1. Introduction
One of the major recent advancements which have
provided hope to patients with leukemia/lymphoma cancer involves
the use of cells from the patient's own body or donor for the
treatment of persisting cancer. T-cells are separated from the
patient's body via leukapheresis. The chimeric antigen receptor
(CAR) cells are engineered hybrid T-cell receptors (TCRs) which are
a combination of antibody and TCRs (1). CAR has a dual function: One is to
bind with the tumor antigen and the other is T-cell activation
functions (2). This artificial
receptor is comprised of a single-chain variable fragment (scFv) of
antibodies, linkers, a transmembrane domain and an intracellular
signaling domain. Normally, T-cells have variable α-chain and
β-chain, and a miniscule quantity of T-cells have γ- or δ-chains
and the antibody consists of two heavy chains and two light chains
with one constant and one variable domains in each chain. The scFv
is a heterodimer of variable heavy (VH) and variable light (VL)
domains (3). Thus, the scFv
antibodies which are used in this construct are produced by the
hybridoma technique, having specificity against a particular cancer
antigen (4).
To genetically modify T-cells, mRNA is isolated from
hybridoma cells, then reverse transcribed into complementary DNA
(cDNA), which then serves as a template for amplification. This
cDNA is amplified by PCR and inserted into the TCR gene of the
isolated T-cell (5). This
construction is performed by delivering the stable CAR expression
gene, either by viral or non-viral mode of gene transfer systems.
The most commonly used expression vectors are lentiviral vectors,
σ-retroviral vector and the transposon system (6). This genetically engineered TCR gene
expresses the CAR, having defined specificity. The replacement of
the both the variable α and β domains of the TCR with VH and VL
immunoglobulin homologs results in the CαVH + CβVL or CαVL + CβVH
immunoglobulin composition. In the 1980s, scientists designed CAR
by the addition of genes coding for artificial T-cell receptor-like
proteins. An effective signal for T-cell activation is transmitted
by the chimeric receptors, being expressed on the T-cells. The
substitution of variable TCR region with the antibody homologs have
been proven effective by endowing antibody type specificity
(7). In 1993, Eshhar et al
(8) constructed the T-cell
receptor genes by combining the antibodies variable domain and
T-cell receptor's constant domain. These chimeric T-cells are
customized according to the target epitope binding in a particular
cancer. They effectively bind to the specific epitope in a human
leucocyte antigen (HLA) in an independent manner, which benefits
patients with a decreased expression of the HLA gene. Upon specific
interaction of the antigen and the CAR T-cell receptor, the
signaling cascade is turned on, resulting in tumor elimination.
This therapy can combat a number of issues associated with
chemotherapy and RNAi technology, or even the issues associated
with the treatment of immunocompromised patients. The overall goal
of developing this therapy is to elucidate an effective immune
response, which is generated by cytokine production (9). Although there are both pros and cons
associated with this therapy, continuous efforts are being made to
neutralize and balance these to make it more suitable for use in
treatment.
2. Reasons for the use of CAR T-cell
immunotherapy
Recently, immunotherapy has been viewed as a useful
and alluring therapeutic strategy in a variety of malignancies,
including colorectal cancer. Chimeric antigen receptor (CAR) T-cell
and CAR-natural killer cell therapy are two immunotherapy
techniques that have had notable success, mostly in the treatment
of hematological malignancies (10). A schematic representation of CAR
T-cell immunotherapy is presented in Fig. 1.
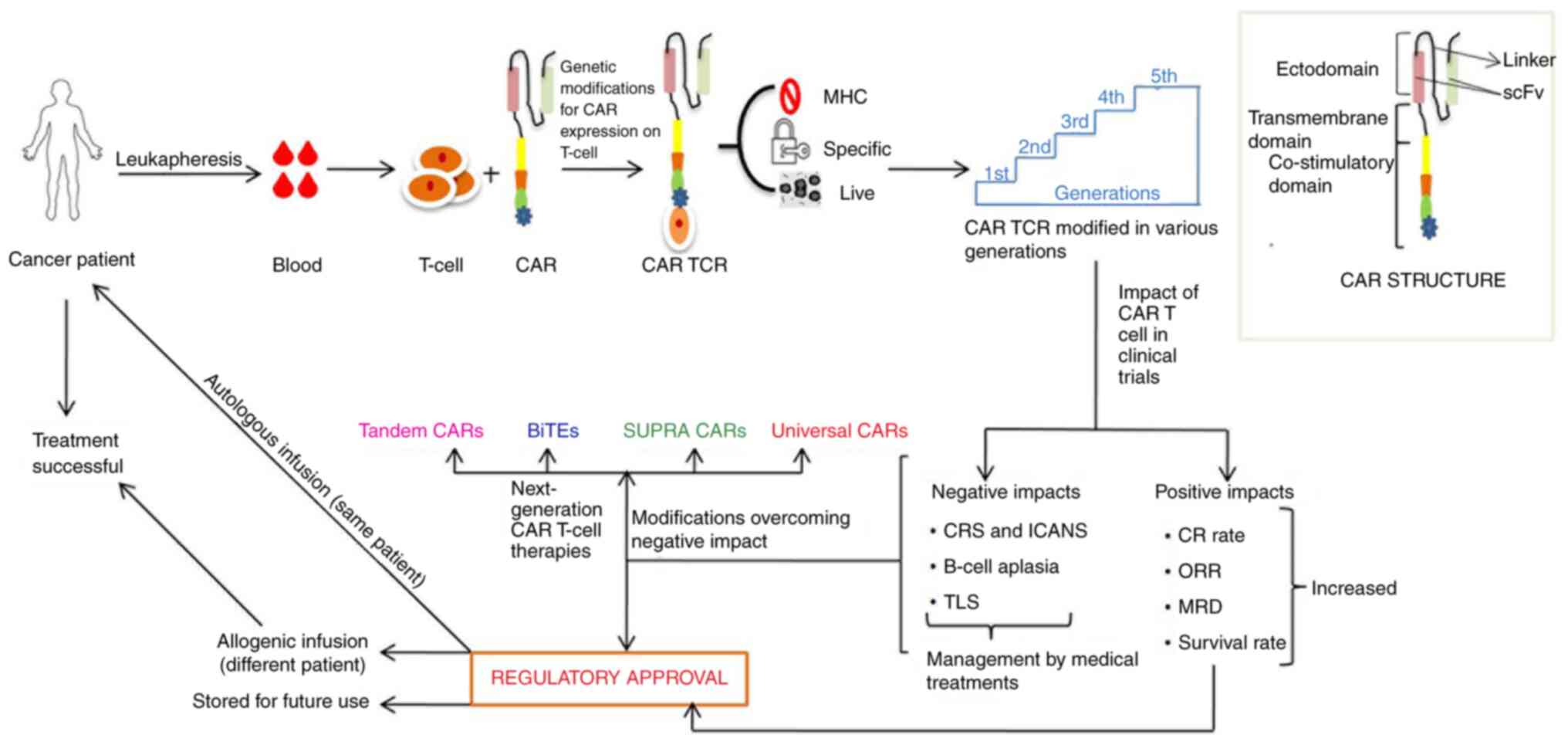 | Figure 1Schematic representation of CAR
T-cell immunotherapy development steps. CAR, chimeric antigen
receptor; TCR, T-cell receptor; MHC, major histocompatibility
complex; CRS, cytokine release syndrome; ICANS, immune effector
cell-associated neurotoxicity syndrome; TLS, tumor lysis syndrome;
CR, complete response; ORR, objective response rate; MRD, minimal
residual disease; scFv, single-chain variable fragment; BiTEs,
bi-specific T-cell engagers. |
CAR T-cell immunotherapy has become a life-saving
approach, highly compatible with biomolecules of the human body, as
drugs from this immunotherapy began to gain Food and Drug
Administration (FDA) approval since 2017(11). Currently, CAR T-cell immunotherapy
has become a more acceptable approach due to the following
reasons:
i) Major histocompatibility complex
(MHC) independence
The T-cell response is only produced when the
