1. Introduction
The immune system plays a dual role in cancer under
the concept of the immune-editing theory (1-3).
As such, the immune system can fight cancer, but can also shape
cancer immunogenicity. In the first phase, the immune system may
eliminate cancer, while in a later phase, such as in the
equilibrium phase, a balance is established between antitumor and
pro-tumor immunity until cancer cells escape the immune control and
acquire metastatic potential (1-3).
This underscores the importance of immunomodulatory therapy in
boosting endogenous defense mechanisms to fight cancer and
infections.
Immunomodulatory drugs include agents that modify
the immune response by increasing (immunostimulators) or decreasing
(immunosuppressives) the production of serum antibodies (4). Cancer-targeted small molecule-based
immunomodulators are classified as inhibitors, agonists, or
degraders (5). Another
classification of cancer immunotherapeutics include checkpoint
inhibitors, chimeric antigen receptor T-cells (CAR T-cells),
monoclonal antibodies, cancer vaccines, cytokines,
radio-immunotherapy and oncolytic virus therapy (6). Immunomodulators, one of four
immunotherapeutic classes, are themselves divided into four
categories based on their mode of action as follows: Checkpoint
inhibitors, cytokines, agonists and adjuvants. To date, 16
different immune-modulators [nine checkpoint inhibitors (7-10)],
four cytokines (11-13)
two adjuvants (5) and a small
molecule with immunomodulatory properties (14) have been approved by the US Food and
Drug Administration (FDA) for the treatment of several major cancer
types. Imiquimod, one of the two approved adjuvants (5), targets Toll-like receptor (TLR)-7 and
activates pathways involved in the innate immune system, which
results in the induction of an adaptive immune response (15,16).
In addition to its topical application for anal and genital warts,
and actinic keratosis (17),
imiquimod is used and approved for subsets of patients with basal
cell carcinoma.
Imidazoquinoxalines, imiquimod analogues, have
outperformed their parent compound and have exhibited potent
antitumor properties with less adverse events in the preclinical
research setting (18,19). In particular, the most promising
analogues, from first and second generations, EAPB0203, EAPB0503
and EABP02303, have been evaluated in different types of cancer,
such as melanoma (20-22),
adult T-cell leukemia/lymphoma (ATL) (19,23),
chronic myeloid leukemia (CML) (24) and acute myeloid leukemia (AML)
(25). In addition, they have
exhibited anti-parasitic activity, specifically against cutaneous
leishmaniasis (26). At the
molecular level, EAPB0203 and EAPB0503 act on tubulin, inhibit its
polymerization, and consequently arrest the cells in the
G2/M phase (19,24,25),
possibly leading to apoptosis (19,22).
However, and unlike the two former compounds, EABP02303 does not
bind to tubulin and its specific mode of action remains to be
elucidated.
In the sections below, an overview of imiquimod is
provided, including a detailed description of the chemical
synthesis of imidazoquinoxalines and their therapeutic applications
to date.
2. Imiquimod
Imiquimod
(1-(2-methylpropyl)-1H-imidazo(4,5-c)
quinolin-4-amine), also known as S-26308 or R-837, is the first
non-osidic (i.e., contain no sugar) nucleoside analogue of the
imidazoquinoline family. It is a tricyclic organic molecule with a
nitrogen (N)-containing heterocyclic compound of four N atoms, a
quinoline component, a 1H-imidazole ring, and a methyl-propyl group
(27). Imiquimod belongs to the
class of immune response modifiers (15,28),
and is commercialized under the name of Aldara® or
Zyclara®. Its topical use was approved by the US FDA in
1997, for the treatment of certain viral infections, such as
perianal and genital human papilloma virus (HPV) disease (genital
warts) by stimulating the host immune system (29,30).
Imiquimod was also the first immune response modifier used for the
treatment of infectious skin conditions due to its potent in
vivo anti-viral and antitumor activities (16). In addition, it was effective as a
topical therapy for certain types of skin cancer, including
superficial basal cell carcinoma, Bowen's disease, superficial
squamous cell carcinoma, certain superficial malignant melanomas
and actinic keratosis (15,31-33).
The systemic administration of imiquimod in mice has been shown to
exert potent antitumor effects against various types of cancer,
such as melanoma, lung sarcoma, mammary, colon, bladder carcinoma
(19,34), basal cell carcinoma (16,35),
actinic keratosis (36) and
cutaneous B-cell lymphoma (19,33,37).
Furthermore, its therapeutic spectrum has been extended to certain
parasitic infections, such as cutaneous leishmaniasis (26,38)
and toxoplasmosis (39). In a
previous study on cutaneous leishmaniasis, imiquimod was clinically
used, in combination with a systemic antimonial, and yielded a cure
rate of 90% in patients with refractory cutaneous leishmaniasis, as
compared to pentavalent antimonial treatment alone (40). In a clinical trial performed in
Peru, Miranda-Verastegui et al (41) demonstrated that this combination
was more effective than the placebo plus pentavalent antimony
(41). Imiquimod has also been
proven to be effective as a first line therapy for cutaneous
leishmaniasis (42).
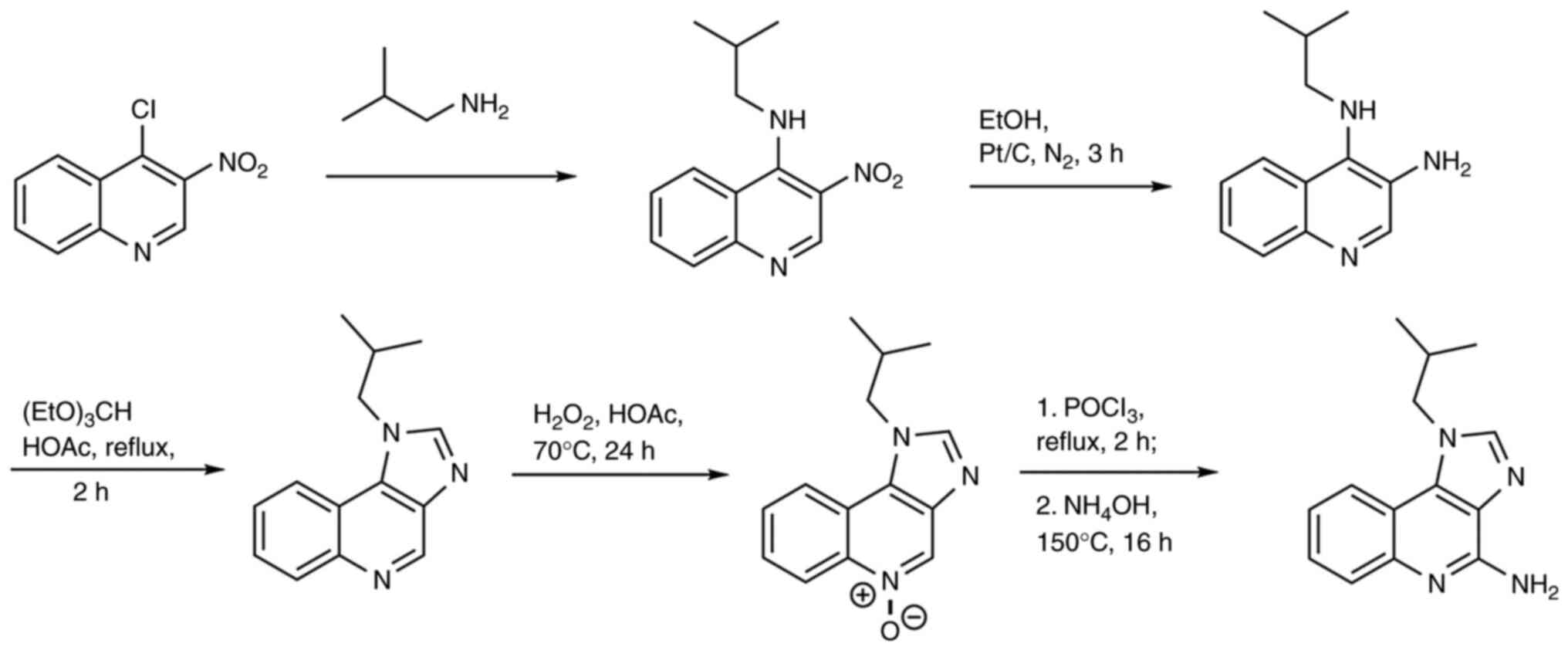
Chemical synthesis of imiquimod
Over the years, the chemical synthesis of imiquimod
was performed using several methods. Conventional synthesis was
based on the addition of imidazole group to the quinoline core
(27) (Fig. 1).
However, this method presented several limitations,
including the numerous and lengthy steps involved in the addition
of N atoms. Thereafter, other strategies were adopted to save time
and cost. One strategy began with 2-bromobenzaldehyde instead of
quinolone (Fig. 2), yet it
resulted in a low yield of 30%. Another approach used, in the first
step, anthranilic acid, a commercially available compound. Several
intermediates including benzoxazine, hydrazoic acid,
tetrazoloquinoline and iminophosphorane were produced, and
imiquimod was formed from iminophosphorane. While this route was
synthetically interesting, it included the formation of a tetrazole
derivative, a potentially explosive product (27).
