Introduction
Systemic lupus erythematosus (SLE) is a complex
systemic autoimmune disease that predominantly affects women and is
characterized by clinical heterogeneity, an unpredictable course
and disease exacerbations (flares). The aim of SLE therapy is to
achieve disease remission, or at least a low disease activity and a
prevention of flares. Therefore, the treatment approaches for SLE
mainly include high-intensity immunosuppressive therapy for an
initial period followed by a less intensive treatment strategy to
prevent relapses for a long period of time. Drug options include
cyclophosphamide, mycophenolate mofetil, azathioprine and
calcineurin inhibitors, in combination with glucocorticoids. For
patients with refractory lupus or life-threatening disease,
biologics, combination regimens, plasma exchange and intravenous
immunoglobulins are adopted (1).
Due to persistent disease activity or flares, a large proportion of
patients with SLE require long-term treatment with corticosteroids
and/or immunosuppressive drugs, eventually leading to the
progressive aggravation of the impairment and adverse outcomes.
However, these therapies are often not sufficiently effective.
B lymphocyte stimulator (BLyS), also known as B-cell
activating factor (BAFF), is a member of the TNF family and plays a
significant role in B-cell survival. It has been reported that BLyS
expression is upregulated in patients with autoimmune diseases,
such as SLE (2,3). Belimumab (BLM), a human IgG
monoclonal antibody, binds with soluble human BLyS to inhibit its
biological activity, thereby promoting autoimmune B cell apoptosis
and reducing the number of new or existing autoimmune B-cell clones
(4,5).
An increasing number of clinical trials have
supported the beneficial effects of BLM in the treatment of SLE.
Therefore, BLM combined with standard of care (SOC) treatment was
approved by the Food and Drug Administration (FDA) in 2011 for the
treatment of patients with active and autoantibody-positive SLE
(6). In addition, BLM has recently
been approved for the treatment of children >5 years of age
suffering from childhood-onset SLE (cSLE). However, the therapeutic
effects and adverse reactions of the aforementioned drug vary with
the extension of the treatment duration. Nevertheless,
comprehensive meta-analyses on the efficacy and safety of BLM in
patients with active SLE remain limited (7-9).
Therefore, the present meta-analysis aimed to systematically review
and summarize these studies to evaluate the efficacy and safety of
the use of BLM in patients with active SLE treated with SOC.
Data and methods
Sources and searches
Electronic literature screening was performed using
the Web of Science, The Cochrane Library, PubMed and Embase
databases with a cut-off date of July, 2021. The Medical Subject
Headings terms used were as follows: ‘lupus’, ‘systemic lupus
erythematosus’, ‘SLE’, ‘belimumab’ and ‘Benlysta’. Only studies
published in English were included. In the case of overlapping
studies from the same authors, the most recent or complete study
was included in the meta-analysis.
Inclusion and exclusion criteria
The inclusion criteria were as follows: i) Patients
diagnosed with SLE according to the American College of
Rheumatology classification criteria (10); ii) randomized controlled trials
(RCTs) or case series studies, including prospective and
retrospective case series; iii) patients with SLE treated with BLM;
iv) patients positive for SLE autoantibodies (antinuclear and/or
anti-dsDNA autoantibodies); v) patients treated with a stable
treatment regimen prior to the initiation of the trial, including
corticosteroids, antimalarials, immunosuppressive and non-steroidal
anti-inflammatory drugs; vi) patients with active SLE, defined by a
Safety of Estrogens in Lupus National Assessment-Systemic Lupus
Erythematosus Disease Activity Index (SELENA-SLEDAI) score of ≥8 or
6. The exclusion criteria were as follows: i) Case report studies
and review articles; ii) studies with no available data; iii)
studies that included patients who were previously treated with
B-lymphocyte-targeting drugs or other novel medications against
SLE, other than corticosteroids.
Data extraction and quality
assessment
Two investigators, XL and JZ independently screened
the titles and abstracts of the included studies, identified the
duplicates studies, reviewed the full articles, decided on the
eligibility of the studies and collected the data. Disagreements
were resolved through discussion. XL designed a standard electronic
format for data collection. The following data were extracted from
the eligible trials: Study design, sample size, age, female
proportion, disease duration, use of other immunosuppressive
agents, medication protocols, outcome characteristics, adverse
events (AEs) and severe AEs (SAEs). The quality of each trial was
evaluated according to the Cochrane Collaboration tool (https://training.cochrane.org/handbook/current).
Statistical analyses
STATA 16.0 (Stata Corp LP) and Review Manager
(version 5.3; The Nordic Cochrane Centre) software were used to
pool and analyze the results, respectively. More specifically, the
response rate of the case series studies was pooled using STATA
16.0 (Stata Corp LP), while additional data analysis was performed
using Review Manager (version 5.3; The Nordic Cochrane Centre). For
binary outcomes, the relative risk (RR) was estimated with a 95%
confidence interval (95% CI). The results are presented as
analytical graphs generated using forest plots. Heterogeneity was
assessed using a χ2-based Q test. Therefore, with an
I²<50% or P>0.10, heterogeneity was considered small and RR
values were pooled in a fixed-effects model. With an I²>50% or
P<0.10, heterogeneity was considered significant and RR values
were pooled in a random effects model. A value of P<0.05 was
considered to indicate a statistically significant difference.
Results
Search results
The flow diagram of trial selection is presented in
Fig. 1. Among a total of 2,377
eligible manuscripts, after screening, seven RCTs and 19 case
series were included in the meta-analysis (11-36).
Characteristics of included
studies
A total of seven RCTs (11-17)
and 19 case series (18-36)
were included in the analysis, including 6,832 patients with SLE.
The details of the aforementioned studies are presented in Tables I and II. As regards the RCTs, six trials
(11-15,17)
included adult patients only, and one (16) trial only children. In addition, in
six trials (11-14,16,17),
BLM was administered intravenously and in one trial subcutaneously
(15).
 | Table ICharacteristics of the randomized
controlled trials included in the meta-analysis. |
Table I
Characteristics of the randomized
controlled trials included in the meta-analysis.
| | | | | | | | Medication
protocols | |
|---|
| Author/(Refs.) | No. of patients
(T/C) | Age, years
(T/C) | Female (%) | Disease duration,
years (T/C) |
Region/nationality | Tw | T | C | Other
immunosuppressive agents |
|---|
| Wallace et
al (11) | 449 (338/111) | 42.1±11.3/
42.2±10.9 | 94 | 9.0±8.2/
8.1±7.4 | American Canada,
African American, Hispanic, Latino | 52 | Belimumab 1, 4, or
10 mg/kg by intravenous on days 0, 14, and 28, then every 28 days
until 52w + SOC | Placebo + SOC | CS, antimalarials,
AZA, MTX, MMF |
| Furie et al
(12) | 819 (544/275) | 40.0±11.4/
40.0±11.9 | 93.3 | 7.2±7.5/
7.4±6.7 | American, Asian
Hispanic, Latino | 76 | Belimumab 1 or 10
mg/kg by intravenous on days 0, 14, and 28, then every 28 days
until 72w + SOC | Placebo + SOC | CS, antimalarials,
AZA, MTX, MMF |
| Navarra et
al (13) | 865 (579/288) | 35.4±10.8/
36.2±11.8 | 94.9 | 5.0±4.8/
5.9±6.2 | Latin America,
Asia-Pacific, Eastern Europe | 52 | Belimumab 1 or 10
mg/kg by intravenous on days 0, 14, and 28, then every 28 days
until 72w + SOC | Placebo + SOC | CS, antimalarials,
AZA, MTX, MMF |
| Zhang et al
(14) | 677 (451/226) | 32.3±9.65/
31.7±9.18 | 92.9 | 6.07±5.04/
5.97±5.19 | China, Japan, South
Korea | 52 | Belimumab 10 mg/kg
intravenously on days 0, 14 and 28, then every 28 days until 48w +
SOC | Placebo + SOC | CS, antimalarials,
Lef, MTX, MMF, traditional Chinese medicine |
| Stohl et al
(15) | 836 (556/280) | 38.1±12.1/
39.6±12.61 | 94.4 | 4.3 (0-35)/ 4.6
(0-38) | America, Europe,
Australia, Asia | 52 | Weekly subcutaneous
belimumab 200 mg for 52w + SOC | Placebo + SOC | CS, antimalarials,
AZA, MTX, MMF |
| Brunner et
al (16) | 93 (53/40) | 14 (12-15)/ 15
(14-16) IQR | 94.6 | 1.48 (0.79-2.46)/
(1.30-3.57) | America, Europe,
Japan | 52 | Belimumab 1 or 10
mg/kg by intravenous on days 0, 14, and 28, then every 28 days
until 72w + SOC | Placebo + SOC | CS,
antimalarials |
| D'Cruz et al
(17) | 496 (331/165) | 38.8±11.42 | 96.9 | N/A | African
American | 52 | Belimumab 1 or 10
mg/kg by intravenous on days 0, 14, and 28, then every 28 days
until 72w + SOC | Placebo + SOC | N/A |
 | Table IICharacteristics of the case series
studies included in the meta-analysis. |
Table II
Characteristics of the case series
studies included in the meta-analysis.
