Introduction
Cerebral cavernous malformations (CCMs) present a
relatively low prevalence (0.16-0.5%), accounting for 5-15% of all
central nervous system vascular malformations (1-3).
The disease is characterized as low-flow vascular malformations
composed of blood-filled sinusoidal locules known as ‘caverns’. At
the histological level, CCM is characterized by the lack of mural
elements of mature vascular structures (3).
The major clinical presentations are epilepsy,
headaches or focal neurological deficits; however 30% of the
patients are asymptomatic or presents non-specific headache
(4). During disease progression,
the growth of vascular malformations is associated with recurrent
hemorrhages (annual hemorrhage rate of 0.6-11%/patient/year)
(3,5), which is considered to be a
consequence of the immature vascular network constituting the CCM
lesions (6). In the management of
this pathology, it is also important to take into account changes
in arterial pressure, which can alter the hemorrhage propensity and
patterns (7). Depending on the
anatomical localization of the CCM, patient management relies on
surgical resection, observation and symptomatic treatment. In
surgical inaccessible lesions, drugs such as statins,
anti-angiogenic agents or vitamin D3 have been tested, although
none have revealed clear benefits (8-11).
The beneficial use of propranolol in childhood
hemangioma, a close pathological counterpart of cavernous
malformations, supports the putative use of propranolol in the
management of patients with symptomatic CCM (12). Propranolol administration in
patients with CCM, although not commonly prescribed, has
effectively been used in children and appears to play a protective
role in the prevention of CCM-derived hemorrhaging in adults
(13-18).
A common feature of propranolol-sensitive vascular
tumors, such as hemangioma and CCM, is the distinctive expression
of CD15-positive ‘vasculogenic zones’ (19-21).
Of note, the in vitro gain of CD15 is followed by embryonic
stem cell differentiation into endothelial cells (ECs) (22), indicating the putative involvement
of endothelial progenitor cells (EPCs) in the development of
vascular malformations. The association between CD15-positive cells
and neo-vessels formation is not novel; it was described, >40
years ago in immature vessels of the placenta (23,24);
the effects of propranolol on the placental regression have also
long been described (25).
The identification of EPC subsets in peripheral
blood (PB) is not yet clear. The authors have previously
demonstrated that in tumors and normal tissues, some ECs
simultaneously express CD14 (monocytic marker) and CD31 (EC
marker), indicating mixed features between monocytes and ECs
(26). More recently, it was
demonstrated that monocytes can differentiate into ECs and be
incorporated into blood vessels (27). These studies indicate the
underestimation of monocytes as a relevant source for vascular
growth. In fact, due to their 2-10% prevalence in PB (28) and compared to the estimated 0.002%
of EPCs proposed by other studies (29-32),
monocytes are putatively the most representative EPC subgroup in
PB. Moreover, some studies have demonstrated that CD15 is also
expressed in monocytes (33,34),
with its levels being increased in pathological conditions
(35). Notably, in tumors, CD14
immune cells are also CD15-positive, clearly indicating a subset of
monocytes/macrophages (36). These
observations indicate that CD15 cells in the ‘vasculogenic zone’
are in fact monocytes functioning as EPCs, contributing for blood
vessel formation in CCM.
Hemangiomas and CCM share phenotypical
characteristics, being both composed of a mixture of abnormal
dilated capillary vessels with disorganized ECs and pericytes
(37-39).
The exact mechanisms that regulate the development of vascular
abnormalities remain poorly understood. It is known that during the
growth phase of hemangiomas, the increased expression of fibroblast
growth factor (FGF) and vascular endothelial growth factor (VEGF)
is associated with ECs and interstitial cell proliferation
(37). The effect of propranolol
in the reversion of vascular malformations is putatively associated
with a decreased expression of FGF and VEGF, impairing EC
migration, proliferation and reorganization, which in turns leads
to vasoconstriction (involution phase) (12,40-42).
Since the natural evolution of CCM is chronic and
unpredictable, the follow-up of patients with CCM involves the
long-term clinical and imagiological evaluation with magnetic
resonance imaging (MRI) (12). In
an attempt to identify a suitable follow-up method, the
monitorization of the levels of CD14+/CD31+
monocytes in the PB of patients with CCM is proposed. Considering
that the authors recently published a study demonstrating that
monocytes are viable EPCs (27),
it was hypothesized that circulating
CD14+/CD31+ monocytes function as EPCs and
contribute to the development of CCM lesions.
Materials and methods
PB processing and cell
characterization
The PB of a 13-year-old Caucasian girl with CCM was
collected and analyzed, between 2013 and 2020, after obtaining
informed consent from her parents at the Neuropediatrics Department
at the Portuguese Institute of Oncology of Lisbon, Francisco Gentil
(IPOLFG; ethics approval was obtained from the IPOLFG Ethics
Committee; UIC-1137); her parents also agreed to the publication of
the case study. The PB was centrifuged at 155 x g for 5 min, at
room temperature, and serum was then stored at -20˚C until further
analysis. The cell pellet was resuspended in 45 ml 1X RBC lysis
buffer (786-1701, G-Biosciences) and incubated for 15 min in the
dark, at room temperature. Subsequently, the resuspended cells were
centrifuged at 155 x g for 5 min, at room temperature, washed twice
with 1X phosphate-buffered saline (PBS) and incubated with
anti-CD14-FITC (1:100; cat. no. 555397, BD Biosciences) and
anti-CD31-APC (1:100; cat. no. FAB3567A, R&D Systems, Inc.)
antibodies in 0.5% bovine serum albumin (BSA; BSAV-RO; Merck
KGaA)-PBS (v/w) at 4˚C for 20 min in the dark. Immunolabelling was
evaluated using a flow cytometer (FACSCalibur, BD Biosciences) and
the data were analyzed using FlowJo X v10.0.7 software (https://www.flowjo.com/). PB cells from healthy blood
donors (at least two by measurement) were used, under consent, as
normal controls. A total of 16 controls, male and female, with an
age range between 18 and 40 years, collected between 2013 and 2020;
sample collection was performed (four donors at each time point of
follow-up) on the same date with the CCM patient blood
collection.
EC culture
Human umbilical vein ECs cells (HUVECs; CRL-1730,
ATCC) were cultured in endothelial cell growth basal medium-2
(EBM-2: CC-3156, Lonza Group, Ltd.) supplemented with EGM-2
SingleQuots Supplements (CC-4176, Lonza Group, Ltd.), which
included 2% fetal bovine serum (FBS- CC4101A, Lonza Group, Ltd.),
and maintained at 37˚C in a humidified atmosphere with 5%
CO2. The cells were used until passage 10 and were
detached with 0.05% Trypsin-EDTA 1X (25300-054, Invitrogen; Thermo
Fisher Scientific, Inc.). For the experimental conditions, the
cells were cultured in the presence or absence of 100 µM
propranolol (P8688; Sigma-Aldrich; Merck KGaA), as previously
described (43).
