Introduction
Pulmonary metastasis secondary to prostate cancer
has been reported in >40% of patients in an autopsy series
(1). By contrast, solitary lung
metastasis from prostate cancer is rare, and only 34 cases have
been reported to date (Table I)
(2-25).
More than half of the patients (22/34) underwent lung resection,
and the majority achieved cancer control and long-term
survival.
 | Table IClinical data of patients with
solitary pulmonary metastasis from prostate cancer obtained from
the literature and the present case. |
Table I
Clinical data of patients with
solitary pulmonary metastasis from prostate cancer obtained from
the literature and the present case.
| No. of cases | Authors/(Refs.) | Year of
publication | Age, years | Initial
characteristics | Initial
treatment | Preoperative PSA
levels | Lung metastasis
characteristics | Solitary lung
metastasis treatment | Outcome |
|---|
| 1 | Varkarakis et
al (2) | 1974 | Details unknown for 1
case |
| 11 | Fabozzi et al
(3) | 1995 | Details unknown for
11 cases |
| 1 | Rockey and Graham
(4) | 1990 | 83 | Low grade | Radiation
therapy | Unknown | Left lower lobe | Orchiectomy | Undetectable |
| 1 | Smith et al
(5) | 1999 | 70 | pT2/GS:4+5 | Radical
prostatectomy | Unknown | Right S7, 2 cm | Lung resection | Undetectable |
| 1 | Hofland and Bagg
(6) | 2000 | 49 | pT3c/GS:4+5 | Radical
prostatectomy | 1 | Left lower lobe | Lobectomy | Brain metastases |
| 1 | Chao et al
(7) | 2004 | 68 | pT2a/GS:4+5 | Radical
prostatectomy | 0.4 | Left lower lobe, 1.2
cm | Wedge resection | 12-year disease- free
follow-up |
| 1 | Pruthi et al
(8) | 2007 | 72 | pT2b/GS:3+3 | Radical
prostatectomy | 4.1 | Left S8, 2 cm | Endocrine therapy and
wedge resection | 3-Year disease- free
follow-up |
| 1 | Khandani et al
(9) | 2009 | 78 | Unknown | Radiation
therapy | 8.5 | Left S10, 5 cm | Lobectomy and
mediastinal LND | Undetectable |
| 1 | Boyer and Boyer
(10) | 2009 | 65 | pT2/GS:3+3 | Radical
prostatectomy | 3 | Left upper lobe, 2.8
cm | Lung resection | Undetectable |
| 1 | Sakai et al
(11) | 2010 | 74 | Unknown | Endocrine + radiation
therapy | 1.24 | Left S8, 2 cm | Wedge resection | 5-Month disease- free
follow-up |
| 1 | Goto et al
(12) | 2010 | 73 | pT4/GS:4+5 | Neoadjuvant endocrine