| Author/(Refs.) | Study design | No. of
patients | Age, years | Female (%) | Disease
duration | Duration of
follow-up | CS dose
(mg/day) | Concurrent
treatment | SLEDAI-2K |
|---|
| Fanouriakis et
al (18) | Prospective
study | 91 | 45.9±12.5 | 94.5 | Median, 9.7
(0.2-36.2) years | 24 M | Median, 10 (range,
0-60) | N/A | Median, 8 (range,
2-28) |
| Iaccarino et
al (19) | Prospective
study | 188 | 40.7±10.1 | 92.5 | 12.7±8.5 years | 17.5±10.6 M | 11.1±7.6 | CS, HCQ, AZA, Tac,
MMF, MTX, Lef | 8.3±3.3 |
| Parodis et
al (20) | Prospective
study | 58 | 41.3 IQR
(31.2-50) | 91.4 | 7.8 IQR (4.3-14.2)
years | 48 M | 10.0 IQR
(7.8-12.5) | CS, HCQ, AZA, MMF,
MTX, CsA | Median, 8 (range,
4-14) |
| Prete et al
(21) | Prospective
study | 20 | 44.15±2.14 | 75.0 | 10.4±6.8 years | 6 M | 19.8±17.5 | N/A | 12.70±5.72 |
| Gatto et al
(22) | Retrospective
study | 466 | 41.4±11.2 | 91.4 | 11.6±8.8 years | 18 M | 10.6±8.6 | CS, HCQ, AZA, MMF,
MTX, CsA, Lef, Tac | 9.3±3.3 |
| Collins et
al (23) | Retrospective
study | 501 | 41.3±12.1 | 89.0 | 1-10 years | 24 M | 19.9±14.39 | CS, HCQ, MTX, MMF,
AZA, CsA, CTX | 12.4±3.62 |
| Babini et al
(24) | Retrospective
study | 81 | 42±12 | 91.0 | 1-10 years | 24 M | 14.59±11.90 | CS, HCQ, AZA | 11.21±6.07 |
| von Kempis et
al (25) | Retrospective
study | 53 | 46.7±13.6 | 81.0 | 1-10 years | 6 M | 11.6 | CS, HCQ, AZA, MMF,
MTX, CsA | 8.0±5.0 |
| Scheinberg and
Golmia (26) | Prospective
study | 20 | 36.0±9.2 | 100.0 | 1-10 years | 6 M | 20.0±7.5 | N/A | 10.2±1.1 |
| Schwarting et
al (27) | Retrospective
study | 102 | 42.5±13.8 | 91.0 | 1-10 years | 6 M | 13.7±13.75 | CS, HCQ, MTX, MMF,
AZA, CsA, Tac | 10.6±6.1 |
| Cortés et al
(28) | Retrospective
study | 64 | 42.7±12.0 | 89.0 | 1-10 years | 6 M | 14.8 | N/A | N/A |
| Anjo et al
(29) | Retrospective
study | 23 | 41.5±10.5 | 100.0 | 171.8±131.1 M | 24 M | 10.2±1.8 | CS, HCQ, AZA, MMF,
MTX, Lef | 9.6±1.6 |
| Hui-Yuen et
al (30) | Prospective
study | 195 | 40.7±13.7 | 92.0 | 11.9±8.1 years | 6 M | 12.2 (range, 5 to
50) | CS, HCQ, AZA, MMF,
MTX | N/A |
| Sthoeger et
al (31) | Retrospective
study | 36 | 41.6±12.2 | 77.8 | 15.7±9.6 years | 2.3±1.7 years | N/A | CS, HCQ, AZA, MMF,
CTX | N/A |
| Scheinberg et
al (32) | Prospective
study | 48 | 32.6 (range,
19-61) | 100.0 | 11.6 (range,
1.5-18) years | 12 M | 30±12.5 | CS, HCQ, MTX, AZA,
MMF, CTX, RTX | 12.0±3.0 |
| Touma et al
(33) | Retrospective
study | 52 | 46.5±10.8 | 94.2 | 1-10 years | 6 M | 13.6±10.0 | CS, HCQ, MMF, AZA,
MTX, CsA | 8.1±3.2 |
| Scheinberg et
al (34) | Prospective
study | 74 | 34 (20-55) | 97.4 | 1-4.4 years | 36 M | 13.6 | CS, HCQ, AZA,
MMF | N/A |
| Iaccarino et
al (35) | Prospective
study | 458 | 43.5±11.3 | N/A | 12.3±8.7 years | 21.2±15.3 M | 10.5±8.1 | N/A | 8.1±3.4 |
| Iaccarino et
al (36) | Prospective
study | 67 | 39.3±10.2 | 91.0 | 12.8±8.3 years | 16.2±9.5 M | 11.2±6.6 | CS, HCQ, AZA, MMF,
CsA | 8.7±3.8 |
Quality evaluation of the
literature
All eligible RCTs included multicenter, randomized,
double-blind and placebo-controlled studies. Among the eligible
trials, four studies (12-14,16)
described the random method, while the other three (11,15,17)
did not mention a specific method. A quality evaluation of the
included studies is presented in Table III.
 | Table IIIAssessment of methodological quality
of included studies. |
Table III
Assessment of methodological quality
of included studies.