Monocyte isolation and culture
Monocytes were isolated from PB collected after
obtaining consent from healthy donors, from 2013 to 2020, at the
Immune-Hemotherapy Department at Portuguese Institute of Oncology
of Lisbon, Francisco Gentil (IPOLFG). Ethics approval was obtained
from the IPOLFG Ethics Committee; UIC-1137). PB mononuclear cells
(PBMCs) from blood samples were separated using Histopaque-1077
(10771, Sigma-Aldrich; Merck KGaA), followed by magnetic monocyte
isolation using the Monocyte isolation kit II (130-091-153, MACS
Technology; MiltenyiBiotec, Inc.), according to the manufacturers'
protocols. Monocytes were cultured in EBM-2 (CC-3156, Lonza Group,
Ltd.) plus EGM-2 SingleQuots Supplements (CC-4176, Lonza Group,
Ltd.) and with 2% FBS (CC4101A, Lonza Group, Ltd.), 50 ng/ml VEGF
(V7259, Sigma-Aldrich; Merck KGaA) and 10 U/ml heparin (H3149,
Sigma-Aldrich; Merck KGaA). The cells were maintained at 37˚C, in a
humidified atmosphere with 0.5% CO2. Hydrogen peroxide
(H2O2; 15 µM; 1.07210.0250, MerckKGaA) was
used as a reactive oxygen species (ROS) generator, as previously
described (27). The inhibitory
effects of propranolol (100 µM; 16 h) on monocyte differentiation
capacity in these particular culture conditions have been
previously published by the authors. The differentiation process
was confirmed by measuring the levels of expression of the
endothelial marker, vWF, as previously reported (43).
Determination of VEGF levels
The concentration of VEGF in PB serum and in the
culture medium conditioned by monocytes, isolated as described
above, was evaluated using the Human VEGF Quantikine ELISA kit
(DVE00, R&D Systems, Inc.), according to the manufacturer's
instructions. PB serum from healthy blood donors (the same donors
used to determine blood cell markers), was used as normal controls
and for cell supernatants, cells under control conditions were
maintained in H2O2 and propranolol-free
media.
Cell proliferation assay
The determination of cell proliferation was
calculated using the ratio of total and Ki67+ nuclei.
Briefly, HUVECs (5x104 cells/well) were cultured on
glass slides coated with 0.2% gelatin and fixed in 2%
paraformaldehyde for 15 min at 4˚C, followed by blocking with 1%
BSA-1X PBS (w/v). The cells were then incubated with anti-Ki67
antibody [1:100 in 1% BSA-0.1% triton X-100-1X PBS (w/v/v); cat.
no. sc-15402, Santa Cruz Biotechnology, Inc.], overnight at 4˚C,
followed by incubation with secondary antibody (Alexa Fluor 488
goat anti-rabbit, 1:1,000 in 1% BSA-0.1% triton x100-PBS; cat. no.
A-11078, Invitrogen; Thermo Fisher Scientific, Inc.), for 2 h at
room temperature. Slides were mounted in VECTASHIELD media with
DAPI (4'-6-diamidino-2-phenylindole; H-1200, Vector Laboratories,
Inc.), and examined by standard fluorescence microscopy using an
Axio Imager. Z1 microscope (Zeiss AG) with CytoVision®
software version 3.9 and analyzed using ImageJ software MacOS X,
with Java 1.8.0_172 (National Institutes of Health).
Wound healing assay
Cells were plated in 24-well plates
(1x105 cells/well) until the formation of a confluent
monolayer. The cells were then incubated with mitomycin-C (M4287,
Sigma-Aldrich; Merck KGaA), an antimitotic agent, for 3 h. A linear
scratch in each monolayer was created using a P200 pipette tip,
creating a gap across the well diameter. The media (EBM-2
supplemented with EGM-2 SingleQuots Supplements, which include 2%
FBS; CC4101A, Lonza Group, Ltd.) was replaced to remove debris and
cells in suspension, and to expose cells to the experimental
conditions. Bright-field images of each well at 0 and 10 h were
acquired using an Olympus IX53 inverted microscope (Olympus
Corporation) and images were analyzed and quantified using ImageJ
software MacOS X, with Java 1.8.0_172 (National Institutes of
Health).
Rat aortic rings sprouting assay
Aortas (thoracic and abdominal segments) were
dissected from male Wistar rats (aortas were collected from
10-week-old rats; n=6; used as controls; the rats were not
submitted to any experimental condition) in the scope of another
project. The study was approved by the Ethics Committee at NOVA
Medical School (Ref. 75/2019/CEFCM). The rats housing conditions,
as well as anesthesia and euthanasia procedures were as previously
described (44). After removing
all extraneous fat, fibrotic tissue and vasa vasorum structures,
the aorta was segmented into rings with a length of ~1 mm. The
rings were transferred to a Petri dish and incubated overnight in
FBS-free culture medium at 37˚C with 5% CO2. On the
following day, the rings were embedded in Matrigel in a 24-well
plate with EBM-2, with or without 100 µM propranolol. The medium
was refreshed every 3-4 days, with the sprouts becoming visible at
7-13 days. Representative images were acquired using an Olympus
IX53 inverted microscope (Olympus Corporation) and the branch
points (intersections between ECs) and number per area were counted
using ImageJ software MacOS X, with Java 1.8.0_172 (National
Institutes of Health). The density of vessel-like structure
formation (branch points number/µm2) was calculated as
the proxy of vascular density.
Statistical analysis
All data were analyzed using a Student's t-test or
one-way ANOVA and Tukey's post hoc test, in GraphPad Prism v7
software (www.graphpad.com/). The assays were
performed with at least three biological replicates per condition.
A value of P<0.05 was considered to indicate a statistically
significant difference.
Results
Clinical case presentation
A 13-year-old Caucasian girl presented complex
partial seizures at the age of 18 months. An MRI scan revealed
>30 brain lesions, some with evidence of recent bleeding,
compatible with cavernomas (lesions were of several sizes, three
with a diameter >10 mm, mainly hemispheric, in the cortical and
subcortical regions). Apart from this, she had no relevant previous
personal or family medical history. Her parents' imaging analyses
did not reveal any notable vascular lesions.
At the time of diagnosis, she underwent surgery and
a bleeding frontal lesion was partially resected; the pathology
report confirmed a cavernoma lesion. Despite treatment with
anti-epileptic drugs, the seizures recurred, usually at the same
time each year. No causal association was established with the
bleeding of the lesions, apart from a single time when bleeding and
perilesional edema were documented in one cavernoma. She had no
targeted therapy for cavernoma prior to her condition being brought
to our attention.
She was examined for the first time in the
aforementioned department at the age of 6 years. Following an
examination, no notable neurological deficits were observed. She
was under valproic acid and carbamazepine treatment. Genetic
analysis revealed the presence of a CCM3-PDCD-10 mutation, one of
the loci associated with CCMs (45,46).
Propranolol therapy was commenced at the dose of 0.16 mg/kg/day and
titrated to a maximum of 20 mg three times a day (0.8 mg/kg/day).
At 6 years of follow-up, treatment with propranolol was
well-tolerated and the seizures were controlled with valproic acid.