Given the limitations of the aforementioned methods,
new strategies are being developed to improve imiquimod synthesis,
and to generate imiquimod derivatives with enhanced efficiency and
reduced adverse effects (discussed below).
Biological activity and mechanisms of
action of imiquimod
The exact mechanisms of action of imiquimod have not
yet been fully explored. Nevertheless, it is well documented that
imiquimod is a TLR-7 agonist (15,43),
which ligates TLR-7 activating immune cells. TLR-7 is expressed on
the endosomal surface of antigen presenting cells and is commonly
involved in pathogen recognition (40). Other cell types activated by
imiquimod include natural killer cells, macrophages, dendritic
cells and B-lymphocytes, leading to the consequent activation of
downstream protein kinases and transcription factors, including
nuclear factor-κB (NF-κB), and the production of pro-inflammatory
cytokines and chemokines, including tumor necrosis factor α
(TNF-α), interleukin (IL)-6, IL-8(44), IL-12, interferon (IFN)-α and IFN-γ
(19,45-53).
Cytokine induction and cellular infiltrates underlying
cell-mediated immune responses result in the regression of warts
caused by HPV infection (54). In
the case of cutaneous leishmaniasis, imiquimod exhibits an
anti-leishmanial activity via the activation of TLRs, the
production of inflammatory cytokines and the induction of nitric
oxide release (26). Recently, the
mechanism of action of imiquimod against acute and chronic
toxoplasmosis was elucidated. Indeed, imiquimod was shown to
upregulate the expression of TLR-7, -11 and -12, and subsequently
mediate the activation of the MyD88 pathway, resulting in the
induction of an immune response (39). There is also evidence to indicate
that imiquimod, when applied to the skin, can lead to the
activation of Langerhans cells, which then migrate to local lymph
nodes to activate the adaptive immune system (40).
Independent of TLR-binding, imiquimod can also boost
pro-inflammatory signaling by restricting the negative feedback on
inflammation. In that sense, imiquimod inhibits adenylyl cyclase
activity and consequently suppresses the adenosine receptor
signaling pathways involved in negative regulation of inflammation
(51,55). Imiquimod has also been shown to
inhibit tumor progression in immunocompromised animals (34), and its topical application potently
inhibits tumor induced-angiogenesis in vivo, in a dose and
time-dependent manner (56). This
effect was associated with the imiquimod-induced production of
several cytokines, including IFN-γ, TNF-α and IL-18. This
constitutes an effective response to the treatment of
HPV-associated benign and premalignant tumor, but also of early
malignant angiogenesis-dependent proliferative tumors (56).
Under the TLR-independent pathway, imiquimod induces
direct apoptotic effects, through the intrinsic mitochondrial-and
Bcl-2-dependent apoptotic pathway (18,50,57),
leading to the translocation of cytochrome c into the
cytosol and the activation of procaspase-3 and -9(58). Notably, imiquimod selectively
induces the apoptosis of transformed keratinocytes and melanoma
cells, without any effect on their normal counterparts (19,49-51,59).
Clinical use of imiquimod and adverse
effects
Imiquimod is approved and commercially available as
a 2.5, 3.75 or 5% cream for the treatment of external genital warts
and superficial basal cell carcinoma. However, it is also used for
off-label conditions as follows: Melanocytic proliferations, such
as lentigo maligna (60-62),
atypical nevi (63), as well as
lichen sclerosus, alopecia areata (64), Kaposi sarcoma (65,66)
and Molluscum contagiosum (67).
Research on imiquimod is still an active area. Indeed, >450
studies were published on the topic over the past year alone (as of
May, 2023). A previous study evaluated the potentiating effect of
imiquimod on dyphencyprone for the topical immunotherapy of
alopecia areata and reported that 77% of patients exhibited an
improvement in symptoms upon the addition of imiquimod (68). Imiquimod was also tested as a
topical treatment in patients with HIV and high-grade squamous
intraepithelial lesions, susceptible to develop into anal cancer
(69). Compared to the active
monitoring group, the rate of anal cancer onset was 57% lower in
the treatment group; suggesting that the treatment of high-grade
squamous intraepithelial lesions with medications such as imiquimod
is protective against anal cancer (69). Another recent study described the
efficacy of imiquimod loaded in phospholipid-free small unilamellar
vesicles targeted to hepatocytes, in the treatment of chronic
hepatitis B (70). In patients
with lentigo maligna, imiquimod was shown to reduce the lentigo
maligna surface after 1 month of treatment and could be prescribed
to prevent the progression of these lesions to carcinogenesis
(71). In a human melanoma cell
line, imiquimod induced cell death by lysosomal membrane
permeabilization and the release of lysosomal proteolytic enzymes,
including cathepsins, which resulted in mitochondrial dysfunction
(72). While imiquimod is an
effective and safe therapy, certain adverse effects have been
reported; these include local inflammatory reactions such as
itching, burning, bleeding, erosions, ulcerations, excoriations,
crusting, induration, edema and pain (73,74).
Some patients have presented with systemic reactions, such as upper
respiratory tract infections and flu-like symptoms, such as fever,
sinusitis, headaches and tiredness (50,74,75).
In the case of periocular skin lesions, imiquimod may cause
conjunctivitis and ocular stinging (61). In addition, imiquimod can affect
vitamin B12 levels (60).
Imiquimod has also been shown to be associated with hypertrophic
lupus erythematosus in an elderly patient (76). Additionally, a case report of
actinic keratosis associated imiquimod with a severe case of
bullous pemphigoid (77) and the
occurrence of lupus-like reactions (78). In another study, when applied to a
to a single lesion of in situ melanoma in a patient,
imiquimod caused debilitating severe fatigue (79). Moreover, imiquimod caused localized
skin ulceration in a patient with type-2 diabetes mellitus
(80). Finally, women of
reproductive age are advised to use contraception upon imiquimod
treatment, due to the absence of definitive data on its teratogenic
potential (74).
3. Quinolines and quinoxalines
Heterocyclic compounds are a cornerstone of current
anticancer drug design due to their favorable pharmacokinetic and
pharmacodynamic properties (higher lipophilicity, adjusted polarity
and other physicochemical features including solubility, ionization
state and hydrogen-bonding capacity). In 2015, ~30% of US
FDA-approved anticancer drugs included one or more cyclic rings
containing a nitrogen or an oxygen (81).
Quinolines and quinoxalines are N-containing
heterocycles found in a number of natural compounds. In both
quinoline and quinoxaline compounds, the main core of imiquimod is
composed of a benzene ring. The difference is that, in
quinoxalines, the pyridine ring of quinoline is replaced by a less
alkaline component, the pyrazine ring (Table I) (82-84).
The quinoline compound has limited applications,
while its derivatives span a wide range of activities, such as
anti-malarial (quinine, quinidine, chloroquine, mefloquine,
amodiaquinine, primaquine, etc.), anti-viral (saquinavir),
anti-bacterial (fluoroquinolones such as ciprofloxacin,
sparfoxacin, gatifloxacin, etc.), anti-fungal/anti-protozoan
(clioquinol), anti-helminthic (oxamniquine), local anesthetic
(dibucaine), anti-asthmatic (montelukast), anticancerous
(camptothecin, irinotecan, topotecan, etc.), anti-psychotic
(aripiprazole, brexpiprazole, etc.), anti-glaucoma (carteolol) and
cardiotonic (vesnarinone) activities. In the particular case of
cancer, quinoline derivatives induce cell cycle arrest and
apoptosis, the inhibition of angiogenesis, the disruption of cell
migration and the modulation of nuclear receptor responsiveness in
cancer cells (85). Similarly,
quinoxaline compounds exibit a wide array of pharmacological
activities, some of which include anti-malarial (86), anti-inflammatory (87), anti-HIV (88) and anticancer activities (89).
4. Imidazoquinoxalines
Imiquimod exerts a selective cytotoxic effect on
cancer cells. However, the imiquimod-induced production of
pro-inflammatory cytokines can have deleterious effects. Hence, a
series of imiquimod analogues belonging to the quinoxaline family
was synthesized, with the ultimate aim of enhancing the antitumor
potential of imiquimod, while rendering it systemically available
and curbing its toxicity profile (18).
A series of three imiquimod analogues were
synthesized: The imidazo[1,2-a]quinoxalines, the
imidazo[1,5-a]quinoxalines and the
pyrazolo[1,5-a]quinoxalines. The present review focuses on
three compounds of the imidazo[1,2-a]quinoxalines series,
namely EAPB0503, EAPB0203 and EAPB02303. The first generation
encompasses EAPB0203 [N-methyl-1-(2-phenylethyl)
imidazo[1,2-a]quinoxalin-4-amine)] as an earlier derivative,
followed by EAPB0503 [1-(3-methoxyphenyl)-N-methyl
imidazo[1,2-a]quinoxalin-4-amine] (Fig. 3), and it paved the way for in
vitro studies revealing considerable anticancer potential for
this class of imidazoquinoxalines (19).
