Introduction
Atopic dermatitis (AD), a heterogeneous skin lesion
with an increasing prevalence worldwide, is recognized as the most
common chronic and relapsing inflammatory disorder among infants,
children and even adults of all races, particularly among families
with a history of related allergic diseases (1,2).
Overall, AD can be divided into the intrinsic and extrinsic
subtypes, which approximately comprises up 20 and 80% of patients,
respectively (3). The disease has
been reported to have a complex causality due to a series of
immunological, genetic and environmental elements, with resultant
comorbidities, such as respiratory diseases, food allergies,
inflammatory skin infections and relative autoimmune disorders
(1,4). As previously reported by Kim et
al, the curative efficacy of the current clinical regimens
against AD is relatively insufficient, along with marked ambiguity
and side-effects (5). Moreover,
complications, including xerosis, pruritus and eczematous skin
ulcers, further complicate the exploration of the pathogenesis and
management of AD (4,6). Thus, considering the increasing
evidence that AD has caused a tremendous burden upon patients and
their caregivers, there is an urgent need for the establishment of
a more systemic therapeutic strategy with which to eliminate the
complex pathophysiology together with the accompanied non-allergic
and allergic comorbidities (2,7).
Mesenchymal stem cells (MSCs) are immunomodulating
cell populations with static adherence and a typically fusiform
morphology, with a high-level expression of mesenchymal-associated
surface markers, and a multi-lineage differentiation capacity
towards adipocytes, osteoblasts and chondroblasts (8). For decades, numerous fundamental
research and clinical applications have collectively demonstrated
the vast prospects of the use of MSCs in regenerative medicine and
the coordinate contributions to the physiological and pathological
microenvironment (8-10).
For instance, researchers have extensively investigated the
cultivate prospects of MSCs in a variety of refractory and
relapsing diseases, such as the sports-related injuries (e.g.,
meniscal injuries) and osteoarthritis (11,12),
nervous system disorders (e.g., Alzheimer's disease and spinal cord
injuries) (13,14), digestive disorders (e.g.,
acute-on-chronic liver failure and fulminant hepatic disease)
(15,16), endocrine disorders (e.g., diabetes
mellitus and related complications) (17,18),
reproductive disorders (e.g., premature ovarian failure and
intrauterine adhesions) (19,20),
haematological disorders (e.g., aplastic anaemia and acute
myelogenous leukaemia) (21,22),
and in particular, immunodysregulation (graft-vs.-host disease and
Crohn's disease) (23,24). Kim et al reported that the
first-in-class clinical trial upon AD administration with human
umbilical cord blood-derived MSCs (hUCB-MSCs), even though hUCB-MSC
infusion was well tolerated without noteworthy adverse events; yet,
the efficiency together with the crucial effects did not yield
statistical significance (5).
Above all, the detailed effects of MSCs upon AD-associated skin
ulcers and vital signs of elderly patient >60 years of age
remain unexplored.
The present study describes the case of a
65-year-old female patient with typical symptoms of severe AD,
including unbearable pruritus, xeroderma and systemic eczema
plaques with refractory foot skin ulcer. With the aid of hormone
and other drug treatments, the pruritus of the elderly patient was
transiently attenuated, but relapsed quickly, whereas the other
complications did not exhibit a visible improvement. Thus, with the
consent of the patient and approval by the relevant ethics
committee, 5x107 human umbilical cord-derived MSCs
(hUC-MSCs) were administered into the body of the patient via
intravenous infusion. Notably, the aforementioned symptoms of AD
and multiple complications of the patient were completely
eliminated without visible side-effects upon examination of
systematic vital signs, and in particular, the severe foot ulcers
in both feet had subsided. Taken together, the findings of the
present study confirm the safety and efficacy of the
proof-of-concept use of hUC-MSCs for the treatment of AD. In
addition, the present study highlights the prospects of the use of
hUC-MSCs for elderly patients with refractory AD-associated severe
skin ulcers.