therapy + pelvic evisceration | Normal range | Right S3, 2 cm | Wedge resection | 10-Month disease-
free follow-up |
| 1 | Pepe et al
(13) | 2010 | 75 | pT3a/GS:4+3 | Radical
prostatectomy | Unknown | Left S6, 2 cm | Segmental
resection | 6-Month disease-
free follow-up |
| 1 | Calais et al
(14) | 2014 | 67 | pT1c/GS:4+4 | Endocrine +
radiation therapy | 3.5 | Right middle lobe,
4.6 cm | Lobectomy | Undetectable |
| 1 | Maebayashi et
al (15) | 2015 | 50 | cT4/GS:4+5 with
NED | Endocrine +
radiation therapy | Normal range | Left S5, 3 cm | Lobectomy and
mediastinal LND | Died after 2.5
years after metastases were detected |
| 1 | Gago et al
(16) | 2016 | 62 | pT3a/GS:7 | Radical
prostatectomy | 4.3 | Left lower
lobe | Wedge
resection | 4-Year disease-
free follow-up |
| 1 | Mortier et
al (17) | 2016 | 82 | pT3/GS:6 | Radical
prostatectomy | 3.32 | Right S7, 2 cm | Lobectomy | 1-Year disease-
free follow-up |
| 1 | Iijima et al
(18) | 2017 | 71 | Unknown | Radical
prostatectomy | 0.521 | Right S3, 2.2
cm | Lobectomy and
ND2a-2 LND | 2-Year and 3-month
disease- free follow-up |
| 1 | Rush et al
(19) | 2017 | 70 | pT4/GS:4+4 | Radical
prostatectomy | 2.9 | Right lower lobe,
4.7 cm | Segmental
resection | 2-Year disease-
free follow-up |
| 1 | Hokamp et al
(20) | 2017 | 63 | Unknown | Radical +
prostatectomy endocrine + radiation therapy | 1.6 | Right S1, 1.1
cm | Lung resection | Undetectable |
| 1 | Boschian et
al (21) | 2018 | 69 | pT3a/GS:4+3 | Radical
prostatectomy | 0.4 | Left lower lobe, 1
cm | Segmental resection
and LND | 3-Year disease-
free follow-up |
| 1 | Polverali et
al (22) | 2019 | 78 | pT2c/GS:4+3 | Radical
prostatectomy | 0.33 | Right S3, 0.7
cm | Wedge resection +
LND | Undetectable |
| 1 | Asano et al
(23) | 2019 | 80 | pT3a/GS:4+5 | Radical
prostatectomy + endocrine + radiation therapy | 0.33 | Right S9, 1.1
cm | Wedge
resection | 1-Year and 7-month
disease- free follow-up |
| 1 | Wu et al
(24) | 2020 | 74 | cT4/GS:4+4 | Chemotherapy +
endocrine therapy | 3 | Right S3, 1.8
cm | Lobectomy | 3-Year disease-
free follow-up |
| 1 | Present case | 2020 | 77 | pT2b/GS:3+4 | Radical
prostatectomy | 0.412 | Left S3, 2.1
cm | Lobectomy and
ND2a-1 LND | 1-Year and 4-month
disease- free follow-up |
| 1 | Yoshitake et
al (25) | 2021 | 83 | Unknown/GS:5 | Radiation
