Introduction
Diabetes mellitus (DM), the foremost
non-communicable disease is a complex metabolic disorder related to
alterations in the metabolism of carbohydrates, proteins and lipids
(1). Type 2 DM (T2DM) is evolving
as the major cause for morbidity and mortality in India and is
considered as the equivalent of cardiovascular disease due to its
principal complication, atherosclerosis, ensuing from dyslipidemia.
As regards the Indian population, epidemiological studies on
dyslipidemia are inadequate (2).
The South Asian population which encompasses Indians, has been
found to have atherogenic dyslipidemia, that is characterized by
decreased levels of high-density lipoprotein cholesterol (HDL-c)
and increased triglycerides (TGL) which are associated with T2DM
(3).
In India, with the rapid increase in the population,
urbanization and lifestyle changes, including an unhealthy diet and
sedentary habits are paving way for dyslipidemia. According to the
ICMR INDIAB phase I study, 79% of the Indian population have
fasting dyslipidemia with low HDL-c levels, accounting for 72.3% of
the population (4). HDL-c
maintains the intracellular cholesterol homeostasis through a
process known as reverse cholesterol transport, through which
excess cholesterol is removed from peripheral tissues. The
important steps involved in the reverse cholesterol pathway are the
following: i) The HDL-c-mediated uptake of cellular cholesterol;
ii) the esterification of cholesterol present in HDL-c-mediated by
lecithin cholesterol acyl transferase; and iii) the confiscation of
cholesterol esters from HDL-c by the liver for excretion through
bile (5). It has been discovered
that a protein, namely ATP-binding cassette transporter A1 (ABCA1),
plays a crucial role in the first step of reverse cholesterol
transport.
ABCA1 belongs to a family of proteins that pair the
hydrolysis of ATP to the binding of a substrate, thereby enabling
its transport through the plasma membrane. The ABCA1 protein is
coded by a gene mapping to chromosome 9 in humans (6). It is made up of two transmembrane
domains, two nucleotide binding domains, two regulatory domains and
two extracellular domains (ECDs). The ECD has a hydrophobic hollow
interior, which serves as a potential passage for the
transportation of lipids to apolipoprotein A-I (ApoA-I) from the
interior of the membrane. Lipid flopping mechanism causes
conformational change, to facilitate the delivery of the lipids to
the ECD from the transmembrane cavity. It is hypothesized that
ABCA1 and the lipid bilayer bind cooperatively with ApoA-I and
deliver the substrate for nascent HDL-c formation (7).
According to a meta-analysis of proteomic and
genomic studies, it was found that an impairment in the ABCA1
pathway attributed to the quantitative decline in HDL-c levels,
causing the intracellular accumulation of lipids (8). Therefore, the aim of the present
study was to determine the association between single nucleotide
polymorphisms (SNPs) of the ABCA1 gene and T2DM.
Patients and methods
A case control study was conducted among 50 patients
with T2DM selected as the cases and 50 age- and sex-matched
patients without comorbidities were selected as the controls. An
ethical clearance was obtained from the Institutional Human Ethics
Committee of the PSG Institute of Medical Sciences and Research,
Coimbatore, India (Ref. no. 15/376). The diagnosis of T2DM was
based on the American Diabetic Association (ADA) criteria (1). Patients satisfying the diagnostic
criteria were provided with an explanation of the study. A written
informed consent was acquired from the patients for the use of
their samples for scientific research, prior to the collection of
blood samples. The samples were collected between June, 2016 to
June, 2017. Both males and females aged >30 years, diagnosed
with T2DM with a maximum duration of 2 years on treatment (oral
drugs/insulin) were included as the cases. Subjects with other
malignancies, type 1 DM, T2DM with microvascular and macrovascular
complications, acute illnesses, genetic malformations and pregnancy
were excluded from the study.
Venous blood samples were collected after following
overnight fasting and were processed for the estimation of routine
parameters, including fasting plasma glucose (FPG), glycated
hemoglobin (HbA1c), total cholesterol, high-density
lipoprotein-cholesterol (HDL-c), low-density
lipoprotein-cholesterol (LDL-c) and triglycerides (TGL). The
samples for DNA extraction were collected in BD
vacutainer® EDTA tubes (BD Biosciences) and transferred
to a labeled, sterile cryovials and stored at -80˚C until analysis.
HbA1c levels were measured using turbidimetric inhibition
immunoassay. Plasma glucose and lipid profile estimations were
performed on a Cobas Integra 400 analyzer (Roche Diagnostics) using
standard methods.
