexpression being predictive of a favorable outcome for
patients with head and neck cancer (Fig. 3).
miRNAs represent important players in the
post-transcriptionally regulation of gene expression. In this
manner, they affect signaling pathways and cellular processes with
implication in cancer progression and development (53-56).
miR-137 has shown to control tumorigenesis, invasion and metastasis
in pancreatic neuroendocrine tumors (56). Moreover, miR-137 exhibits crucial
developmental roles in neuronal differentiation (57). In addition, miR-137, by modulating
SLC1A5-dependent glutamine uptake, is involved in the progression
of head and neck squamous cell carcinoma (58,59).
The significant role of miR-137 in the progression, diagnosis and
prognosis of hepatocellular carcinoma has also been documented
(60). The therapeutic potential
of targeting miR-137 in non-small cell lung cancer (NSCLC) has been
recently proposed (61). By
retrospectively analyzing tumor patient data, the task of
identifying potential druggable genes and miRNAs related to the
process of epithelial mesenchymal plasticity and exhibiting
deregulated expression levels in different malignancies has been
set forth. Moreover, the data presented in the present study
justify the affordability of identifying common druggable cancer
biomarkers applicable to various tumors, in order to proceed
thereafter, through the pharmacological assessment, to the
development of successful anticancer therapeutics. Of note, HOXB9
and miR-137 were found to be deregulated in all types of
malignancies that were analyzed. However, both were expressed in
very low levels compared to other genes and/or miRNAs. Therefore,
careful examination is required upon attempting to further
clinically validate their usefulness and therapeutic applicability,
as several DE genes and miRNAs related to EMT are also shared by
different types of cancer that were analyzed herein. To this end,
the regulatory network of miRNAs in EMT plasticity has been
previously evaluated in breast cancer (62). Moreover, the EMT regulatory network
includes a number of EMT-related transcription factors (e.g., the
Snail and Zeb family) and epigenetic collaborative regulators
(e.g., miR-34 for Snail and miR-200s for Zeb). Such interplay
drives the well-orchestrated epithelial-mesenchymal transcriptional
program, thus mediating the downstream biological effects (6). In the present study, the
bioinformatics analysis focused on EMT-related miRNAs and genes
dysregulated in various origin tumor patient samples and thus the
findings obtained highlight only such conclusions. Whether or not
there exists any functional involvement between the miRNAs and the
genes identified, needs to be experimentally validated. It is
interesting, however, to note that recent data highlight the yet
unexplored role of miRNAs as regulators of Hox genes in
hematopoiesis, through the elucidation of the role of miR-708 as a
novel regulator of the Hoxa9 program in leukemia myeloid cells
(63).
Overall, the analysis approach, is further
strengthening the previously published data regarding the
modulation of EMT in tumors and proposes that targeted research
efforts focused on identifying common biomarkers could provide
effective anticancer drugs. The results obtained support the notion
that as such, druggable biomarkers could be considered the HOXB9
gene and/or miR-137, irrespective of the cured tumor type, although
further clinical and experimental studies are also needed.
Importantly, however, such a direction is expected to provide
valuable therapeutic interventions in malignancies by contributing
toward overcoming the existed cellular and genomic heterogeneity
(inter- and intra-tumoral) and the differential pharmacological
response seen among patients. In particular, the data provided
herein support the notion of identifying druggable biomarkers that
impinge on the fundamental cancer cell traits that provide the
needed advantageous capacity of tumor cell metabolism to abrogate
the molecular balance, as well as the existing physiological
restriction signals between differentiation, apoptosis and
proliferation. This notion of the pan-cancer clinical intervention
and the implementation of informed clinical decisions are based on
the profiling of genomic signatures and molecular biomarkers in
cancer patients; the origin and type of histology of the tumor have
already begun to be left. Indeed, the development of therapeutics
showing pan-cancer capabilities present a new revolutionary
therapeutic era, an approach mentioned as ‘tumor-agnostic
therapies’. Complementary to this, the ability to identify and
clinically validate cancer biomarkers working irrespectively of the
tumor type, permits the implementation of personalized cancer
therapy in the clinical setting. The already marketed anticancer
drugs, pembrolizumab, larotrectinib and entrectinib, belong to the
class of tumor-agnostic therapies, by successfully receiving
approval and being clinical used in patients with various types of
tumor bearing common molecular features (64). Furthermore, the ability to
implement cancer therapy with pharmacogenomics-guided therapeutic
decisions offers the needed precision in clinical practice for the
practical utilization of molecular profiling and biomarkers, as
well as the outcome improvement in patients (65).