therapy | Normal range | Right S4 | Lobectomy and
LND | 5-Year disease-
free follow-up |
The present study reports the 35th rare case of
isolated lung metastasis from prostate cancer with normal
prostate-specific antigen (PSA) levels 14 years after radical
prostatectomy. In addition, a review of former case series was
performed.
Case report
A 77-year-old male who was a previous smoker with
low-grade fever was referred to Kansai Medical University Hospital.
The patient had a medical history of primary prostate cancer,
pathological stage T2bN0 [Gleason score (GS): 3+4] organ-confined
disease. He underwent radical prostatectomy at a former hospital
when he was 63 years of age. Following surgical treatment, the
patient was followed-up without additional treatment. The patient's
PSA level fell to undetectable levels for 11 years post-surgery.
During his consultation, the patient's PSA level gradually
increased and exceeded 0.2 ng/ml on two occasions without evidence
of macroscopic recurrence. This condition is identified as
biochemical recurrence. The gradual increase in the PSA levels
continued on for a further 2 years. When the levels reached 0.412
ng/ml, the doubling time was determined to be 2 years, and the
imaging examination of metastasis prior to salvage radiotherapy was
considered at the former hospital. Upon referral to Kansai Medical
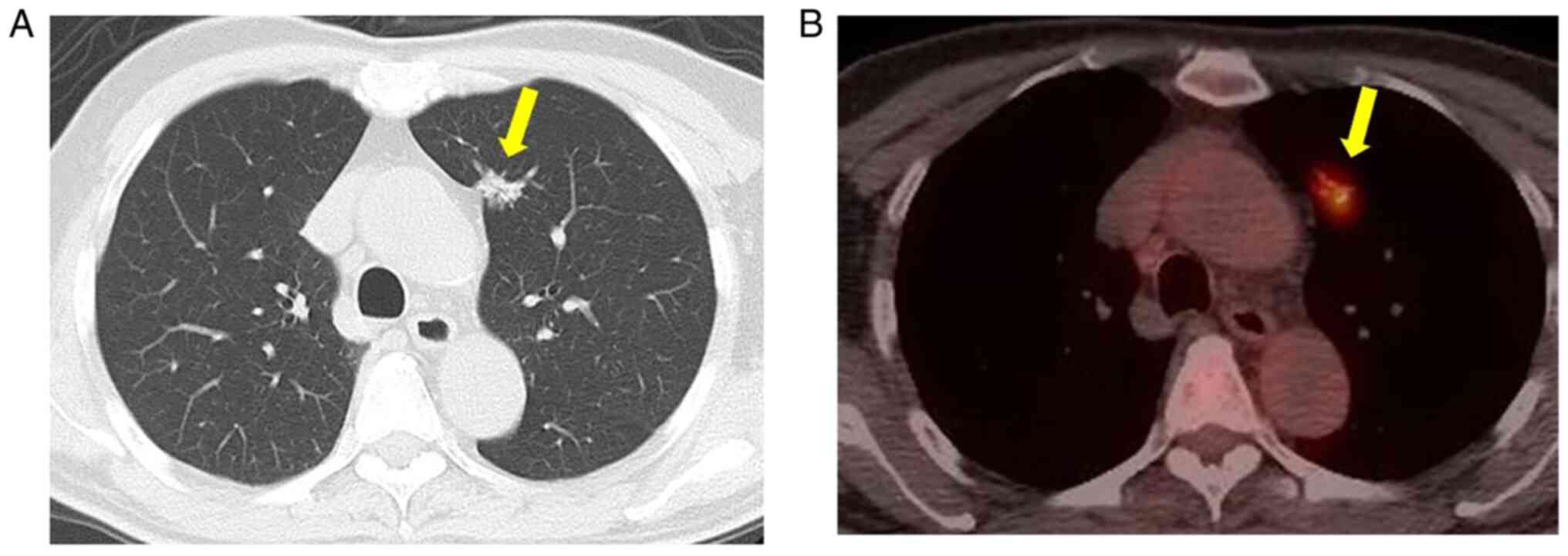
University Hospital due to low-grade fever, computed tomography
(CT) revealed a small nodule (1.5 cm in diameter) with a spiculated
morphology and pleural indentation in the upper lobe of the left
lung (Fig. 1A). Subsequently,
18-fluoro-2-deoxyglucose positron emission tomography
(18F-FDG-PET) revealed highly selective accumulation in
the nodule, strongly suggestive of a malignant tumor (maximum
standardized uptake value, 3.6) (Fig.
1B). The CT scan and 18F-FDG-PET did not reveal any
abnormal accumulation in the other organs. Primary lung cancer
(clinical T1bN0M0) was highly suspected. The levels of serum tumor
biomarkers for primary lung cancer were within the normal ranges:
Carcinoembryonic antigen, <1.0 ng/ml (normal range, <5.0
ng/ml); neuron-specific enolase, 8.2 ng/ml (<16.3 ng/ml); and
pro-gastrin-releasing peptide, 50.9 pg/ml (<81.0 pg/ml). A