SNP genotyping
The SNPs in the ABCA1 gene, namely R219K (rs2230806)
and C69T (rs1800977) were selected for investigation in the present
study based on population genetics. The mutation in the R219K
polymorphism was (G→A), whereas for C69T it was (C→T). DNA
extraction was performed using a kit from Bio Basic, Inc. (EZ-10
Spin Column Genomic DNA Minipreps kit). The quality of the DNA was
assessed using 0.8% agarose gel electrophoresis and viewed using a
Gel Doc system (Syngene). DNA was quantified using a NanoDrop UV
absorption spectrophotometer (Thermo Fisher Scientific, Inc.) at
A260.
The nucleotide sequences of the two SNPs were
determined, and forward and reverse primers for the flanking
sequence of these nucleotide sequences were designed and were as
follows: R219K forward, 5'-CCTCTTGTGCTTGTCTCTCTTTGCATG-3' and
reverse, 5'-TTGGCTTCAGGATGTCCATGTTGG-3'; C69T forward,
5'-CAGCGCTTCCCGCGCGTCTTA-3' and reverse,
5'-CCACTCACTCTCGTCCGCAATTAC-3'. DNA amplification by polymerase
chain reaction (PCR) was carried out in a DNA Thermal Cycler
(Eppendorf). Reactions were performed with 1 µmol of each of the
primers of R219K and with 10 µmol of each of the primers of C69T.
The total reaction volume was 20 µl as follows: Forward primer, 1
µl; reverse primer, 1 µl; Taq DNA Polymerase master mix RED
(Ampliqon A/S), 10 µl; DNA, 0.8 µl; and milli Q water (Merck KGaA),
7.2 µl. The annealing temperature was standardized at
60˚C for the primers of R219K and at 63.5˚C for the
primers of C69T. The PCR program was performed as follows: Initial
denaturation at 94˚C for 2 min, cycle denaturation at 94˚C for 30
sec, annealing for 45 sec, extension at 72˚C for 45 sec, and a
final extension at 72˚C for 2 min. A total of 35 cycles were run,
and the PCR product was held at 4˚C till it was removed.
PCR amplification products were confirmed using 2% agarose gel
electrophoresis. The PCR product size was determined [211 base
pairs (bp) for SNP R219K and 345 bp for SNP C69T] using the Gel Doc
system (Syngene) by comparing with a 100-bp DNA ladder (Invitrogen;
Thermo Fisher Scientific, Inc.).
For restriction fragment length polymorphism, 1 µl
of PCR product was mixed with 0.8 µl of restriction enzyme, 5 µl of
10X NE buffer and the reaction volume was made to 50 µl with milli
Q water. The PCR product of SNP R219K was digested with
EcoNI (XagI) and incubated at 37˚C for 1 h and was
inactivated at 65˚C for 20 min. The PCR product of SNP
C69T was digested with BsmAI and incubated at 55˚C for 1 h
with no inactivation. Samples along with the 100 bp ladder and
undigested PCR product were run on a 15% polyacrylamide gel for the
analysis of restriction fragments. The gel was stained using
ethidium bromide and the fragments were visualized using a
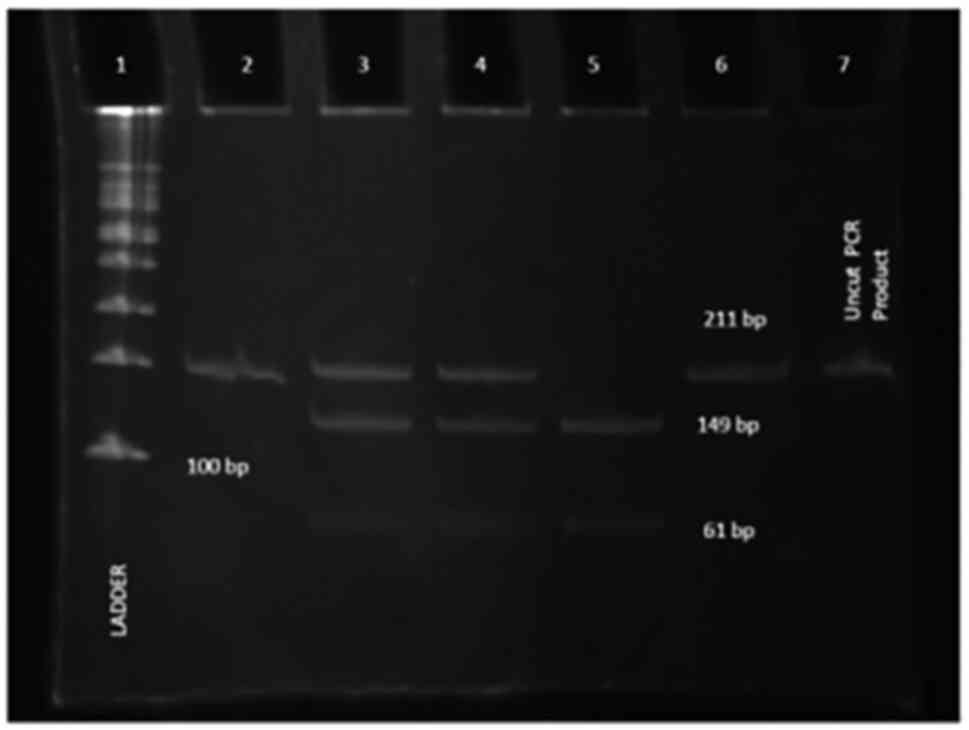
Chemiluminescence Gel Doc System (Figs. 1 and 2).
