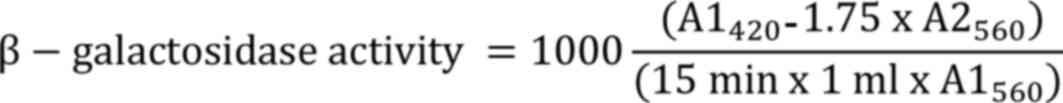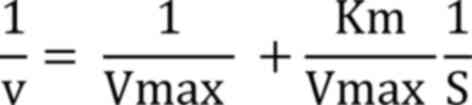Introduction
Lactic acid bacteria (LAB), which are employed as
starters in the manufacturing of dairy products, are critical
variables in the fermentation and preservation of fermentative
foods, improving the texture and flavor of food products (1). Fermented foods containing LAB are
traditionally used every day as food in several parts of India.
Curd, prepared by the fermentation of milk with an inoculum of
previously prepared curd, is used in the majority of households in
India, where it constitutes an integral part of the daily diet. The
LAB that ferment the milk are likely to differ slightly in each
household, as there is no standardized starter culture used to
prepare curd. Although curd is believed to have probiotic
properties, the documentation of this is limited of this in the
literature (2). The present study
was undertaken to evaluate the LAB from homemade curd in southern
India for its probiotic properties. Rural symbolizes the ethnicity
of food culture, having indigenous microbial culture affording to
improve the general health of consumers. The intention of
collecting samples from such a region was to obtain the bacteria
which are prototrophs. Although there are not many differences as
regards homes or locations, the diversity of LAB perhaps yields
novel enzyme production.
β-galactosidase (EC 3.2.1.23) is the most essential
enzyme in the dairy sector for the production of low-lactose foods
to address lactose intolerance. It is produced commercially from
microorganisms, such as bacteria, yeast and fungus. Bacterial
enzymes are preferred over synthetic ones due to their high
activity and stability (3,4). β-galactosidase is employed in the
food industry to hydrolyze lactose into glucose and galactose,
preventing lactose crystallization and enhancing the sweetness and
milk product solubility. In addition, β-galactosidase has two
distinct enzymatic functions: It breaks down lactose and also
cleaves cellobiose, cellotriose, and cellulose to a lesser extent
(5). The majority of chemically
synthesized β-galactosidase is not approved for use in food, as it
is expensive and less efficient for industrial usage. Therefore,
there is an urgent need to address the gap by selecting
microorganisms which are safe for consumption and capable of
producing β-galactosidase. It has been observed that, for
functional food applications, LAB and Bifidobacterium, are
safe organisms and the most desirable sources of β-galactosidase
(6).
β-galactosidase has various applications in the
fields of medicine and industry. A biosensor can be developed using
the β-galactosidase enzyme to determine the lactose in milk, and it
is also used in the diagnosis and treatment of lactose intolerance.
The inability to digest lactose in the small intestine due to a low
β-galactosidase activity known as lactose intolerance, is
associated with clinical symptoms such as nausea, bloating,
flatulence and/or stomach discomfort after consuming lactose or
dietary components containing lactose; this may affect everyday
activities and the quality of life of individuals with this
condition (5,7). The encapsulation of the enzyme may be
the strategy for the production of lactose-hydrolyzed milk
(8,9). The first stage in isolating the
intracellular enzyme, β-galactosidase, from LAB, is to interrupt
the cell by various mechanical and chemical methods (10). In addition, the enzyme extracted
from Aspergillus niger (A. niger) is useful in the
commercial production of an array of sugars, such as glucose,
galactose, heteropolysaccharides, galacto-oligosaccharide. The
β-galactosidase enzyme from A. niger has undergone analyses
regarding comparative modeling, structural annotation, domain
identification and structural comparison. Additionally, the
catalytic sites, Glu200 and Glu298, with β-D-galactose were docked
independently (11-14).
The main objective of the present study was to
produce β-galactosidase from LAB and a selection of
Lactiplantibacillus isolates producing potential
β-galactosidase. By biochemical analysis [with
5-bromo-4-chloro-3-indolyl β-D-galactopyranoside (X-Gal) and
O-nitrophenyl-β-D-galactopyranoside (ONPG) as substrates]
and sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE), the enzyme was characterized. For enzyme optimization,
physical conditions (pH and temperature), the effects of metal ions
and kinetics with various ONPG substrate concentrations were
examined. In addition, the construction of a 3D structure,
structural annotation and structural comparison of the
β-galactosidase enzyme was determined.
Materials and methods
Isolation and screening of LAB. A total of 16
homemade curd samples were collected from rural areas in Davangere,
Karnataka, India. The de Man, Rogosa and Sharpe (MRS) agar medium
(Himedia Laboratories Pvt., Ltd.) was used for the isolation of LAB
and incubated at 37˚C for 24 to 48 h. The isolated colonies were
purified by several successive streaks on MRS medium and the
selected bacterial isolates were preserved at 4˚C on MRS slants
until further use. The ability of the bacterial isolate to produce
β-galactosidase was examined using the X-Gal (Himedia Laboratories
Pvt., Ltd.) substrate supplemented with MRS medium and isopropyl
β-D-1-thiogalactosidase (IPTG) (Himedia Laboratories Pvt., Ltd.) as
an inducer for β-galactosidase. The production of blue-colored
colonies indicated the production of β-galactosidase and were
selected for further analyses. In another study (15), the authors isolated and screened 27
β-galactosidase-producing isolates, further subjected to
morphological and physiological characterization. Furthermore, the
eight most potential probiotic β-galactosidase-producing LAB were
identified using 16S rRNA gene sequencing. All the eight isolates
belong to the phylum Firmicutes, exhibiting the highest similarity
with Lactobacillus spp. Amongst these, four LAB producing
high concentrations of β-galactosidase were selected for use in the
present study.
