Introduction
Blood culture is essential for the microbiological
diagnosis of bacteremia, infective endocarditis, fever of unknown
origin and other infectious diseases (1). The results of blood cultures are
directly linked to appropriate treatment; therefore, the
corresponding tests need to be highly accurate (2). The calculation and monitoring of the
positive blood culture rate, the number of blood culture sets per
1,000 patient days, the multiple set collection rate and the
contamination rate (hereinafter referred to as ‘the four
indicators’) have been proposed as indicators for the assessment of
the suitability of blood culture (3-5).
However, it is difficult to set international unified standard
values as medical conditions differ between countries.
Japanese reference values must be calculated to
assess the state of blood culture in a general hospital. Relevant
Japanese studies include a field survey of blood culture in six
Japanese healthcare facilities from 2007 to 2010(6), a limited assessment of indicators in
five healthcare facilities in 2014(7), and limited studies by the All-Japan
Hospital Association (8) and the
Japan Hospital Association (9).
Moreover, the Japan Surveillance for Infection Prevention and
Healthcare Epidemiology (J-SIPHE), a joint infection control
platform led by the Antimicrobial Resistance (AMR) Clinical
Reference Center within the National Center for Global Health and
Medicine, tabulated basic data that served as indicators (10). However, this report was not a
stratified analysis that accounted for hospital size and therefore
did not specifically demonstrate the four indicators according to
hospital size.
A valid assessment of the suitability of blood
culture in healthcare facilities requires clear domestic reference
values by hospital size based on data from a multicenter study
(6). Additionally, the positive
blood culture rate differs based on blood samples drawn following
antibiotic administration and on blood culture test systems
(11). Furthermore, the antiseptic
used when collecting blood for blood culture affects the culture
contamination rates (12).
Identifying the factors associated with these blood culture
indicators may lead to more suitable blood collection
protocols.
In the present study, in order to help improve the
suitability of blood culture throughout Japan, information related
to blood culture was obtained from >200 hospitals to investigate
domestic reference values by hospital size. The findings of the
present study may lay the foundation for the establishment of a
standard protocol for the appropriate collection of blood in Japan
and similar regions.
Patients and methods
Methods
The present study was a multicenter, retrospective,
descriptive, epidemiological statistical study conducted from
January, 2017 to December, 2018. Beginning with 3,129 hospitals
throughout Japan with ≥200 beds as of 2018, hospitals were sampled
from a corporate database (Landscape Co., Ltd.). Hospitals which
are ranked among those with the highest number of beds in each
prefecture (top 10%; 1,000 hospitals in total, nationwide) were
sampled and their cooperation with the study was requested. The
hospitals provided information related to blood culture, excluding
any personal information of patients.
Outcome measures
Outcome measures were based on a previous study
(6). As the basic characteristics
reflecting the attributes of healthcare facilities, the hospital
size (number of beds), the total number of hospitalized patients by
year and additional reimbursement obtained for infection prevention
(hereafter termed ‘additional reimbursement’) paid by the Ministry
of Health, Labor and Welfare of Japan when certain infection
control standards are met (in other words, infection control is
guaranteed to a certain standard) were surveyed. The total number
of hospitalized patients (patient days) and the number of patients
hospitalized every day at midnight were defined as their respective
totals over a year.
The following data related to blood culture were
collected in order to calculate the four indicators: The total
number of sets, number of solitary sets, number of solitary sets in
pediatric settings, number of positive sets, number of positive
samples in only one set, the blood culture test system manufacturer
and the antiseptics used for blood collection.
Positive blood culture rate
A positive blood culture rate indicates the
suitability of blood collection timing for culture. A positive case
was defined as a positive blood culture result in a solitary set,
regardless of the bacterial strain detected. However, in the case
that the same strain was detected in the same patient more than
once within a period of 30 days (with the date of the first
positive result defined as day 0), all detections from the second
detection were considered invalid, and the case was defined as a
single positive case. In the case that multiple strains of bacteria
were detected in the same sample, each strain detected was defined
as an independent case. The positive blood culture rate was
calculated as follows: Positive blood culture rate = number of
positive sets/total number of collected sets x100.
Number of sets per 1,000 patient
days
The number of sets per 1,000 patient days indicates
whether blood culture sets were collected without failure from
patients who required blood culture. This number was divided by the
total number of hospitalized patients to minimize bias in set
collection rates associated with the differences among hospitals in
the number of hospitalized patients. This was calculated as
follows: Number of sets per 1,000 patient days = total number of
collected sets/total number of hospitalized patients x1,000.
Multiple set collection rate
The multiple set collection rate is an indicator of
accuracy management that contributes to the accurate diagnosis of
bloodstream infections. A solitary blood culture set was defined as
the sample obtained from a single venipuncture. The submission of
multiple blood culture sets was defined as samples from multiple
sets dispensed from multiple punctures on the same date; if the
date was the same, the duration between set collections was
irrelevant. Based on this definition, solitary set submission was
defined as the collection of only one blood culture set on a given
date (5). The multiple set
collection rate was calculated as follows: Multiple set collection
rate = (total number of collected sets - number of solitary
sets)/total number of collected sets x100.
Contamination rate
The contamination rate indicates the education level
of hospital staff members collecting blood and the condition of the
blood collection environment. The contamination rate was defined as
the total number of cases in which only one blood culture set of ≥2
sets submitted on the same day tested positive for
coagulase-negative staphylococci, Propionibacterium acnes,
Micrococcus spp., Viridans streptococci,
Corynebacterium spp. and Bacillus spp. (13). The contamination rate was
calculated as follows: Contamination rate = number of positive
samples in only one set/(total number of collected sets - number of
solitary sets) x100.
Statistical analysis
A descriptive epidemiological analysis was conducted
for all values. The freely available EZR v1.50 software (Jichi
Medical University, Saitama, Japan) was used for statistical
analyses (14). To determine
whether the means obtained from the samples were equal to the means
of the total hospitalized population, a one-sample t-test was used
and the 95% confidence interval (95% CI) values were
calculated.
