Introduction
Stable isotope labeling plays a staple role in
biological research. As such studies are commonly linked to the
kinetic features of biochemical reactions, the kinetic isotope
effect (KIE) on the growth and metabolism of an organism should be
considered (1,2). An isotope is a species of an element
with a different mass due to the different number of neutrons. The
isotope effect refers to the phenomenon that the physical and
chemical properties of isotopic atoms differ due to the
dissimilarities in their nuclear properties (3,4).
For decades, various efforts have been made to
explore the biology isotope effects of biogenic elements. Despite
the increasing knowledge of the biological isotope effect, a
comprehensive understanding of the effects in the biological
context has not yet been achieved (2,5-9).
Among the main biogenic elements (C, H, O and N), deuterium has
been investigated in a number of studies and its effects on
organisms have been demonstrated (5-9).
However, the carbon isotope effects of bioactive compounds have yet
not been investigated, at least to the best of our knowledge,
although such studies are important to the biology development in
theory and application. Additionally, fewer isotope effect studies
on human cells have been conducted compared with other organism
(10).
In the present study, testosterone, a typical
androgen, was isotopically modified. It was demonstrated that the
level of isotope enrichment potentially influenced its isotope
effect, and it was classified as high (>50%) and low (<10%)
level (11,12). Herein, carbon-13 (13C)
was enriched in testosterone with a low enrichment level
(13C/12C, 6.7%) in order to investigate the
carbon isotope effect on human osteoblasts, aortic endothelial
cells and umbilical vein endothelial cells. Androgen is involved in
the regulation of a number of physiological processes, including
bone development (13,14) and the modulation of vascular
behavior (15-17).
Osteoblasts and vascular endothelial cells express androgen
receptor and are targets for the action of androgen (18-20).
Several studies have demonstrated that testosterone enhances the
proliferation of human osteoblasts (13), human primary aortic endothelial
cells (15,20) and human umbilical vein endothelial
cells (21). The present study
examined the isotope effect of 13C-enriched testosterone
at various concentrations on the growth of the aforementioned cell
types. The alkaline phosphatase (ALP) level and osteocalcin (OC)
secretion of osteoblasts were examined. To the best of our
knowledge, the present study is the first investigate the carbon
isotope effect of a bioactive compound on normal human cells.
The aim of the present study was to highlight the
importance of isotope effects in stable isotope-based research by
investigating the carbon isotope effect of 13C-enriched
testosterone on the growth of human cells. The concentration
effects at physiological (10-10 and 10-8
mol/l) (22) and
supraphysiological (10-6 and 10-5 mol/l)
levels were investigated using an in vitro model.
Materials and methods
Materials and reagents
Human osteoblasts (cat no. 4610), human primary
aortic endothelial cells (cat no. H-6052) and human umbilical vein
endothelial cells (cat no. C0035C) were obtained from ScienCell
Research Laboratories, Inc., Cell Biologics, Inc. and Thermo Fisher
Scientific, Inc., respectively. Ascorbic acid, glycerol-2-phosphate
and dexamethasone were purchased from Sigma-Aldrich; Merck KGaA.
Dulbecco's modified Eagle's medium (DMEM) with low glucose,
penicillin-streptomycin, trypsin-ethylenediaminetetraacetic acid
(EDTA), fetal bovine serum (FBS) and phosphate-buffered saline
(PBS) were obtained from Thermo Fisher Scientific, Inc.