Production of β-galactosidase enzyme. For the
production of β-galactosidase, MRS culture medium was supplemented
with 4% lactose. The selected bacterial culture was inoculated in
100 ml MRS medium and incubated at 37˚C for 48 h (200 x g). The
bacterial cells were further harvested at 12,000 x g for 5 min at
4˚C and suspended in phosphate buffer (pH 6.8) to measure the
activity of β-galactosidase, as previously described (16).
Optimization of β-galactosidase using different
carbon and nitrogen sources. The effects of media composition
on β-galactosidase production were determined by using different
carbon sources, such as arabinose, fructose, glucose, galactose,
lactose, sucrose, mannose and xylose. Additionally, ammonium
chloride, ammonium nitrate, ammonium sulfate, peptone, casein,
urea, yeast extract, beef extract, sodium and potassium nitrate
(Himedia Laboratories Pvt., Ltd.) were used as nitrogen sources for
the optimization of production media.
Extraction and partial purification of
intracellular β-galactosidase. The extraction of enzyme was
performed using the cell permeabilized method with the
toluene/acetone solvent system. A total of 10 ml bacterial culture
was treated with 0.1 ml toluene/acetone (1:9 v/v) (Himedia
Laboratories Pvt., Ltd.) and vortexed for 7 min, as previously
described (15). The cell-free
supernatant was precipitated with ammonium sulfate (Himedia
Laboratories Pvt., Ltd.) using a magnetic stirrer until it reached
a saturation level of 70% (w/v). That precipitate was then
centrifuged at 12,000 x g, for 10 min at 4˚C using 0.1 M phosphate
buffer (Na2HPO4 x 7H2O/40 mM
NaH2PO4 buffer pH 7.0) and the obtained
precipitate was dialyzed overnight against a phosphate buffer
(Himedia Laboratories Pvt., Ltd.).
SDS-PAGE. A 12% SDS-PAGE was used as the
resolving gel for the enzyme analysis. A pre-stained protein ladder
(MBT092-10LN) was purchased from HiMedia Laboratories Pvt., Ltd.
The gel was run for 4 h at 110 V and the enzyme bands were
visualized by staining with 0.25% Coomassie brilliant blue G-250 at
28˚C for 1 h (Himedia Laboratories Pvt., Ltd.) and de-stained with
a mixture of 10% acetic acid and 40% methanol (Himedia aboratories
Pvt., Ltd.).
β-Galactosidase assay. For the
β-galactosidase assay, the reaction mixture consisted of 200 µl
ONPG and 100 µl of the extracted enzyme with 900 µl of 60 mM
phosphate buffer (Himedia Laboratories Pvt., Ltd.) (pH 7).
Subsequently, 0.5 ml of 1 M sodium carbonate (NaCO3) was
added to the reaction mixture and incubated for 15 min at 37˚C to
terminate the reaction, which was then measured at 420 and 560 nm
using a NanoDrop 2000C UV-Spectrophotometer (Thermo Fisher
Scientific, Inc.) and β-galactosidase activity was represented in
Miller units as follows:
where, A1560 was the absorbance value of
the reaction mixture prior to incubation, and A1420 and
A2560 were the absorbance values of the reaction mixture
following incubation.
Characterization of β-galactosidase
enzyme
Effects of pH and temperature. The effects of
pH and temperature on intracellular β-galactosidase were determined
using ONPG as a substrate. The reaction mixture containing
partially purified β-galactosidase with 15 mM ONPG and various pH
levels (pH 5.0 to 9.0) was adjusted (pH 5 0.1 M sodium acetate
buffer, pH 6-7 0.1 M sodium phosphate buffer, and pH 8-9 0.1 M Tris
HCl buffer), and further incubated at 37˚C for 15 min. Similarly,
β-galactosidase was incubated for 15 min at various temperatures
30, 35, 37, 40, 45 and 50˚C, set up to determine the maximum enzyme
activity.
Effects of various metal ions. To examine the
effects of various cations on β-galactosidase activity (release of
O-nitrophenol from ONPG), the enzyme samples were assayed
with ONPG in the presence 1.0 mM of various metal ions [sodium
chloride (NaCl), potassium chloride (KCl), silver nitrate
(AgNO3), magnesium sulfate (MgSO4), calcium
chloride (CaCl2), copper sulfate (CuSO4),
manganese(II) chloride (MnCl2) and ferrous sulfate
(FeSO4) compound form] and the enzyme activity was
determined as mentioned above.
Kinetics analysis. Enzyme kinetics were
determined using ONPG as a substrate range between 1-22 mM at pH
7.0, and Lineweaver-Burk plot (1/V against 1/S) was used to measure
the maximum velocity (Vmax) and kinetic constant
(Km) using following equation:
3D modeling and structural annotation of
β-galactosidase
Sequence retrieval. The National Centre for
Biotechnology Information's GenPept database was used to identify
the amino acid sequence of the β-galactosidase enzyme from
Lactiplantibacillus plantarum in FASTA format (L.
plantarum; http://www.ncbi.nlm.nih.gov/protein/; https://www.ncbi.nlm.nih.gov/genbank/fastaformat/).
Comparative modeling and structural
annotation. An important and promising field of study is the
prediction of protein structure via comparative modeling (13), using the 3D jigsaw server for the
comparative modeling of the target protein, and the SAS-sequence
annotated by the structure server (http://www.ebi.ac.uk/thornton-srv/databases/sas/),
which was used to characterize the structural annotation. The
predicted structure was visualized using the PyMol program.
(https://pymol.org/2/).
ProFunc, a database of protein functions from the
Thornton Structure Server (http://www.ebi.ac.uk/thornton-srv/databases/ProFunc/),
and PDBsum, a visual representation of 3D protein structures from
the Protein Data Bank server (http://www.ebi.ac.uk/pdbsum/), are both accessible
through the European Bioinformatics Institute was also used.
