Introduction
Amniotic fluid embolism (AFE), a rare and severe
obstetric complication, is a significant cause of maternal
mortality worldwide (1). AFE
occurs in a fetal antigen dose-dependent (mechanical obstruction
subtype) or -independent manner (anaphylactic and anaphylactoid
reaction subtype) (2). The former
subtype is believed to occur when amniotic fluid disastrously
enters the maternal circulation, resulting in the physical
obstruction of pulmonary circulation. The latter subtype is
predominantly mediated by the first exposure to amniotic fluid
components that may cause an anaphylactoid reaction (3). Although AFE remains unpredictable
based on current diagnostic strategies, recent advances in
molecular biology have facilitated analysis of its pathogenesis.
Advances in biochemistry experiments have enabled the
identification of novel serum biomarkers of amniotic fluid passage
into the maternal circulation (4-13).
For example, Zinc-coproporphilin-1 (ZnCP1) (4), sialyl Tn (STN) (5,6) and
insulin-like growth factor binding protein 1 (IGFBP1) (12) have been reported as substances that
are extremely abundant in amniotic fluid and present in trace
amounts in maternal blood. In 2012, the authors newly identified
squamous cell carcinoma antigen (SCCA) as a substance with a very
high amniotic fluid/maternal blood concentration ratio (10). SCCA is a member of the serine
protease inhibitor family of proteins, serpin, physiologically
found in the spinous and granular layers of normal squamous
epithelium (3,14). SCCA is typically expressed in
neoplastic cells of epithelial or endodermal origins, such as
advanced squamous cell carcinomas of the cervix (14). SCCA inhibits cell damage caused by
lysosomal cathepsins; however, the regulation of SCCA gene
expression, its biological function and localization in each organ
remain unclear (15). SCCA is
present in trace amounts in peripheral blood even in non-pregnant
women (14). Therefore, SCCA is
not an amniotic fluid specific substance, but is characterized by
the highest amniotic fluid/maternal blood concentration ratio,
indicating that SCCA is suitable for serodiagnosis of AFE (10). This fact suggests that SCCA is a
more valuable and reliable marker for serodiagnosis of AFE than
ZnCP1, STN and IGFBP1 (9-11).
In 2017, the authors reported extremely high levels of SCCA in the
maternal serum of 4 patients with autopsy-proven AFE (13).
It was previously demonstrated that amniotic fluid
enters the maternal circulation, particularly in the uterine
vasculature, in a variety of obstetric disorders (16). Maternal intravascular fetal
material during peripartum hysterectomy for the treatment of
uterine rupture, abruption, uterine atony, placenta previa,
placenta accreta, coagulopathy and retained placenta has been found
to be present in one-third of patients (16). Furthermore, the fetal-to-maternal
transfer of cell-free fetal DNA and fetal blood through the
placenta and uterine vasculature may be common at some point during
pregnancy (17). Therefore, the
entry of amniotic fluid and fetal material into the maternal
circulation and uterine vasculature is necessary (minimum
requirement) for the onset of AFE, although it is not sufficient
(2). Anaphylactic and
anaphylactoid reactions can occur at the first exposure to amniotic
fluid components (2).
The aim of the present study was to determine the
dynamic changes in serum SCCA levels before and after delivery in
relation to the mode of delivery and the potential origin of
amniotic fluid SCCA. Maternal serum SCCA levels were measured
before and after delivery to determine whether amniotic fluid
components enter the maternal circulation only in patients with AFE
or even in women with normal labor. Knowing whether amniotic fluid
gains access to the systemic circulation during normal vaginal
delivery is important for understanding the pathophysiology of
AFE.
Patients and methods
Patient information
The collection of blood, amniotic fluid, urine and
tissue samples was approved by the Institutional Review Board for
Human Research at Nara Medical University (no. 579, 579-2, 873 and
873-2). Informed consent was obtained from all participants. The
present prospective first cohort study was conducted at the Nara
Medical University Hospital, Kashihara, Japan, from December, 2011
to October, 2015 to measure the dynamic changes in maternal serum
SCCA levels before and after delivery in relation to the mode of
delivery. Japanese pregnant women who had been admitted to the
labor ward or operation room were recruited. All women had
singleton pregnancies. Women with all levels of parity were
included. Women undergoing instrumental delivery and those with
pregnancy-related complications, including hypertensive disorders
of pregnancy or preeclampsia were excluded. No participant had any
malignant disease. Women who underwent normal vaginal delivery
(n=339), planned cesarean section without labor (n=97), or
emergency cesarean section after the onset of labor (n=28) were
included. The second cohort study was conducted to determine the
potential origin of SCCA in amniotic fluid from September, 2017 to
December, 2019 (described below in 'Sample collection').