Case report
General description of the
patient
In March, 2019, the 65-year-old female patient in
question was diagnosed with influenza and a cough, when then
subsided, apart from the occasional itch and sporadic eczema spots
on the left foot. The patient was treated by an oral administration
of a routine dose of levofloxacin according to the drug
instructions as suggested by the doctors at the General Outpatient
Department, People's Hospital of Shangrao Economic and
Technological Development Zone, Jiangxi Health-Biotech Medical
Development Co., Ltd. However, in the middle of April, the area of
the original eczema began to gradually increase from 1.0x1.5 to
2.5x5.5 cm, and the local skin turned purple and was accompanied by
a persistent itch. After 1 month, a clinical examination revealed a
urinary tract infection in the patient. However, a significant
remission in symptoms was observed following the administration of
norfloxacin (for eczema) for 3 days. Even though the urinary tract
infection was temporarily eliminated with the aid of levofloxacin
treatment, yet a secondary infection was detected and this became
more frequent and severe by mid-July. For the purpose of
alleviating the refractory infection and the accompanied erythema
in the body, an oral cefixime-based remedy was conducted for 2
weeks. Unexpectedly, the erythema in the double lower limbs
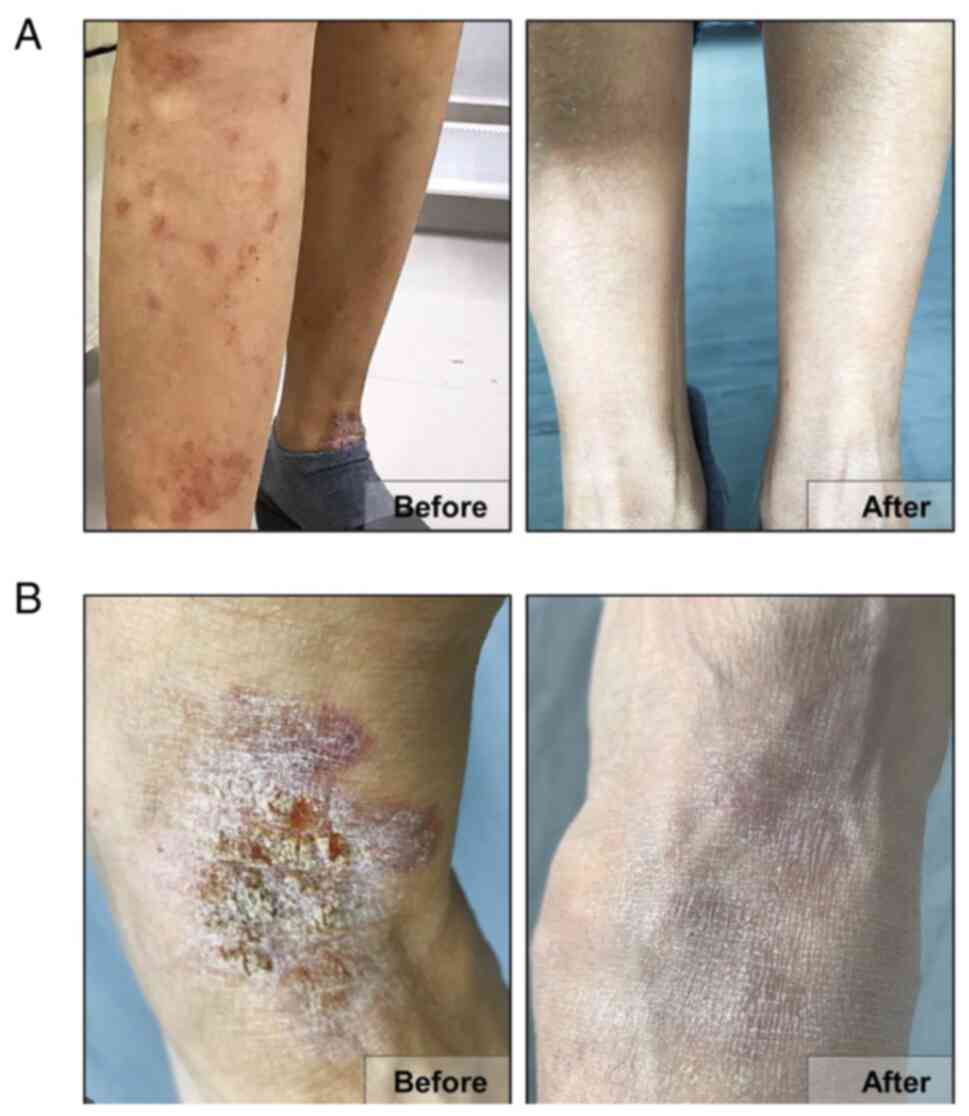
diffused and increased gradually, and spread upward from the crus
without signs of subsiding at all (Fig. 1A). In addition, ulcerations began
to form on the feet and these expanded into the epidermis and
dermis (Fig. 1B).