Structural comparison. The similarities and
contrasts of related homologous-3D structures are frequently
emphasized using protein structural comparisons. Although they
share a common ancestor protein, homologous proteins, although
multiplied, develop along different pathways, and are throughout
time (13,14). In order to identify comparable
structures in other organisms, a structural comparison of the
modeled structure was carried out using the Dali server (http://ekhidna2.biocenter.helsinki.fi/dali/) in the
PDB database (14).
Statistical analysis. All results are
reported as the mean ± standard error of mean and GraphPad Prism 9
software (GraphPad Software; Dotmatics) was used to compare data
using one-way ANOVA with post hoc Tukey's multiple comparisons
test. 3D structure analyses were performed using EMBL-EBI database
software.
Results
A total of 190 LAB were isolated from 16 different
homemade curd samples and screened for β-galactosidase production
using X-Gal substrate. A total of 27 isolates exhibited blue
coloration on MRS agar medium supplemented with the X-Gal
substrate. The data presented in Table
I revealed that L. plantarum GV54 (56.32±0.6 Miller
units; P<0.05) and L. plantarum GV64 (48.35±1.63 Miller
units; P<0.05) significantly increased β-galactosidase
production by utilizing lactose as a carbon source compared with
other different carbon sources tested. L. plantarum GV54
exhibited maximum enzyme production by utilizing ammonium sulfate
and beef extract (31.12±0.05 and 28.45±0.95 Miller units; P<0.05
respectively) as two nitrogen sources compared with other different
nitrogen sources tested (Table
II). β-galactosidase was extracted using the cell
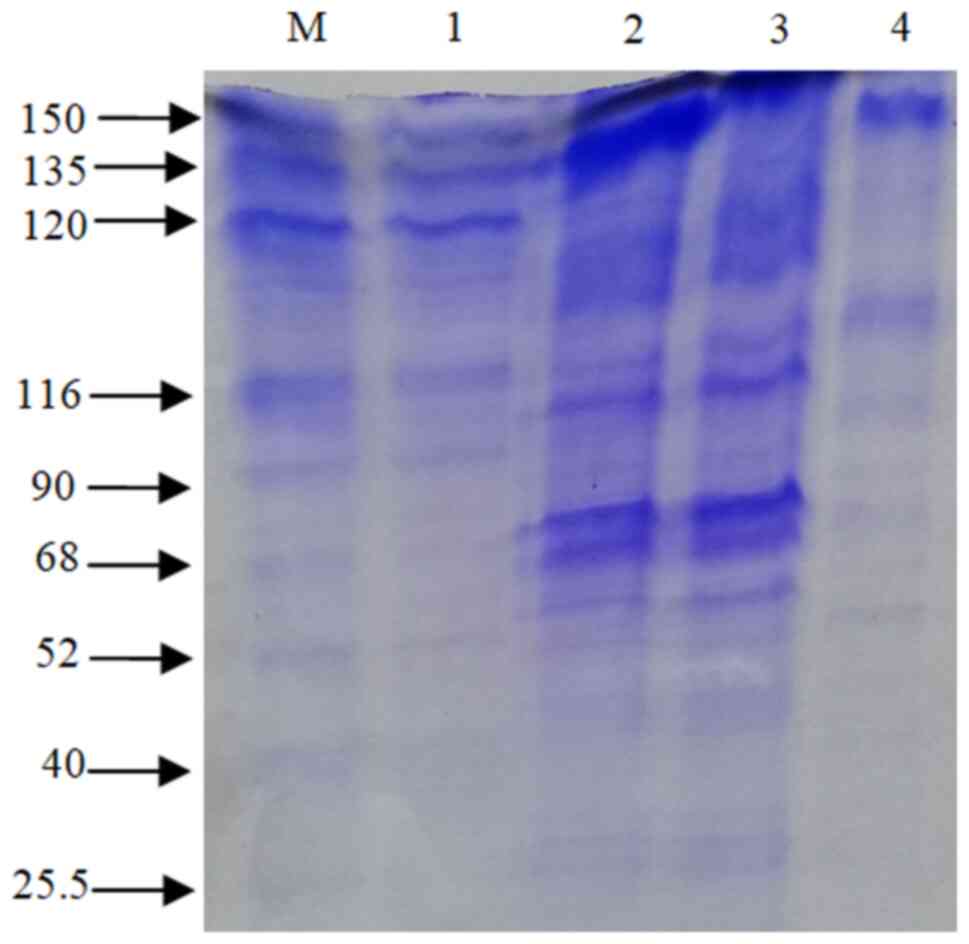
permeabilization method with toluene/acetone solvents. SDS-PAGE
(12%) was used for band separation and the molecular weight was
determined (~116 kDa) (Fig. 1).