Sample collection
In the first cohort study, a 9-ml venous blood
sample was collected from each participant. Maternal serum samples
were obtained at baseline (time of admission), at 2 h postpartum
and on postpartum day 3. In the second cohort study, placental
tissue and amniotic fluid samples were obtained during cesarean
deliveries. Urine samples (n=7) of neonates without complications
were obtained within 2 days after birth at term. Serum and urine
samples were separated by centrifugation at 1,400 x g for 5 min at
room temperature and stored at -80˚C until tested. A pairwise
comparison of SCCA measurements between maternal serum and neonatal
urine could not be performed as they were obtained from separate
cases. Amniotic fluid samples were aseptically retrieved by
amniotomy following uterine muscle layer incision. These samples
were divided into 2 groups as follows: Control samples with clear
amniotic fluid (n=27) and samples of meconium-stained amniotic
fluid (n=4). Formalin-fixed, paraffin-embedded tissue sections from
3 full-term placentas and 3 cervical cancer cases from the
Department of Obstetrics and Gynecology of Nara Medical University
Hospital were obtained from the pathology archives. A commercially
available fetal skin tissue slide obtained from a 35-week fetus was
purchased (Pantomics, Inc.). SCCA protein expression was assessed
by immunohistochemistry.
Preparation of amniotic fluid
samples
Amniotic fluid contains fetal skin keratinocytes,
amnion epithelial cells, epithelioid cells, fibroblasts and
pluripotent stem cells. Approximately 20 ml amniotic fluid was
obtained during surgery. As previously described (18), cell block sections of amniotic
fluid samples were prepared using the sodium alginate method and
subjected to hematoxylin and eosin (H&E) and
immunohistochemical staining. Cellular pellets were fixed with 10%
paraformaldehyde for 12 h at room temperature. Formalin-fixed
pellets were then embedded in paraffin. The unstained sections
(3-µm-thick) were stained with hematoxylin for 5 min and with eosin
(Sakura Finetek Japan Co., Ltd.) for 2 min at room temperature. The
slides were examined and photographed using an Olympus CX41
microscope (Olympus Corporation).
SCCA measurements
The serum SCCA levels were measured by competitive
Luminex immunoassay using a commercially available kit from BML
according to the manufacturer's instructions. SCCA levels were
calculated using their average optical densities based on standard
curves. The detection limit was 0.1 ng/ml.
Immunohistochemistry
SCCA is a member of the ovalbumin serpin
(ov-serpin)/clade B serpin family, which is composed of SCCA1
(SERPINB3) and SCCA2 (SERPINB4) (19,20).
Routine H&E staining followed by immunohistochemical staining
was performed to detect SCCA protein expression. For
immunohistochemistry, paraffin sections (3-µm-thick) were mounted
on poly-L-lysine-coated slides. Samples, including fetal skin,
placental and cervical cancer tissues were incubated for 1 h at
room temperature with a mouse-specific antibody against
pan-cytokeratin AE1/AE3 (ab27988; Abcam; 1:100 dilution),
monoclonal antibodies against SCCA1/A2 (sc-28384; Santa Cruz
Biotechnology, Inc.; 1:100 dilution), or normal rabbit IgG (#3900;
Cell Signaling Technology, Inc.; 1:100 dilution). The sections were
placed for 30 min at room temperature with amplification reagent
(Simple Stain MAX PO; Nichirei Biosciences), followed by incubation
for 5 min with diaminobenzidine (Simple Stain DAB Solution,
Nichirei Bioscience).
Statistical analysis
Statistical analyses were performed using SPSS
Statistics version 25 (IBM Japan). Data distribution was verified
by the Shapiro-Wilk test, indicating that all groups exhibited
non-normal distribution (data not shown). Data were analyzed using
the Kruskal-Wallis test followed by post hoc analysis with
Bonferroni correction to evaluate differences among the 3 groups in
maternal age, gestational age of the fetus and SCCA level between
baseline, 2 h postpartum and 3 day postpartum. Pearson's
Chi-squared test was performed to compare parity among the 3
groups. P-values of <0.05 were considered to indicate
statistically significant differences.