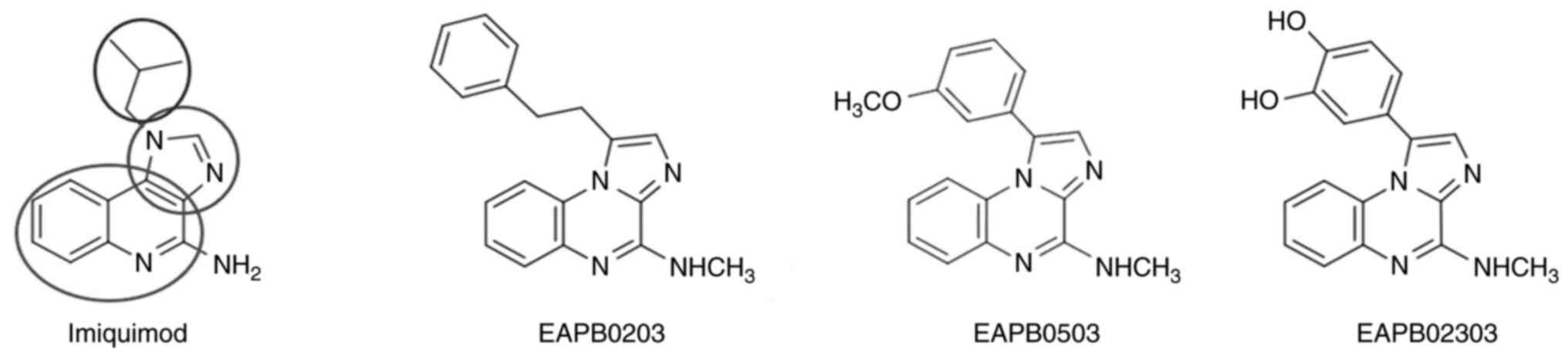 | Figure 3Structure of imiquimod, EAPB0203,
EAPB0503 and EAPB02303. The heterocyclic scaffold platform of
imiquimod is comprised of a quinoline component (a six-membered
ring, lowermost ellipse) and a 1H-imidazole ring (a five-membered
ring, middle ellipse). Analogue design is based on a modified
heterocyclic scaffold, named imidazoquinoxaline, comprised of a
quinoxaline (bicycle with two nitrogen atoms) fused on one of its
face to an imidazole, leading to the presence of a bridgehead
nitrogen which slightly modifies the general planarity of the
molecule (90). The isopropyl
group in imiquimod (uppermost ellipse) is substituted by a
phenethyl in EAPB0203, a 3-methoxyphenyl in EAPB0503 or a
3,4-dihydroxyphenyl group in the new derivative EAPB02303. In the
three imidazoquinoxaline analogues, the amino group of imiquimod is
replaced by a methylamino group. |
Recently, a newer imidazoquinoxaline derivative,
EAPB02303 [1-(3,4-dihydroxyphenyl)imidazo[1,2-a]quinoxaline]
(Fig. 3, right panel), as the lead
compound of the second generation, was synthesized; this
demonstrated a higher potency in a melanoma cell line model, with a
50% inhibitory concentration (IC50) of 3 nM, as compared
with IC50 of 383 nM for EAPB0503(90).
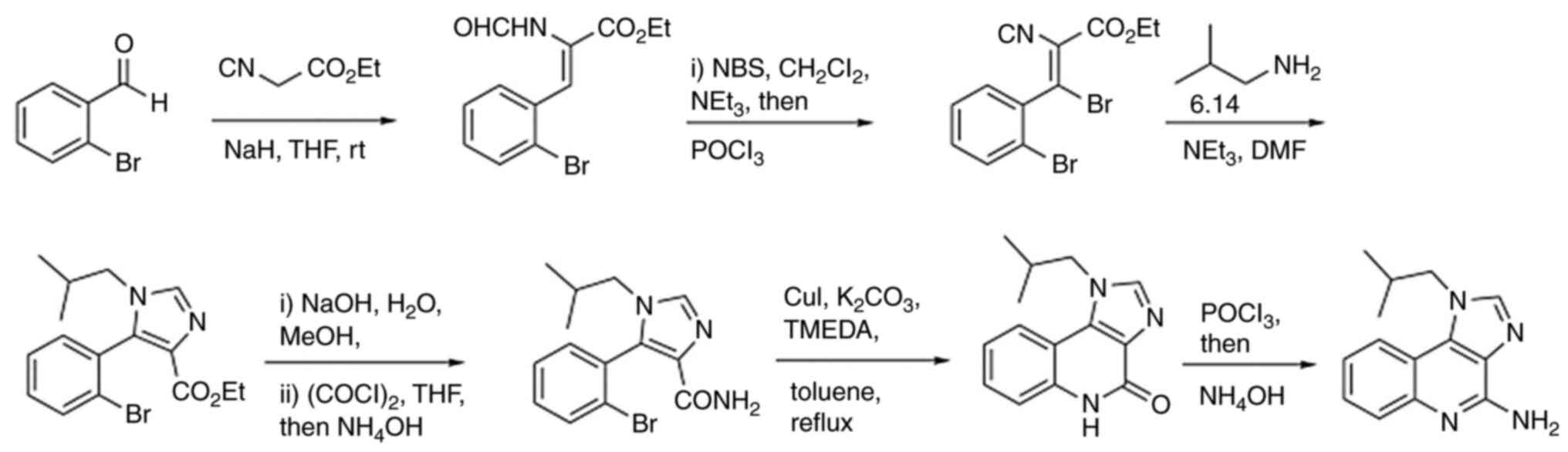
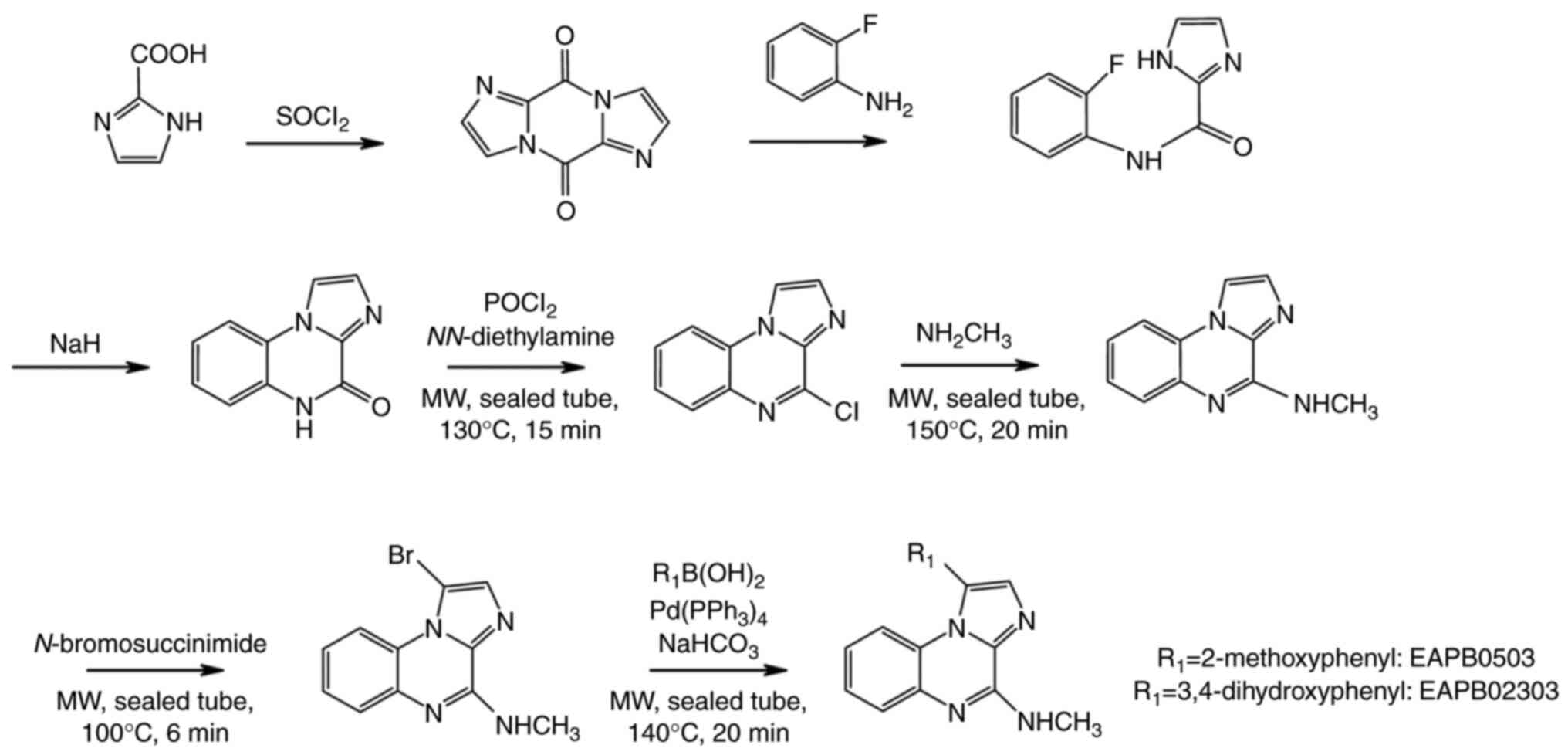
Chemical synthesis of EAPB0203,
EABP0503 and EAPB02303
EAPB0203 was first synthesized using the traditional
method, which consists of the condensation of two imidazole
derivatives coupled to ortho-fluoroaniline, followed by an
intramolecular cyclisation and substitution with the appropriate
amines (19). The synthesis of
EAPB0503 is based on EAPB0203 synthesis, but was modified and
optimized using the Suzuki reaction under basic conditions in a
microwave-assisted reaction to yield greater purity with shorter
synthesis time (Fig. 4). In
summary, EAPB0503 was generated by replacing the phenethyl group in
EAPB0203 with 3-methoxyphenyl (Fig.