The β-galactosidase activity of L. plantarum GV54 was
comparably high in both qualitative and quantitative assays. The
maximum enzyme activity (162.96±0.36 Miller units; P<0.05) was
exhibited by the L. plantarum GV54 isolate compared to the
other isolates tested (Table
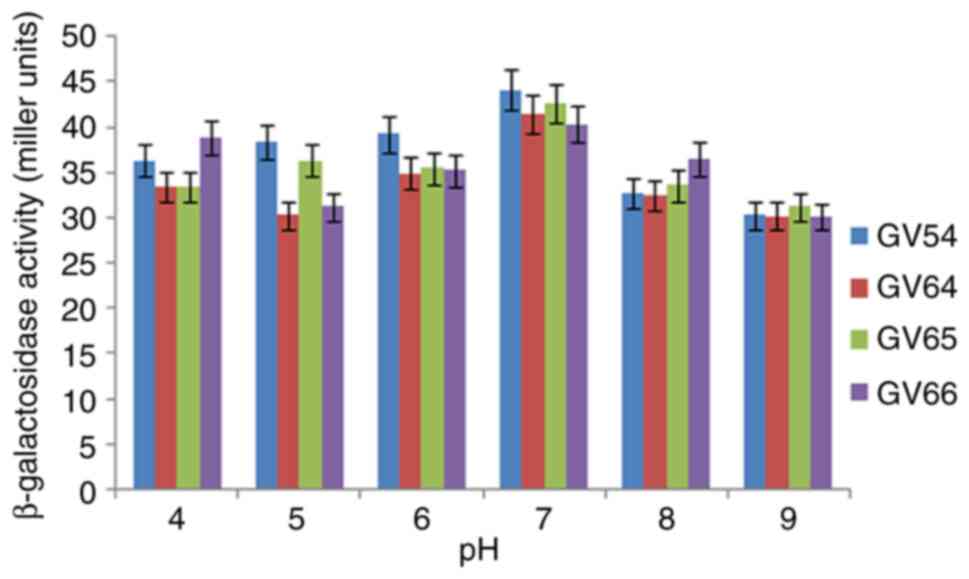
III). As illustrated in Figs.
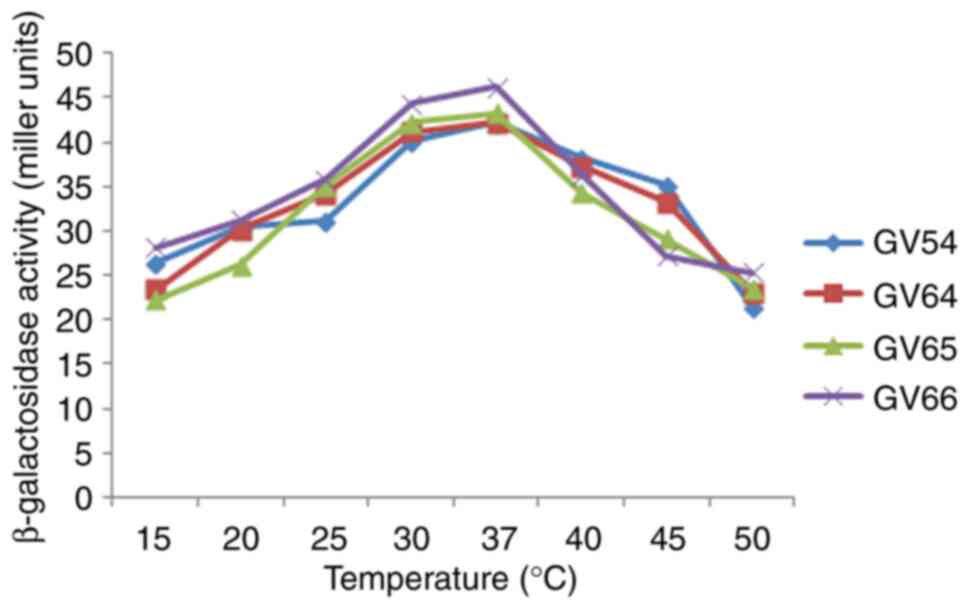
2 and 3, β-galactosidase
activity was high at pH 7.0 and 37˚C. The potential of various
cations to either stimulate or inhibit the enzyme activity was
investigated. As per the data presented in Table IV, the addition of
Mg2+, Mn2+, and Fe2+ had an
indirect effect on the relative enzymatic activity, and
Mn2+ was shown to lead to the maximum relative enzymatic
activity. However, the addition of Ag+ decreased the
enzyme activity. However, these results need to be validated in the
future using in vivo animal model studies or human clinical
trials in collaboration with health departments.
 | Table IEffect of carbon sources on
intracellular β-galactosidase production by
Lactiplantibacillus isolates. |
Table I
Effect of carbon sources on
intracellular β-galactosidase production by
Lactiplantibacillus isolates.
| | β-galactosidase
activity (Miller units) |
|---|
| Carbon sources | GV54 | GV64 | GV65 | GV66 |
|---|
| Lactose | 56.32±0.6 | 48.35±1.63 | 43.48±1.43 | 43.85±1.33 |
| Sucrose | 38.35±0.55 | 30.22±1.69 | 36.33±1.66 | 31.20±1.46 |
| Glucose | 39.24±1.18 | 34.91±1.31 | 35.48±1.32 | 35.28±1.34 |
| Galactose | 20.12±0.92 | 21.45±1.30 | 22.51±0.72 | 24.32±0.75 |
| Fructose | 32.65±1.05 | 22.43±1.36 | 23.54±1.65 | 16.44±0.56 |
| Mannose | 28.25±0 .20 | 27.20±1.32 | 14.22±1.65 | 16.07±1.35 |
| Xylose | 25.35±1.90 | 23.45±1.39 | 19.14±1.90 | 17.15±1.56 |
| Arabinose | 28.50±1.85 | 23.10±0.65 | 23.50±0.56 | 15.30±0.56 |
 | Table IIEffect of nitrogen sources on
intracellular β-galactosidase production by
Lactiplantibacillus isolates |
Table II
Effect of nitrogen sources on
intracellular β-galactosidase production by
Lactiplantibacillus isolates
| | β-galactosidase
activity (Miller units) |
|---|
| Nitrogen
sources | GV54 | GV64 | GV65 | GV66 |
|---|
| Yeast extract | 22.12±0.4 | 15.45±1.53 | 19.28±1.23 | 16.18±1.23 |
| Peptone | 24.45±0.75 | 25.60±1.86 | 23.20±1.46 | 25.10±1.56 |
| Casein | 27.34±0.78 | 22.45±1.30 | 21.28±1.12 | 19.28±1.30 |
| Urea | 15.54±0.42 | 15.50±0.90 | 17.30±0.81 | 17.20±0.80 |
| Beef extract | 28.45±0.95 | 18.57±1.26 | 16.34±1.46 | 15.27±0.76 |
|
NH4SO4 | 31.12±0 .05 | 29.30±1.25 | 18.20±1.74 | 12.50±1.55 |
|
NH4NO3 | 18.25±1.50 | 16.91±1.96 | 13.21±1.86 | 12.31±1.66 |
|
NH4Cl | 19.50±1.45 | 16.00±0.60 | 19.20±0.43 | 13.30±0.36 |
 | Table IIIβ-galactosidase activity of
Lactiplantibacillus isolates. |
Table III
β-galactosidase activity of
Lactiplantibacillus isolates.
