Introduction
Aconitine exerts pharmacological effects, such as
dispelling wind and dampness, and easing menstrual cramps and pain
(1). In China, it is considered
common practice to soak Radix aconitiagrestis and Radix
aconiti sinensisin wine or to add Kusnezoff monkshood root
slices when cooking food. However, the majority of plants of the
genus Aconitum are poisonous. Aconitine is the main toxic
component of Aconitum plants. It exerts effects mainly on
the heart and nervous system, causing a variety of arrhythmias and
leading to death (1). Its
therapeutic dose is close to the poisonous or lethal dose, and it
is a typical representative of poisonous plants with medicinal
value (2). The oral administration
of 0.2 mg aconitine can be poisonous, and a dose of 3-5 mg can
cause death. During use, aconitine can easily cause poisoning and
death due to individual differences in drug tolerance, improper
usage or misuse and deliberate poisoning (3). The cause of death is mostly malignant
arrhythmia, and the hospital mortality rate in China is 5.5%
(3). In China, medicinal liquor
made from aconitine root soaked in wine is mostly poisoned.
At present, reliable detoxification drugs are not
available. In addition to inducing vomiting, gastric lavage and
catharsis, the key to treating aconitine poisoning is to correct
arrhythmia in a timely and effective manner, stabilize myocardial
cells, and maintain the stability of vital signs (4). The majority of patients are treated
with atropine, lidocaine, or amiodarone, which can effectively
control arrhythmia and cure patients. However, some severely ill
patients have severe arrhythmia, such as an electrical storm, and
thus do not survive due to ineffective treatment with
antiarrhythmic drugs. Temporary pacemakers enable pacing and
biphasic defibrillation, which can pace the heart in a short period
of time, and also defibrillate, restore autonomous heart rhythm,
prevent malignant arrhythmia, and ensure blood supply to vital
organs (5). To the best of our
knowledge, no study to date has used temporary pacemakers for the
treatment of malignant arrhythmias induced by aconitine poisoning.
Therefore, the present study used a temporary pacemaker to treat 12
patients who were admitted to the Department of Emergency Medicine
of Yan'an Hospital in Kunming from June, 2017 to April, 2021 due to
an electrical storm caused by aconitine poisoning, and all were
cured.
Patients and methods
Patient information
From June, 2017 to April, 2021, 12 patients were
admitted to the Department of Emergency Medicine of Yan'an Hospital
in Kunming due to aconitine poisoning. Among them, 8 patients were
males, and 4 patients were females. The patients' age ranged from
33 to 65 years, with an average age of 45 years. Poisoning was
induced in 2 patients due to the ingestion of herbal black powder,
and in 10 patients due to the consumption of medicated wine.
Symptoms occurred after 10-30 min, and they all visited the
Department of Emergency Medicine within 1-2 h following the onset
of symptoms. The present study was approved by the First Affiliated
Hospital of Kunming Medical University Research Ethics Boards.
Written informed consent for participation in the study was
obtained from the parent or relative of the participants.
Inclusion criteria
The inclusion criteria were as follows: i) All the
12 patients met the diagnostic criteria for ventricular tachycardia
electrical storm: Patients who suffered from two or more
spontaneous ventricular tachycardias or ventricular fibrillation
within 24 h, sharp and severe disturbance of cardiac electrical
activity, rapid ventricular tachycardia, and ventricular
fibrillation that can occur repeatedly requiring repeated
electrical cardioversion; ii)treatment with atropine, lidocaine, or
amiodarone was ineffective; iii) electrical cardioversion therapy
was ineffective; iv) suffered from combined high-grade
atrioventricular block; v) suffered from QT syndrome with torsades
de pointes; vi) patients and relatives agreed to the use of
temporary pacemakers.
Exclusion criteria
The exclusion criteria were as follows: i) Patients
in which a ventricular tachycardia electrical storm was caused by
non-aconitine poisoning; ii) treatment with atropine, lidocaine, or
amiodarone was effective; iii) electrical cardioversion was
effective; iv) patients and relatives did not agree to the use of
temporary pacemakers.
