Introduction
A number of atypical pneumonia cases first reported in Wuhan, China in December, 2019 were later discovered to be caused by a novel coronavirus. This virus was officially named by The World Health Organization (WHO) as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the infection caused by the virus was referred to as coronavirus disease 2019 (COVID-19). Since the initial outbreak, COVID-19 has rapidly spread worldwide, and by March, 2020 the WHO officially declared a global pandemic (1,2). As of August 3, 2021, globally, there have been 198,229,562 confirmed cases of COVID-19 and ~4,223,339 fatalities, with a total of 2,848,252 confirmed cases and 240,906 fatalities reported in Mexico at the time of the writing the present study (3).
One of the primary public health measures necessary to mitigate the spread of COVID-19 is the early identification of infected patients. However, the clinical symptoms of patients with COVID-19 can be unspecific, ranging from asymptomatic to highly symptomatic cases suffering from fever, cough, headache, tightness of chest and dyspnea, amongst other symptoms. As the number of cases increased, so did the urgency to identify effective treatments. Since no specific therapy was previously known, various therapeutic management strategies have been adopted, both in ambulatory and hospital settings to reduce complications and mortality rates. The pharmacological tools used for patients with COVID-19 include the following: Antiviral agents (remdesivir, lopinavir and chloroquine), antibiotics (azithromycin), anti-parasitics (ivermectin), anti-SARS-CoV-2 antibody products (monoclonal antibodies, convalescent plasma and immunoglobulins), immunomodulators (corticosteroids, colchicine and immunoglobulins) and certain supplements (vitamins C and D, and zinc) (4). Supportive strategies are also implemented, including oxygen supplementation when required, by using high-flow devices, non-invasive ventilation, mechanical ventilation and extracorporeal membrane oxygenation (ECMO) (4).
With no specific drug treatments previously available for COVID-19, increased attention has been paid to optimizing the use of supportive therapies, such as oxygen delivery, with numerous researchers focusing on early intervention. Hyperbaric oxygen therapy (HBOT) has recently gained attention for its potential for use in patients with COVID-19. HBOT consists of a treatment using 100% oxygen administered at a pressure >1 absolute atmosphere (ATA), usually between 1.5 and 3 ATA for 60-120 min, administered in a compression vessel known as a ‘hyperbaric chamber’ (5,6). The therapeutic effects achieved with HBOT can be attributed to three main mechanisms: i) The reversal of the dysfunction in hypoxic tissues; ii) the increase in arterial oxygen tension; and iii) the elimination of inert gas from tissue promoted by hydrostatic pressure (5,6).
The initial indication for the use of HBOT was revealed during the treatment of decompression sickness, a complication often observed in divers and, subsequently, HBOT has been accepted for use in numerous other conditions, such as air or gas embolisms, carbon monoxide poisoning, necrotizing soft tissue infections and wound healing. Several of the favorable indications for the use of HBOT are based on its anti-inflammatory effects and its modification of metabolic pathways, as well as the reversal of hypoxia (5,6). Taking these effects into consideration, clinicians have examined HBOT and its possible application in patients with COVID-19, which may address some of the key pathophysiological aspects of the infection, such as through its anti-inflammatory and antithrombotic effects, as well as causing the reduction of tissue hypoxia, production of viricidal reactive oxygen intermediates, and modulation of stem cells and cytokines (6-8).
The first reported use of HBOT in patients with COVID-19 was in Wuhan, China, where 5 patients with severe-to-critical symptoms who required intubation received HBOT. At the end of the trial, the patients experienced a resolution of the hypoxia-associated symptoms and hypoxemia correction, with no reported HBOT-related complications (9). In the study conducted by Thibodeaux et al (7), 5 patients with COVID-19 required mechanical ventilation and received HBOT applied at 2 ATA for 90 min each day, with an average of five sessions required per patient; it was revealed that all these patients were able to avoid the use of mechanical ventilation and exhibited a rapid improvement in oxygen saturation and resolution of tachypnea, as well as a reduction in D-dimer levels. Guo et al (10) also reported the use of HBOT in severe cases of COVID-19, where the patients underwent sessions of 1.5 ATA for 60 min, once a day over a period of 1 week, and these authors observed an alleviation of the symptomology from the first therapy session. At the end of that study, the patients presented an improvement in arterial gas oxygenation and leukocyte count, and a reduction of D-dimer and cholinesterase values (10). Despite the sample size of these studies being limited, the results of studies on the use of HBOT are promising. Thus, HBOT warrants further investigation for its potential use in the treatment of patients with COVID-19. The present study thus aimed to provide further insight into the use of HBOT in the treatment of patients with COVID-19.