Ethical considerations
The study protocol was approved by the Institutional
Review Board of Saiseikai Yokohamashi Tobu Hospital (2018107). The
present study: i) was an observational study that used only
existing information and is not categorized under specified
clinical research; ii) used existing specimen information; iii) was
conducted solely by Saiseikai Yokohamashi Tobu Hospital; iv) did
not involve any intervention; v) did not use human samples; vi)
used anonymized sample information; vii) did not use medical
history or other personal information requiring special care; viii)
received sample information from other hospitals; ix) may have
immense social significance; and x) simplified explanations and
consent. Informed consent was not required due to the retrospective
nature of the study.
Results
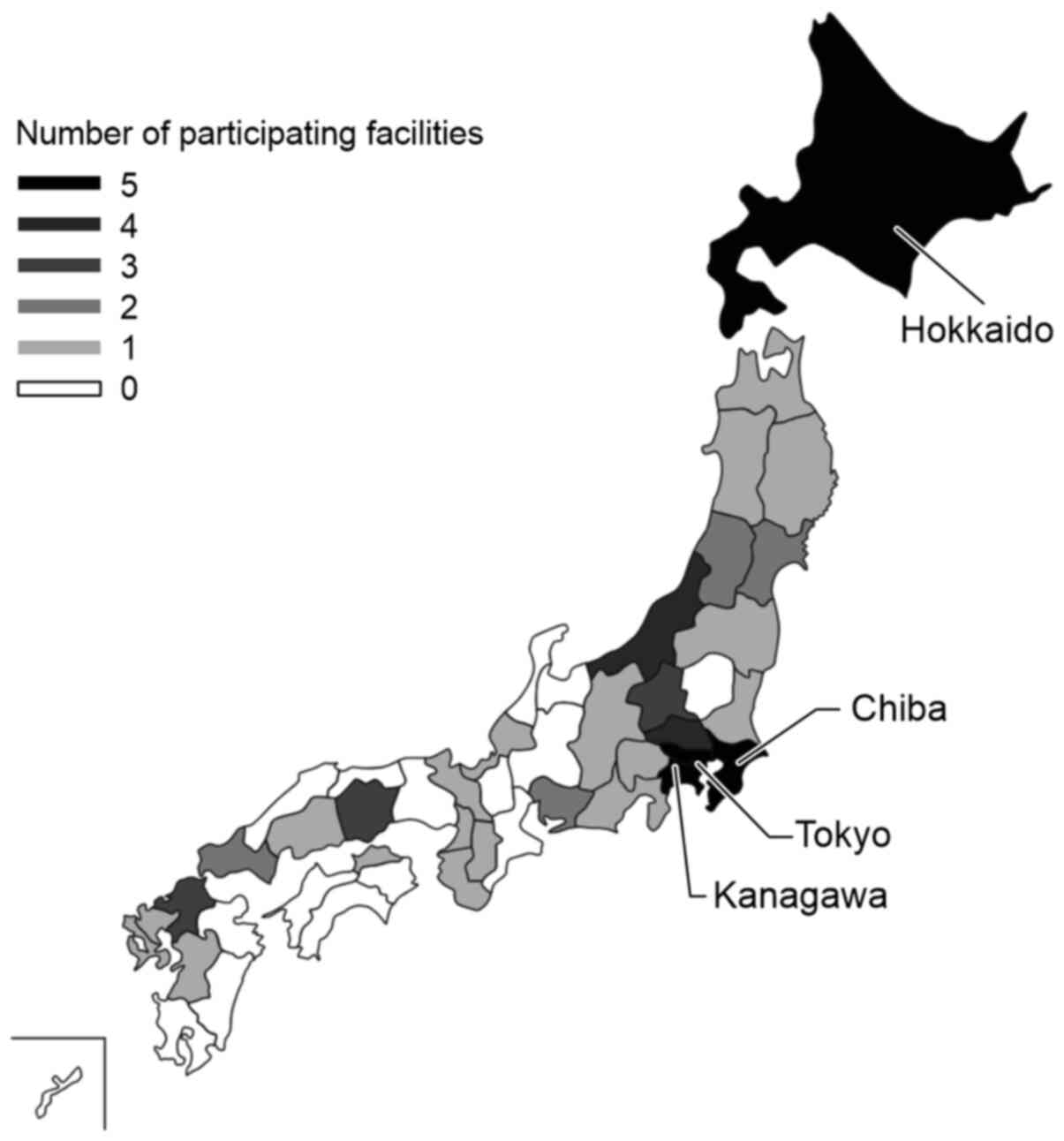
The geographical status of the participating
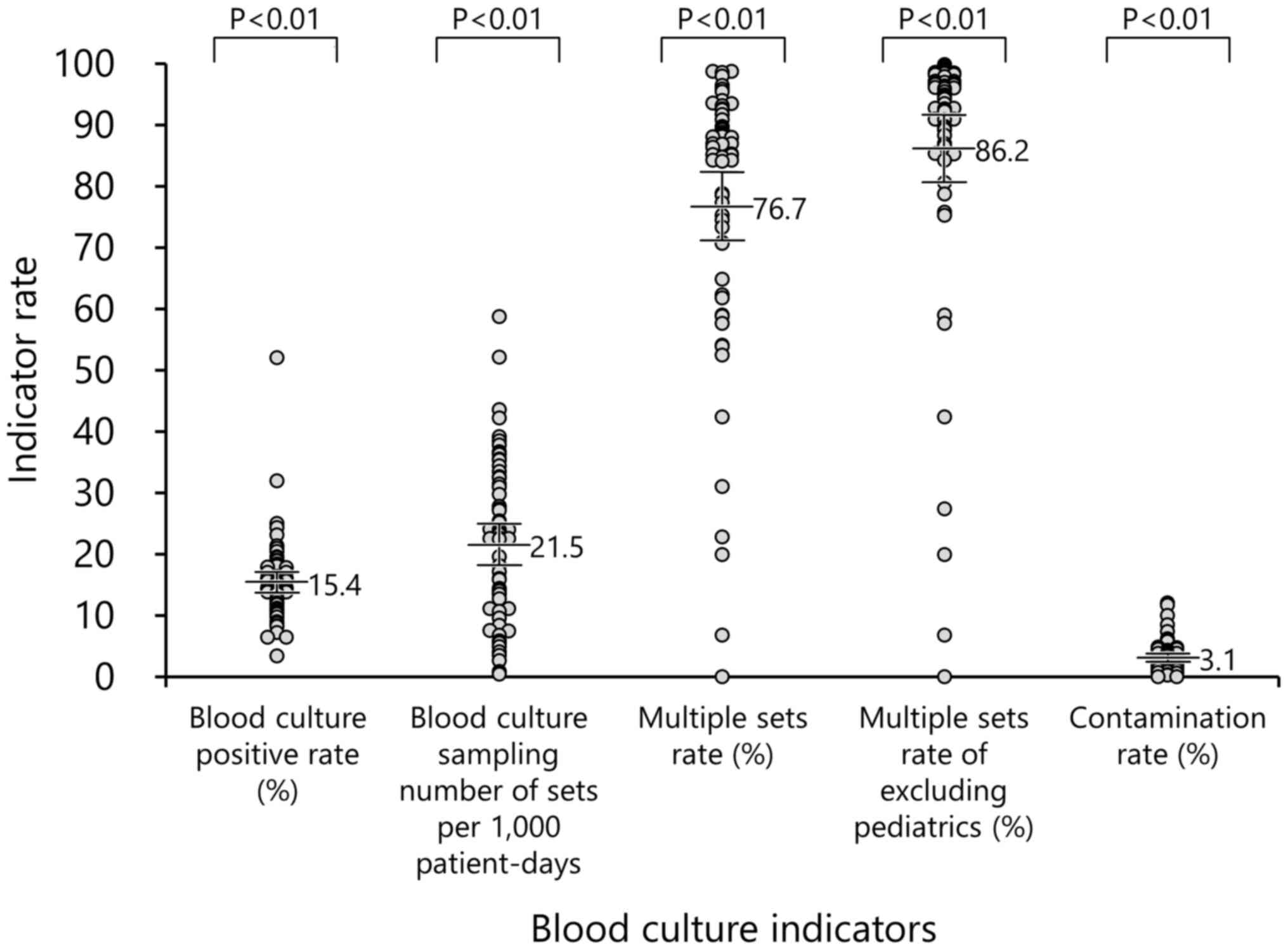
hospitals by prefecture is illustrated in Fig. 1. The 2-year total data for the four
indicators are presented in Fig.
2. In addition, the data stratified by year and the number of
beds are presented in Fig. 3. The
number of sets per 1,000 patient days was affected by the total
number of hospitalized inpatients and was therefore stratified by
the total number of hospitalized patients rather than by the number
of beds.
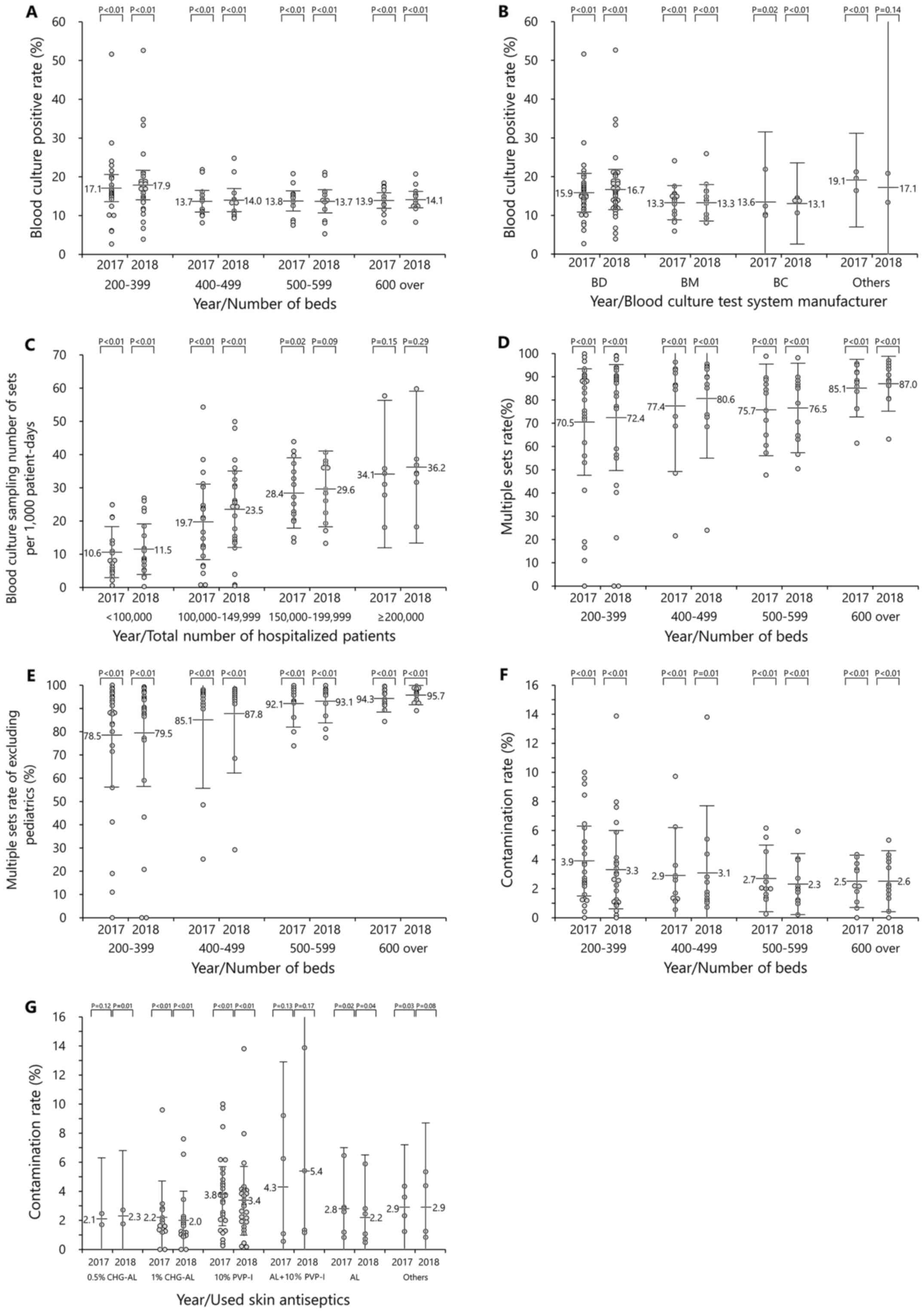 | Figure 3Blood culture-associated rates. (A)