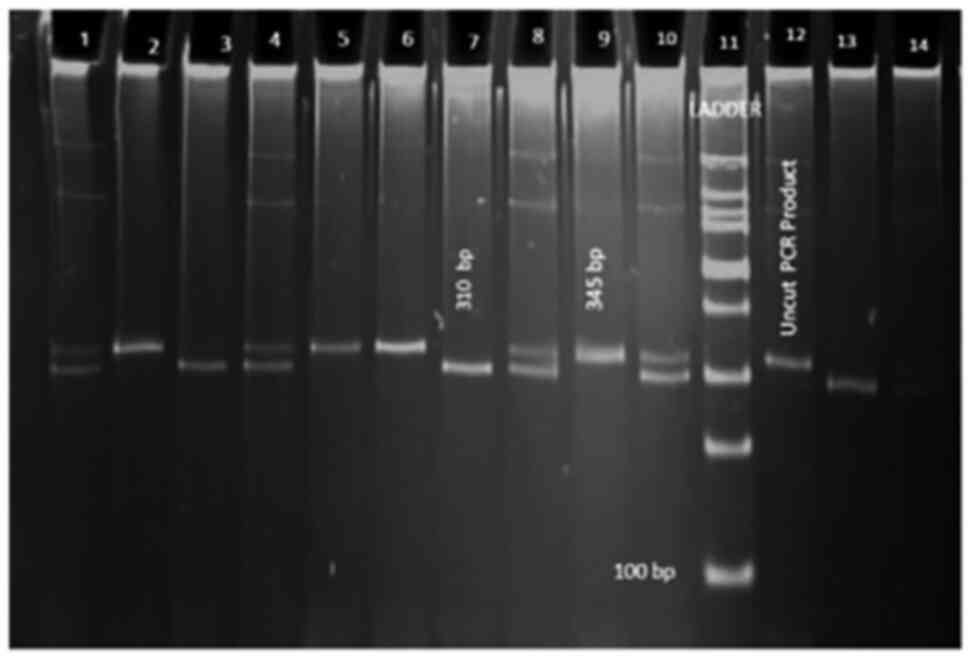 | Figure 2Gel image depicting the restriction
fragments of the C69T single nucleotide polymorphism. Lanes 1, 4,
8, 10 and 14, CT; lanes 2, 5, 6 and 9, CC; lanes 3, 7 and 13, TT;
lane 11, DNA ladder; lane 12, uncut PCR product. |
Restriction did not occur when the recessive G
allele was present, yielding a 211-bp fragment (GG-homozygous
wild-type), whereas restriction occurred in the presence of the
dominant A allele, yielding 149- and 61-bp fragments (AA-homozygous
mutant) and in the presence of both the A and G allele, yielding
fragments of 211 + 149 + 61 bp (AG-heterozygous wild-type).
Restriction did not occur when the recessive C
allele was present, yielding a 345-bp fragment (CC-homozygous
wild-type), whereas restriction occurred in the presence of the
dominant T allele, yielding a 310-bp fragment (TT-homozygous
mutant), and in the presence of both the C and T allele, yielding
fragments of 310 + 35 bp (CT-heterozygous wild-type).
Statistical analysis
Statistical analysis was performed using R i386
3.6.3 software for Windows. Continuous variables are presented as
the mean ± standard deviation. Categorical variables are presented
as frequency tables. Continuous data were compared using an
independent t-test/Welch's t-test or the Mann-Whitney U-test.