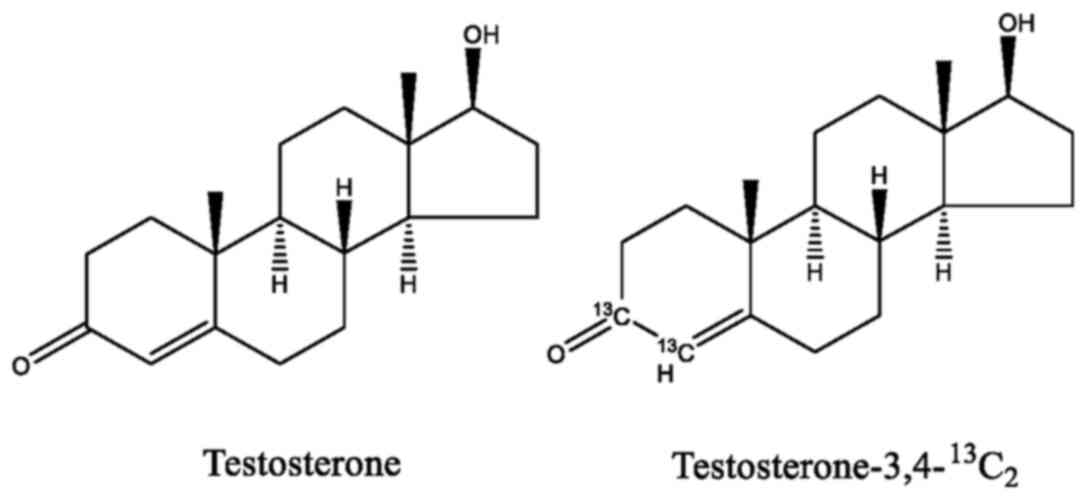
Testosterone and testosterone-3,4-13C2 were
purchased from Alta Scientific. Co., Ltd.
Preparation of 13C-enriched
testosterone
Testosterone and
testosterone-3,4-13C2 were both dissolved in
ethanol and then mixed together at a ratio of 1:1 (mole), by which
13C-enriched testosterone was obtained. The carbon
isotopic composition of 13C-enriched testosterone was
calculated to be 13C/12C=6.7%. The chemical
structures of testosterone and
testosterone-3,4-13C2 are presented in
Fig. 1.
Cell culture and compound
intervention
Human osteoblasts, human primary aortic endothelial
cells and human umbilical vein endothelial cells were thawed and
cultured in low-glucose DMEM supplemented with 10% FBS and 1%
penicillin-streptomycin with 5% CO2 at 37˚C. The cells
were dissociated with trypsin-EDTA and seeded in 96-well tissue
culture plates at the density of 1x104 cells/well. The
culture media were then changed to media with low-glucose DMEM, 10%
FBS, 1% penicillin-streptomycin, 50 mg/ml ascorbic acid, 0.01 mol/l
glycerol-2-phosphate and 100 nmol/l dexamethasone. The cells were
cultured in the culture medium containing either testosterone or
13C-enriched testosterone at concentrations of 0,
10-10, 10-8, 10-6 and
10-5 mol/l. An untreated (no drugs; 0 mol/l) was used as
a blank control. The morphology of the cells treated with
testosterone and 13C-enriched testosterone at the
concentration of 10-5 mol/l was also observed. The
observation was conducted using an inverted microscope (XDS-500C,
Shanghai Caikon Optical Instrument Co., Ltd.).
Measurement of cell proliferative
activity
The proliferative activities of human osteoblasts,
human primary aortic endothelial cells and human umbilical vein
endothelial cells were determined using
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
(MTS) assay (MTS cell proliferation colorimetric assay kit, AmyJet
Scientific, Inc.) when the cells were cultured for 48 h at 37˚C.
Following the manufacturer's instructions, MTS and phenazine
methosulfate (PMS) solution were mixed (MTS:PMS=20:1). The culture
medium and the mixed solution of MTS and PMS were added to the test
well of a 96-well plate and were incubated at 37˚C for 2 h. The
light absorbance of the formazan product was measured at a 490-nm
wavelength using a spectrophotometer (BioTek Instruments, Inc.).
The measurement for each concentration was repeated 6 times, and
the average optical density (OD) value was recorded.
Measurement of the ALP level of
osteoblasts
In order to further investigate the isotope effect
of 13C-enriched testosterone on osteoblasts, the ALP
level in osteoblasts was determined. An elevation in ALP levels
serves as a marker of osteogenic differentiation (23,24).