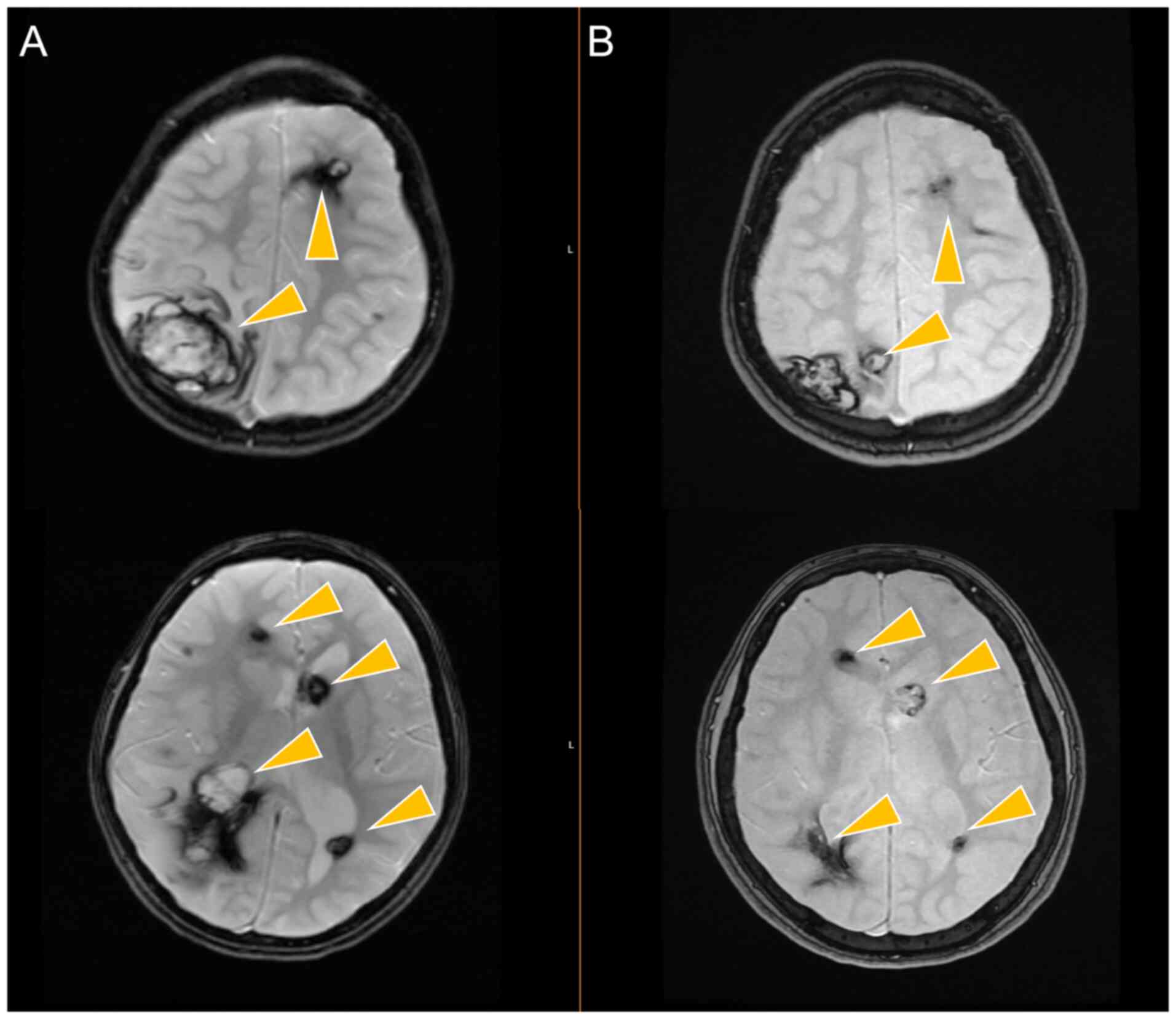
Accordingly, vascular lesions were more exuberant before
propranolol treatment (Fig. 1A),
and MRI scans over the years revealed the spontaneous involution of
some lesions and the stability of the others, without new bleeding
events, as observed following a 6-year follow-up period (Fig. 1B).
Cellular and molecular effects
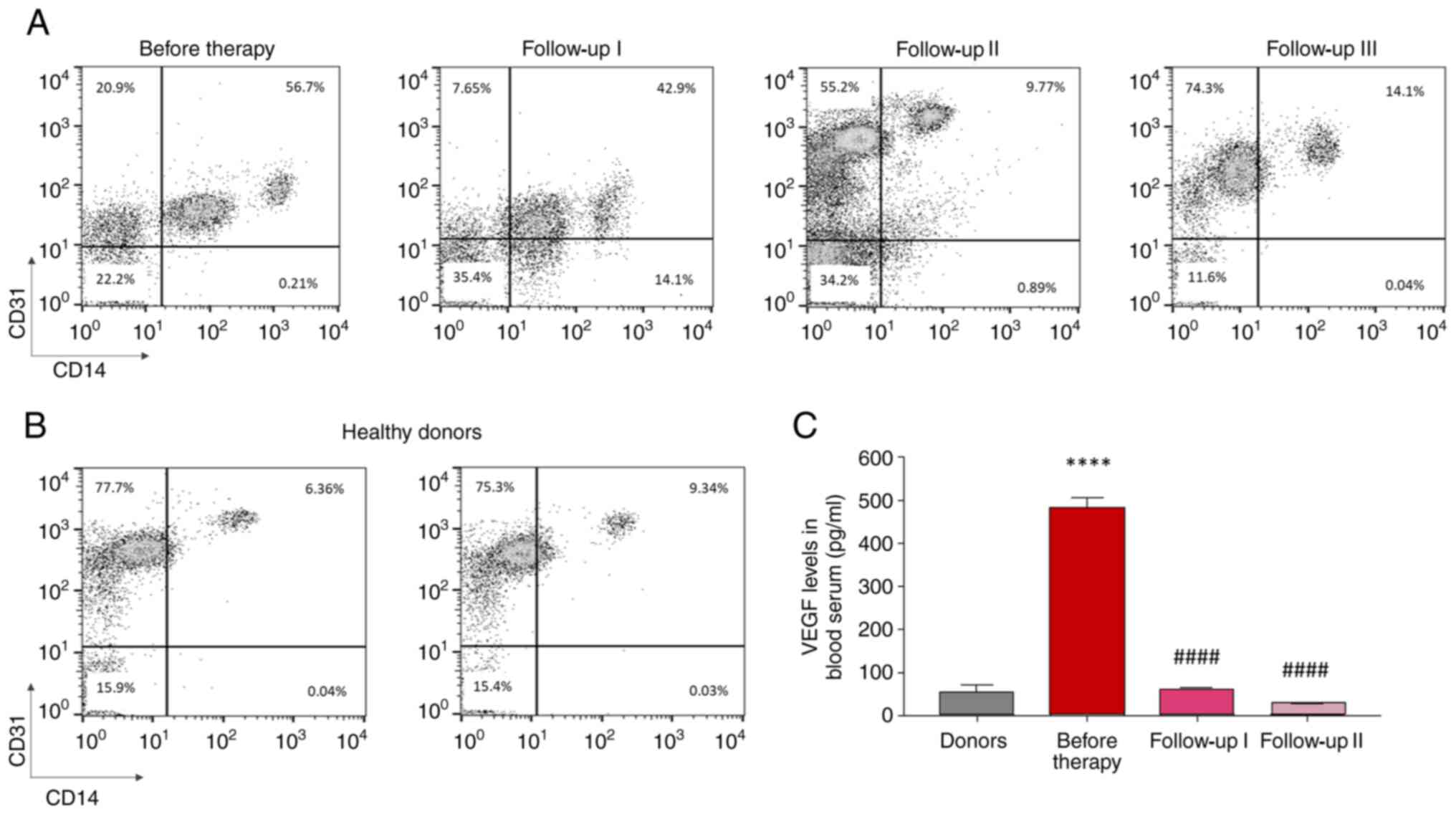
In the PB of the child patient with CCM prior to
propranolol administration, the percentage of double-positive
CD14+/CD31+ cells was higher than that in PB
from healthy blood donors (Fig. 2A
and B). Of note, during the
follow-up period with propranolol administration, a decrease in
CD14+/CD31+ levels in PB was observed, with
the levels being similar to those of the normal controls (Fig. 2A and B).