antigen is processed and presented by the protein known as MHC or
MHC molecules. Following the recognition of the antigen, a triad
complex, comprising of antigenic peptide, MHC and T-cells forms,
which activates the signaling cascade to eliminate the tumor;
however, defects exist in the machinery, which downregulates the
signaling and hence, allow for tumor escape (12). To overcome this issue,
immunotherapy needs to be MHC-independent, and this therapy follows
the same route of independence that benefits the MHC
downregulation.
ii) CD4+ and
CD8+ T-cell redirection to CAR TCR
The T-cell subsets, CD4+ and
CD8+, can be redirected for target cell recognition by
detouring both the classes of MHC molecule restriction.
CD4+ CAR and CD8+ CAR T-cells are potent
cytotoxic cells against defined opted targets, although
CD8+ CAR T-cells are considered more potent (13).
iii) Live drugs
CAR T-cell immunotherapy is known as a ‘living drug
as the specific T-cells used in this therapy are first obtained by
leukapheresis then modified into a robust receptor and
subsequently, infused into the patient with leukemia/lymphoma. In
the case that the infusion is performed in the same patient from
whom the T-cells were obtained, this is termed autologous infusion,
whereas in the case that the cells are administered to a different
patient, this is termed allogenic infusion. In the whole process of
CAR T-cell generation, the T-cells do not lose their potency. This
CAR TCR recognizes and kills the cancer cells possessing a specific
antigen on their surface. Their persistence is high due to their
capacity for proliferation, signal initiation and adequate killing
of cancer cells until the antigen is present on its surface
(14). CAR T-cells have a high
proliferative capacity, which enables maximum interaction and
accessibility with the tumor antigen for more efficient tumor
clearance.
iv) Specificity
The immune response is initiated, only upon the
specific interaction of the CAR and the target cell, which results
in the production of IFN-γ, IL-6 and IL-15(15). This specificity is acquired due to
the presence of the single-chain variable fragment (scFv) region of
the antibody. The basic structure of CAR has a single ζ-chain,
which produces cytokines, thus rendering these CAR T-cells more
specific towards their target, and various generations of CAR
T-cells are developed. A summary of CAR T-cell immunotherapy drugs
of various generations and trial phases is presented Table I.
 | Table ICAR-T Cell Immunotherapy drugs of
various generations in trial phases. |
Table I
CAR-T Cell Immunotherapy drugs of
various generations in trial phases.
| Generations of CAR
T-cells | Targeted
antigen | Clinical trial
ID | Type of cancer | Phase | (Refs.) |
|---|
| First | FR-α | NCT00019136 | Ovarian cancer | I | (50) |
| | CAIX | DDHK97-29 | Renal
carcinoma | II | (51,52) |
| | L1-CAM | NCT00006480 | Neuroblastoma | I | (53) |
| | IL13Rα2 | NCT00730613 | Glioblastoma | I | (23) |
| | | NCT01082926 | Glioblastoma | I | (54) |
| | GD2 | NCT00085930 | Neuroblastoma | I (Active, not
recruiting) | (55) |
| Second | MSLN | NCT01355965 | Malignant pleural
mesothelioma | I | (56,57) |
| | HER2 | NCT01109095 | Glioblastoma
multiforme | I | (58) |
| | CEA | NCT01373047 | Liver
metastases | I | (59,60) |
| | | NCT02416466 | Liver
metastases | I | (61) |
| | FAP | NCT01722149 | Malignant pleural
mesothelioma | I | (62) |
| | MUC16ecto | NCT02498912 | Solid tumors | I | (63) |
| Third | EGFRvIII | NCT01454596 | Glioma,
glioblastoma, brain tumor | I/II | (64) |
| | GD2 | NCT01822652 | Neuroblastoma | I (Active, not
recruiting) | (65) |
| | | NCT01953900 | Sarcomas | I (Active, not
recruiting) | (66) |
| | | NCT02107963 | Osteosarcoma,
neuroblastoma, melanoma | I | (67) |
| Fourth | FR-α | NCT03185468 | Urothelial
cancer | I/II
(recruiting) | (68) |
| | GD2 | NCT02765243 | Neuroblastoma | II (suspended) | (69) |
| | PSMA | NCT03185468 | Bladder cancer | I(recruiting) | (70) |
3. The CAR T-cell generations
The CAR T-cell is classified into different
generations depending on the type and number of co-stimulatory
domains attached to the construct. These domains are altered to
elevate the level of cytokine production for optimal tumor cell
killing; cytokines are small protein or peptide or glycoprotein
messengers released on the specific interaction between cell to
cell or cell to a receptor. They have a broad anti-tumor spectrum,
as it recruits immune effector cells at the tumor site, enhances
tumor cell recognition, recruits natural killer cells, inhibits p53
tumor-suppressor function and enhances T cell function (16). The response generated by the CAR
TCR is solely dependent upon the activation domain. The
generational classification is illustrated in Fig. 2A.