Results
The patient demographic characteristics are
presented in Table I. There were
significant differences between the vaginal delivery and the 2
cesarean section groups as regards maternal age, gestational age
and parity (all P<0.001). Maternal age was lower, whereas
gestational age was higher in the vaginal delivery group than in
the cesarean section groups. In the cesarean section with labor
group, 71.4% of mothers (20/28) were nulliparous compared with
51.3% (174/339) in the vaginal delivery group and 24.7% (24/97) in
the cesarean section without labor group. The numbers of serum
samples at baseline, at 2 h postpartum and on postpartum day 3 are
presented in Table II. The serum
SCCA levels in all groups were significantly altered during the
study period. In the vaginal delivery group, the SCCA levels
significantly increased from baseline to 2 h postpartum, and
decreased 3 day postpartum. The SCCA levels were lowest at 3 days
postpartum. In both cesarean section groups, the serum SCCA levels
at baseline and 2 h postpartum did not differ significantly
(P=0.999 and 0.999, respectively). All groups exhibited a marked
decrease in SCCA levels from admission or 2 h postpartum to 3 days
postpartum (P<0.001 or P<0.001 for the vaginal delivery
group, P=0.002 or P=0.003 for the cesarean section with labor
group, and P<0.001 or P<0.001 for the cesarean section
without labor group; Table
II).
 | Table IDemographic characteristics of the
patients in the present study. |
Table I
Demographic characteristics of the
patients in the present study.
| Characteristic | Normal vaginal
delivery | CS with labor | CS without labor | P-value |
|---|
| Number of
deliveries | 339 | 28 | 97 | |
| Maternal age
(years) | | | | <0.001 |
|
Mean ±
SD |
32.1±5.6a |
34.8±4.9b |
34.3±4.4c | |
|
Median
(range) | 33.0 (17-46) | 35.0 (26-43) | 34.0 (22-46) | |
| Gestational age
(weeks) | | | | <0.001 |
|
Mean ±
SD |
39.1±1.5d |
38.4±2.3e |
37.6±1.5f | |
|
Median
(range) | 39 (26-41) | 38.0 (29-41) | 38.0 (29-41) | |
| Parity | | | | <0.001 |
|
0 | 174 | 20 | 24 | |
|
≥1 | 165 | 8 | 73 | |
 | Table IISCCA levels for each delivery mode at
the time of admission, at 2 h postpartum, and on postpartum day
3. |
Table II
SCCA levels for each delivery mode at
the time of admission, at 2 h postpartum, and on postpartum day
3.
| Delivery mode | Admission | 2 h postpartum | Postpartum day 3 | P-value |
|---|
| Vaginal (n) | 231 | 277 | 150 | <0.001 |
|
Mean ±
SD |
1.83±1.86a |
4.15±2.42b |
0.96±0.99c | |
|
Median
(range) | 1.60
(0.40-19.30) | 3.70
(0.60-18.80) | 0.8 (0.40-11.80) | |
| CS with labor
(n) | 23 | 14 | 8 | 0.001 |
|
Mean ±
SD |
1.78±1.35d |
1.83±1.05e |
0.78±0.16f | |
|
Median
(range) | 1.40 (0.70-6.80) | 1.60 (0.80-4.20) | 0.85 (0.50-0.90) | |
| CS without labor
(n) | 70 | 60 | 53 | <0.001 |
|
Mean ±
SD |
1.58±1.82g |
1.43±0.56h |
0.85±0.53i | |
|
Median
(range) | 1.30
(0.60-16.10) | 1.30 (0.60-3.40) | 0.80 (0.40-3.70) | |
The origin of SCCA in amniotic fluid was then
determined. No significant differences were observed in the SCCA
levels between the clear amniotic fluid, meconium-stained amniotic
fluid and neonatal urine samples, all of which were extremely high
(Table III). Finally, the
present study investigated whether SCCA was expressed in the
placenta, amniotic fluid cells and fetal skin. Full-term placentas,
amniotic fluid cell blocks, fetal skin and cervical cancer tissues
were examined by immunostaining for cytokeratin and SCCA (Fig. 1). Cytokeratin is widely used as a
marker for the identification and characterization of epithelial
cells, trophoblasts and cervical cancer cells (Fig. 1A, D, G and
J). For SCCA immunostaining,
cervical cancer tissues were used as the positive control (Fig. 1K). The samples were reviewed by
experienced pathologists (Tomoko Uchiyama and Chiho Ohbayashi,
Department of Diagnostic Pathology, Nara Medical University,
Kashihara, Japan) to determine the expression of cytokeratin, SCCA
and nonimmune IgG. The amniotic membrane, and trophoblast cells and
mesenchymal cells in the placenta were not stained for SCCA
(Fig. 1B). Diffuse cytokeratin
immunostaining was strong in the majority of amniotic fluid cells
(Fig. 1D), whereas SCCA expression
was scattered and weakly positive (Fig. 1E). Fetal skin epidermal cells were
positively labeled for cytokeratin (Fig. 1G), but SCCA expression was limited
(Fig. 1H). In fetal skin, SCCA was
localized to scattered cells of the keratinization area (stratum
corneum) and hair matrix cells (Fig.