The concentration of VEGF in PB serum and in the
culture medium conditioned by monocytes, isolated as previously
described (27), was evaluated
using ELISA. PB serum from healthy blood donors was used as normal
controls and for cell supernatants, cells under control conditions
were maintained in H2O2 and propranolol-free
media.
The levels of VEGF in PB were higher prior to
treatment with propranolol and decreased towards normal levels
during follow-up (Fig. 2C). As
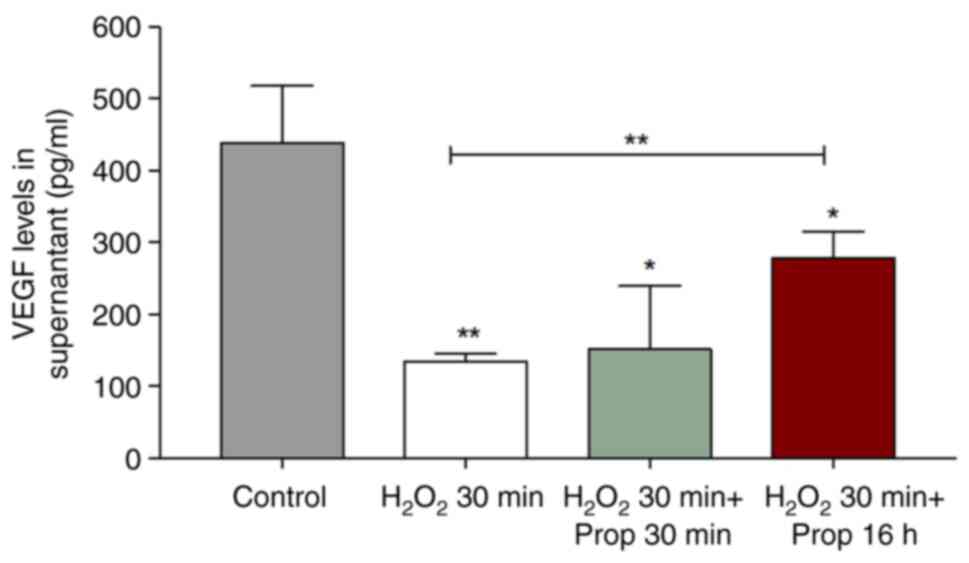
regards monocytes exposed to H2O2, it was
found that ROS decreased the VEGF levels in the culture media;
however, a long exposure time to propranolol reverted this tendency
(Fig. 3). Possibly, upon ROS
generation, monocytes undergo an EC differentiation route and
during this process, they lose the capacity of producing VEGF.
Thereafter, the exposure of monocytes to propranolol increased VEGF
production and accumulation in the culture medium.
The effects of propranolol on EC properties, such as
proliferation (percentage Ki67+ nuclei/total nuclei) and
migration (wound healing assay) were evaluated in vitro
using HUVECs. Propranolol (100 µM) impaired EC angiogenic
properties through a decrease in EC proliferation (Fig. 4A) and migration (Fig. 4B). The ex vivo effects of
propranolol on EC activation and further vessel-like structures
formation were evaluated using the rat aortic ring sprouting assay,
in which it was proven that propranolol completely abrogated EC
sprouting (Fig. 4C and D).
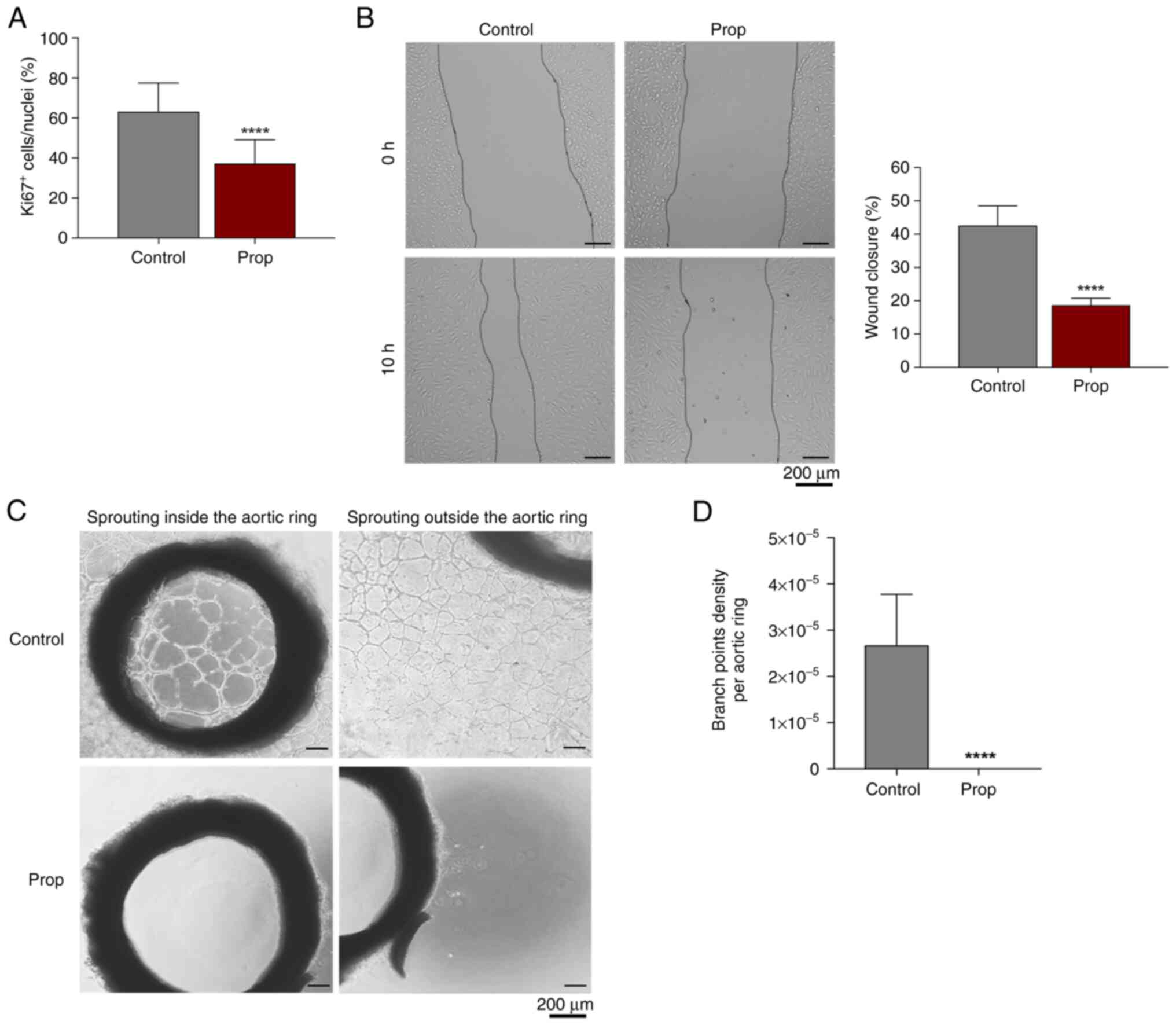 | Figure 4Propranolol decreases endothelial
cell proliferation and migration, and impairs the capacity to form
vessel-like structures. (A) Proliferation analysis based on the
percentage of Ki67+ nuclei/total nuclei of HUVECs
cultured with and without Prop (100 µM), for 16 h. (B) Migration
rate of HUVECs, previously exposed to mitomycin-C (3 h, 5 µg/ml) to
inhibit cell proliferation, in the absence and in presence of Prop,
at time 0 h and after 10 h (C) Representative images at day 13 of
aortic ring sprouting assay and the quantification of branch points
density (D), in the presence or absence of propranolol. Assays with
HUVECs were performed as previously described by Lopes-Coelho et
al (43). Aortic rings
sprouting assay was developed with aortas (thoracic and abdominal
segments) dissected from male Wistar rats (10 weeks old) and
cleaned to remove external tissue. After removing all extraneous
fat, fibrotic tissue and vasa vasorum structures, the aorta was
segmented into rings with a length of approximately 1 mm. For
fluorescence and bright filed microscopy, representative images
were acquired using an Olympus IX53 inverted microscope. The
quantification of Ki67+ nuclei, wound healing area and
aortic rings branch points (intersections between ECs) was
performed using ImageJ software. All data were analyzed using the
Student's t-test with GraphPad Prism v7 software. The assays were
performed with at least three biological replicates per condition.
****P<0.0001, statistically significant difference
vs. control. Prop, propranolol; HUVECs, human umbilical vein
endothelial cells. |
Discussion
Propranolol is a non-selective
β-adrenergic blocker commonly used in the control of
anxiety and cardiovascular conditions, such as hypertension,
myocardial infarction and angina pectoris. Over the past decade,
propranolol was re-discovered as an effective drug in the treatment
of certain vascular tumors, inducing the rapid involution to
quiescent residual lesions in 80% of cases (12,47-50).
Its use in the treatment of infantile hemangiomas, the most common
