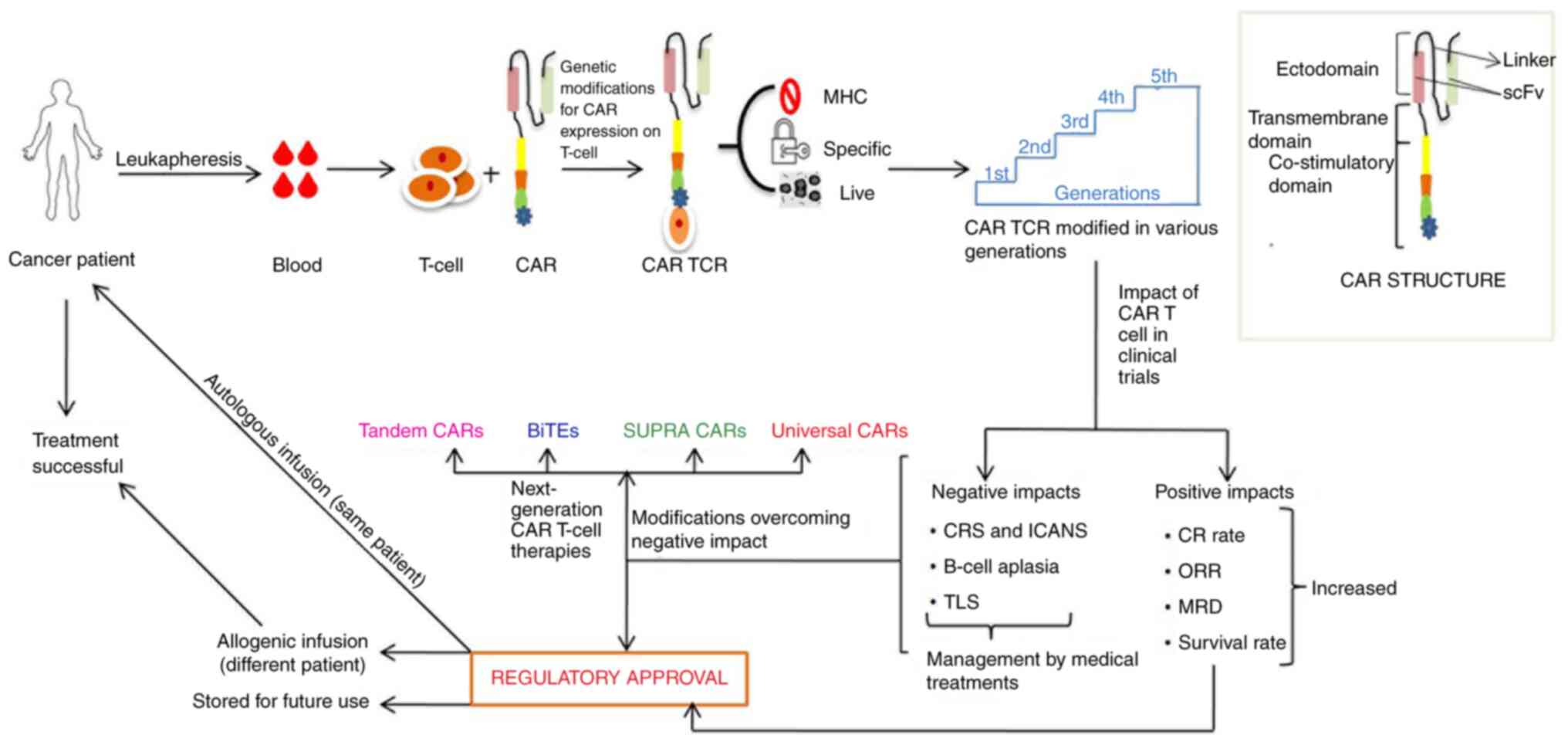
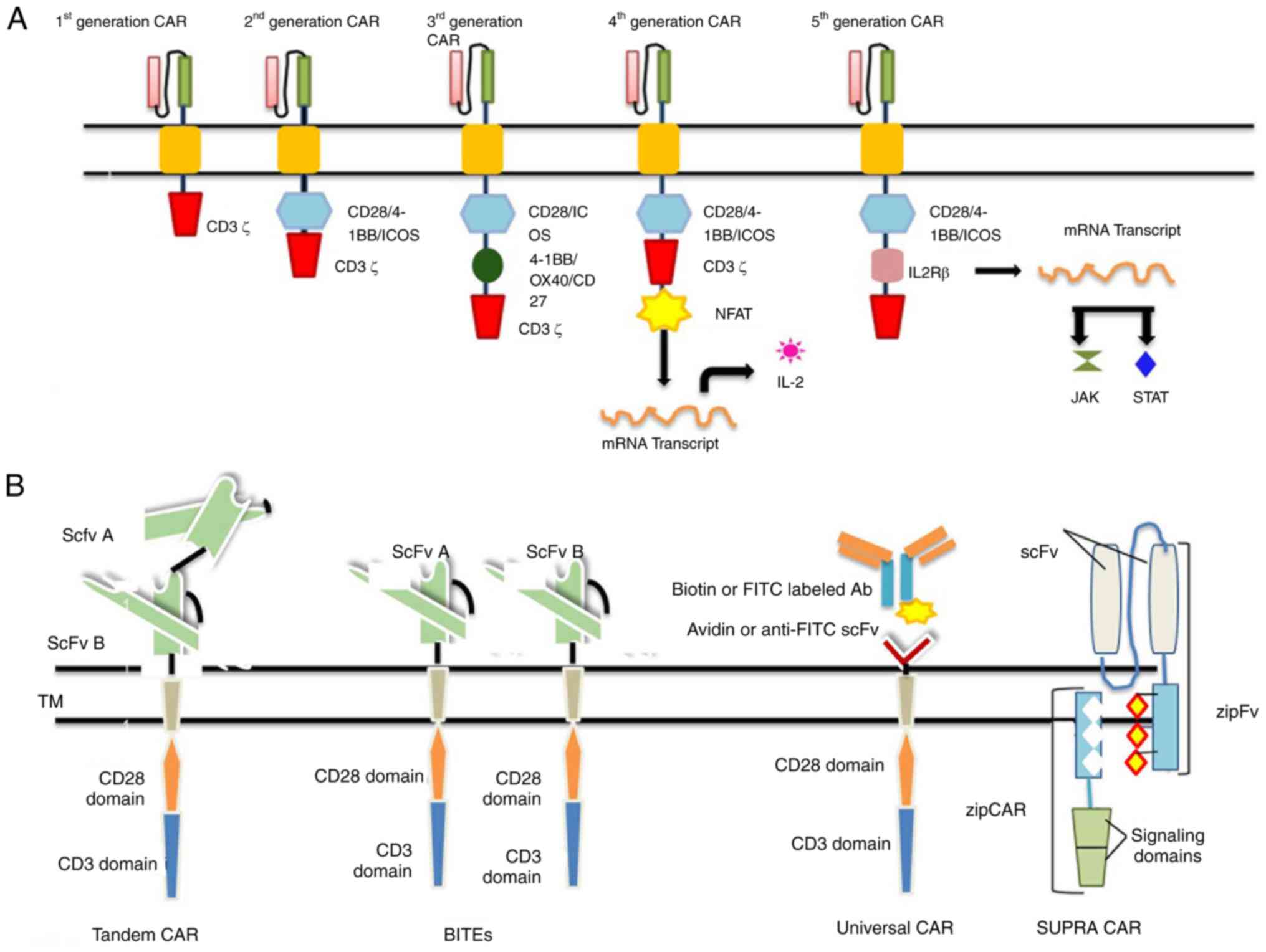
|
1
|
Poondla N, Sheykhhasan M, Akbari M, Samadi
P, Kalhor N and Manoochehri H: The Promise of CAR T-Cell therapy
for the treatment of cancer stem cells: A short review. Curr Stem
Cell Res Ther. 17:400–406. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Maus MV and Levine BL: Chimeric Antigen
Receptor T-Cell Therapy for the Community Oncologist. Oncologist.