1H).
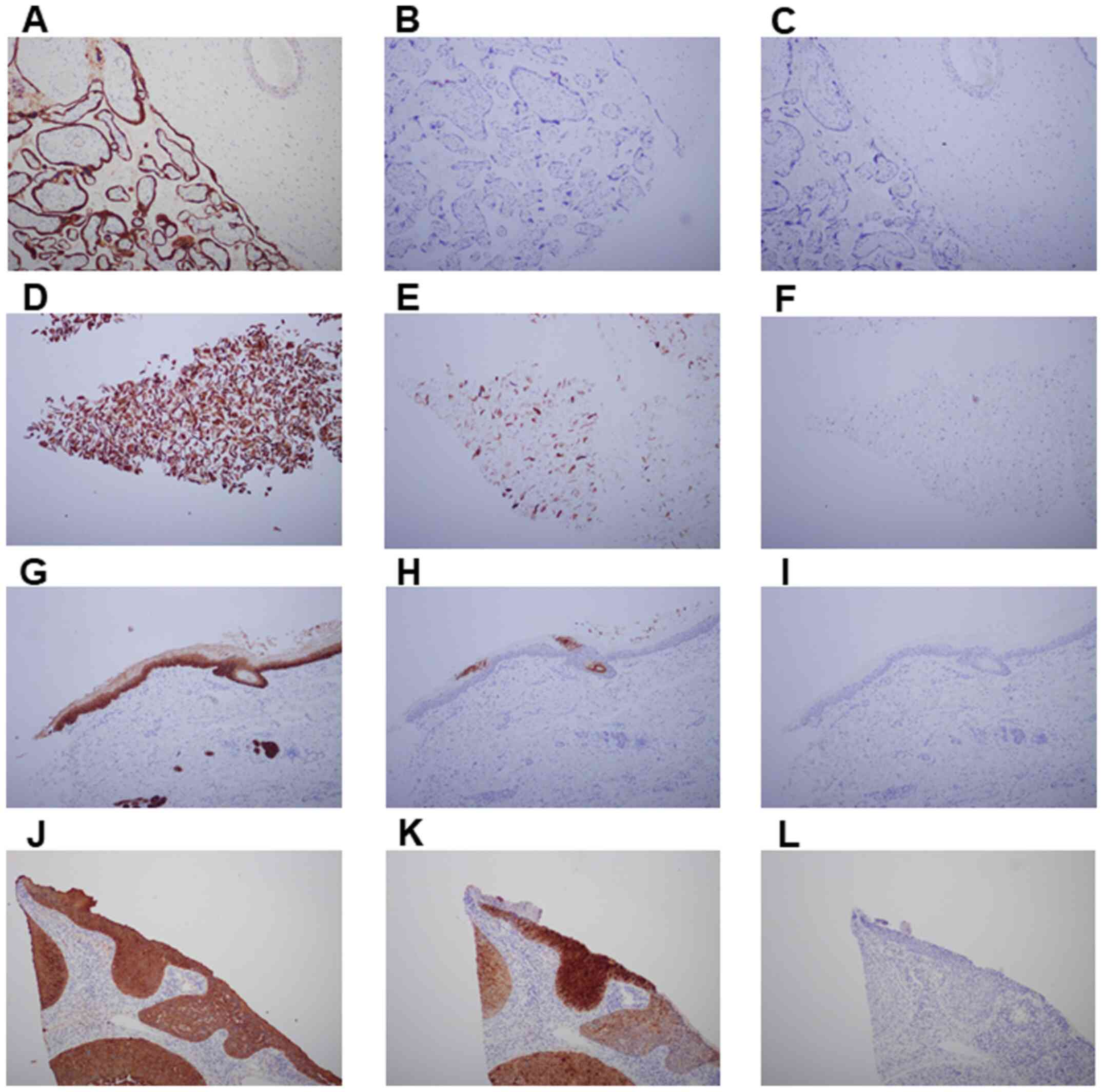 | Figure 1Distribution of cytokeratin and
squamous cell carcinoma antigen (SCCA) in full-term placentas,
amniotic fluid cell blocks, fetal skin, and cervical cancer
tissues. The distribution of (A, D, G and J) cytokeratin, (B, E, H
and K) SCCA, and (C, F, I and L) normal rabbit IgG in the (A, B and
C) full-term placentas, (D, E and F) amniotic fluid cell blocks,
(G, H and I) fetal skin and (J, K and L) cervical cancer tissues is
shown by representative immunostaining. The full-term placenta was
(A) positive for cytokeratin AE1/AE3 and (B) negative for SCCA
(magnification, x100). Cytokeratins were present in the epidermis
and all kinds of trophoblasts. Amniotic fluid cell blocks exhibited
(D) intense labeling for AE1/AE3, but (E) weak and scattered
staining for SCCA (magnification, x100). Fetal skin epidermal cells
were negatively labeled for SCCA. SCCA was localized in scattered
cells of the keratinization area and hair matrix cells. As positive
controls for immunostaining, cervical cancer tissues were
positively labeled for (J) cytokeratin and (K) SCCA (magnification,
x100). |
 | Table IIISCCA levels in amniotic fluid and
neonatal urine. |
Table III
SCCA levels in amniotic fluid and
neonatal urine.
| SCCA levels (mean ±
SD) Clear amniotic fluid (n=27) | Meconium-stained
amniotic fluid (n=4) | Neonatal urine
(n=7) | P-value |
|---|
| 569.37±432.32 | 378.08±57.82 | 322.83±427.74 | 0.147 |
Discussion
The present study suggests, for the first time, to
the best of our knowledge, that amniotic fluid components
containing SCCA enter the maternal circulation during normal
vaginal delivery, and that amniotic fluid SCCA may originate from