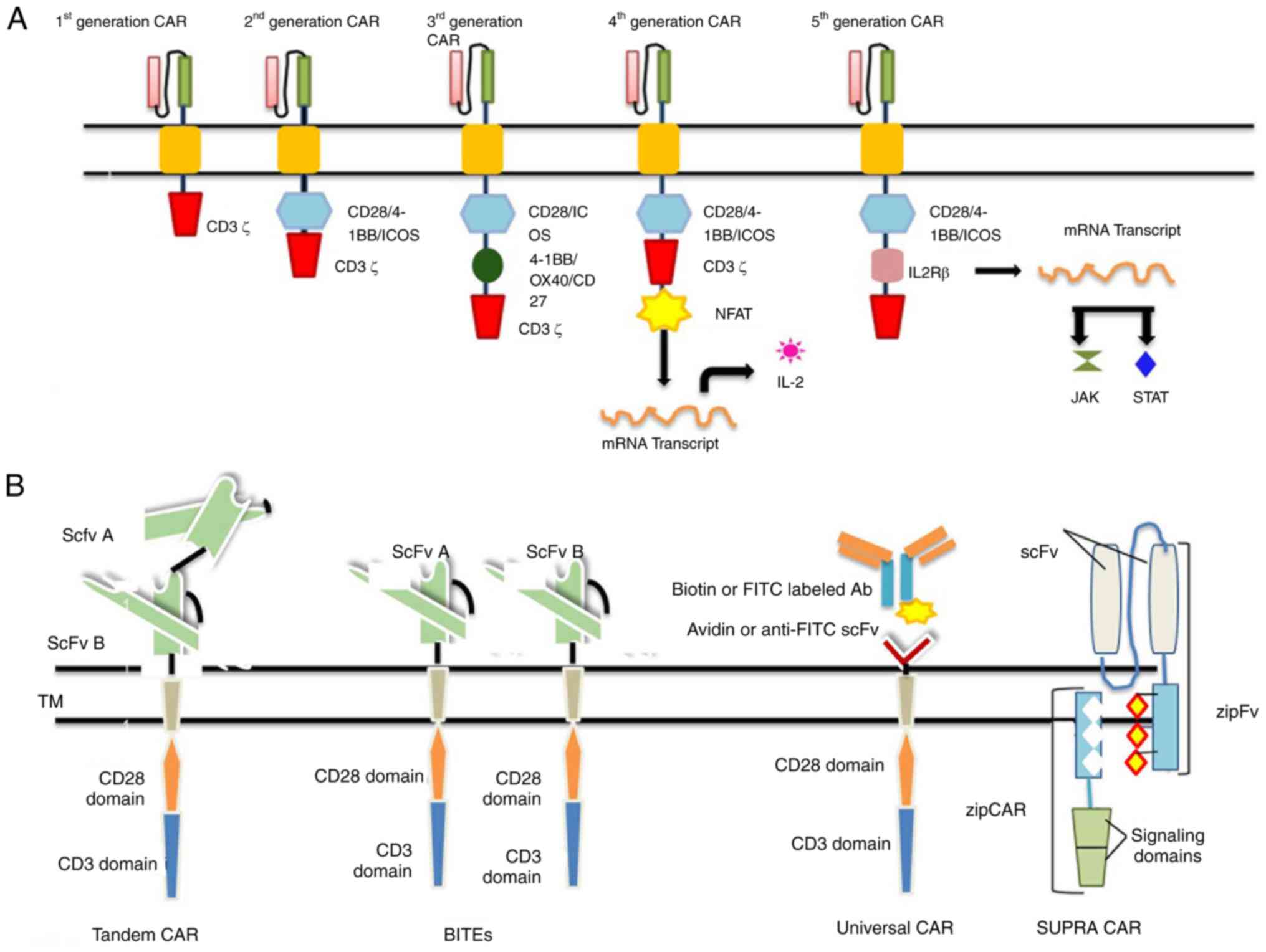 | Figure 2(A) CAR T-cell generations. (B)
Next-generation CARs. CAR, chimeric antigen receptor; NFAT, nuclear
factor of activated T-cells; STAT, signal transducer and activator
of transcription; scFv, single-chain variable fragment; BiTEs,
bi-specific T-cell engagers; CAR, chimeric antigen receptor; NFAT,
nuclear factor of activated T-cells; STAT, signal transducer and
activator of transcription; scFv, single-chain variable fragment;
BiTEs, bi-specific T-cell engagers. |
First-generation CARs
The first-generation CARs have an intracellular
signaling CD3ζ domain or FcRγ coupled with scFv domain.
Experimentally, it has been determined that ζ-chain signaling is
insufficient for CAR T-cell persistence and a lasting response
(8).
Second-generation CARs
The lack of an extra co-stimulatory domain results
in T-cell apoptosis; thus, second-generation CARs are required. To
increase the level of cytokine production, co-stimulatory signaling
segments, such as CD28 and 4-1BB (or CD137) are used for the
construction of second-generation CAR T-cells. The CAR TCR
acknowledges the antigenic peptide MHC complex, and the signal is
then transduced from the co-stimulatory domain, which renders the
generation of cytokine IL-2 to activate the T-cells (17). Second-generation CAR drugs, such as
tisagenlecleucel (Kymriah) and axicabtagene (Yescarta) ciloleucel
have been approved by US FDA respectively (18,19).