The measurement of the ALP level is based on the ALP-mediated
conversion of p-nitrophenol phosphate (PNPP) to nitrophenol in an
alkaline buffer (25). The product
nitrophenol exhibits the light absorption at a 405-nm wavelength.
In the present study, the ALP levels of human osteoblasts cultured
on the 5th day with 13C-enriched testosterone at the
concentrations of 0, 10-10, 10-8,
10-6 and 10-5 mol/l were determined.
Following the instructions of the Alkaline Phosphatase Assay kit
(TW-Reagent Industrial Co., Ltd.), the cells were lysed in 600 µl
lysis buffer and the lysate was centrifuged at 1,000 x g for 20 min
at 20˚C. The supernatant and PNPP solution were added to the well
of a tissue culture plate and then incubated at 37˚C for 1 h. After
the stop solution provided with the Alkaline Phosphatase Assay kit
was added, the light absorbance was measured at wavelength of 405
nm using a microplate reader (Synergy LX, Bio-Tek Instruments,
Inc.). The values of the ALP level (U/l) were recorded. The test
was repeated six times, and the average value was recorded. The
data were normalized to the control.
Measurement of OC secretion levels of
osteoblasts
In order to investigate the isotope effect of
13C-enriched testosterone on the OC secretion of
osteoblasts, the OC level of osteoblasts was examined. OC, an
osteoblast-specific secreted protein, is synthesized by osteoblasts
during bone formation (26). It
plays key roles in both the biological and mechanical functions of
bone (13,27). As a biochemical marker of
osteoblast activity, the OC level reflects the rate of bone