thoracoscopic left upper lobectomy with hilar and mediastinal lymph
node dissection was performed as a radical surgery for primary lung
cancer. The duration of the surgery was 161 min and blood loss was
106 ml. An intraoperative frozen section was not submitted for
examination due to the high index of suspicion for primary lung
cancer based on the preoperative imaging and serum PSA levels
within the normal range, and the limitations brought about by the
coronavirus disease 2019 pandemic.
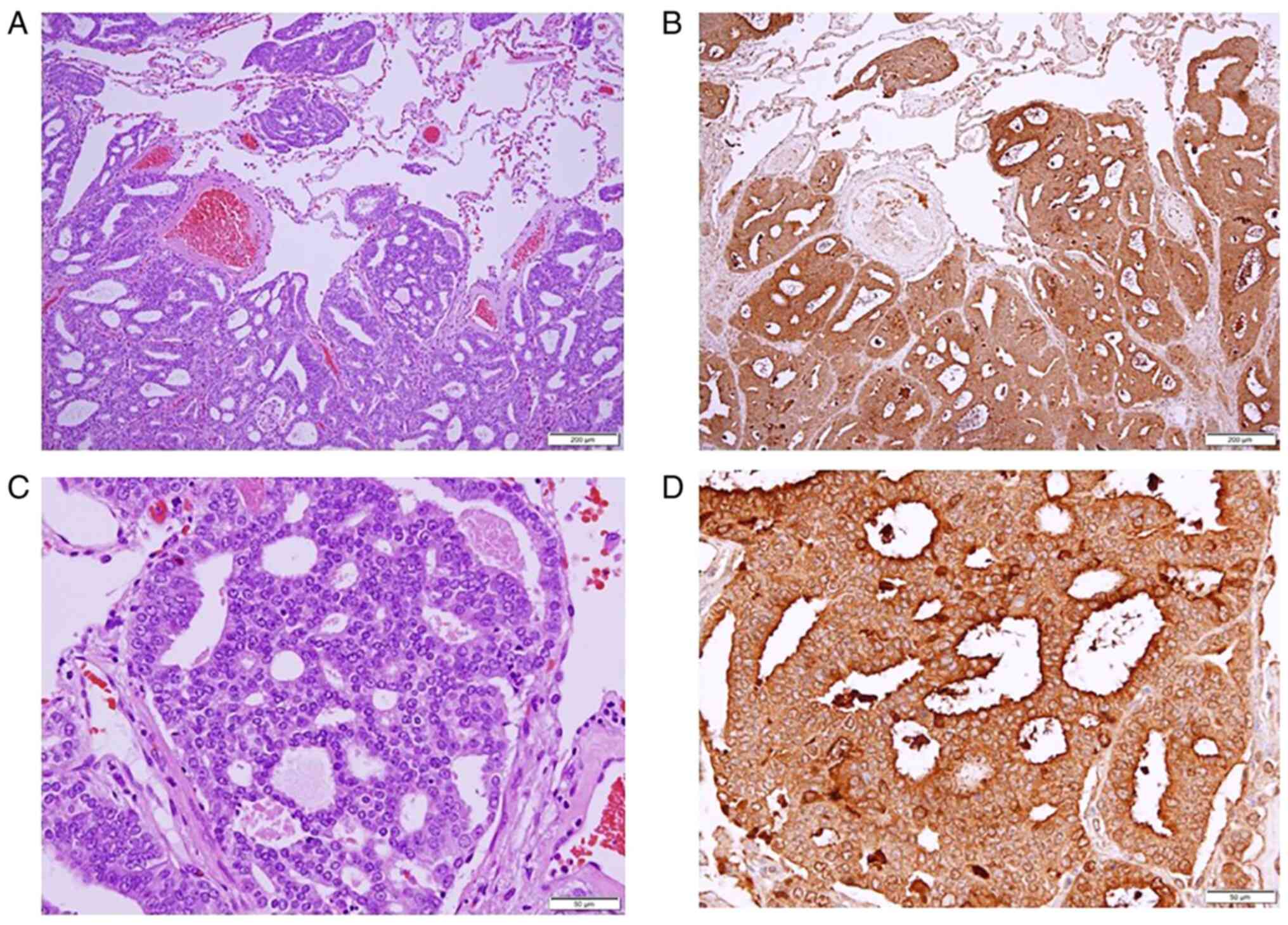
A post-operative histological examination revealed
adenocarcinoma. The tumor exhibited crowded small glands with an
amphophilic cytoplasm and enlarged nuclei with visible nucleoli
(Fig. 2A and C). Immunohistochemical staining was then
performed using sections at a thickness of 3-4 µm. The primary
antibodies used were the following: Anti-thyroid transcription
factor-1 monoclonal mouse antibody (clone 8G7G3/1; cat. no. IR056;
Dako; Agilent Technologies, Inc.), hepatocyte nuclear factor 4α
monoclonal mouse antibody (clone H1415; cat. no. PP-H1415-00,
Perseus Proteomics, Inc.), anti-human α-methylacyl-CoA racemase
monoclonal rabbit antibody (clone 13H4; cat. no. M361629-2, Dako;
Agilent Technologies, Inc.). The secondary reaction was performed
using a Ventana OptiView DAB universal kit (111427; Roche
Diagnostics K.K.). Antibody incubations were performed at 72˚C for
16 min. No counterstain was used. The sections were examined under
an Olympus BX51 microscope (Olympus Corporation).
Immunohistochemical staining revealed thyroid transcription factor
1 (-), hepatocyte nuclear factor 4α (-), PSA (+) and
α-methylacyl-CoA racemase (+) in the tumor cells (Fig. 2B and D). The tumor was diagnosed as
differentiated adenocarcinoma metastatic from prostate cancer,
based on hematoxylin and eosin staining and immunohistochemistry.
Following surgery, the patient's recovery remained uneventful, and
he was discharged on post-operative day 9. His serum PSA levels
decreased to 0.07 ng/ml. He was closely followed-up at the
Department of Urology in Kansai Medical University Hospital for 1
year and 4 months without any additional post-operative treatment.
No recurrent signs, including an elevation in PSA levels, have been
detected thus far.