| Study | Random
allocation | Blind method | Hidden
distribution | The completeness of
the result data | Selective reporting
of results | Other bias |
|---|
| Wallace et
al (11) | Mention random | Double-blind | Not clear | Yes | No | Not clear |
| Furie et al
(12) | Central interactive
voice response system | Double-blind | Yes | Yes | No | Not clear |
| Navarra et
al (13) | Central interactive
voice response system | Double-blind | Yes | Yes | No | Not clear |
| Zhang et al
(14) | Validated
software | Double-blind | Yes | Yes | No | Not clear |
| Stohl et al
(15) | Mention random | Double-blind | Not clear | Yes | No | Not clear |
| Brunner et
al (16) | Interactive
response system | Double-blind | Yes | Yes | No | Not clear |
| D'Cruz et al
(17) | Mention random | Double-blind | Not clear | Yes | No | Not clear |
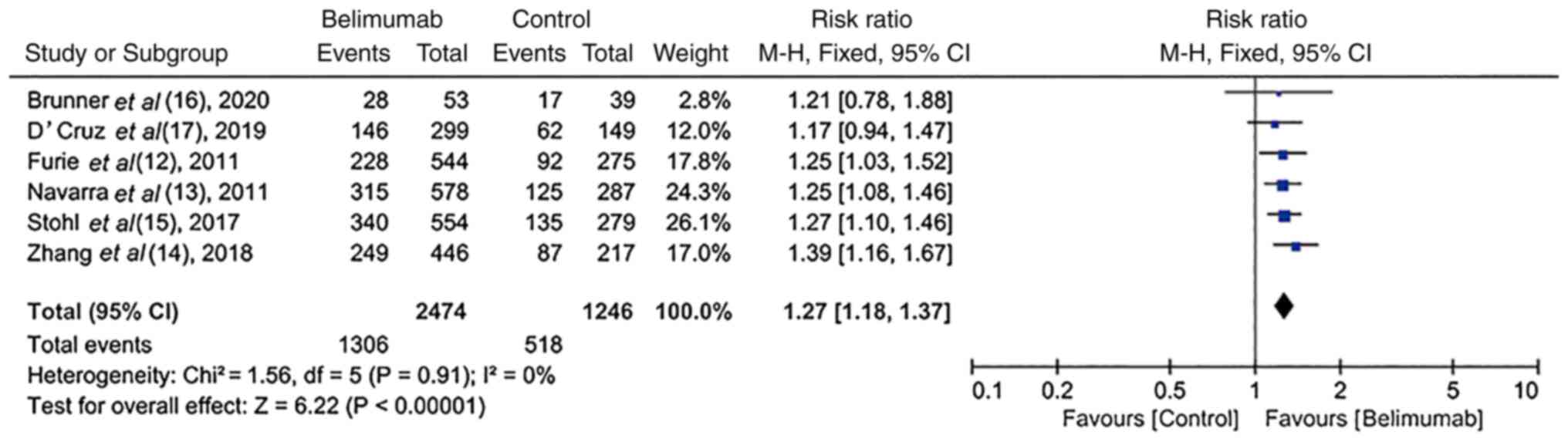
SLE responder index (SRI) rate in
RCTs
In six RCTs (12-17),
a SRI-4 response rate was reported at the end of each study. There
was homogeneity among these studies (I2=0%; P=0.91).
Compared with the control group, treatment with BLM resulted in a
significantly increased SRI-4 response rate [52.8% (1,306/2,474)
vs. 41.6% (518/1,246); RR, 1.27; 95% CI, 1.18-1.37; P<0.00001;
Fig. 2].
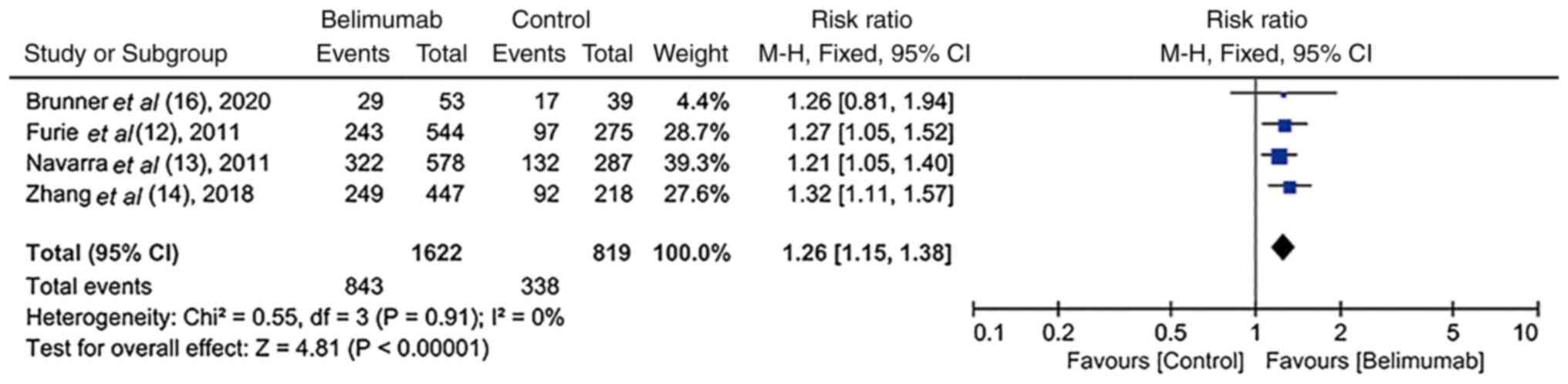
SELENA-SLEDAI score ≥4-point reduction
in RCTs
In four RCTs (12-14,16),
the SELENA-SLEDAI score was reduced by at least 4 points in
patients with SLE. In addition, there was homogeneity among these
studies (I2=0%; P=0.91). The number of patients who
achieved at least a 4-point reduction in the SELENA-SLEDAI score
was significantly increased in the BLM group compared with the
control group [52.0% (843/1,622) vs. 41.3% (338/819); RR, 1.26; 95%
CI, 1.15-1.38); P<0.00001; Fig.
3].
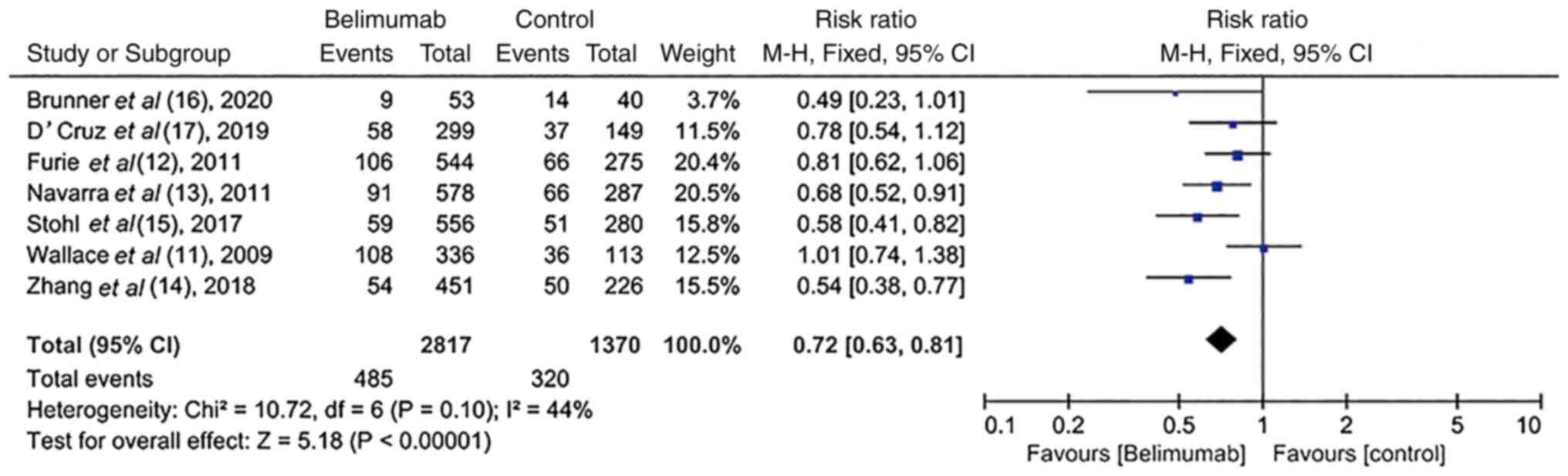
Change in severe flares in RCTs
Based on the modified SLE flare index (37-39),
severe flares were reported in all trials (11-17).
However, the heterogeneity among these studies was poor
(I2=44%; P=0.10). Patients treated with BLM exhibited a
lower rate of severe flares compared with the placebo group [17.2%
(485/2,817) vs. 23.4% (320/1,370); RR, 0.72; 95% CI, 0.63-0.81);
P<0.00001; Fig. 4].