Patients and methods
Patients and treatment
A total of 36 patients diagnosed with moderate-to-severe COVID-19 (23 males and 13 females; average age, 54.071±1.97 years; age range, 25-72 years), who had received medical care at the Naval Specialties Hospital (Veracruz, Mexico) between June, 2020 and September, 2020, were referred to the Hyperbaric Medicine Service to determine the efficacy of the use of HBOT to help improve hypoxemia. Patients were diagnosed using the positive fluorescence-based reverse transcription-quantitative PCR detection (RT-qPCR) of SARS-CoV-2 nucleic acid performed at the State Laboratory of the Secretary of Health of Veracruz (Veracruz, Mexico). All patients were administered a standardized pharmacological treatment consisting of dexamethasone 6 mg/8 h, azithromycin 500 mg/24 h and ceftriaxone 1 g/12 h for 14 days, as well as acetaminophen 1 g/6 h, vitamin C 1 g/8 h and acetylcysteine 300 mg/6 h up to 5 days. Patients with comorbidities received their previously established treatment. The present study was approved by the Institutional Bioethics Committee of the Naval Specialties Hospital of Veracruz with the approval no. 537/2020 and was performed in accordance with the principles outlined in the Declaration of Helsinki. Written informed consent was provided by all participants prior to the study.
The beneficiaries of the health services at the Naval Specialties Hospital are active or retired members of the naval service. The patients included in the study were diagnosed with COVID-19, regardless of their sex or age. Moreover, some of the patients had hypertension, diabetes, or were smokers. All patients were obese or overweight. A full clinical evaluation was performed before candidates underwent HBOT. The exclusion criteria were as follows: i) Refusal to participate and/or denial to sign an informed consent to receive HBOT; ii) acute congestive heart failure; iii) claustrophobia; and iv) pneumothorax. Patients were excluded from the study if they decided to abandon or interrupt treatment, or if they missed >5 sessions.
In addition to the indicated pharmacological treatment, the participants received one session per day of HBOT in a multiplace hyperbaric chamber. On average, treatment was initiated 5 days following the initial onset of symptoms. The transfer of the patient from their hospital bed to the hyperbaric chamber was performed through the exclusive controlled circuit for hospitalized patients with COVID-19, and prior to their transfer, an evaluation of their vital signs and quantification of oxygen saturation were conducted. A total of 30 patients were able to withstand transfer to the chamber without oxygen (5-7 min, oxygen saturation, >85%), while patients with an oxygen saturation <85% were transferred to the chamber with supplemental oxygen (6 patients) prior to the HBOT session. Oxygen saturation measurements before and after HBOT were made in room air. The infectious-contagious control measures outside the chamber were the same as those in the hospital room, and the sanitization measures for the hyperbaric chamber were the same as those followed in the COVID-19 hospital areas. As well as achieving a significant improvement in oxygen saturation and symptomatology, HBOT has been observed to diminish inflammation, as evidenced by laboratory inflammation markers. For instance, Guo et al (10) evaluated the difference in oxygen saturation levels in patients subjected to HBOT and measured the lymphocyte count, and found that the immune function gradually recovered following HBOT; they also observed that D-dimer levels and serum cholinesterase concentrations were improved. In another study, Chen et al (11) measured the lymphocyte count, fibrinogen and D-dimer levels, and all these parameters were found to be significantly decreased following HBOT. Moreover, Gorenstein et al (12) evaluated troponin, ferritin, D-dimer, C-reactive protein and lactate dehydrogenase (LDH) levels as prognostic indicators in the treatment of patients with COVID-19 undergoing HBOT; by the end of their study, the group treated with HBOT exhibited a significant improvement in oxygen saturation and in mortality compared with the control group. In another study, the levels of procalcitonin and C-reactive protein, differential and total white blood cell count, as well as the levels of IL-6, ferritin, D-dimer and LDH, were measured as inflammatory markers (13).
Patients were provided with a mask that formed a component of a closed circuit, delivering a continuous 100% oxygen flux, and was installed in the corresponding hyperbaric chamber. The entrance of the chamber was sealed, and the HBOT session began with a compression (descent) of ~15 min until reaching iso-pressure (bottom; 2.0 ATA) that was maintained for 90 min, with intervals of 30 min and two 5-min rest intervals. Subsequently, a decompression (ascent) period of ~15 min was initiated. During three 30-min periods, patients breathed 100% oxygen through closed circuit masks, while during the 5-min rest intervals they were administered compressed air. The treatment lasted for ~130 min per HBOT session. At the end of the session, partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) was evaluated again, and vital signs were recorded on the data collection monitoring sheet of each patient; the blue code was activated for the transfer of patients to a hospital room if they were stable. In case of any adverse reaction to saturation or clinical deterioration due to the transfer, patients were immediately referred to the Emergency Department of the Naval Specialties Hospital Veracruz (Veracruz, Mexico) and were accompanied by a physician and a nurse for immediate attention. The most frequently observed adverse effects were nasal irritation, epistaxis, anxiety, dizziness and headaches. The less frequently observed events included cerebral arterial embolism, barotrauma and pneumothorax.