hUC-MSC infusion and safety
outcomes
On the basis of the aforementioned information, the
clinical expert panel consist of the doctors at the General
Outpatient Department, People's Hospital of Shangrao Economic and
Technological Development Zone, Jiangxi Health-Biotech Medical
Development Co., Ltd. reached the following consensus: The elderly
patient was diagnosed with refractory AD accompanied by pruritus,
diffuse erythema in the body, severe skin ulcers on the feet and
the incorporated urinary tract infection (Fig. 1A and B, and Table
SI). Having considered the inefficacious and even cumulative
outcomes of the traditionally comprehensive treatment, the patient
then received an intravenous administration of 5x107
clinical grade hUC-MSCs (Jiangxi Research Centre of Stem Cell
Engineering, Jiangxi Health-Biotech Stem Cell Technology Co. Ltd.;
product lot no. 201909JF03) via a sterile blood transfusion needle
at a rate of 45 drops per min on September 15th, 2020 in 100 ml
0.9% saline after obtaining the ethical approval of the Ethics
Committee of People's Hospital of Shangrao Economic and
Technological Development Zone, Jiangxi Health-Biotech Medical
Development Co., Ltd. and the informed consent of the patient.
During the transfusion process, no acute adverse effects or
drug-related events, such as infusion-related or allergic reactions
(e.g., polypnea, slight trembling, or instantaneous low fever) were
observed in the patient with AD. Subsequently, no delayed
hypersensitivity, secondary infection, or life-threatening events
were observed within the 14-month period of observation as
well.
Complete remission of the patient with
AD administered the hUC-MSCs
To assess the therapeutic effects of the hUC-MSC
administration, the clinical symptoms of the patient with AD were
initially evaluated. Generally, the size and crimson colour of the
diffuse erythema were collectively and gradually alleviated within
the first 10 days and eventually disappeared after 1 month, which
indicated the effectiveness of the infusion of MSCs upon
AD-associated eczema. Moreover, the unbearable itch and refractory
urinary tract infection were also alleviated from the patient with
AD. Notably, during the 14-month follow-up visits, no recrudescent
AD-associated pathophysiological events, including itch and
erythema, and in particular ulcers on the foot, were observed in
the body of the elderly patient (Fig.
1A). Additionally, with only a single hUC-MSC administration,
the patient with AD was satisfied with the treatment effects (with
the smooth skin) and felt well in body and mind as well, which
collectively revealed the improvement of the quality of life
(Fig. 1B).
