Introduction
Almost two years have passed since the World Health
Organization (WHO) declared the severe acute respiratory syndrome
coronavirus 2 (SARS-CoV-2) outbreak a global pandemic (1). The infection rate became markedly
stable and some authors, considering the interactions between
coronavirus disease 2019 (COVID-19) and other non-communicable
diseases, refer to this situation as a ‘syndemic’ (2,3).
Up to January 31, 2022, >380 million cases of
SARS-CoV-2 infection were reported and over five million
individuals succumbed to the disease worldwide. To date, a total of
11 million cases have been reported in Italy, 147,000 of which have
not survived (4). It is now clear
that an uncontrolled systemic inflammation represents a critical
element in the progression of COVID-19 to acute respiratory
distress syndrome (ARDS) (5). This
non-specific and deleterious inflammatory response appears to lead
to alveolar damage due to inflammatory cell infiltration, pulmonary
edema and endothelial impairment along with microvascular
thrombosis, playing a key role in the development of severe
COVID-19 (6,7).
Of note, dexamethasone treatment has been shown to
be associated with improved outcomes in patients with severe
COVID-19(8). The IL-6 cascade has
already been proposed as a potential target for immunomodulatory
therapy to moderate systemic hyper-inflammation during SARS-CoV-2
infection (9,10).
Scientific literature and international guidelines
(11,12) suggest the use of tocilizumab, a
recombinant monoclonal antibody, in addition to standard of care
treatment, due to its ability to lower the risk of respiratory
deterioration, thus reducing the mortality rate. In the case that
tocilizumab is not available, sarilumab, a human monoclonal
antibody targeting IL-6 soluble receptors, which is already
approved for the treatment of rheumatoid arthritis, represents a
valid alternative for IL-6 blockade (13). Considering that IL-6 has multiple
pathways with varying effects, both positive and negative on
inflammation and homeostatic control, it is arduous for clinicians
to determine the correct therapeutic window for the administration
of anti-IL-6 drugs (13).
The present study describes cases of 4 patients with
severe pulmonary forms of COVID-19, requiring high-flow nasal
oxygenation along with dexamethasone administration, who were
successfully discharged following sarilumab administration.
Case report
Case 1
Upon admission to the ARNAS Garibaldi Hospital,
Catania, Italy, the patient was feverish (temperature, 38.5˚C),
blood pressure (BP) was 125/60 mmHg, heart rate (HR) was 62 bpm,
and oxygen saturation was 91% in room air. He was administered
O2 therapy with a Venturi mask (VM) at 12 l/min
(fraction of inspired oxygen (FiO2, 60%). Blood tests
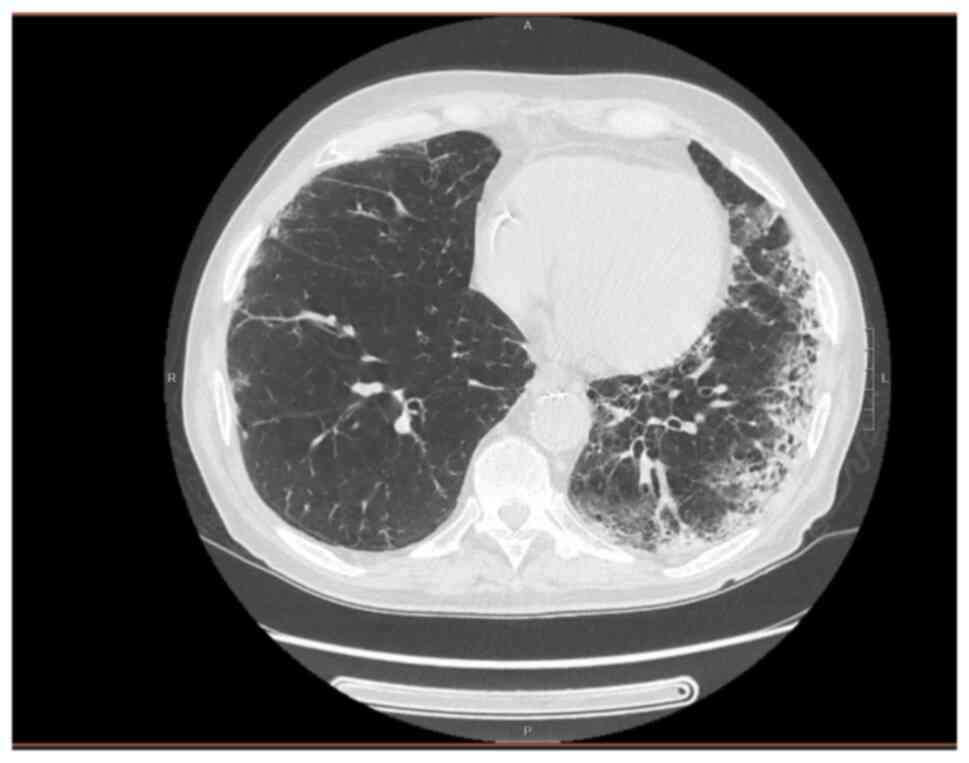
revealed elevated inflammatory marker levels (Table I) and a thoracic CT scan revealed
bilateral interstitial pneumonia (Fig.