Clinical manifestations
All 12 cases exhibited symptoms, such as numbness in
the mouth, tongue, face and limbs, and other symptoms including
nausea, vomiting, dizziness, sweating, chest tightness and
palpitations. Among the patients, there were two cases of
convulsions with unconsciousness during medical treatment, six
cases of syncope, two cases of convulsions with facial cyanosis,
and two cases of whole-body numbness and a ‘feeling of dying’
(Table I).
 | Table IClinical data of the 12 patients
before treatment with the temporary pacemaker. |
Table I
Clinical data of the 12 patients
before treatment with the temporary pacemaker.
| Patient no. | Sex | Age, years | Type of poison
ingested | Clinical
manifestations and electrocardiogram | Treatment | Effect |
|---|
| 1 | Male | 41 | Traditional Chinese
medicine (Radix aconiti agrestis) | Chest tightness,
vomiting, perioral numbness, convulsions, etc.; electrocardiogram:
Multi-source premature ventricular contractions, short bursts of
ventricular tachycardia, ventricular fibrillation; blood pressure:
78/49 mmHg | Amiodarone, dopamine,
lidocaine, a tropine, bedside gastric lavage, electrical
defibrillation, symptomatic treatment | Treatment
ineffective |
| 2 | Male | 51 | Medicinal wine
(Radix aconitiagrestis + wine) | Palpitation,
sweating, dizziness, syncope, etc.; ECG chart: Polymorphic
ventricular tachycardia, ventricular premature contraction; blood
pressure: 89/57 mmHg | Amiodarone,
lidocaine, atropine, dopamine, electrical defibrillation, bedside
gastric lavage, symptomatic treatment | Treatment
ineffective |
| 3 | Female | 35 | Medicinal wine
(Radix aconitiagrestis + wine) | Chest tightness,
general numbness, convulsions with facial cyanosis; ECG:
Ventricular fibrillation, supraventricular tachycardia, frequent
ventricular premature contractions; blood pressure: 61/38 mmHg | Electric
defibrillation, lidocaine, atropine, dopamine, potassium and
magnesium supplements, symptomatic treatment | Treatment
ineffective |
| 4 | Female | 48 | Medicinal wine
(Radix aconitiagrestis + wine) | Chest tightness,
palpitations, sweating, syncope, etc.; ECG: Torsades de pointes,
ventricular tachycardia; blood pressure: 91/52 mmHg | Electric
defibrillation, lidocaine, amiodarone, dopamine, potassium and
magnesium supplements, symptomatic treatment | Treatment
ineffective |
| 5 | Female | 44 | Traditional Chinese
medicine (Radix aconitiagrestis) | Whole-body numbness
and a ‘feeling of dying’, sweating, etc.; ECG: Multi-source
ventricular premature contractions, torsades de pointes ventricular
tachycardia; blood pressure: 95/61 mmHg | Electric
defibrillation, lidocaine, amiodarone, dopamine, potassium and
magnesium supplements, symptomatic treatment | Treatment
ineffective |
| 6 | Male | 47 | Medicinal wine
(Radix aconitiagrestis + wine) | Whole-body numbness