Discussion
Prostate cancer has a high risk of biochemical
recurrence, with the incidence ranging from 27 to 53% (26). It has been demonstrated that the
mean interval between biochemical recurrence (PSA re-elevation
>0.2 ng/ml twice following radical prostatectomy) and clinical
recurrence (macroscopic appearance of the recurrent lesion that can
be identified by imaging or histological examination) is ~8 years,
suggesting that the long-term observation is required for patients
with prostate cancer (27). For
the patient presented herein, the possibility of a clinical
recurrence should have been suspected when the CT scan revealed a
lung nodule, as biochemical recurrence following radical
prostatectomy was already observed in the previous 3 years.
However, it was not suspected due to the following reasons: The CT
findings of this case included a solitary lesion with a spiculated
morphology, which is more typical of primary lung cancer, rather
than a metastatic lung tumor, and the serum PSA level was still
within the normal range. Additionally, solitary lung metastasis
from prostate cancer is extremely rare. Previously, radiological
evidence of pulmonary metastases following surgery, chemotherapy,
or radiotherapy for prostate cancer was detected in 48 out of 1,290
patients (3.6%) while a solitary pulmonary nodule was detected in
only 11 out of 1,290 patients (0.85%) (3). For these reasons, the clinical
recurrence of prostate cancer was not strongly suspected
preoperatively in the present case.
A search of PubMed and Ichuushi-web (http://www.jamas.or.jp) identified 24 articles and 34
patients with solitary lung metastasis from prostate cancer. A
total of 22 articles described their cases in detail. The clinical
characteristics of 23 cases were reviewed, including 22 from the
literature and the present case (Table
I). A review of a previous case series (4-25)
helped to identify pitfalls which are prone to misinterpretation
errors with significant consequences for patients.
First, the concept of the biochemical recurrence of
prostate cancer is not a commonly known issue in the fields of
surgery other than urology. However, considering the possibility of
clinical recurrence from serum PSA fluctuations, even in patients
with normal values as in the case presented herein, is crucial. It
has been demonstrated that metastases from prostate cancer with
normal serum PSA levels suggest high-grade cancer (GS≥8), small
cell carcinoma, neuroendocrine tumor, or neuroendocrine
differentiation (28). Among the
prior 23 cases, high-grade cancer or neuroendocrine differentiation
was found in 9 patients, and in the majority of cases (8/9), the
serum PSA levels were within the normal range. However, even among
patients with low-grade cancer, normal serum PSA levels were found
in more than half of the cases (6/10). Of all the cases, 74%
(17/23) had isolated lung metastasis with normal PSA levels,
regardless of the histology.
The standard procedure for pulmonary metastases is
partial resection aimed at the preservation of lung parenchyma.
Among the reviewed 23 cases with solitary lung metastasis, 22
patients had undergone lung resection and 14 of them reported
satisfactory outcomes. Lobectomy was performed in 9 patients,
including the present case. The reasons for this procedure in some
cases are highly suggestive. In four cases, intraoperative frozen
section examinations were unable to determine whether the tumor was
a metastasis or primary lung adenocarcinoma (6,15,18,25).
Considering these previous case studies, there is a high likelihood
of the misdiagnosis of prostatic adenocarcinoma with lung
metastasis as a primary pulmonary neoplasm in clinical practice,
and this is considered to be a second pitfall. Copeland et
al (29) reported the presence
of a tubule-papillary or a carcinoid-like histologic pattern in a
pulmonary tumor which was not associated with the histological
features of prostate carcinoma (29). Thus, in clinical practice, it is
prudent to consider solitary lung metastasis from prostate cancer
as a differential diagnosis for patients previously treated for
prostate cancer.
However, the surgical resection of isolated lung
metastasis from prostate cancer can be justified by its
satisfactory outcomes. Pulmonary metastatic lesions which underwent
metastasectomies have been closely related to survival in various
types of cancer, including colon and uterine cervical cancer
(30,31). The number of metastases from
prostate cancer is considered an independent prognostic factor
affecting the 5-year cancer-specific survival rate, of which
characteristic is the same as other malignancies previously
mentioned. The 5-year cancer-specific survival rate for patients
with one metastasis is 90%, compared to 32% for those with two or
more (32). Surgical resection,
particularly for solitary pulmonary lesions, is associated with
favorable outcomes in patients with prostate cancer.