fetal urine.
First, SCCA levels in amniotic fluid were reportedly
much higher than those in maternal serum, suggesting the potential
clinical utility of serum SCCA level as a marker for AFE diagnosis
(10,13). This fact suggests that amniotic
fluid components enter the maternal circulation in patients with
AFE. However, it remains unclear whether normal delivery affects
the influx of amniotic fluid components into the maternal
circulation. The present study therefore determined the dynamic
changes in maternal serum SCCA levels before and after delivery in
relation to the mode of delivery. In vaginal delivery, serum SCCA
levels increased significantly from baseline to 2 h postpartum and
decreased 3 days postpartum (Table
II). The decline in serum SCCA levels 3 days after delivery may
be due to metabolism of SCCA in the kidney and its short half-life
(2). The disappearance curve of
serum SCCA levels following resection of malignant tumor has
exhibited an average half-life of 2.2 h (21). It was found that amniotic fluid
components entered the maternal circulation in all cases of vaginal
delivery, but no women exhibited cardiorespiratory symptoms
suggestive of AFE.
Second, it was hypothesized that during a cesarean
section, maternal serum SCCA levels should increase as the influx
of amniotic fluid into the maternal circulation is inevitable.
Unexpectedly, maternal serum SCCA levels remained unaltered at 2 h
postpartum compared with those at baseline in both cesarean
patients with or without labor. However, the SCCA levels in
patients with normal delivery were significantly higher than those
of planned cesarean patients without labor (1.83±1.86 ng/ml vs.