Acknowledgements
The statistical methods used in the present study
were reviewed by Angelis Eleftherios, Professor of Statistics and
Information Systems, Head of the School of Informatics, Faculty of
Sciences, Aristotle University of Thessaloniki, Greece.
Funding
The present study was funded in the context of the
project ‘Molecular signatures analysis of three-dimensional cell
cultures and circulating tumor cells in the treatment of cancer’
(MIS 5004622) under the call for proposals ‘Supporting researchers
with emphasis on new researchers’ (EDULLL 34). The project was
co-financed by Greece and the European Union (European Social
Fund-ESF) by the Operational Programme Human Resources Development,
Education and Lifelong Learning 2014-2020.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
KAK was involved in the acquisition of data,
analysis and interpretation of data, drafting the article and
providing final approval. MGA was involved in the interpretation of
the data, cell culture experiments and providing final approval.
LPNG was involved in the interpretation of the data, drafting the
article and providing final approval. NGG was involved in the
interpretation of the data, revising the article and providing
final approval. ISV conceived and designed the study, drafting the
article, critical revision and provided final approval.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
NGG declares ownership of Biogenea Pharmaceuticals
Ltd. The company had no role in the design or outcomes of the
study. The remaining authors declare that they have no competing
interests.
References
|
1
|
Bocci F, Gearhart-Serna L, Boareto M,
Ribeiro M, Ben-Jacob E, Devi GR, Levine H, Onuchic JN and Jolly MK:
Toward understanding cancer stem cell heterogeneity in the tumor
microenvironment. Proc Natl Acad Sci USA. 116:148–157.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vizirianakis IS, Miliotou AN, Mystridis
GA, Andriotis EG, Andreadis II, Papadopoulou LC and Fatouros DG:
Tackling pharmacological response heterogeneity by PBPK modeling to
advance precision medicine productivity of nanotechnology and
genomics therapeutics. Expert Rev Precis Med Drug Dev. 4:139–151.
2019.
|
|
3
|
Brabletz T, Kalluri R, Nieto MA and
Weinberg RA: EMT in cancer. Nat Rev Cancer. 18:128–134.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Roche J: The epithelial-to-mesenchymal
transition in cancer. Cancers (Basel). 10(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Culig Z: Epithelial mesenchymal transition
and resistance in endocrine-related cancers. Biochim Biophys Acta
Mol Cell Res. 1866:1368–1375. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lu W and Kang Y: Epithelial-mesenchymal
plasticity in cancer progression and metastasis. Dev Cell.
49:361–374. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Williams ED, Gao D, Redfern A and Thompson
EW: Controversies around epithelial-mesenchymal plasticity in
cancer metastasis. Nat Rev Cancer. 19:716–732. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kyrodimou M, Andreadis D, Drougou A,
Amanatiadou EP, Angelis L, Barbatis C, Epivatianos A and
Vizirianakis IS: Desmoglein-3/γ-catenin and E-cadherin/ß-catenin
differential expression in oral leukoplakia and squamous cell
carcinoma. Clin Oral Investig. 18:199–210. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cancer Genome Atlas Research Network.
Weinstein JN, Collisson EA, Mills GB, Shaw KR, Ozenberger BA,
Ellrott K, Shmulevich I, Sander C and Stuart JM: The cancer genome
atlas pan-cancer analysis project. Nat Genet. 45:1113–1120.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Zhao M, Kong L, Liu Y and Qu H: dbEMT: An
epithelial-mesenchymal transition associated gene resource. Sci
Rep. 5(11459)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Colaprico A, Silva TC, Olsen C, Garofano
L, Cava C, Garolini D, Sabedot TS, Malta TM, Pagnotta SM,
Castiglioni I, et al: TCGAbiolinks: An R/Bioconductor package for
integrative analysis of TCGA data. Nucleic Acids Res.
44(e71)2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Anders S, Pyl PT and Huber W: HTSeq-a
Python framework to work with high-throughput sequencing data.