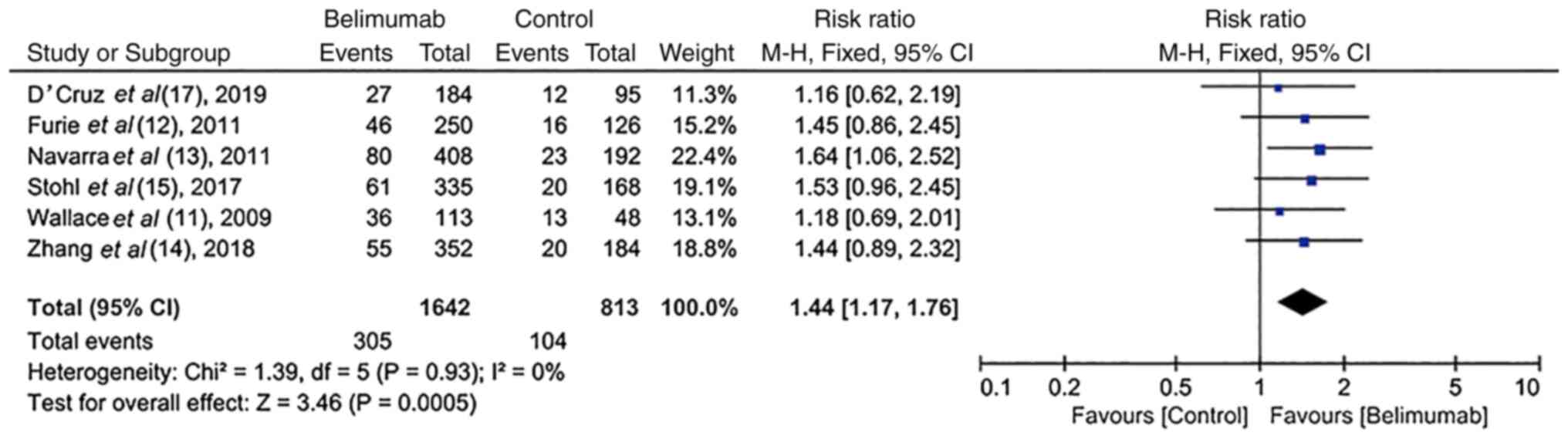
Corticosteroid dosage reduction in
RCTs
In six RCTs (11-15,17),
the average dose of corticosteroids was reduced by ≥25 or 50% to
≤7.5 mg/day during weeks 40-52 in the majority of patients treated
with BLM. There was significant homogeneity among these studies
(I2=0%; P=0.93). More specifically, the majority of
patients in the BLM group [18.6% (305/1,642)] were treated with a
reduced dose of corticosteroids (reduced by ≥25 or 50% to ≤7.5
mg/day) at weeks 40-52 compared with those in the control group
[12.8% (104/813); RR, 1.44; 95% CI, 1.17-1.76); P=0.0005; Fig. 5].
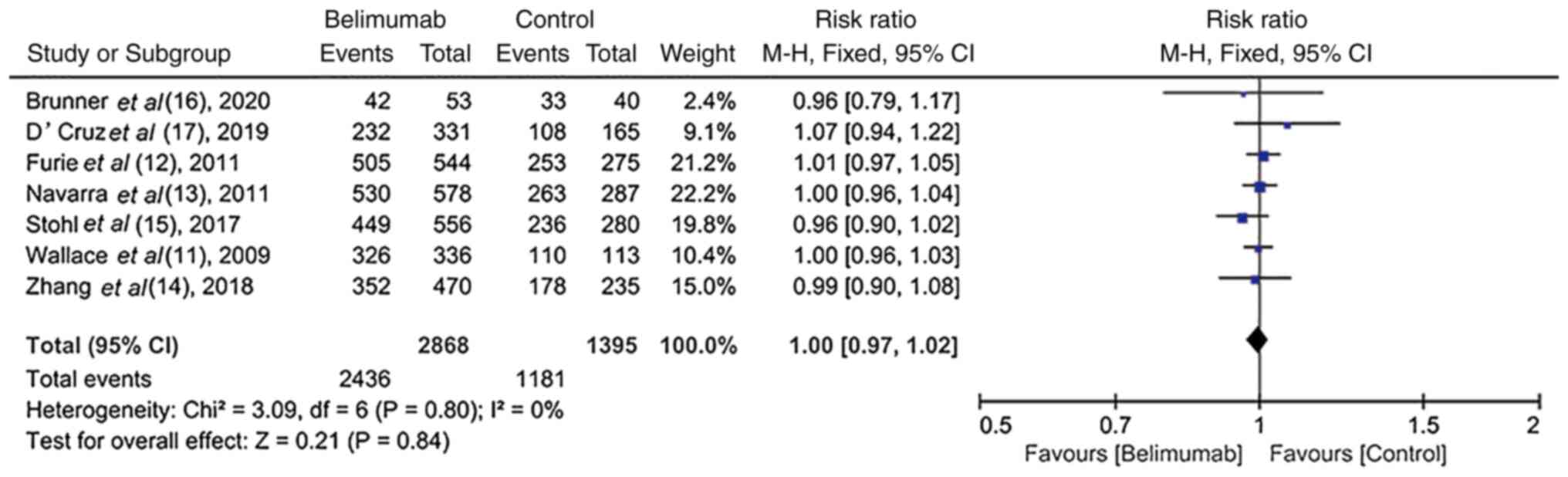
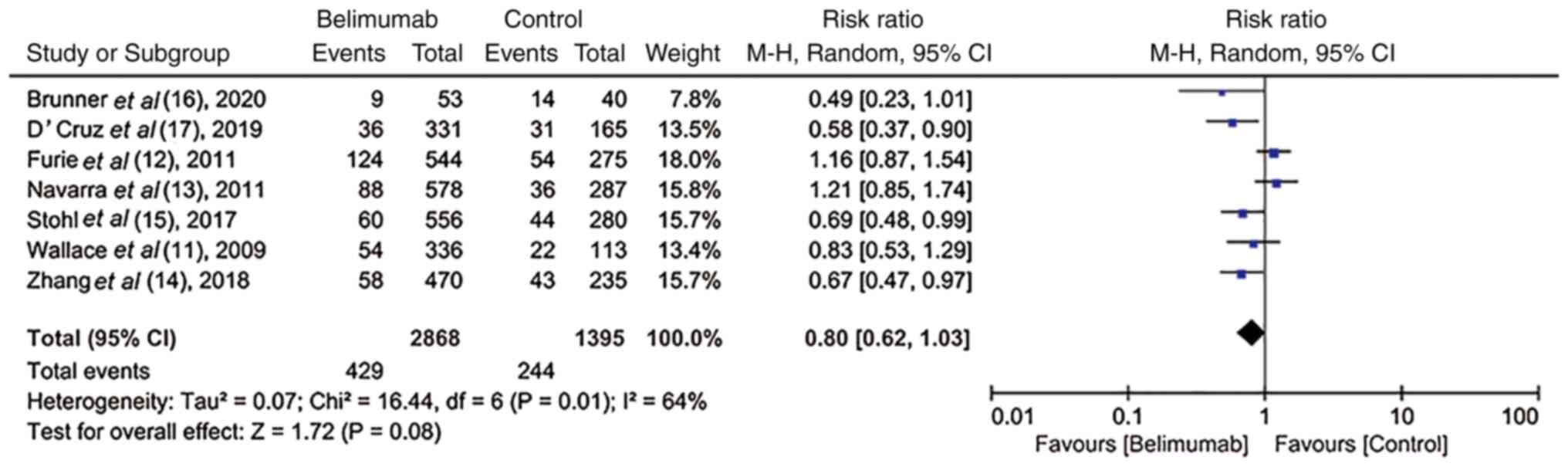
Safety and tolerability of BLM in
RCTs
All trials (11-17)
recorded the AEs and SAEs. The incidence of AEs and SAEs, including
arthralgia, fatigue, mortality and infection, was similar between
the BLM and control groups (RR, 1.00; 95% CI, 0.97-1.02; P=0.84 vs.
