Introduction
Neoadjuvant chemotherapy for the treatment of breast
cancer is well-established as a therapeutic modality for locally
advanced disease, inflammatory cancers, large tumours, for selected
high-risk cases and for patients who may otherwise require a
mastectomy due to the small size of the breast or the position of
the tumour within it. The use of neoadjuvant chemotherapy provides
patients with large tumours, the possibility of surgical resection
or breast-conserving surgery rather than mastectomy due to the
shrinkage of the tumour. Neoadjuvant chemotherapy allows for the
active monitoring of the response and the discontinuation of
inactive therapy in the event of disease progression.
The optimal timing for neoadjuvant chemotherapy in
breast cancer has long been debated and studied. The optimal time
interval between the end of neoadjuvant chemotherapy and definitive
surgery remains unclear. However, some large randomized clinical
trials have demonstrated no significant differences in disease-free
and overall survival between patients receiving chemotherapy in the
adjuvant and the neoadjuvant settings (1-4).
Some published studies have investigated the proper
timing for the initiation of adjuvant chemotherapy following
surgery in patients with breast cancer. Available data from these
trials revealed that there is a decrease in the efficacy of
adjuvant systemic therapy when the delay was >12 weeks
post-surgery (3,4). The Current European Society of
Medical Oncology (ESMO) guidelines suggest that systemic adjuvant
treatment should preferably commence within 2-6 weeks following
surgery (5). However, no
recommendation regarding the most effective time interval between
neoadjuvant chemotherapy and surgery has yet been made.
To date, at least to the best of our knowledge, no
large randomized control trials on neoadjuvant systemic therapy
have addressed the issue associated with the delays in time to
surgery (TS) following the completion of neoadjuvant chemotherapy
and its effect on survival outcomes.
The increasing need for multidisciplinary input,
genetic testing and counselling for reconstructive procedures,
including contralateral prophylactic surgery is known to contribute
to delays in these high-risk patients. The general consensus is to
perform surgery as soon as possible, when the neutropenic window is
overcome and following the resolution of any short-term
chemotherapy toxicities. Surgical intervention is typically
performed within 4-6 weeks following the completion of neoadjuvant
chemotherapy. This allows patients to recover sufficiently from the
side-effects of neoadjuvant chemotherapy, and theoretically
prevents potential tumour regrowth. However, the acceptable maximum
time interval is yet unknown.
Prolonged intervals without exposure to chemotherapy
and without surgical intervention may allow tumour
neo-angiogenesis, tumour growth and a possible theoretical
increased risk of recurrence. A more effective response to
neoadjuvant chemotherapy predicts decreased rates of recurrence and
an improved overall survival rate. In addition, it was described in
the literature that significant reductions in Ki-67 expression
levels following neoadjuvant chemotherapy may be associated with
decreased recurrence rates.
The timing of surgery following neoadjuvant
chemotherapy is crucial and three previous studies have
investigated its effect on the survival outcomes of these high-risk
patients. Sanford et al (6)
demonstrated that patients with neoadjuvant chemotherapy to surgery
intervals of up to 8 weeks had equivalent overall, recurrence-free
and locoregional recurrence-free survival rates. Another published
study suggested that significantly worse overall and
recurrence-free survival rates were associated with a TS >21
days (7). In addition, it has been
reported that patients who had undergone surgery >40 days
following neoadjuvant chemotherapy had lesser reductions in Ki-67
levels, potentially indicating tumour regrowth and predicting a
worse oncological outcome (8).
As per the authors' observation, the delays were
mainly due to chemotherapy side-effects, post-chemotherapy scans,
delayed multi-disciplinary team (MDT) discussion, clinical
appointments, long waiting lists and patient's decision to postpone
surgery. Unnecessary delays can affect the survival outcomes of
these high-risk patients. The Breast Unit Multidisciplinary team at
Medway Hospital is in agreement that an ideal interval from
neoadjuvant chemotherapy to surgery of ≤28 days or less would allow
a reasonable time to recover from side-effects and would also allow
adequate time for surgical planning, yet not compromising the
survival outcomes. Thus, the present study aimed to evaluate the
efficacy of TS of ≥28 days in post-neoadjuvant chemotherapy breast
cancer patients and to determine its effect on survival
outcomes.
Patients and methods
The present study was retrospective study on female
patients who had received neoadjuvant chemotherapy followed by
breast-conserving surgery or mastectomy for breast cancer
(inclusion criteria) between January 1, 2012 and December 31, 2016
at Medway Hospital, Gillingham, Kent, UK. Patients with recurrent
breast cancer, those with distant metastases and male patients were
excluded from the study. UK Research Ethics Committees (RECs) and
HRA approval for the present study was obtained (REC reference:
17/NS/0007 and IRAS project ID: 222885).