Apart from the subjective and objective changes in
the general physical examination of the patient, multifaceted
assessments, including vital signs, and detailed clinical and
laboratory examinations were collectively conducted upon the
patient with AD. Firstly, from the routine urine test and the
microscopic examination of leucocytes (R-WBC), a marked decline in
the numbers of leucocytes was observed, and a decrease was also
observed the content of nitrite in the urine (Tables SI and SII). Secondly, according to blood cell
test results of the patient, both blood cell components (e.g.,
neutrophils, lymphocytes and granulocytes) and biochemical indexes
(e.g., bilirubin, alanine aminotransferase, cholesterol and
high-density lipoprotein cholesterol) were within the normal
ranges, without anomalous changes before treatment and after
complete recovery, while the contents of total cholesterol and
low-density lipoprotein cholesterol were slightly increased
(Tables SI and SII). In addition, following MSC
treatment, the dysregulated other blood parameters, such as Casson
viscosity, whole blood shear rate 50 and 100, and erythrocyte
sedimentation rate were also ameliorated (Tables SI and SII). Above all, as shown by the test
results, none of the well-known cancer-associated parameters with
abnormal elevations were observed in the patient with AD, including
carcinoembryonic antigen (CEA), alpha fetoprotein (AFP),
carbohydrate antigens (e.g., CA-125, CA-133, CA-199, CA-242 and
CA-724), neuron-specific enolase (NSE), cytokeratin 19 (CK-19) and
squamous cell carcinoma antigen (SCCA) (Tables SI and SII).
Discussion
State-of-the-art renewal has enlightened the
promising prospects of MSCs in the management of refractory and
relapsing disorders. This is mainly attribute to their unique
hematopoietic-supporting and immunoregulatory properties (21,25,26).
However, their potential application in elderly patients with
refractory allergic dermatitis with multiple complications are
largely unknown. The present study describes the case of a
65-year-old female patient with AD accompanied by an unbearable
itch, extensive eczema, severe foot ulcers, urinary tract
infection, and in particular, resistance to conventional drug
therapy. With the aid of only one single systemic hUC-MSC
injection, the major symptoms of AD were effectively relieved
within 10 days following the MSC administration. Finally, a
positive outcome without palindromia was observed with the patient
during the 14-month follow-up visit, including the significant
elimination of pathophysiological manifestations and persistent
improvement in the quality of life.
The pathogenesis of AD typically manifests in
epidermal barrier disruption, dysbiosis of the skin microbiota and
the overactivation of the helper T lymphocyte subpopulations (Th1,
Th2, Th17 and Th22), as well as increased eosinophils and IgE in
blood (3,27). However, the detailed underlying
mechanisms, as well as the absolute magnitude of the risks of AD
remain largely unknown (2,3,27).
For instance, the impact of gene-environment interactions upon
epidermal barrier destruction and the resultant variations in
clinical presentations have not yet been clearly demonstrated
(5). In addition, AD is considered
to be an initiating factor for other atopic disorders, including
food allergies, allergic asthma and rhinitis, which can continue
for a long period of time, maintaining a relapsing-remitting status
in affected patients (6). In
general, the systematic and precise clarification of AD-associated
epidemiologic features, clinical phenotypes, genetic subtype,
clinical remission and the underlying pathogenic mechanisms are not
yet fully understood (5,27). As a consequence, even though
current treatment modalities, including pharmacological-associated
interventions alone or in combination with non-pharmacological
strategies may be able to relieve pain in patients with moderate to
severe AD, their effectiveness is not satisfactory due to the
complexity and indeterminacy of the underlying pathogenesis. For
example, a recent advancement has been made in the monoclonal
antibody (mAb), dupilumab, which has exhibited satisfactory
effectiveness against AD with a remission rate of 85% via a
subcutaneous administration (11 times) by blocking IL-3 and
IL-13(28). However, in spite of
its effectiveness, the accompanied adverse reactions, inconvenience
and unexpected clinical outcomes (e.g., headache, injection-site
reaction and conjunctivitis), would largely hinder its use in the
rational and effective application in patients with AD (29,30).
Distinguishing from the traditional or late-model
drug-based strategies, MSC-based cytotherapy has been proven to be
safe and effective against multiple disorders associated with
immune abnormalities (31-34).