formation (28). In the present
study, the OC levels of human osteoblasts cultured on the 5th day
with 13C-enriched testosterone at the concentrations of
0, 10-10, 10-8, 10-6 and
10-5 mol/l were determined. Following the instructions
of the ELISA kit, the OC levels in the supernatant of the culture
medium were analyzed using an OC ELISA kit (cat. no. RAB1073-1KT;
Sigma-Aldrich; Merck KGaA). The measurement of the OC level (µg/l)
was repeated six times, and the average value was recorded. The
data were normalized to the control.
Statistics analysis
Data were analyzed using IBM SPSS Statistics v26
software (IBM Corp.). The distribution of the variables was
examined using the Shapiro-Wilk test, and the variance was
determined using Levene's test. When the distribution was found to
be parametric, the differences were assessed using one-way ANOVA
with the Bonferroni post hoc test. P-values <0.05 were
considered to indicate statistically significant differences. The
numerical variables are presented as the mean ± SD (n=6 repeated
experiments).
Results
Cell proliferative activity
The proliferative activities of the human
osteoblasts, human primary aortic endothelial cells and human
umbilical vein endothelial cells treated with testosterone and
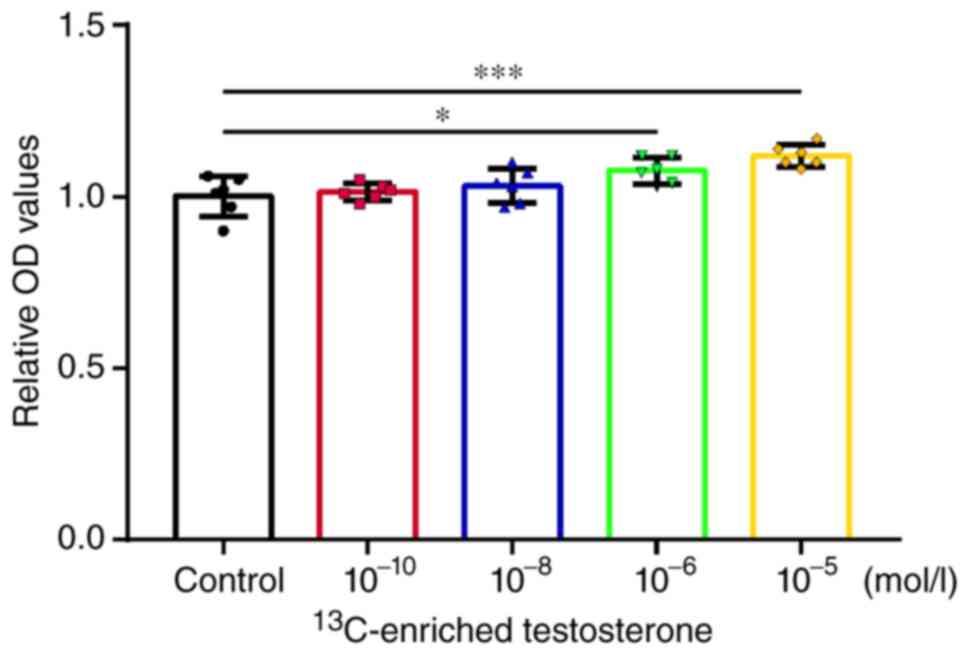
13C-enriched testosterone were analyzed. The measured OD
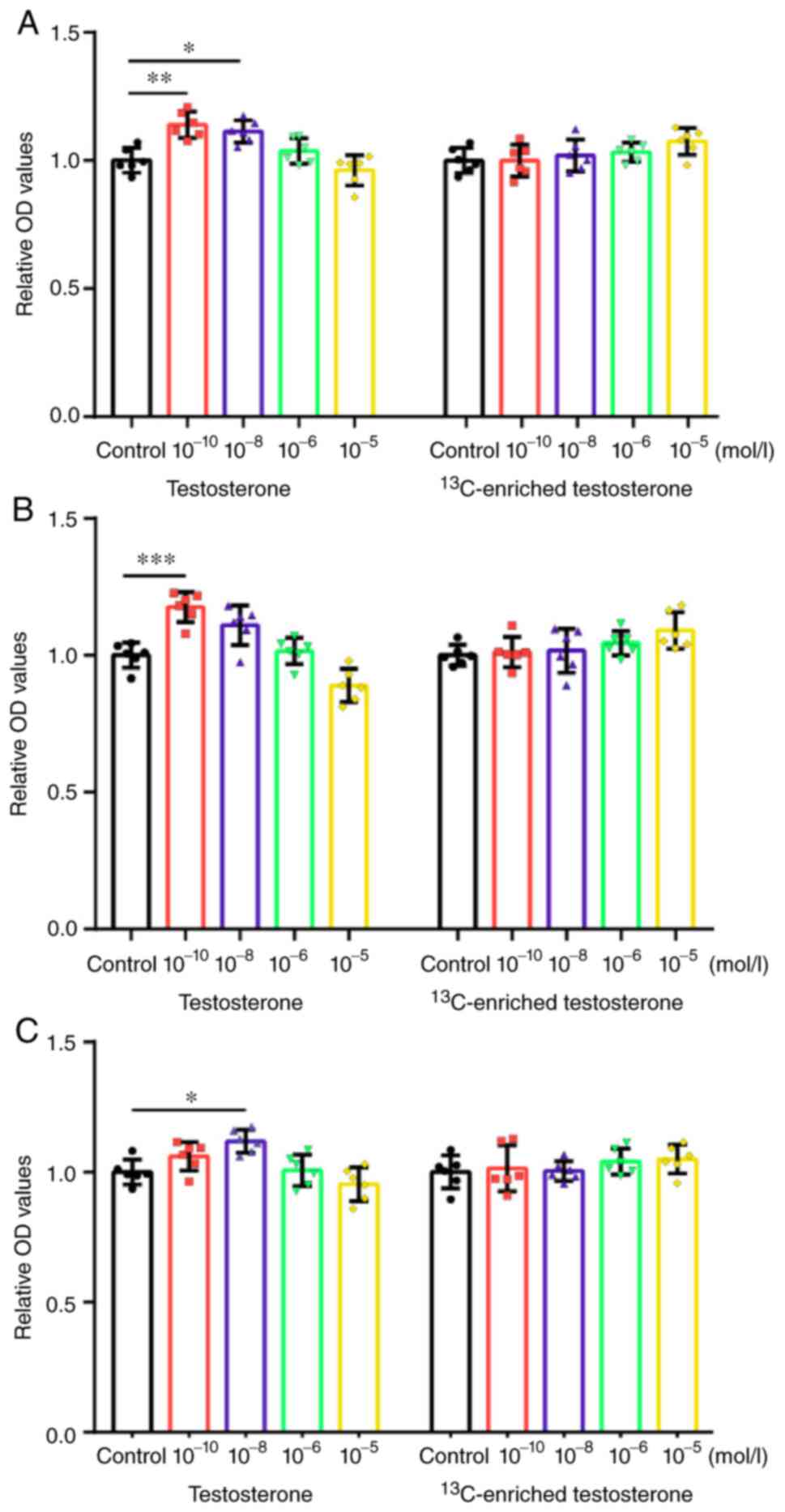
values were normalized and plotted (Fig. 2). The morphology of the cells
treated with testosterone and 13C-enriched testosterone
at the concentration of 10-5 mol/l is illustrated in
Fig. 3.