and a ‘feeling of dying’, sweating, etc.; ECG: Multi-source
ventricular premature beats, ventricular tachycardia, ventricular
fibrillation; blood pressure 95/61 mmHg | Electric
defibrillation, lidocaine, amiodarone, dopamine, potassium and
magnesium supplements, symptomatic treatment | Treatment
ineffective |
| 7 | Male | 55 | Medicinal wine
(Radix aconitiagrestis + wine) | Palpitation,
whole-body numbness and a ‘feeling of dying’, vomiting, etc.; ECG:
Multi-source premature ventricular contractions, polymorphic
ventricular tachycardia; blood pressure: 66/40 mmHg | Lidocaine,
amiodarone, dopamine, potassium and magnesium supplements,
symptomatic treatment | Treatment
ineffective |
| 8 | Male | 53 | Medicinal wine
(Radix aconitiagrestis + wine) | Perioral, tongue,
facial numbness, vomiting, syncope with convulsions; ECG:
Polymorphic ventricular tachycardia, short bursts of ventricular
tachycardia; blood pressure: 71/38 mmHg | Electric
defibrillation, lidocaine, dopamine, potassium and magnesium
supplements, symptomatic treatment | Treatment
ineffective |
| 9 | Female | 48 | Medicinal wine
(Radix aconitiagrestis + wine) | Dizziness, vomiting,
chest tightness, general numbness, etc.; ECG: Polymorphic
ventricular tachycardia, short bursts of ventricular tachycardia,
ventricular fibrillation; blood pressure: 99/49 mmHg | Electric
defibrillation, amiodarone, lidocaine, bedside gastric lavage | Treatment
ineffective |
| 10 | Male | 51 | Medicinal wine
(Radix aconitiagrestis + wine) | Chest tightness,
palpitations, sweating, dizziness, vomiting, etc.; ECG: Polymorphic
ventricular tachycardia, polygenic premature ventricular
contractions; blood pressure: 82/53 mmHg | Lidocaine,
defibrillation, dopamine, potassium and magnesium | Treatment
ineffective |
| 11 | Male | 38 | Medicinal wine
(Radix aconitiagrestis + wine) | Dizziness, vomiting,
chest tightness, general numbness, etc.; ECG: High atrioventricular
block, short bursts of ventricular tachycardia, multi-source
ventricular premature contractions; blood pressure: 67/54 mmHg | Amiodarone, atropine,
lidocaine, bedside gastric lavage | Treatment
ineffective |
| 12 | Male | 61 | Medicinal wine
(Radix aconitiagrestis + wine) | Chest tightness,
palpitations, sweating, syncope, vomiting, etc.; ECG: Polymorphic
ventricular tachycardia, QT syndrome with torsades de pointes;
blood pressure: 91/53 mmHg | Atropine, lidocaine,
bedside gastric lavage | Treatment
ineffective |
Electrocardiogram (ECG)
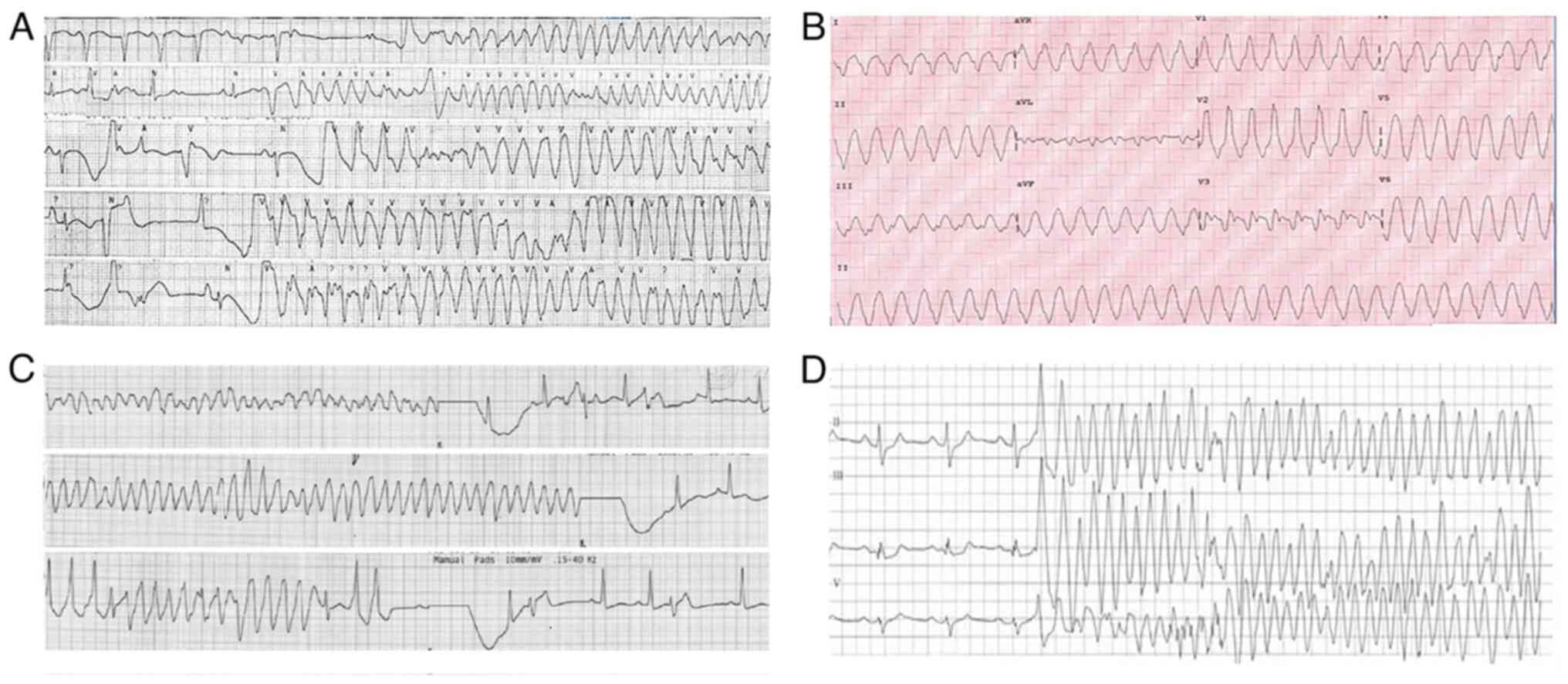
The first ECG or ECG monitoring revealed five cases
of multi-source ventricular premature beats, six cases of
polymorphic ventricular tachycardia, four cases of ventricular
fibrillation, and three cases of torsades de pointes ventricular
tachycardia. All 12 cases had varying degrees of hypotension
(Table I). A typical ECG is
illustrated in Fig. 1.