Categorical data were compared using Chi squared test and the data
that did not follow the assumptions of Chi squared test were
analyzed using Fisher's exact test. Odds ratios (ORs) with 95%
confidence intervals (95% CIs) were used to assess the strength of
the association between the R219K and C69T ABCA1 SNPs, and T2DM.A
value of P<0.05 was considered to indicate a statistically
significant difference.
Results
A total of 100 subjects consisting of 50 patients in
each group were used in the present study. The demographic,
anthropometric and laboratory data of the cases and controls are
presented in Table I. The mean age
of the subjects in the case and control group was 54.08 and 53.9
years, respectively. The mean body mass index (BMI) of the subjects
in the case and control group was 26.51 and 27.48 kg/m2,
respectively. The mean FPG levels were significantly higher in the
cases than in the controls and the distribution of HbA1c differed
significantly between the case and control group. In addition, the
mean LDL-c levels were significantly higher in the cases compared
with the controls.
 | Table ISummary of the demographic and lipid
profile data of the study participants. |
Table I
Summary of the demographic and lipid
profile data of the study participants.
| Parameter | Cases (n=50) | Controls (n=50) | P-value |
|---|
| Age (years) | 54.08±10.82 | 53.9±10.99 |
0.9344T |
| Sex | | | |
| Male | 28 (56%) | 29 (58%) |
0.8399C |
| Female | 22 (44%) | 21 (42%) | |
| BMI
(kg/m2) | 26.51±3.48 | 27.48±2.63 |
0.1209T |
| WHR | 0.91±0.09 | 0.92±0.08 |
0.4502T |
| FPG (mg/dl) | 137.36±32.68 | 100.78±8.8 |
<0.0001M |
| HbA1c (%) | 7.64±1.5 | 5.77±0.38 |
<0.0001M |
| Total cholesterol
(mg/dl) | 186.36±40.15 | 186.8±28.01 |
0.9494T |
| HDL-c (mg/dl) | 41.44±8.67 | 42.28±10.12 |
0.6567T |
| TGL (mg/dl) | 134.62±48.16 | 130.3±48.59 |
0.6562T |
| LDL-c (mg/dl) | 127.46±32.96 | 116.26±21.3 |
0.0234WTO |
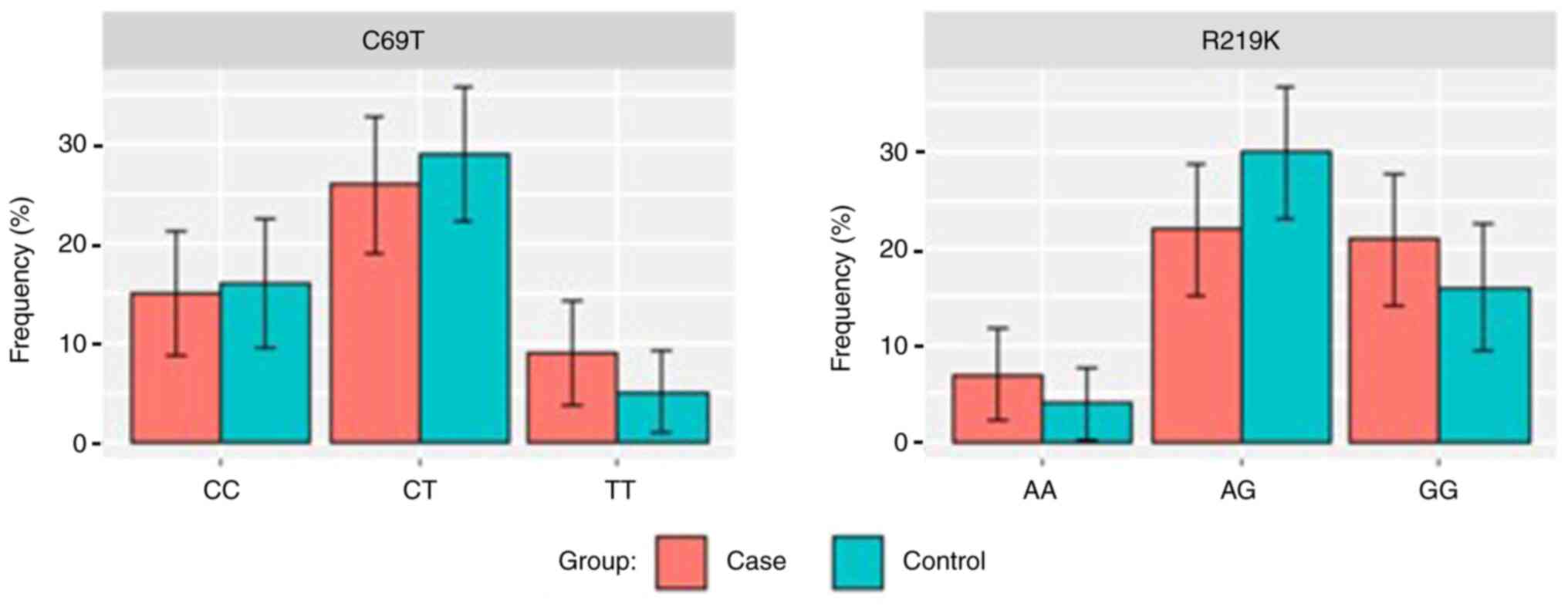
The present study found that the distribution of the
CC, CT and TT variants of the C69T genotype was 30, 52 and 18% in
the case group, and 32, 58 and 10% in the control group,
respectively. In addition, the distribution of the AA, AG and GG
variants of the R219K genotype was 14, 44 and 42% in the case
group, and 8, 60 and 32% in the control group, respectively. Using
the Chi-squared test, it was noted that the distribution of the
C69T and R219K SNPs did not differ significantly between the two
groups (Fig. 3 and Table II).