Statistical analysis
All data were analyzed using GraphPad Prism® version 3.10 (GraphPad Software, Inc.). A paired Student's t-test was used to compare differences before and after HBOT. Data are presented as the mean ± SEM. P<0.05 was considered to indicate a statistically significant difference. BMI analysis was performed using one-way ANOVA, followed by Dunnett's multiple comparisons test. Bars in the figures represent the mean ± SEM.
Results
Sociodemographic data of patients with COVID-19
The present study was performed at the Naval Specialties Hospital (Veracruz, Mexico), and included 36 patients, 23 males and 13 females (mean age, 54.071±1.97 years; age range, 25-72 years) who met the selection criteria to receive HBOT sessions at 2 ATA for 130 min. The average weight of the patients was 80.3±2.37 kg and the average height was 1.65±0.017 m. Moreover, the average BMI was 29.02±0.65 kg/m2, with 18 patients classified as overweight (BMI ≥25 and <30 kg/m2) and 12 patients as obese (BMI ≥30 kg/m2). The number of patients with comorbidities associated with more severe COVID-19 symptoms were as follows: 19 patients with diabetes, 11 patients with arterial hypertension and 10 active smokers (Table I).
 |
Table I
Sociodemographic data of the patients diagnosed with COVID-19 in the present study (n=36).
|
Table I
Sociodemographic data of the patients diagnosed with COVID-19 in the present study (n=36).
| Characteristic |
Values |
| Sex, n |
|
| Male |
23 |
| Female |
13 |
| Age, years (range) |
54.071±1.97 (25-72) |
| Weight, kg |
80.3±2.37 |
| Height, m |
1.65±0.017 |
| BMI, kg/m2 |
29.02±0.65 |
| Normal (n=6) |
23.7±0.43 |
| Overweight (n=18) |
27.1±0.18 |
| Obese (n=12) |
33.1±0.80 |
| Comorbidities, n |
|
| Diabetes |
19 |
| Hypertension |
11 |
| Active smoking |
10 |
Overweight and obesity as risk factors in COVID-19
When analyzing the BMI results of the 36 patients, it was observed that 6 patients presented an average BMI of 23.7±0.43 kg/m2, which was considered to be within the normal range, while 18 patients were classified as overweight (27.1±0.18 kg/m2) and 12 patients were obese (33.1±0.80 kg/m2). The statistical analysis revealed a significant difference (P<0.01) when comparing the overweight and obesity groups to the normal weight group (Fig. 1).
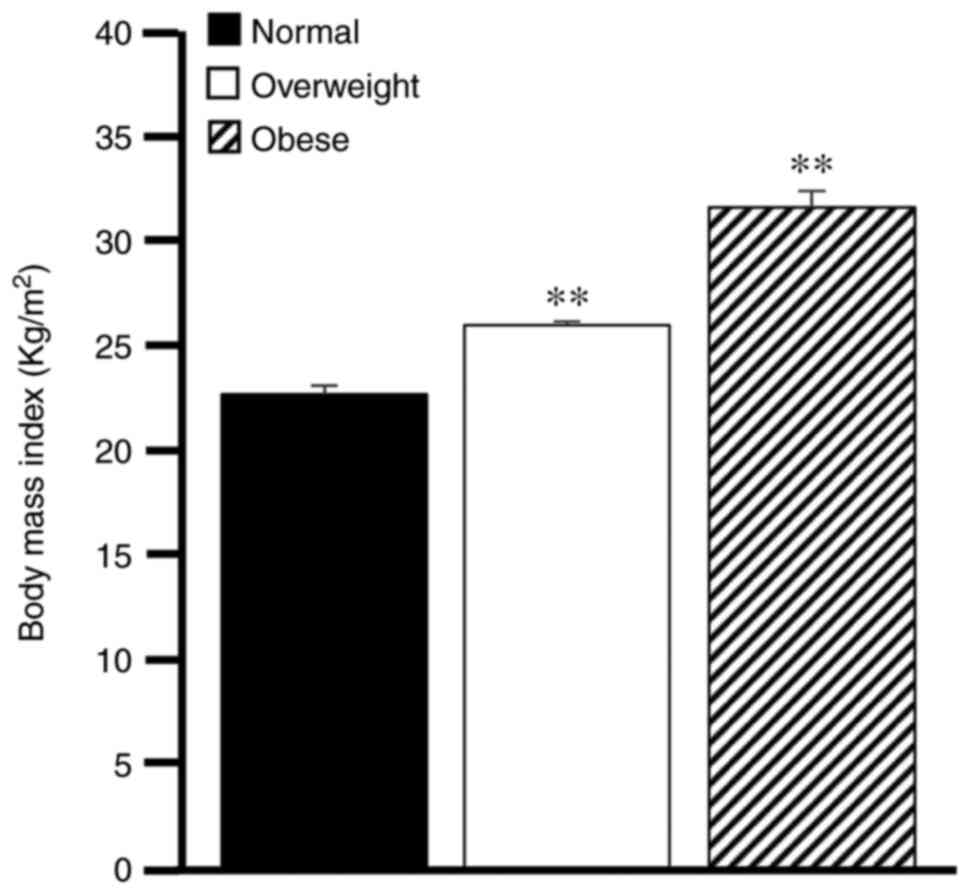 |
Figure 1
BMI of patients with COVID-19. BMI measurements were conducted for the patients with COVID-19 participating in the study. BMI was classified as normal, overweight and obese. Comparisons were performed using one-way ANOVA, followed by Dunnett's multiple test. **P<0.01, overweight or obese groups vs. normal group. Bars represent the mean ± SEM. BMI, body mass index; COVID-19, coronavirus 2019.