21:608–617. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stoiber S, Cadilha BL, Benmebarek MR,
Lesch S, Endres S and Kobold S: Limitations in the design of
chimeric antigen receptors for cancer therapy. Cells.
8(472)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ahmad ZA, Yeap SK, Ali AM, Ho WY, Alitheen
NB and Hamid M: scFv Antibody: Principles and clinical application.
Clin Dev Immunol. 2012(980250)2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li D, Li X, Zhou WL, Huang Y, Liang X,
Jiang L, Yang X, Sun J, Li Z, Han WD and Wang W: Genetically
engineered T cells for cancer immunotherapy. Signal Transduct
Target Ther. 4(35)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao L and Cao YJ: Engineered T cell
therapy for cancer in the clinic. Front Immunol.
10(2250)2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gross G, Gorochov G, Waks T and Eshhar Z:
Generation of effector T cells expressing chimeric T cell receptor
with antibody type-specificity. Transplant Proc. 21:127–130.
1989.PubMed/NCBI
|
|
8
|
Eshhar Z, Waks T, Gross G and Schindler
DG: Specific activation and targeting of cytotoxic lymphocytes
through chimeric single chains consisting of antibody-binding
domains and the gamma or zeta subunits of the immunoglobulin and
T-cell receptors. Proc Natl Acad Sci USA. 90:720–724.
1993.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhylko A, Winiarska M and Graczyk-Jarzynka
A: The great war of today: Modifications of CAR-T cells to
effectively combat malignancies. Cancers (Basel).
12(2030)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maalej KM, Merhi M, Inchakalody VP,
Mestiri S, Alam M, Maccalli C, Cherif H, Uddin S, Steinhoff M,
Marincola FM and Dermime S: CAR-cell therapy in the era of solid
tumor treatment: Current challenges and emerging therapeutic
advances. Mol Cancer. 22(20)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
FDA approval brings first gene therapy to
the United States. https://www.fda.gov/news-events/fda-newsroom/press-announcements,
Accessed February 15, 2021.
|
|
12
|
Chmielewski M, Hombach AA and Abken H:
Antigen-specific T-cell activation independently of the MHC:
Chimeric antigen receptor-redirected T Cells. Front Immunol.
4(371)2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lustgarten J, Waks T and Eshhar Z: CD4 and
CD8 accessory molecules function through interactions with major
histocompatibility complex molecules which are not directly
associated with the T cell receptor-antigen complex. Eur J Immunol.
21:2507–2515. 1991.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Miliotou AN and Papadopoulou LC: CAR
T-cell Therapy: A New Era in cancer immunotherapy. Curr Pharm
Biotechnol. 19:5–18. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
van der Schans JJ, van de Donk NWCJ and
Mutis T: Dual targeting to overcome current challenges in multiple
myeloma CAR T-Cell treatment. Front Oncol. 10(1362)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kany S, Vollrath JT and Relja B: Cytokines
in inflammatory disease. Int J Mol Sci. 20(6008)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ramos CA, Savoldo B and Dotti G: CD19-CAR
trials. Cancer J. 20:112–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Diehn M, Alizadeh AA, Rando OJ, Liu CL,
Stankunas K, Botstein D, Crabtree GR and Brown PO: Genomic
expression programs and the integration of the CD28 costimulatory
signal in T cell activation. Proc Natl Acad Sci USA.
99:11796–11801. 2002.PubMed/NCBI View Article : Google Scholar
|
|
19
|
US Food, Drug Administration: KYMRIAH
(tisagenlecleucel). https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel.