 | Table IIComparison of genotypes of R219K and
C69T polymorphisms among the study groups. |
Table II
Comparison of genotypes of R219K and
C69T polymorphisms among the study groups.
| Genotype | Sub-category | Cases, n (% ) | Controls, n (%) | P-value |
|---|
| C69T | CC | 15 (30%) | 16 (32%) | 0.512 |
| | CT | 26 (52%) | 29 (58%) | |
| | TT | 9 (18%) | 5 (10%) | |
| R219K | AA | 7 (14%) | 4 (8%) | 0.2561 |
| | AG | 22 (44%) | 30 (60%) | |
| | GG | 21 (42%) | 16 (32%) | |
Using Fisher's exact test, it was also that the
dominant T allele of the C69T genotype and the dominant A allele of
the R219K genotype were not significantly associated with the case
group in the different additive models (Table III).
 | Table IIIEffects of dominant allele of
genotypes of SNP R219K and SNP C69T among the study groups. |
Table III
Effects of dominant allele of
genotypes of SNP R219K and SNP C69T among the study groups.
| Allele and model | Cases, n (%) | Controls, n (%) | OR (95% CI) | P-value |
|---|
| C69T additive model
1 | | | | |
| TT | 9 (18%) | 5 (10%) | 1.98
(0.61-6.38) | 0.3881 |
| CT + CC | 41 (82%) | 45 (90%) | | |
| C69T additive model
2 | | | | |
| CT + TT | 35 (70%) | 34 (68%) | 0.91
(0.39-2.13) | >0.99 |
| CC | 15 (30%) | 16 (32%) | | |
| R219K additive
model 1 | | | | |
| AA | 7 (14%) | 4 (8%) | 1.87
(0.51-6.85) | 0.5246 |
| AG + GG | 43 (86%) | 46 (92%) | | |
| R219K additive
model 2 | | | | |
| AA + AG | 29 (58%) | 34 (68%) | 1.54
(0.68-3.49) | 0.4076 |
| GG | 21 (42%) | 16 (32%) | | |
In addition, it was observed that the mean levels of
total cholesterol, HDL-c, TGL and LDL-c did not differ
significantly between the mutant and non-mutant variants of both
the C69T and R219K genotypes in the case and control groups
(Table IV).
 | Table IVComparison of lipid profile
parameters of the R219K and C69Tgenotypes among the cases and
controls. |
Table IV
Comparison of lipid profile
parameters of the R219K and C69Tgenotypes among the cases and
controls.
