|
1
|
Mok C, Hamijoyo L, Kasitanon N, Chen DY,
Chen S, Yamaoka K, Oku K, Li MT, Zamora L, Bae SC, et al: The
Asia-pacific league of associations for rheumatology consensus
statements on the management of systemic lupus erythematosus.
Lancet Rheumatol. 3:e517–e531. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Cheema GS, Roschke V, Hilbert DM and Stohl
W: Elevated serum B lymphocyte stimulator levels in patients with
systemic immune-based rheumatic diseases. Arthritis Rheum.
44:1313–1319. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Groom J, Kalled SL, Cutler AH, Olson C,
Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway
J, et al: Association of BAFF/BLyS overexpression and altered B
cell differentiation with Sjögren's syndrome. J Clin Invest.
109:59–68. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Mosak J and Furie R: Breaking the ice in
systemic lupus erythematosus: Belimumab, a promising new therapy.
Lupus. 22:361–371. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nardelli B, Moore PA, Li Y and Hilbert DM:
B lymphocyte stimulator (BLyS): A therapeutic trichotomy for the
treatment of B lymphocyte diseases. Leuk Lymphoma. 43:1367–1373.
2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Vilas-Boas A, Morais SA and Isenberg DA:
Belimumab in systemic lupus erythematosus. RMD Open.
1(e000011)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borba HHL, Wiens A, Correr CJ and
Pontarolo R: Efficacy and safety of belimumab for the treatment of
systemic lupus erythematosus. Value Health. 16:PA725–PA726.
2013.
|
|
8
|
Lee YH and Song GG: Comparative efficacy