Positive blood culture rate per year, per number of beds. Gray
circles represent medical facilities. (B) Positive blood culture
per year, per blood culture test manufacturer. BD, BD Biosciences;
BM, bioMérieux; BC, Beckman Coulter, Inc. (C) Blood culture
sampling number per 1,000 patient days. (D) Multiple set collection
rate per year, per number of beds. (E) Multiple set collection rate
(excluding pediatric settings) per year, per number of beds. (F)
Contamination rate per year, per number of beds. (G) Contamination
rate per skin antiseptic. CHG-AL, chlorhexidine gluconate alcohol;
PVP-I, povidone-iodine; AL+10% PVP-I, 70% alcohol, and 10%
povidone-iodine use. The results of each index will serve as a
reference for blood cultures in medical facilities with >200
beds in Japan. The authors hope that on-site interventions and
awareness-raising activities will be used in order to improve the
blood culture status of each facility. The gray dots represent the
values for each facility. The long bar represents the average of
the indicator rate. The error bar indicates the 95% confidence
intervals. |
Status of participating
facilities
Out of 3,129 facilities with at least 200 beds, 63
hospitals gave their consent to participate in the study. A total
of 31 of the 47 prefectures of Japan were analyzed. The prefectures
with the highest number of participating facilities were Hokkaido,
Chiba, Tokyo and Kanagawa, with five facilities each (Fig. 1).