Third-generation CARs
The early exhaustion of second-generation CARs has
led to the development of third-generation CARs, which are
constructed by uniting several co-stimulatory domains.
CD3ζ-CD28-41BB or CD3ζ-CD28-OX40 is used in combination to increase
cytokine production and exhaustion (20); to enhance the antitumor properties,
fourth-generation CARs have been designed.
Fourth-generation CARs
The fourth-generation CAR T-cells are termed T-cells
redirected for universal cytokine-mediated killing (TRUCKs). These
CAR T-cells are modified by the integration of the desired gene
construct in the promoter region, which alters the expression of
the TCR gene. This desired gene codes for cytokines that will be
delivered to the tumor site. When the CAR binds to the antigenic
epitope on tumor cells, it initiates the CD3ζ chain downstream
signaling that causes the phosphorylation of nuclear factor of
activated T-cells (NFAT), a T cell transcription factor; thus, NFAT
is activated, which in turn activates the TCR that attaches to the
NFAT-response element-IL-2 promoter. This results in the production
of the transgenic proteins (i.e., cytokines) which accumulate at
the target site to eliminate cancer cells with minimal toxicity to
normal cells (21).
Fifth-generation CARs
The need for fifth-generation CAR T-cells emerged
due to insufficient tumor elimination and the partial activation of
antitumor T-cell functions. For optimal tumor elimination, proper
cytokine engagement is a crucial step for improved T-cell
activation and proliferation. This generation differs, it has a
novel CAR design with a supplementary IL-2Rβ activation domain
between the CD28 and CD3ζ domains of the CAR. Furtermore, by the
addition of a tyrosine motif by site-directed mutagenesis at the
C-terminus of the CD3ζ domain, the signal transducer and activator
of transcription (STAT) transcription factor is recruited as the
antigen binds to the CAR T-cell. The signal is then transduced for
the recruitment of STAT, which promotes CAR T-cell proliferation
and promotes the activation of programmed death ligand in tumor
cells. Ultimately, this will result in tumor eradication to a
maximum extent (22). The drugs
used in this immunotherapy belonging to all the aforementioned five
generations are markedly efficient in blood cancers and pave the
way towards their use in the treatment of solid cancers as well.
Drugs for the treatment of solid cancers are currently in clinical
trials, as presented in Table
I.
4. Positive impact of CAR T-cell
immunotherapy on patients
CAR T-cell immunotherapy, as a modern-day
innovation, is customized according to the selected target present
on the cancer cell. The selected targets are proteins, which are
widely expressed on the malignant B-cell surface, benefiting the
specific CAR and target interaction. The target antigen should be
cancer-specific; particularly, CD19, CD22, CD33, CD38, CD5, CD7,
IL3A, SDCI, MS4A1, NCAM1 and ULBPI are used as prominent targets.
The main antigens targeted in solid cancers are Her2, CEA, GD2,
PSMA and CAIX (23). Thus, the
impacts of these targeted drugs are listed as follows:
High complete remission (CR) rate
CR indicates the disappearance of all the signs of
cancer in response to the therapy. The efficiency of this therapy
is summarized in Table II,
demonstrating increased CR rates in approved drugs. The
conventional chemotherapy had only a 20-40% CR rate or symptom
reduction; however, this immunotherapy treatment results in a
68-93% CR rate, which is improved compared with other available
immunotherapies (24,25).
 | Table IICharacteristics of approved drugs for
CAR T-cell immunotherapy. |
Table II
Characteristics of approved drugs for
CAR T-cell immunotherapy.
| Sr. no. | CAR-T approved
drugs | Type of
lymphoma | Scfv | Costimulatory
domain | Other names | Trial study | Cells extracted
(defined) | ORR (%) | CR (%) | Clinical trial
ID | (Refs.) |
|---|
| 1 | Axicabtagene
ciloleucel | Adult DLBCL | FMC63 | CD28 | KTE-C19,
Axi-cel | ZUMA-1 | No | 82 | 54 | NCT03391466 | (71,72) |
| 2 |
Tisagenlecleucel | Adult DLBCL | FMC63 | 4-1BB CD137 | CTL019,
CART-19 | JULIET | No | 54 | 40 | NCT02445248 | (73) |
| 3 | Lisocabtagene
maraleucel | Adult DLBCL | FMC63 | 4-1BB CD137 | Liso-cel,
JCAR017 | TRANSCEND-001 | CD4:CD8
(fixed) | 73 | 53 | NCT02631044 | (74) |
| 4 | Idecabtagene
vicleucel | RR-MM | Anti-BCMA | CD137 4-1BB and
CD3-ζ | Ide-cel,
bb2121 | KarMMa | CD4:CD8
(variable) | 73 | 45 | NCT03361748 | (75) |
| 5 | Brexucabtagene
autoleucel | MCL | Anti-CD19 | CD28 and CD3-
ζ | Tecartus,
KTE-X19 | ZUMA-2 | No | 87 | 62 | NCT02601313 | (76) |
High objective response rate
(ORR)
ORR indicates the percentage of patients with a
reduced tumor size within a specific period. The ORR following CAR
T-immunotherapy was found in the range of 58-87%, which indicates a
sufficient amount of tumor size reduction in patients (26).
Elevated minimal residual disease
(MRD)
MRD indicates the number of cancer cells remaining
either during therapy or following therapy. An elevated
MRD-negative CR was obtained in the range of 75-93% (27,28).
Higher survival rate
As previously demonstrated, 75 to 90% of patients
with a specific type of leukemia such as acute lymphoblastic
leukemia, chronic lymphocytic leukemia, Hodgkin's lymphoma and
B-cell non-Hodgkin's lymphoma recovered following this
immunotherapy; this thus indicates a high survival rate of patients
treated with CAR T-cell immunotherapy (29,30).
Easily permeable in the blood-brain
barrier process
Drugs usually lack the ability to cross the
blood-brain barrier or to penetrate in the tumor tissue to access
the antigen in solid tumors; however, CAR constructs have the
potential to activate existing T-cells inside the body that can
even cross the blood-brain barrier.