3) (22). As regards
EAPB02303, it was obtained by the substitution of 3-methoxyphenyl
group at R1 position in EAPB0503 by 3,4-dihydroxyphenyl group on
the 4-methylaminoimidazo(1,2-a)quinoxaline heterocyclic
platform (Fig. 3) (90).
Pharmacokinetic and pharmacodynamic
properties of EAPB0203, EAPB0503 and EAPB02303
The modifications introduced into the
imidazoquinoxalines, EAPB0203, EAPB0503 and EAPB02303(19), aimed to improve their polarity and
binding properties.
Imidazo[1,2-a]quinoxalines, mostly EAPB0203
and EAPB0503, their metabolites, and their major oxygenated
derivatives, were characterized and identified using nuclear
magnetic resonance and liquid chromatography-mass spectrometry
studies (91). Briefly, EAPB0203
and EAPB0503 are metabolized inside liver microsomes of numerous
species, including mouse, human, dog and rat. Both EAPB0203 and
EAPB0503 metabolites exhibit an increased polarity compared to
their parent compounds, and have mostly shorter half-lives, apart
from one metabolite (92).
EAPB0203 and EAPB0503 biotransformation began with a demethylation
step, yielding their two main metabolites EAPB0202 and EAPB0603,
respectively. These metabolites are less potent than their parent
molecule (92). Furthermore, ~98%
of EAPB0203 and EAPB0503 bind to human serum albumin in the plasma,
with very little compound remaining in the free form. Both
compounds are eliminated from the plasma in two phases: an initial
rapid decline phase, followed by a prolonged terminal phase. The
terminal half-life is ~2 h. However, the systemic availability of
EAPB0203 and EAPB0503, upon intraperitoneal administration is
relatively modest, at 22.7 and 35% respectively (92); this motivates further research to
enhance bioavailability and pave the way for more preclinical (and
ultimately clinical) studies.
5. Immunomodulatory effect
A total of 22 imidazo(I,2-a)quinoxalines
derivatives have been proven to be potent inhibitors of nucleotide
phosphodiesterase enzyme 4 extracted from human alveolar epithelial
cells (93). It is considered that
by inhibiting phosphodiesterase enzyme 4 activity, these compounds
could lead to cAMP accumulation and transcription factor cAMP
response element-binding protein activation inside the cell
(31). In addition, since they are
able to decrease TNF-α levels (19,93)
they can consequently, modulate cytokine synthesis and/or
production by immune cells, exerting therefore their
anti-inflammatory activity (93).
Moreover, it has been shown that imidazo[1,2-a]quinoxalines
activate the p38 MAPK pathway and inhibit the PI3K pathway in a
murine fibroblastic cell line (93).
6. Antitumor properties of EAPB0203,
EAPB0503 and EAPB02303
Melanoma
Melanoma is the most aggressive form of skin cancer
and is associated with a poor prognosis (94). Standard of care begins with
surgical excision (when the tumor is resectable), systemic
treatment (chemotherapy and/or immunotherapy), or radiation therapy
for unresectable tumors or as adjuvant therapy (21). However, the mortality rates remain
very high, urging the need for the identification of novel
therapeutic targets. The activity of imiquimod and some of its
derivatives was previously tested in melanoma cells in vitro
and in animal models in vivo (50,95).
EAPB0203 was 50-fold more potent than imiquimod and exhibited a
remarkable cytotoxic activity against A375 melanoma cell line
(19,96). EAPB0203 was also 110-fold more
potent than fotemustine, a major treatment for metastatic melanoma
(19,97). Of note, as previously demonstrated
EAPB0503 exhibited a 7-9-fold greater antitumor and cytotoxic
activity than EAPB0203 in the same cell line, and induced apoptosis
by activating the NF-κB pathway (92). Indeed, both EAPB0203 and EAPB0503
induced the intrinsic apoptotic pathway characterized by the loss
of mitochondrial membrane potential, the release of cytochrome
c, the activation of caspases and the subsequent
fragmentation of DNA. The potent cytotoxic activity of EAPB0503 was
also observed in an in vivo mouse model of xenograft
melanoma (19,96). In this regard, the antitumor
activity of APB0503 was attributed to anti-proliferative and
anti-mitotic effects, mediated by the inhibition of tubulin
polymerization (18,22). This compound interacts with tubulin
via a colchicine-binding site (18), with a 52% higher potency than
colchicine, a well-known natural inhibitor of tubulin
polymerization. Unlike imiquimod, the effect of EAPB0503 on tubulin
polymerization in melanoma cells is not associated with its TLR-7
agonist activity, even at concentrations >300 µM (18). An EAPB0503 derivative,
1-(2-hydroxy-3-methoxyphenyl)-N-methylimidazo
[1,2-a]quinoxalin-4-amine, exhibited a greater inhibition of
tubulin polymerization in melanoma A375 cells (22).
Given encouraging data on melanoma in vitro
and in vivo models, first-generation imidazoquinoxalines
were evaluated in models of hematological malignancies (25) .
The next-generation derivative, EAPB02303, was
unable to bind to tubulin at the colchicine site (90). Notably, EAPB02303 was still able to
inhibit the growth of A375 melanoma cells in vitro at a
nanomolar concentration range, independent of the inhibition of
tubulin polymerization. The growth inhibition in vitro was,
dose-dependently, associated with a reduction in size and weight of
mouse-xenograft A375 melanoma cell tumors. In addition, growth
inhibition was associated with a lower mitotic index, but not with
necrosis (90). Transcriptomic
analysis, in comparison with first-generation imidazoquinoxalines
and a panel of 12 well-known anticancer drugs, suggested that
EAPB02303 acts through a different mechanism. However, the exact
mechanistic pathway remains to be elucidated (90).
Of note, the second-generation derivatives,
EAPB02302 and EAPB02303, were even more cytotoxic on A375 cells,
with IC50 values of 60 and 10 nM, respectively, versus
an IC50 value of 1,570 and 200 nM for EAPB0203 and
EAPB0503, respectively (96).
ATL
ATL is a rare and aggressive blood malignancy due to
the transformation of T-cells by human lymphotropic virus type I.
ATL is associated with a poor prognosis, due to chemo-resistance
and immunosuppression (98).
Following more than four decades of research, the therapeutic
management of ATL remains intricate and a cure for ATL is still out
of reach in the majority of patients. The current strategies
include the watch-and-wait policy, conventional chemotherapy,
allogeneic hematopoietic cell transplantation (allo-HCT), and a
combination of two antiviral agents, zidovudine and IFN-α (99-103).
Although indolent ATL and a fraction of acute ATL exhibit a
long-term survival subsequent to antiviral therapies and one third
of patients with aggressive ATL undergo allo-HCT and <10% of
those who received chemotherapy could have disease control for
>5 years.
Several targeted therapies have been tested in ATL.
Among these, EAPB0203 has been shown to induce growth inhibition,
cell cycle arrest and apoptosis, selectively in ATL cell lines,
while no effect has been reported in normal resting or
PHA-activated peripheral blood mononuclear cells taken from two
healthy donors (23). EAPB0203
induces apoptosis by decreasing the levels of the anti-apoptotic
proteins, c-IAP-1 and Bcl-xL, thus triggering the intrinsic
apoptotic pathway. In addition, EAPB0203 treatment stabilizes the
tumor suppressor proteins p21 and p53 in a dose-dependent manner
(19).
CML
CML is a clonal myeloproliferative disorder
resulting from a reciprocal translocation between the abl
proto-oncogene on chromosome 9 and the breakpoint cluster region
(bcr) on chromosome 22, resulting in a shortened chromosome
22, known as the Philadelphia (Ph) chromosome. This translocation
produces the recombined bcr-abl gene, encoding for BCR-ABL,
a constitutively activated tyrosine kinase, responsible for CML
leukemogenesis, maintenance and progression of the disease from
chronic to blast phase, indicating a poor prognosis. The initial
treatments for CML included busulfan and hydroxyurea. These were
later replaced with IFN-α (104).
In 2001, CML treatment witnessed a revolution with the use of
imatinib mesylate (imatinib), the first tyrosine kinase inhibitor
(TKI) (105-107).
However, this treatment was associated with certain drawbacks,
including intolerance to treatment, the lack of a therapeutic
response or resistance, and this has motivated the development of
second- and third-generation TKIs.
While TKIs significantly revolutionized CML
treatment and prolonged survival of patients, CML cure is still out
of reach, and bone marrow transplantation remains the only
long-term curative option for CML patients. Both EAPB0203 and
EAPB0503 were evaluated in an in vitro model of human blast
crisis CML cell lines. The compounds induced cell cycle arrest at
the G2/M phase, accompanied by an increased level of
histone 3 phosphorylation. Growth inhibition was more pronounced in
three chronic-phase CML cell lines treated with EAPB0503 as
compared to EAPB0203(24), and was
enhanced by the induction of the intrinsic apoptosis pathway.