Human osteoblasts
Testosterone promoted the proliferation of human
osteoblasts at the concentrations of 10-10 (P<0.01)
and 10-8 mol/l (P<0.05). Following treatment with
supraphysiological concentrations (10-5 mol/l) of
testosterone, the human osteoblasts exhibited a decreasing trend in
proliferative activity (Fig.
2A).
13C-enriched testosterone did not promote
the proliferation of human osteoblasts at the concentrations of
10-10-10-6 mol/l. Among these concentration
groups, there were no marked differences in the effects of
13C-enriched testosterone, which suggested that the
effect had no association with the concentration at the level of
10-10-10-6 mol/l. It was noted that the human
osteoblasts exhibited an increasing trend in proliferative activity
when treated with supraphysiological concentrations
(10-5 mol/l) of 13C-enriched testosterone,
although no significant differences were found (Fig. 2A).
Human primary aortic endothelial
cells
Testosterone significantly promoted the
proliferation of human primary aortic endothelial cells at the
concentration of 10-10 mol/l (P<0.001). Following
treatment with supraphysiological concentrations (10-5
mol/l) of testosterone, the human primary aortic endothelial cells
exhibited a decreasing trend in proliferative activity (Fig. 2B).
13C-enriched testosterone did not promote
the proliferation of human primary aortic endothelial cells at the
concentrations of 10-10-10-6 mol/l. Among
these concentration groups, there were no differences in the
effects of 13C-enriched testosterone, which indicated
that the effect had no association with the concentration at the
level of 10-10-10-6 mol/l. It was noted that
the human primary aortic endothelial cells exhibited an increasing
trend in proliferative activity when treated with a high
concentration (10-5 mol/l) of 13C-enriched
testosterone, although no significant differences were found
(Fig. 2B).
Human umbilical vein endothelial
cells
Testosterone significantly promoted the
proliferation of human umbilical vein endothelial cells at the
concentration of 10-8 mol/l (P<0.05). Following
treatment with a high concentration (10-5 mol/l) of
testosterone, the human umbilical vein endothelial cells exhibited
a decreasing trend in proliferative activity (Fig. 2C).
13C-enriched testosterone did not
increase the proliferation of human vein endothelial cells at the
concentrations of 10-10-10-5 mol/l. Among
these concentration groups, there were no significant differences
in the effects of 13C-enriched testosterone, which
indicated that the effect had no association with the concentration
at the level of 10-10-10-5 mol/l (Fig. 2C).
ALP level of human osteoblasts
Compared with the control group,
13C-enriched testosterone did not enhance the ALP level
of human osteoblasts at the concentrations of 10-10 and
10-8 mol/l. The human osteoblasts exhibited an
increasing trend in ALP levels when treated with a high
concentration (10-6 and 10-5 mol/l) of
13C-enriched testosterone, although no significant
differences were found (Fig.
4).