1.58±1.82 ng/ml, P=0.033; Table
II). Furthermore, the SCCA levels were extremely high in those
who reached full dilatation and delivered vaginally (4.15±2.42
ng/ml). These data suggest that the onset of labor is a major
factor for elevated SCCA levels, and that amniotic fluid can enter
the maternal circulation from a micro-laceration at the amniotic
fluid-maternal blood barrier when the cervix is fully opened.
Benson et al suggested (16) that amniotic fluid, fetal cells,
hair and other debris can enter the maternal circulation by
breaching the placenta-uterine vascular circulation barrier during
full cervical dilatation. That is, both the onset of labor and full
cervical dilatation are the causes of entry of amniotic fluid
components into the maternal circulation.
Third, the present study investigated the origin of
amniotic fluid SCCA. There was no significant difference in SCCA
levels between amniotic fluid with and without meconium staining
(Table III), suggesting that
meconium is not a source of amniotic fluid SCCA. The placenta was
negative for SCCA immunostaining. Okawa et al reported that
the cytoplasm of adult skin epidermal cells was positively stained
for SCCA (22), whereas SCCA was
not expressed in the granular, superficial spinous, deep spinous
and basal layers of fetal skin. This difference may be due to
insufficient differentiation of fetal skin epidermal cells. With
careful observation, SCCA-positive cells can be focally detected in
the keratinized layer of fetal skin. Furthermore, the
immunostaining of amniotic fluid cells revealed scattered SCCA
positivity, which may indicate that SCCA-positive keratinocytes are
released into the amniotic fluid. These results suggest that
amniotic fluid SCCA is unlikely to be derived from placenta,
amniotic fluid cells and meconium. SCCA levels in amniotic fluid
may originate from fetal urine, as neonatal urine SCCA levels were
nearly as high as those in amniotic fluid samples (Table III). However, the exact origin of
SCCA in fetal urine remains unknown. Thus, studies on the origin of
SCCA suggest that keratinized fetal skin, placenta and meconium are
not primary sources, whereas the fetal urine is more likely.
However, it does not deny the possibility other than the above.
Fourth, serum biomarkers including ZnCP-1, STN,
IGFBP1 and SCCA are not used widely in clinical practice due to
several limitations including low sensitivity, lack of
standardization of methods, difficulty of measuring markers during
the acute phase of an episode, and the fact that its use remains
experimental (4-13).
The diagnostic significance of SCCA in AFE has been well-documented
recently (13). The authors
previously reported that serum SCCA levels were 25-fold higher in
women with autopsy-proven AFE (mean, 112.0 ng/ml) than in healthy
controls with normal deliveries (mean, 4.4 ng/ml) (13). The SCCA test can improve
discriminatory power in predicting AFE (sensitivity, 60.0%;
specificity, 89.2%) (13). The
disastrous amniotic fluid entry into the maternal circulation may
lead to dramatic sequelae of clinical events including
cardiopulmonary collapse and coagulopathy in autopsy-proven AFE.
The association between amniotic fluid entry into the maternal
circulation and a rapid onset of anaphylaxis may explain the
underlying mechanism of the pathogenesis of AFE. In the present
study, however, despite the reliable amniotic fluid entry into the
maternal circulation during normal vaginal delivery, no case of
concomitant anaphylactic reaction was found. However, the reasons
why they did not develop a fatal anaphylactic shock are not clear.
There are two possibilities for this: AFE does not develop unless a
certain amount of amniotic fluid enters the maternal circulation
and an idiosyncrasy may be related to AFE onset. Amniotic fluid
SCCA may become a useful marker for identifying amniotic fluid
entry into the maternal circulation rather than supporting the
potential diagnosis of AFE (23).
Notwithstanding these limitations, serum SCCA levels are abnormally
elevated among women with established AFE (13). Currently, serodiagnosis of AFE
based on amniotic fluid-specific markers is not internationally
accepted.
Finally, there are two limitations to the present
study. The first is that as this was a single-center study, it may
have been subjected to unpredictable selection bias. In addition,
our hospital is a university hospital; therefore, there may have
been bias regarding more serious disease cases being present.
Secondly, the number of emergency cesarean section cases was
relatively small, which needs to be investigated in future
large-scale studies.