Accessed February, 2021.
|
|
20
|
Weinkove R, George P, Dasyam N and
McLellan AD: Selecting costimulatory domains for chimeric antigen
receptors: Functional and clinical considerations. Clin Transl
Immunology. 8(e1049)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Zhang L, Kerkar SP, Yu Z, Zheng Z, Yang S,
Restifo NP, Rosenberg SA and Morgan RA: Improving adoptive T cell
therapy by targeting and controlling IL-12 expression to the tumor
environment. Mol Ther. 19:751–759. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kagoya Y, Tanaka S, Guo T, Anczurowski M,
Wang CH, Saso K, Butler MO, Minden MD and Hirano N: A novel
chimeric antigen receptor containing a JAK-STAT signaling domain
mediates superior antitumor effects. Nat Med. 24:352–359.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Wei J, Han X, Bo J and Han W: Target
selection for CAR-T therapy. J Hematol Oncol. 12(62)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang Z, Guo Y and Han W: Current status
and perspectives of chimeric antigen receptor modified T cells for
cancer treatment. Protein Cell. 8:896–925. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Maude SL, Frey N, Shaw PA, Aplenc R,
Barrett DM, Bunin NJ, Chew A, Gonzalez VE, Zheng Z, Lacey SF, et
al: Chimeric antigen receptor T cells for sustained remissions in
leukemia. N Engl J Med. 371:1507–1517. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Aykan NF and Ozatlı T: Objective response
rate assessment in oncology: Current situation and future
expectations. World J Clin Oncol. 11:53–73. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Davila ML, Riviere I, Wang X, Bartido S,
Park J, Curran K, Chung SS, Stefanski J, Borquez-Ojeda O, Olszewska
M, et al: Efficacy and toxicity management of 19-28z CAR T cell
therapy in B cell acute lymphoblastic leukemia. Sci Transl Med.
6(224ra25)2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gardner RA, Finney O, Annesley C, Brakke
H, Summers C, Leger K, Bleakley M, Brown C, Mgebroff S,
Kelly-Spratt KS, et al: Intent-to-treat leukemia remission by CD19
CAR T cells of defined formulation and dose in children and young
adults. Blood. 129:3322–3331. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wang Z, Wu Z, Liu Y and Han W: New
development in CAR-T cell therapy. J Hematol Oncol.
10(53)2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wei G, Hu Y, Pu C, Yu J, Luo Y, Shi J, Cui
Q, Wu W, Wang J, Xiao L, et al: CD19 targeted CAR-T therapy versus
chemotherapy in re-induction treatment of refractory/relapsed acute
lymphoblastic leukemia: Results of a case-controlled study. Ann
Hematol. 97:781–789. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pacenta HL, Laetsch TW and John S: CD19
CAR T cells for the treatment of pediatric Pre-B cell acute
lymphoblastic leukemia. Paediatr Drugs. 22:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Qu C, Ping N, Kang L, Liu H, Qin S, Wu Q,
Chen X, Zhou M, Xia F, Ye A, et al: Radiation priming chimeric
antigen receptor T-Cell therapy in relapsed/refractory diffuse
large B-Cell lymphoma with high tumor Burden. J Immunother.
43:32–37. 2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee DW, Gardner R, Porter DL, Louis CU,
Ahmed N, Jensen M, Grupp SA and Mackall CL: Current concepts in the
diagnosis and management of cytokine release syndrome. Blood.
24:188–195. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yanez L, Alarcon A, Sanchez Escamilla M
and Perales MA: How I treat adverse effects of CAR-T cell therapy.