| Isolate name | β-galactosidase
activity (Miller units) |
|---|
| GV54 |
162.96±0.36a |
| GV55 |
124.91±0.15a |
| GV57 |
127.78±0.01a |
| GV64 |
158.16±2.12a |
| GV65 |
117.26±0.37a |
| GV66 |
112.64±0.6a |
| GV68 |
78.79±0.56a |
| GV69 | 68.5±0.14 |
| GV254 | 75±2.5a |
| GV418 |
93.94±6a |
| GV419 |
96.59±11.9a |
 | Table IVEffect of metals ions on
β-galactosidase activity by Lactiplantibacillus
isolates. |
Table IV
Effect of metals ions on
β-galactosidase activity by Lactiplantibacillus
isolates.
| | β-galactosidase
activity (Miller units) |
|---|
| Metal ions | GV54 | GV64 | GV65 | GV66 |
|---|
| NaCl | 76.40±3.46 | 75.25±1.90 | 77.15±2.75 | 66.25±2.0 |
| KCl | 91.20±3.50 | 97.34±3.21 | 85.54±2.80 | 78.24±2.31 |
|
AgNO3 | 23.12±3.25 | 35.76±2.50 | 35.44±1.80 | 26.56±1.90 |
|
MgSO4 | 114.30±3.78 | 142.54±3.53 | 135.23±2.33 | 123.54±2.13 |
|
CaCl2 | 71.30±3.96 | 60.30±2.75 | 65.30±2.90 | 56.40±2.4 |
|
CuSO4 | 33.90±2.50 | 43.51±1.40 | 56.12±1.20 | 34.41±0.4 |
|