A total of 61 breast cancer patients who had
received neoadjuvant chemotherapy fulfilled the inclusion and
exclusion criteria. The data elements extracted were patient age,
tumour type, grade, hormone receptor status, HER2 status,
lymphovascular invasion and evidence of lymph node involvement, the
time interval between the completion of the last cycle of
neoadjuvant chemotherapy to surgery, any evidence of local or
regional recurrence in the post-operative follow-up period,
evidence of distant metastasis in the post-operative follow-up
period and mortality in the post-operative follow-up period.
These patients were categorised into two cohorts
based on the TS following the completion of neoadjuvant
chemotherapy based on the multidisciplinary team agreement of the
Breast Unit of Medway Hospital on the ideal TS. The patients in
group 1 (n=8) had a TS of ≤28 days, and those in group 2 had a TS
of ≥29 days (n=53). Overall and locoregional recurrence-free
survival were compared between both groups. Locoregional
recurrence-free and overall survival curves were constructed using
the Kaplan-Meier method for both cohorts and P-values
calculated.
Statistical analysis
The overall survival and locoregional
recurrence-free survival of the patient cohorts was analysed using
Kaplan-Meier analysis with the log-rank test for statistical
significance. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
Details of age distribution, the type of breast
cancer, receptor positivity and type of surgery performed in the
study groups are presented in Table
I. A total of 61 breast cancer patients who had received
neoadjuvant chemotherapy and satisfied the inclusion criteria were
included in the study. In total, 13.1% of the patients in the study