In conclusion, as demonstrated in the present study,
there are some pitfalls of solitary lung metastasis from prostate
cancer leading to misdiagnosis as a primary lung cancer and
occasionally they were treated with surgical resection including
lobectomy. However, the favorable prognosis in previous case series
suggests that surgical resection may be justified.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or data analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
NM and TM wrote the manuscript. NM and TM performed
the surgery (a thoracoscopic left upper lobectomy with hilar and
mediastinal lymph node dissection). NM, TU, HM, YT, TS, HH and TM
determined the treatment plan. AO and KT performed the pathological
diagnosis and contributed to the drafting of the pathological
findings section of the manuscript. NM and HH confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Kansai Medical University Hospital (approval no.
2015630). The patient provided written informed consent.
Patient consent for publication
The patient provided consent for the publication of
his data and associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bubendorf L, Schopfer A, Wagner U, Sauter
G, Moch H, Willi N, Gasser TC and Mihatsch MJ: Metastatic patterns
of prostate cancer: An autopsy study of 1589 patients. Hum Pathol.
31:578–583. 2000.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Varkarakis MJ, Winterberger AR, Gaeta J,
Moore RH and Murphy GP: Lung metastases in prostatic carcinoma.
Clinical significance. Urology. 3:447–452. 1974.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fabozzi SJ, Schellhammer PF and El-Mahdi
AM: Pulmonary metastases from prostate cancer. Cancer.
75:2706–2709. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rockey KE and Graham TE: Prostate
adenocarcinoma metastatic to the lung. Postgrad Med. 87:199–205.
1990.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Smith CP, Sharma A, Ayala G, Cagle P and
Kadmon D: Solitary pulmonary metastasis from prostate cancer. J
Urol. 162(2102)1999.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hofland CA and Bagg MD: An isolated
pulmonary metastasis in prostate cancer. Mil Med. 165:973–974.
2000.PubMed/NCBI
|
|
7
|
Chao DH, Higgins JP and Brooks JD:
Biochemical remission after resection of prostate cancer lung
metastasis. Urology. 63:584–585. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pruthi RS, Hubbard JS, Kouba E and Wallen
E: Androgen-independent prostate cancer treated with resection of
the solitary metastatic site. Urol Int. 79:371–373. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Khandani AH, Funkhouser WK, Feins R and
Socinski MA: Simultaneous FDG PET +/Glut1+ lung and FDG
PET-/Glut1-subcarinal lymph node metastases from prostate cancer.
Ann Nucl Med. 23:595–597. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Boyer BP and Boyer MJ: An elusive tumor in
a man who has evidence of prostate cancer metastasis. JAAPA.
22:22–25. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sakai T, Kimura D, Hatanaka R, Yamada Y,
Tsushima T, Fukuda I and Kamata Yl: Surgical treated pulmonary
metastasis from prostatic cancer: Report of a case. Kyobu Geka.
63:340–343. 2010.PubMed/NCBI(In Japanese).
|
|
12
|
Goto T, Maeshima A, Oyamada Y and Kato R:
Solitary pulmonary metastasis from prostate sarcomatoid cancer.
World J Surg Oncol. 8(101)2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pepe P, Fraggetta F, Tornabene F, Nicolosi
M and Aragona F: Solitary lung metastasis after radical
prostatectomy in presence of undetectable PSA. Arch Ital Urol
Androl. 84:208–210. 2010.PubMed/NCBI
|
|
14
|
Calais J, Lussato D, Menard J, Kerviler
ED, Mongiat-Artus P, Castier Y and Merlet P: Resection of a
solitary pulmonary metastasis from prostatic Adenocarcinoma
misdiagnosed as a Bronchocele: Usefulness of 18F-choline and
18F-FDG PET/CT. J Thorac Oncol. 12:1826–1829. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Maebayashi T, Abe K, Aizawa T, Sakaguchi
M, Ishibashi N, Fukushima S, Honma T, Kusumi Y, Matsui T and Kawata
N: Solitary pulmonary metastasis from prostate cancer with
neuroendocrine differentiation: A case report and review of
relevant cases from the literature. World J Surg Oncol. 13:173–180.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gago JP, Câmara G, Dionísio J and Opinião
A: Pulmonary metastasis as sole manifestation of relapse in
previously treated localized prostate cancer: Three exceptional
case reports. Ecancermedicalscience. 10(645)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Mortier D, Baten E, Vandeurzen K and van
Renterghem K: The benefit of a surgical resection of a solitary
pulmonary metastasis of prostate cancer after radical
prostatectomy. Curr Urol. 20:210–212. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Iijima Y, Kinoshita H, Nakajima Y, Akiyama
H, Uramoto H and Hirata T: Isolated solitary lung metastasis 12
years after radical prostatectomy for prostate cancer without
elevation of the serum prostate-specific antigen level. Jpn J Chest
Surg. 31:648–652. 2017.(In Japanese).