Availability
This therapy has been approved to treat two groups
of patients, one is adults and the other is children, as well as
young adults <25 year of age. There are various ongoing studies
evaluating the effectiveness of CAR T-cell therapy in pregnant
women (31). CAR T-cell therapy
can be administered to patients with any stage of cancer, although
if the tumor burden is very high it is used in combination with
chemotherapy or radiation priming (32). Independence from HLA recognition
makes it easier for any patient with a downregulated or very low
HLA gene expression to opt for this type of treatment.
5. Post-therapy challenges and their
management
The success of the CAR T-cell therapy is notable;
however, in some patients, there are associated side-effects that
can be managed with various medical treatments, and are either
resolved on their own or require further modification in the CAR
T-cell construct. Some of these are as follows:
Cytokine release syndrome (CRS) and
immune effector cell-associated neurotoxicity syndrome (ICANS)
CRS is a systemic inflammatory response caused by
the interaction between CAR T-cells and the mononuclear phagocyte
lineage cells. This is commonly known as the ‘cytokine storm’,
which is the response produced due to a high level of cytokine
production. These effects can be controlled by blocking the
cytokine receptors (33). The
second most acute toxicity associated with CAR T-cell immunotherapy
is ICANS, which presents with symptoms, such as aphasia, confusion
and difficulty in finding words, which may lead to depression,
seizures, coma, motor weakness and cerebral edema (34). Neurotoxicity can be managed by the
addition of more specificity in the receptor genes. These
toxicities have been graded into groups as presented in Table III.
 | Table IIIToxicity level of CAR T-cell
immunotherapy in B-cell lymphoma with CD19 as the target. |
Table III
Toxicity level of CAR T-cell
immunotherapy in B-cell lymphoma with CD19 as the target.
| Sr. no. | Drug study
name | JULIET (%) | TRANSCEND (%) | ZUMA-1 (%) | (Ref.) |
|---|
| 1 | CRS (all
grades) | 58 | 37 | 93 | (77) |
| 2 | CRS (grade ≥3) | 22 | 1 | 13 | |
| 3 | Neurotoxicity (all
grades) | 21 | 25 | 65 | |
| 4 | Grade ≥3 (cytopenia
ongoing on day 30) | 32 | N/A | 28 | |
B-cell aplasia (off the tumor and on
target)
B-cell aplasia is the destruction of normal B-cells
or when anti-CD19 CAR T-cells inadvertently damage normal
B-lymphocytes that express CD19, which causes
hypogammaglobulinemia; consequently, patients are typically at a
high risk of developing infections. Despite the death of 1 patient
from influenza A, the infections have been shown to be manageable
with a low mortality rate. This can be controlled by intravenous
immunoglobulin replacement therapy which increases the antibody
levels in the body (35,36).
Tumor lysis syndrome (TLS) and
anaphylaxis
As a result of this immunotherapy, tumor cells are
broken down, which release constituents into the bloodstream that
leads to the development of hypocalcemia, hyperkalemia,
hyperphosphatemia and hyperuricemia (37). TLS can be managed carefully by
maintaining fluid uptake, balancing the electrolytes, etc.
Anaphylaxis is a severe allergic reaction caused by the domains of
the murine antibody origin (38);
efforts are being made to improvise the construct into the
humanized source.
6. Next-generation CAR T-cell therapies
Tandem CARs (Tan-CARs)
Tan-CARs have a unique structure, comprising two
domains joined by a linker in a tandem orientation to the
intracellular signaling domain; both are expressed together as one
single unit of CAR on the cell surface. This synchronized targeting
of both antigens enhances the therapeutic potential of this
immunotherapy by elucidating an effective immune response. This
increases their avidity and therapeutic potential (39). Grada et al (40) constructed and studied the trivalent
CAR T-cells that co-targeted multiple antigens one after the other,
as a result of which glioblastoma tumor was cleared with 100%
efficiency.
Bi-specific T-cell engagers
To overcome the cancer relapse due to tumor escape,
bispecific or dual CARs are synthesized. Bi-specific T-cell
engagers (BiTEs) are recombinant bispecific proteins that have two
linked scFvs of monoclonal antibodies. The only drug to gain FDA
approval on March 29, 2018 was Blinatumomab (BLINCYTO®;
Amgen, Inc.) for the treatment of acute lymphoblastic leukemia,
which belongs to the BiTEs generation of CARs (41). One end of scFv targets CD19 and the
other targets CD3. This drug drives a synergistic cascade of
effector molecules by activating CD4+ and
CD8+ T-effector memory cells; as a result, the optimal
killing of tumor cells is achieved (42).
Universal CARs
To increase the antigen specificity, scalability and
widen the antigen recognition spectrum, improved CARs are designed.
The universal CARs are constructed using a ‘third party’, which
could be biotin or anti-fluorescein isothiocyanate (FITC) scFv
region (43). The extracellular
region is composed of avidin, joined with an intracellular T-cell
region. The biotin attached to the CARs is recognized by the
biotin-binding immune receptors, which switches on the signaling
cascade; hence, the tumor is eliminated. CD19-uCAR-T cells
(NCT03229876) are under clinical trials for the treatment of acute
lymphoblastic leukemia and non-Hodgkin's lymphoma (44).
SUPRA CARs
A system having two constituents was invented to
improve the controllability and flexibility of CAR T-cells. One of
the two is the universal receptor termed zip-CAR, and the other
scFv adaptor segment which targets the tumor is termed zipFv. zipFv
is a combination of the leucine zipper and scFv domain of the
antibody and zip-CAR is another leucine zipper, that is attached to
the CAR expressed on T-cells (45). The targeted antigen binds to the
scFv domain, and the zipFv binds to the zipCAR that activates the
T-cells and interferons are produced in response to it. To alter
the level of T-cell response, binding affinity can be altered,
these alterations can be used either to upregulate or downregulate
the T-cell activation; even a zipFv with no antigen specificity can
terminate the toxicity. The SUPRA CAR model allows for multiple
targeting without any genetic manipulations (46). These CAR systems can be used to
elevate tumor recognition precision. The next-generation CAR
T-cells are illustrated in Fig.