benign tumor of the skin, has been discovered accidentally and it
was verified that propranolol administration is highly efficient in
inducing tumor regression with very few adverse effects (40). Thus far, the beneficial effects of
propranolol have been observed in the treatment of neonatal
hemangiomatosis (51,52), placental chorioangioma (53) and CCM (13).
In the present study, the administration of
propranolol decreased the percentage of double-positive
CD14+/CD31+ cells (monocytes) in the PB of
the patient with CCM, reaching the levels presented by healthy
blood donors during the follow-up period (Fig. 2A and B). The observed normalization of the
CD14+/CD31+ cell levels upon propranolol
administration suggested that the levels of circulating cells,
sharing monocytic and EC features, are involved in CCM pathogenesis
and are propranolol-sensitive. Moreover, it was hypothesized that
circulating monocytes sharing EC features
(CD14+/CD31+) function as EPCs, as was
recently described (27),
contributing to CCM progression by being incorporated into CCM
neovessels.
The exact mechanisms through which propranolol
interferes with angiogenesis are not yet known; however, some
studies have indicated that its anti-angiogenic effects are
mediated by the downregulation of VEGF and FGF levels (12,40-42).
The dynamics of VEGF were also addressed in the present study, in
an attempt to clarify whether the VEGF levels are linked to CCM
regression. In the patient described herein, a decrease in the
levels of VEGF in the PB was observed upon propranolol treatment
(Fig. 2C); the levels were similar
to the values observed in healthy donors (Fig. 2C). Notably, in vitro,
monocytes appear to use more VEGF upon H2O2
exposure, decreasing its free levels in conditioned culture medium;
however, a longer exposure to propranolol, rescued the observed
decrease in VEGF levels due to H2O2 exposure
(Fig. 3). This observation
suggests that propranolol, apart from affecting the levels of
circulating VEGF, can also affect the way monocytes use VEGF in
vitro, thus decreasing the overall pro-angiogenic capacity.
According to the decreased stimulation of monocyte differentiation
into ECs, it was also observed that propranolol affected the
proliferation (Fig. 4A) and
migration (Fig. 4B) of mature ECs.
These observations are in agreement with recently published data by
the authors demonstrating that propranolol also impairs vessel-like
structure formation by ECs (43).
Accordingly, the exposure to propranolol disrupted vessel-like
sprouting in aortic rings (Fig. 4C
and D). Since the VEGF levels may
also be involved in monocyte recruitment (54-56),
the decreased levels of VEGF, upon propranolol treatment, may be
responsible, at least in part, by the decrease in the levels of
circulating CD14+/CD31+ cells and CCM
regression. However, further studies are required to elucidate the
mechanisms through which propranolol affects VEGF dynamics in
monocytes and ECs.
In zebrafish, the lack of CCM1, 2 or 3 constitutes
them a reliable CCM animal model, since it results in abnormal EC
sprouting and thin-walled vessels (46,57).
Therefore, through a murine and an embryonic zebrafish model, Li
et al (58) demonstrated
that propranolol ameliorated cavernous malformation, possibly
through the inhibition of β1-adrenergic receptor, once
the silencing of this receptor prevented vascular abnormalities.
Additionally, several research groups have already demonstrated
that VEGF levels are regulated by the catecholamines' pathway,
since its levels are proportional to β-adrenergic
receptors expression and can be inhibited by
β-adrenergic receptor antagonists (59-61).
