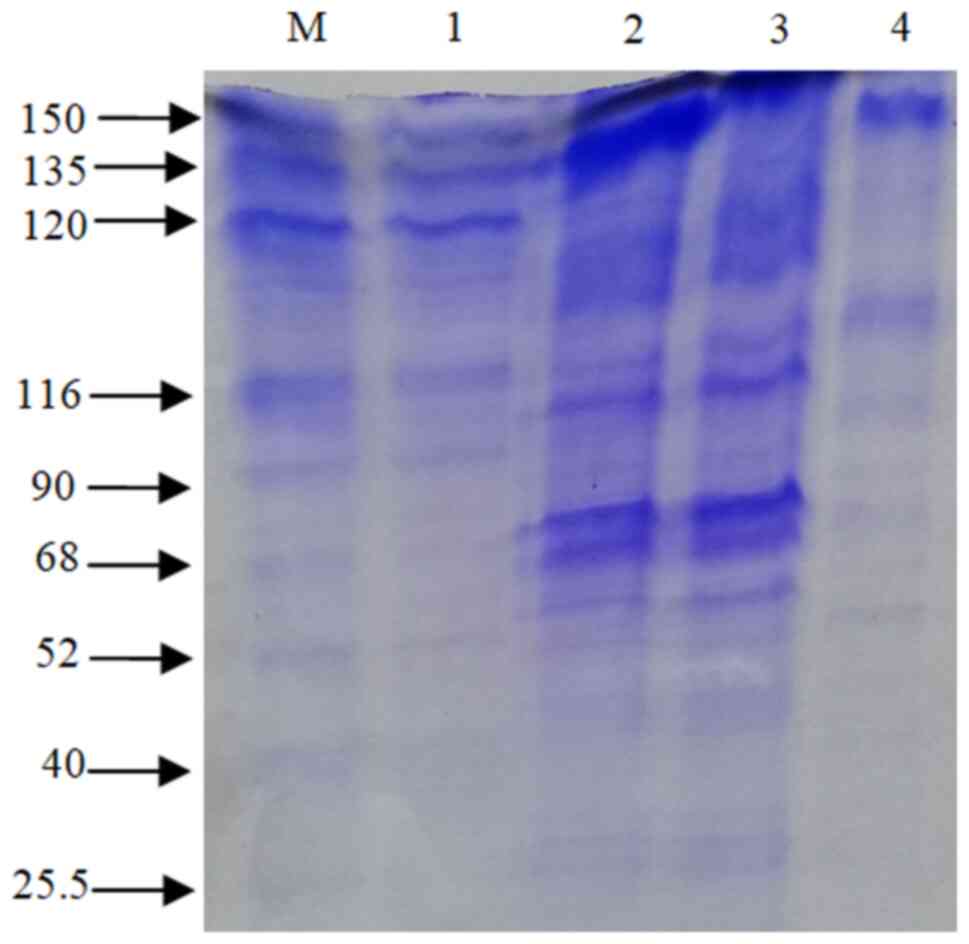
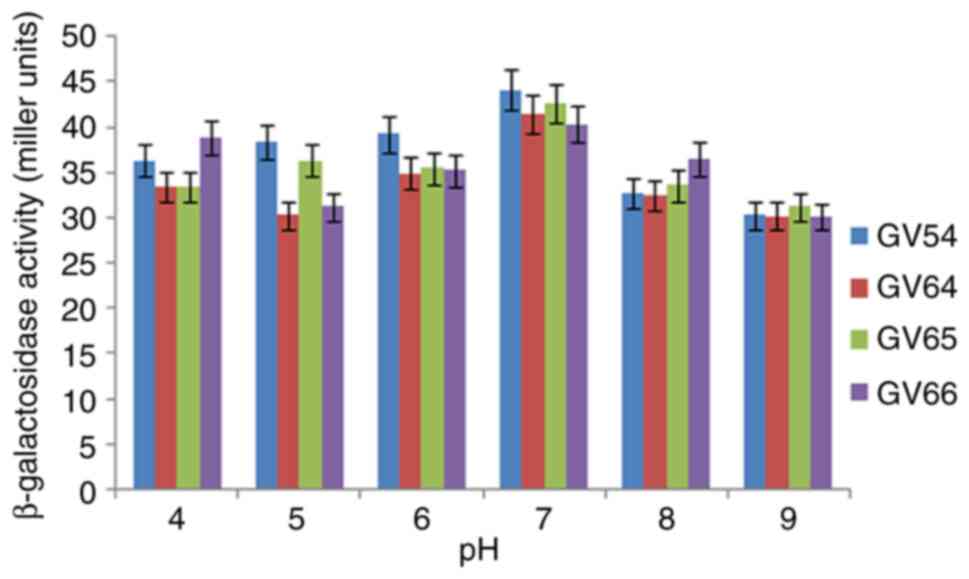
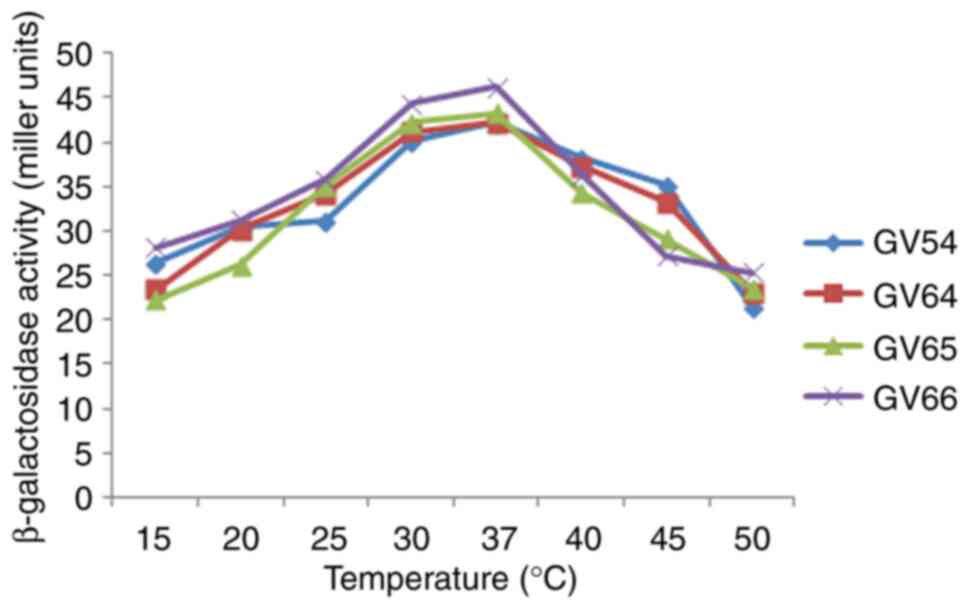
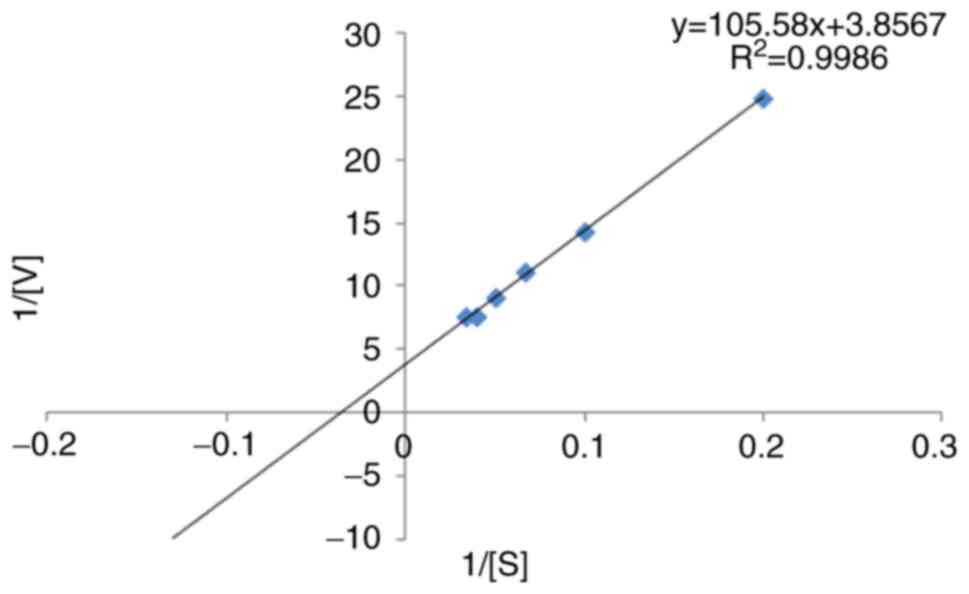
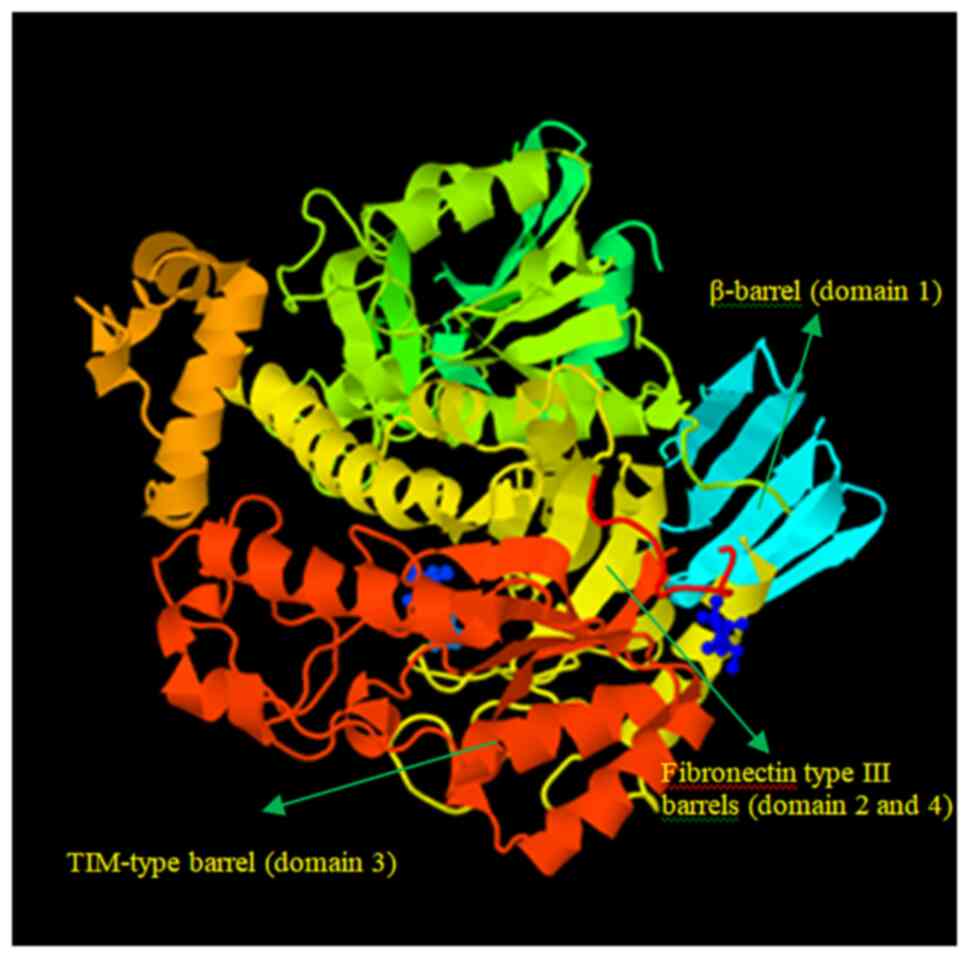
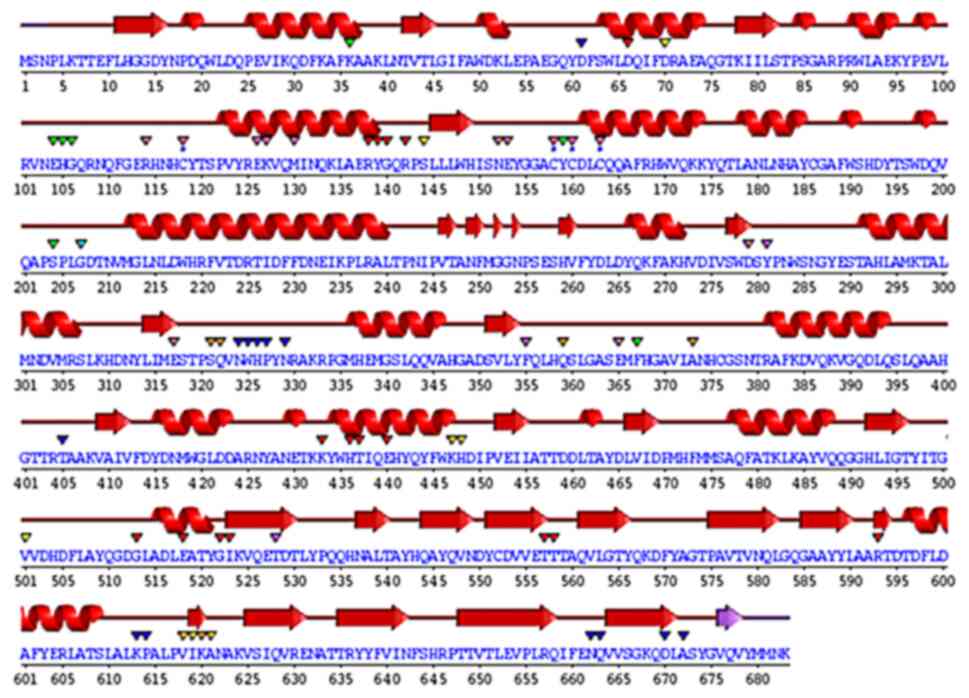
|
1
|
Elmira G, Fariba H, Bahareh KS, Jamileh N
and Farahnaz M: Study on β-galactosidase enzyme produced by
isolated lactobacilli from milk and cheese. Afri J Microbiol Res.
4:454–458. 2010.
|
|
2
|