1). Treatment with prophylactic enoxaparin, dexamethasone and
piperacillin/tazobactam was commenced.
 | Table IDemographics, clinical
characteristics at the time of admission, treatment and outcomes of
the 4 patients with COVID-19. |
Table I
Demographics, clinical
characteristics at the time of admission, treatment and outcomes of
the 4 patients with COVID-19.
|
Characteristics | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
|---|
| Age, years | 75 | 73 | 84 | 73 |
| Sex | Male | Male | Female | Female |
| SARS-CoV-2 vaccine
(no. of doses) | No | Yes (2 doses of
BNT162b2) | No | No |
| Comorbidities | Hypertension,
coronary disease with previous percutaneous revascularization. | Atrial
fibrillation, diabetes mellitus, COPD | Hypertension | Hypertension, GERD,
major depressive disorder |
| Home therapy | Clopidogrel,
bisoprolol, ramipril, amlodipine, cardioaspirin, omeprazole | Methimazole,
insulin, pantoprazole, amlodipine, propranolol, apixaban | None | Candesartan,
alprazolam, pantoprazole |
| Days between
admission and HFNC treatment initiation | 4 | 4 | 3 | 2 |
| Chest CT
findings | Bilateral
honeycombing pattern, fibrotic reticulation, and
consolidations | Bilateral
interstitial pneumonia along with consolidations and bilateral
pleural effusion | Bilateral
interstitial pneumonia along with vast consolidations | Bilateral
interstitial pneumonia, and consolidations within
bronchiectasis |
| Days between the
onset of symptoms and hospital admission | 15 | 7 | 12 | 7 |
| Laboratory finding;
unit (reference range) | | | | |
|
WBC,
cells/mmc (4,000-10,000) | 7,100 | 6,000 | 12,900 | 8,400 |
|
Neutrophils,
% (40-75) | 80.5 | 63.8 | 86 | 83.8 |
|
Lymphocytes,
% (25-50) | 10.7 | 26.2 | 9.9 | 10.6 |
|
Monocytes, %
(2-10) | 8.6 | 8.8 | 3.9 | 5.5 |
|
Platelets,
cells/mmc x103 (150-400) | 230 | 85 | 321 | 172 |
|
Hzemoglobin,
g/dl (12-16) | 13.3 | 13 | 13.6 | 13.9 |
|
AST, UI/l
(15-35) | 29 | 45 | 40 | 73 |
|
ALT, UI/l
(15-35) | 24 | 16 | 27 | 107 |
|
LDH, UI/l
(80-250) | 362 | 411 | 430 | 526 |
|
Creatinine,
mg/dl (0.8-1.2) | 1.28 | 0.84 | 0.6 | 0.57 |
|
CRP, mg/dl
(0-0.5) | 9.95 | 12.34 | 10.04 | 12.99 |
|
ESR, mm/h
(0-10) | 54 | 29 | 56 | 66 |
|
D-dimer,
ng/ml (<250) | 2,013 | 883 | 680 | 18,847 |
|
Ferritin,
ng/ml (20-200) | 814 | 522 | 796 | 1,118 |
|
Lowest
PaO2/FiO2 ratio | 103 | 98 | 94 | 93 |
| Antibiotic therapy
(duration) |
Piperacillin/tazobactam (12 days),
levofloxacin (7 days) |
Piperacillin/tazobactam (12 days) |
Piperacillin/tazobactam (10 days)
levofloxacin (7 days) |
Piperacillin/tazobactam (11 days) |
| Others therapies
(duration) | Enoxaparin 6,000 UI
s.c. (18 days), dexamethasone 6 mg i.v. (18 days) | Dexamethasone 6 mg
i.v. (22 days) | Enoxaparin 6,000 UI
s.c. (28 days), dexamethasone 6 mg i.v. (26 days) | Enoxaparin 6,000 UI
s.c. (18 days), dexamethasone 6 mg i.v. (16 days) |
| Days on HFNC | 8 | 9 | 11 | 9 |
| Sarilumab dose
(number of doses) | 400 mg i.v. (single
dose) | 400 mg i.v. (single
dose) | 400 mg i.v. (single
dose) | 400 mg i.v. (single
dose) |
| Days from admission
to sarilumab | 5 | 5 | 4 | 3 |
| Time to hospital
discharge (days) | 18 | 24 | 28 | 18 |
On the 4th day, due to the worsening of oxygen
saturation, an arterial blood analysis was performed, revealing
partial pressure of oxygen (PaO2) levels of 62.3 mmHg,
partial pressure of carbon dioxide (PCO2) levels of 30.2
mmHg, pH 7.39 and a PaO2/FiO2 ratio of
103.
Oxygen ventilation with a high flow nasal cannula
(HFNC; Optiflow™ Nasal High Flow Therapy delivered by AIRVO™ 2;
Fisher & Paykel Healthcare), 60 l/min, FiO2 60% was
administered, and on the same day, the patient was administered a
single dose of sarilumab at 400 mg intravenously. Furthermore,
levofloxacin 500 mg/day was empirically added to extend the
antibiotic coverage.