RR, 0.80; 95% CI, 0.62-1.03; P=0.08; Figs. 6 and 7), thus indicating that BLM was well
tolerated.
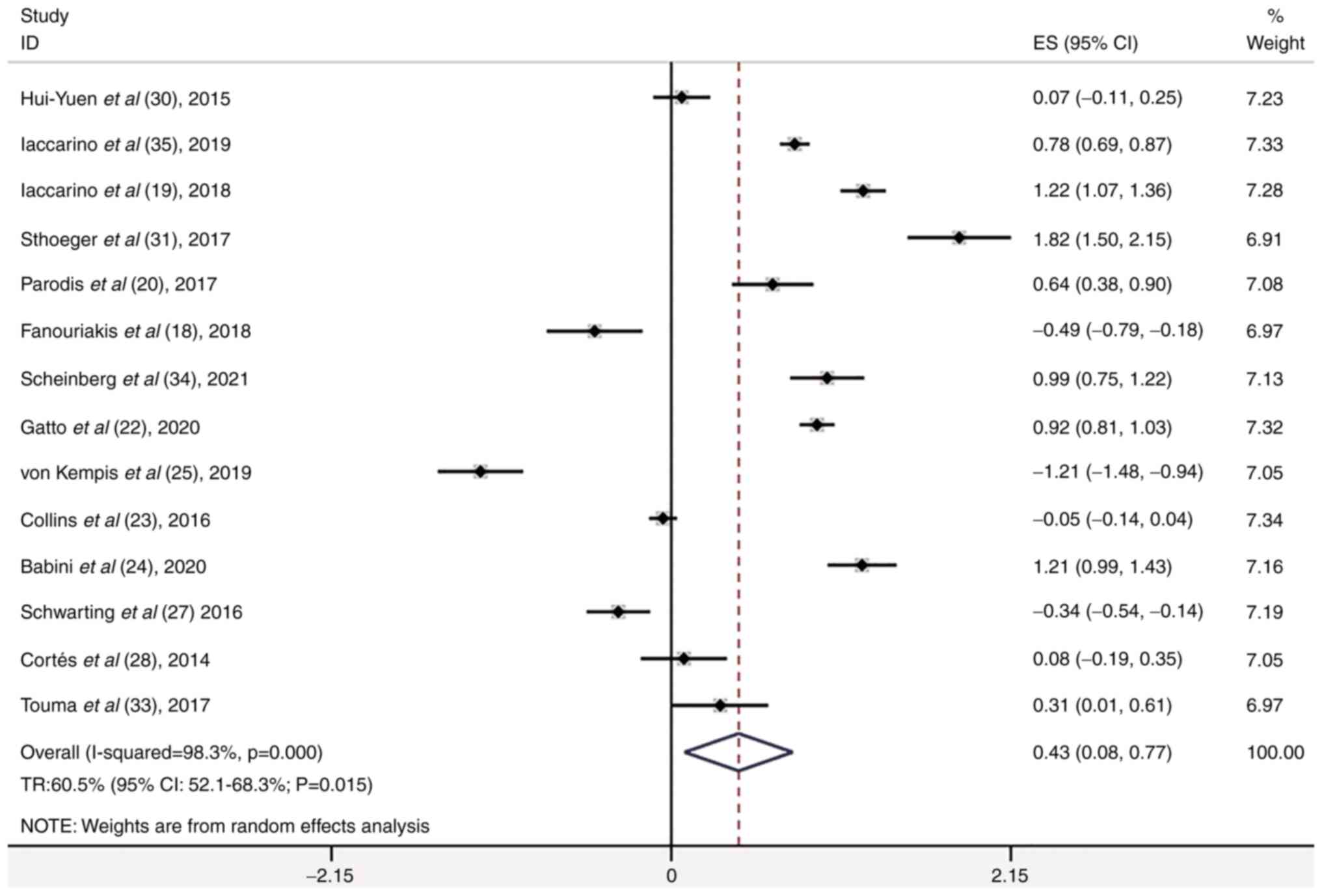
Meta-analysis of the case series
studies
The 19 case series included 2,597 patients with
active SLE. A total of 14 of the 19 case series studies involved
disease response and the total remission (TR) rate was 60.5% (95%
CI, 52.1-68.3%; P=0.015; Fig. 8).
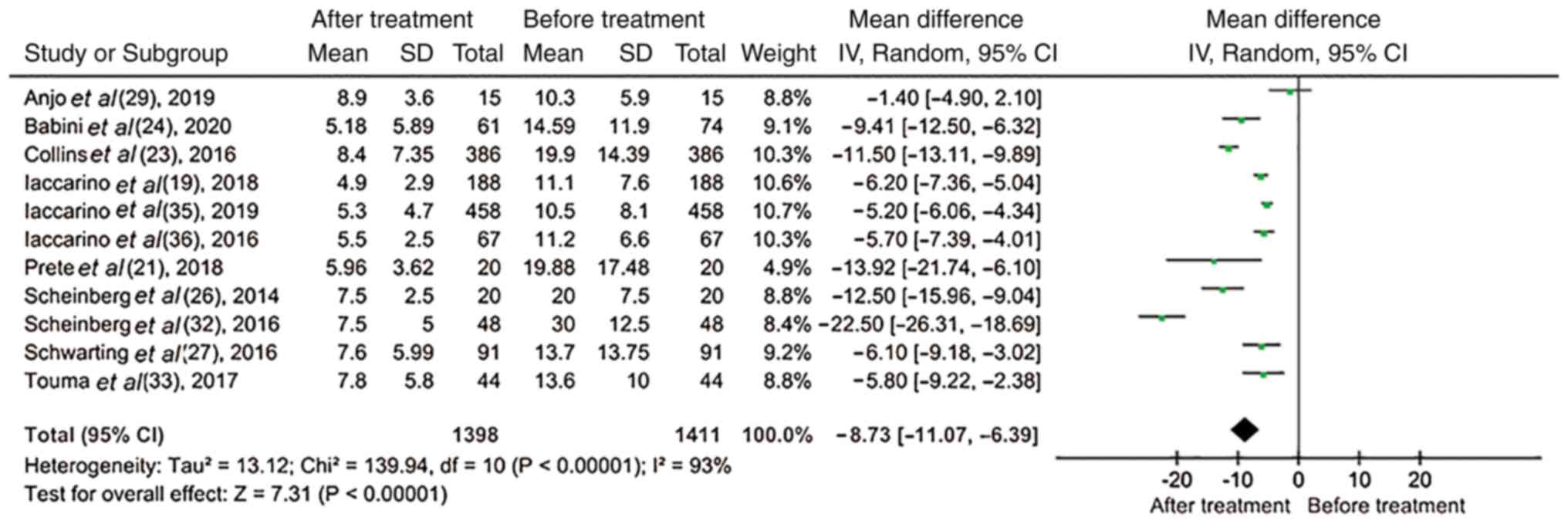
Among all the case series studies, 11 (19,21,23,24,26,27,29,32,33,35,36)
reported changes in the dose of corticosteroids. Therefore, the
results revealed that following the treatment of patients with BLM,
the use of corticosteroids was significantly decreased (mean
difference, -8.73; 95% CI, -11.07 to -6.39; P<0.00001; Fig. 9).
Discussion
SLE is a complex disease and its pathogenesis
remains poorly understood. However, it is widely accepted that the
activation of autoreactive B- and T-cells, particularly that of
B-cells, which may lead to a loss of immune tolerance, plays a
crucial role in the pathogenesis of SLE. During the progression of
the disease, the activation of cells can promote their
proliferation and differentiation into pathogenic cells that
produce pathogenic autoantibodies. Therefore, targeting B-cells and
their related cytokines may be considered as a significant
treatment approach. BLyS, a B cell survival factor, plays an
essential role in the development of autoimmune SLE by promoting B
cell survival, differentiation and maturation (40). Therefore, compared with healthy
individuals, patients with SLE exhibit higher BLyS serum
concentrations. It has been reported that the plasma levels of BLyS
are positively associated with SLE activity (41). BLyS binds to three particular BAFF
receptors, namely the transmembrane activator, cyclophilin ligand
interactor and B-cell maturation antigen. BLM is a fully humanized
monoclonal antibody that specifically binds to soluble trimeric
BAFF, thus preventing its interaction with its corresponding
receptors, eventually causing autoimmune B-cell apoptosis and
reducing new or existing autoimmune B cell clones (4,5).