|
Effectiveness of HBOT as a therapeutic tool for the treatment of COVID-19
When SARS-CoV-19 enters the lungs of patients, it can reach the circulation and destroy hemoglobin, which is responsible for transporting oxygen to the tissues. As a consequence, generalized hypoxia (lack of oxygen), inflammation, pneumonitis and, in severe cases, respiratory failure, may develop. These are extreme complications of the disease that may lead to a fatal outcome (4).
In the present study, the 36 patients with COVID-19 administered HBOT had dyspnea, shortness of breath, cough and fever. The treatment outcomes were as follows: i) These symptoms were relieved immediately in 7 patients after the first HBOT session; ii) in 22 other patients the symptoms were markedly relieved after 7.57±0.63 days of HBOT; and iii) an additional 7 patients exhibited a slow recovery from shortness of breath after performing activity, suggesting that they should continue with drug treatment and HBOT, as well as pulmonary rehabilitation therapy.
In order to determine the clinical progression and aid in the staging of the patients, the current study evaluated the oxygen saturation levels and also compared initial and end CT scans. As presented in Fig. 2, HBOT significantly increased the oxygen levels in patients with COVID-19. Of note, 12 patients had oxygen saturation levels <85% prior to treatment; however, HBOT increased these levels to >95%. On the other hand, 27 patients (75%) exhibited varying degrees of improvement in the morphological lesions shown on CT images obtained at the beginning compared with those at the end of the therapy, while the remaining 25% of the patients did not exhibit changes in their CT scans by the end of the therapy (Fig. 3).
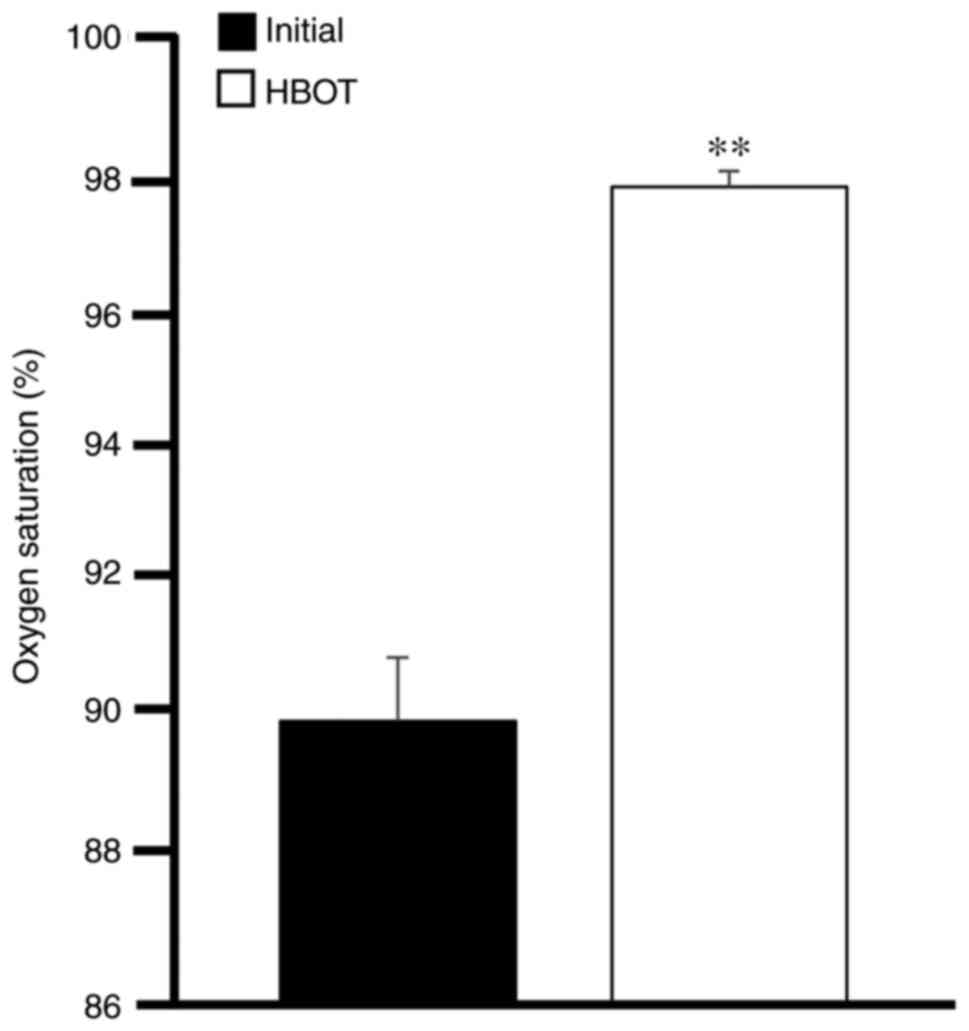 |
Figure 2
Oxygen saturation in patients with COVID-19. The levels of oxygen saturation were measured at the beginning and at the end of HBOT. Data were analyzed using a paired Student's t-test. **P<0.01, vs. initial levels, prior to treatment. Bars represent the average mean ± SEM. COVID-19, coronavirus 2019; HBOT, hyperbaric oxygen therapy.