In conclusion, the present study demonstrates that
dynamic changes in maternal serum SCCA levels during labor indicate
that amniotic fluid components can enter the maternal circulation
during normal vaginal delivery. The onset of labor and full
cervical dilatation are the main causes of entry of amniotic fluid
components into the maternal circulation. The origin of SCCA may be
fetal urine, but it does not rule out other possibilities. Amniotic
fluid SCCA, which may be derived from fetal urine, can enter the
maternal circulation during vaginal delivery. The onset of labor
and full cervical dilatation are the main causes of entry of
amniotic fluid components into the maternal circulation.
Acknowledgements
The authors acknowledge the outstanding histological
assistance of Dr Takeshi Nishikawa, Dr Sumire Sugimoto and Dr
Tomoko Uchiyama (Department of Diagnostic Pathology, Nara Medical
University, Kashihara, Japan).
Funding
The present study was supported by a Grant-in-Aid
for Scientific Research from the Ministry of Education, Science,
and Culture of Japan to the Department of Obstetrics and
Gynecology, Nara Medical University (JSPS KAKENHI grant no.
JP16K11150; JSPS KAKENHI grant no. JP 15K10682; JSPS KAKENHI grant
no. JP 17K11292, and JSPS KAKENHI grant no. JP 26462497). In
addition, the present study was supported by AMED under grant no.
17gk0210005h0003 and by the Tohoku Bureau of Economy, Trade and
Industry (grant no. Tohoku 1607028) and the Kanzawa Medical
Research Foundation (grant no. 30-28).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
HK, NK and KN contributed to the conception and
design of the study. KN, NK and YY collected patient data and serum
and tissue samples. KN, NK and YY measured the ELISA samples. The
samples were reviewed by experienced pathologists (LL and CO) to
determine the expression of cytokeratin and SCCA. KN, NK and HK
were involved in the drafting of the manuscript or revising it
critically for important intellectual content. The final version of
the manuscript has been read and approved by all authors.
Ethics approval and consent to
participate
All procedures involving human participants were in
accordance with the ethical standards of the institutional research
committee and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. The protocols were
approved by the ethics committee of Nara Medical University
(approval numbers 579, 579-2, 873, and 873-2). The present study is
a retrospective observational study, carried out by the opt-out
method of our hospital website. Written informed consent was
obtained from each study subject, and all subjects consented to
donate samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Conde-Agudelo A and Romero R: Amniotic
fluid embolism: An evidence-based review. Am J Obstet Gynecol.
201:445.e1–445.e13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kobayashi H: Amniotic fluid embolism:
Anaphylactic reactions with idiosyncratic adverse response. Obstet
Gynecol Surv. 70:511–517. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Schneider SS, Schick C, Fish KE, Miller E,
Pena JC, Treter SD, Hui SM and Silverman GA: A serine proteinase
inhibitor locus at 18q21.3 contains a tandem duplication of the
human squamous cell carcinoma antigen gene. Proc Natl Acad Sci USA.
92:3147–3151. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kanayama N, Yamazaki T, Naruse H, Sumimoto
K, Horiuchi K and Terao T: Determining zinc coproporphyrin in
maternal plasma - a new method for diagnosing amniotic fluid
embolism. Clin Chem. 38:526–529. 1992.PubMed/NCBI
|
|
5
|
Kobayashi H, Ohi H and Terao T: A simple,
noninvasive, sensitive method for diagnosis of amniotic fluid
embolism by monoclonal antibody TKH-2 that recognizes NeuAc alpha
2-6GalNAc. Am J Obstet Gynecol. 168:848–853. 1993.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kobayashi H, Ooi H, Hayakawa H, Arai T,
Matsuda Y, Gotoh K and Tarao T: Histological diagnosis of amniotic
fluid embolism by monoclonal antibody TKH-2 that recognizes NeuAc
alpha 2-6GalNAc epitope. Hum Pathol. 28:428–433. 1997.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Oi H, Kobayashi H, Hirashima Y, Yamazaki
T, Kobayashi T and Terao T: Serological and immunohistochemical
diagnosis of amniotic fluid embolism. Semin Thromb Hemost.