Bioinformatics. 31:166–169. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Love MI, Huber W and Anders S: Moderated
estimation of fold change and dispersion for RNA-seq data with
DESeq2. Genome Biol. 15(550)2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Shaffer JP: Multiple hypothesis testing.
Annu Rev Psychol. 46:561–584. 1995.
|
|
15
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43(e47)2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Therneau TM and Grambsch PM: Modeling
survival data: Extending the Cox model. Springer-Verlag New York,
2000.
|
|
17
|
Therneau T: A package for survival
analysis in R. R package version. 3:2–7. 2020.
|
|
18
|
Kassambara A, Kosinski M and Biecek P:
survminer: Drawing survival curves using‘ggplot2’, 2017.
|
|
19
|
Tseligka ED, Rova A, Amanatiadou EP,
Calabrese G, Tsibouklis J, Fatouros DG and Vizirianakis IS:
Pharmacological development of target-specific delocalized
lipophilic cation-functionalized carboranes for cancer therapy.
Pharm Res. 33:1945–1958. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akrivou MG, Demertzidou VP, Theodoroula
NF, Chatzopoulou FM, Kyritsis KA, Grigoriadis N, Zografos AL and
Vizirianakis IS: Uncovering the pharmacological response of novel
sesquiterpene derivatives that differentially alter gene expression
and modulate the cell cycle in cancer cells. Int J Oncol.
53:2167–2179. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Uhlen M, Zhang C, Lee S, Sjöstedt E,
Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, et
al: A pathology atlas of the human cancer transcriptome. Science.
357(eaan2507)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Liang H, Fu Z, Jiang X, Wang N, Wang F,
Wang X, Zhang S, Wang Y, Yan X, Guan WX, et al: miR-16 promotes the
apoptosis of human cancer cells by targeting FEAT. BMC Cancer.
15(448)2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Qu Y, Liu H, Lv X, Liu Y, Wang X, Zhang M,
Zhang X, Li Y, Lou Q, Li S and Li H: MicroRNA-16-5p overexpression
suppresses proliferation and invasion as well as triggers apoptosis
by targeting VEGFA expression in breast carcinoma. Oncotarget.
8:72400–72410. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ruan L and Qian X: MiR-16-5p inhibits
breast cancer by reducing AKT3 to restrain NF-κB pathway. Biosci
Rep. 39(BSR20191611)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nilsson S, Möller C, Jirström K, Lee A,
Busch S, Lamb R and Landberg G: Downregulation of miR-92a is
associated with aggressive breast cancer features and increased
tumour macrophage infiltration. PLoS One. 7(e36051)2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zearo S, Kim E, Zhu Y, Zhao JT, Sidhu SB,
Robinson BG and Soon PS: MicroRNA-484 is more highly expressed in
serum of early breast cancer patients compared to healthy
volunteers. BMC Cancer. 14(200)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Volinia S and Croce CM: Prognostic
microRNA/mRNA signature from the integrated analysis of patients
with invasive breast cancer. Proc Natl Acad Sci USA. 110:7413–7417.
2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ye FG, Song CG, Cao ZG, Xia C, Chen DN,
Chen L, Li S, Qiao F, Ling H, Yao L, et al: Cytidine deaminase axis
modulated by miR-484 differentially regulates cell proliferation
and chemoresistance in breast cancer. Cancer Res. 75:1504–1515.
2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Thompson EW, Reich R, Shima TB, Albini A,
Graf J, Martin GR, Dickson RB and Lippman ME: Differential
regulation of growth and invasiveness of MCF-7 breast cancer cells
by antiestrogens. Cancer Res. 48:6764–6768. 1988.PubMed/NCBI
|
|
31
|
Gjerdrum C, Tiron C, Høiby T, Stefansson
I, Haugen H, Sandal T, Collett K, Li S, McCormack E, Gjertsen BT,
et al: Axl is an essential epithelial-to-mesenchymal
transition-induced regulator of breast cancer metastasis and
patient survival. Proc Natl Acad Sci USA. 107:1124–1129.