2B.
7. Challenges and limitations associated
with CAR T-cell therapy
The therapeutic application of CAR T-cell therapy
for solid tumors has advanced significantly. However, there are a
number of obstacles to CAR T-cell therapy in solid tumors that are
related to the tumor microenvironment, including the absence of
tumor-specific antigen, a poor CAR T-cell trafficking efficiency,
migration into tumor locations and the presence of an
immunosuppressive tumor microenvironment (47).
Tumor resistance to single-antigen targeting CAR
constructions is one of the most difficult limitations of CAR
T-cell treatment. The malignant cells of a sizable fraction of
patients treated with these CAR T-cells exhibit either a partial or
complete loss of target antigen expression, despite the fact that
single-antigen targeting CAR T-cells initially have the potential
to produce high response rates; this is known as antigen escape
(48). Another limitation is the
selective pressure of the CAR T-cells that can cause tumor cells to
downregulate antigens. On-target off-tumor effects can still occur
even with proper antigen targeting, leading to related toxicity
(49).
8. Conclusions and future perspectives
Genetic engineering has played a vital role in the
diagnosis and treatment of various ailments, one of the major
achievements being CAR T-cell immunotherapy. This immunotherapy is
becoming the most effective and prominent treatment for various
types of cancer, with an improved survival rate along with fewer
post-therapy side-effects in comparison to other therapies
available for cancer treatments. These are the new generation
treatment options with better persistence, proliferation and tumor
elimination. These CAR T-cells are on the way to being stored in
reservoirs serving as off-the-shelf therapeutic cells. The race to
design the most efficient CARs with the least possible
side-effects, has come a long way from the development of new CAR
T-cells from the laboratory to the therapeutic approach. Continuous
improvements have been made to cover all types of cancers,
including solid cancers. CAR T-cell immunotherapy drugs developed
for solid cancers are currently undergoing clinical trials for
approval; once approved these drugs will open a new door for cancer
treatment. Modifications are required to construct humanized CAR
T-cells which can cover and treat almost all types of cancer with a
maximum survival rate and minimal post-therapeutic challenges.
Acknowledgements
The authors would like to express their sincere
gratitude to Professor Javed Musarrat, the Hon'ble Vice-Chancellor
of Integral University Lucknow, India, for his motivational support
and for providing the necessary facility. The authors would also
like to give special thanks to Dr Saif Khan (Department of Basic
Dental and Medical Sciences, College of Dentistry, University of
Ha'il, Ha'il 2440, Saudi Arabia) for supporting the conception of
the present review.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
All the authors contributed equally to the
preparation and design of the manuscript. AA was involved in the
conception and design of the study. MH was involved in the
articulation the contents, and in drafting and editing the
manuscript. SAK was involved in data mining for data to be included
in the present review. FK was involved in editing the manuscript.
NA was involved in editing the technical part of the manuscript. SK
was involved in the organization of the data to be included in the
review. SJ was involved in the design the figures and tables. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Poondla N, Sheykhhasan M, Akbari M, Samadi
P, Kalhor N and Manoochehri H: The Promise of CAR T-Cell therapy
for the treatment of cancer stem cells: A short review. Curr Stem
Cell Res Ther. 17:400–406. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maus MV and Levine BL: Chimeric Antigen
Receptor T-Cell Therapy for the Community Oncologist. Oncologist.
21:608–617. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stoiber S, Cadilha BL, Benmebarek MR,
Lesch S, Endres S and Kobold S: Limitations in the design of
chimeric antigen receptors for cancer therapy. Cells.
8(472)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen
NB and Hamid M: scFv Antibody: Principles and clinical application.
Clin Dev Immunol. 2012(980250)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li D, Li X, Zhou WL, Huang Y, Liang X,
Jiang L, Yang X, Sun J, Li Z, Han WD and Wang W: Genetically
engineered T cells for cancer immunotherapy. Signal Transduct
Target Ther. 4(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao L and Cao YJ: Engineered T cell
therapy for cancer in the clinic. Front Immunol.
10(2250)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gross G, Gorochov G, Waks T and Eshhar Z:
Generation of effector T cells expressing chimeric T cell receptor
with antibody type-specificity. Transplant Proc. 21:127–130.
1989.PubMed/NCBI
|
|
8
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and
T-cell receptors. Proc Natl Acad Sci USA. 90:720–724.
1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhylko A, Winiarska M and Graczyk-Jarzynka
A: The great war of today: Modifications of CAR-T cells to
effectively combat malignancies. Cancers (Basel).
12(2030)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maalej KM, Merhi M, Inchakalody VP,
Mestiri S, Alam M, Maccalli C, Cherif H, Uddin S, Steinhoff M,
Marincola FM and Dermime S: CAR-cell therapy in the era of solid
tumor treatment: Current challenges and emerging therapeutic
advances. Mol Cancer. 22(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
FDA approval brings first gene therapy to
the United States. https://www.fda.gov/news-events/fda-newsroom/press-announcements,
Accessed February 15, 2021.
|
|
12
|
Chmielewski M, Hombach AA and Abken H:
Antigen-specific T-cell activation independently of the MHC:
Chimeric antigen receptor-redirected T Cells. Front Immunol.
4(371)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lustgarten J, Waks T and Eshhar Z: CD4 and
CD8 accessory molecules function through interactions with major
histocompatibility complex molecules which are not directly
associated with the T cell receptor-antigen complex. Eur J Immunol.
21:2507–2515. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miliotou AN and Papadopoulou LC: CAR
T-cell Therapy: A New Era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van der Schans JJ, van de Donk NWCJ and
Mutis T: Dual targeting to overcome current challenges in multiple
myeloma CAR T-Cell treatment. Front Oncol. 10(1362)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kany S, Vollrath JT and Relja B: Cytokines
in inflammatory disease. Int J Mol Sci. 20(6008)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramos CA, Savoldo B and Dotti G: CD19-CAR
trials. Cancer J. 20:112–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Diehn M, Alizadeh AA, Rando OJ, Liu CL,
Stankunas K, Botstein D, Crabtree GR and Brown PO: Genomic
expression programs and the integration of the CD28 costimulatory
signal in T cell activation. Proc Natl Acad Sci USA.