Treatment method
The 12 patients were immediately administered a
thorough gastric lavage, activated charcoal plus 250 ml of mannitol
oral catharsis, continuous ECG monitoring, oxygen inhalation,
routine fluid replacement, intravenous supplementation of potassium
and magnesium polarized fluids, correction of acidosis and
electrolyte disorders, and 102 mg of intravenous atropine.
Furosemide (Shaanxi Jingxi Pharmaceutical Co., Ltd.; 20 mg) is an
intravenous diuretic. Lidocaine (Zhejiang Chengxin Pharmaceutical
Co., Ltd.; 100 mg) was injected intravenously every 15-30 min.
Amiodarone (Shandong Fangming Pharmaceutical Group Co., Ltd.) was
used when lidocaine (Zhejiang Chengxin Pharmaceutical Co., Ltd.)
was ineffective. All patients were administered a slow intravenous
bolus of 150 mg of amiodarone (Shandong Fangming Pharmaceutical
Group Co., Ltd.), followed by continuous micropump pumping of 1
mg/min amiodarone (Shandong Fangming Pharmaceutical Group Co.,
Ltd.). The aforementioned ‘rescue’ methods were not effectivein 10
patients, who manifested a loss of consciousness, shock,
ventricular fibrillation and Adams-Stokes syndrome. The patients
were immediately administered electric shock defibrillation, chest
compressions and emergency bedside temporary cardiac pacing for
rescue.
Equipment
The equipment used included a Medtronic Temporary
Pacemaker T10 (Shanghai Zanzhuo Medical Instrument Co., Ltd.), no.
18 thin-walled puncture needle and J-shaped guide wire (Shenzhen
Yixinda Medical New Technology Co., Ltd.), 6F bipolar electrode
catheter (Beijing Wandong Medical Equipment Co., Ltd.), 6F venous
dilator and venous sheath (Beijing Wandong Medical Equipment Co.,
Ltd.), a photoelectric electrocardiograph and a Minadray 9000 ECG
monitor (Nanjing Baden Medical Co., Ltd.).
Operation method
For the procedure, the patient was placed on his/her
back and monitored using an ECG. Following local infiltration
anesthesia, the left subclavian vein was punctured by Seldinger's
method, and the pacing electrode was successfully inserted. Bedside
ECG monitoring was used for temporary cardiac pacing. After the
puncture was successful, the outer sheath was rapidly fed into the
6F electrode catheter with a slightly curved tip end, and the
electrode catheter was fed 20-25 cm. A temporary pacemaker was
connected. The pacemaker voltage and sensitivity were adjusted to 3
and 0.8 mV, respectively. The pacing frequency was 60 or 10
beats/min higher than the patient's own frequency. Once the main
wave in leads II, III and aVF appeared downward, and lead V1
presented a pacing pattern with left bundle branch block wide
malformation, the ECG was traced. The pacing threshold <1.0 V
was determined, and the lead was fixed and placed locally. Finally,
the operation was complete.