2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Theodossiou TA, Ali M, Grigalavicius M,
Grallert B, Dillard P, Schink KO, Olsen CE, Wälchli S, Inderberg
EM, Kubin A, et al: Simultaneous defeat of MCF7 and MDA-MB-231
resistances by a hypericin PDT-tamoxifen hybrid therapy. NPJ Breast
Cancer. 5(13)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sha L, Dong L, Lv L, Bai L and Ji X: HOXB9
promotes epithelial-to-mesenchymal transition via transforming
growth factor-β1 pathway in hepatocellular carcinoma cells. Clin
Exp Med. 15:55–64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bhatlekar S, Fields JZ and Boman BM: Role
of HOX genes in stem cell differentiation and cancer. Stem Cells
Int. 2018(3569493)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Hayashida T, Takahashi F, Chiba N,
Brachtel E, Takahashi M, Godin-Heymann N, Gross KW, Vivanco Md,
Wijendran V, Shioda T, et al: HOXB9, a gene overexpressed in breast
cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad
Sci USA. 107:1100–1105. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
De Braekeleer E, Douet-Guilbert N, Basinko
A, Le Bris MJ, Morel F and De Braekeleer M: Hox gene dysregulation
in acute myeloid leukemia. Futur Oncol. 10:475–495. 2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eoh KJ, Kim HJ, Lee JY, Nam EJ, Kim S, Kim
SW and Kim YT: Dysregulated expression of homeobox family genes may
influence survival outcomes of patients with epithelial ovarian
cancer: Analysis of data from the cancer genome atlas. Oncotarget.
8:70579–70585. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Song J, Wang T, Xu W, Wang P, Wan J, Wang
Y, Zhan J and Zhang H: HOXB9 acetylation at K27 is responsible for
its suppression of colon cancer progression. Cancer Lett.
426:63–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhan J, Niu M, Wang P, Zhu X, Li S, Song
J, He H, Wang Y, Xue L, Fang W and Zhang H: Elevated HOXB9
expression promotes differentiation and predicts a favourable
outcome in colon adenocarcinoma patients. Br J Cancer. 111:883–893.
2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Chiba N, Comaills V, Shiotani B, Takahashi
F, Shimada T, Tajima K, Winokur D, Hayashida T, Willers H, Brachtel
E, et al: Homeobox B9 induces epithelial-to-mesenchymal
transition-associated radioresistance by accelerating DNA damage
responses. Proc Natl Acad Sci USA. 109:2760–2765. 2012.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Carbone C, Piro G, Simionato F, Ligorio F,
Cremolini C, Loupakis F, Alì G, Rossini D, Merz V, Santoro R, et
al: Homeobox B9 mediates resistance to anti-VEGF therapy in
colorectal cancer patients. Clin Cancer Res. 23:4312–4322.
2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zhang G, Fan E, Yue G, Zhong Q, Shuai Y,
Wu M, Feng G, Chen Q and Gou X: Five genes as a novel signature for
predicting the prognosis of patients with laryngeal cancer. J Cell
Biochem, Oct 31, 2019 (Online ahead of print).
|
|
43
|
Xu H, Wu S, Shen X, Wu D, Qin Z, Wang H,
Chen X and Sun X: Silencing of HOXB9 suppresses cellular
proliferation, angiogenesis, migration and invasion of prostate
cancer cells. J Biosci. 45(40)2020.PubMed/NCBI
|
|
44
|
Fu D, He C, Wei J, Zhang Z, Luo Y, Tan H
and Ren C: PGK1 is a potential survival biomarker and invasion
promoter by regulating the HIF-1α-mediated epithelial-mesenchymal
transition process in breast cancer. Cell Physiol Biochem.
51:2434–2444. 2018.PubMed/NCBI View Article : Google Scholar
|
|
45
|
He Y, Luo Y, Zhang D, Wang X, Zhang P, Li
H, Ejaz S and Liang S: PGK1-mediated cancer progression and drug
resistance. Am J Cancer Res. 9:2280–2302. 2019.PubMed/NCBI
|
|
46
|
Sambri I, Capasso R, Pucci P, Perna AF and
Ingrosso D: The microRNA 15a/16-1 cluster down-regulates protein
repair isoaspartyl methyltransferase in hepatoma cells:
Implications for apoptosis regulation. J Biol Chem.
286:43690–43700. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Dong L, Li Y, Xue D and Liu Y: PCMT1 is an
unfavorable predictor and functions as an oncogene in bladder
cancer. IUBMB Life. 70:291–299. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Schosserer M, Minois N, Angerer TB, Amring
M, Dellago H, Harreither E, Calle-Perez A, Pircher A, Gerstl MP,
Pfeifenberger S, et al: Methylation of ribosomal RNA by NSUN5 is a
conserved mechanism modulating organismal lifespan. Nat Commun.