and safety of intravenous or subcutaneous belimumab in combination
with standard therapy in patients with active systemic lupus
erythematosus: A Bayesian network meta-analysis of randomized
controlled trials. Lupus. 27:112–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kandala NB, Connock M, Grove A, Sutcliffe
P, Mohiuddin S, Hartley L, Court R, Cummins E, Gordon C and Clarke
A: Belimumab: A technological advance for systemic lupus
erythematosus patients? Report of a systematic review and
meta-analysis. BMJ Open. 3(e002852)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hochberg MC: Updating the American college
of rheumatology revised criteria for the classification of systemic
lupus erythematosus. Arthritis Rheum. 40(1725)1997.PubMed/NCBI View Article : Google Scholar
|
|
11
|
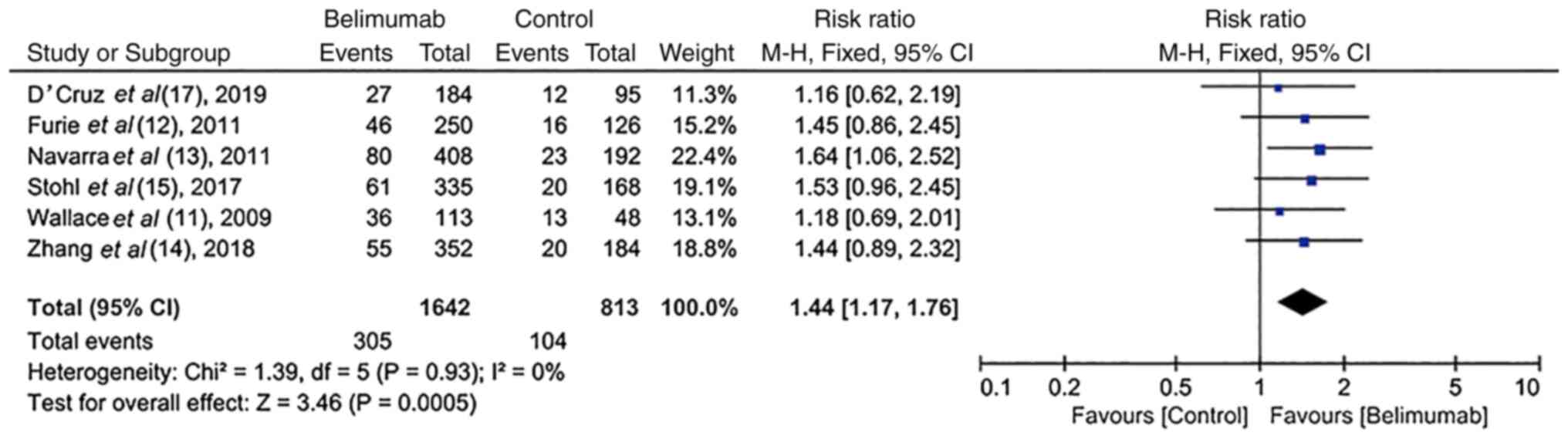
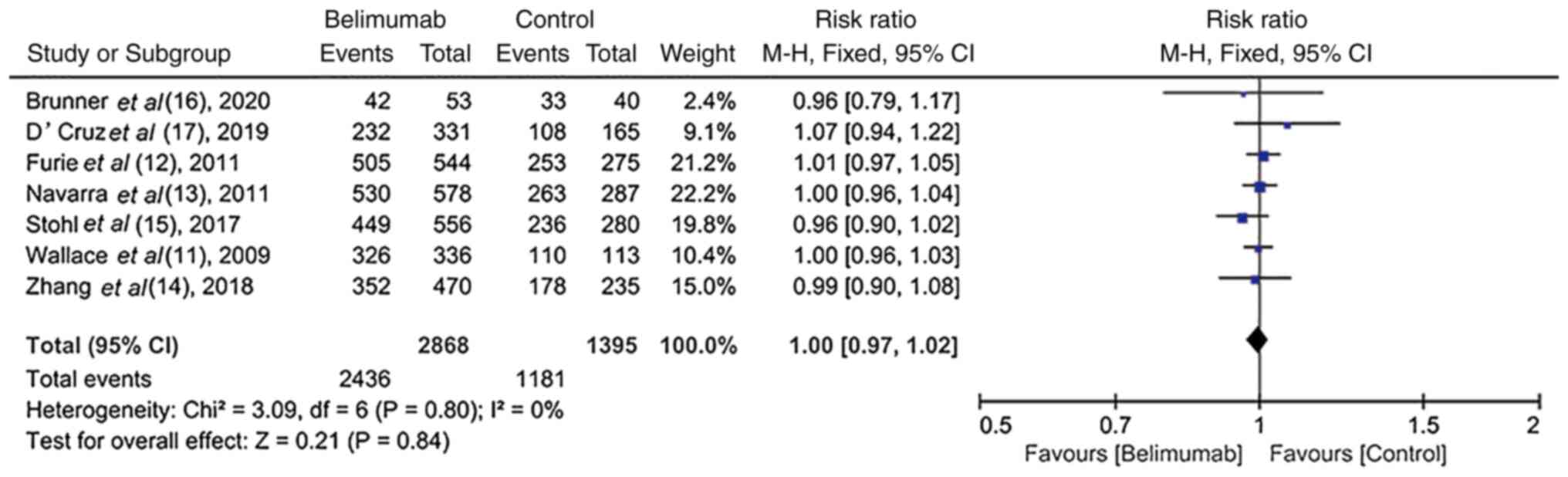
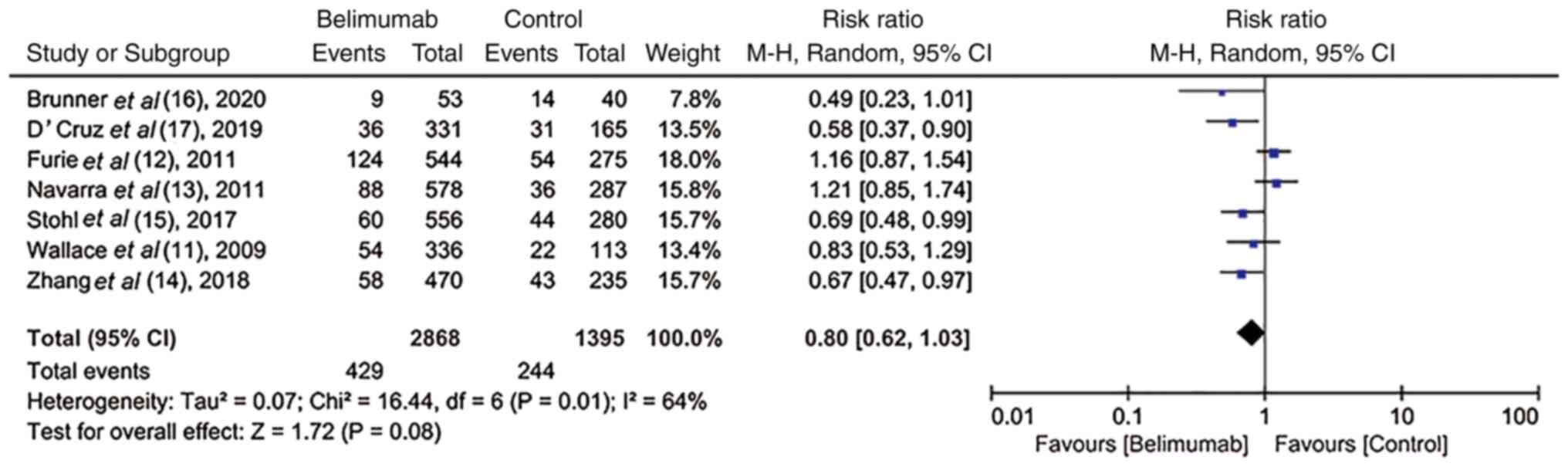
Wallace DJ, Stohl W, Furie RA, Lisse JR,
McKay JD, Merrill JT, Petri MA, Ginzler EM, Chatham WW, McCune WJ,
et al: A phase II, randomized, double-blind, placebo-controlled,
dose-ranging study of belimumab in patients with active systemic
lupus erythematosus. Arthritis Rheum. 61:1168–1178. 2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Furie R, Petri M, Zamani O, Cervera R,
Wallace DJ, Tegzová D, Sanchez-Guerrero J, Schwarting A, Merrill
JT, Chatham WW, et al: A phase III, randomized, placebo-controlled
study of belimumab, a monoclonal antibody that inhibits B
lymphocyte stimulator, in patients with systemic lupus
erythematosus. Arthritis Rheum. 63:3918–3930. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Navarra SV, Guzmán RM, Gallacher AE, Hall