Results
Effect of temporary pacemaker on wind
burst induced by severe acute aconitine poisoning
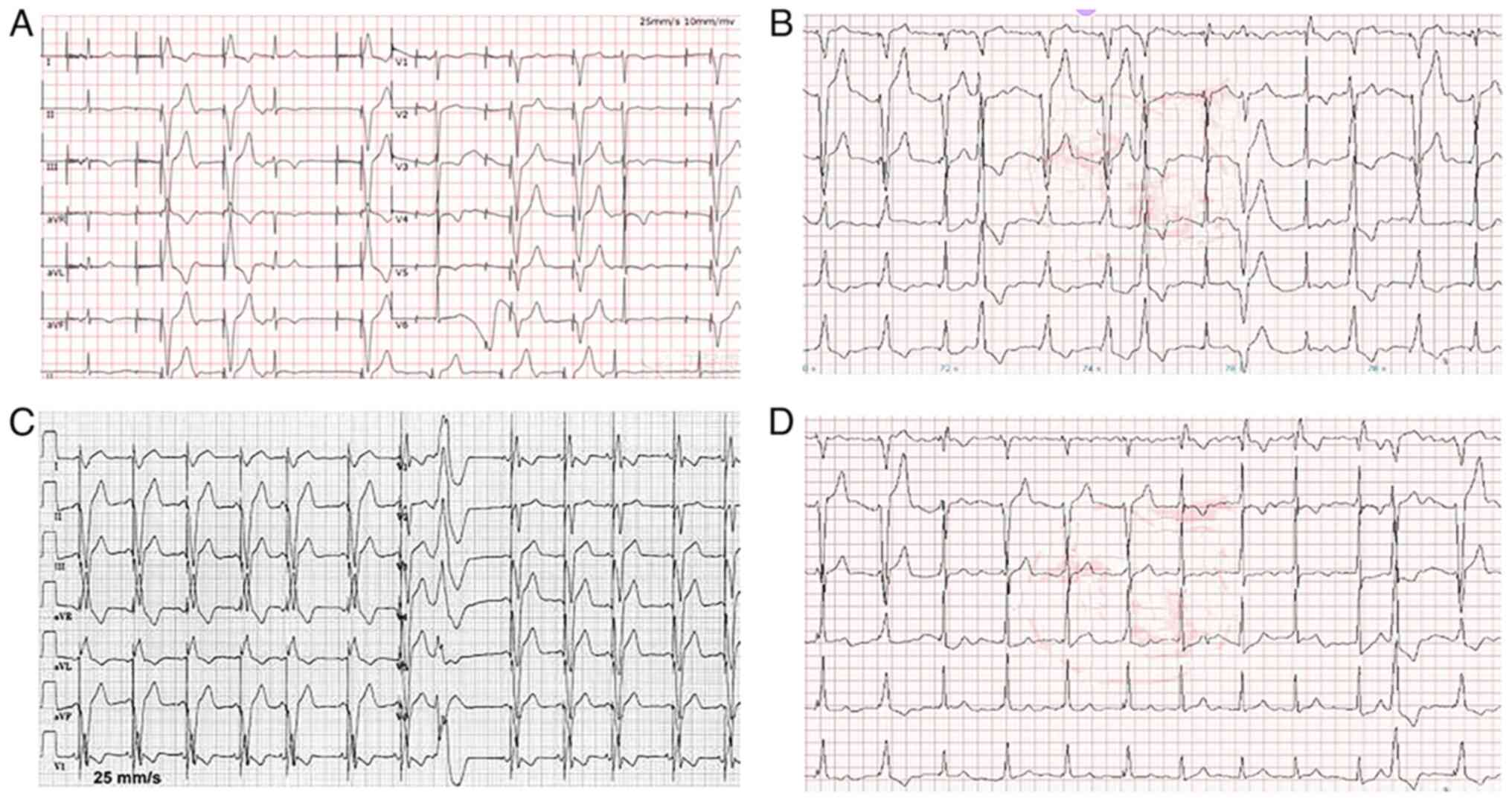
Temporary pacing was successful in all 12 patients.
The electrode placement rate was 100%, and the effective cardiac
pacing rate was 100%. The patients' blood pressure gradually
returned to normal. Ventricular tachycardia and high-grade
atrioventricular block were terminated. For atrial premature beats
and ventricular premature beats, the sinus rhythm was restored
following continuous amiodarone pumping (Fig. 2). At the same time, fluid
supplementation and control supportive treatment were administered.