Within 24 h from sarilumab administration, his
clinical condition ameliorated, and arterial blood analysis
improved (PaO2/FiO2 ratio of 140). The levels
of serum inflammatory markers decreased [C-reactive protein (CRP)
levels, 0.44 mg/dl]. HFNC ventilation was continued for a further 7
days (8 days overall), and the patient was gradually weaned off of
O2 therapy following a further 5 days.
Case 2
Upon admission (to the same hospital as mentioned
above), the patient was afebrile, BP was 135/60 mmHg, HR was 110
bpm, and oxygen saturation was 96% with a nasal cannula (NC)
3l/min. Blood tests revealed high inflammatory marker levels
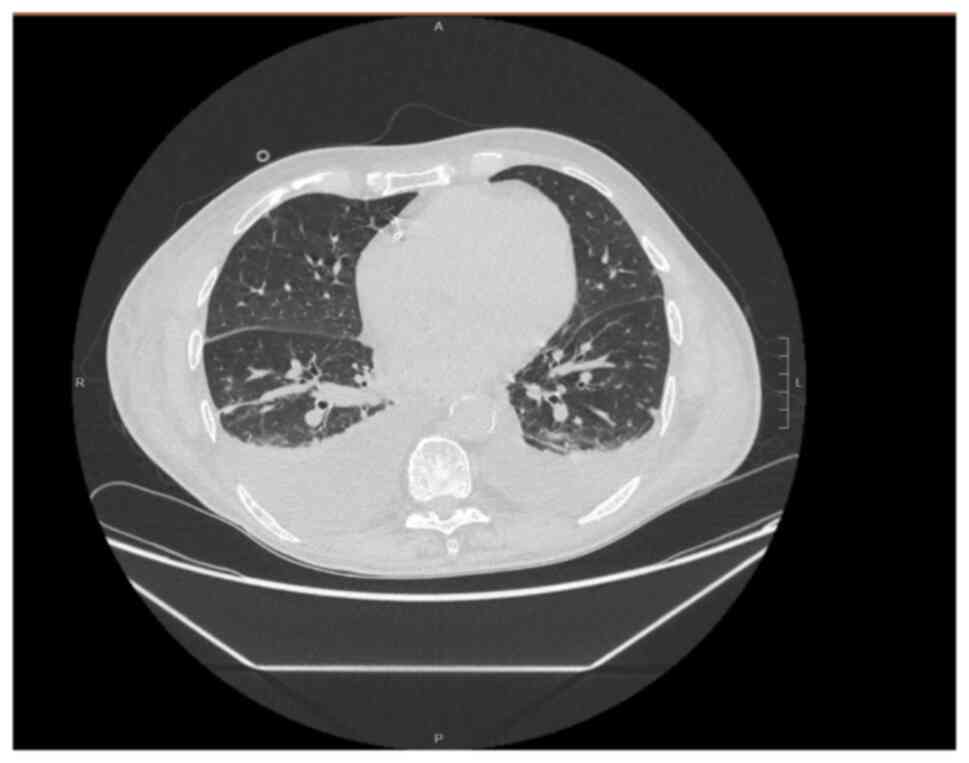
(Table I). A thoracic CT scan
revealed bilateral interstitial pneumonia (Fig. 2). Treatment with dexamethasone and
piperacillin/tazobactam was commenced.
Despite pharmacologic treatment and O2
supplementation, his respiratory functions continued to
deteriorate. Blood gas analysis with the VM at 12 l/min,
Fi02 60%, revealed PaO2 at 59 mmHg,
PCO2 at 39 mmHg, pH 7.47 and a
PaO2/FiO2 ratio of 98. HFNC ventilation (60
l/min, FiO2 60%) was commenced on the 4th day from
admission, and sarilumab at 400 mg intravenously was administered
within 24 h.
Within 48 h from the sarilumab administration, his
clinical condition, laboratory tests (CRP levels 1.32 mg/dl), and
blood gas analysis (PaO2/FiO2, 121) improved. HFNC
treatment was steadily reduced; this was switched to a VM at 9 days
following HFNC initiation, and the patient was also gradually
weaned off this as well.