S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al:
Efficacy and safety of belimumab in patients with active systemic
lupus erythematosus: A randomised, placebo-controlled, phase 3
trial. Lancet. 377:721–731. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang F, Bae SC, Bass D, Chu M, Egginton
S, Gordon D, Roth DA, Zheng J and Tanaka Y: A pivotal phase III,
randomised, placebo-controlled study of belimumab in patients with
systemic lupus erythematosus located in China, Japan and South
Korea. Ann Rheum Dis. 77:355–363. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stohl W, Schwarting A, Okada M, Scheinberg
M, Doria A, Hammer AE, Kleoudis C, Groark J, Bass D, Fox NL, et al:
Efficacy and safety of subcutaneous belimumab in systemic lupus
erythematosus: A fifty-two-week randomized, double-blind,
placebo-controlled study. Arthritis Rheumatol. 69:1016–1027.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Brunner HI, Abud-Mendoza C, Viola DO,
Calvo Penades I, Levy D, Anton J, Calderon JE, Chasnyk VG,
Ferrandiz MA, Keltsev V, et al: Safety and efficacy of intravenous
belimumab in children with systemic lupus erythematosus: Results
from a randomised, placebo-controlled trial. Ann Rheum Dis.
79:1340–1348. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
D'Cruz D, Maksimowicz-Mckinnon K, Oates J,
Santiago MB, Bass D, Burriss S, Gilbride J, Groark J, Miller M and
Ji B: 200 Efficacy and safety of belimumab in patients of black
race with systemic lupus erythematosus: Results from the EMBRACE
study. Lupus Sci Med. 6 (Suppl 1):A1–A227. 2019.