The patients were hospitalized for 4-6 days. The patients' symptoms
disappeared, their vital signs were stable and their ECG exhibited
normal findings. No abnormalities in the ECG were observed for 2
days, and the patients were discharged for follow-up.
Side-effects of the temporary
pacemaker
In total, 1 patient experienced pacing dysfunction
caused by temporary pacemaker displacement. Pacemaker malfunction
was corrected after adjusting the position of the pacemaker.
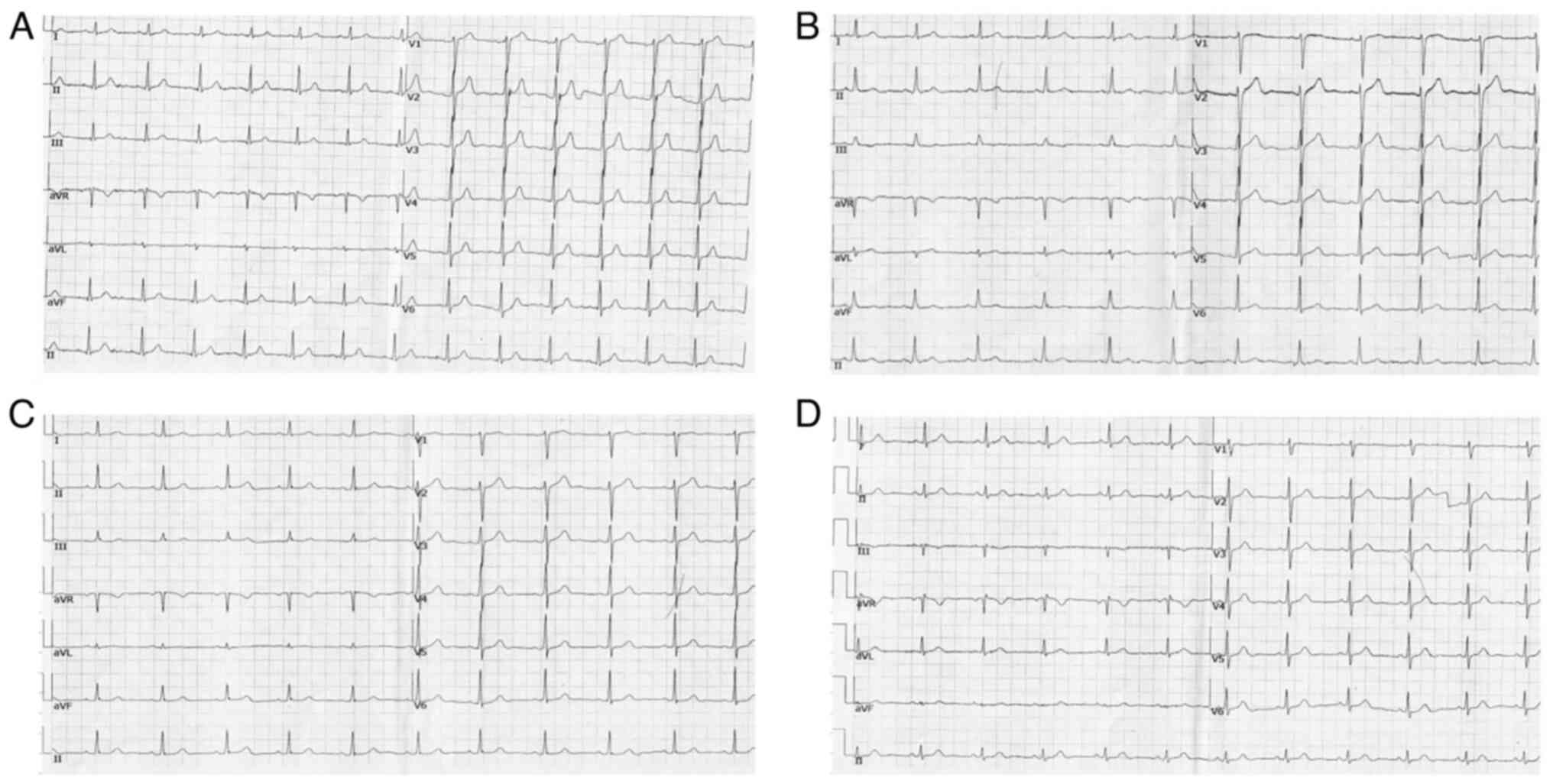
Patient follow-up
The 12 patients were followed-up for 1 week
following discharge without any discomfort, and the ECG did not
reveal any abnormalities (Fig.