Balamurugan R, Chandragunasekaran AS,
Chellappan G, Rajaram K, Ramamoorthi G and Ramakrishna BS:
Probiotic potential of lactic acid bacteria present in homemade
curd in southern India. Indian J Med Res. 140:345–355.
2014.PubMed/NCBI
|
|
3
|
Jurado E, Camacho F, Luzon G and Vicaria
JM: A new kinetic model proposed for enzymatic hydrolysis of
lactose by a β-galactosidase from Kluyveromyces fragilis. Enzyme
Microb Technol. 31:300–309. 2002.
|
|
4
|
Sriphannam W, Lumyong S, Niumsap P, Ashida
H, Yamamoto K and Khanongnuch C: A selected probiotic strain of
Lactobacillus fermentum CM33 isolated from breast-fed infants as a
potential source of β-galactosidase for prebiotic oligosaccharide
synthesis. J Microbiol. 50:119–126. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Heyman MB: The committee on nutrition.
Lactose intolerance in infants, children, and adolescents. J
Pediatrics. 118:1297–1286. 2006.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oliveira C, Guimarães PM and Domingues L:
Recombinant microbial systems for improved β-galactosidase
production and biotechnological applications. Biotechnol Adv.
29:600–609. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Vasiljevic T and Jelen P: Production of
β-galactosidase for lactose hydrolysis in milk and products using
thermophilic lactic acid bacteria. J Innov Food Sci Emerg Technol.