Since the first separation and identification in the 1960s, MSCs
have been successfully isolated from adult tissues, perinatal
tissues and even derived from human pluripotent stem cells
(35-37).
Of these, the natural bone marrow- and umbilical cord-segregated
MSCs are acknowledged with the most popular and proliferative
characteristics, respectively (21,23,24).
MSCs function mainly via cytokine paracrine mechanisms [e.g.,
prostaglandin E2 (PGE2) and transforming growth factor (TGF)-β1],
the inhibition of inflammatory factor production [e.g., interferon
(IFN)-γ and tumour necrosis factor (TNF)-α], the recruitment and
regulation of other cell constituents (e.g., mast cells, T
lymphocytes) and direct differentiation, together with providing a
favourable microenvironment (9,38-40).
As for the management of AD, by administering a high-dose of
hUCB-MSCs (5x107) via local subcutaneous injection, Kim
et al reported few adverse events and a certain improvement
of patients with AD with an overall response rate of 55% at week
12(40).
However, the systemic infusion and long-term effects
of MSCs against AD remain unknown. To the best of our knowledge,
the present study demonstrates for the first time that hUC-MSCs
were effective in the treatment of a patient with AD with other
complications, including foot ulcers and a urinary tract infection,
leading to a full, rather than partial remission, via a single and
convenient intravenous injection. In particular, the severe ulcers
and refractory urinary tract infection in the elderly patient with
AD were completely eliminated without recrudesce during the
14-month follow-up visit. Simultaneously, according to the
systematic clinical and biochemical examinations, and in
particular, the tumour-related indicators, the consistent safety
and continuous efficacy of MSC-based cytotherapy for AD was
confirmed. Nevertheless, more patients with AD need to be enrolled
and examined in order to evaluate the reliability and validity of
hUC-MSC infusion via intravenous injection. Overall, the data
presented herein provide a paradigm and prospect for further
investigations towards AD treatment, and may help to predominantly
ameliorate the refractory disorder in the future.
Supplementary Material
Parameters of clinical and laboratory
examinations (prior to treatment).
Parameters of clinical and laboratory
examinations (post-treatment).
Acknowledgements
The authors would like to thank Health-Biotech
(Tianjin) Stem Cell Research Institute Co., Ltd. for their kind
support.
Funding
The present study was supported by the National
Science and Technology Major Projects of China
(2014ZX09508002-003), the project funded by the China Postdoctoral
Science Foundation (2019M661033), the project Youth Fund supported
by Shandong Provincial Natural Science Foundation (ZR2020QC097),
the Key project funded by the Department of Science and Technology
of Shangrao City (2020XGFY05), the Key project funded by the
Department of Science and Technology of Shangrao City (2021, to
JYa), the Jiangxi Key New Product Incubation Program funded by the
Technical Innovation Guidance Program of Shangrao city (2021, to
LZ) and the Natural Science Foundation of Fujian Province
(2020J01649).
Availability of data and materials
The data used to support the findings of the present
study are included in the article. Additional data related to the
study are available from the corresponding author. In addition, a
trial registration has been made as follows: Clinical
Classification, Epidemiological Investigation and Mechanism of Skin
Barrier Dysfunction of Hand Eczema in Outpatient Clinics of
Hospitals in China, ChiCTR1800018943. Registered October 17, 2018
(http://www.chictr.org.cn/showproj.aspx?proj=31989;
prospectively registered); and ‘Human mesenchymal stem cell in the
treatment of wounds in partial-thickness skin donor site in burn
patients: A randomized controlled trial’, ChiCTR2000038275.
Registered September 15, 2020 (http://www.chictr.org.cn/showproj.aspx?proj=56515;
prospectively registered).