group (n=8) had a TS of ≤28 days and comprised group 1 and 86.9% of
the patients (n=53) with a TS of ≥29 days comprised group 2. The
rate of breast-conserving surgery in the study group was 62%, with
100% of the patients in group 1 and 56% in group 2, respectively.
The follow-up period was 13-73 months. The median follow-up time
was 29 months. The TS and patient characteristics including age,
nuclear grade, histology, lymphovascular invasion, tumour biology
(HER2- positive, triple-negative, or hormone-receptor status), the
presence of pathological complete response, and surgery type
(breast-conserving surgery or mastectomy) were not compared between
the two groups, as there were no observed differences.
 | Table IDetails of patient age distribution,
type of breast cancer, receptor positivity and type of surgery
performed. |
Table I
Details of patient age distribution,
type of breast cancer, receptor positivity and type of surgery
performed.
| Parameter | No. of patients |
|---|
| Age groups | |
|
31-50
years | 33 |
|
51-70
years | 24 |
|
71-80
years | 4 |
| Tumour type | |
|
Invasive
ductal carcinoma | 55 |
|
Invasive
lobular carcinoma | 5 |
|
Basal
type | 1 |
| Tumour grade | |
|
Grade 1 | 0 |
|
Grade 2 | 19 |
|
Grade 3 | 42 |
| Receptor status (ER,
PR, HER2) | |
|
Triple-negative
(ER-, PR-, HER2-) | 22 |
|
ER+,
PR+, HER2- | 11 |
|
HER2 only
positive (ER-, PR-, HER2+) | 12 |
|
ER+,
PR-, HER2+ | 3 |
|
ER only
positive (ER+, PR-, HER2-) | 9 |
|
Triple-positive
(ER+, PR+, HER2+) | 4 |
| Type of surgery
performed | |
|
Mastectomy | 23 |
|
Wide local
excision with no wire localisation | 14 |
|
Wide local
excision with wire localisation | 24 |
The Kaplan-Meier estimates of survival in both
cohorts were calculated for overall survival and locoregional