Basic characteristics of the
participating facilities
The basic characteristics of the participating
facilities are presented in Table
I. The majority of facilities had obtained additional
reimbursement for the prevention of infection, which was granted by
the Ministry of Health, Labour and Welfare when a hospital clears a
certain standard related to the control of nosocomial infections.
The total number of hospitalized patients was over eight million.
The most common hospital size was 200-399 beds, whereas the number
of hospitals with 400-499, 500-599 and ≥600 beds was identical. The
smallest number of beds in any hospital was 200, whereas the
largest number was 1,435.
 | Table IStatus of participating hospitals by
prefecture. |
Table I
Status of participating hospitals by
prefecture.
| | Year |
|---|
| Characteristic | 2017 | 2018 |
|---|
| Number of additional
reimbursements for infection prevention, no. (%) | | |
|
First | 58 (92.1) | 58 (92.1) |
|
Second | 5 (7.9) | 4 (6.3) |
|
Unacquired | 0 (0.0) | 1 (1.6) |
| Total no. of
hospitalized patients | | |
|
Number | 87,13,567 | 86,81,663 |
|
Average
(range) | 1,38,310.6
(61,304-464,314) | 1,37,804.1
(57,533-472,534) |
| No. of beds, no.
(%) | | |
|
200-399 | 27 (42.9) | 27 (42.9) |
|
400-499 | 12 (19.0) | 12 (19.0) |
|
500-599 | 12 (19.0) | 12 (19.0) |
|
>600 | 12 (19.0) | 12 (19.0) |
| Blood culture system
manufacturer, no. (%) | | |
|
BD
Biosciences | 39 (61.9) | 41 (65.1) |
|
bioMérieux | 17 (27.0) | 17 (27.0) |
|
Beckman
Coulter, Inc. | 4 (6.3) | 3 (4.8) |
|
Others | 3 (4.8) | 2 (3.2) |
| Recommended skin
antiseptics | | |
|
0.5% CHG-AL,
no. (%) | 2 (3.2) | 2 (3.2) |
|
1%
CHG-AL | 15 (23.8) | 18 (28.6) |
|
10%
PVP-I | 31 (49.2) | 28 (44.4) |
|
AL + 10%
PVP-I | 4 (6.3) | 4 (6.3) |
|
AL | 7 (11.1) | 7 (11.1) |
|
Others | 4 (6.3) | 4 (6.3) |
The most commonly used blood culture test system was
from BD Biosciences, followed by bioMérieux and Beckman Coulter,
Inc. The antiseptic most commonly used prior to blood collection
was 10% povidone-iodine (PVP-I), followed by 1% chlorhexidine
gluconate/alcohol (CHG-AL) and alcohol (AL).
Positive blood culture rate
The positive blood culture rate (95% CI) during the
study period was 15.4% (13.7-17.1%, P<0.05) (Table II). The hospital size with the
largest mean positive blood culture rate was 200-399 beds, with no
marked differences among other hospital sizes (Fig. 3A). According to the blood culture
test system manufacturer, the mean positive blood culture rate
tended to be higher with BD Biosciences than with the other two
manufacturers (Fig. 3B).
 | Table IIBasic data of participating
hospitals. |
Table II
Basic data of participating
hospitals.