For instance, melanoma cell lines exposed to norepinephrine, an
adrenergic receptor agonist, have been shown to exhibit increased
VEGF levels (62). However, other
studies have yielded contradictory results, demonstrating that the
anti-angiogenic effect of propranolol is independent of its
β-blocker action. Sasaki et al (63) demonstrated that both β
blockade by active S(-)- and inactive R(+)-propranolol enantiomers
were able to downregulate the expression of angiopoietin like 4, an
angiogenesis regulator, leading to the impairment of hemangioma
growth in vitro. In fact, besides its effects on
differentiated cells, Seebauer et al (64) demonstrated that the treatment of a
murine xenograft model with the R(+) enantiomer inhibited the
differentiation of hemangioma stem cells to ECs and further vessel
formation. Moreover, recently, the authors demonstrated that
propranolol exerted an anti-angiogenic effect through an
antioxidant mechanism accounting for the inhibition of a
ferroptosis-like mechanism, which in turn, impaired EC activation
and the formation of vessel-like structures (43). Therefore, propranolol may present
diverse mechanisms of action to impair vascular growth.
In summary, propranolol, apart from promoting the
regression of CCM, impairs CD14+/CD31+ cell
circulation (Fig. 2A), in part by
the decreased VEGF levels (Fig.
2C). It was also observed in vitro, that stimulation
with a prooxidant (H2O2) tended to promote
the differentiation of monocytes in cell culture medium towards ECs
(27), with decreased levels of
VEGF. The underlying mechanism may involve the control of monocyte
differentiation into ECs and how these cells are phenotypically
altered. As recently demonstrated by the authors, oxidative stress
promotes monocyte differentiation into ECs, and this process is
reversed by propranolol, which appears to attenuate oxidative
stress (43). Furthermore, VEGF is
essential during the differentiation of monocytes into ECs
(27); however, when monocytes
differentiate into macrophage-like cells, they become
VEGF-producing cells (56,65). This switch from macrophages to ECs
explains the dynamics of VEGF in cell culture media. Considering
that monocytes functioning as EPCs may favor the development of CCM
lesions and given that VEGF is pivotal for monocytic
differentiation into ECs, the increased circulating levels of VEGF
observed in the patient with CCM without treatment may be crucial
for potentiating the EC differentiation route and further, for
preventing CCM pathogenesis.
Although the propranolol mechanisms of action in CCM
are not yet fully understood, the lack of a better therapeutic
option for patients with surgically inaccessible CCM and the
notable responses in a few patients suggest that it may be of value
to explore the exact efficacy of propranolol in the treatment of
CCM, as well as the associated adverse side-effects (12). In accordance with this, randomized
prospective clinical trials with propranolol vs. placebo/nothing
groups [phase 1 trial NCT03523650, phase 2 trials NCT03474614 and
NCT03589014(66)] are currently
ongoing. The findings of the present study reinforce the use of
propranolol in the clinical management of CCM and points out the
monitorization levels of monocytes
(CD14+/CD31+) and VEGF in PB as useful tools
which may be used to predict treatment efficacy.
Acknowledgements
The authors would like to thank the Serviço de
Imuno-Hemoterapia from Instituto Português de Oncologia de Lisboa
Francisco Gentil (IPOLFG EPE) for providing the buffy coats from
healthy donors.
Funding
Funding: The present study was funded by IPOLFG EPE and by
iNOVA4Health (UID/Multi/04462/2019) a program financially supported
by Fundação para a Ciência e Tecnologia (FCT)/Ministério da
Educação e Ciência, through national funds. The PhD fellowship of
FLC was funded by FCT (PD/BD/128337/2017).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (FLC, SN, FM, AH, SGF, GD, BFM, JFS,
SVC, SAP, SV, DS and JS) participated in the conception and design
of the study, and read and discussed the submitted and the accepted
for publication versions of the manuscript. In addition, the
authors contributed clinically and technically in the different
stages of the study. FLC performed the analysis of the patient
samples analysis, the analysis of the in vitro and ex
vivo experiments, and prepared the first draft of the
manuscript. FM, AH, SGF and GD performed the in vitro
experiments. BFM and JFS performed the ex vivo experiments.
SN, SV and DS were responsible for the clinical management of the
patient. SAP and SVC coordinated the in vitro and ex
vivo experiments. JS coordinated the whole project and was
responsible for funding acquisition. FLC and JS confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The PB of the child patient with cerebral cavernous
malformation was collected after obtaining informed consent from
the parents at the Neuropediatrics Department at Portuguese
Institute of Oncology of Lisboa, Francisco Gentil (IPOLFG; ethics
approval was obtained from the IPOLFG Ethics Committee; UIC-1137).
Monocytes were isolated from PB collected after obtaining consent
from healthy donors at Immuno-Hemotherapy Department at Portuguese
Institute of Oncology of Lisboa, Francisco Gentil (IPOLFG). Ethics
approval was obtained from the IPOLFG Ethics Committee; UIC-1137).
The use of animals was approved by the Ethics Committee at NOVA
Medical School (Ref. 75/2019/CEFCM).
Patient consent for publication
The consent from parents of the patient with
cerebral cavernous malformation was obtained stating that they
agreed for the data of their child to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Akers A, Al-Shahi Salman R, A Awad I,
Dahlem K, Flemming K, Hart B, Kim H, Jusue-Torres I, Kondziolka D,
Lee C, et al: Synopsis of guidelines for the clinical management of
cerebral cavernous malformations: Consensus recommendations based
on systematic literature review by the angioma alliance scientific
advisory board clinical experts panel. Neurosurg. 80:665–680.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Flemming KD, Graff-Radford J, Aakre J,
Kantarci K, Lanzino G, Brown RD Jr, Mielke MM, Roberts RO, Kremers
W, Knopman DS, et al: Population-based prevalence of cerebral
cavernous malformations in older adults: Mayo clinic study of
aging. JAMA Neurol. 74:801–805. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Goldstein HE and Solomon RA: Epidemiology
of cavernous malformations. Handb. Clin Neurol. 143:241–247.
2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gross BA, Lin N, Du R and Day AL: The
natural history of intracranial cavernous malformations. Neurosurg
Focus. 30(E24)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Batra S, Lin D, Recinos PF, Zhang J and
Rigamonti D: Cavernous malformations: Natural history, diagnosis
and treatment. Nat Rev Neurol. 5:659–670. 2009.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cox EM, Bambakidis NC and Cohen ML:
Pathology of cavernous malformations. Handb Clin Neurol.
143:267–277. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Louis N and Marsh R: Simultaneous and
sequential hemorrhage of multiple cerebral cavernous malformations:
A case report. J Med Case Rep. 10(36)2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Leblanc GG, Golanov E, Awad IA and Young
WL: Biology of Vascular Malformations of the Brain NINDS Workshop
Collaborators. Biology of vascular malformations of the brain.
Stroke. 40:e694–e702. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wüstehube J, Bartol A, Liebler SS, Brütsch
R, Zhu Y, Felbor U, Sure U, Augustin HG and Fischer A: Cerebral
cavernous malformation protein CCM1 inhibits sprouting angiogenesis
by activating DELTA-NOTCH signaling. Proc Natl Acad Sci USA.
107:12640–12645. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Gibson CC, Zhu W, Davis CT, Bowman-Kirigin
JA, Chan AC, Ling J, Walker AE, Goitre L, Delle Monache S, Retta
SF, et al: Strategy for identifying repurposed drugs for the
treatment of cerebral cavernous malformation. Circulation.