Authors' contributions
JYa was involved in the collection and assembly of
data and in manuscript writing. XH, JYe and SY were involved in the
collection and assembly of data. LZ and ZH were involved in the
conception and design of the study, and in manuscript writing and
revision. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The treatment of the patient followed the
internationally recognized guidelines and the principles of the
Declaration of Helsinki. Ethical approval for the research was
signed by the Ethics Committee of Jiangxi Health-Biotech
Development Co., Ltd., China (approval no. EC-2019-01). The patient
signed an informed consent to the publication of her case
report.
Patient consent for publication
The patient signed an informed consent to the
publication of her case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torres T, Ferreira EO, Gonçalo M,
Mendes-Bastos P, Selores M and Filipe P: Update on atopic
dermatitis. Acta Med Port. 32:606–613. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cabanillas B, Brehler AC and Novak N:
Atopic dermatitis phenotypes and the need for personalized
medicine. Curr Opin Allergy Clin Immunol. 17:309–315.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Suárez-Fariñas M, Dhingra N, Gittler J,
Shemer A, Cardinale I, de Guzman Strong C, Krueger JG and
Guttman-Yassky E: Intrinsic atopic dermatitis shows similar TH2 and
higher TH17 immune activation compared with extrinsic atopic
dermatitis. J Allergy Clin Immunol. 132:361–370. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Leung DY, Nicklas RA, Li JT, Bernstein IL,
Blessing-Moore J, Boguniewicz M, Chapman JA, Khan DA, Lang D, Lee
RE, et al: Disease management of atopic dermatitis: An updated
practice parameter. Joint Task Force on Practice Parameters. Ann
Allergy Asthma Immunol. 93 (3 Suppl 2):S1–S21. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kim HS, Lee JH, Roh KH, Jun HJ, Kang KS
and Kim TY: Clinical trial of human umbilical cord blood-derived
stem cells for the treatment of moderate-to-severe atopic
dermatitis: Phase I/IIa studies. Stem Cells. 35:248–255.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Schneider L, Tilles S, Lio P, Boguniewicz
M, Beck L, LeBovidge J, Novak N, Bernstein D, Blessing-Moore J,
Khan D, et al: Atopic dermatitis: A practice parameter update 2012.
J Allergy Clin Immunol. 131:295–299.e1-27. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Silverberg NB and Duran-McKinster C:
Special considerations for therapy of pediatric atopic dermatitis.
Dermatol Clin. 35:351–363. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Huo J, Zhang L, Ren X, Li C, Li X, Dong P,
Zheng X, Huang J, Shao Y, Ge M, et al: Multifaceted
characterization of the signatures and efficacy of mesenchymal
stem/stromal cells in acquired aplastic anemia. Stem Cell Res Ther.
11(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shi Y and Wang Y, Li Q, Liu K, Hou J, Shao
C and Wang Y: Immunoregulatory mechanisms of mesenchymal stem and
stromal cells in inflammatory diseases. Nat Rev Nephrol.
14:493–507. 2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Menasche P: Cell therapy trials for heart
regeneration-lessons learned and future directions. Nat Rev
Cardiol. 15:659–671. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matas J, Orrego M, Amenabar D, Infante C,
Tapia-Limonchi R, Cadiz MI, Alcayaga-Miranda F, González PL, Muse
E, Khoury M, et al: Umbilical cord-derived mesenchymal stromal
cells (MSCs) for knee osteoarthritis: Repeated MSC dosing is
superior to a single MSC dose and to hyaluronic acid in a
controlled randomized phase I/II trial. Stem Cells Transl Med.
8:215–224. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hidalgo Perea S, Lyons LP, Nishimuta JF,
Weinberg JB and McNulty AL: Evaluation of culture conditions for in
vitro meniscus repair model systems using bone marrow-derived
mesenchymal stem cells. Connect Tissue Res. 61:322–337.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Reza-Zaldivar EE, Hernández-Sapiéns MA,
Minjarez B, Gutiérrez-Mercado YK, Márquez-Aguirre AL and
Canales-Aguirre AA: Potential effects of MSC-derived exosomes in
neuroplasticity in Alzheimer's disease. Front Cell Neurosci.