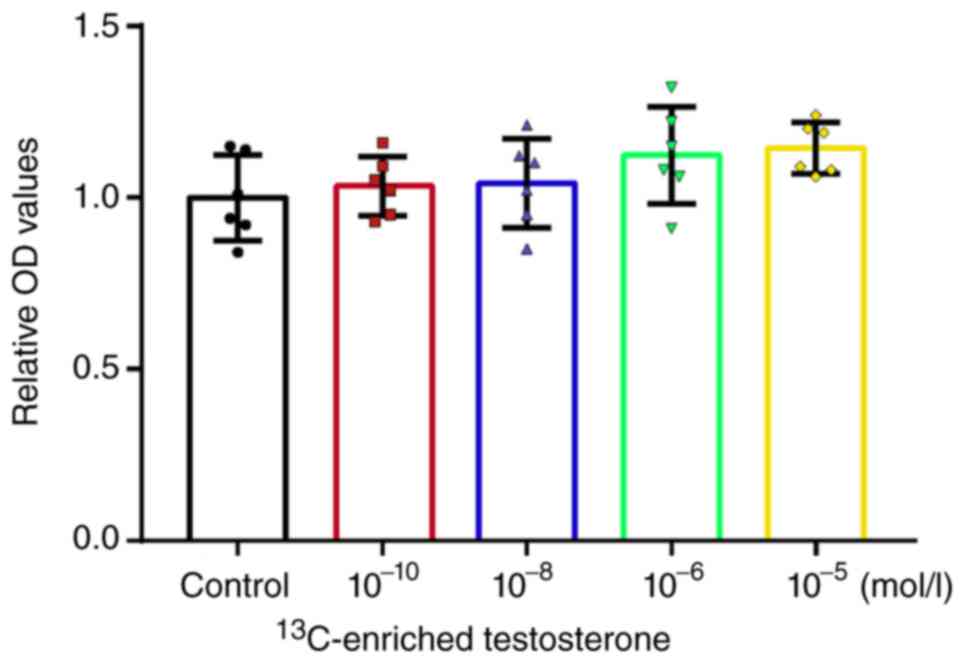
Osteocalcin secretion of human
osteoblasts
Compared with the control group,
13C-enriched testosterone significantly enhanced the OC
level in human osteoblasts at the concentrations of 10-6
(P<0.05) and 10-5 mol/l (P<0.001) (Fig. 5).
Discussion
Among the biogenic elements composing 96% of the
human body (29), carbon is a
vital element and composes the backbone of biological
macromolecules. As biochemical reactions are often accompanied by
the cleavage or formation of carbon-carbon bonds, the change in
carbon isotope composition potentially influences the biochemical
reaction rate and exerts a biological isotope effect. Even though
the carbon isotope effect is crucial to biological research and
medicine, related studies are very limited (30). The present study demonstrated that
there was a difference in the biological effect between
13C-enriched testosterone and testosterone. In future
studies, the authors aim to elucidate the mechanisms responsible
for this phenomenon by investigating other biomarkers secreted by
cells and the related signaling pathways.
The growth rate is arguably the most profound
phenotypic parameter that defines the existence of an organism. It
integrates multiple aspects of the physiological state of a cell,
and is often associated with how cells respond to drugs (31). Previous studies have established
that testosterone exerts effects on cell proliferation in a
concentration-dependent manner (13,15,20,21),
which was supported by the findings of the present study. Of note,
the effects of 13C-enriched testosterone were
concentration-independent at 10-10-10-6 mol/l
concentrations. This finding suggested that 13C
enrichment in a drug may alter the pharmacological properties of
the drug.
The concentration gradient used in the present study
was designed according to the physiological levels of testosterone,
which was beneficial to observe the effects in a physiological and
supraphysiological state. However, higher gradients of testosterone
concentration need to be applied in future studies to supplement
the current findings, since a higher gradient may result in a more
significant difference in the effects between testosterone and
13C-enriched testosterone.
It is considered that the enrichment of a heavy
isotope attenuates the biochemical reaction rate and delays the
growth of an organism due to the kinetic isotope effect (2,32-34).
This view was supported by the findings of the present study using
13C-enriched testosterone at physiological
concentrations; however, this view was challenged by the results
obtained when using supraphysiological concentrations.