|
|
18
|
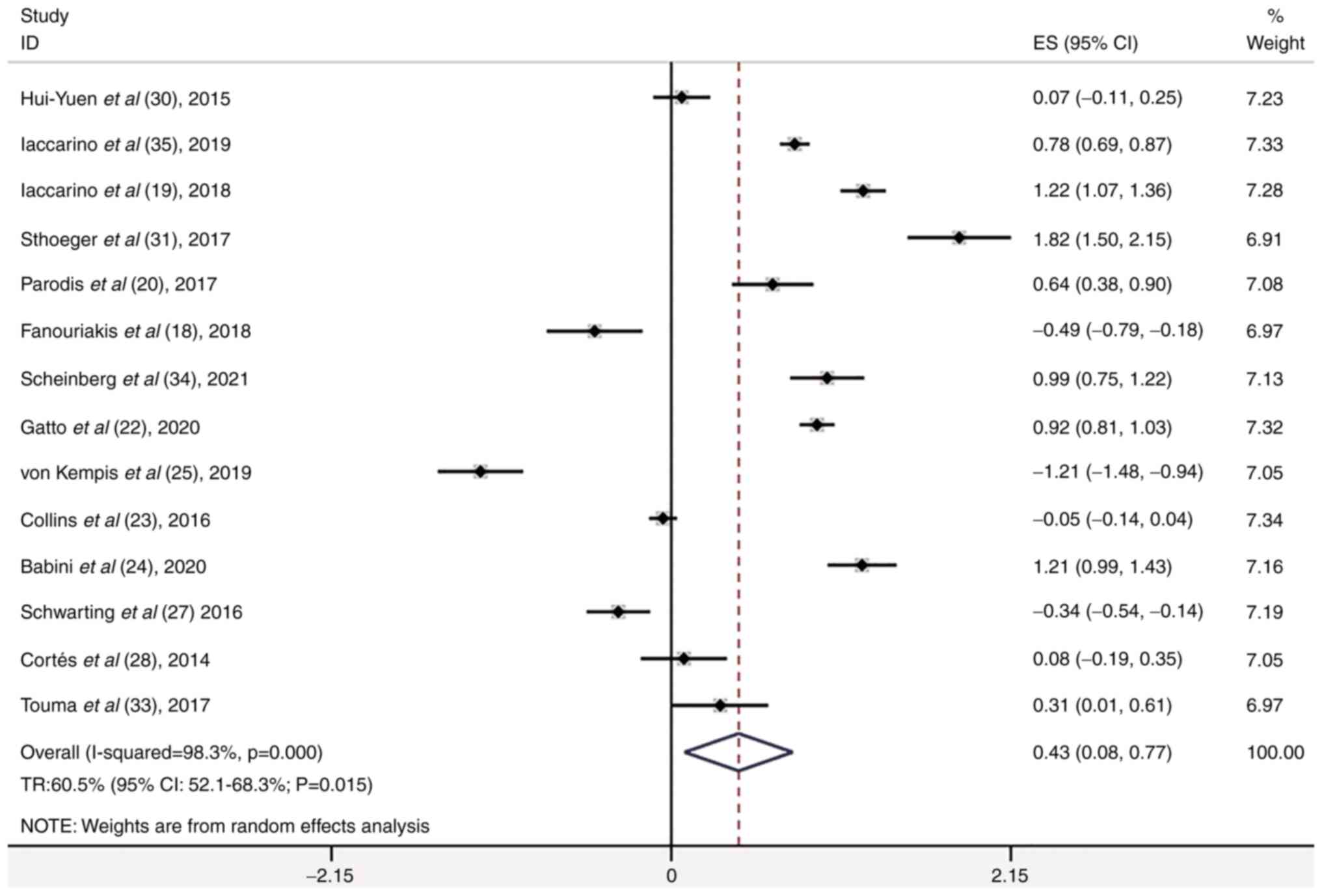
Fanouriakis A, Adamichou C, Koutsoviti S,
Panopoulos S, Staveri C, Klagou A, Tsalapaki C, Pantazi L, Konsta
S, Mavragani CP, et al: Low disease activity-irrespective of
serologic status at baseline-associated with reduction of
corticosteroid dose and number of flares in patients with systemic
lupus erythematosus treated with belimumab: A real-life
observational study. Semin Arthritis Rheum. 48:467–474.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Iaccarino L, Andreoli L, Bocci EB,
Bortoluzzi A, Ceccarelli F, Conti F, De Angelis R, De Marchi G, De
Vita S, Di Matteo A, et al: Clinical predictors of response and
discontinuation of belimumab in patients with systemic lupus
erythematosus in real life setting. Results of a large,
multicentric, nationwide study. J Autoimmun. 86:1–8.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Parodis I, Sjöwall C, Jönsen A, Ramsköld
D, Zickert A, Frodlund M, Sohrabian A, Arnaud L, Rönnelid J,
Malmström V, et al: Smoking and pre-existing organ damage reduce
the efficacy of belimumab in systemic lupus erythematosus.