In the present meta-analysis, seven selected RCTs
and 19 case series studies were selected to evaluate the efficacy
and safety of BLM plus SOC in patients with active SLE. In the
included RCTs, BLM increased the SRI-4 response rate. Furthermore,
the SELENA-SLEDAI score, the incidence of severe flare and
corticosteroid dosage were significantly decreased in patients with
active SLE treated with BLM. Both adults and children treated with
intravenous or subcutaneous BLM in combination with SOC exhibited a
significant improvement compared with those who received placebo
treatment. Furthermore, BLM combined with SOC was well tolerated
from patients with SLE, while no significant differences were
observed in the occurrence of AEs and SAEs between the BLM and
placebo groups. In the case series studies, the TR was 60.5% and
the use of corticosteroids was significantly reduced following BLM
treatment.
SRI is a composite method for evaluating the
biological treatment of SLE. This method combines the
SELENA-SLEDAI, British Isles Lupus Assessment Group (BILAG) and
Physician Global Assessment (PGA) scores, thus offering a more
comprehensive assessment of SLE (33). The SRI-4 is defined by a ≥4-point
reduction in SELENA-SLEDAI score, no new BILAG A organ domain
score, no >1 new BILAG B score and no worsening (increase
<0.3) in PGA score vs. baseline. In the present meta-analysis,
the included studies demonstrated that compared with the control
group, treatment with BLM notably increased the SRI-4 response
rate. Due to the relative strict standards of SRI-4, studies can
underestimate the effectiveness of BLM in ‘real’ world. The OBSErve
study, focusing on real-world effectiveness of BLM, verified that
BLM could improve the clinical manifestations of SLE (42), thus further supporting the efficacy
of BLM in the treatment of SLE.
Currently, glucocorticoids remain the cornerstone of
treatment in SLE, particularly when several organs are affected.
However, emerging evidence has suggested that high doses and the
high intensity of glucocorticoid use in patients with SLE can
increase the risk of bacterial infection along with other
non-infectious complications, such as osteoporosis, sleep disorders
and cushingoid syndrome (43).
Therefore, the long-term administration of corticosteroids can be
burdensome for patients, resulting in a low rate of patient
satisfaction and poor compliance (43). Minimizing or even terminating
glucocorticoid administration during the treatment of SLE is
considered a major goal for scientists. In the present
meta-analysis, although none of the six RCTs revealed a
statistically significant reduction in glucocorticoid
administration, the meta-analysis of the included RCTs revealed
that BLM significantly reduced the dose of glucocorticoids
administered. Consistent with the RCT analysis, the case series
analysis also revealed the same results. In ‘real-world’ studies,
OBSErve demonstrated that the majority of patients could reduce or
discontinue oral glucocorticoid use 6 months following BLM
treatment. Overall, the aforementioned findings indicated that
treatment with BLM effectively reduced glucocorticoid
administration in patients with SLE, thus attenuating
glucocorticoid-related morbidity and the irreversible damage caused
by their use.
The incidence of AEs and SAEs was similar between
the BLM and placebo groups. The majority of AEs included infections
and infestations. The most common infections in the BLM groups were
upper respiratory tract infections, cellulitis, pneumonia and
urinary tract infections. As regards the reaction rate at the
infusion site, the majority of studies reported that these were the
same between both groups. However, a previous study demonstrated
that hypersensitivity in the site of infusion was more common in
the BLM group compared with the placebo group (14-16 vs. 10%)
(12). Nevertheless, all infusion
and hypersensitivity reactions were improved following treatment
with antihistamine, prednisone or epinephrine. Psychological
effects should be also taken into consideration. In a previous
study, a patient who was treated with 1 mg/kg BLM committed
suicide, although this event was not associated with the drug
itself (11). In addition, three
patients in the BLM group had suicidal intentions, but no one
attempted suicide. Additionally, 4 patients in the placebo groups
also had suicidal intentions or behavior. Depression was recorded
more frequently in patients treated with BLM compared with the
placebo group (6-7 vs. 4%) (12).
Furthermore, the incidence of malignant disease was numerically
higher in the BLM group compared with the placebo group (9 vs. 2
patients) (11,12,14).
However, whether there was an association between BLM and cancer
should be further investigated. An extensive study lasting 8 years
(BEL112234), also reported a stable safety profile without new
safety signals (44). The
aforementioned results suggested that BLM was generally well
tolerated.
cSLE is less common than adult SLE. However, cSLE is
characterized by an enhanced disease activity and immediate
neurological, renal and hematological damage (45,46).
The trial by Brunner et al (16) revealed that the benefits and risk
profile of BLM treatment in children was similar and consistent
with those observed in adult patients. Therefore, BLM could be
considered as a novel therapeutic approach for treating cSLE. In
patients with SLE, disease activity can persist even after the
initiation of dialysis. However, the effect of BLM on treating SLE
after dialysis has not been previously investigated, at least to
the best of our knowledge. The case report study by Karasawa et
al (47) demonstrated that SLE
activity was attenuated in a patient with SLE treated with BLM
following hemodialysis. Another case study on a patient with SLE
who was treated with BLM during peritoneal dialysis revealed that
the clinical symptoms were significantly improved (48). However, the safety of BLM treatment
during dialysis needs to be further evaluated.
The RCTs included four interventions, one study
compared treatment with 1, 4 and 10 mg/kg BLM vs. the placebo; two
studies, 1 and 10 mg/kg BLM vs. the placebo; three studies, 10
mg/kg BLM vs. the placebo; and one study, 200 mg BLM subcutaneous
vs. the placebo. The network meta-analysis suggested that the
administration of 10 mg/kg BLM exhibited the highest efficacy in
the treatment of active SLE, followed by 1 mg/kg BLM, 200 mg
subcutaneous BLM and placebo (8).
Although BLM has emerged as a promising regimen for the treatment
of patients with active SLE, in the present meta-analysis, 52.8 and
60.5% of all patients reached the primary endpoint in the RCTs and
case series, respectively. The pathogenesis of SLE is complex and
BLM functions by specifically targeting a BAFF. CD20+
cells also play a crucial role in the pathogenesis of SLE.
Currently, the BEAT Lupus study (trial registration no.
ISRCTN47873) aims to investigate the safety and efficacy of BLM in
patients treated with rituximab, a B-cell depletion therapy
(49). This strategy could provide
a novel approach for the treatment of SLE. On the other hand, the
follow-up time in the present meta-analysis was relatively short,
52-76 weeks for the RCTs and 6-36 months for the case series. To
date, the longest in duration study evaluating the safety and
efficacy of BLM was conducted by Wallace et al (50) over the course of 13 years as part
of a phase II trial. That study revealed that the percentage of
patients who achieved a SRI response, increased from 32.8% at year
1 to 75.6% in those who remained under treatment for 12 years. In
addition, BLM was well tolerated with no new safety concerns.