12(317)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wu GH, Shi HJ, Che MT, Huang MY, Wei QS,
Feng B, Ma YH, Wang LJ, Jiang B, Wang YQ, et al: Recovery of
paralyzed limb motor function in canine with complete spinal cord
injury following implantation of MSC-derived neural network tissue.
Biomaterials. 181:15–34. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Huang B, Cheng X, Wang H, Huang W, la Ga
Hu Z, Wang D, Zhang K, Zhang H, Xue Z, Da Y, et al: Mesenchymal
stem cells and their secreted molecules predominantly ameliorate
fulminant hepatic failure and chronic liver fibrosis in mice
respectively. J Transl Med. 14(45)2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lin BL, Chen JF, Qiu WH, Wang KW, Xie DY,
Chen XY, Liu QL, Peng L, Li JG, Mei YY, et al: Allogeneic bone
marrow-derived mesenchymal stromal cells for hepatitis B
virus-related acute-on-chronic liver failure: A randomized
controlled trial. Hepatology. 66:209–219. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wang L, Liu T, Liang R, Wang G, Liu Y, Zou
J, Liu N, Zhang B, Liu Y, Ding X, et al: Mesenchymal stem cells
ameliorate beta cell dysfunction of human type 2 diabetic islets by
reversing beta cell dedifferentiation. EBioMedicine.
51(102615)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li B, Luan S, Chen J, Zhou Y, Wang T, Li
Z, Fu Y, Zhai A and Bi C: The MSC-derived exosomal lncRNA H19
promotes wound healing in diabetic foot ulcers by upregulating PTEN
via MicroRNA-152-3p. Mol Ther Nucleic Acidrs. 19:814–826.
2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ding L, Yan G, Wang B, Xu L, Gu Y, Ru T,
Cui X, Lei L, Liu J, Sheng X, et al: Transplantation of UC-MSCs on
collagen scaffold activates follicles in dormant ovaries of POF
patients with long history of infertility. Sci China Life Sci.
61:1554–1565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu F, Hu S, Yang H, Li Z, Huang K, Su T,
Wang S and Cheng K: Hyaluronic acid hydrogel integrated with
mesenchymal stem cell-secretome to treat endometrial injury in a
rat model of Asherman's syndrome. Adv Healthc Mater.
8(e1900411)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wei Y, Zhang L, Chi Y, Ren X, Gao Y, Song
B, Li C and Han Z, Zhang L and Han Z: High-efficient generation of
VCAM-1(+) mesenchymal stem cells with multidimensional
superiorities in signatures and efficacy on aplastic anaemia mice.
Cell Prolif: e12862, 2020.
|
|
22
|
Song K, Li W and Li M: Acute promyelocytic
leukemia following autologous bone marrow-derived mesenchymal stem
cell transplantation for traumatic brain injury: A case report.
Oncol Lett. 10:2905–2908. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao Q, Zhang L, Wei Y, Yu H, Zou L, Huo
J, Yang H, Song B, Wei T, Wu D, et al: Systematic comparison of
hUC-MSCs at various passages reveals the variations of signatures
and therapeutic effect on acute graft-versus-host disease. Stem
Cell Res Ther. 10(354)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hou H, Zhang L, Duan L, Liu Y, Han Z, Li Z
and Cao X: Spatio-temporal metabolokinetics and efficacy of human
placenta-derived mesenchymal stem/stromal cells on mice with
refractory Crohn's-like enterocutaneous fistula. Stem Cell Rev Rep.
16:1292–1304. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Golchin A, Seyedjafari E and
Ardeshirylajimi A: Mesenchymal Stem Cell Therapy for COVID-19:
Present or Future. Stem Cell Rev Rep. 16:427–433. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kabat M, Bobkov I, Kumar S and Grumet M:
Trends in mesenchymal stem cell clinical trials 2004-2018: Is
efficacy optimal in a narrow dose. range? Stem Cells Transl Med.