Molecular analysis revealed that EAPB0503-treated CML cells
exhibited decreased BCR-ABL oncoprotein levels, which could explain
growth inhibition. Notably, EAPB0503 appeared to circumvent
imatinib resistance in vitro, presenting it as a potentially
attractive therapeutic option in CML. The combination of imatinib
(CML standard-of-care) and EAPB0503 synergized to inhibit CML cell
line growth and proliferation in vitro (24). Collectively, these findings suggest
that EAPB0503 is a potential therapeutic agent capable of
inhibiting CML cell growth, synergizing with imatinib to alleviate
the tumor burden, and overcoming resistance to imatinib and maybe
other TKIs.
AML
AML is a complex and heterogeneous hematological
malignancy, characterized by the excessive proliferation of
undifferentiated myeloid precursors, resulting in an impaired
hematopoiesis and bone marrow failure. AML is associated with a
highly variable, yet frequently poor prognosis, based on somatic
genetic alterations (108). Due
to the genetic complexity and heterogeneity of AML, treatment
strategies remain variable among patients, apart from the AML
subtype, acute promyelocytic leukemia and core binding-factor (CBF)
AML, which have their own standard of treatment (109,110). In general, AML treatment remained
unaltered for more than three decades, and relied on aggressive
chemotherapy administered in two phases: An induction phase to
eliminate AML blasts, and a consolidation phase to prevent relapse
(111). Hematopoietic stem cell
transplantation was used independently or sequentially, depending
on the availability of stem cell donors and the overall clinical
fitness of the patient (112).
Genome-wide studies, including gene and microRNA
expression profiles, single nucleotide polymorphisms and gene copy
number studies, have identified a growing number of recurrent
genetic mutations of AML (113).
Among the identified genes, mutations of nucleophosmin-1
(NPM-1) and fms-like tyrosine kinase 3 (FLT3) are
prognostic markers of AML. NPM-1 is one of the most frequently
mutated genes in AML (114).
NPM-1 mutations alter the C-terminal DNA-binding domain of the
protein, resulting in an aberrant nuclear export sequence and its
retention in the cytoplasm, hence the NPM-1c nomenclature,
referring to its cytosolic localization (115).
The pre-clinical efficacy of EAPB0503 and EAPB0203
has been evaluated in AML. EAPB0503 was shown to significantly and
selectively inhibit the growth of NPM-1c-positive cells, at lower
concentrations than EAPB0203(25).
Growth inhibition was associated with EAPB0503-induced early
apoptosis in NPM-1c-, but not wild-type NPM-1-AML cells through
caspase activation and p53 activation (25). More recently, EAPB0503 was shown to
prolong the survival of mice with NPM-1c AML xenografts and a novel
mechanism of action of this compound was found. Indeed, EAPB0503
induced NPM-1c SUMOylation, concomitant with the downregulation of
the de-SUMOylase, sentrin/SUMO specific peptidase 3, and paralleled
by the upregulation of alternative reading frame (ARF) in
NPM-1c-expressing cells (116).
These results implicate EAPB0503 in the post-translational
modifications of NPM-1c, demonstrating that therapies modulating
NPM-1c post-translational modifications can be introduced to the
management of NPM1c AML.
In conclusion, EAPB0503 displays a greater
anti-leukemic activity than EAPB0203 in both CML and AML. This is
hypothesized to be due to the ethyl linker that separates the
imidazoquinoxaline heterocycle from the phenyl group in EAPB0203,
but not in EAPB0503(24).
7. Anti-parasitic activity of EAPB0503
The anti-parasitic activity of imidazoquinoxalines,
was first reported for imiquimod (26). In the context of cutaneous
leishmaniasis, imiquimod exerted its anti-amastigote activity via
TLR-7 upregulation, leading to NF-κB activation and
pro-inflammatory cytokine production. However, the effect of
EAPB0503 on TLR-7 was less prominent (26). Noteworthy, findings suggest that
EAPB0503 may act not only via TLR-7, but also through other TLRs
(26). Moreover, the levels of
macrophage inflammatory protein-1α and β, monocyte chemoattractant
protein and some pro-inflammatory cytokines, such as IL-1β, IL-1,
IL-6, IL-12 and TNF-α, which appear to be associated with
resistance against leishmaniasis, are increased upon exposure to
EAPB0503. This increase appears to be crucial for the clearance of
cutaneous leishmaniasis and for the protective role of imiquimod
derivatives against cutaneous leishmaniasis (26). Moreover, the production of the
inducible nitric oxide synthase, normally induced in response to
pro-inflammatory cytokines, increased in response to EAPB0503
further enhancing the leishmanicidal activity of macrophages
infected with Leishmania spp. By contrast, anti-inflammatory
cytokine levels, such as IL-10 and IL-4, are usually associated
with disease progression (117),
decreased by 4- and 15-fold, respectively, upon EAPB0503 exposure
versus 4-fold upon exposure to imiquimod in L.
tropica-infected macrophages (26). Moreover, EAPB0503 exhibited a more
prominent anti-leishmanicidal activity than the clinically used
glucantime, whether alone or combined with imiquimod (26)
8. Conclusions and future perspectives
As an immune response modifier, imiquimod has shed
light on a new class of potential anti-cancer agents.
Imidazoquinoxalines, imiquimod analogues, were synthesized in an
attempt to mitigate the deleterious effects of the
imiquimod-induced production of pro-inflammatory cytokines and to
improve its antitumor properties. Earlier agents, EAPB0203 and
EAPB0503, exhibited notable antitumor properties, mediated by the
inhibition of tubulin polymerization, the inhibition of growth and
the induction of apoptosis, in melanoma and/or leukemia models. In
addition, they exhibited a potential to synergize with
standard-of-care treatments. The next-generation derivative,
EAPB02303, exhibited a more potent cytotoxic activity against
melanoma cells, through a mechanism, which is independent of
tubulin polymerization and which remains to be elucidated. A
summary of the mechanisms of action of imiquimod and its
analogues/derivatives is provided in Table II. Currently, imidazoquinoxalines
are actively assessed in preclinical models of diseases, mainly
cancer, in order to provide sufficient scientific evidence and
promote their translation from bench to bedside.
 | Table IIMechanisms of action of imiquimod,
EAPB0203, EAPB0503 and EAPB02303. |
Table II
Mechanisms of action of imiquimod,
EAPB0203, EAPB0503 and EAPB02303.
| Compound | Disease/disease
model | Mechanisms of
action | (Refs.) |
|---|
|
Imiquimoda | Genital warts | TLR-7: Innate and
adaptive immune response activation | (15,43) |
| | Cutaneous | and cytokines
secretion | (38) |
| | Leishmaniasis | Apoptosis:
Bcl-2-dependent intrinsic apoptotic pathway | (26) |
| | Toxoplamosis | Nitric oxide
production in response to pro-inflammatory cytokine | (39) |
| EAPB0203 | ATL | Growth inhibition:
Inhibition of tubulin polymerization | (19) |
| | Melanoma | Apoptosis:
Anti-apoptotic proteins c-IAP-1 and Bcl-xL, downregulation
triggering the intrinsic apoptotic pathway | (19) |
| EAPB0503 | AML | Growth inhibition:
Inhibition of tubulin polymerization | (25) |
| | CML | Apoptosis:
Intrinsic apoptotic pathway | (24) |
| | Melanoma | | (22) |
| EAPB02303 | Melanoma | Growth inhibition:
Unknown mechanism; independent from tubulin binding | (90) |
Acknowledgements
The authors would like to acknowledge Miss
Christa-Maria El-Khoury, Miss Nesrine Dagher and Miss Reem Hamze
for contributing to the literature review, which was in partial
fulfilment of their graduate research projects, at the Department
of Biology, Faculty of Sciences, Lebanese University, Beirut,
Lebanon.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
JO wrote the manuscript. Authors AS, HEH, CDM, PAB,
MES and JS reviewed the different drafts, contributed to the
writing and synthesized the available literature. JS designed the
review and oversaw the writing process. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dunn GP, Old LJ and Schreiber RD: The
three Es of cancer immunoediting. Annu Rev Immunol. 22:329–360.
2004.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Abbott M and Ustoyev Y: Cancer and the
immune system: The history and background of immunotherapy. Semin
Oncol Nurs. 35(150923)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kennedy LB and Salama AKS: A review of
cancer immunotherapy toxicity. CA Cancer J Clin. 70:86–104.
2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Avorn J: Learning about the safety of
drugs-a half-century of evolution. N Engl J Med. 365:2151–2153.
2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wu Y, Yang Z, Cheng K, Bi H and Chen J:
Small molecule-based immunomodulators for cancer therapy. Acta
Pharm Sin B. 12:4287–4308. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kumar AR, Devan AR, Nair B, Vinod BS and
Nath LR: Harnessing the immune system against cancer: Current
immunotherapy approaches and therapeutic targets. Mol Biol Rep.
48:8075–8095. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Liu SV, Reck M, Mansfield AS, Mok T,
Scherpereel A, Reinmuth N, Garassino MC, Carpeno JD, Califano R,
Nishio M, et al: Updated overall survival and PD-L1 subgroup
analysis of patients with extensive-stage small-cell lung cancer
treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133).