| | 2017 | 2018 | Total |
|---|
| Characteristic | Value | 95% CI | P-value | Value | 95% CI | P-value | Value | 95% CI | P-value |
|---|
| Blood culture
positive rate % | 15.2 | 13.5-16.9 | <0.01 | 15.6 | 13.8-17.5 | <0.01 | 15.4 | 13.7-17.1 | <0.01 |
| No. of beds | | | | | | | | | |
|
200-399 | 17.1 | 13.5-20.6 | <0.01 | 17.9 | 14.0-21.7 | <0.01 | 17.3 | 13.8-20.9 | <0.01 |
|
400-499 | 13.7 | 10.9-16.5 | <0.01 | 14.0 | 11.1-17.0 | <0.01 | 13.9 | 11.1-16.6 | <0.01 |
|
500-599 | 13.8 | 11.3-16.4 | <0.01 | 13.7 | 10.7-16.7 | <0.01 | 13.7 | 11.0-16.5 | <0.01 |
|
>600 | 13.9 | 11.8-15.9 | <0.01 | 14.1 | 12.0-16.2 | <0.01 | 14.0 | 12.0-16.0 | <0.01 |
| Blood culture
system manufacturer | | | | | | | | | |
|
BD
Biosciences | 15.9 | 13.4-18.4 | <0.01 | 16.7 | 14.1-19.3 | <0.01 | 16.2 | 13.7-18.7 | <0.01 |
|
bioMérieux | 13.3 | 11.1-15.5 | <0.01 | 13.3 | 10.9-15.6 | <0.01 | 13.3 | 11.0-15.5 | <0.01 |
|
Beckman
Coulter, Inc. | 13.6 | 4.6-22.6 | 0.02 | 13.1 | 7.9-18.4 | <0.01 | 13.0 | 7.1-18.9 | <0.01 |
|
Others | 19.1 | 13.0-25.1 | <0.01 | 17.1 | -30.0-64.1 | 0.14 | 19.6 | 14.5-24.7 | <0.01 |
| Blood culture
sampling number of sets per 1,000 patient days | 20.8 | 17.4-24.1 | <0.01 | 22.3 | 18.9-25.8 | <0.01 | 21.5 | 18.2-24.9 | <0.01 |
| Total number of
hospitalized patients | | | | | | | | | |
|
<100,000 | 10.6 | 6.7-14.4 | <0.01 | 11.5 | 7.7-15.3 | <0.01 | 11.3 | 7.3-15.2 | <0.01 |
|
100,000-149,999 | 19.7 | 14.0-25.4 | <0.01 | 23.5 | 17.7-29.2 | <0.01 | 20.3 | 14.6-25.9 | <0.01 |
|
150,000-199,999 | 28.4 | 23.1-33.7 | 0.02 | 29.6 | 23.9-35.3 | 0.09 | 29.5 | 24.2-34.8 | <0.01 |
|
≥200,000 | 34.1 | 23.0-45.2 | 0.15 | 36.2 | 24.8-47.7 | 0.29 | 35.2 | 24.0-46.4 | <0.01 |
| Multiple sets rate
% | 75.6 | 69.8-81.4 | <0.01 | 77.5 | 71.9-83.2 | <0.01 | 76.7 | 71.1-82.3 | <0.01 |
|
No. of
beds | | | | | | | | | |
|
200-399 | 70.5 | 59.1-82.0 | <0.01 | 72.4 | 61.0-83.8 | <0.01 | 71.7 | 60.5-83.0 | <0.01 |
|
400-499 | 77.4 | 63.3-91.5 | <0.01 | 80.6 | 67.8-93.4 | <0.01 | 79.1 | 65.8-92.4 | <0.01 |
|
500-599 | 75.7 | 65.8-85.5 | <0.01 | 76.5 | 66.9-86.1 | <0.01 | 76.1 | 66.5-85.7 | <0.01 |
|
>600 | 85.1 | 78.9-91.3 | <0.01 | 87.0 | 81.1-92.9 | <0.01 | 86.1 | 80.1-92.1 | <0.01 |
| Multiple sets rate
of excluding pediatrics % | 85.3 | 79.8-90.9 | <0.01 | 86.8 | 81.2-92.3 | <0.01 | 86.2 | 80.6-91.7 | <0.01 |
|
Number of
beds | | | | | | | | | |
|
200-399 | 78.5 | 67.3-89.7 | <0.01 | 79.5 | 68.0-91.1 | <0.01 | 79.2 | 67.9-90.5 | <0.01 |
|
400-499 | 85.1 | 70.3-99.8 | <0.01 | 87.8 | 75.0-100.6 | <0.01 | 86.5 | 72.9-100.1 | <0.01 |
|
500-599 | 92.1 | 87.0-97.2 | <0.01 | 93.1 | 88.4-97.7 | <0.01 | 92.6 | 87.7-97.5 | <0.01 |
|
>600 | 94.3 | 91.4-97.2 | <0.01 | 95.7 | 93.7-97.8 | <0.01 | 95.0 | 92.6-97.5 | <0.01 |
| Contamination rate
% | 3.2 | 2.6-3.8 | <0.01 | 2.9 | 2.2-3.6 | <0.01 | 3.1 | 2.4-3.8 | <0.01 |
|
200-399 | 3.9 | 2.7-5.1 | <0.01 | 3.3 | 1.9-4.6 | <0.01 | 3.7 | 2.4-5.0 | <0.01 |
|
400-499 | 2.9 | 1.3-4.6 | <0.01 | 3.1 | 0.8-5.4 | <0.01 | 3.0 | 1.0-5.0 | <0.01 |
|
500-599 | 2.7 | 1.5-3.8 | <0.01 | 2.3 | 1.3-3.4 | <0.01 | 2.5 | 1.4-3.6 | <0.01 |
|
>600 | 2.5 | 1.6-3.4 | <0.01 | 2.5 | 1.5-3.6 | <0.01 | 2.6 | 1.6-3.5 | <0.01 |
| Skin antiseptics
used | | | | | | | | | |
|
0.5%
CHG-AL | 2.1 | -3.0-7.2 | 0.12 | 2.3 | -3.5-8.0 | 0.01 | 2.2 | -3.6-7.9 | 0.13 |
|
1%
CHG-AL | 2.2 | 0.9-3.4 | <0.01 | 2.0 | 1.0-3.0 | <0.01 | 2.2 | 1.0-3.4 | <0.01 |
|
10%
PVP-I | 3.8 | 2.8-4.7 | <0.01 | 3.4 | 2.2-4.5 | <0.01 | 3.8 | 2.7-4.8 | <0.01 |
|
AL + 10%
PVP-I | 4.3 | -2.3-10.9 | 0.13 | 5.4 | -4.1-15.0 | 0.17 | 4.9 | -3.2-12.9 | 0.15 |
|
AL | 2.8 | 0.7-4.9 | 0.02 | 2.2 | 0.1-4.4 | 0.04 | 2.5 | 0.4-4.5 | 0.03 |
|
Others | 2.9 | 0.7-5.0 | 0.03 | 2.9 | -0.7-6.5 | 0.08 | 2.9 | 0.0-5.8 | 0.05 |
Sets per 1,000 patient days
The number of sets per 1,000 patient days (95% CI)
during the study period was 21.5 (18.2-24.9, P<0.05) (Table II). The mean number of sets among
the total number of hospitalized patients increased proportionally
with the total number of hospitalized patients (Fig. 3C).