ESMO Open. 4(Suppl 4)(e000746)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cordeiro A, Bezerra ED, Hirayama AV, Hill
JA, Wu QV, Voutsinas J, Sorror ML, Turtle CJ, Maloney DG and Bar M:
Late events after treatment with CD19-Targeted chimeric antigen
receptor modified T cells. Biol Blood Marrow Transplant. 26:26–33.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Park JH, Romero FA, Taur Y, Sadelain M,
Brentjens RJ, Hohl TM and Seo SK: Cytokine release Syndrome grade
as a predictive marker for infections in patients with relapsed or
refractory B-Cell acute lymphoblastic leukemia treated with
chimeric antigen receptor T cells. Clin Infect Dis. 67:533–540.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Abu-Alfa AK and Younes A: Tumor lysis
syndrome and acute kidney injury: Evaluation, prevention, and
management. Am J Kidney Dis. 55 (Suppl 3):S1–S19; quiz S14-9.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Curran KJ, Pegram HJ and Brentjens RJ:
Chimeric antigen receptors for T cell immunotherapy: Current
understanding and future directions. J Gene Med. 14:405–415.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Schneider D, Xiong Y, Wu D, Nӧlle V,
Schmitz S, Haso W, Kaiser A, Dropulic B and Orentas RJ: A tandem
CD19/CD20 CAR lentiviral vector drives on-target and off-target
antigen modulation in leukemia cell lines. J Immunother Cancer.
5(42)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Grada Z, Hegde M, Byrd T, Shaffer DR,
Ghazi A, Brawley VS, Corder A, Schönfeld K, Koch J, Dotti G, et al:
TanCAR: A novel bispecific chimeric antigen receptor for cancer
immunotherapy. Mol Ther Nucleic Acids. 2(e105)2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Malard F and Mohty M: Acute lymphoblastic
leukaemia. Lancet. 395:1146–1162. 2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Bargou R, Leo E, Zugmaier G, Klinger M,
Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, et
al: Tumor regression in cancer patients by very low doses of a T
cell-engaging antibody. Science. 321:974–977. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lohmueller JJ, Ham JD, Kvorjak M and Finn
OJ: mSA2 affinity-enhanced biotin-binding CAR T cells for universal
tumor targeting. Oncoimmunology. 7(e1368604)2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Shi H, Sun M, Liu L and Wang Z: Chimeric
antigen receptor for adoptive immunotherapy of cancer: Latest
research and future prospects. Mol Cancer. 13(219)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cho JH, Collins JJ and Wong WW: Universal
chimeric antigen receptors for multiplexed and logical control of T
cell responses. Cell. 173:1426–1438.e11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chen YY: Increasing T cell versatility
with SUPRA CARs. Cell. 173:1316–1317. 2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wang H and Pan W: Challenges of chimeric
antigen receptor-T/natural killer cell therapy in the treatment of
solid tumors: Focus on colorectal cancer and evaluation of
combination therapies. Mol Cell Biochem. 478:967–980.
2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Hossain N, Sahaf B, Abramian M, Spiegel
JY, Kong K, Kim S, Mavroukakis S, Oak J, Natkunam Y, Meyer EH, et
al: Phase I experience with a Bi-specific CAR targeting CD19 and
CD22 in adults with B-cell malignancies. Blood. 132 (Suppl
1)(S490)2018.
|
|
49
|
Sterner RC and Sterner RM: CAR-T cell
therapy: Current limitations and potential strategies. Blood Cancer
J. 11(69)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Kershaw MH, Westwood JA, Parker LL, Wang
G, Eshhar Z, Mavroukakis SA, White DE, Wunderlich JR, Canevari S,
Rogers-Freezer L, et al: A phase I study on adoptive immunotherapy
using gene-modified T cells for ovarian cancer. Clin Cancer Res.
12:6106–6115. 2006.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Lamers CH, Sleijfer S, van Steenbergen S,
van Elzakker P, van Krimpen B, Groot C, Vulto A, den Bakker M,
Oosterwijk E, Debets R and Gratama JW: Treatment of metastatic
renal cell carcinoma with CAIX CAR-engineered T cells. Clinical
evaluation and management of on-target toxicity. Mol Ther.