| C69T |
|---|
| | Cases | Controls |
|---|
| Lipid profile
parameters | Mutant | Non-mutant | P-value | Mutant | Non-mutant | P-value |
|---|
| Total cholesterol
(mg/dl) | 179.78±33.07 | 187.80±41.76 |
0.5922T | 177.00±25.14 | 187.89±28.35 |
0.4151T |
| HDL-c (mg/dl) | 44.11±9.87 | 40.85±8.40 |
0.3121T | 41.80±8.17 | 42.33±10.39 |
0.9123T |
| TGL (mg/dl) | 133.00±53.48 | 134.98±47.63 |
0.9126T | 104.60±7.99 | 133.16±50.39 |
0.2071M |
| LDL-c (mg/dl) | 121.89±19.83 | 128.68±35.27 |
0.5808T | 121.4±15.21 | 115.69±21.93 |
0.3003M |
| R219K |
| | Cases | Controls |
| Lipid profile
parameters | Mutant | Non-mutant | P-value | Mutant | Non-mutant | P-value |
| Total cholesterol
(mg/dl) | 177.86±49.27 | 187.74±38.98 |
0.5511T | 192.75±31.57 | 186.28±28.00 |
0.6624T |
| HDL-c (mg/dl) | 38±.05 | 42±8.85 |
0.2617T | 47±6.22 | 41.87±10.33 |
0.3359T |
| TGL (mg/dl) | 122.14±39.52 | 136.65±49.53 |
0.4655T | 135.50±59.12 | 129.85±48.32 |
0.7206M |
| LDL-c (mg/dl) | 122.29±32.63 | 128.30±33.32 |
0.6589T | 100.5±5.07 | 117.63±21.64 |
0.124T |
Discussion
The global prevalence of T2DM has markedly
increased; thus, it is critical to carry out research to assess the
risk factors and multigenetic factors associated with this
condition. The most severe complication of T2DM is atherogenesis
ensuing from dyslipidemia (9). One
of the aberrations in lipid metabolism commonly encountered in T2DM
is a decrease in HDL-c levels. The association between the genetic
polymorphism in the ABCA1 gene and the decrease in HDL-c levels has
been demonstrated in recent years (10). A systematic review and
meta-analysis on studies that determined the association between
the ABCA1 gene polymorphism and T2DM, revealed inconsistent results
(11). The divergence may be due
to the influence of ethnicity, genetic susceptibility, and
environmental factors.
One of the contributing factors for dyslipidemia is
obesity and the association between obesity and dyslipidemia is
illustrious. Recently, there has been a revision in the cut-off
value for obesity for Asian Indians, and as per this revision, a
BMI ≥25.0 kg/m2 is defined as obese (12). According to this criterion, both
the patients with T2DM and the controls in the present study fell
under the obese category, as reflected by their mean BMI values
(Table I). In addition to this,
the mean waist-to-hip ratio of both the groups was above the
cut-off value for obesity as per the WHO (13) (Table
I). This reveals the high prevalence of obesity among the
Indian population, which is in agreement with the study conducted
by Ahirwar and Mondal (14).
In the present study upon assessing the lipid
profile parameters between the cases and controls, it was found
that the mean LDL-c values were significantly higher in the cases
than in the controls (Table I).
This may be explained by the decrease in the insulin-mediated
expression of LDL-c cell surface receptors in T2DM, which causes a
decrease in the clearance of LDL-c, thereby contributing to these
increased levels (15).
According to the National Cholesterol Education
Program (NCEP) ATP III guidelines, the optimal HDL-c level is 50
mg/dl (16). When the levels of
HDL-c decrease, it is considered as a risk factor for the
development of cardiovascular disease (17). In the present study, the HDL-c
levels were low in both the cases and the controls (Table I). It is often considered that this
decrease in the levels of HDL-c is due to the action of cholesterol
ester transfer protein (CETP), which enhances the formation of
TGL-rich HDL-c, thereby priming for HDL-c catabolism (18). However, CETP is only partially
responsible for the decrease in HDL-c levels, as it has been
previously reported that genetic variations in the ABCA1 gene are
associated with decreased serum HDL-c levels (19).
In the present study, the genotypic distribution of
the ABCA1 gene for the R219K and C69T SNPs did not reveal any
statistically significant differences in the distribution of
homozygous mutants (AA and TT) among the cases when compared to the
controls (Table II).
Additionally, additive models for ORs were used to determine
whether the presence of the dominant allele alone (the A allele in
R219K and the T allele in C69T) is sufficient to cause pathological
changes in HDL-c metabolism in T2DM (Table III). No statistically significant
differences were observed between the case and control groups,
depicting that there is a possibility of an inherent disruption in
HDL-c metabolism among the Indian population. A previous
meta-analysis conducted by Shim et al (11), reported on the inconsistencies in
lipid levels and ABCA1 variants. The association between the two
SNPs and lipid profiles was assessed in the present study, and no
substantial association (P>0.05) was found for these parameters
and the genotype distribution (Table
IV). This was in accordance with the studies of Alharbi et
al (20) and Ergen et
al (21).
The lack of significant difference in HDL-c levels,
the genotype and allele distribution of the ABCA1 gene among the
cases and control subjects in the present study may be due to a
defective ABCA1 genotype in the Indian population, thereby
predisposing these individuals to a decrease in HDL-c levels.