Multiple set collection rate
The multiple set collection rate (95% CI) during the
study period was 76.7% (71.1-82.3%, P<0.05) (Table II). The hospital sizes with the
lowest and highest rates were 200-399 beds and ≥600 beds,
respectively (Fig. 3D). The
multiple set collection rate, excluding pediatric settings (95% CI)
was 86.2% (80.6-91.7%, P<0.05) (Table II). In terms of hospital size, the
multiple set collection rate increased proportionally with the
number of beds (Fig. 3E).
Contamination rate
The contamination rate (95% CI) during the study
period was 3.1% (2.4-3.8%, P<0.05) (Table II). In terms of the hospital size,
the mean contamination rate decreased as the number of beds
increased (Fig. 3F). With the use
of antiseptics prior to blood collection, the mean contamination
rate was high with PVP-I and low with CHG-AL (Fig. 3G).
Discussion
In 63 Japanese hospitals, it was found that the
positive blood culture rate, number of sets per 1,000 patient days,
multiple set collection rate and contamination rate (95% CI) were
15.4% (13.7-17.1%), 21.5 (18.2-24.9), 76.7% (71.1-82.3%) and 3.1%
(2.4-3.8%), respectively.
Although a positive blood culture rate of 5-15% is
considered appropriate (3), the
rate in the present study exceeded 15%. In 2019, the J-SIPHE found
a positive blood culture rate (n=276) of 13.3%, which was lower
than that in the present study (10), suggesting that the positive rate
exceeded 15% in the present study for specific reasons. This was
affected by the fact that there were two facilities which were
unique compared with the others (top 2 grey dots indicated in the
blood culture positive rate illustrated in Fig. 2), with positive blood culture rates
of 52.0 and 31.9% (with the total number of collected sets of 148
and 1,129, respectively). This was likely due to the fact that the
total number of collected sets of blood cultures in these
facilities was small. Additionally, of the eight facilities with a
positive blood culture rate of >20%, three also had a
contamination rate of >10%, suggesting that frequent
contamination may have increased positive rates. Furthermore, 29 of
the 63 facilities had a positive blood culture rate of over 15%,
indicating that many facilities excessively limited the number of
patients for whom blood cultures were performed.
As for positive blood culture rates by blood culture
test system manufacturer (a secondary endpoint), only BD
Biosciences (16.2%) was associated with a rate >15%. This high
positive rate occurred as the two facilities with a blood culture
positive rate >30% (top 2 grey dots indicated in the blood
culture positive rate illustrated in Fig. 2.) used BD Biosciences blood culture
test systems (when these two facilities were excluded, the positive
rate with BD Biosciences decreased to 14.8%). However, even when
these two facilities were excluded, the positive blood culture rate
with BD Biosciences test systems remained higher than the rates for
test systems of other manufacturers. In a Chinese study that
compared positive blood culture rates among blood culture systems
manufactured by BD Biosciences, bioMérieux and Beckman Coulter,
Inc., antibiotic treatment prior to blood collection resulted in a
higher positive rate with the BD Biosciences system than with the
other two systems (15). The
present study may also have included cases in which antibiotic
agents were administered prior to blood collection, indicating that
the differences in positive rates among systems may have included
cases in which antibiotics were administered prior to blood
collection.
American guidelines recommend 103-188 sets per 1,000
patient days (3). Here, the mean
number (21.5) was considerably lower than the recommended range.
However, in Europe, the number of sets per 1,000 patient days
varies widely by country, from 5.3 to 206.3(16). Moreover, the number of sets per
1,000 patient days is affected by the mean length of hospital
duration, thereby precluding simple comparisons. In the 2019
J-SIPHE survey of 255 healthcare facilities, the median number of
sets per 1,000 patient days was 23.8(10). However, the number of sets per
1,000 patient days in the present study was lower than the
reference value. Infectious endocarditis and other severe
infections require blood culture tests to assess the progress of
treatment, which necessarily leads to a large number of collections
(17). Patients with severe
infections are often treated in hospitals with a higher number of
beds (total number of hospitalized patients), such as core
hospitals and university hospitals. Herein, the number of sets
increased proportionally with the total number of hospitalized
patients. In fact, a number of the facilities exhibited a low total
number of hospitalized patients, which may have reduced the overall
number of sets per 1,000 patient days.
In a fiscal 2019 report by the All-Japan Hospital
Association, the rate of blood culture implementation upon the
administration of broad-spectrum antibiotics in 27 facilities was
26.5% (8). In the Japan Hospital
Association's Quality Indicator Project 2018, the mean blood
culture implementation rate across 355 facilities was 34.5%
(9), suggesting a disconcerting
situation in which Japanese healthcare facilities do not
sufficiently implement blood culture, despite the fundamental need
to do so. In Europe, the surveillance of blood culture indicators
has been conducted in all countries (16). In Japan, it is hoped that J-SIPHE
will construct a nationwide surveillance system, conduct analyses
by hospital size and enact other improvements to assess the quality
of blood culture.
In general, when blood is collected, multiple sets
should be collected (18). The
multiple set collection rate indicates the suitability of blood
collection and should therefore be as close to 100% as possible.
However, in the diagnosis of infective endocarditis and the
assessment of treatment, solitary sets are sometimes collected for
several consecutive days; thus, cases of 100% multiple set
collection are rare in clinical practice. In the Japan Hospital
Association's Quality Indicator Project 2018, the multiple set
collection rate across 355 healthcare facilities was 60.4%
(9); the present study had a
higher rate (76.7%). Although the reason for the high multiple set
collection rate cannot be clearly identified, the number of
participating facilities and differences in the methods of
calculating multiple set collection rates (the numerator in the
formula) may have played a role.
A multiple set collection rate of 10-19% has been
reported in a Japanese pediatric clinical setting (19). Ensuring that a sufficient volume of
blood is collected from children poses an issue, and multiple sets
of collections have not progressed in pediatric settings. In 2019,
J-SIPHE reported a rather low multiple set collection rate of 4.9%
in children aged <15 years at 178 Japanese healthcare facilities
(10). In the present study, the
multiple set collection rate increased by ~10% when children were
excluded. Therefore, the assessments of multiple set collection
rates should consider special circumstances in pediatric
settings.