J Clin Oncol. 39:619–630. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Vaddepally RK, Kharel P, Pandey R, Garje R
and Chandra AB: Review of indications of FDA-approved immune
checkpoint inhibitors per NCCN guidelines with the level of
evidence. Cancers (Basel). 12(738)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chen XY, Li YD, Xie Y, Cao LQ, Ashby CR
Jr, Zhao H and Chen ZS: Nivolumab and relatlimab for the treatment
of melanoma. Drugs Today (Barc). 59:91–104. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kang C: Retifanlimab: First approval.
Drugs. 83:731–737. 2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Di Trolio R, Simeone E, Di Lorenzo G,
Grimaldi AM, Romano A, Ayala F, Caracò C, Mozzillo N and Ascierto
PA: Update on PEG-interferon α-2b as adjuvant therapy in melanoma.
Anticancer Res. 32:3901–3909. 2012.PubMed/NCBI
|
|
12
|
Qureshi YA, Karp CL and Dubovy SR:
Intralesional interferon alpha-2b therapy for adnexal Kaposi
sarcoma. Cornea. 28:941–943. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rallis KS, Corrigan AE, Dadah H, George
AM, Keshwara SM, Sideris M and Szabados B: Cytokine-based cancer
immunotherapy: Challenges and opportunities for IL-10. Anticancer
Res. 41:3247–3252. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lamb YN: Pexidartinib: First Approval.
Drugs. 79:1805–1812. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hemmi H, Kaisho T, Takeuchi O, Sato S,
Sanjo H, Hoshino K, Horiuchi T, Tomizawa H, Takeda K and Akira S:
Small anti-viral compounds activate immune cells via the TLR7
MyD88-dependent signaling pathway. Nat Immunol. 3:196–200.
2002.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Kamath P, Darwin E, Arora H and Nouri K: A
review on imiquimod therapy and discussion on optimal management of
basal cell carcinomas. Clin Drug Investig. 38:883–899.
2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Tyring S: Imiquimod applied topically: A
novel immune response modifier. Skin Therapy Lett. 6:1–4.
2001.PubMed/NCBI
|
|
18
|
Courbet A, Bec N, Constant C, Larroque C,
Pugniere M, Messaoudi SE, Zghaib Z, Khier S, Deleuze-Masquefa C and
Gattacceca F: Imidazoquinoxaline anticancer derivatives and
imiquimod interact with tubulin: Characterization of molecular
microtubule inhibiting mechanisms in correlation with cytotoxicity.
PLoS One. 12(e0182022)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moarbess G, Deleuze-Masquefa C, Bonnard V,
Gayraud-Paniagua S, Vidal JR, Bressolle F, Pinguet F and Bonnet PA:
In vitro and in vivo anti-tumoral activities of
imidazo[1,2-a]quinoxaline, imidazo[1,5-a]quinoxaline, and
pyrazolo[1,5-a]quinoxaline derivatives. Bioorg Med Chem.
16:6601–6610. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Deleuze-Masquefa C, Moarbess G, Khier S,
David N, Gayraud-Paniagua S, Bressolle F, Pinguet F and Bonnet PA:
New imidazo[1,2-a]quinoxaline derivatives: Synthesis and in vitro
activity against human melanoma. Eur J Med Chem. 44:3406–3411.
2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kwong A, Sanlorenzo M, Rappersberger K and
Vujic I: Update on advanced melanoma treatments: Small molecule
targeted therapy, immunotherapy, and future combination therapies.
Wien Med Wochenschr. 169:314–322. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zghaib Z, Guichou JF, Vappiani J, Bec N,
Hadj-Kaddour K, Vincent LA, Paniagua-Gayraud S, Larroque C,
Moarbess G, Cuq P, et al: New imidazoquinoxaline derivatives:
Synthesis, biological evaluation on melanoma, effect on tubulin
polymerization and structure-activity relationships. Bioorg Med
Chem. 24:2433–2440. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moarbess G, El-Hajj H, Kfoury Y, El-Sabban
ME, Lepelletier Y, Hermine O, Deleuze-Masquéfa C, Bonnet PA and
Bazarbachi A: EAPB0203, a member of the imidazoquinoxaline family,
inhibits growth and induces caspase-dependent apoptosis in T-cell
lymphomas and HTLV-I-associated adult T-cell leukemia/lymphoma.
Blood. 111:3770–3777. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saliba J, Deleuze-Masquéfa C, Iskandarani
A, El Eit R, Hmadi R, Mahon FX, Bazarbachi A, Bonnet PA and Nasr R:
EAPB0503, a novel imidazoquinoxaline derivative, inhibits growth
and induces apoptosis in chronic myeloid leukemia cells. Anticancer
Drugs. 25:624–632. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nabbouh AI, Hleihel RS, Saliba JL, Karam
MM, Hamie MH, Wu HCJM, Berthier CP, Tawil NM, Bonnet PAA,
Deleuze-Masquefa C and El Hajj HA: Imidazoquinoxaline derivative
EAPB0503: A promising drug targeting mutant nucleophosmin 1 in
acute myeloid leukemia. Cancer. 123:1662–1673. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
El Hajj R, Youness HB, Lachaud L, Bastien
P, Masquefa C, Bonnet PA, El Hajj H and Khalifeh I: EAPB0503: An
Imiquimod analog with potent in vitro activity against cutaneous
leishmaniasis caused by Leishmania major and Leishmania tropica.
PLoS Negl Trop Dis. 12(e0006854)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Baumann M and Baxendale IR: An overview of
the synthetic routes to the best selling drugs containing
6-membered heterocycles. Beilstein J Org Chem. 9:2265–2319.
2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Rudy SJ: Imiquimod (Aldara): Modifying the
immune response. Dermatol Nurs. 14:268–270. 2002.PubMed/NCBI
|
|
29
|
Miller RL, Gerster JF, Owens ML, Slade HB
and Tomai MA: Imiquimod applied topically: A novel immune response
modifier and new class of drug. Int J Immunopharmacol. 21:1–14.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Smith KJ, Hamza S and Skelton H: The
imidazoquinolines and their place in the therapy of cutaneous
disease. Expert Opin Pharmacother. 4:1105–1119. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Deleuze-Masquefa C, Gerebtzoff G, Subra G,
Fabreguettes JR, Ovens A, Carraz M, Strub MP, Bompart J, George P
and Bonnet PA: Design and synthesis of novel
imidazo[1,2-a]quinoxalines as PDE4 inhibitors. Bioorg Med Chem.
12:1129–1139. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Del Rosso JQ: Topical imiquimod therapy
for actinic keratosis: Is long-term clearance a realistic benefit?
J Clin Aesthet Dermatol. 1:44–47. 2008.PubMed/NCBI
|
|
33
|
Oumata N, Nguyen PH, Beringue V, Soubigou
F, Pang Y, Desban N, Massacrier C, Morel Y, Paturel C, Contesse MA,
et al: The toll-like receptor agonist imiquimod is active against
prions. PLoS One. 8(e72112)2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sidky YA, Borden EC, Weeks CE, Reiter MJ,
Hatcher JF and Bryan GT: Inhibition of murine tumor growth by an
interferon-inducing imidazoquinolinamine. Cancer Res. 52:3528–3533.
1992.PubMed/NCBI
|
|
35
|
Sauder DN, Skinner RB, Fox TL and Owens
ML: Topical imiquimod 5% cream as an effective treatment for
external genital and perianal warts in different patient
populations. Sex Transm Dis. 30:124–128. 2003.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yokogawa M, Takaishi M, Nakajima K,
Kamijima R, Digiovanni J and Sano S: Imiquimod attenuates the
growth of UVB-induced SCC in mice through Th1/Th17 cells. Mol
Carcinog. 52:760–769. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Spaner DE, Miller RL, Mena J, Grossman L,
Sorrenti V and Shi Y: Regression of lymphomatous skin deposits in a
chronic lymphocytic leukemia patient treated with the toll-like
receptor-7/8 agonist, imiquimod. Leuk Lymphoma. 46:935–939.
2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Raman VS, Duthie MS, Fox CB, Matlashewski
G and Reed SG: Adjuvants for Leishmania vaccines: From models to
clinical application. Front Immunol. 3(144)2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hamie M, Najm R, Deleuze-Masquefa C,
Bonnet PA, Dubremetz JF, El Sabban M and El Hajj H: Imiquimod
targets toxoplasmosis through modulating host toll-like
receptor-MyD88 signaling. Front Immunol. 12(629917)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Arevalo I, Ward B, Miller R, Meng TC,
Najar E, Alvarez E, Matlashewski G and Llanos-Cuentas A: Successful
treatment of drug-resistant cutaneous leishmaniasis in humans by
use of imiquimod, an immunomodulator. Clin Infect Dis.
33:1847–1851. 2001.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Miranda-Verastegui C, Tulliano G, Gyorkos
TW, Calderon W, Rahme E, Ward B, Cruz M, Llanos-Cuentas A and
Matlashewski G: First-line therapy for human cutaneous
leishmaniasis in Peru using the TLR7 agonist imiquimod in
combination with pentavalent antimony. PLoS Negl Trop Dis.
3(e491)2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Arevalo I, Tulliano G, Quispe A, Spaeth G,
Matlashewski G, Llanos-Cuentas A and Pollack H: Role of imiquimod
and parenteral meglumine antimoniate in the initial treatment of
cutaneous leishmaniasis. Clin Infect Dis. 44:1549–1554.