Autoimmun Rev. 16:343–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prete M, Leone P, Frassanito MA, Desantis
V, Marasco C, Cicco S, Dammacco F, Vacca A and Racanelli V:
Belimumab restores Treg/Th17 balance in patients with refractory
systemic lupus erythematosus. Lupus. 27:1926–1935. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gatto M, Saccon F, Zen M, Regola F, Fredi
M, Andreoli L, Tincani A, Urban ML, Emmi G, Ceccarelli F, et al:
Early disease and low baseline damage as predictors of response to
belimumab in patients with systemic lupus erythematosus in a
real-life setting. Arthritis Rheumatol. 72:1314–1324.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Collins CE, Dall'Era M, Kan H, Macahilig
C, Molta C, Koscielny V and Chang DJ: Response to belimumab among
patients with systemic lupus erythematosus in clinical practice
settings: 24-Month results from the OBSErve study in the USA. Lupus
Sci Med. 3(e000118)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Babini A, Cappuccio AM, Caprarulo C,
Casado G, Eimon A, Figueredo H, Garcia MA, Magri S, Mannucci P,
Perez Rodriguez S, et al: Evaluation of belimumab treatment in
patients with systemic lupus erythematosus in a clinical practice
setting: Results from a 24-month OBSErve study in Argentina. Lupus.
29:1385–1396. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
von Kempis J, Duetsch S, Reuschling N,
Villiger R, Villiger PM, Vallelian F, Schaer DJ and Mueller RB:
Clinical outcomes in patients with systemic lupus erythematosus
treated with belimumab in clinical practice settings: A
retrospective analysis of results from the OBSErve study in
Switzerland. Swiss Med Wkly. 149(w20022)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Scheinberg M and Golmia R: Real life
experience on the effect of belimumab in patients with active
systemic lupus. Springerplus. 3(758)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Schwarting A, Schroeder JO, Alexander T,
Schmalzing M, Fiehn C, Specker C, Perna A, Cholmakow-Bodechtel C,
Koscielny VB and Carnarius H: First real-world insights into
belimumab use and outcomes in routine clinical care of systemic
lupus erythematosus in germany: Results from the OBSErve Germany
study. Rheumatol Ther. 3:271–290. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cortés J, Andreu JL, Calvo J,
García-Aparicio AM, Coronell CG and Díaz-Cerezo S: Evaluation of
use of belimumab in clinical practice settings (observe study) in
spain: Health resource utilization and labour absenteeism. Value
Health. 17(A534)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Anjo C, Mascaró JM Jr, Espinosa G and
Cervera R: Effectiveness and safety of belimumab in patients with
systemic lupus erythematosus in a real-world setting. Scand J
Rheumatol. 48:469–473. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hui-Yuen JS, Reddy A, Taylor J, Li X,
Eichenfield AH, Bermudez LM, Starr AJ, Imundo LF, Buyon J, Furie
RA, et al: Safety and efficacy of belimumab to treat systemic lupus
erythematosus in academic clinical practices. J Rheumatol.
42:2288–2295. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sthoeger Z, Lorber M, Tal Y, Toubi E,
Amital H, Kivity S, Langevitz P, Asher I, Elbirt D and Agmon Levin
N: Anti-BLyS treatment of 36 Israeli systemic lupus erythematosus
patients. Isr Med Assoc J. 19:44–48. 2017.PubMed/NCBI
|
|
32
|
Scheinberg M, de Melo FF, Bueno AN, Costa
CM, de Azevedo Bahr ML and Reis ER: Belimumab for the treatment of
corticosteroid-dependent systemic lupus erythematosus: From
clinical trials to real-life experience after 1 year of use in 48
Brazilian patients. Clin Rheumatol. 35:1719–1723. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Touma Z, Sayani A, Pineau CA, Fortin I,
Matsos M, Ecker GA, Chow A and Iczkovitz S: Belimumab use, clinical
outcomes and glucocorticoid reduction in patients with systemic
lupus erythematosus receiving belimumab in clinical practice
settings: Results from the OBSErve Canada study. Rheumatol Int.
37:865–873. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Scheinberg MA, Golmia AP, Golmia RP, de
Souza Molotievschi RN and Dos Santos Cortada AP: Lupus low disease
activity (SLE) in patients treated with belimumab: A single-center
real-life experience (2016-2019). Clin Rheumatol. 40:923–927.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Iaccarino L, Saccon F, Mathieu A, Piga M,
Ceribelli A, Selmi C, Cardinaletti P, Gabrielli A, Di Matteo A, De
Angelis R, et al: FRI0199 effectiveness and safety of belimumab in
patientswith active systemic lupus erythematosus: Results from a
large, nationwide, multicentric study. Ann Rheum Dis. 78 (Suppl
2):S778–S779. 2019.
|
|
36
|
Iaccarino L, Bettio S, Reggia R, Zen M,
Frassi M, Andreoli L, Gatto M, Piantoni S, Nalotto L, Franceschini
F, et al: Belimumab decreases flare rate and hinders the expected
damage progression in patients with active systemic lupus
erythematosus. Arthritis Care Res. 69:115–123. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Buyon JP, Petri MA, Kim MY, Kalunian KC,
Grossman J, Hahn BH, Merrill JT, Sammaritano L, Lockshin M, Alarcón
GS, et al: The effect of combined estrogen and progesterone hormone
replacement therapy on disease activity in systemic lupus
erythematosus: A randomized trial. Ann Intern Med. 142:953–962.