MnCl2 | 143.23±1.24 | 123.30±0.40 | 120.10±0.15 | 110.00±0.01 |
|
FeSO4 | 128.34±3.45 | 108.0±3.71 | 129.12±3.25 | 101.0±3.12 |
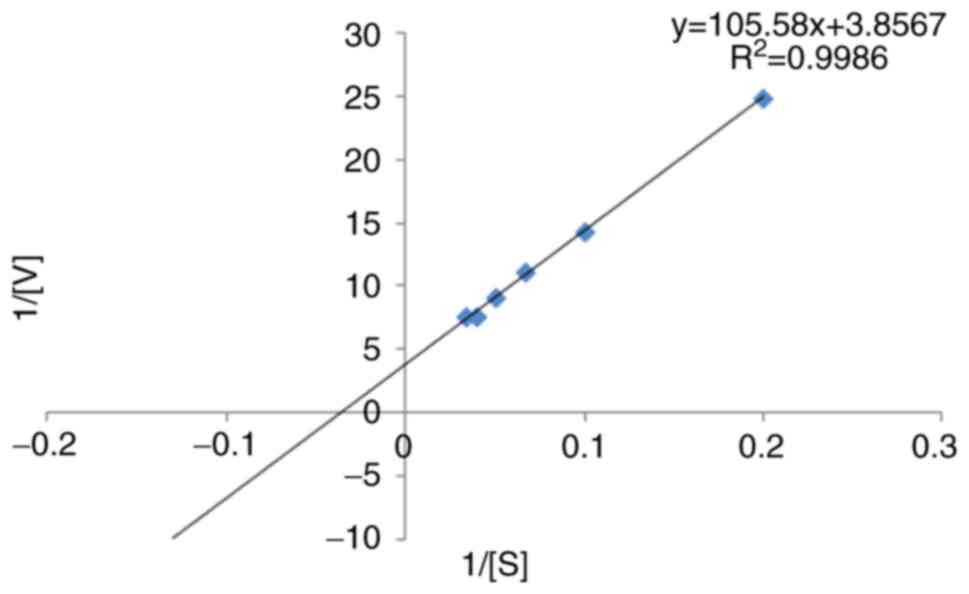
The kinetic parameters of β-galactosidase with the
ONPG substrate were determined using the Lineweaver-Burk plot. The
Km was found to be 27.3757 mM, and its Vmax
was 0.2592 U/min (Fig. 4). Using
the FASTA format, the amino acid sequence of β-galactosidase
(accession no. KZD98639.1) was retrieved, which was isolated from
L. plantarum. By employing a comparison modeling method, the
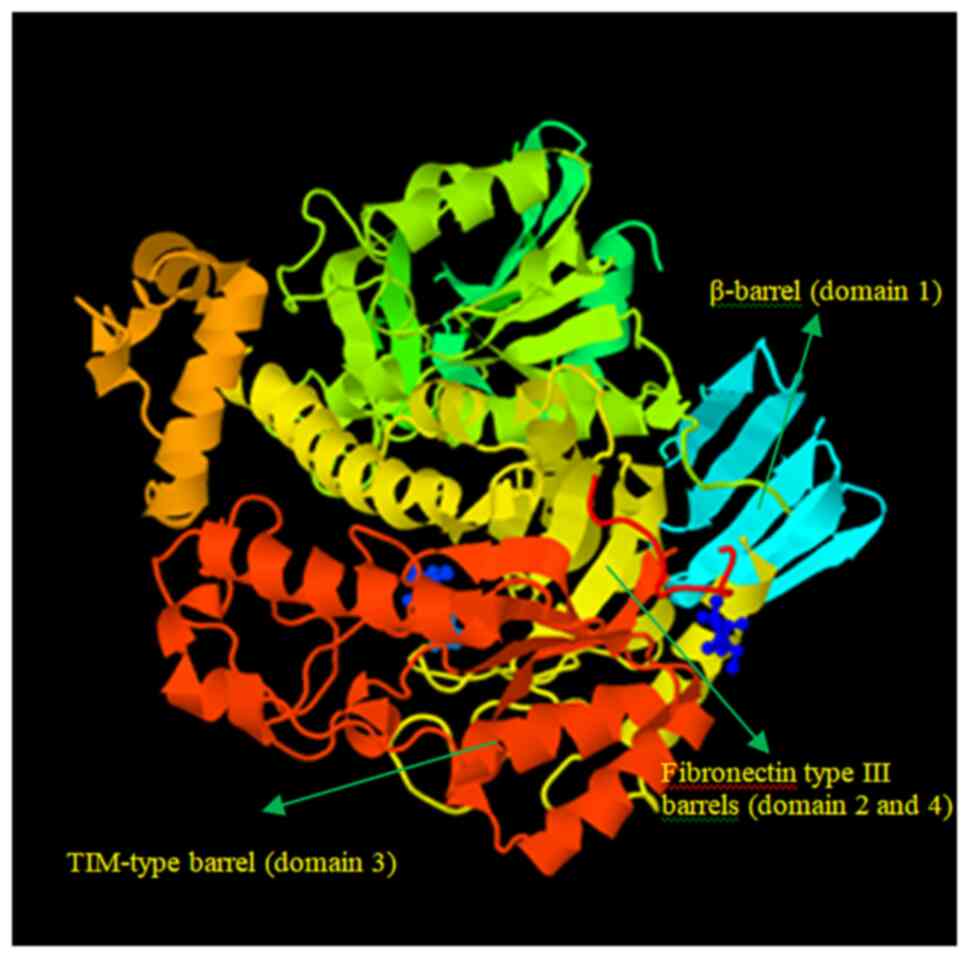
3D jigsaw server produced the 3D structure of the target protein.
The PyMOL application was used to view the anticipated structure
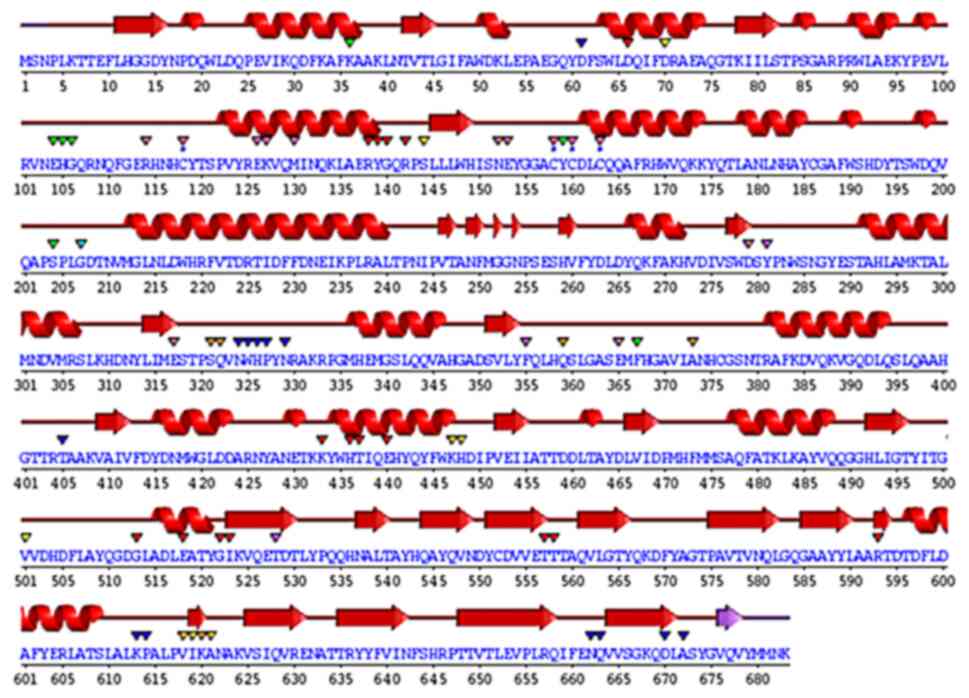
(Fig. 5). The wiring diagram
presented in Fig. 6 was produced
by the SAS server. The crystal structure of Bifidobacterium
bifidum β-galactosidase in the PDB database shared the most
similarities with the modeled structure when structural comparisons
were made.