3).
Discussion
Aconitine mainly damages the circulatory and central
nervous systems. The effects of aconitine poisoning on the heart
excite the vagus nerve, causing the postganglionic fibers to
release a large amount of acetylcholine, thereby reducing the
autonomy and conductivity of the sinus node. Aconitine also has a
direct effect on the myocardium, which renders the excitatory
conduction and refractory period of each part of the myocardium
inconsistent. Moreover, repolarization is not synchronized to form
re-entry, resulting in severe ventricular arrhythmia. Ventricular
tachycardia is the most common and likely cause of death. Aconitine
can directly act on ventricular muscles, producing high-frequency
ectopic rhythms and causing ventricular fibrillation, cardiogenic
shock and Adams-Stokes syndrome (6). Therefore, malignant arrhythmia
induced by acute aconitine poisoning can be life-threatening if not
corrected in a timely manner.
Temporary cardiac pacemakers have biphasic
defibrillation and pacing functions and play an important role in
rescuing patients undergoing cardiac arrest and bradyarrhythmia
(7). It has been shown that the
defibrillation rate of temporary cardiac pacing can reach 100%, and
the pacing success rate of patients with bradycardia is >80%
(8). In addition, the energy
required for biphasic defibrillation is minimal, the damage to the
patient's myocardium is minor, and the time it takes for the heart
to restore sinus rhythm after defibrillation is short. Temporary
cardiac pacing therapy can stabilize the patients' hemodynamics and
reduce the recurrence rate of cardiac arrest (9,10).
In the present study, 12 patients were administered temporary
pacemaker therapy, which controlled ventricular tachycardia and
electrical storms in a timely manner, and maintained the vital
signs of the patients. In addition to the temporary pacemaker,
fluid replacement and antiarrhythmic treatment with amiodarone were
continued. All 12 patients were cured.
The more common complications of temporary cardiac
pacing include pacing disorder, arrhythmia, myocardial perforation,
pericardial tamponade, pneumothorax, infection and puncture
complications (such as subcutaneous hematoma and venous thrombosis)
(11-13).
In the present study, 1 patient experienced pacing dysfunction
caused by temporary pacemaker displacement. Pacemaker malfunction
was corrected after adjusting the position of the pacemaker.
Therefore, temporary cardiac pacing may be an
effective treatment for malignant arrhythmias caused by severe
acute aconitine poisoning. However, the present study has some
limitations which are as follows: The present study involved a very
small number of cases, and the data provided are thus limited.
Therefore, further studies with larger sample sizes are required,
and farther randomized, controlled multi-center studies are also
warranted to confirm the current findings. There are reports on the
implantation of a temporary external transvenous implantable
cardioverter-defibrillator as a bridge for reimplantation following
infected device extraction (14,15).
Those temporarily implanted devices with a dual-coil ICD lead can
deliver shocks (16); however,
they were not used in the present study. A temporary pacemaker
cannot treat ventricular tachycardia/ventricular fibrillation with
biphasic cardioversion or defibrillation. Advanced life
support/advanced cardiac life support for hemodynamically unstable
patients with malignant ventricular arrhythmias is always
electrical cardioversion, or CPR and defibrillation in case of
cardiac arrest. Temporary pacemakers may be useful for maintaining
stable cardiac output after effective defibrillation/cardioversion
or they can provide overdrive pacing that can terminate fast
ventricular rhythms. However, they are not a treatment of choice
for patients with an electrical storm where external
defibrillation/cardioversion is a priority. A more in-depth
analysis of the side-effects which may occur prior to large-scale
implementation is thus essential.
Acknowledgements
Not applicable.
Funding
Funding: The present study received funding via a grant provided
by the National Natural Science Foundation (grant no.
81960350).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FC, LH and MWL made contributions to the acquisition
and analysis of the data. FC and MWL contributed to the
interpretation of the data. FC, LH and MWL made contributions to
the conception and design of the study and drafted the manuscript.