2007.PubMed/NCBI View
Article : Google Scholar
|
|
43
|
Walter A, Schäfer M, Cecconi V, Matter C,
Urosevic-Maiwald M, Belloni B, Schönewolf N, Dummer R, Bloch W,
Werner S, et al: Aldara activates TLR7-independent immune defence.
Nat Commun. 4(1560)2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kono T, Kondo S, Pastore S, Shivji GM,
Tomai MA, McKenzie RC and Sauder DN: Effects of a novel topical
immunomodulator, imiquimod, on keratinocyte cytokine gene
expression. Lymphokine Cytokine Res. 13:71–76. 1994.PubMed/NCBI
|
|
45
|
Weber A, Zimmermann C, Mausberg AK,
Kieseier BC, Hartung HP and Hofstetter HH: Induction of
pro-inflammatory cytokine production in thymocytes by the immune
response modifiers Imiquimod and Gardiquimod. Int Immunopharmacol.
17:427–431. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wolf IH, Kodama K, Cerroni L and Kerl H:
Nature of inflammatory infiltrate in superficial cutaneous
malignancies during topical imiquimod treatment. Am J
Dermatopathol. 29:237–241. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wong JG, Toole JWP, Demers AA, Musto G and
Wiseman MC: Topical 5% imiquimod in the treatment of lentigo
maligna. J Cutan Med Surg. 16:245–249. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Schon M and Schon MP: The antitumoral mode
of action of imiquimod and other imidazoquinolines. Curr Med Chem.
14:681–687. 2007.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Schon MP and Schon M: The small-molecule
immune response modifier imiquimod-its mode of action and clinical
use in the treatment of skin cancer. Expert Opin Ther Targets.
10:69–76. 2006.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bilu D and Sauder DN: Imiquimod: Modes of
action. Br J Dermatol. 149 (Suppl 66):5–8. 2003.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Levine E.N.a.V., Role of Topical Therapy:
Imiquimod. 2017.
|
|
52
|
Wagstaff AJ and Perry CM: Topical
imiquimod: A review of its use in the management of anogenital
warts, actinic keratoses, basal cell carcinoma and other skin
lesions. Drugs. 67:2187–2210. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Megyeri K, Au WC, Rosztoczy I, Raj NB,
Miller RL, Tomai MA and Pitha PM: Stimulation of interferon and
cytokine gene expression by imiquimod and stimulation by Sendai
virus utilize similar signal transduction pathways. Mol Cell Biol.
15:2207–2218. 1995.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Sauder DN: Immunomodulatory and
pharmacologic properties of imiquimod. J Am Acad Dermatol.
43:S6–S11. 2000.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Schon MP, Schon M and Klotz KN: The small
antitumoral immune response modifier imiquimod interacts with
adenosine receptor signaling in a TLR7- and TLR8-independent
fashion. J Invest Dermatol. 126:1338–1347. 2006.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Majewski S, Marczak M, Mlynarczyk B,
Benninghoff B and Jablonska S: Imiquimod is a strong inhibitor of
tumor cell-induced angiogenesis. Int J Dermatol. 44:14–19.
2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Denning DP and Hirose T: Anti-tubulins
DEPendably induce apoptosis. Nat Cell Biol. 16:741–743.
2014.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Schön MP and Schön M: Immune modulation
and apoptosis induction: Two sides of the antitumoral activity of
imiquimod. Apoptosis. 9:291–298. 2004.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Bong AB, Bonnekoh B, Franke I, Schön MP,
Ulrich J and Gollnick H: Imiquimod, a topical immune response
modifier, in the treatment of cutaneous metastases of malignant
melanoma. Dermatology. 205:135–138. 2002.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Heikkinen AK and Susitaival P: Severe
systemic reaction to topical imiquimod. Acta Derm Venereol.
91:594–595. 2011.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Cannon PS, O'Donnell B, Huilgol SC and
Selva D: The ophthalmic side-effects of imiquimod therapy in the
management of periocular skin lesions. Br J Ophthalmol.
95:1682–1685. 2011.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Benson E: Imiquimod: Potential risk of an
immunostimulant. Australas J Dermatol. 45:123–124. 2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Somani N, Martinka M, Crawford RI, Dutz JP
and Rivers JK: Treatment of atypical nevi with imiquimod 5% cream.
Arch Dermatol. 143:379–385. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Hanna E, Abadi R and Abbas O: Imiquimod in
dermatology: An overview. Int J Dermatol. 55:831–844.
2016.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Rosen T: Limited extent AIDS-related
cutaneous Kaposi's sarcoma responsive to imiquimod 5% cream. Int J
Dermatol. 45:854–856. 2006.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Ezzell TI, Fromowitz JS and Ramos-Caro FA:
Recurrent pyogenic granuloma treated with topical imiquimod. J Am
Acad Dermatol. 54 (5 Suppl):S244–S245. 2006.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Barba AR, Kapoor S and Berman B: An open
label safety study of topical imiquimod 5% cream in the treatment
of Molluscum contagiosum in children. Dermatol Online J.
7(20)2001.PubMed/NCBI
|
|
68
|
Díaz-Guimaraens B, Saceda-Corralo D,
Hermosa-Gelbard A, Moreno-Arrones ÓM, Dominguez-Santas M,
Suarez-Valle A and Vañó-Galván S: Imiquimod-enhanced immunotherapy
with diphencyprone for patients with alopecia areata. Dermatol
Ther. 35(e15516)2022.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Palefsky JM, Lee JY, Jay N, Goldstone SE,
Darragh TM, Dunlevy HA, Rosa-Cunha I, Arons A, Pugliese JC, Vena D,
et al: Treatment of anal high-grade squamous intraepithelial
lesions to prevent anal cancer. N Engl J Med. 386:2273–2282.
2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Al Fayez N, Rouhollahi E, Ong CY, Wu J,
Nguyen A, Böttger R, Cullis PR, Witzigmann D and Li SD:
Hepatocyte-targeted delivery of imiquimod reduces hepatitis B virus
surface antigen. J Control Release. 350:630–641. 2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Daude M, Dinulescu M, Nguyen JM, Maillard
H, Duff FL, Machet L, Beylot-Barry M, Legoupil D,
Wierzbicka-Hainaut E, Bedane C, et al: Efficacy of imiquimod in the
management of lentigo maligna. J Eur Acad Dermatol Venereol.
27(10.1111/jdv.19141)2023.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Chang SH, Lin PY, Wu TK, Hsu CS, Huang SW,
Li ZY, Liu KT, Kao JK, Chen YJ, Wong TW, et al: Imiquimod-induced
ROS production causes lysosomal membrane permeabilization and
activates caspase-8-mediated apoptosis in skin cancer cells. J
Dermatol Sci. 107:142–150. 2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Urquhart JL and Weston WL: Treatment of
multiple trichoepitheliomas with topical imiquimod and tretinoin.
Pediatr Dermatol. 22:67–70. 2005.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Cantisani C, Lazic T, Richetta AG, Clerico
R, Mattozzi C and Calvieri S: Imiquimod 5% cream use in
dermatology, side effects and recent patents. Recent Pat Inflamm
Allergy Drug Discov. 6:65–69. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Pasadyn SR and Cain R: Topical imiquimod
induces severe weakness and myalgias after three applications: A
case report. J Clin Aesthet Dermatol. 12:58–59. 2019.PubMed/NCBI
|
|
76
|
Safadi MG, Hassan S, Patel V, Viglione M
and Zahner SL: Imiquimod-induced hypertrophic lupus
erythematosus-like reaction. Dermatol Online J.
28(10.5070/D328458526)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Li HO, Aw M and Glassman SJ:
Imiquimod-induced bullous pemphigoid: A case report. SAGE Open Med
Case Rep. 11(2050313x231164222)2023.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Arias NM, Bonino CB, Feal PP, Rico MLP,
Peñaranda JMS and Osorio IV: Lupus-like reaction following
imiquimod treatment for actinic keratoses. Dermatol Ther.
35(e15700)2022.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Raman J, Bisbee E, Missall TA and Saikaly
SK: A case of topical imiquimod induced fatigue. J Dermatolog
Treat. 33:3202–3204. 2022.PubMed/NCBI View Article : Google Scholar
|
|
80
|
McKinzie AH and Christman MA:
Imiquimod-associated localized skin ulceration in a patient with
uncontrolled diabetes. Obstet Gynecol. 140:316–319. 2022.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Martins P, Jesus J, Santos S, Raposo LR,
Roma-Rodrigues C, Baptista PV and Fernandes AR: Heterocyclic
anticancer compounds: Recent advances and the paradigm shift
towards the use of nanomedicine's tool box. Molecules.
20:16852–16891. 2015.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Kumar S, Bawa S and Gupta H: Biological
activities of quinoline derivatives. Mini Rev Med Chem.
9:1648–1654. 2009.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chu XM, Wang C, Liu W, Liang LL, Gong KK,
Zhao CY and Sun KL: Quinoline and quinolone dimers and their
biological activities: An overview. Eur J Med Chem. 161:101–117.