According to the study conducted by Salinas et al (22), it was found that apart from HDL-c
metabolism, ABCA1 plays a role in cholesterol homeostasis in
β-cells of pancreas and adipocytes. Hence, a defective ABCA1
genotype causes obesity due to enlarged adipocytes and T2DM due to
decreased insulin secretion (22).
This predisposes the population to a greater risk of developing
dyslipidemia. Similar genetic variations in ABCA1 genotypes leading
to dyslipidemia and subsequent cardiovascular diseases have been
reported in studies conducted across South Asia and Saudi Arabia
(17,19,20,23).
Dyslipidemia due to a decrease in the levels of
HDL-c is merely one end of the spectrum. On the other end, the
functional capacity of HDL-c is equally attributed to dyslipidemia
in recent years. In addition to reverse cholesterol transport,
HDL-c also has anti-inflammatory, antioxidant, antithrombotic and
endothelial cell maintenance functions. A decrease in HDL-c levels
causes a decrease in cholesterol sequestration from foam cells.
This results in an inflammatory state, which causes a modification
in the HDL-c structure, resulting in a detrimental effect on its
function and thereby rendering it dysfunctional (24). This produces a vicious cycle of
decreased HDL-c levels, an inflammatory state and dysfunctional
HDL-c. In the Indian population, this inflammatory state is
aggravated by obesity due to the release of inflammatory mediators,
paving the way for a pro-inflammatory state and oxidative stress
(25). The increase in the
prevalence of T2DM in India may be ascribed to dysfunctional HDL-c
and obesity. Overall, there is an increased risk of developing
atherosclerosis associated with T2DM at an early age. In the
present study, the levels of HDL-c were below the optimum in both
the cases and the controls, most likely due to the defective ABCA1
genotype. The present study, however, did not evaluate the
functional capacity of HDL-c due to a lack of availability of
diagnostic methods. The identification of ABCA1 gene polymorphisms
leading to a disruption in HDL-c metabolism may aid in the early
assessment of the risk of developing T2DM and its associated
complications (26).
A limitation of the present study was the small
sample size. Hence, it is recommended that further proteomics and
genomics studies are conducted in the future to evaluate the
association of ABCA1 variants with T2DM in larger sample sizes, in
order to assess the contribution of dysfunctional HDL-c on overall
cardiovascular disease risk in T2DM in the Indian population.
However, the findings of the present study, may shed light into the
association between these polymorphisms and T2DM and may aid future
studies on this topic.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Dr. Anandhi
Gopal Joshi PSG PRIME Grant - intramural research grant for the
year 2016 received from the PSG Institute of Medical Sciences and
Research, Coimbatore.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
DB was involved in the conception and design of the
study, in the screening of the patients, in the selection and
recruitment of the study participants, the provision of informed
consent, laboratory investigations, laboratory report
interpretations, data collection and the monitoring of data,
interpretation of the data, statistical analysis and
interpretation, maintaining a master file of project and in
drafting the final manuscript. GB was involved in the conception
and design of the study, in the drafting of the final manuscript,
laboratory report interpretations, data collection and in the
monitoring of data and interpretation of data. Both authors have
read and approved the final manuscript. DB and GB confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by Institutional
Human Ethics Committee of the PSG Institute of Medical Sciences and
Research, Coimbatore, India (Ref. no. 15/376). Consent was obtained
from all the participants after explaining the objectives of the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 32 (Suppl
1):S62–S67. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mohan V, Venkatraman JV and Pradeepa R:
Epidemiology of cardiovascular disease in type 2 diabetes: The
Indian scenario. J Diabetes Sci Technol. 4:158–170. 2010.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bilen O, Kamal A and Virani SS:
Lipoprotein abnormalities in South Asians and its association with
cardiovascular disease: Current state and future directions. World
J Cardiol. 8:247–257. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Joshi SR, Anjana RM, Deepa M, Pradeepa R,
Bhansali A, Dhandania VK, Joshi PP, Unnikrishnan R, Nirmal E,
Subashini R, et al: Prevalence of dyslipidemia in urban and rural
India: The ICMR-INDIAB study. PLoS One. 9(e96808)2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ohashi R, Mu H, Wang X, Yao Q and Chen C:
Reverse cholesterol transport and cholesterol efflux in
atherosclerosis. QJM. 98:845–856. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Dean M: The genetics of ATP-binding
cassette transporters. Methods Enzymol. 400:409–429.
2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Qian H, Zhao X, Cao P, Lei J, Yan N and
Gong X: Structure of the human lipid exporter ABCA1. Cell.