99:11796–11801. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
US Food, Drug Administration: KYMRIAH
(tisagenlecleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel.
Accessed February, 2021.
|
|
20
|
Weinkove R, George P, Dasyam N and
McLellan AD: Selecting costimulatory domains for chimeric antigen
receptors: Functional and clinical considerations. Clin Transl
Immunology. 8(e1049)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S,
Restifo NP, Rosenberg SA and Morgan RA: Improving adoptive T cell
therapy by targeting and controlling IL-12 expression to the tumor
environment. Mol Ther. 19:751–759. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kagoya Y, Tanaka S, Guo T, Anczurowski M,
Wang CH, Saso K, Butler MO, Minden MD and Hirano N: A novel
chimeric antigen receptor containing a JAK-STAT signaling domain
mediates superior antitumor effects. Nat Med. 24:352–359.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wei J, Han X, Bo J and Han W: Target
selection for CAR-T therapy. J Hematol Oncol. 12(62)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Z, Guo Y and Han W: Current status
and perspectives of chimeric antigen receptor modified T cells for
cancer treatment. Protein Cell. 8:896–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Aykan NF and Ozatlı T: Objective response
rate assessment in oncology: Current situation and future
expectations. World J Clin Oncol. 11:53–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6(224ra25)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gardner RA, Finney O, Annesley C, Brakke
H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S,
Kelly-Spratt KS, et al: Intent-to-treat leukemia remission by CD19
CAR T cells of defined formulation and dose in children and young
adults. Blood. 129:3322–3331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol.
10(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei G, Hu Y, Pu C, Yu J, Luo Y, Shi J, Cui
Q, Wu W, Wang J, Xiao L, et al: CD19 targeted CAR-T therapy versus
chemotherapy in re-induction treatment of refractory/relapsed acute
lymphoblastic leukemia: Results of a case-controlled study. Ann
Hematol. 97:781–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pacenta HL, Laetsch TW and John S: CD19
CAR T cells for the treatment of pediatric Pre-B cell acute
lymphoblastic leukemia. Paediatr Drugs. 22:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q,
Chen X, Zhou M, Xia F, Ye A, et al: Radiation priming chimeric
antigen receptor T-Cell therapy in relapsed/refractory diffuse
large B-Cell lymphoma with high tumor Burden. J Immunother.
43:32–37. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
24:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yanez L, Alarcon A, Sanchez Escamilla M
and Perales MA: How I treat adverse effects of CAR-T cell therapy.
ESMO Open. 4(Suppl 4)(e000746)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cordeiro A, Bezerra ED, Hirayama AV, Hill
JA, Wu QV, Voutsinas J, Sorror ML, Turtle CJ, Maloney DG and Bar M:
Late events after treatment with CD19-Targeted chimeric antigen
receptor modified T cells. Biol Blood Marrow Transplant. 26:26–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Park JH, Romero FA, Taur Y, Sadelain M,
Brentjens RJ, Hohl TM and Seo SK: Cytokine release Syndrome grade
as a predictive marker for infections in patients with relapsed or
refractory B-Cell acute lymphoblastic leukemia treated with
chimeric antigen receptor T cells. Clin Infect Dis. 67:533–540.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abu-Alfa AK and Younes A: Tumor lysis
syndrome and acute kidney injury: Evaluation, prevention, and
management. Am J Kidney Dis. 55 (Suppl 3):S1–S19; quiz S14-9.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schneider D, Xiong Y, Wu D, Nӧlle V,
Schmitz S, Haso W, Kaiser A, Dropulic B and Orentas RJ: A tandem
CD19/CD20 CAR lentiviral vector drives on-target and off-target
antigen modulation in leukemia cell lines. J Immunother Cancer.
5(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grada Z, Hegde M, Byrd T, Shaffer DR,
Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al:
TanCAR: A novel bispecific chimeric antigen receptor for cancer
immunotherapy. Mol Ther Nucleic Acids. 2(e105)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Malard F and Mohty M: Acute lymphoblastic
leukaemia. Lancet. 395:1146–1162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bargou R, Leo E, Zugmaier G, Klinger M,
Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et
al: Tumor regression in cancer patients by very low doses of a T
cell-engaging antibody. Science. 321:974–977. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lohmueller JJ, Ham JD, Kvorjak M and Finn
OJ: mSA2 affinity-enhanced biotin-binding CAR T cells for universal
tumor targeting. Oncoimmunology. 7(e1368604)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi H, Sun M, Liu L and Wang Z: Chimeric
antigen receptor for adoptive immunotherapy of cancer: Latest
research and future prospects. Mol Cancer. 13(219)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cho JH, Collins JJ and Wong WW: Universal
chimeric antigen receptors for multiplexed and logical control of T
cell responses. Cell. 173:1426–1438.e11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen YY: Increasing T cell versatility
with SUPRA CARs. Cell. 173:1316–1317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang H and Pan W: Challenges of chimeric
antigen receptor-T/natural killer cell therapy in the treatment of
solid tumors: Focus on colorectal cancer and evaluation of
combination therapies. Mol Cell Biochem. 478:967–980.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hossain N, Sahaf B, Abramian M, Spiegel
JY, Kong K, Kim S, Mavroukakis S, Oak J, Natkunam Y, Meyer EH, et
al: Phase I experience with a Bi-specific CAR targeting CD19 and
CD22 in adults with B-cell malignancies. Blood. 132 (Suppl
1)(S490)2018.
|
|
49
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immunotherapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lamers CH, Sleijfer S, van Steenbergen S,
van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M,
Oosterwijk E, Debets R and Gratama JW: Treatment of metastatic
renal cell carcinoma with CAIX CAR-engineered T cells. Clinical
evaluation and management of on-target toxicity. Mol Ther.
21:904–912. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lamers CH, Langeveld SC, Groot-van Ruijven
CM, Debets R, Sleijfer S and Gratama JW: Gene-modified T cells for
adoptive immunotherapy of renal cell cancer maintain
transgene-specific immune functions in vivo. Cancer Immunol
Immunother. 56:1875–1883. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR and Jensen MC: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al:
Antitumour activity and long-term fate of chimeric antigen
receptor-positive T cells in patients with neuroblastoma. Blood.