Discussion
The present study revealed that
Lactiplantibacillus isolates may be a promising source of
β-galactosidase enzyme. The isolate stabilized throughout a pH
range of 4 to 9 and exhibited the highest β-galactosidase activity
at pH 7. The different types and quantities of carbon and nitrogen
sources in the culture media play a crucial role in β-galactosidase
production. Gomaa (17) reported
that the maximum output of β-galactosidase was produced utilizing
wheat flour as the carbon source for the β-galactosidase synthesis
by Lactobacillus delbrueckii and Lactobacillus
reuteri. Lactobacillus delbrueckii generated the most
enzymes from beef extract (24.25 U/ml), while Lactobacillus
reuteri produced the most β-galactosidase (20.20 U/ml) when
peptone was present, while β-galactosidase activity was reported at
40˚C and pH 7. Nguyen et al (18) demonstrated that the optimal pH
range for lactose hydrolysis and the temperature for ONPG
β-galactosidase activity were 6.5-8 and 55˚C, respectively. Genetic
variations among distinct enzyme-producing microbial strains may
account for the variations in their dietary needs (19). It was evident that certain
biochemical molecules are perhaps essential for the high-level
production of this enzyme. A number of biomolecules, such as amino
acids, nucleic acids and enzymes are produced by a vast group of
microorganisms by the metabolism of both inorganic/organic forms
nitrogen (20). β-galactosidase is
a tetrameric enzyme with four identical subunits and a molecular
weight of 116 kDa (21). In the
present study, using the SDS-PAGE technique, the β-galactosidase
enzyme exhibited an intense ~116 kDa protein band, which is in
agreement with the findings of other studies. Elmira et al
(1) demonstrated that the 116 kDa
protein band was found in Lactobacillus delbrueckii, using
SDS-PAGE. Nguyen et al (18), using gel electrophoresis, revealed
that the enzyme was a heterodimer comprised of two subunits of 35
and 72 kDa and the activity of β-galactosidase was 158 U/ml
extracted from Lactobacillus reuteri L103 compared with
Lactobacillus casei subsp. rhamnosus (22.7 U/ml). Elmira
et al (1) demonstrated that
the Lactobacillus delbrueckii had the highest enzymatic
value (1,966 U/ml). Furthermore, a pH 7 has been shown to be ideal
for mesophilic β-galactosidase production (22). In the present study, the
Km value of β-galactosidase activity was found to be
27.37 mM, estimated from L. plantarum GV54 compared with
other values.
Gomaa (17) also
reported that the optimum β-galactosidase activity was recorded at
15 mM ONPG at 40˚C and pH 7. The enzyme activity was further
enhanced in the presence of Mg2+, Mn2+and
Fe2+, while it was decreased by Ag+ and
Ni+ for both the Lactobacillus delbrueckii and
Lactobacillus reuteri strains. In the present study,
Mn2+ was shown to have the highest relative enzymatic
activity, and the addition of Mg2+, Mn2+ and
Fe2+ had an indirect effect on the relative enzymatic
activity. The enzyme activity was nonetheless reduced by the
presence of Ag+. In the present study, using the
Lineweaver-Burk plot, the kinetic parameters of β-galactosidase
using the ONPG substrate were determined. It was found that the
Km was 27.3757 mM and the Vmax was 0.2592
U/min. Nguyen et al (18)
deemonstrated that, in terms of D-glucose released per minute per
mg of protein, lactose and O-nitrophenol had Km
and Vmax values of 4.04±0.26 mM and 28.8±0.2 mmol, and
0.73±0.07 mM and 361 12 mmol, respectively. Di Serio et al
(23) demonstrated the kinetics of
β-galactosidase from Kluyveromices marxianus lactis, and a
kinetic model based on the Michaelis-Menten mechanism was created,
and the associated parameters were calculated.
β-galactosidase is used as a biosensor to estimate
lactose present in milk and in the production of lactose-hydrolyzed
milk for the treatment of lactose intolerance (8,9).
Bioinformatics is an essential tool for the construction of the
β-galactosidase structure, identification, localization, function
and modification (12). In the
present study, the 3D structure of β-galactosidase was constructed
by amino acid sequences retrieved from L. plantarum
exhibiting maximum resemblance with the structure of
β-galactosidase from Bifidobacterium bifidum. This led to
the obvious conclusion that the functional range of the L.
plantarum enzyme perhaps expanded. Additionally, it has been
well-documented that the enzyme in L. plantarum functions as
a ligand for β-D-galactose. Ghosh et al (12) reported that the high-resolution
experimental structure of the same enzyme from Penicillium
sp. was similar to the modeled structure of β-galactosidase
from A. niger. Thus, it can be inferred that the functioning
of the A. niger enzyme may be expanded. Additionally, the
enzyme in A. niger responds to β-D-galactose as a ligand
(12). The modeled structure had
five domains that were located in various residue locations. In the
first domain of the modeled structure, Glu200 and Glu298 were found
to be two catalytic residues. The structure was molecularly docked
with β-D-galactose as well (12).
Bifidobacterium bifidum is widely used as a
commercial probiotic in the food industry. The ability of this
bacterial group to utilize short-chain β-galactosides, such as
galacto-oligosaccharides (GOS) as an energy source is a key factor
for the upregulation of the bacterial population levels in the
human intestine (14). In fact,
>8% of the genome of Bifidobacteria is related to carbohydrate
metabolism; however, the molecular level understanding of the
complexity of their metabolism remains elusive. In the process of
hydrolysis, a water molecule plays the role of the electron
acceptor necessary for break the covalent bond. Eventually, the
acceptor of the reaction can also be the hydroxyl group of another
sugar, such as glucose, for example, leading to the formation of
GOS (22). The latter saccharides
are classified as prebiotic sugars due to their ability to promote
the growth of beneficial bacteria in the gut (9). In the human gastrointestinal tract,
GOS are the fermentable substrates for probiotic organisms.
Bifidobacteria compose almost 4.5% of the total gastrointestinal
microbiota, and are known for their ability to utilize GOS,
fructooligosaccharides, pectin and mucin as substrates (14). The crystal structures of the GH42
β-galactosidase BbgII from Bifidobacterium sp, a probiotic
bacteria, for D-α-galactose, docking, as well as molecular dynamics
simulations have elucidated that the enzyme is recognized,
specifically and tightly bound to D-α-galactose (14). Using these data, the 3D structure
of the amino acid sequence of β-galactosidase from L.
plantarum was constructed. Considering the aforementioned
characteristics, L. plantarum GV54 was considered the most
efficient β-galactosidase producer for food and dairy technological
applications. However, as the structure of β-galactosidase is
predictive and not yet experimentally determined, there is thus a
need to determine the protein sequence analysis of L.
plantarum GV54.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MV executed the experimental work, prepared the
manuscript and was involved in the interpretation and analysis of
the data. DG conceptualized and designed the study, and was
involved in the acquisition, analysis, or interpretation of data
for the study. DG drafted the manuscript and revised it critically
for important intellectual content. MV and DG confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Elmira G, Fariba H, Bahareh KS, Jamileh N
and Farahnaz M: Study on β-galactosidase enzyme produced by
isolated lactobacilli from milk and cheese. Afri J Microbiol Res.