6(6158)2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Heissenberger C, Liendl L, Nagelreiter F,
Gonskikh Y, Yang G, Stelzer EM, Krammer TL, Micutkova L, Vogt S,
Kreil DP, et al: Loss of the ribosomal RNA methyltransferase NSUN5
impairs global protein synthesis and normal growth. Nucleic Acids
Res. 47:11807–11825. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
de Melo IS, Iglesias C, Benítez-Rondán A,
Medina F, Martínez-Barberá JP and Bolívar J: NOA36/ZNF330 is a
conserved cystein-rich protein with proapoptotic activity in human
cells. Biochim Biophys Acta. 1793:1876–1885. 2009.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Willis S, Sun Y, Abramovitz M, Fei T,
Young B, Lin X, Ni M, Achua J, Regan MM, Gray KP, et al: High
expression of FGD3, a putative regulator of cell morphology and
motility, is prognostic of favorable outcome in multiple cancers.
JCO Precis Oncol 1: PO.17.00009, 2017.
|
|
52
|
Renda I, Bianchi S, Vezzosi V, Nori J,
Vanzi E, Tavella K and Susini T: Expression of FGD3 gene as
prognostic factor in young breast cancer patients. Sci Rep.
9(15204)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Vidigal JA and Ventura A: The biological
functions of miRNAs: Lessons from in vivo studies. Trends Cell
Biol. 25:137–147. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Peng Y and Croce CM: The role of microRNAs
in human cancer. Signal Transduct Target Ther.
1(15004)2016.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Bartel DP: Metazoan MicroRNAs. Cell.
173:20–51. 2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Michael IP, Saghafinia S and Hanahan D: A
set of microRNAs coordinately controls tumorigenesis, invasion, and
metastasis. Proc Natl Acad Sci USA. 116:24184–24195.
2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Cherone JM, Jorgji V and Burge CB:
Cotargeting among microRNAs in the brain. Genome Res. 29:1791–1804.
2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Dong J, Xiao D, Zhao Z, Ren P, Li C, Hu Y,
Shi J, Su H, Wang L, Liu H, et al: Epigenetic silencing of
microRNA-137 enhances ASCT2 expression and tumor glutamine
metabolism. Oncogenesis. 6(e356)2017.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhang Z, Liu R, Shuai Y, Huang Y, Jin R,
Wang X and Luo J: ASCT2 (SLC1A5)-dependent glutamine uptake is
involved in the progression of head and neck squamous cell
carcinoma. Br J Cancer. 122:82–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Wu P, Xiao Y, Guo T, Wang Y, Liao S, Chen
L and Liu Z: Identifying miRNA-mRNA pairs and novel miRNAs from
hepatocelluar carcinoma mirnomes and TCGA database. J Cancer.
10:2552–2559. 2019.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Nuzzo S, Catuogno S, Capuozzo M, Fiorelli
A, Swiderski P, Boccella S, de Nigris F and Esposito CL:
Axl-targeted delivery of the oncosuppressor miR-137 in
non-small-cell lung cancer. Mol Ther Nucleic Acids. 17:256–263.
2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Drago-García D, Espinal-Enríquez J and
Hernández-Lemus E: Network analysis of EMT and MET micro-RNA
regulation in breast cancer. Sci Rep. 7(13534)2017.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Schneider E, Pochert N, Ruess C, MacPhee
L, Escano L, Miller C, Krowiorz K, Delsing Malmberg E,
Heravi-Moussavi A, Lorzadeh A, et al: MicroRNA-708 is a novel
regulator of the Hoxa9 program in myeloid cells. Leukemia.
34:1253–1265. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Looney AM, Nawaz K and Webster RM:
Tumor-agnostic therapies. Nat Rev Drug Discov. 19:383–384.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Astras G, Papagiannopoulos CI, Kyritsis
KA, Markitani C and Vizirianakis IS: Pharmacogenomic testing to
guide personalized cancer medicine decisions in private oncology
practice: A case study. Front. Oncol. 10(521)2020.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Gu Z, Eils R and Schlesner M: Complex
heatmaps reveal patterns and correlations in multidimensional
genomic data. Bioinformatics. 32:2847–2849. 2016.PubMed/NCBI View Article : Google Scholar
|