131:289–299. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shenkar R, Shi C, Austin C, Moore T,
Lightle R, Cao Y, Zhang L, Wu M, Zeineddine HA, Girard R, et al:
RhoA kinase inhibition with fasudil versus simvastatin in murine
models of cerebral cavernous malformations. Stroke. 48:187–194.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Apra C, Dumot C, Bourdillon P and
Pelissou-Guyotat I: Could propranolol be beneficial in adult
cerebral cavernous malformations? Neurosurg Rev. 42:403–408.
2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moschovi M, Alexiou GA, Stefanaki K,
Tourkantoni N and Prodromou N: Propranolol treatment for a giant
infantile brain cavernoma. J Child Neurol. 25:653–655.
2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dotan M and Lorber A: Congestive heart
failure with diffuse neonatal hemangiomatosis-case report and
literature review. Acta Paediatr. 102:e232–e238. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Berti I, Marchetti F, Skabar A, Zennaro F,
Zanon D and Ventura A: Propranolol for cerebral cavernous
angiomatosis: A magic bullet. Clin Pediatr (Phila). 53:189–190.
2014.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Miquel J, Bruneau B and Dupuy A:
Successful treatment of multifocal intracerebral and spinal
hemangiomas with propranolol. J Am Acad Dermatol. 70:e83–e84.
2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cavalheiro S, Campos HG and Silva da Costa
MD: A case of giant fetal intracranial capillary hemangioma cured
with propranolol. J Neurosurg Pediatr. 17:711–716. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Reinhard M, Schuchardt F, Meckel S, Heinz
J, Felbor U, Sure U and Geisen U: Propranolol stops progressive
multiple cerebral cavernoma in an adult patient. J Neurol Sci.
367:15–17. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ritter MR, Reinisch J, Friedlander SF and
Friedlander M: Myeloid cells in infantile hemangioma. Am J Pathol.
168:621–628. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Navarrete MG, Hernández AD, Collado-Ortiz
MA, Salinas-Lara C and Tena-Suck ML: Brain vascular lesions: A
clinicopathologic, immunohistochemistry, and ultrastructural
approach. Ann Diagn Pathol. 18:193–198. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Seidmann L, Suhan T, Unger R, Gerein V and
Kirkpatrick CJ: Transient CD15-positive endothelial phenotype in
the human placenta correlates with physiological and pathological
fetoplacental immaturity. Eur J Obstet Gynecol Reprod Biol.
180:172–179. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yue W, Pi QM, Zhang WJ, Zhou GD, Cui L,
Liu W and Cao Y: Platelet endothelial cell adhesion molecule-1,
stage-specific embryonic antigen-1, and Flk-1 mark distinct
populations of mouse embryonic stem cells during differentiation
toward hematopoietic/endothelial cells. Stem Cells Dev.
19:1937–1948. 2010.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Reed RL, Cheney CB, Fearon RE, Hook R and
Hehre FW: Propranolol therapy throughout pregnancy: A case report.
Anesth Analg. 53(214)1974.PubMed/NCBI
|
|
24
|
Cottrill CM, McAllister RG Jr, Gettes L
and Noonan JA: Propranolol therapy during pregnancy, labor, and
delivery: Evidence for transplacental drug transfer and impaired
neonatal drug disposition. J Pediatr. 91:812–814. 1977.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schoenfeld N, Epstein O, Nemesh L, Rosen M
and Atsmon A: Effects of propranolol during pregnancy and
development of rats. I. Adverse effects during pregnancy. Pediatr
Res. 12:747–750. 1978.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Domingues G, Gouveia-Fernandes S, Salgado
D, et al: Monocytes/macrophages in cancer, from tumor aggressors to
vascular components-a new insight for anti-angiogenic therapy. In:
EACR-AACR-SIC special conference on anticancer drug action and drug
resistance from cancer biology to the clinic, pp98-99, 2015.
|
|
27
|
Lopes-Coelho F, Silva F, Gouveia-Fernandes
S, Martins C, Lopes N, Domingues G, Brito C, Almeida AM, Pereira SA
and Serpa J: Monocytes as endothelial progenitor cells (EPCs),
another brick in the wall to disentangle tumor angiogenesis. Cells.
9(107)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Curry CV: Differential blood count:
Reference range, interpretation, collection and panels. Medscape,
2015.
|
|
29
|
Coffelt SB, Tal AO, Scholz A, De Palma M,
Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y and
Lewis CE: Angiopoietin-2 regulates gene expression in
TIE2-expressing monocytes and augments their inherent proangiogenic
functions. Cancer Res. 70:5270–5280. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Richardson MR and Yoder MC: Endothelial
progenitor cells: Quo vadis? J Mol Cell Cardiol. 50:266–272.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yoder MC: Human endothelial progenitor
cells. Cold Spring Harb Perspect Med. 2(a006692)2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kaur S, Sehgal R, Shastry SM, McCaughan G,
McGuire HM, Fazekas St de Groth B, Sarin S, Trehanpati N and Seth
D: Circulating endothelial progenitor cells present an inflammatory
phenotype and function in patients with alcoholic liver cirrhosis.
Front Physiol. 9(556)2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakayama F, Nishihara S, Iwasaki H, Kudo
T, Okubo R, Kaneko M, Nakamura M, Karube M, Sasaki K and Narimatsu
H: CD15 expression in mature granulocytes is determined by alpha
1,3-fucosyltransferase IX, but in promyelocytes and monocytes by
alpha 1,3-fucosyltransferase IV. J Biol Chem. 276:16100–16106.
2001.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Martin AW: Chapter 6-immunohistology of
non-hodgkin lymphoma. In: Dabbs DJ (ed), Diagnostic
Immunohistochemistry. 3rd edition. Philadelphia: W.B. Saunders,
pp156-188, 2011.
|
|
35
|
Chung JW, Park CJ, Cha CH, Cho YU, Jang S,
Chi HS, Seo EJ, Lee JH, Lee JH, Lee KH, et al: A combination of
CD15/CD10, CD64/CD33, CD16/CD13 or CD11b flow cytometric
granulocyte panels is sensitive and specific for diagnosis of
myelodysplastic syndrome. Ann Clin Lab Sci. 42:271–280.
2012.PubMed/NCBI
|
|
36
|
Elliott LA, Doherty GA, Sheahan K and Ryan
EJ: Human tumor-infiltrating myeloid cells: Phenotypic and
functional diversity. Front Immunol. 8(86)2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Frieden IJ, Haggstrom AN, Drolet BA,
Mancini AJ, Friedlander SF, Boon L, Chamlin SL, Baselga E, Garzon
MC, Nopper AJ, et al: Infantile hemangiomas: Current knowledge,
future directions. Proceedings of a research workshop on infantile
hemangiomas, April 7-9, 2005, Bethesda, Maryland, USA. Pediatr
Dermatol. 22:383–406. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kim J: Introduction to cerebral cavernous
malformation: A brief review. BMB Rep. 49:255–262. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ganmore I and Achiron A: Cerebral
cavernous malformations. N Engl J Med. 377(71)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Léauté-Labrèze C, Dumas de la Roque E,
Hubiche T, Boralevi F, Thambo JB and Taïeb A: Propranolol for
severe hemangiomas of infancy. N Engl J Med. 358:2649–2651.