|
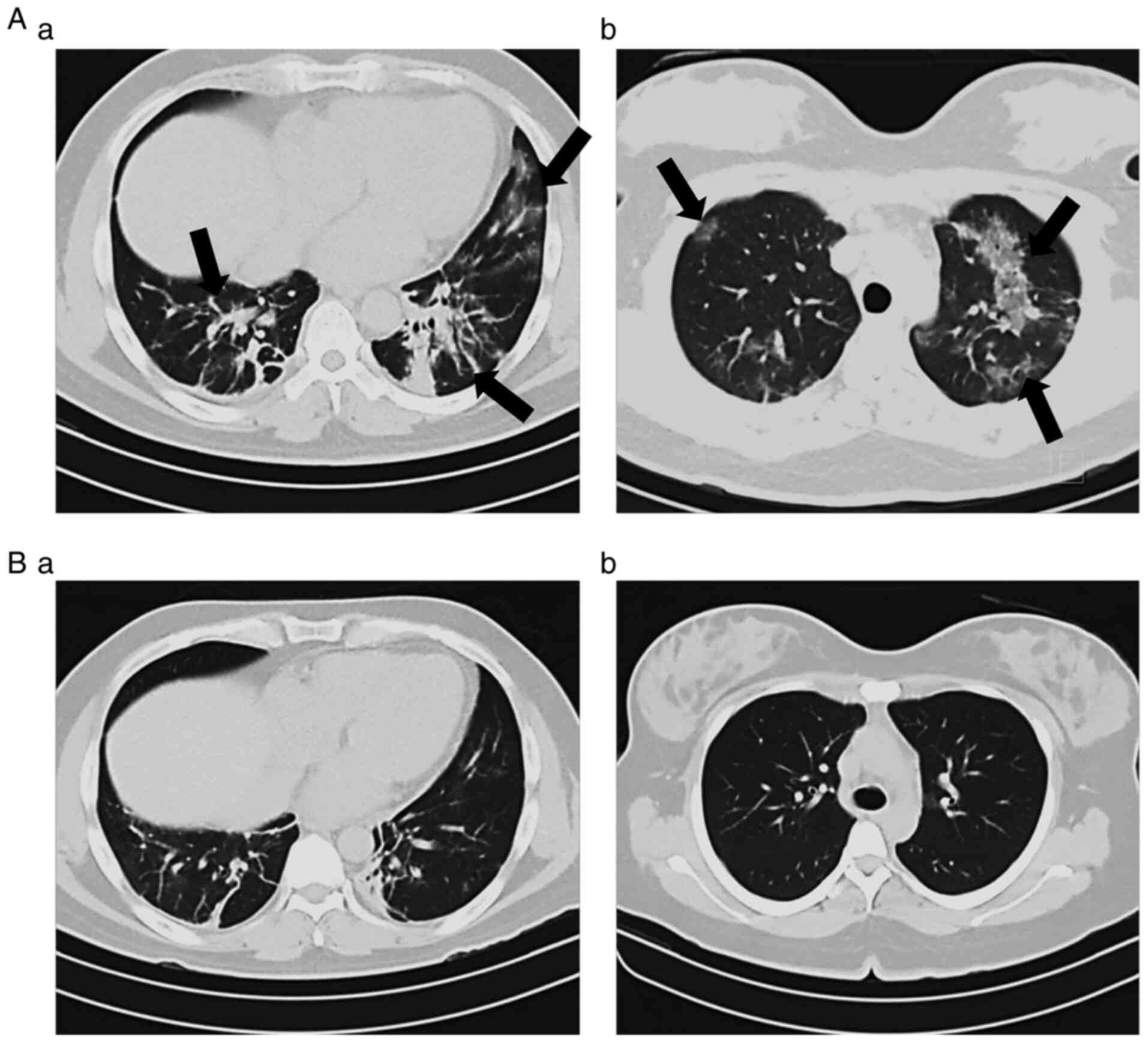 |
Figure 3
Chest computed tomography images from patients with COVID-19. Two examples (A and B) of images obtained at the (a) beginning and at the (b) end of the HBOT treatment. The characteristic features observed in the tomography scans at the beginning of the treatment had changed by the end of the therapy. COVID-19, coronavirus 2019; HBOT, hyperbaric oxygen therapy.