2:75–85. 2001.
|
|
8
|
Staiano M, Bazzicalupo P, Rossi M and
D'Auria S: Glucose biosensors as models for the development of
advanced proteinbased biosensors. Mol Biosyst. 1:354–362.
2005.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Mlichova´ Z and Rosenberg M: Current
trends of β-galactosidase application in food technology. J Food
Nutr Res. 2:47–54. 2006.
|
|
10
|
Selvarajan E and Mohanasrinivasan V:
Kinetic studies on exploring lactose hydrolysis potential of β
galactosidase extracted from Lactobacillus plantarum HF571129. J
Food Sci Technol. 52:6206–6217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Holm L and Rosenström P: Dali server:
Conservation mapping in 3D. Nucleic Acids Res. 38:W545–W549.
2010.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghosh SK, Pandey A, Arora S and Dwivedi
VD: Comparative modeling and docking studies of β-galactosidase
from Aspergillus niger. Netw Model Anal Health Inform Bioinform.
2:297–302. 2013.
|
|
13
|
Dwivedi VD, Arora S and Pandey A:
Computational analysis of physico-chemical properties and homology
modeling of carbonic anhydrase from Cordyceps militaris. Netw Model
Anal Health Inform Bioinforma. 2:209–212. 2013.
|
|
14
|
Godoy AS, Camilo CM, Kadowaki MA, Muniz
HD, Santo MS, Murakami MT, Nascimento AS and Polikarpov I: Crystal
structure of β1→ 6-galactosidase from Bifidobacterium bifidum S17:
trimeric architecture, molecular determinants of the enzymatic
activity and its inhibition by α-galactose. FEBS J. 283:4097–4112.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Vasudha M, Prashantkumar CS, Mallika B,
Vishwas K and Gayathri D: Probiotic potential of
β-galactosidase-producing Lactic Acid Bacteria for Lactose
intolerance from fermented milk and their molecular
characterization. Biomed Rep (In Press).
|
|
16
|
Vinderola CG and Reinheimer JA: Lactic
acid starter and probiotic bacteria: A comparative ‘in vitro’ study
of probiotic characteristics and biological barrier resistance.
Food Res Int. 36:895–904. 2003.
|
|
17
|
Gomaa EZ: β-galactosidase from
Lactobacillus delbrueckii and Lactobacillus reuteri: Optimization,
characterization and formation of galactooligosaccharides. Indian J
Biotechnol. 17:407–415. 2018.
|
|
18
|
Nguyen TH, Splechtna B, Steinböck M,
Kneifel W, Lettner HP, Kulbe KD and Haltrich D: Purification and
characterization of two novel β-galactosidases from Lactobacillus
reuteri. J Agric Food Chem. 54:4989–4998. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hall AB, Tolonen AC and Xavier RJ: Human
genetic variation and the gut microbiome in disease. Nat Rev Genet.
18:690–699. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Akcan N: High-level production of
extracellular β-galactosidase from Bacillus licheniformis ATCC
12759 in submerged fermentation. Afr J Microbiol Res. 5:4615–4621.
2011.
|
|
21
|
Białkowska AM, Cieśliński H, Nowakowska
KM, Kur J and Turkiewicz M: A new β-galactosidase with a low
temperature optimum isolated from the Antarctic Arthrobacter
sp. 20B: Gene cloning, purification and characterization. Arch
Microbiol. 191:825–835. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iqbal S, Nguyen TH, Nguyen HA, Nguyen TT,
Maischberger T, Kittl R and Haltrich D: Characterization of a
heterodimeric GH2 β-galactosidase from Lactobacillus sakei Lb790
and formation of prebiotic galacto-oligosaccharides. J Agric Food
Chem. 59:3803–3811. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Di Serio M, Maturo C, De Alteriis E,
Parascandola P, Tesser R and Santacesaria E: Lactose hydrolysis by
immobilized β-galactosidase: The effect of the supports and the
kinetics. Catalysis Today. 79:333–339. 2003.
|