21:904–912. 2013.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lamers CH, Langeveld SC, Groot-van Ruijven
CM, Debets R, Sleijfer S and Gratama JW: Gene-modified T cells for
adoptive immunotherapy of renal cell cancer maintain
transgene-specific immune functions in vivo. Cancer Immunol
Immunother. 56:1875–1883. 2007.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Park JR, Digiusto DL, Slovak M, Wright C,
Naranjo A, Wagner J, Meechoovet HB, Bautista C, Chang WC, Ostberg
JR and Jensen MC: Adoptive transfer of chimeric antigen receptor
re-directed cytolytic T lymphocyte clones in patients with
neuroblastoma. Mol Ther. 15:825–833. 2007.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Louis CU, Savoldo B, Dotti G, Pule M, Yvon
E, Myers GD, Rossig C, Russell HV, Diouf O, Liu E, et al:
Antitumour activity and long-term fate of chimeric antigen
receptor-positive T cells in patients with neuroblastoma. Blood.
118:6050–6056. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pule MA, Savoldo B, Myers GD, Rossig C,
Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, et al:
Virus-specific T cells engineered to coexpress tumour-specific
receptors: Persistence and antitumour activity in individuals with
neuroblastoma. Nat Med. 14:1264–1270. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Beatty GL, Haas AR, Maus MV, Torigian DA,
Soulen MC, Plesa G, Chew A, Zhao Y, Levine BL, Albelda SM, et al:
Mesothelin-specific chimeric antigen receptor mRNA-engineered T
cells induce anti-tumour activity in solid malignancies. Cancer
Immunol Res. 2:112–120. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Maus MV, Haas AR, Beatty GL, Albelda SM,
Levine BL, Liu X, Zhao Y, Kalos M and June CH: T cells expressing
chimeric antigen receptors can cause anaphylaxis in humans. Cancer
Immunol. 1:26–31. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ahmed N, Brawley V, Hegde M, Bielamowicz
K, Wakefield A, Ghazi A, Ashoori A, Diouf O, Gerken C, Landi D, et
al: Autologous HER2 CMV bispecific CAR T cells are safe and
demonstrate clinical benefit for glioblastoma in a Phase I trial. J
Immunother Cancer. 3 (Suppl 2)(S011)2015.
|
|
59
|
Katz SC, Burga RA, McCormack E, Wang LJ,
Mooring W, Point GR, Khare PD, Thorn M, Ma Q, Stainken BF, et al:
Phase I hepatic immunotherapy for metastases study of
intra-arterial chimeric antigen receptor-modified T-cell therapy
for CEA+ liver metastases. Clin Cancer Res. 21:3149–3159.
2015.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Saied A, Licata L, Burga RA, Thorn M,
McCormack E, Stainken BF, Assanah EO, Khare PD, Davies R, Espat NJ,
et al: Neutrophil: lymphocyte ratios and serum cytokine changes
after hepatic artery chimeric antigen receptor-modified T-cell
infusions for liver metastases. Cancer Gene Ther. 21:457–462.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Katz SC, Point GR, Cunetta M, Thorn M,
Guha P, Espat NJ, Boutros C, Hanna N and Junghans RP: Regional
CAR-T cell infusions for peritoneal carcinomatosis are superior to
systemic delivery. Cancer Gene Ther. 23:142–148. 2016.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Petrausch U, Schuberth PC, Hagedorn C,
Soltermann A, Tomaszek S, Stahel R, Weder W and Renner C:
Re-directed T cells for the treatment of fibroblast activation
protein (FAP)-positive malignant pleural mesothelioma (FAPME-1).
BMC Cancer. 12(615)2012.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Koneru M, O'Cearbhaill R, Pendharkar S,
Spriggs DR and Brentjens RJ: A phase I clinical trial of adoptive T
cell therapy using IL-12 secreting MUC-16(ecto) directed chimeric
antigen receptors for recurrent ovarian cancer. J Transl Med.