|
|
19
|
Rush J, Pai R and Parikh RA: Complete
biochemical response after pulmonary metastasectomy in prostate
adenocarcinoma. Exp Hematol Oncol. 6(25)2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hokamp NG, Kobe C, Linzenich E, Maintz D
and Drzezga A: Solitary PSMA-positive pulmonary metastasis in
biochemical relapse of prostate cancer. Clin Nucl Med. 42:406–407.
2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Boschian R, Rizzo M, Zandona L, Trombetta
C and Liguori G: Pulmonary recurrence from prostate cancer and
biochemical remission after metastasis directed therapy. A case
report. Arch Ital Urol Androl. 90:74–75. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Polverari G, Ceci F, Allen-Auerbach M,
Gupta P, Fishbein MC, Reiter RE, Lee JM, Hope TA, Carroll RM,
Czernin J and Calais J: Solitary mucinous prostate adenocarcinoma
lung metastasis detected by 68Ga-PSMA-11 PET/CT. Clin
Genitourin Cancer. 17:e53–e55. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Asano H, Arakawa S, Kato D, Mori S, Nakada
T and Ohtsuka T: A case of single lung metastasis with normal serum
PSA level after prostate cancer surgery. Jpn Chest Surg. 33:66–69.
2019.(In Japanese).
|
|
24
|
Wu LX, Lei L, Zhu YC, Du KQ, Li XF, Chen
HF, Wang WX and Xu CW: A prostate cancer patient with isolated lung
metastases: A case report. Transl Cancer Res. 9:2064–2068.
2020.
|
|
25
|
Yoshitake H, Oura S, Yamaguchi T and
Makimoto S: Solitary lung metastasis of prostate cancer with a long
disease-free interval and normal prostate-specific antigen level.
Case Rep Oncol. 14:284–289. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Heidenreich A, Bastian PJ, Bellmunt J,
Bolla M, Joniau S, van der Kwast T, Mason M, Matveev V, Wiegel T,
Zattoni F, et al: EAU guidelines on prostate cancer. Part II:
Treatment of advanced, relapsing, and castration-resistant prostate
cancer. Eur Urol. 65:467–479. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tourinho-Barbosa R, Srougi V, Nunes-Silva
I, Baghdadi M, Rembeyo G, Eiffel SS, Barret E, Rozet F, Galiano M,
Cathelineau X and Sanchez-Salas R: Biochemical recurrence after
radical prostatectomy: What does it mean? Int Braz J Urol.
44:14–21. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Leibovici D, Spiess PE, Agarwal PK, Tu SM,
Pettaway CA, Hitszhusen K, Millikan RE and Pisters L: Prostate
cancer progression in the presence of undetectable or low serum
prostate-specific antigen level. Cancer. 109:198–204.
2007.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Copeland JN, Amin BD, Humphrey PA, Tamboli
P, Ro JY and Gal AA: The morphologic spectrum of metastatic
prostatic adenocarcinoma to the lung. Am J Clin Pathol.
117:552–557. 2002.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Onaitis MW, Petersen RP, Haney JC, Salts
L, Park B, Flores R, Rizk N, Bains MS, Dycoco J, D'Amico TA, et al:
Prognostic factors for recurrence after pulmonary resection of
colorectal cancer metastases. Ann Thorac Surg. 87:1684–1688.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yamamoto K, Yoshikawa H, Shiromizu K,
Saito T, Kuzuya K, Tsunematsu R and Kamura T: Pulmonary
metastasectomy for uterine cervical cancer: A multivariate
analysis. Ann Thorac Surg. 77:1179–1182. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ost P, Decaestecker K, Lambert B, Fonteyne
V, Delrue L, Lumen N, Ameye F and De Meerleer G: Prognostic factors
influencing prostate cancer-specific survival in non-castrate
patients with metastatic prostate cancer. Prostate. 74:297–305.
2014.PubMed/NCBI View Article : Google Scholar
|