Introduction
The association between severe acute respiratory
syndrome-coronavirus 2 (SARS-CoV-2) infection and the occurrence of
autoimmune cytopenia has become a matter of concern during the
coronavirus disease 2019 (COVID-19) pandemic. Since the early
spread of the virus, pulmonary impairment has appeared to be the
main feature of SARS-CoV-2 infection, in accordance with its
primary transmission mode via droplets, not differing from the main
characteristics of SARS-CoV (1,2).
However, numerous cases of thrombocytopenia secondary to COVID-19
have appeared almost immediately, alongside several other different
complications (3-8).
The present study describes the case of a female
patient admitted to the Infectious Diseases Unit in ‘Gaetano
Martino’ Hospital, Messina, Italy for severe anaemia and COVID-19;
while the respiratory symptoms were absent during hospitalization,
anaemia appeared to be the preponderant feature during the
infection. The possible association between SARS-CoV-2 infection
and autoimmune anaemia has to be considered as one of the potential
complications of COVID-19, just as COVID-19 has already been
recognized as having an important association with thrombocytopenia
(9). Autoimmune haemolytic anaemia
(AIHA) represents a heterogeneous group of pathologies
characterised by the abnormal formation of autoantibodies that bind
to antigens on the membrane of red blood cells (RBCs) and determine
their destruction. Among the factors that may contribute to the
development of AIHA, there are viral infections and
inflammation.
In order to further evaluate the association between
AIHA and SARS-CoV-2 infection, the present study performed a search
of the literature for other reported cases of COVID-19-related
autoimmune anaemia. The association between viral infections and
autoimmune haematological disorders is well known. The development
of numerous variants of the virus has posed the need for further
investigations of COVID-19 (10,11).
Case Report
The present study describes the case of a Caucasian
61-year-old female patient, weighing 67 kg; she had a clinical
history of hypertension, asthma, a previous episode of
leishmaniasis, a previous cerebral stroke, and thyroid cancer
treated with a total thyroidectomy; at the time of admission, she
was in treatment with levothyroxine. There was no history of
autoimmune diseases or anaemia.
Symptoms, such as coughing and throat ache commenced
in late November, 2020, 1 month prior to hospitalisation;
therefore, she began therapy with levofloxacin and betamethasone.
At 1 week after the onset of the first symptoms, her coughing and
throat ache disappeared; however, she tested positive to two
nasopharyngeal swabs (via RT-PCR) for SARS-CoV-2. After 3 weeks,
she presented with jaundice and fatigue, which led the patient to
visit the ‘Gaetano Martino’ Hospital in Messina, Italy, on December
30, 2020. Blood tests revealed severe anaemia with haemoglobin (Hb)
levels of 5.3 g/dl. The patient was admitted to the Infectious
Disease Unit in ‘Gaetano Martino’ Hospital, after another
nose-pharyngeal swab tested positive for SARS-CoV-2. Chest X-rays
revealed basal bilateral parenchymal thickening and apical
micronodules in the right lung.
Further blood tests were requested, which were
performed by the Laboratory of Clinical Pathology, Microbiology and
Immunometry of ‘Gaetano Martino’ Hospital. Initial blood test
results confirmed anaemia with Hb levels of 5.1 g/dl (normal range,
12.1-15.1 g/dl). Other altered values were present at the blood
count: RBCs, 1,540,000; mean corpuscular volume, 101 (normal range,
80-100); haematocrit, 15% (normal range, 38-46%); platelets,
183,000 (normal range, 150,000-450,000). In addition, increased
levels of erythropoietin (Epo; 90 IU/ml; normal range, 2.6-18.5
IU/ml) were observed, which can indicate low O2 levels
in the blood, anaemia from bone marrow failure, thalassemia or iron
deficiency. Ferritin levels were also high (614,00 ng/ml; normal
range, 12-150 ng/ml). Total bilirubin levels were also increased
5.01 mg/dl (normal range, 0.2-1 mg/dl), as well as direct bilirubin
levels (1.14 mg/dl); biomarkers of cholestasis usually increase in
haemolytic anaemia due to the high rate of RBC destruction and the
degradation of heme to bilirubin; lactate-dehydrogenase levels were
also high (501 U/L; normal range, 150-460 U/L). The levels of this
enzyme are usually increased in plasma when there is tissue damage,
in various types of anaemia, and during acute infections and
inflammation in general. DAT was positive; thus, the patient was
diagnosed with severe AIHA. Reticulocytosis (7% with a range of
0.5-2.5% of the erythrocyte count) and a reduction in haptoglobin
levels were also observed (2 mg/dl with a range of 30-200 mg/dl).