4:454–458. 2010.
|
|
2
|
Balamurugan R, Chandragunasekaran AS,
Chellappan G, Rajaram K, Ramamoorthi G and Ramakrishna BS:
Probiotic potential of lactic acid bacteria present in homemade
curd in southern India. Indian J Med Res. 140:345–355.
2014.PubMed/NCBI
|
|
3
|
Jurado E, Camacho F, Luzon G and Vicaria
JM: A new kinetic model proposed for enzymatic hydrolysis of
lactose by a β-galactosidase from Kluyveromyces fragilis. Enzyme
Microb Technol. 31:300–309. 2002.
|
|
4
|
Sriphannam W, Lumyong S, Niumsap P, Ashida
H, Yamamoto K and Khanongnuch C: A selected probiotic strain of
Lactobacillus fermentum CM33 isolated from breast-fed infants as a
potential source of β-galactosidase for prebiotic oligosaccharide
synthesis. J Microbiol. 50:119–126. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heyman MB: The committee on nutrition.
Lactose intolerance in infants, children, and adolescents. J
Pediatrics. 118:1297–1286. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oliveira C, Guimarães PM and Domingues L:
Recombinant microbial systems for improved β-galactosidase
production and biotechnological applications. Biotechnol Adv.
29:600–609. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vasiljevic T and Jelen P: Production of
β-galactosidase for lactose hydrolysis in milk and products using
thermophilic lactic acid bacteria. J Innov Food Sci Emerg Technol.
2:75–85. 2001.
|
|
8
|
Staiano M, Bazzicalupo P, Rossi M and
D'Auria S: Glucose biosensors as models for the development of
advanced proteinbased biosensors. Mol Biosyst. 1:354–362.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Mlichova´ Z and Rosenberg M: Current
trends of β-galactosidase application in food technology. J Food
Nutr Res. 2:47–54. 2006.
|
|
10
|
Selvarajan E and Mohanasrinivasan V:
Kinetic studies on exploring lactose hydrolysis potential of β
galactosidase extracted from Lactobacillus plantarum HF571129. J
Food Sci Technol. 52:6206–6217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Holm L and Rosenström P: Dali server:
Conservation mapping in 3D. Nucleic Acids Res. 38:W545–W549.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghosh SK, Pandey A, Arora S and Dwivedi
VD: Comparative modeling and docking studies of β-galactosidase
from Aspergillus niger. Netw Model Anal Health Inform Bioinform.
2:297–302. 2013.
|
|
13
|
Dwivedi VD, Arora S and Pandey A:
Computational analysis of physico-chemical properties and homology
modeling of carbonic anhydrase from Cordyceps militaris. Netw Model
Anal Health Inform Bioinforma. 2:209–212. 2013.
|
|
14
|
Godoy AS, Camilo CM, Kadowaki MA, Muniz
HD, Santo MS, Murakami MT, Nascimento AS and Polikarpov I: Crystal
structure of β1→ 6-galactosidase from Bifidobacterium bifidum S17:
trimeric architecture, molecular determinants of the enzymatic
activity and its inhibition by α-galactose. FEBS J. 283:4097–4112.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vasudha M, Prashantkumar CS, Mallika B,
Vishwas K and Gayathri D: Probiotic potential of
β-galactosidase-producing Lactic Acid Bacteria for Lactose
intolerance from fermented milk and their molecular
characterization. Biomed Rep (In Press).
|
|
16
|
Vinderola CG and Reinheimer JA: Lactic
acid starter and probiotic bacteria: A comparative ‘in vitro’ study
of probiotic characteristics and biological barrier resistance.
Food Res Int. 36:895–904. 2003.
|
|
17
|
Gomaa EZ: β-galactosidase from
Lactobacillus delbrueckii and Lactobacillus reuteri: Optimization,
characterization and formation of galactooligosaccharides. Indian J
Biotechnol. 17:407–415. 2018.
|
|
18
|
Nguyen TH, Splechtna B, Steinböck M,
Kneifel W, Lettner HP, Kulbe KD and Haltrich D: Purification and
characterization of two novel β-galactosidases from Lactobacillus
reuteri. J Agric Food Chem. 54:4989–4998. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hall AB, Tolonen AC and Xavier RJ: Human
genetic variation and the gut microbiome in disease. Nat Rev Genet.
18:690–699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akcan N: High-level production of
extracellular β-galactosidase from Bacillus licheniformis ATCC
12759 in submerged fermentation. Afr J Microbiol Res. 5:4615–4621.
2011.
|
|
21
|
Białkowska AM, Cieśliński H, Nowakowska
KM, Kur J and Turkiewicz M: A new β-galactosidase with a low
temperature optimum isolated from the Antarctic Arthrobacter
sp. 20B: Gene cloning, purification and characterization. Arch
Microbiol. 191:825–835. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iqbal S, Nguyen TH, Nguyen HA, Nguyen TT,
Maischberger T, Kittl R and Haltrich D: Characterization of a
heterodimeric GH2 β-galactosidase from Lactobacillus sakei Lb790
and formation of prebiotic galacto-oligosaccharides. J Agric Food
Chem. 59:3803–3811. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Di Serio M, Maturo C, De Alteriis E,
Parascandola P, Tesser R and Santacesaria E: Lactose hydrolysis by
immobilized β-galactosidase: The effect of the supports and the
kinetics. Catalysis Today. 79:333–339. 2003.
|