13C-enriched testosterone was found to promote cell
proliferation at a high concentration (10-5 mol/l).
Furthermore, 13C-enriched testosterone enhanced the OC
secretion of human osteoblasts at high concentrations
(10-6 and 10-5 mol/l). These findings
demonstrated the polytropic characteristics of the biological
isotope effects, which should be taken into account in stable
isotope-based research.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XW and WY contributed to the conception of the
study. MZ and TZ performed the experiments. XW, MZ and WY performed
the data analyses and wrote the manuscript. XW, MZ and WY confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Iotti S, Raff L and Sabatini A: Chemical
and biochemical thermodynamics: Is it time for a reunification?
Biophys Chem. 221:49–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Andriukonis E and Gorokhova E: Kinetic
15N-isotope effects on algal growth. Sci Rep.
7(44181)2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Buchachenko AL: Mass-independent isotope
effects. J Phys Chem B. 117:2231–2238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Basov A, Fedulova L, Vasilevskaya E and
Dzhimak S: Possible mechanisms of biological effects observed in
living systems during 2H/1H isotope
fractionation and deuterium interactions with other biogenic
isotopes. Molecules. 24(4101)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rodin S, Rebellato P, Lundin A and Zubarev
RA: Isotopic resonance at 370 ppm deuterium negatively affects
kinetics of luciferin oxidation by luciferase. Sci Rep.
8(16249)2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kozin S, Skrebitsky V, Kondratenko R,
Kravtsov A, Butina E, Moiseev A, Malyshko V, Baryshev M, Elkina A
and Dzhimak S: Electrophysiological activity and survival rate of
rats nervous tissue cells depends on D/H isotopic composition of
medium. Molecules. 26(2036)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Li X and Snyder MP: Yeast longevity
promoted by reversing aging-associated decline in heavy isotope
content. NPJ Aging Mech Dis. 2(16004)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li X and Snyder MP: Can heavy isotopes
increase lifespan? Studies of relative abundance in various
organisms reveal chemical perspectives on aging. Bioessays.
38:1093–1101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hill S, Hirano K, Shmanai VV, Marbois BN,
Vidovic D, Bekish AV, Kay B, Tse V, Fine J, Clarke CF and
Shchepinov MS: Isotope-reinforced polyunsaturated fatty acids
protect yeast cells from oxidative stress. Free Radic Biol Med.
50:130–138. 2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zlatskiy IA, Zlatska AV, Antipova NV,
Dolenko SA, Gordiienko IM, Gubar OS, Vasyliev RG, Zubov DA,
Novikova SN and Syroeshkin AV: Comparative analysis of the
different dyes' potential to assess human normal and cancer cell
viability in vitro under different D/H ratios in a culture medium.
ScientificWorldJournal. 2020(2373021)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xie XS and Zubarev RA: Effects of
low-level deuterium enrichment on bacterial growth. PLoS One.
9(e102071)2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Andreyev AY, Tsui HS, Milne GL, Shmanai
VV, Bekish AV, Fomich MA, Pham MN, Nong Y, Murphy AN, Clarke CF and
Shchepinov MS: Isotope-reinforced polyunsaturated fatty acids
protect mitochondria from oxidative stress. Free Radic Biol Med.
82:63–72. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wu XC and Zhang MQ: Effects of androgen
and progestin on the proliferation and differentiation of
osteoblasts. Exp Ther Med. 16:4722–4728. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Vandewalle S, Van Caenegem E, Craen M,
Taes Y, Kaufman JM and T'Sjoen G: Growth, sexual and bone
development in a boy with bilateral anorchia under testosterone
treatment guided by the development of his monozygotic twin. J
Pediatr Endocrinol Metab. 31:361–367. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Campelo AE, Cutini PH and Massheimer VL:
Cellular actions of testosterone in vascular cells: Mechanism
independent of aromatization to estradiol. Steroids. 77:1033–1040.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lorigo M, Mariana M, Lemos MC and Cairrao
E: Vascular mechanisms of testosterone: The non-genomic point of
view. J Steroid Biochem Mol Biol. 196(105496)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Littleton-Kearney M and Hurn PD:
Testosterone as a modulator of vascular behavior. Biol Res Nurs.