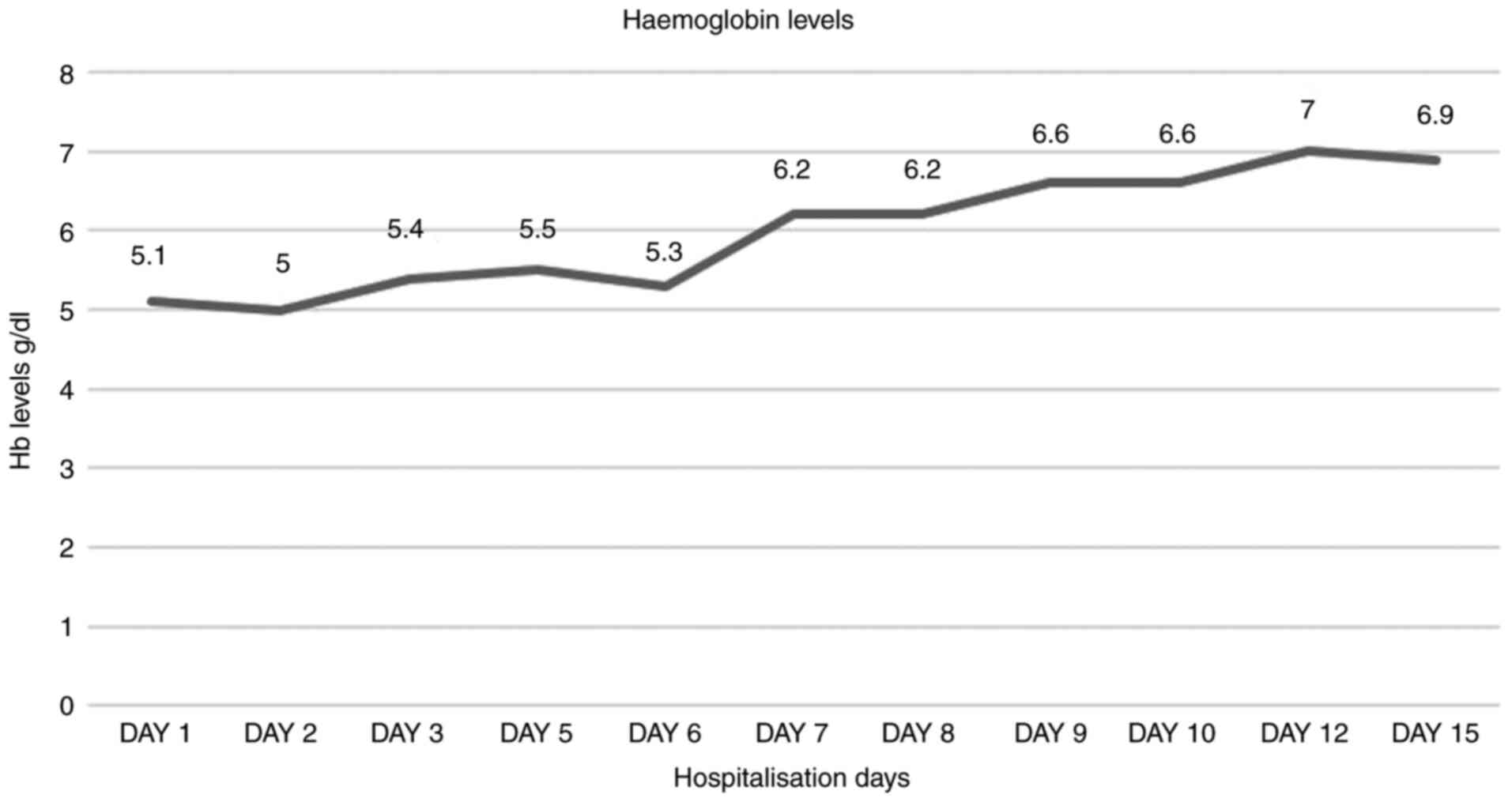
The Hb trends during the period of hospitalisation are presented in
Fig. 1.
During the period of hospitalisation, an abdomen
ultrasound was performed to exclude conditions that may have
resulted in an alteration of haemolytic markers: No sign of
obstruction of the biliary tract was observed, and there were no
alterations in the liver parenchyma and no splenomegaly. There was
also no evidence of overt bleeding. The test results for
antinuclear antibody, antineutrophil cytoplasmic antibody,
anti-smooth muscle antibody, antiphospholipid antibodies and
anti-dsDNA-Ab were negative. Screening for HBV, HCV and leishmania
was also performed, and the results were negative. Mild
hypothyroidism was found in the blood tests for thyroid function,
tested using electrochemiluminescence immunoassay (ECLIA; with a
COBAS 8000 analyser; Roche Diagnostics; cat. nos. 07027397190,
07027362190, 08443432190). The results were as follows: Free
thyroxine, 16.30 pmol/l (normal range, 12-30 pmol/l); free
triiodothyronine, 0.9 pg/ml (normal range, 2.3-4.1 pg/ml); and
thyroid stimulating hormone, 6.06 u(IU)/ml (normal range, 0.35-5.5
uIU/ml). There was a mild prevalence in IgG levels (384 mg/dl),
with normal IgA and IgM levels (78 and 40 mg/dl, respectively)
(performed using the Abcam Human IgM/IgG/IgA ELISA kit; cat. no.
ab195215, Abcam). Complement factors were normal with a C3 level of
89 mg/dl and a C4 level of 11 mg/dl. There were no temperature
peaks or a need for O2 therapy during the
hospitalisation period. In the first few days since her admission,
the Hb levels continued to slowly decrease (Fig. 1). A haematology consultation was
requested to investigate the anaemia of the patient and to select a
treatment.