24:479–484. 1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oi H, Naruse K, Noguchi T, Sado T, Kimura
S, Kanayama N, Terao T and Kobayashi H: Fatal factors of clinical
manifestations and laboratory testing in patients with amniotic
fluid embolism. Gynecol Obstet Invest. 70:138–144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tsunemi T, Oi H, Sado T, Naruse K, Noguch
T and Kobayashi H: An overview of amniotic fluid embolism: Past,
present and future directions. Open Womens Health J. 6:24–29.
2012.
|
|
10
|
Naruse K, Noguchi T, Yoshida S, Tsunemi T,
Shigetomi H, Oi H and Kobayashi H: Identification of interleukin-6
(IL-6) and squamous cell carcinoma (SCC) as amniotic fluid-specific
markers. Open J Obstet Gynecol. 2:147–150. 2012.
|
|
11
|
Iwai K, Oi H, Tsunemi T, Naruse K, Noguchi
T, Sado T and Kobayashi H: Sialyl Tn and Zinc coproporphyrin 1 as
potential serum markers of amniotic fluid embolism. Adv Obstet
Gynecol. 63:483–487. 2011.(In Japanese).
|
|
12
|
Legrand M, Rossignol M, Dreux S, Luton D,
Ventré C, Barranger E, Laribi S, Payen D and Muller F: Diagnostic
accuracy of insulin-like growth factor binding protein-1 for
amniotic fluid embolism. Crit Care Med. 40:2059–2063.
2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Koike N, Oi H, Naruse K, Kanayama N and
Kobayashi H: Squamous cell carcinoma antigen as a novel candidate
marker for amniotic fluid embolism. J Obstet Gynaecol Res.
43:1815–1820. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kato H: Expression and function of
squamous cell carcinoma antigen. Anticancer Res. 16:2149–2153.
1996.PubMed/NCBI
|
|
15
|
Ullman E, Pan JA and Zong WX: Squamous
cell carcinoma antigen 1 promotes caspase-8-mediated apoptosis in
response to endoplasmic reticulum stress while inhibiting necrosis
induced by lysosomal injury. Mol Cell Biol. 31:2902–2919.
2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Benson MD, Cheema N, Kaufman MW,
Goldschmidt RA and Beaumont JL: Uterine intravascular fetal
material and coagulopathy at peripartum hysterectomy. Gynecol
Obstet Invest. 73:158–161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Karakas B, Qubbaj W, Al-Hassan S and
Coskun S: Noninvasive digital detection of fetal DNA in plasma of
4-week-pregnant women following in vitro fertilization and embryo
transfer. PLoS One. 10(e0126501)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kase S, Namba K, Iwata D, Mizuuchi K,
Kitaichi N, Tagawa Y, Okada-Kanno H, Matsuno Y and Ishida S:
Diagnostic efficacy of cell block method for vitreoretinal
lymphoma. Diagn Pathol. 11(29)2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cataltepe S, Gornstein ER, Schick C,
Kamachi Y, Chatson K, Fries J, Silverman GA and Upton MP:
Co-expression of the squamous cell carcinoma antigens 1 and 2 in
normal adult human tissues and squamous cell carcinomas. J
Histochem Cytochem. 48:113–122. 2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Izuhara K, Yamaguchi Y, Ohta S, Nunomura
S, Nanri Y, Azuma Y, Nomura N, Noguchi Y and Aihara M: Squamous
cell carcinoma antigen 2 (SCCA2, SERPINB4): An emerging biomarker
for skin inflammatory diseases. Int J Mol Sci.
19(1102)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yoshimasu T, Maebeya S, Suzuma T, Bessho
T, Tanino H, Arimoto J, Sakurai T and Naito Y: Disappearance curves
for tumor markers after resection of intrathoracic malignancies.
Int J Biol Markers. 14:99–105. 1999.PubMed/NCBI
|
|
22
|
Okawa T, Yamaguchi Y, Kou K, Ono J, Azuma
Y, Komitsu N, Inoue Y, Kohno M, Matsukura S, Kambara T, et al:
Serum levels of squamous cell carcinoma antigens 1 and 2 reflect
disease severity and clinical type of atopic dermatitis in adult
patients. Allergol Int. 67:124–130. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kobayashi H: The entry of fetal and
amniotic fluid components into the uterine vessel circulation leads
to sterile inflammatory processes during parturition. Front
Immunol. 3(321)2012.PubMed/NCBI View Article : Google Scholar
|