|
The most frequently observed findings in CT imaging of the patients prior to the initiation of HBOT were as follows: Ground glass opacity (nebulous areas with slightly increased density in the lungs without darkening of the bronchial and vascular margins), reticular pattern (thickening of lung interstitial structures, interlobular septa and intralobular lines, manifests as a collection of innumerable small linear opacities), ‘crazy-paving’ pattern (resulting from alveolar edema and interstitial inflammation of acute lung injury, irregular cobblestone appearance) and air bronchogram [pattern of air-filled bronchi (low attenuation) on a cloudy lung fundus (high attenuation) without air]. As indicated by the arrows on the representative tomographic images presented in Fig. 3A-a and B-a, severe injury to the lung parenchyma occurred in patients with COVID-19 and these lesions were reversed following HBOT, as illustrated in Fig. 3A-b and B-b.
Discussion
The effectiveness of HBOT as a supportive treatment was evaluated by examining the clinical characteristics, oxygen saturation levels and CT scan images. The majority of the patients reported a significant relief in symptomology after an average of seven sessions, with 7 patients reporting an improvement immediately after the first session, which was consistent with previous studies (7,9-12). Oxygen saturation levels also improved significantly, from 90% at the beginning to 98% by the end of the trial. The CT patterns observed in the patients with COVID-19 are in line with those reported in the literature (14). The comparison of the initial CT imaging features and those observed at the end of the study revealed a significant clearance of lung pathology, with complete reversal of the atypical findings in some patients.
A relevant aspect of the present study was the high prevalence of comorbidities in the enrolled population and the fact that the majority of patients exhibited a significant improvement in all evaluated parameters at the end of the study, with no progression in respiratory deterioration or requirement for mechanical ventilation. A well-established predictor for disease severity in patients with COVID-19 is age; the patients in the present study had a mean age of 54 years. It has been shown in the majority of the previous studies that patients with severe COVID-19 have an age range of 52-66 years (15-17). Other primary predictors of severity were also taken into consideration in the present study, such as obesity, arterial hypertension, diabetes mellitus and smoking, all of which can lead to the requirement of mechanical ventilation (15).
Multiple studies have revealed that patients recovering from COVID-19 often experience symptoms, such as shortness of breath, tiredness, pain and muscle weakness, hyperglycemia, severe pneumonia, cardiovascular problems, kidney failure and severe anemia due to the cytokine storm and inflammation induced by SARS-CoV-2 (18-23). Hypoxemia in patients with COVID-19 has been shown to be caused by various mechanisms, mainly hypoventilation, impaired diffusion, shunting in hypoventilated areas and a ventilation-perfusion mismatch. The primary cause of hypoxemia is ventilation-perfusion mismatch due to a lack of ventilation in certain areas, even with adequate blood perfusion of the region (24). Additionally, microemboli are a known complication in patients with COVID-19, which can compromise lung perfusion to regions with a low ventilation-perfusion ratio (24-26). As the level of hypoxemia has been shown to be associated with mortality in hospitalized patients with COVID-19, it may serve as a prognostic indicator (24,27,28).
For over five decades, HBOT has exhibited an adequate safety range, with limited absolute contraindications and side-effects (5). Although a significant proportion of the global population has now received vaccination against COVID-19, with 4,042,645,108 doses administered globally as of August 3, 2021(3), and with the number of vaccinations increasing daily, in certain geographical areas the availability of vaccines is scarce. Moreover, with no specific pharmacological treatment, the management of patients with COVID-19 remains focused on vital organ function support, with HBOT serving as an attractive alternative to other oxygen delivery methods, such as invasive ventilation, particularly in severe cases. Additionally, HBOT presents an advantage over other ventilation methods by delivering oxygen at an elevated partial pressure, increasing the oxygen diffusion rate and reverting tissue hypoxia. With the added benefits of HBOT exerting anti-inflammatory effects and possessing viricidal properties, it should be considered as a valuable tool in the management of patients with COVID-19 (6-12). HBOT has been proposed as a useful treatment in a variety of conditions, particularly in those with a significant inflammatory component. In an animal study, HBOT was shown to diminish serum and tissue inflammatory cytokine levels (including TNF-α and IL-1β) and increase IL-10 levels (an anti-inflammatory cytokine) in tissues subjected to radiotherapy (29). During the cytokine storm, TNF-α and IL-1β have been shown to play a crucial role in enhancing the severity of the pathophysiology of COVID-19 infection, and elevated blood levels of both these factors have been observed in severe cases of COVID-19(30). Thus, HBOT may be able to mitigate inflammation in severe COVID-19 cases by reducing circulating TNF-α and IL-1β levels.