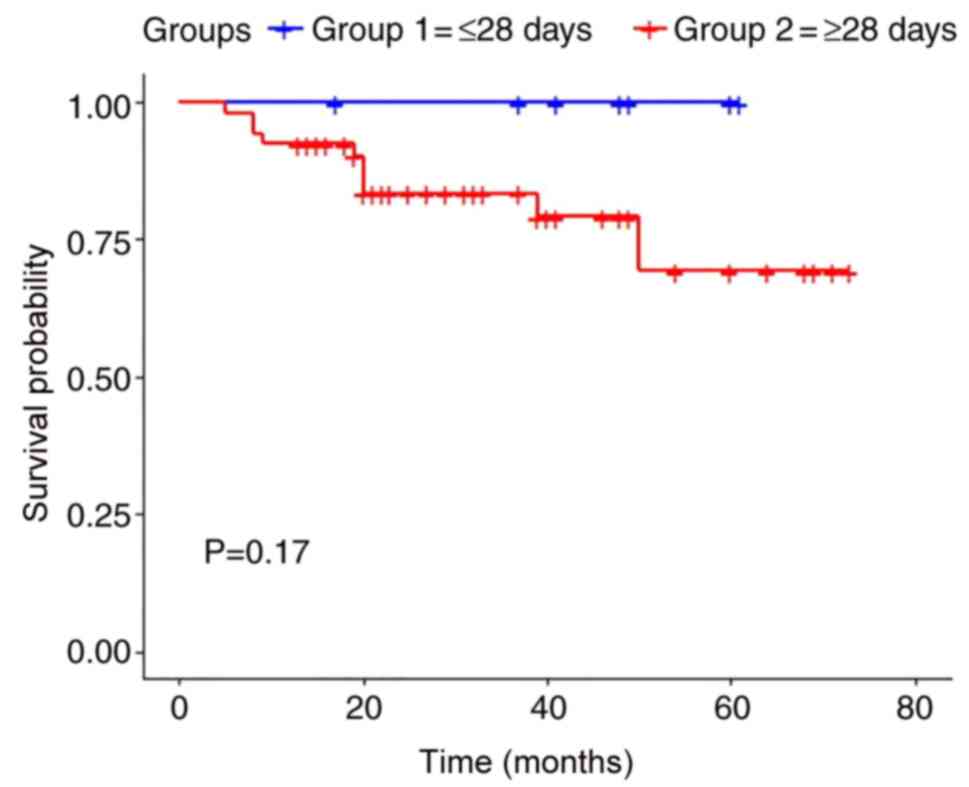
recurrence-free survival. A P-value of 0.17 was estimated for
overall survival with no statistically significant difference
(Fig. 1). The estimated P-value
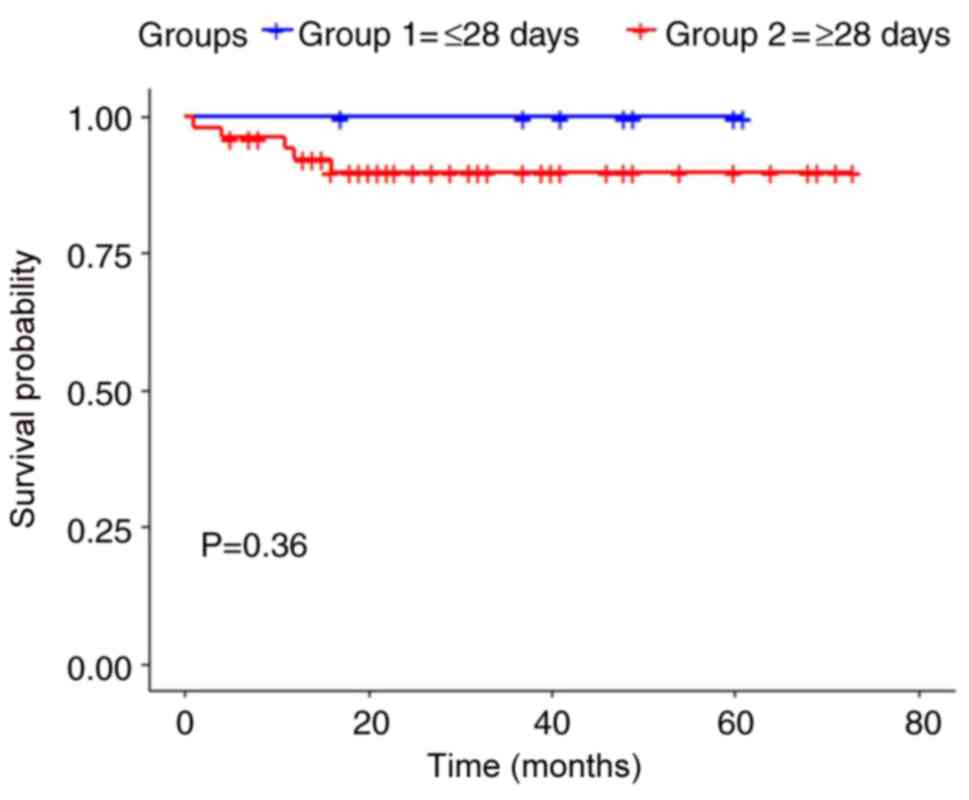
for locoregional recurrence-free survival was 0.36 with no
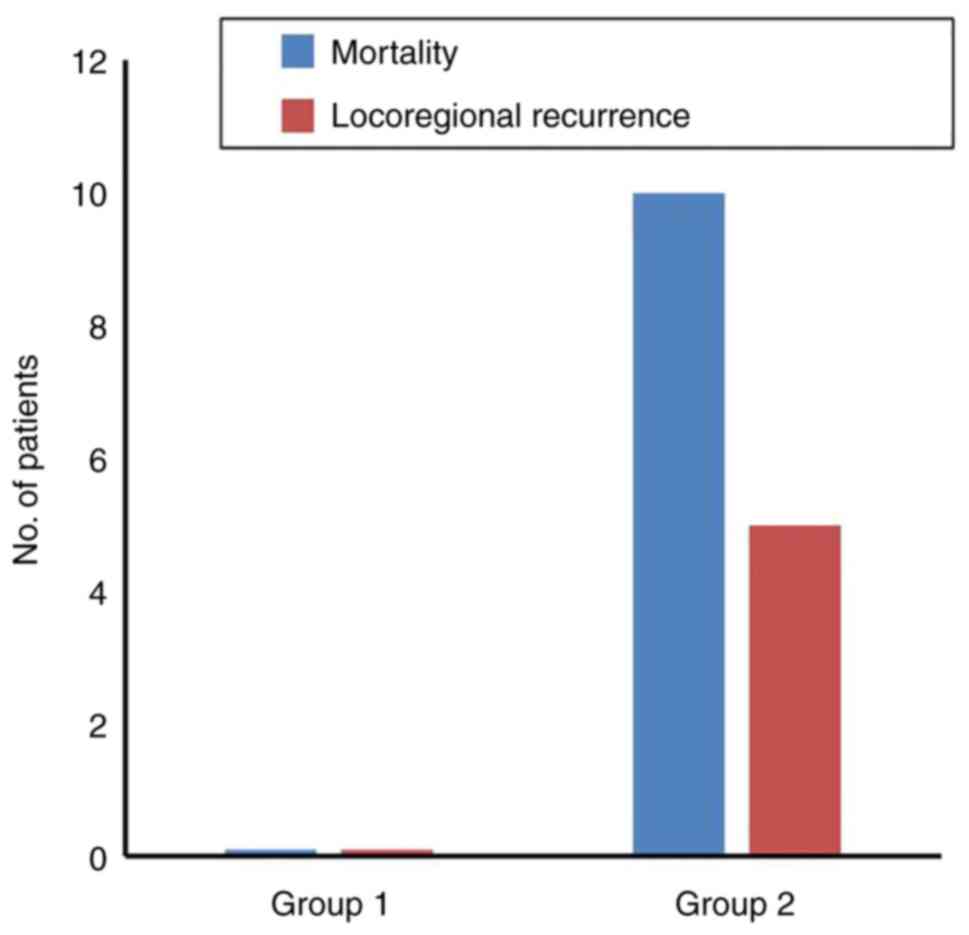
statistically significant difference (Fig. 2). The mortality rates were zero in
group 1 and 18.9% in group 2. Locoregional recurrence rates were
zero in group 1 and 9.4% in group 2 (Fig. 3).
Discussion
The optimal time interval between the completion of
neoadjuvant chemotherapy and definitive surgery in breast cancer
patients remains unclear. To date, no large randomized clinical
trials on neoadjuvant systemic therapy have addressed the TS delays
and the effect on survival outcomes in patients receiving
neoadjuvant chemotherapy. The general consensus is to perform
surgery following the resolution of chemotherapy-induced
neutropenia and short-term chemotherapy-related toxicities.
Omarini et al (7) evaluated the association between TS
following neoadjuvant chemotherapy and survival outcomes. They
concluded that the patients with breast cancer who underwent
surgery within 21 days experienced maximal benefit from the
previous treatment and this advantage was consistent and maintained
over time. Sanford et al (6) investigated the association between
the time interval from neoadjuvant chemotherapy to surgery and
survival outcomes at the MD Anderson Centre in the time period
between years 1995-2007. They suggested that those patients with
neoadjuvant chemotherapy to surgery intervals of up to 8 weeks had
equivalent overall, recurrence-free and locoregional
recurrence-free survival rates.