169:1228–1239.e10. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yoon HY, Lee MH, Song Y, Yee J, Song G and
Gwak HS: ABCA1 69C>T polymorphism and the risk of type 2
diabetes mellitus: A systematic review and updated meta-analysis.
Front Endocrinol (Lausanne). 12(639524)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Martín-Timón I, Sevillano-Collantes C,
Segura-Galindo A and Del Cañizo-Gómez FJ: Type 2 diabetes and
cardiovascular disease: Have all risk factors the same strength?
World J Diabetes. 5:444–470. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jacobo-Albavera L, Domínguez-Pérez M,
Medina-Leyte DJ, González-Garrido A and Villarreal-Molina T: The
role of the ATP-binding cassette A1 (ABCA1) in human disease. Int J
Mol Sci. 22(1593)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Shim SY, Yoon HY, Yee J, Han JM and Gwak
HS: Association between ABCA1 gene polymorphisms and plasma lipid
concentration: A systematic review and meta-analysis. J Pers Med.
11(883)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mahajan K and Batra A: Obesity in adult
Asian Indians-the ideal BMI cut-off. Indian Heart J.
70(195)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Waist circumference and waist-hip ratio:
Report of a WHO expert consultation. (2011, May 16). https://www.who.int/publications/i/item/9789241501491.
|
|
14
|
Ahirwar R and Mondal PR: Prevalence of
obesity in India: A systematic review. Diabetes Metab Syndr.
13:318–321. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vergès B: Pathophysiology of diabetic
dyslipidaemia: Where are we? Diabetologia. 58:886–899.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Expert Panel on Detection, Evaluation and
Treatment of High Blood Cholesterol in Adults. Executive summary of
the third report of the national cholesterol education program
(NCEP) expert panel on detection, evaluation, and treatment of high
blood cholesterol in adults (adult treatment panel III). JAMA.
285:2486–2497. 2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Haghvirdizadeh P, Ramachandran V, Etemad
A, Heidari F, Ghodsian N, Bin Ismail N and Ismail P: Association of
ATP-binding cassette transporter A1 gene polymorphisms in type 2
diabetes mellitus among malaysians. J Diabetes Res.
2015(289846)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vergès B: Lipid modification in type 2
diabetes: The role of LDL and HDL. Fundam Clin Pharmacol.
23:681–685. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Daimon M, Kido T, Baba M, Oizumi T, Jimbu
Y, Kameda W, Yamaguchi H, Ohnuma H, Tominaga M, Muramatsu M and
Kato T: Association of the ABCA1 gene polymorphisms with type 2 DM
in a Japanese population. Biochem Biophys Res Commun. 329:205–210.
2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Alharbi KK, Khan IA, Al-Daghri NM, Munshi
A, Sharma V, Mohammed AK, Wani KA, Al-Sheikh YA, Al-Nbaheen MS,
Ansari MG and Syed R: ABCA1 C69T gene polymorphism and risk of type
2 diabetes mellitus in a Saudi population. J Biosci. 38:893–897.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ergen HA, Zeybek U, Gök O and Karaali ZE:
Investigation of ABCA1 C69T polymorphism in patients with type 2
diabetes mellitus. Biochem Med (Zagreb). 22:114–120.
2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Salinas CA, Cruz-Bautista I, Mehta R,
Villarreal-Molina MT, Pérez FJ, Tusié-Luna MT and
Canizales-Quinteros S: The ATP-binding cassette transporter
subfamily A member 1 (ABC-A1) and type 2 diabetes: An association
beyond HDL cholesterol. Curr Diabetes Rev. 3:264–267.
2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhao TY, Lei S, Huang L, Wang YN, Wang XN,
Zhou PP, Xu XJ, Zhang L, Xu LW and Yang L: Associations of genetic
variations in ABCA1 and lifestyle factors with coronary artery
disease in a southern Chinese population with dyslipidemia: A
nested case-control study. Int J Environ Res Public Health.
16(786)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Feingold KR and Grunfeld C: Effect of
inflammation on HDL structure and function. Curr Opin Lipidol.
27:521–530. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ellulu MS, Patimah I, Khaza'ai H, Rahmat A
and Abed Y: Obesity and inflammation: The linking mechanism and the
complications. Arch Med Sci. 13:851–863. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haerian BS, Haerian MS, Roohi A and
Mehrad-Majd H: ABCA1 genetic polymorphisms and type 2 diabetes
mellitus and its complications. Meta Gene. 13:104–114. 2017.
|