2008.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Annabi B, Lachambre MP, Plouffe K,
Moumdjian R and Béliveau R: Propranolol adrenergic blockade
inhibits human brain endothelial cells tubulogenesis and matrix
metalloproteinase-9 secretion. Pharmacol Res. 60:438–445.
2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lamy S, Lachambre MP, Lord-Dufour S and
Béliveau R: Propranolol suppresses angiogenesis in vitro:
Inhibition of proliferation, migration, and differentiation of
endothelial cells. Vascul Pharmacol. 53:200–208. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lopes-Coelho F, Martins F, Hipólito A,
Mendes C, Sequeira CO, Pires RF, Almeida AM, Bonifácio VDB, Pereira
SA and Serpa J: The activation of endothelial cells relies on a
ferroptosis-like mechanism: Novel perspectives in management of
angiogenesis and cancer therapy. Front Oncol.
11(656229)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sacramento JF, Olea E, Ribeiro MJ,
Prieto-Lloret J, Melo BF, Gonzalez C, Martins FO, Monteiro EC and
Conde SV: Contribution of adenosine and ATP to the carotid body
chemosensory activity in ageing. J Physiol. 597:4991–5008.
2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Bergametti F, Denier C, Labauge P, Arnoult
M, Boetto S, Clanet M, Coubes P, Echenne B, Ibrahim R, Irthum B, et
al: Mutations within the programmed cell death 10 gene cause
cerebral cavernous malformations. Am J Hum Genet. 76:42–51.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
46
|
Uebelhoer M, Boon LM and Vikkula M:
Vascular anomalies: From genetics toward models for therapeutic
trials. Cold Spring Harb. Perspect Med. 2(a009688)2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zabramski JM, Kalani MYS, Filippidis AS
and Spetzler RF: Propranolol treatment of cavernous malformations
with symptomatic hemorrhage. World Neurosurg. 88:631–639.
2016.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Storch CH and Hoeger PH: Propranolol for
infantile haemangiomas: Insights into the molecular mechanisms of
action. Br J Dermatol. 163:269–274. 2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Al-Majed AA, Bakheit AHH, Abdel Aziz HA,
Alajmi FM and AlRabiah H: Propranolol. Profiles Drug Subst Excip
Relat Methodol. 42:287–338. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rotter A and de Oliveira ZNP: Infantile
hemangioma: Pathogenesis and mechanisms of action of propranolol. J
Dtsch Dermatol Ges. 15:1185–1190. 2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhou H, Li QF, Cao GL, Ling HZ, Dai HR and
Chen XH: Successful treatment of diffuse neonatal hemangiomatosis
with propranolol: a case report. J Dtsch Dermatol Ges. 12:625–628.
2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mazereeuw-Hautier J, Hoeger PH, Benlahrech
S, Ammour A, Broue P, Vial J, Ohanessian G, Léauté-Labrèze C,
Labenne M, Vabres P, et al: Efficacy of propranolol in hepatic
infantile hemangiomas with diffuse neonatal hemangiomatosis. J
Pediatr. 157:340–342. 2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Padys P, Fouque L, Le Duff M, D'Hervé D
and Poulain P: Propranolol during pregnancy for large
chorioangioma. Med Hypotheses. 85:513–514. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Lee HW, Choi HJ, Ha SJ, Lee KT and Kwon
YG: Recruitment of monocytes/macrophages in different tumor
microenvironments. Biochim Biophys Acta. 1835:170–179.
2013.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wheeler KC, Jena MK, Pradhan BS, Nayak N,
Das S, Hsu CD, Wheeler DS, Chen K and Nayak NR: VEGF may contribute
to macrophage recruitment and M2 polarization in the decidua. PLoS
One. 13(e0191040)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Jaipersad AS, Lip GY, Silverman S and
Shantsila E: The role of monocytes in angiogenesis and
atherosclerosis. J Am Coll Cardiol. 63:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Zhu Y, Wu Q, Xu JF, Miller D, Sandalcioglu
IE, Zhang JM and Sure U: Differential angiogenesis function of CCM2
and CCM3 in cerebral cavernous malformations. Neurosurg Focus.
29(E1)2010.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Li W, Shenkar R, Detter MR, Moore T,
Benavides C, Lightle R, Girard R, Hobson N, Cao Y, Li Y, et al:
Propranolol inhibits cavernous vascular malformations by β1
adrenergic receptor antagonism in animal models. J Clin Invest.
131(e144893)2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Ciccarelli M, Sorriento D, Cipolletta E,
Santulli G, Fusco A, Zhou RH, Eckhart AD, Peppel K, Koch WJ,
Trimarco B and Iaccarino G: Impaired neoangiogenesis in
β2-adrenoceptor gene-deficient mice: Restoration by intravascular
human β2-adrenoceptor gene transfer and role of NFκB and CREB
transcription factors. Br J Pharmacol. 162:712–721. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ji Y, Chen S, Li K, Xiao X, Zheng S and Xu
T: The role of β-adrenergic receptor signaling in the proliferation
of hemangioma-derived endothelial cells. Cell Div.
8(1)2013.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Madden KS, Szpunar MJ and Brown EB:
β-Adrenergic receptors (β-AR) regulate VEGF and IL-6 production by
divergent pathways in high β-AR-expressing breast cancer cell
lines. Breast Cancer Res Treat. 130:747–758. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Yang EV, Kim SJ, Donovan EL, Chen M, Gross
AC, Webster Marketon JI, Barsky SH and Glaser R: Norepinephrine
upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor
cell lines: Implications for stress-related enhancement of tumor
progression. Brain Behav Immun. 23:267–275. 2009.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Sasaki M, North PE, Elsey J, Bubley J, Rao
S, Jung Y, Wu S, Zou MH, Pollack BP, Kumar J, et al: Propranolol
exhibits activity against hemangiomas independent of beta blockade.
NPJ Precis Oncol. 3(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Seebauer CT, Graus MS, Huang L, McCann A,
Wylie-Sears J, Fontaine F, Karnezis T, Zurakowski D, Staffa SJ,
Meunier F, et al: Non-beta blocker enantiomers of propranolol and
atenolol inhibit vasculogenesis in infantile hemangioma. J Clin
Invest. 132(e151109)2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ruan Q, Zhao C, Ye Z, Ruan J, Xie Q and
Xie W: Effect and possible mechanism of monocyte-derived VEGF on
monocyte-endothelial cellular adhesion after electrical burns.
Burns. 41:825–832. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Lanfranconi S, Scola E, Bertani GA, Zarino
B, Pallini R, d'Alessandris G, Mazzon E, Marino S, Carriero MR,
Scelzo E, et al: Propranolol for familial cerebral cavernous
malformation (Treat_CCM): Study protocol for a randomized
controlled pilot trial. Trials. 21(401)2020.PubMed/NCBI View Article : Google Scholar
|