LH and MWL prepared Fig. 1,
Fig. 2 and Fig. 3. FC, LH and MWL confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the First
Affiliated Hospital of Kunming Medical University Research Ethics
Boards. Written informed consent for participation in the study was
obtained from the parent or relative of participants.
Patient consent for publication
Consent for patient data to be published was
provided by the patients themselves or their relatives.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tomlinson B, Chan TY, Chan JC and
Critchley JA: Herb-induced aconitine poisoning. Lancet.
341:370–371. 1993.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jeon SY, Jeong W, Park JS, You Y, Ahn HJ,
Kim S, Kim D, Park D, Chang H and Kim SW: Clinical relationship
between blood concentration and clinical symptoms in aconitine
intoxication. Am J Emerg Med. 40:184–187. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jin R, Wang YG and Zhang B: Establishment
and verification of risk assessment scale for clinical safety
medication of aconitine. Zhongguo Zhong Yao Za Zhi. 43:222–226.
2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
4
|
Cho YS, Choi HW, Chun BJ, Moon JM and Na
JY: Quantitative analysis of aconitine in body fluids in a case of
aconitine poisoning. Forensic Sci Med Pathol. 16:330–334.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Chatzidou S, Kontogiannis C, Tsilimigras
DI, Georgiopoulos G, Kosmopoulos M, Papadopoulou E, Vasilopoulos G
and Rokas S: Propranolol versus metoprolol for treatment of
electrical storm in patients with implantable
cardioverter-defibrillator. J Am Coll Cardiol. 71:1897–1906.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pan MC, Zhou XW, Liu Y, Wang YN, Qiu XG,
Wu SF and Liu Q: Research progress on the molecular mechanisms of
toxicology of ethanol-aconitine induced arrhythmia. Fa Yi Xue Za
Zhi. 36:115–119. 2020.PubMed/NCBI View Article : Google Scholar : (In English,
Chinese).
|
|
7
|
He WW and Chu YJ: Clinical investigation
of temporary heart pacemaker for emergency treatment of patients
with acute and severe cardiovascular diseases. Zhonghua Yi Xue Za
Zhi. 96:2644–2647. 2016.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
8
|
Sert A, Aypar E, Odabas D and Aygul MU:
Temporary cardiac pacemaker in the treatment of junctional rhythm
and hypotension due to imipramine intoxication. Pediatr Cardiol.
32:521–524. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee MS, Nguyen H and Shlofmitz R:
Incidence of bradycardia and outcomes of patients who underwent
orbital atherecto-my without a temporary pacemaker. J Invasive
Cardiol. 29:59–62. 2017.PubMed/NCBI
|
|
10
|
Kowey PR, Mullan DF and Wetstein L:
Pacemaker therapy. Surg Clin North Am. 65:595–611. 1985.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Metkus TS, Schulman SP, Marine JE and Eid
SM: Complications and outcomes of temporary transvenous pacing: An
analysis of > 360,000 patients from the national inpatient
sample. Chest. 155:749–757. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhou X, Ze F, Li D, Li X and Wang B:
Outcomes of temporary pacing using active fixation leads and
externalized permanent pacemakers in patients with cardiovascular
implantable electronic device infection and pacemaker dependency. J
Cardiovasc Electrophysiol. 32:3051–3056. 2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Buttigieg J, Asciak R and Azzopardi CM:
Pacemaker lead-associated thrombosis in cardiac resynchronisation
therapy. BMJ Case Rep. 2015(bcr2015210314)2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dębski M, Ząbek A, Boczar K,
Urbańczyk-Zawadzka M, Lelakowski J and Małecka B: Temporary
external implantable cardioverter-defibrillator as a bridge to
reimplantation after infected device extraction. J Arrhythm.
34:77–80. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Da Rosa MR, Sapp JL, Howlett JG, Falkenham
A and Légaré JF: Implantable cardioverter-defibrillator
implantation as a bridge to cardiac transplantation. J Heart Lung
Transpl. 26:1336–1339. 2007.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kumar P, Baker M and Gehi AK: Comparison
of single-coil and dual-coil implantable defibrillators: A
meta-analysis. JACC Clin Electrophysiol. 3:12–19. 2017.PubMed/NCBI View Article : Google Scholar
|