118:6050–6056. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumour-specific
receptors: Persistence and antitumour activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Beatty GL, Haas AR, Maus MV, Torigian DA,
Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al:
Mesothelin-specific chimeric antigen receptor mRNA-engineered T
cells induce anti-tumour activity in solid malignancies. Cancer
Immunol Res. 2:112–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Maus MV, Haas AR, Beatty GL, Albelda SM,
Levine BL, Liu X, Zhao Y, Kalos M and June CH: T cells expressing
chimeric antigen receptors can cause anaphylaxis in humans. Cancer
Immunol. 1:26–31. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ahmed N, Brawley V, Hegde M, Bielamowicz
K, Wakefield A, Ghazi A, Ashoori A, Diouf O, Gerken C, Landi D, et
al: Autologous HER2 CMV bispecific CAR T cells are safe and
demonstrate clinical benefit for glioblastoma in a Phase I trial. J
Immunother Cancer. 3 (Suppl 2)(S011)2015.
|
|
59
|
Katz SC, Burga RA, McCormack E, Wang LJ,
Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al:
Phase I hepatic immunotherapy for metastases study of
intra-arterial chimeric antigen receptor-modified T-cell therapy
for CEA+ liver metastases. Clin Cancer Res. 21:3149–3159.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Saied A, Licata L, Burga RA, Thorn M,
McCormack E, Stainken BF, Assanah EO, Khare PD, Davies R, Espat NJ,
et al: Neutrophil: lymphocyte ratios and serum cytokine changes
after hepatic artery chimeric antigen receptor-modified T-cell
infusions for liver metastases. Cancer Gene Ther. 21:457–462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Katz SC, Point GR, Cunetta M, Thorn M,
Guha P, Espat NJ, Boutros C, Hanna N and Junghans RP: Regional
CAR-T cell infusions for peritoneal carcinomatosis are superior to
systemic delivery. Cancer Gene Ther. 23:142–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Petrausch U, Schuberth PC, Hagedorn C,
Soltermann A, Tomaszek S, Stahel R, Weder W and Renner C:
Re-directed T cells for the treatment of fibroblast activation
protein (FAP)-positive malignant pleural mesothelioma (FAPME-1).
BMC Cancer. 12(615)2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Koneru M, O'Cearbhaill R, Pendharkar S,
Spriggs DR and Brentjens RJ: A phase I clinical trial of adoptive T
cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric
antigen receptors for recurrent ovarian cancer. J Transl Med.
13(102)2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Morgan RA, Johnson LA, Davis JL, Zheng Z,
Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, et
al: Recognition of glioma stem cells by genetically modified T
cells targeting EGFRvIII and development of adoptive cell therapy
for glioma. Hum Gene Ther. 23:1043–1053. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Heczey A, Liu D, Tian G, Courtney AN, Wei
J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, et al: Invariant NKT
cells with chimeric antigen receptor provide a novel platform for
safe and effective cancer immunotherapy. Blood. 124:2824–2833.
2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tanaka M, Tashiro H, Omer B, Lapteva N,
Ando J, Ngo M, Mehta B, Dotti G, Kinchington PR, Leen AM, et al:
Vaccination Targeting native receptors to enhance the function and
proliferation of chimeric antigen receptor (CAR)-Modified T Cells.
Clin Cancer Res. 23:3499–3509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Stroncek DF, Lee DW, Ren J, Sabatino M,
Highfill S, Khuu H, Shah NN, Kaplan RN, Fry TJ and Mackall CL:
Elutriated lymphocytes for manufacturing chimeric antigen receptor
T cells. J Transl Med. 15(59)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
ClinicalTrials.gov: Intervention of Bladder Cancer by
CAR-T. https://clinicaltrials.gov/ct2/show/NCT03185468,
Accessed March 13, 2021.
|
|
69
|
ClinicalTrials.gov: Anti-GD2 4th Generation Chimeric
Antigen Receptor-modified T Cells (4SCAR-GD2) Targeting Refractory
and/or Recurrent Neuroblastoma. https://clinicaltrials.gov/ct2/show/NCT02765243,
Accessed March 13, 2021 (2016).
|
|
70
|
ClinicalTrials.gov: Intervention of Advanced or
Metastatic Urothelial Bladder Cancer by 4SCAR-T Cell Therapies,
https://clinicaltrials.gov/ct2/show/NCT03185468,
Accessed March 5, 2021 (2017).
|
|
71
|
Jain MD, Bachmeier CA, Phuoc VH and Chavez
JC: Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy
for the treatment of relapsed/refractory aggressive B-cell
non-Hodgkin's lymphoma. Ther Clin Risk Manag. 14:1007–1017.
2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Vairy S, Garcia JL, Teira P and
Bittencourt H: CTL019 (tisagenlecleucel): CAR-T therapy for
relapsed and refractory B-cell acute lymphoblastic leukemia. Drug
Des Devel Ther. 12:3885–3898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Abramson JS, Palomba ML, Gordon LI,
Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG,
Andreadis C, et al: Lisocabtagene maraleucel for patients with
relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001):
A multicentre seamless design study. Lancet. 396:839–852.
2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rodríguez-Lobato LG, Ganzetti M, Fernandez
de Larrea C, Hudecek M, Einsele H and Danhof S: CAR T-Cells in
Multiple Myeloma: State of the Art and Future Directions. Front
Oncol. 10(1243)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Clinical Trials Arena. Tecartus
(brexucabtagene autoleucel) for the Treatment of Mantle Cell
Lymphoma (MCL). https://www.clinicaltrialsarena.com/projects/tecartus-brexucabtagene-autoleucel/.
Accessed April, 2021 (2020).
|
|
77
|
Neelapu SS: Managing the toxicities of CAR
T-cell therapy. Hematol Oncol. 37 (Suppl 1):S48–S52.
2019.PubMed/NCBI View Article : Google Scholar
|
