Case 3
Upon admission (to the same hospital as mentioned
above), the patient was feverish (temperature, 39.5˚C), BP was
150/75 mmHg, HR was 100 bpm, the oxygen saturation rate was 95%
with a VM at 8 l/min and a FiO2 of 35%. Blood tests
revealed elevated inflammatory marker levels along with
neutrophilic leukocytosis (Table
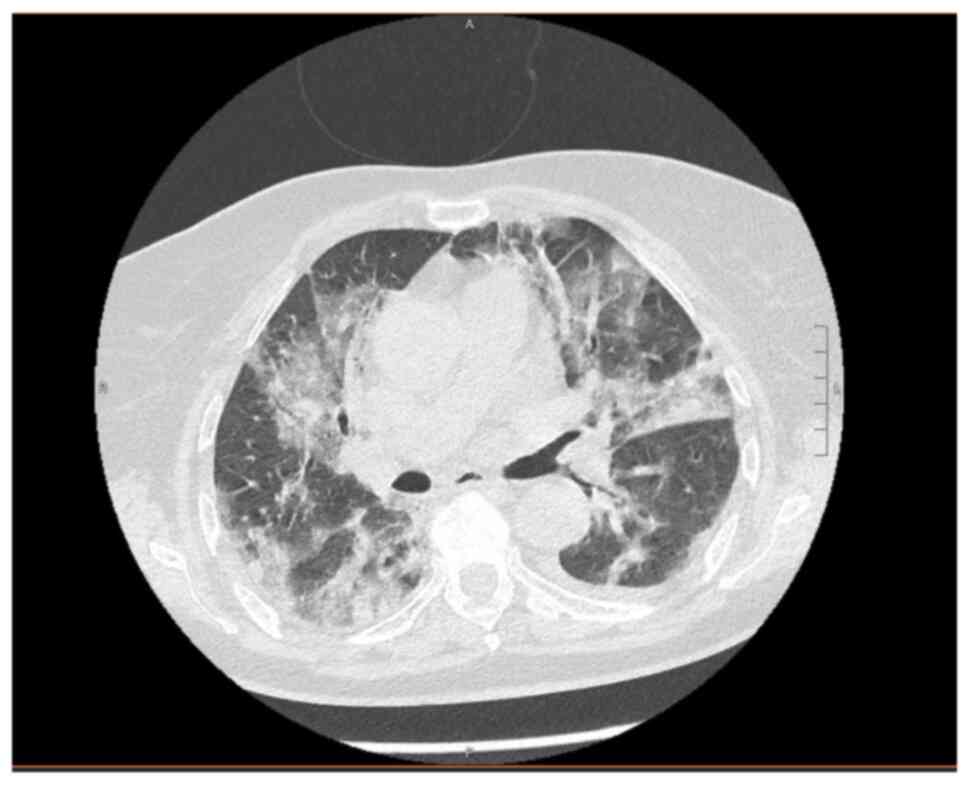
I). A chest CT scan revealed bilateral interstitial pneumonia
(Fig. 3). Enoxaparin 6,000 U/day,
dexamethasone 6 mg/day and piperacillin/tazobactam 4.5 g/three
times/day were administered.
On the 3rd day, arterial blood analysis revealed a
PaO2 of 49 mmHg, PCO2 of 28.5 mmHg, pH 7.45
and a PaO2/FiO2 ratio of 139. HFNC was
commenced at 60 l/min, and a FiO2 of 60% was arranged.
The following day, sarilumab was administered intravenously at a
single dose of 400 mg.
Within 48 h, arterial blood analysis revealed
progressive amelioration in the patient's condition
(PaO2/FiO2 was 122). Although serum
inflammatory marker levels began to decrease (CRP levels, 0.29
mg/dl), blood tests revealed leukopenia with neutropenia, reaching
a nadir 9 days following sarilumab infusion (2,000/mmc, 12.1%
neutrophils). Neutropenia resolved within 7 days without treatment.
HFNC ventilation was terminated within 11 days. Subsequently, a VM
was used for a further 5 days, and this was gradually reduced over
this period of time.
Case 4
Upon admission (to the same hospital as mentioned
above), the patient was feverish (temperature, 39.5˚C), BP was
145/55 mmHg, HR was 100 bpm, oxygen saturation was 97% with a VM at
6 l/min and FiO2 of 31%. Blood tests revealed high
inflammatory marker levels along with high D-dimer levels and
hypertransaminasemia (Table I). A
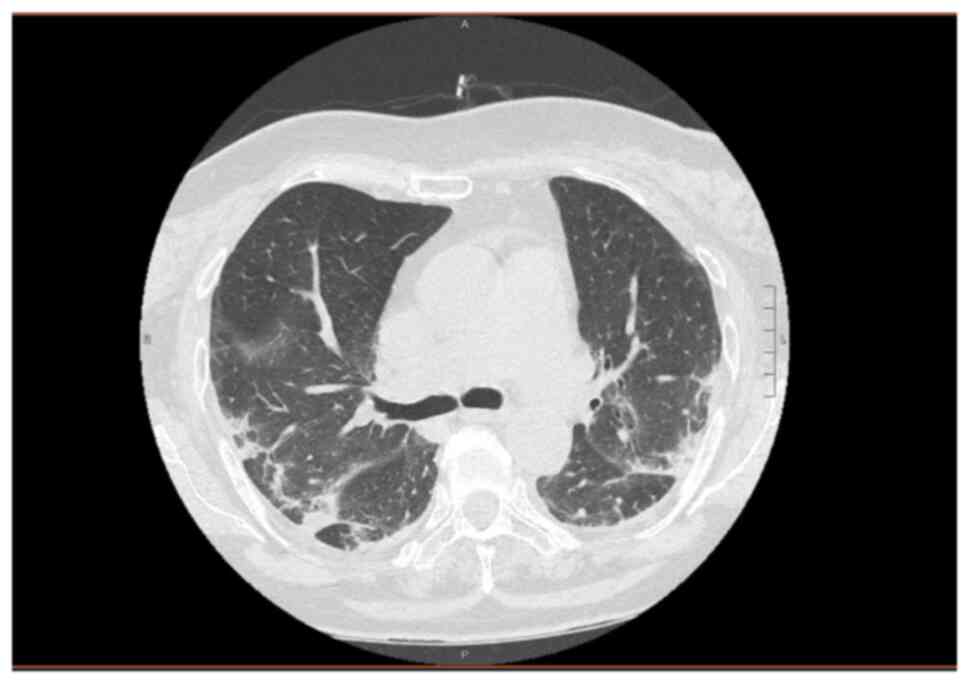
chest CT scan revealed bilateral interstitial pneumonia (Fig. 4). The patient was commenced on
treatment with enoxaparin 6,000 U/day, dexamethasone 6 mg/day and
piperacillin/tazobactam 4.5 g/three times/day.