9:17–27. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Guttman-Yassky E, Nograles KE and Krueger
JG: Contrasting pathogenesis of atopic dermatitis and
psoriasis-part II: Immune cell subsets and therapeutic concepts. J
Allergy Clin Immunol. 127:1420–1432. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Beck LA, Thaçi D, Hamilton JD, Graham NM,
Bieber T, Rocklin R, Ming JE, Ren H, Kao R, Simpson E, et al:
Dupilumab treatment in adults with moderate-to-severe atopic
dermatitis. N Engl J Med. 371:130–139. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Ou Z, Chen C, Chen A, Yang Y and Zhou W:
Adverse events of Dupilumab in adults with moderate-to-severe
atopic dermatitis: A meta-analysis. Int Immunopharmacol.
54:303–310. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Blauvelt A, de Bruin-Weller M, Gooderham
M, Cather JC, Weisman J, Pariser D, Simpson EL, Papp KA, Hong HC,
Rubel D, et al: Long-term management of moderate-to-severe atopic
dermatitis with dupilumab and concomitant topical corticosteroids
(LIBERTY AD CHRONOS): A 1-year, randomised, double-blinded,
placebo-controlled, phase 3 trial. Lancet. 389:2287–2303.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cho J, D'Antuono M, Glicksman M, Wang J
and Jonklaas J: A review of clinical trials: Mesenchymal stem cell
transplant therapy in type 1 and type 2 diabetes mellitus. Am J
Stem Cells. 7:82–93. 2018.PubMed/NCBI
|
|
32
|
Gonzaga VF, Wenceslau CV, Lisboa GS, Frare
EO and Kerkis I: Mesenchymal stem cell benefits observed in bone
marrow failure and acquired aplastic anemia. Stem Cells Int.
2017(8076529)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dalal J, Gandy K and Domen J: Role of
mesenchymal stem cell therapy in Crohn's disease. Pediatr Res.
71:445–451. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pourgholaminejad A, Aghdami N, Baharvand H
and Moazzeni SM: The effect of pro-inflammatory cytokines on
immunophenotype, differentiation capacity and immunomodulatory
functions of human mesenchymal stem cells. Cytokine. 85:51–60.
2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wei Y, Hou H, Zhang L, Zhao N, Li C, Huo
J, Liu Y, Zhang W, Li Z, Liu D, et al: JNKi- and DAC-programmed
mesenchymal stem/stromal cells from hESCs facilitate hematopoiesis
and alleviate hind limb ischemia. Stem Cell Res Ther.
10(186)2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yao J, Chen N, Wang X, Zhang L, Huo J, Chi
Y, Li Z and Han Z: Human supernumerary teeth-derived apical
papillary Stem Cells possess preferable characteristics and
efficacy on hepatic fibrosis in mice. Stem Cells Int.
2020(6489396)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Du W, Li X, Chi Y, Ma F, Li Z, Yang S,
Song B, Cui J, Ma T, Li J, et al: VCAM-1+ placenta
chorionic villi-derived mesenchymal stem cells display potent
pro-angiogenic activity. Stem Cell Res Ther. 7(49)2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan XL, Zhang Y, Li X and Fu QL:
Mechanisms underlying the protective effects of mesenchymal stem
cell-based therapy. Cell Mol Life Sci. 77:2771–2794.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kfoury Y and Scadden DT: Mesenchymal cell
contributions to the stem cell niche. Cell Stem Cell. 16:239–253.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kim HS, Yun JW, Shin TH, Lee SH, Lee BC,
Yu KR, Seo Y, Lee S, Kang TW, Choi SW, et al: Human umbilical cord
blood mesenchymal stem cell-derived PGE2 and TGF-β1 alleviate
atopic dermatitis by reducing mast cell degranulation. Stem Cells.
33:1254–1266. 2015.PubMed/NCBI View Article : Google Scholar
|