Prolonged TS intervals may theoretically increase
the risk of recurrence by allowing tumour neo-angiogenesis and
tumour growth. Gabordi et al (8) evaluated the association between TS
from the completion of neoadjuvant chemotherapy and the reduction
in Ki-67 levels. They reported that patients who underwent surgery
within 40 days from the completion of neoadjuvant chemotherapy
experienced a greater reduction in Ki-67 levels. Patients
undergoing surgical intervention >40 days following neoadjuvant
chemotherapy had lesser reductions in Ki-67, potentially indicating
tumour regrowth and predicting a worse oncologic outcome.
The breast multidisciplinary team at Medway Hospital
is in agreement that an ideal TS interval of ≤28 days, allows for a
reasonable time to recover from side-effects and allows adequate
surgical planning. It is considered ≤21 days is too short to be
practically achievable and does not allow sufficient time to
recover from the side-effects of chemotherapy.
In the present study, 86.8% of the patients (group
2) underwent surgery at ≥29 days following the completion of
neoadjuvant chemotherapy. In total, 13.1% of the patients (group 1)
had surgery at ≤28 days following the completion of neoadjuvant
chemotherapy. Kaplan-Meier estimates of overall survival (P-value
of 0.17) and locoregional recurrence-free survival (P-value of
0.36) revealed no statistically significant differences. However, a
trend towards superior survival outcomes with a TS interval of ≤28
days was observed.
It should be noted however, that the present study
has certain limitations due to the small samples size and its
retrospective nature.
In conclusion, the analysis of the data in the
present study suggests a trend towards favourable survival outcomes
following neoadjuvant chemotherapy if the TS is ≤28 days. A target
interval of 28 days is achievable and also favours adequate
recovery from the side-effects of chemotherapy. The findings of the
present study support a shorter TS following neoadjuvant
chemotherapy for an optimal survival outcome in these high-risk
patients.
Acknowledgements
The authors would like to extend their special
gratitude to Dr Maher Hadaki, Consultant medical oncologist, Medway
Hospital for providing access to the trust oncology database and
for his valuable assistance during the data collection phase.
Funding
Funding: No funding was received.
Availability of data and materials
The availability of datasets used and/or analysed
during the current study are available only from the corresponding
author on reasonable request subjected to prior approval by Medway
hospital ethics and clinical governance committee.
Authors' contributions
BSP was involved in the conception and design of the
study, as well as in data collection and data analysis, and in the
writing, revising and reviewing of the manuscript. DH, AK, CA and
IA were involved in the conception and design of the study, and in
the revising and reviewing of the manuscript. BSP and IA confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
NHS Research Ethics Committees (RECs) and HRA
approval for the present study was obtained (REC reference:
17/NS/0007 and IRAS project ID: 222885).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deo SV, Bhutani M, Shukla NK, Raina V,
Rath GK and Purkayasth J: Randomized trial comparing neo-adjuvant
versus adjuvant chemotherapy in operable locally advanced breast
cancer (T4b N0-2 M0). J Surg Oncol. 84:192–197. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mauri D, Pavlidis N and Ioannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kupstas AR, Hoskin TL, Day CN, Habermann
EB and Boughey JC: Effect of surgery type on time to adjuvant
chemotherapy and impact of delay on breast cancer survival: A
national cancer database analysis. Ann Surg Oncol. 26:3240–3249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai L, Tong Y, Zhu X, Shen K, Zhu J and
Chen X: Prolonged time to adjuvant chemotherapy initiation was
associated with worse disease outcome in triple negative breast
cancer patients. Sci Rep. 10(7029)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
European Society for Medical Oncology
(ESMO): ESMO Clinical Practice Guidelines: Breast cancer. ESMO,
Lugano, 2020. https://www.esmo.org/guidelines/breast-cancer.
|
|
6
|
Sanford RA, Lei X, Barcenas CH, Mittendorf
EA, Caudle AS, Valero V, Tripathy D, Giordano SH and
Chavez-MacGregor M: Impact of time from completion of neoadjuvant
chemotherapy to surgery on survival outcomes in breast cancer
patients. Ann Surg Oncol. 23:1515–1521. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Omarini C, Guaitoli G, Noventa S,
Andreotti A, Gambini A, Palma E, Papi S, Tazzioli G, Balduzzi S,
Dominici M, et al: Impact of time to surgery after neoadjuvant
chemotherapy in operable breast cancer patients. Eur J Surg Oncol.
43:613–618. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gabordi RC, Huth J, Rivers A, Hendrix AA,
Wooldridge R, Leitch , et al: Optimal time interval to
surgery after neoadjuvant chemotherapy in breast cancer. Annual
Meeting, The American Society of Breast Surgeons, April 30-May 4,.
2014, Las Vegas, NV.
|