Since arterial blood analysis revealed
PaO2 of 57 mmHg, PCO2 of 31 mmHg, pH 7.5, and
a PaO2/FiO2 ratio of 93, along with the
deterioration of chest imaging, HFNC (60 l/min; FiO2
60%) was commenced. Sarilumab was administered intravenously at 400
mg within 24 h after the initiation of HFNC.
Within 48 h, the patient's respiratory performances
and blood arterial analysis began to improve
(PaO2/FiO2 ratio was 136). Inflammatory
marker levels also began to decrease. However, 7 days following the
administration of sarilumab the patient's laboratory tests revealed
leukopenia with neutropenia (2,700/mmc, 18.9% neutrophils).
Neutropenia resolved within 7 days without treatment.
HFNC treatment was gradually reduced and switched to
VM first and then NC, until hospital discharge.
Discussion
To date, SARS-CoV-2 infection pathophysiology
remains mostly unclear, arguably as it is not attributable only to
the virus; in fact, both immune and inflammatory responses appear
to play a key role in disease development and duration,
particularly as regards its severe form.
In order to avoid disease progression, intervention
can take place during the first phase of infection (viral phase)
with certain antiviral drugs (remdesivir, molnupiravir,
nirmatrelvir/ritonavir) or monoclonal antibodies (sotrovimab),
administering these within the correct time lapse. However, the
second phase (inflammatory phase) is more severe, and thus it is
difficult to treat due to its severity, complexity and lack of
standardized treatments (14).
It has been reported that COVID-19 causes cytokine
release syndrome (CRS), leading to a dysregulated immune response,
culminating in ARDS, which is also determined by the up- and
downregulation of the expression of several genes directly
stimulated by SARS-CoV-2 infection (15).
CRS is considered an immune system overreaction
response developing in an unregulated manner, the same pathogenetic
mechanism which underlines autoimmune and hematological diseases
(16,17). As confirmation, drugs to treat this
phase are also administered against autoimmune disorders (16).
Various proinflammatory cytokines have been
investigated as the cause of CRS and, among these, IL-6 is one of
the most extensively studied due to its crucial role in
inflammatory pathways. IL-6 has been studied as both an
inflammatory/prognostic marker, since its levels are associated
with the inflammatory state and a therapeutic target (13). Monoclonal antibodies targeting IL-6
and IL-6 receptors are recommended by Italian and American
guidelines for the treatment of patients with severe and critical
COVID-19(14).
Sarilumab, a humanized monoclonal antibody (IgG1
subtype), specifically binds both soluble and membrane-attached
IL-6 receptors (IL6-Rα); it inhibits IL6-mediated pathways
involving glycoprotein 130 (gp130) along with STAT-3. Sarilumab has
been investigated in a few studies, the results of which are not
conclusive (13). Della-Torre
et al (18) reported data
from 28 patients with COVID-19 treated with a single dose of
sarilumab intravenously, and demonstrated a decrease in recovery
time; however, no statistically significant differences in terms of
mortality and overall improvement between patients treated with
standard of care were reported.
Gremese et al (19) studied 53 patients treated with
sarilumab, almost all of which received a second infusion; 14 of
these patients were from the intensive care unit (ICU) and
exhibited an improvement in clinical conditions along with a
reduction in oxygen supplementation therapy; in addition, more than
half of the ICU patients exhibited clinical amelioration following
sarilumab administration. Although on a smaller sample size,
similar results were demonstrated in the study by Benucci et
al (20) on 8 patients treated
with sarilumab.
To date, only a few randomized controlled trials
(13) have been published on
sarilumab administration in patients with COVID-19 and no specific
meta-analyses have been performed to date, at least to the best of
our knowledge. In the study performed by the REMAP-CAP
collaborative group (21), 48
patients were assigned to one dose of 400 mg sarilumab intravenous
administration; the results revealed that sarilumab improved
in-hospital survival compared with usual care.
A larger study, performed by Lescure et al
(12) on 420 subjects, did not
demonstrate the efficacy of sarilumab as regards the outcomes and
survival rates of patients hospitalized with severe COVID-19 and
receiving supplemental oxygen, despite an improved recovery
time.