Compared with a previous meta-analysis (7), the present study was more
comprehensive, including seven RCTs and 19 case series. In addition
to the research of RCTs, case series studies based on the ‘real’
world have more reference significance. A notable finding of the
present study was that the use of BLM reduced the use of
glucocorticoids. This discovery was not mentioned in the
aforementioned previous meta-analysis. Although the present study
was an integrated meta-analysis of the clinical research, there
were several potential shortcomings. Firstly, the number of
patients included in several studies was small and the observation
time varied among these studies, potentially leading to a certain
degree of uncertainty in the estimation of the TR. Secondly, the
possibility of selection and information bias, and uncertain
confounders could not be entirely ruled out. Thirdly, in the
present meta-analysis, the longest follow-up time was 76 weeks,
which was too short to evaluate long-term efficacy. Therefore,
further studies with a larger sample size and higher quality,
including patients of various ethnicities, undergoing dialysis or
during pregnancy need to be performed in the future to further
clarify the efficacy and safety of BLM.
In conclusion, the present meta-analysis revealed
that BLM therapy may provide significant clinical efficacy and was
well tolerated by patients with active SLE. Additionally, treatment
with BLM could reduce the use of glucocorticoids.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ contributed to the conception, design and
modification of the study. JZ, XL and YuX reviewed the articles,
extracted the data and organized the database search. XL performed
the statistical analysis. JZ wrote the first draft of the
manuscript. YaX guided and assisted in the statistical analysis.
YaX and HL also contributed to the conception and design of the
study. YaX, HL and YuX confirm the authenticity of all the data.
All authors contributed to manuscript revision, read, and approved
the submitted version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mok C, Hamijoyo L, Kasitanon N, Chen DY,
Chen S, Yamaoka K, Oku K, Li MT, Zamora L, Bae SC, et al: The
Asia-pacific league of associations for rheumatology consensus
statements on the management of systemic lupus erythematosus.
Lancet Rheumatol. 3:e517–e531. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheema GS, Roschke V, Hilbert DM and Stohl
W: Elevated serum B lymphocyte stimulator levels in patients with
systemic immune-based rheumatic diseases. Arthritis Rheum.
44:1313–1319. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Groom J, Kalled SL, Cutler AH, Olson C,
Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway
J, et al: Association of BAFF/BLyS overexpression and altered B
cell differentiation with Sjögren's syndrome. J Clin Invest.
109:59–68. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Mosak J and Furie R: Breaking the ice in
systemic lupus erythematosus: Belimumab, a promising new therapy.
Lupus. 22:361–371. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nardelli B, Moore PA, Li Y and Hilbert DM:
B lymphocyte stimulator (BLyS): A therapeutic trichotomy for the
treatment of B lymphocyte diseases. Leuk Lymphoma. 43:1367–1373.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vilas-Boas A, Morais SA and Isenberg DA:
Belimumab in systemic lupus erythematosus. RMD Open.
1(e000011)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borba HHL, Wiens A, Correr CJ and
Pontarolo R: Efficacy and safety of belimumab for the treatment of
systemic lupus erythematosus. Value Health. 16:PA725–PA726.
2013.
|
|
8
|
Lee YH and Song GG: Comparative efficacy
and safety of intravenous or subcutaneous belimumab in combination
with standard therapy in patients with active systemic lupus
erythematosus: A Bayesian network meta-analysis of randomized
controlled trials. Lupus. 27:112–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kandala NB, Connock M, Grove A, Sutcliffe
P, Mohiuddin S, Hartley L, Court R, Cummins E, Gordon C and Clarke
A: Belimumab: A technological advance for systemic lupus
erythematosus patients? Report of a systematic review and
meta-analysis. BMJ Open. 3(e002852)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hochberg MC: Updating the American college
of rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40(1725)1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wallace DJ, Stohl W, Furie RA, Lisse JR,
McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ,
et al: A phase II, randomized, double-blind, placebo-controlled,
dose-ranging study of belimumab in patients with active systemic
lupus erythematosus. Arthritis Rheum. 61:1168–1178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furie R, Petri M, Zamani O, Cervera R,
Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill
JT, Chatham WW, et al: A phase III, randomized, placebo-controlled
study of belimumab, a monoclonal antibody that inhibits B
lymphocyte stimulator, in patients with systemic lupus
erythematosus. Arthritis Rheum. 63:3918–3930. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Navarra SV, Guzmán RM, Gallacher AE, Hall
S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al:
Efficacy and safety of belimumab in patients with active systemic
lupus erythematosus: A randomised, placebo-controlled, phase 3
trial. Lancet. 377:721–731. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang F, Bae SC, Bass D, Chu M, Egginton
S, Gordon D, Roth DA, Zheng J and Tanaka Y: A pivotal phase III,
randomised, placebo-controlled study of belimumab in patients with
systemic lupus erythematosus located in China, Japan and South
Korea. Ann Rheum Dis. 77:355–363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stohl W, Schwarting A, Okada M, Scheinberg
M, Doria A, Hammer AE, Kleoudis C, Groark J, Bass D, Fox NL, et al:
Efficacy and safety of subcutaneous belimumab in systemic lupus
erythematosus: A fifty-two-week randomized, double-blind,
placebo-controlled study. Arthritis Rheumatol. 69:1016–1027.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brunner HI, Abud-Mendoza C, Viola DO,
Calvo Penades I, Levy D, Anton J, Calderon JE, Chasnyk VG,
Ferrandiz MA, Keltsev V, et al: Safety and efficacy of intravenous
belimumab in children with systemic lupus erythematosus: Results
from a randomised, placebo-controlled trial. Ann Rheum Dis.
79:1340–1348. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
D'Cruz D, Maksimowicz-Mckinnon K, Oates J,
Santiago MB, Bass D, Burriss S, Gilbride J, Groark J, Miller M and
Ji B: 200 Efficacy and safety of belimumab in patients of black
race with systemic lupus erythematosus: Results from the EMBRACE
study. Lupus Sci Med. 6 (Suppl 1):A1–A227. 2019.
|
|
18
|
Fanouriakis A, Adamichou C, Koutsoviti S,
Panopoulos S, Staveri C, Klagou A, Tsalapaki C, Pantazi L, Konsta
S, Mavragani CP, et al: Low disease activity-irrespective of
serologic status at baseline-associated with reduction of
corticosteroid dose and number of flares in patients with systemic
lupus erythematosus treated with belimumab: A real-life
observational study. Semin Arthritis Rheum. 48:467–474.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iaccarino L, Andreoli L, Bocci EB,
Bortoluzzi A, Ceccarelli F, Conti F, De Angelis R, De Marchi G, De
Vita S, Di Matteo A, et al: Clinical predictors of response and
discontinuation of belimumab in patients with systemic lupus
erythematosus in real life setting. Results of a large,
multicentric, nationwide study. J Autoimmun. 86:1–8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parodis I, Sjöwall C, Jönsen A, Ramsköld
D, Zickert A, Frodlund M, Sohrabian A, Arnaud L, Rönnelid J,
Malmström V, et al: Smoking and pre-existing organ damage reduce
the efficacy of belimumab in systemic lupus erythematosus.
Autoimmun Rev. 16:343–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prete M, Leone P, Frassanito MA, Desantis
V, Marasco C, Cicco S, Dammacco F, Vacca A and Racanelli V:
Belimumab restores Treg/Th17 balance in patients with refractory
systemic lupus erythematosus. Lupus. 27:1926–1935. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gatto M, Saccon F, Zen M, Regola F, Fredi
M, Andreoli L, Tincani A, Urban ML, Emmi G, Ceccarelli F, et al:
Early disease and low baseline damage as predictors of response to
belimumab in patients with systemic lupus erythematosus in a
real-life setting. Arthritis Rheumatol. 72:1314–1324.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Collins CE, Dall'Era M, Kan H, Macahilig
C, Molta C, Koscielny V and Chang DJ: Response to belimumab among
patients with systemic lupus erythematosus in clinical practice
settings: 24-Month results from the OBSErve study in the USA. Lupus
Sci Med. 3(e000118)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Babini A, Cappuccio AM, Caprarulo C,
Casado G, Eimon A, Figueredo H, Garcia MA, Magri S, Mannucci P,
Perez Rodriguez S, et al: Evaluation of belimumab treatment in
patients with systemic lupus erythematosus in a clinical practice
setting: Results from a 24-month OBSErve study in Argentina. Lupus.