2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Balderas-Renteria I, Gonzalez-Barranco P,
Garcia A, Banik BK and Rivera G: Anticancer drug design using
scaffolds of β-lactams, sulfonamides, quinoline, quinoxaline and
natural products. Drugs advances in clinical trials. Curr Med Chem.
19:4377–4398. 2012.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Afzal O, Kumar S, Haider MR, Ali MR, Kumar
R, Jaggi M and Bawa S: A review on anticancer potential of
bioactive heterocycle quinoline. Eur J Med Chem. 97:871–910.
2015.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Bonilla-Ramirez L, Rios A, Quiliano M,
Ramirez-Calderon G, Beltrán-Hortelano I, Franetich JF, Corcuera L,
Bordessoulles M, Vettorazzi A, de Cerain AL, et al: Novel
antimalarial chloroquine- and primaquine-quinoxaline 1,4-di-N-oxide
hybrids: Design, synthesis, Plasmodium life cycle stage profile,
and preliminary toxicity studies. Eur J Med Chem. 158:68–81.
2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Burguete A, Pontiki E, Hadjipavlou-Litina
D, Ancizu S, Villar R, Solano B, Moreno E, Torres E, Pérez S,
Aldana I and Monge A: Synthesis and biological evaluation of new
quinoxaline derivatives as antioxidant and anti-inflammatory
agents. Chem Biol Drug Des. 77:255–267. 2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Fabian L, Porro MT, Gómez N, Salvatori M,
Turk G, Estrin D and Moglioni A: Design, synthesis and biological
evaluation of quinoxaline compounds as anti-HIV agents targeting
reverse transcriptase enzyme. Eur J Med Chem.
188(111987)2020.PubMed/NCBI View Article : Google Scholar
|
|
89
|
El Newahie AMS, Nissan YM, Ismail NSM, El
Ella DA, Khojah SM and Abouzid KAM: Design and synthesis of new
quinoxaline derivatives as anticancer agents and apoptotic
inducers. Molecules. 24(1175)2019.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Patinote C, Deleuze-Masquéfa C, Kaddour
KH, Vincent LA, Larive R, Zghaib Z, Guichou JF, Assaf MD, Cuq P and
Bonnet PA: Imidazo[1,2-a]quinoxalines for melanoma treatment with
original mechanism of action. Eur J Med Chem.
212(113031)2021.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Lafaille F, Banaigs B, Inguimbert N,
Enjalbal C, Doulain PE, Bonnet PA, Masquefa C and Bressolle FMM:
Characterization of a new anticancer agent, EAPB0203, and its main
metabolites: nuclear magnetic resonance and liquid
chromatography-mass spectrometry studies. Anal Chem. 84:9865–9872.
2012.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Khier S, Deleuze-Masquéfa C, Moarbess G,
Gattacceca F, Margout D, Solassol I, Cooper JF, Pinguet F, Bonnet
PA and Bressolle FMM: Pharmacology of EAPB0203, a novel
imidazo[1,2-a]quinoxaline derivative with anti-tumoral activity on
melanoma. Eur J Pharm Sci. 39:23–29. 2010.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Morjaria S, Deleuze-Masquefa C, Lafont V,
Gayraud S, Bompart J, Bonnet PA and Dornand J: Impairment of
TNF-alpha production and action by imidazo[1,2- alpha]
quinoxalines, a derivative family which displays potential
anti-inflammatory properties. Int J Immunopathol Pharmacol.
19:525–538. 2006.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Lideikaitė A, Mozūraitienė J and
Letautienė S: Analysis of prognostic factors for melanoma patients.
Acta Med Litu. 24:25–34. 2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Drobits B, Holcmann M, Amberg N, Swiecki
M, Grundtner R, Hammer M, Colonna M and Sibilia M: Imiquimod clears
tumors in mice independent of adaptive immunity by converting pDCs
into tumor-killing effector cells. J Clin Invest. 122:575–585.
2012.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Chouchou A, Patinote C, Cuq P, Bonnet PA
and Deleuze-Masquéfa C: Imidazo[1,2-a]quinoxalines derivatives
grafted with amino acids: Synthesis and evaluation on A375 melanoma
cells. Molecules. 23(2987)2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Quereux G and Dreno B: Fotemustine for the
treatment of melanoma. Expert Opin Pharmacother. 12:2891–2904.
2011.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hermine O, Ramos JC and Tobinai K: A
review of new findings in adult T-cell leukemia-lymphoma: A focus
on current and emerging treatment strategies. Adv Ther. 35:135–152.
2018.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Tsukasaki K, Hermine O, Bazarbachi A,
Ratner L, Ramos JC, Harrington W Jr, O'Mahony D, Janik JE,
Bittencourt AL, Taylor GP, et al: Definition, prognostic factors,
treatment, and response criteria of adult T-cell leukemia-lymphoma:
A proposal from an international consensus meeting. J Clin Oncol.
27:453–459. 2009.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Bazarbachi A, Suarez F, Fields P and
Hermine O: How I treat adult T-cell leukemia/lymphoma. Blood.
118:1736–1745. 2011.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Cook LB, Fuji S, Hermine O, Bazarbachi A,
Ramos JC, Ratner L, Horwitz S, Fields P, Tanase A, Bumbea H, et al:
Revised adult T-cell leukemia-lymphoma international consensus
meeting report. J Clin Oncol. 37:677–687. 2019.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Tsukasaki K, Marçais A, Nasr R, Kato K,
Fukuda T, Hermine O and Bazarbachi A: Diagnostic approaches and
established treatments for adult T cell leukemia lymphoma. Front
Microbiol. 11(1207)2020.PubMed/NCBI View Article : Google Scholar
|
|
103
|
El Hajj H, Tsukasaki K, Cheminant M,
Bazarbachi A, Watanabe T and Hermine O: Novel treatments of adult T
cell leukemia lymphoma. Front Microbiol. 11(1062)2020.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Hehlmann R, Heimpel H, Hasford J, Kolb HJ,
Pralle H, Hossfeld DK, Queisser W, Löffler H, Hochhaus A and Heinze
B: Randomized comparison of interferon-alpha with busulfan and
hydroxyurea in chronic myelogenous leukemia. The german CML study
group. Blood. 84:4064–4077. 1994.PubMed/NCBI
|
|
105
|
Bisen A and Claxton DF: Tyrosine kinase
targeted treatment of chronic myelogenous leukemia and other
myeloproliferative neoplasms. Adv Exp Med Biol. 779:179–196.
2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Krause DS and Van Etten RA: Bedside to
bench: Interfering with leukemic stem cells. Nat Med. 14:494–495.
2008.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Okimoto RA and Van Etten RA: Navigating
the road toward optimal initial therapy for chronic myeloid
leukemia. Curr Opin Hematol. 18:89–97. 2011.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Lagunas-Rangel FA, Chávez-Valencia V,
Gómez-Guijosa MA and Cortes-Penagos C: Acute myeloid
leukemia-genetic alterations and their clinical prognosis. Int J
Hematol Oncol Stem Cell Res. 11:328–339. 2017.PubMed/NCBI
|
|
109
|
Yilmaz M, Kantarjian H and Ravandi F:
Acute promyelocytic leukemia current treatment algorithms. Blood
Cancer J. 11(123)2021.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Borthakur G and Kantarjian H: Core binding
factor acute myelogenous leukemia-2021 treatment algorithm. Blood
Cancer J. 11(114)2021.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Molica M, Breccia M, Foa R, Jabbour E and
Kadia TM: Maintenance therapy in AML: The past, the present and the
future. Am J Hematol. 94:1254–1265. 2019.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Kassim AA and Savani BN: Hematopoietic
stem cell transplantation for acute myeloid leukemia: A review.
Hematol Oncol Stem Cell Ther. 10:245–251. 2017.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Lin WY, Fordham SE, Hungate E, Sunter NJ,
Elstob C, Xu Y, Park C, Quante A, Strauch K, Gieger C, et al:
Genome-wide association study identifies susceptibility loci for
acute myeloid leukemia. MedRxiv.
2021(2021.07.22.21259893)2021.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Falini B, Brunetti L, Sportoletti P and
Martelli MP: NPM1-mutated acute myeloid leukemia: from bench to
bedside. Blood. 136:1707–1721. 2020.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wang AJ, Han Y, Jia N, Chen P and Minden
MD: NPM1c impedes CTCF functions through cytoplasmic
mislocalization in acute myeloid leukemia. Leukemia. 34:1278–1290.
2020.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Skayneh H, Jishi B, Hleihel R, Hamie M, El
Hajj R, Deleuze-Masquefa C, Bonnet PA, El Sabban M and El Hajj H:
EAPB0503, an imidazoquinoxaline derivative modulates SENP3/ARF
mediated SUMOylation, and induces NPM1c degradation in NPM1 mutant
AML. Int J Mol Sci. 23(3421)2022.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Sacks D and Noben-Trauth N: The immunology
of susceptibility and resistance to Leishmania major in mice. Nat
Rev Immunol. 2:845–858. 2002.PubMed/NCBI View
Article : Google Scholar
|
|
118
|
Ley SV and Thomas AW: Modern synthetic
methods for copper-mediated C(aryl)(bond)O, C(aryl)[bond]N, and
C(aryl)[bond]S bond formation. Angew Chem Int Ed Engl.
42:5400–5449. 2003.PubMed/NCBI View Article : Google Scholar
|