The CORIMUNO19 group performed an open-label,
randomized, controlled trial with 148 patients randomly assigned to
sarilumab or standard of care, with half of the patients in the
sarilumab group treated with a second dose (22). That trial did not highlight any
effect of sarilumab in patients with moderate to severe COVID-19 in
terms of mortality rate nor on the decreasing proportion of
patients requiring non-invasive ventilation.
The cases reported in the present study developed
severe pulmonary disease due to SARS-CoV2 between 10 and 15 days
from infection, probably due to an excessive proinflammatory
response. All the patients described herein had multiple risk
factors for severe COVID-19 (an age >70 years, multiple
comorbidities and polypharmacy). The results of CT scans revealed
the presence of bilateral interstitial lung involvement and
arterial blood examination displayed gradual respiratory parameters
deterioration.
Among the patients in the present study, only one
had received two doses of the anti-SARS-CoV-2 vaccine. Each patient
received oxygen administration through HFNC, achieving a better
peripheral saturation along with blood gas analysis amelioration
(23,24). Furthermore, a single dose of
sarilumab was intravenously administered to each patient, in
accordance with Italian guidelines (AIFA) and IDSA guidelines
(11,14).
Prior to the sarilumab administration, all the
patients in the present study were screened for latent or active
infections such as hepatitis C virus, human immunodeficiency virus
and hepatitis B virus (25-31).
Within 48-72 h following anti-IL-6 treatment, the patients'
respiratory conditions began to improve, along with improved blood
gas examination and clinical parameters. CRP levels at a cut-off
value of 75 mg/l were used as a surrogate marker of systemic
inflammation to guide the sarilumab administration and to assess
the patients' conditions.
Antibiotic therapy was selected based on local
bacterial epidemiology, the patients' previous antibiotic
treatments and the thoracic CT scan results (31-34).
Furthermore, standard of care therapy (enoxaparin and
dexamethasone) was administered upon admission, following Italian
guidelines.
Dexamethasone administration has been found to be
highly variable across studies on sarilumab therapy, in contrast to
trials on tocilizumab administration, in which the anti-IL-6 drug
has been almost always administered together with corticosteroids.
This difference may explain the diverse results between tocilizumab
and sarilumab in favor of the former drug in previous studies;
however, these data need to be clarified in larger-scale trials
(35).
As regards the scientific literature on the adverse
reactions associated with the use of sarilumab, although with some
limitations (the absence of a control group, single-center setting,
concomitant treatments), Gremese et al (19) did not register severe adverse
events or any secondary infections related to treatment with
sarilumab. In the study by Lescure et al (12), the occurrence of adverse events of
varying severity was similar between both the treatment and the
placebo group.
No severe adverse events were reported in the
REMAP-CAP study (21), and the
CORIMUNO19 group (22) reported a
few cases of temporary neutropenia that is a common side-effect of
all IL-6 blockers. In the same study, a non-statistically
significant increase in the number of bacterial infections was
reported in the sarilumab group (12 patients) compared to the
control group (7 patients).
In the present study, 2 of the patients described
developed neutropenia following the sarilumab administration, which
resolved within a few days without sequelae. Although it is
limited, the authors' experience with sarilumab administration is
in accordance with the findings in the literature as regards the
percentage of recovery, time to oxygen weaning, safety of sarilumab
and time to discharge (12-15).
In August 2020, the authors published the results of
a small case series on tocilizumab administration in patients on
HFNC (23); that study yielded
promising results that have since then been confirmed by larger
trials (35); larger randomized
controlled trials together with meta-analyses are required to
determine the specific effects of sarilumab administration in
patients with COVID-19 and to determine whether other contemporary
therapies, such as dexamethasone, may lead to improvements in
outcomes.
In conclusion, the present study demonstrates that
sarilumab is a safe drug with good clinical outcomes in patients
with COVID-19 and, hence, may be an alternative regimen for the
treatment of patients with SARS-CoV2 severe pulmonary involvement.
Further prospective and well-designed clinical studies with larger
sample sizes and long-term follow up are warranted to assess the
efficacy and the safety of this therapeutic approach to achieve
improved outcomes of patients with COVID-19.
Acknowledgements
The authors would like to thank Dr Pietro Leanza,
Department of Clinical and Experimental Medicine, University of
Catania, Catania, Italy, for his kind English revision of the
manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analyzed during the current study.
Authors' contributions
All authors (AMa, EC, MC, LL, CB, CM, AMu, BMC, GN
and BC) contributed to the study conception and design. AM wrote
the manuscript. EC, MC, CB, CM revised the literature and searched
for relevant references. LL and MC provided clinical assistance to
the patients. AMu was responsible for the laboratory tests and
pharmacological treatments. BMC, GN and BC revised the manuscript.
GN and BC confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all individual
participants included in the present case report study.
Patient consent for publication
Written informed consent was obtained from all the
patients for publication of the present care report study and
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhu N, Zhang D, Wang W, Li X, Yang B, Song
J, Zhao X, Huang B, Shi W, Lu R, et al: A novel coronavirus from
patients with pneumonia in China, 2019. N. Engl J Med. 382:727–733.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Horton R: Offline: COVID-19 is not a
pandemic. Lancet. 396(935)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Musumeci G: Effects of COVID-19 Syndemic
on Sport Community. J Funct Morphol Kinesiol. 7(19)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ministero della Salute. Covid-19,
situation in Italy. https://www.salute.gov.it/portale/nuovocoronavirus/dettaglioContenutiNuovoCoronavirus.jsp?area=nuovoCoronavirus&id=5367&lingua=english&menu=vuoto.