29:1385–1396. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
von Kempis J, Duetsch S, Reuschling N,
Villiger R, Villiger PM, Vallelian F, Schaer DJ and Mueller RB:
Clinical outcomes in patients with systemic lupus erythematosus
treated with belimumab in clinical practice settings: A
retrospective analysis of results from the OBSErve study in
Switzerland. Swiss Med Wkly. 149(w20022)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Scheinberg M and Golmia R: Real life
experience on the effect of belimumab in patients with active
systemic lupus. Springerplus. 3(758)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schwarting A, Schroeder JO, Alexander T,
Schmalzing M, Fiehn C, Specker C, Perna A, Cholmakow-Bodechtel C,
Koscielny VB and Carnarius H: First real-world insights into
belimumab use and outcomes in routine clinical care of systemic
lupus erythematosus in germany: Results from the OBSErve Germany
study. Rheumatol Ther. 3:271–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cortés J, Andreu JL, Calvo J,
García-Aparicio AM, Coronell CG and Díaz-Cerezo S: Evaluation of
use of belimumab in clinical practice settings (observe study) in
spain: Health resource utilization and labour absenteeism. Value
Health. 17(A534)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anjo C, Mascaró JM Jr, Espinosa G and
Cervera R: Effectiveness and safety of belimumab in patients with
systemic lupus erythematosus in a real-world setting. Scand J
Rheumatol. 48:469–473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hui-Yuen JS, Reddy A, Taylor J, Li X,
Eichenfield AH, Bermudez LM, Starr AJ, Imundo LF, Buyon J, Furie
RA, et al: Safety and efficacy of belimumab to treat systemic lupus
erythematosus in academic clinical practices. J Rheumatol.
42:2288–2295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sthoeger Z, Lorber M, Tal Y, Toubi E,
Amital H, Kivity S, Langevitz P, Asher I, Elbirt D and Agmon Levin
N: Anti-BLyS treatment of 36 Israeli systemic lupus erythematosus
patients. Isr Med Assoc J. 19:44–48. 2017.PubMed/NCBI
|
|
32
|
Scheinberg M, de Melo FF, Bueno AN, Costa
CM, de Azevedo Bahr ML and Reis ER: Belimumab for the treatment of
corticosteroid-dependent systemic lupus erythematosus: From
clinical trials to real-life experience after 1 year of use in 48
Brazilian patients. Clin Rheumatol. 35:1719–1723. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Touma Z, Sayani A, Pineau CA, Fortin I,
Matsos M, Ecker GA, Chow A and Iczkovitz S: Belimumab use, clinical
outcomes and glucocorticoid reduction in patients with systemic
lupus erythematosus receiving belimumab in clinical practice
settings: Results from the OBSErve Canada study. Rheumatol Int.
37:865–873. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Scheinberg MA, Golmia AP, Golmia RP, de
Souza Molotievschi RN and Dos Santos Cortada AP: Lupus low disease
activity (SLE) in patients treated with belimumab: A single-center
real-life experience (2016-2019). Clin Rheumatol. 40:923–927.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Iaccarino L, Saccon F, Mathieu A, Piga M,
Ceribelli A, Selmi C, Cardinaletti P, Gabrielli A, Di Matteo A, De
Angelis R, et al: FRI0199 effectiveness and safety of belimumab in
patientswith active systemic lupus erythematosus: Results from a
large, nationwide, multicentric study. Ann Rheum Dis. 78 (Suppl
2):S778–S779. 2019.
|
|
36
|
Iaccarino L, Bettio S, Reggia R, Zen M,
Frassi M, Andreoli L, Gatto M, Piantoni S, Nalotto L, Franceschini
F, et al: Belimumab decreases flare rate and hinders the expected
damage progression in patients with active systemic lupus
erythematosus. Arthritis Care Res. 69:115–123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Buyon JP, Petri MA, Kim MY, Kalunian KC,
Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcón
GS, et al: The effect of combined estrogen and progesterone hormone
replacement therapy on disease activity in systemic lupus
erythematosus: A randomized trial. Ann Intern Med. 142:953–962.
2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Petri M, Buyon J and Kim M: Classification
and definition of major flares in SLE clinical trials. Lupus.
8:685–691. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Petri M, Kim MY, Kalunian KC, Grossman J,
Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM,
Askanase AD, et al: Combined oral contraceptives in women with
systemic lupus erythematosus. N Engl J Med. 353:2550–2558.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vincent FB, Saulep-Easton D, Figgett WA,
Fairfax KA and Mackay F: The BAFF/APRIL system: Emerging functions
beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev.
24:203–215. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee WS and Amengual O: B cells targeting
therapy in the management of systemic lupus erythematosus. Immunol
Med. 43:16–35. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Collins CE, Cortes-Hernández J, Garcia MA,
von Kempis J, Schwarting A, Touma Z, Kurtinecz M and Gairy K:
Real-world effectiveness of belimumab in the treatment of systemic
lupus erythematosus: Pooled analysis of multi-country data from the
OBSErve studies. Rheumatol Ther. 7:949–965. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen HL, Shen LJ, Hsu PN, Shen CY, Hall SA
and Hsiao FY: Cumulative burden of glucocorticoid-related adverse
events in patients with systemic lupus erythematosus: Findings from
a 12-year longitudinal study. J Rheumatol. 45:83–89.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
van Vollenhoven RF, Navarra SV, Levy RA,
Thomas M, Heath A, Lustine T, Adamkovic A, Fettiplace J, Wang ML,
Ji B and Roth D: Long-term safety and limited organ damage in
patients with systemic lupus erythematosus treated with belimumab:
A phase III study extension. Rheumatology (Oxford). 59:281–291.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kamphuis S and Silverman ED: Prevalence
and burden of pediatric-onset systemic lupus erythematosus. Nat Rev
Rheumatol. 6:538–546. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Silva CA, Avcin T and Brunner HI: Taxonomy
for systemic lupus erythematosus with onset before adulthood.
Arthritis Care Res (Hoboken). 64:1787–1793. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Karasawa K, Ogura S, Takabe T, Miyabe Y,
Iwabuchi Y, Akiyama K, Sato M, Moriyama T, Uchida K and Nitta K:
Successful treatment with belimumab in a patient with refractory
systemic lupus erythematosus after initiation of hemodialysis:
Considering the synergistic effect of belimumab and immunological
burn-out phenomenon in end-stage renal disease patients on
hemodialysis. Blood Purif: Apr 23, 2021 (Epub ahead of print). doi:
10.1159/000512585.
|
|
48
|
Binda V, Trezzi B, Del Papa N, Beretta L,
Frontini G, Porata G, Fabbrini P, Pozzi MR, Messa P, Sinico RA and
Moroni G: Belimumab may decrease flare rate and allow
glucocorticoid withdrawal in lupus nephritis (including dialysis
and transplanted patient). J Nephrol. 33:1019–1025. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jones A, Muller P, Dore CJ, Ikeji F,
Caverly E, Chowdhury K, Isenberg DA, Gordon C and Ehrenstein MR:
Belimumab after B cell depletion therapy in patients with systemic
lupus erythematosus (BEAT Lupus) protocol: A prospective
multicentre, double-blind, randomised, placebo-controlled, 52-week
phase II clinical trial. BMJ Open. 9(e032569)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wallace DJ, Ginzler EM, Merrill JT, Furie
RA, Stohl W, Chatham WW, Weinstein A, McKay JD, McCune WJ, Petri M,
et al: Safety and efficacy of belimumab plus standard therapy for
up to thirteen years in patients with systemic lupus erythematosus.
Arthritis Rheumatol. 71:1125–1134. 2019.PubMed/NCBI View Article : Google Scholar
|