The present study demonstrated that HBOT improved symptoms in patients with COVID-19; however, a limitation of this study was that all patients received HBOT and no control subjects were included. Other studies that have investigated the application of HBOT in the treatment of patients with COVID-19 that included a control group reported similar results as those demonstrated in the current study. For instance, one of these previous studies was conducted using a group of 20 patients with COVID-19 aged 30-79 years who were treated with HBOT at 2.0 ATAs for up to five sessions of 90 min each (12). The initial oxygen requirements of these patients ranged from 2-15 l and by the end of the trial, 90% of the patients did not require mechanical ventilation. This previous study also reported that only two cases required mechanical ventilation and ultimately succumbed to the disease, whereas in the propensity-matched control group, 65% did not require mechanical ventilation and 21% succumbed to the disease. It is important to note that, although the sample size was small, this study demonstrated the safety profile of administering HBOT to patients with COVID-19 with only minor adverse events, and that it significantly decreased inpatient mortality and the need for mechanical ventilation (12). Another currently ongoing study involving 200 patients, with 100 in the HBOT group and 100 in the control group, is testing a similar HBOT regimen as that in the present study, although the results are still pending (13). Recently, Yanagawa (31) analyzed the findings of five studies that reported the benefits of HBOT in patients with COVID-19, mainly in the treatment of hypoxemia, as a possible method to avoid invasive mechanical ventilation, such as ventilator support or ECMO. Even though the present study did not include a control group, the results further support the findings obtained in earlier reports. Thus, it was proposed that HBOT plays an important role in providing a more effective treatment, including via its long-term anti-inflammatory effects (18-23,32).
The ability to keep patients with COVID-19 off mechanical ventilation has a significant benefit as mechanical ventilation can contribute to the induction of the cytokine storm. HBOT may limit ventilation use, and at present, portable and affordable HBOT chambers are available to hospitals for COVID-19 therapy.
To the best of our knowledge, no previous study conducted to date in Mexico has investigated the efficacy of HBOT for increasing oxygen availability in the lungs and body and decreasing inflammation, thereby promoting recovery from the COVID acute phase. The present study demonstrated the utility of HBOT as a supportive treatment in patients with COVID, avoiding the need for mechanical ventilation and ameliorating their respiratory symptoms.
In conclusion, the present study demonstrated that, in addition to the current pharmacological treatment against COVID-19, HBOT may be a useful adjuvant treatment. HBOT can significantly shift the outcome of the illness when implemented in the early stages of the disease and may become a long-term aid to improve symptoms in post-COVID cases. Furthermore, since the COVID-19 pandemic is not currently receding, novel and promising therapeutic approaches, such as HBOT, merit further in-depth study.
Acknowledgements
The publication of the present study is required by JAGR to obtain the title of Specialist in Underwater and Hyperbaric Medicine at the Universidad Naval, Escuela de Posgrados en Sanidad Naval, Secretaría de Marina Armada de México to whom he is grateful for the instructions received during his research.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JAGR analyzed and interpreted the patient data. SGL and JCRN recruited patients and performed the hyperbaric oxygen therapy. HSC, LMM, EFS, BS and BSRM analyzed and interpreted the patient data. HSC, LMM and EFS were major contributors in the writing of the manuscript. JAGR and EFS confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the institutional Ethics Committee of the Naval Specialties Hospital, Veracruz, and the committee's reference no. is 537/2020. Informed consent for participation in the study was obtained from all participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 382:727–733. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Coronavirus Disease (COVID-19) Weekly Epidemiological Update and Weekly Operational Update. 2021. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed July 27, 2021.
|
|
3
|
Dong E, Du H and Gardner L: An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 20:533–534. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
National Institutes of Health: COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. https://www.covid19treatmentguidelines.nih.gov/. Accessed July 28, 2021.
|
|
5
|
Bennett MH and Mitchell SJ: Emerging indications for hyperbaric oxygen. Curr Opin Anaesthesiol. 32:792–798. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paganini M, Bosco G, Perozzo FAG, Kohlscheen E, Sonda R, Bassetto F, Garetto G, Camporesi EM and Thom SR: The role of hyperbaric oxygen treatment for COVID-19: A review. Adv Exp Med Biol. 1289:27–35. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Thibodeaux K, Speyrer M, Raza A, Yaakov R and Serena TE: Hyperbaric oxygen therapy in preventing mechanical ventilation in COVID-19 patients: A retrospective case series. J Wound Care. 29 (Sup5a):S4–S8. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baugh MA: HIV: Reactive oxygen species, enveloped viruses and hyperbaric oxygen. Med Hypotheses. 55:232–238. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhong X, Tao X, Tang Y and Chen R: The Outcomes of Hyperbaric Oxygen Therapy to Retrieve Hypoxemia of Severe Novel Coronavirus Pneumonia: First Case Report. Chin J Naut Med Hyperb Med. 27:E001. 2020.DOI: 10.3760/cma.j.issn.1009-6906.2020.0001. http://rs.yiigle.com/yufabiao/1187201.htm.