5:276–285. 2004.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Mohamad NV, Soelaiman IN and Chin KY: A
concise review of testosterone and bone health. Clin Interv Aging.
11:1317–1324. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Russell PK, Clarke MV, Cheong K, Anderson
PH, Morris HA, Wiren KM, Zajac JD and Davey RA: Androgen receptor
action in osteoblasts in male mice is dependent on their stage of
maturation. J Bone Miner Res. 30:809–823. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cai J, Hong Y, Weng C, Tan C,
Imperato-McGinley J and Zhu YS: Androgen stimulates endothelial
cell proliferation via an androgen receptor/VEGF/cyclin A-mediated
mechanism. Am J Physiol Heart Circ Physiol. 300:H1210–H1221.
2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ling S, Dai A, Williams MRI, Myles K,
Dilley RJ, Komesaroff PA and Sudhir K: Testosterone (T) enhances
apoptosis-related damage in human vascular endothelial cells.
Endocrinology. 143:1119–1125. 2002.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Clark RV, Wald JA, Swerdloff RS, Wang C,
Wu FCW, Bowers LD and Matsumoto AM: Large divergence in
testosterone concentrations between men and women: Frame of
reference for elite athletes in sex-specific competition in sports,
a narrative review. Clin Endocrinol (Oxf). 90:15–22.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lee W, Eo SR, Choi JH, Kim YM, Nam MH and
Seo YK: The osteogenic differentiation of human dental pulp stem
cells through G0/G1 arrest and the p-ERK/Runx-2 pathway by sonic
vibration. Int J Mol Sci. 22(10167)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Sun Y, Liu J, Han Y and Yan J:
MicroRNA-346-5p regulates differentiation of bone marrow-derived
mesenchymal stem cells by inhibiting transmembrane protein 9.
Biomed Res Int. 2020(8822232)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun J, Zhao J, Bao X, Wang Q and Yang X:
Alkaline phosphatase assay based on the chromogenic interaction of
diethanolamine with 4-aminophenol. Anal Chem. 90:6339–6345.
2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mizokami A, Kawakubo-Yasukochi T and
Hirata M: Osteocalcin and its endocrine functions. Biochem
Pharmacol. 132:1–8. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bailey S, Karsenty G, Gundberg C and
Vashishth D: Osteocalcin and osteopontin influence bone morphology
and mechanical properties. Ann NY Acad Sci. 1409:79–84.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Wada S, Fukawa T and Kamiya S: Osteocalcin
and bone. Clin Calcium. 17:1673–1677. 2007.PubMed/NCBI
|
|
29
|
Xie X and Zubarev RA: Isotopic resonance
hypothesis: Experimental verification by Escherichia coli growth
measurements. Sci Rep. 5(9215)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gregg CT, Hutson JY, Prine JR, Ott DG and
Furchner JE: Substantial replacement of mammalian body carbon with
carbon-13. Life Sci. 13:775–782. 1973.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kopf SH, Sessions AL, Cowley ES, Reyes C,
Van Sambeek L, Hu Y, Orphan VJ, Kato R and Newman DK: Trace
incorporation of heavy water reveals slow and heterogeneous
pathogen growth rates in cystic fibrosis sputum. Proc Natl Acad Sci
USA. 113:E110–E116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gorokhova E: Shifts in rotifer life
history in response to stable isotope enrichment: Testing theories
of isotope effects on organismal growth. R Soc Open Sci.
4(160810)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie X and Zubarev RA: On the effect of
planetary stable isotope compositions on growth and survival of
terrestrial organisms. PLoS One. 12(e0169296)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pomytkin IA and Kolesova OE: Relationship
between natural concentration of heavy water isotopologs and rate
of H2O2 generation by mitochondria. Bull Exp Biol Med. 142:570–572.
2006.PubMed/NCBI View Article : Google Scholar
|