Accessed February 24, 2022.
|
|
5
|
Osuchowski MF, Winkler MS, Skirecki T,
Cajander S, Shankar-Hari M, Lachmann G, Monneret G, Venet F, Bauer
M, Brunkhorst FM, et al: The COVID-19 puzzle: Deciphering
pathophysiology and phenotypes of a new disease entity. Lancet
Respir Med. 9:622–642. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Grasselli G, Tonetti T, Protti A, Langer
T, Girardis M, Bellani G, Laffey J, Carrafiello G, Carsana L,
Rizzuto C, et al: Pathophysiology of COVID-19-associated acute
respiratory distress syndrome: A multicentre prospective
observational study. Lancet Respir Med. 8:1201–1208.
2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
RECOVERY Collaborative Group. Horby P, Lim
WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N,
Brightling C, Ustianowski A, et al: Dexamethasone in Hospitalized
patients with Covid-19. N Engl J Med. 384:693–704. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Nissen CB, Sciascia S, de Andrade D,
Atsumi T, Bruce IN, Cron RQ, Hendricks O, Roccatello D, Stach K,
Trunfio M, et al: The role of antirheumatics in patients with
COVID-19. Lancet Rheumatol. 3:e447–e459. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Angriman F, Ferreyro BL, Burry L, Fan E,
Ferguson ND, Husain S, Keshavjee SH, Lupia E, Munshi L, Renzi S, et
al: Interleukin-6 receptor blockade in patients with COVID-19:
Placing clinical trials into context. Lancet Respir Med. 9:655–664.
2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bhimraj A, Morgan R, Shumaker A, Lavergne
V, Baden L, Cheng VC-C, Edwards KM, Gandhi R, Gallagher J, Muller
WJ, et al: IDSA Guidelines on the treatment and management of
patients with COVID-19. Published by IDSA on 4/11/2020. Last
updated, 3/23/2022. Available: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
|
|
11
|
Bassetti M, Giacobbe DR, Bruzzi P,
Barisione E, Centanni S, Castaldo N, Corcione S, De Rosa FG, Di
Marco F, Gori A, et al: Clinical management of adult patients with
COVID-19 outside intensive care units: Guidelines from the Italian
society of anti-infective therapy (SITA) and the Italian Society of
Pulmonology (SIP). Infect Dis Ther. 10:1837–1885. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lescure FX, Honda H, Fowler RA, Lazar JS,
Shi G, Wung P, Patel N and Hagino O: Sarilumab COVID-19 Global
Study Group. Sarilumab in patients admitted to hospital with severe
or critical COVID-19: A randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Respir Med. 9:522–532.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zizzo G, Tamburello A, Castelnovo L, Laria
A, Mumoli N, Faggioli PM, Stefani I and Mazzone A: Immunotherapy of
COVID-19: Inside and beyond IL-6 signalling. Front Immunol.
13(795315)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bhimraj A, Morgan RL, Shumaker AH,
Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Gallagher J,
Muller WJ, et al: Infectious Diseases Society of America Guidelines
on the treatment and management of patients with COVID-19. Clin
Infect Dis: Apr 27, 2020 (Epub ahead of print). doi:
10.1093/cid/ciaa478.
|
|
15
|
Nunnari G, Sanfilippo C, Castrogiovanni P,
Imbesi R, Li Volti G, Barbagallo I, Musumeci G and Di Rosa M:
Network perturbation analysis in human bronchial epithelial cells
following SARS-CoV2 infection. Exp Cell Res.
395(112204)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Que Y, Hu C, Wan K, Hu P, Wang R, Luo J,
Li T, Ping R, Hu Q, Sun Y, et al: Cytokine release syndrome in
COVID-19: A major mechanism of morbidity and mortality. Int Rev
Immunol. 41:217–230. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cosentino F, Moscatt V, Marino A,
Pampaloni A, Scuderi D, Ceccarelli M, Benanti F, Gussio M, Larocca
L, Boscia V, et al: Clinical characteristics and predictors of
death among hospitalized patients infected with SARS-CoV-2 in
Sicily, Italy: A retrospective observational study. Biomed Rep.
16(34)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Della-Torre E, Campochiaro C, Cavalli G,
De Luca G, Napolitano A, La Marca S, Boffini N, Da Prat V, Di
Terlizzi G, Lanzillotta M, et al: Interleukin-6 blockade with
sarilumab in severe COVID-19 pneumonia with systemic
hyperinflammation: An open-label cohort study. Ann Rheum Dis.