13(102)2015.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Morgan RA, Johnson LA, Davis JL, Zheng Z,
Woolard KD, Reap EA, Feldman SA, Chinnasamy N, Kuan CT, Song H, et
al: Recognition of glioma stem cells by genetically modified T
cells targeting EGFRvIII and development of adoptive cell therapy
for glioma. Hum Gene Ther. 23:1043–1053. 2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Heczey A, Liu D, Tian G, Courtney AN, Wei
J, Marinova E, Gao X, Guo L, Yvon E, Hicks J, et al: Invariant NKT
cells with chimeric antigen receptor provide a novel platform for
safe and effective cancer immunotherapy. Blood. 124:2824–2833.
2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Tanaka M, Tashiro H, Omer B, Lapteva N,
Ando J, Ngo M, Mehta B, Dotti G, Kinchington PR, Leen AM, et al:
Vaccination Targeting native receptors to enhance the function and
proliferation of chimeric antigen receptor (CAR)-Modified T Cells.
Clin Cancer Res. 23:3499–3509. 2017.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Stroncek DF, Lee DW, Ren J, Sabatino M,
Highfill S, Khuu H, Shah NN, Kaplan RN, Fry TJ and Mackall CL:
Elutriated lymphocytes for manufacturing chimeric antigen receptor
T cells. J Transl Med. 15(59)2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
ClinicalTrials.gov: Intervention of Bladder Cancer by
CAR-T. https://clinicaltrials.gov/ct2/show/NCT03185468,
Accessed March 13, 2021.
|
|
69
|
ClinicalTrials.gov: Anti-GD2 4th Generation Chimeric
Antigen Receptor-modified T Cells (4SCAR-GD2) Targeting Refractory
and/or Recurrent Neuroblastoma. https://clinicaltrials.gov/ct2/show/NCT02765243,
Accessed March 13, 2021 (2016).
|
|
70
|
ClinicalTrials.gov: Intervention of Advanced or
Metastatic Urothelial Bladder Cancer by 4SCAR-T Cell Therapies,
https://clinicaltrials.gov/ct2/show/NCT03185468,
Accessed March 5, 2021 (2017).
|
|
71
|
Jain MD, Bachmeier CA, Phuoc VH and Chavez
JC: Axicabtagene ciloleucel (KTE-C19), an anti-CD19 CAR T therapy
for the treatment of relapsed/refractory aggressive B-cell
non-Hodgkin's lymphoma. Ther Clin Risk Manag. 14:1007–1017.
2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Neelapu SS, Locke FL, Bartlett NL, Lekakis
LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T,
Lin Y, et al: Axicabtagene Ciloleucel CAR T-Cell Therapy in
Refractory Large B-Cell Lymphoma. N Engl J Med. 377:2531–2544.
2017.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Vairy S, Garcia JL, Teira P and
Bittencourt H: CTL019 (tisagenlecleucel): CAR-T therapy for
relapsed and refractory B-cell acute lymphoblastic leukemia. Drug
Des Devel Ther. 12:3885–3898. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Abramson JS, Palomba ML, Gordon LI,
Lunning MA, Wang M, Arnason J, Mehta A, Purev E, Maloney DG,
Andreadis C, et al: Lisocabtagene maraleucel for patients with
relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001):
A multicentre seamless design study. Lancet. 396:839–852.
2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rodríguez-Lobato LG, Ganzetti M, Fernandez
de Larrea C, Hudecek M, Einsele H and Danhof S: CAR T-Cells in
Multiple Myeloma: State of the Art and Future Directions. Front
Oncol. 10(1243)2020.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Clinical Trials Arena. Tecartus
(brexucabtagene autoleucel) for the Treatment of Mantle Cell
Lymphoma (MCL). https://www.clinicaltrialsarena.com/projects/tecartus-brexucabtagene-autoleucel/.
Accessed April, 2021 (2020).
|
|
77
|
Neelapu SS: Managing the toxicities of CAR
T-cell therapy. Hematol Oncol. 37 (Suppl 1):S48–S52.
2019.PubMed/NCBI View Article : Google Scholar
|