Blood culture contamination, a phenomenon in which
microorganisms normally not present in blood are detected in blood
culture (20), is highly likely to
lead to inappropriate antibiotic treatments and needs to be
prevented as much as possible. In a 1998 American study, a 2009
study including six Japanese healthcare facilities and the 2019
J-SIPHE report (n=276), the contamination rates were 2.5% (21), 1.8% (6) and 1.4% (10), respectively. The contamination rate
in the present study was 3.1%, which was higher than that reported
in previous studies. The higher contamination rate in the present
study was likely due to differences in medical backgrounds. In the
USA, blood collection specialist teams are often used; the two
Japanese studies involved only medium- and large-sized hospital,
and the contamination rate in the present study decreased to 2.5%
when limited to hospitals with ≥500 beds. The high contamination
rate in small hospitals obtained in the present study may be due to
the fact that small hospitals often do not have clinical laboratory
technicians or other staff familiar with blood culture, and
therefore lack education in appropriate blood collection.
As regards the association between antiseptics used
prior to blood collection (a secondary endpoint) and the
contamination rate, the contamination rates were lower with CHG-AL
and AL than with PVP-I. A 2011 meta-analysis reported that the
blood culture contamination rate was 67% lower with CHG-AL than
with PVP-I (22). Although PVP-I
was associated with a high contamination rate, it was used by
>40% of facilities examined in the present study. This
concerning frequent use of PVP-I could lead to incorrect antibiotic
treatment.
A limitation of the present study is that it is
unclear whether the results reflect the overall population due to
the small number of samples, with only 63 participating facilities.
Furthermore, the distribution of participating hospitals suggests a
high participation rate in densely populated areas, and the
accumulation of additional data is warranted for the generalization
of the results. In addition, as facilities with <200 beds were
not eligible for participation in the study, healthcare facilities
could not be sampled from a population throughout Japan.
Furthermore, as the characteristics of medical care in each
healthcare facility (such as stratified evaluation of clinical
departments) were not analyzed, a bias is expected in the positive
blood culture rate specific to clinical departments.
Despite such a bias in the present study, the
results of the four indicators (95% CI) may be used as reference
values for blood cultures in healthcare facilities with ≥200 beds
in Japan, which may guide the assessment of the quality of blood
culture in each facility. The authors also aim to approach these
values in a hospital and hope that on-site interventions and
educational activities will be used to improve the state of blood
culture at apiece hospital as much as possible.
Acknowledgements
The authors would like to express their deep
gratitude to the following individuals for the provision of data
for the study: Ms. Sakiko Igawa of JCHO Gunma Chuo Hospital, Mr.
Yudai Watanabe of Asahi Chuo Hospital, Ms. Kumiko Okuda of
Ichinomiyanishi Hospital, Ms. Chisumi Mukai of Iwate Prefectural
Central Hospital, Dr Kenji Yamamoto of Utano National Hospital, Mr.
Tadashi Ohno of Omuta Tenryo Hospital, Mr. Masaaki Sasano of
Okazaki City Hospital, Ms. Miwaka Tomo of Kaisei Hospital, Mr.
Kazuki Niwa of Kanto Rosai Hospital, Mr. Atsushi Yasuda of Kitami
Red Cross Hospital, Ms. Akiko Iwama of Kimitsu Chuo Hospital, Mr.
Tomohiro Hamashima of Kumamoto Rosai Hospital, Ms. Masami Fujita of
Kurashiki Heisei Hospital, Ms. Yoriko Ueno of Kurihara Central
Hospital, Ms. Saki Matsumoto of Konan Medical Center; Mr. Tatsuya
Uchida of Obama Municipal Hospital, Ms. Momoyo Miyata of
International University of Health and Welfare Hospital, Mr.
Takeshi Ogino of Saiseikai Kawaguchi General Hospital, Ms. Chieko
Tsuji of Saiseikai Kurihashi Hospital, Mr. Kosuke Yasui of
Saiseikai Chuwa Hospital, Ms. Yuri Shoji of Saiseikai Yamagata
Saisei Hospital, Ms. Mayumi Takase of Saiseikai Maebashi Hospital,
Ms. Kiyoko Yamamoto of Saiseikai Yamaguchi General Hospital, Ms.
Akane Kamiya of Saiseikai Yokohamashi Nanbu Hospital, Mr. Shinzo
Kawasaki of Saiseikai Wakayama Hospital, Ms. Tatsuko Okuda of
Saiseikai Hiroshima Hospital, Ms. Yukari Sano of Saga-ken Medical
Centre Koseikan, Ms. Kana Fujita of Asahikawa City Hospital, Mr.
Kazumasa Koga of Tamagawa Hospital, Ms. Tomoko Fudeyasu of Tsuyama
Chuo Hospital, Ms. Hisae Fukuyama of The Fraternity Memorial
Hospital, Mr. Tetsuyoshi Taneichi and Ms. Yuki Matsubara of Tokatsu
Hospital, Mr. Masami Kurokawa of JCHO Tokyo Shinjuku Medical
Center, Ms. Fumi Togashi of Tohoku Kosai Hospital, Ms. Mina Takano
of Nagaoka Red Cross Hospital, Ms. Ayako Yamamoto of Niigata
Prefectural Central Hospital, Mr. Yuudai Kanazawa of Hachinohe City
Hospital, Mr. Takahiro Suzuki of Hitachi General Hospital, Ms.
Kumiko Mandokoro of Fukuoka Mirai Hospital, Dr Kiyohito Ishikawa of
Fujita Health University Hospital, Mr. Masakazu Yoshida of Maebashi
Red Cross Hospital, Ms. Yurika Notsu of Mizushima Kyodo Hospital,
Dr Masaru Amishima MD of NHO Hokkaido Medical Center, Ms. Risa Kato
of NHO Niigata National Hospital, Mr. Satoshi Shinomiya of Minoh
City Hospital, Ms. Toko Kimura of Yamagata City Hospital Saiseikan,
Ms. Tomoko Fujiwara of Yamaguchi Prefectural Medical Center, Ms.
Yuka Shimokawa of Yokosuka Kyosai Hospital, and Ms. Mayumi Sato of
Yokohama Minami Kyousai Hospital. All affiliations and names were
current at the time of participation in the study.
Funding
The present study was supported by the Social
Welfare Organization Saiseikai Imperial Gift Foundation, Inc.