2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Petri M, Buyon J and Kim M: Classification
and definition of major flares in SLE clinical trials. Lupus.
8:685–691. 1999.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Petri M, Kim MY, Kalunian KC, Grossman J,
Hahn BH, Sammaritano LR, Lockshin M, Merrill JT, Belmont HM,
Askanase AD, et al: Combined oral contraceptives in women with
systemic lupus erythematosus. N Engl J Med. 353:2550–2558.
2005.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Vincent FB, Saulep-Easton D, Figgett WA,
Fairfax KA and Mackay F: The BAFF/APRIL system: Emerging functions
beyond B cell biology and autoimmunity. Cytokine Growth Factor Rev.
24:203–215. 2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Lee WS and Amengual O: B cells targeting
therapy in the management of systemic lupus erythematosus. Immunol
Med. 43:16–35. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Collins CE, Cortes-Hernández J, Garcia MA,
von Kempis J, Schwarting A, Touma Z, Kurtinecz M and Gairy K:
Real-world effectiveness of belimumab in the treatment of systemic
lupus erythematosus: Pooled analysis of multi-country data from the
OBSErve studies. Rheumatol Ther. 7:949–965. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen HL, Shen LJ, Hsu PN, Shen CY, Hall SA
and Hsiao FY: Cumulative burden of glucocorticoid-related adverse
events in patients with systemic lupus erythematosus: Findings from
a 12-year longitudinal study. J Rheumatol. 45:83–89.
2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
van Vollenhoven RF, Navarra SV, Levy RA,
Thomas M, Heath A, Lustine T, Adamkovic A, Fettiplace J, Wang ML,
Ji B and Roth D: Long-term safety and limited organ damage in
patients with systemic lupus erythematosus treated with belimumab:
A phase III study extension. Rheumatology (Oxford). 59:281–291.
2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Kamphuis S and Silverman ED: Prevalence
and burden of pediatric-onset systemic lupus erythematosus. Nat Rev
Rheumatol. 6:538–546. 2010.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Silva CA, Avcin T and Brunner HI: Taxonomy
for systemic lupus erythematosus with onset before adulthood.
Arthritis Care Res (Hoboken). 64:1787–1793. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Karasawa K, Ogura S, Takabe T, Miyabe Y,
Iwabuchi Y, Akiyama K, Sato M, Moriyama T, Uchida K and Nitta K:
Successful treatment with belimumab in a patient with refractory
systemic lupus erythematosus after initiation of hemodialysis:
Considering the synergistic effect of belimumab and immunological
burn-out phenomenon in end-stage renal disease patients on
hemodialysis. Blood Purif: Apr 23, 2021 (Epub ahead of print). doi:
10.1159/000512585.
|
|
48
|
Binda V, Trezzi B, Del Papa N, Beretta L,
Frontini G, Porata G, Fabbrini P, Pozzi MR, Messa P, Sinico RA and
Moroni G: Belimumab may decrease flare rate and allow
glucocorticoid withdrawal in lupus nephritis (including dialysis
and transplanted patient). J Nephrol. 33:1019–1025. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Jones A, Muller P, Dore CJ, Ikeji F,
Caverly E, Chowdhury K, Isenberg DA, Gordon C and Ehrenstein MR:
Belimumab after B cell depletion therapy in patients with systemic
lupus erythematosus (BEAT Lupus) protocol: A prospective
multicentre, double-blind, randomised, placebo-controlled, 52-week
phase II clinical trial. BMJ Open. 9(e032569)2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wallace DJ, Ginzler EM, Merrill JT, Furie
RA, Stohl W, Chatham WW, Weinstein A, McKay JD, McCune WJ, Petri M,
et al: Safety and efficacy of belimumab plus standard therapy for
up to thirteen years in patients with systemic lupus erythematosus.
Arthritis Rheumatol. 71:1125–1134. 2019.PubMed/NCBI View Article : Google Scholar
|