79:1277–1285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gremese E, Cingolani A, Bosello SL,
Alivernini S, Tolusso B, Perniola S, Landi F, Pompili M, Murri R,
Santoliquido A, et al: Sarilumab use in severe SARS-CoV-2
pneumonia. EClinicalMedicine. 27(100553)2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Benucci M, Giannasi G, Cecchini P, Gobbi
FL, Damiani A, Grossi V, Infantino M and Manfredi M: COVID-19
pneumonia treated with Sarilumab: A clinical series of eight
patients. J Med Virol. 92:2368–2370. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
REMAP-CAP Investigators. Gordon AC,
Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D,
Beane A, van Bentum-Puijk W, et al: Interleukin-6 receptor
antagonists in Critically Ill patients with Covid-19. N Engl J Med.
384:1491–1502. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
CORIMUNO-19 Collaborative group. Sarilumab
in adults hospitalised with moderate-to-severe COVID-19 pneumonia
(CORIMUNO-SARI-1): An open-label randomised controlled trial.
Lancet Rheumatol. 4:e24–e32. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Marino A, Pampaloni A, Scuderi D,
Cosentino F, Moscatt V, Ceccarelli M, Gussio M, Celesia BM, Bruno
R, Borraccino S, et al: High-flow nasal cannula oxygenation and
tocilizumab administration in patients critically ill with
COVID-19: A report of three cases and a literature review. World
Acad Sci J. 2(1)2020.
|
|
24
|
Ceccarelli M, Marino A, Cosentino F,
Moscatt V, Celesia BM, Gussio M, Bruno R, Rullo EV, Nunnari G and
Cacopardo BS: Post-infectious ST elevation myocardial infarction
following a COVID-19 infection: A case report. Biomed Rep.
16(10)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Marino A, Cosentino F, Ceccarelli M,
Moscatt V, Pampaloni A, Scuderi D, D'Andrea F, Rullo EV, Nunnari G,
Benanti F, et al: Entecavir resistance in a patient with
treatment-naïve HBV: A case report. Mol Clin Oncol.
14(113)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Celesia BM, Marino A, Borracino S,
Arcadipane AF, Pantò G, Gussio M, Coniglio S, Pennisi A, Cacopardo
B and Panarello G: Successful extracorporeal membrane oxygenation
treatment in an acquired immune deficiency syndrome (AIDS) patient
with acute respiratory distress syndrome (ARDS) complicating
pneumocystis jirovecii pneumonia: A challenging case. Am J Case
Rep. 21(e919570)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Celesia BM, Marino A, Del Vecchio RF,
Bruno R, Palermo F, Gussio M, Nunnari G and Cacopardo B: Is it safe
and cost saving to defer the CD4+ cell count monitoring
in stable patients on ART with more than 350 or 500 cells/µl?
Mediterr J Hematol Infect Dis. 11(e2019063)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Marino A, Zafarana G, Ceccarelli M,
Cosentino F, Moscatt V, Bruno G, Bruno R, Benanti F, Cacopardo B
and Celesia BM: Immunological and clinical impact of DAA-mediated
HCV eradication in a cohort of HIV/HCV Coinfected patients:
Monocentric Italian experience. Diagnostics.
11(2336)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Micali C, Russotto Y, Caci G, Ceccarelli
M, Marino A, Celesia BM, Pellicanò GF, Nunnari G and Venanzi Rullo
E: Loco-regional treatments for hepatocellular carcinoma in people
living with HIV. Infect Dis Rep. 14:43–55. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Marino A, Scuderi D, Locatelli ME, Gentile
A, Pampaloni A, Cosentino F, Ceccarelli M, Celesia BM, Benanti F,
Nunnari G, et al: Modification of serum brain-derived neurotrophic
factor levels following anti-HCV therapy with direct antiviral
agents: A new marker of neurocognitive disorders. Hepat Mon.
20(e95101)2020.
|
|
31
|
Burgaletto C, Brunetti O, Munafò A,
Bernardini R, Silvestris N, Cantarella G and Argentiero A: Lights
and shadows on managing immune checkpoint inhibitors in oncology
during the COVID-19 Era. Cancers. 13(1906)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Marino A, Munafò A, Zagami A, Ceccarelli
M, Di Mauro R, Cantarella G, Bernardini R, Nunnari G and Cacopardo
B: Ampicillin plus ceftriaxone regimen against Enterococcus
faecalis endocarditis: A literature review. J Clin Med.
10(4594)2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Erdem H, Hargreaves S, Ankarali H,
Caskurlu H, Ceviker SA, Bahar-Kacmaz A, Meric-Koc M, Altindis M,
Yildiz-Kirazaldi Y, Kizilates F, et al: Managing adult patients
with infectious diseases in emergency departments: International
ID-IRI study. J Chemother. 33:302–318. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
El-Sokkary R, Uysal S, Erdem H, Kullar R,
Pekok AU, Amer F, Grgić S, Carevic B, El-Kholy A, Liskova A, et al:
Profiles of multidrug-resistant organisms among patients with
bacteremia in intensive care units: An international ID-IRI survey.
Eur J Clin Microbiol Infect Dis. 40:2323–2334. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
RECOVERY Collaborative Group. Tocilizumab
in patients admitted to hospital with COVID-19 (RECOVERY): A
randomised, controlled, open-label, platform trial. Lancet.
397:1637–1645. 2021.PubMed/NCBI View Article : Google Scholar
|