Medical/Welfare Joint Subsidy Financing Systems.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
TO was the chief investigator and responsible for
the data analysis and organization. MO, EK and KI collected the
data and confirm the authenticity of all the raw data. All authors
contributed to the writing of the final manuscript. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was approved by the Institutional
Review Board of Saiseikai Yokohamashi Tobu Hospital (2018107).
Informed consent was not required due to the retrospective nature
of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stefani S: Diagnostic techniques in
bloodstream infections: Where are we going? Int J Antimicrob
Agents. 34 (Suppl 4):S9–S12. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Opota O, Croxatto A, Prod'hom G and Greub
G: Blood culture-based diagnosis of bacteraemia: State of the art.
Clin Microbiol Infect. 21:313–322. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Baron EJ, Weinstein MP, Dunne WM, Yagupsky
P, Welch DF and Wilson DM: Cumitech 1C, Blood cultures IV. In:
American Society for Microbiology. Baron EJ (ed). ASM Press,
Washington, DC, 2005.
|
|
4
|
Sánchez-Sánchez MM, Arias-Rivera S,
Fraile-Gamo P, Jareño-Collado R, López-Román S, Vadillo-Obesso P,
García-González S, Pulido-Martos MT, Sánchez-Muñoz EI, Cacho-Calvo
J, et al: Effect of a training programme on blood culture
contamination rate in critical care. Enferm Intensiva. 29:121–127.
2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Novis DA, Dale JC, Schifman RB, Ruby SG
and Walsh MK: Solitary blood cultures: A College of American
Pathologists Q-probes study of 132,778 blood culture sets in 333
small hospitals. Arch Pathol Lab Med. 125:1290–1294.
2001.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ohmagari N, Takakura S, Matsumura Y,
Sugiyama T, Takeshita N, Takahashi M, Ainoda Y, Goto A, Chibana N,
Ohshiro T, et al: A questionnaire survey of blood culture among
Japanese hospitals: A pilot study. J Jpn Soc Clin Microbiol.
22:13–19. 2012.(In Japanese).
|
|
7
|
Akemi K, Kazuhiko T, Hidekazu N, Hitomi M,
Manami I, Yoshibumi A and Yoshihito O: Difference of the bacteria
isolated using BACTEC and BacT/ALERT for automatic blood culture
analysis. Jpn J Med Technol. 63:24–28. 2014.
|
|
8
|
All-Japan Hospital Association: Fiscal
2018 blood culture implementation (blood culture implementation
rate). https://www.ajha.or.jp/hms/qualityhealthcare/pdf/2019/all/2019all_outcome_55.pdf.
Accessed June 23, 2020.
|
|
9
|
Japan Hospital Association: Quality
Indicator Project 2018. https://www.hospital.or.jp/pdf/06_20191120_01.pdf.
|
|
10
|
National Center for Global Health and
Medicine, AMR Clinical Reference Cen ter: Japan Surveillance for
Infection Prevention and Healthcare Epidemiology (J-SIPHE) Annual
Report 2019. J-SIPHE, Tokyo, 2019. https://j-siphe.ncgm.go.jp/files/JSIPHE_AnnualReport_2019en.pdf.
Accessed June 23, 2020.
|
|
11
|
Zadroga R, Williams DN, Gottschall R,
Hanson K, Nordberg V, Deike M, Kuskowski M, Carlson L, Nicolau DP,
Sutherland C, et al: Comparison of 2 blood culture media shows
significant differences in bacterial recovery for patients on
antimicrobial therapy. Clin Infect Dis. 56:790–797. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Suwanpimolkul G, Pongkumpai M and
Suankratay C: A randomized trial of 2% chlorhexidine tincture
compared with 10% aqueous povidone-iodine for venipuncture site
disinfection: Effects on blood culture contamination rates. J
Infect. 56:354–359. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schifman RB, Bachner P and Howanitz PJ:
Blood culture quality improvement: A College of American
Pathologists Q-Probes study involving 909 institutions and 289,572
blood culture sets. Arch Pathol Lab Med. 120:999–1002.
1996.PubMed/NCBI
|
|
14
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li G, Sun J, Pan S, Li W, Zhang S, Wang Y,
Sun X, Xu H and Ming L: Comparison of the performance of three
blood culture systems in a Chinese tertiary-care hospital. Front
Cell Infect Microbiol. 9(285)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
European Centre for Disease Prevention and
Control (ECDC): Surveillance of antimicrobial resistance in Europe
2018. ECDC, Stockholm, 2018. https://www.ecdc.europa.eu/sites/default/files/documents/surveillance-antimicrobial-resistance-Europe-2018.pdf.
Accessed June 23, 2020.
|
|
17
|
Nakatani S, Ohara T, Ashihara K, Izumi C,
Iwanaga S, Eishi K, Okita Y, Daimon M, Kimura T, Toyoda K, et al:
Japanese Circulation Society Joint Working Group: JCS 2017
Guideline on Prevention and Treatment of Infective Endocarditis.
Circ J. 83:1767–1809. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lee A, Mirrett S, Reller LB and Weinstein
MP: Detection of bloodstream infections in adults: How many blood
cultures are needed? J Clin Microbiol. 45:3546–3548.
2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kasai M, Shime N, Saitou A, Funaki T,
Shoji K and Miyairi I: Investigating blood culture collection in a
Japanese pediatric clinical setting. Kansenshogaku Zasshi.
87:620–623. 2013.PubMed/NCBI View Article : Google Scholar : (In Japanese).
|
|
20
|
Hall KK and Lyman JA: Updated review of
blood culture contamination. Clin Microbiol Rev. 19:788–802.
2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schifman RB, Strand CL, Meier FA and
Howanitz PJ: Blood culture contamination: A College of American
Pathologists Q-Probes study involving 640 institutions and 497,134
specimens from adult patients. Arch Pathol Lab Med. 122:216–221.
1998.PubMed/NCBI
|
|
22
|
Caldeira D, David C and Sampaio C: Skin
antiseptics in venous puncture-site disinfection for prevention of
blood culture contamination: Systematic review with meta-analysis.
J Hosp Infect. 77:223–232. 2011.PubMed/NCBI View Article : Google Scholar
|