|
|
10
|
Guo D, Pan S, Wang M and Guo Y: Hyperbaric oxygen therapy may be effective to improve hypoxemia in patients with severe COVID-2019 pneumonia: Two case reports. Undersea Hyperb Med. 47:181–187. 2020.PubMed/NCBI View Article : Google Scholar : Second-Quarter.
|
|
11
|
Chen R, Zhong X, Tang Y, Liang Y, Li B, Tao X and Liao C: The outcomes of hyperbaric oxygen therapy to severe and critically ill patients with COVID-19 pneumonia. https://oxycamaras.com.br/wp-content/uploads/2020/04/Outcome-of-HBOT-to-COVID19.
|
|
12
|
Gorenstein SA, Castellano ML, Slone ES, Gillette B, Liu H, Alsamarraie C, Jacobson AM, Wall SP, Adhikari S, Swartz JL, et al: Hyperbaric oxygen therapy for COVID-19 patients with respiratory distress: Treated cases versus propensity-matched controls. Undersea Hyperb Med. 47:405–413. 2020.PubMed/NCBI View Article : Google Scholar : Third-Quarter.
|
|
13
|
Kjellberg A, Douglas J, Pawlik MT, Kraus M, Oscarsson N, Zheng X, Bergman P, Frånberg O, Kowalski JH, Nyren SP, et al: Randomised, controlled, open label, multicentre clinical trial to explore safety and efficacy of hyperbaric oxygen for preventing ICU admission, morbidity and mortality in adult patients with COVID-19. BMJ Open. 11(e046738)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ye Z, Zhang Y, Wang Y, Huang Z and Song B: Chest CT manifestations of new coronavirus disease 2019 (COVID-19): A pictorial review. Eur Radiol. 30:4381–4389. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
El Aidaoui K, Haoudar A, Khalis M, Kantri A, Ziati J, El Ghanmi A, Bennis G, El Yamani K, Dini N and El Kettani C: Predictors of severity in Covid-19 patients in Casablanca, Morocco. Cureus. 12(e10716)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, et al: Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected Pneumonia in Wuhan, China. JAMA. 323:1061–1069. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, et al: Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 8:475–481. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhang Y and Ma ZF: Impact of the COVID-19 pandemic on mental health and quality of life among local residents in Liaoning Province, China: A cross-sectional study. Int J Environ Res Public Health. 17(2381)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sheehy LM: Considerations for postacute rehabilitation for survivors of COVID-19. JMIR Public Health Surveill. 6(e19462)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vindegaard N and Benros ME: COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 89:531–542. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: The need for an interdisciplinary approach. Aging Clin Exp Res. 32:1613–1620. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Weerahandi H, Hochman KA, Simon E, Blaum C, Chodosh J, Duan E, Garry K, Kahan T, Karmen-Tuohy SL, Karpel HC, et al: Post-discharge health status and symptoms in patients with severe COVID-19. J Gen Intern Med. 36:738–745. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, et al: 6-month consequences of COVID-19 in patients discharged from hospital: A cohort study. Lancet. 397:220–232. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Allado E, Poussel M, Valentin S, Kimmoun A, Levy B, Nguyen DT, Rumeau C and Chenuel B: The fundamentals of respiratory physiology to manage the COVID-19 pandemic: An overview. Front Physiol. 11(615690)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Altemeier WA, Robertson HT, McKinney S and Glenny RW: Pulmonary embolization causes hypoxemia by redistributing regional blood flow without changing ventilation. J Appl Physiol (1985). 85:2337–2343. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Connors JM and Levy JH: COVID-19 and its implications for thrombosis and anticoagulation. Blood. 135:2033–2040. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Xie J, Covassin N, Fan Z, Singh P, Gao W, Li G, Kara T and Somers VK: Association between hypoxemia and mortality in patients with COVID-19. Mayo Clin Proc. 95:1138–1147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wei YY, Wang RR, Zhang DW, Tu YH, Chen CS, Ji S, Li CX, Li XY, Zhou MX, Cao WS, et al: Risk factors for severe COVID-19: Evidence from 167 hospitalized patients in Anhui, China. J Infect. 81:e89–e92. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Arıcıgil M, Dündar MA, Yücel A, Arbağ H, Arslan A, Aktan M, Fındık S and Kılınç İ: Anti-inflammatory effects of hyperbaric oxygen on irradiated laryngeal tissues. Braz J Otorhinolaryngol. 84:206–211. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, et al: The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The perspectives of clinical immunologists from China. Clin Immunol. 214(108393)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yanagawa Y: Current status of hyperbaric oxygen therapy for COVID-19. Acute Med Surg. 8(e678)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Demeco A, Marotta N, Barletta M, Pino I, Marinaro C, Petraroli A, Moggio L and Ammendolia A: Rehabilitation of patients post-COVID-19 infection: A literature review. J Int Med Res. 48(300060520948382)2020.PubMed/NCBI View Article : Google Scholar
|

