On the third day of hospitalisation, treatment with
methylprednisolone at 20 mg twice a day was commenced, according to
haematologist's advice and given that steroids are considered
standard clinical practice for AIHA. In addition, the patient was
treated with low molecular weight heparin (LMWH) at 4,000 UI once a
day for COVID-19 thromboembolic prophylaxis, as stated in the
recommendations by the Italian Society on Thrombosis and
Haemostasis of April, 2020(12).
Proton pump inhibitors were also added to the treatment regimen due
to the increased risk of developing peptic ulcers caused by
steroids, as well as the supplementation of cyanocobalamin and
folic acid since erythropoiesis increases in response to
haemolysis, which results in a greater demand for folate and
cyanocobalamin (13).
During hospitalisation, a progressive improvement in
the woman's clinical conditions and Hb levels were observed, with
the normalisation of haptoglobin and cholestasis levels as the
patient continued the treatment. Jaundice disappeared progressively
with the normalization of bilirubin levels.
On the 13th day of hospitalisation, a nasopharyngeal
swab for SARS-CoV-2 tested negative, and the final blood test
results revealed an improvement in Hb levels and cholestasis
markers. In addition, a reduction in the levels of reticulocytes
and haptoglobin was observed. Upon agreement, on the 16th day of
hospitalisation, the patient was transferred to the haematology
unit for further assistance and monitoring for a further 6 days.
Therapy with methylprednisolone was continued and progressively
tapered; a gradual improvement in her clinical and laboratory
conditions was observed. The woman was discharged on the 22th day
following admission, in January, 2021, with Hb levels of 11.4 g/dl,
a complete resolution of jaundice and fatigue, and with the
indication to continue steroid therapy, switching from
methylprednisolone to prednisone 25 mg, twice a day.
Discussion
In the present study, a search of the literature was
also performed using ‘COVID-19 + anaemia’, ‘SARS-CoV-2 + anaemia’,
‘warm autoimmune haemolytic anaemia + COVID-19 OR SARS-CoV-2’,
‘cold agglutinin disease + COVID-19 OR SARS-CoV-2’, ‘autoimmune
anaemia + SARS-CoV-2’ as key words. The articles were mainly
obtained from PubMed and PMC. Among the articles identified, only
the ones in the English language, studies on humans, and cases
about patients with anaemia concomitant with COVID-19 and a
positive direct antiglobulin test (DAT) and no previous history of
AIHA were considered. For the majority of cases, the entire article
was read; in a few cases, only the abstract was read. After
selecting the articles which fulfilled these criteria, a few more
articles about anaemia with COVID-19 and a positive DAT were found
in the references and these were included as well.
While pulmonary involvement remains the main feature
of SARS-CoV-2, there are a number of other manifestations caused by
the viral infection and COVID-19-related complications that may
lead to a worse outcome, such as neurological involvement or
electrolytes impairment (14,15).
The patient described herein presented with features
suggestive of haemolysis and was diagnosed with AIHA following a
positive DAT. Other cases of AIHA in patients with COVID-19
reported in literature were also identified (Table I). The history of a patient with
AIHA should include the following: Symptoms of anaemia
(breathlessness, fatigue, chest pain), symptoms suggestive of
intravascular haemolysis (dark urine, loin pain), recent infection,
symptoms of cold-induced acrocyanosis, symptoms suggestive of
underlying lymphoproliferative disorder (weight loss, night sweats,
lymphadenopathy), recent transfusions to exclude delayed haemolytic
reactions, a previous medical history stating if the patient is a
solid organ or stem cell transplant recipient and a detailed drug
history (16). There are numerous
causes of AIHA (13). These
include autoimmune, viral, lymphoproliferative disorders and
immunodeficiency states. In the patient in the present study, there
were no signs that may have suggested a new onset of cancer or a
reactivation of her previous one, since the thyroid cancer had been
successfully removed with the whole gland, and she had been in
follow-up ever since, with the final check-up performed 1 month and
a half prior to admission with no radiologic evidence of residual
tissue. Moreover, there were no other causes that could have
explained the features of AIHA or episodes prior to the one
described. It is considered that hypothyroidism could have
justified the acute anaemia, particularly since the patient was
still in treatment with levothyroxine and had been for years prior
to the onset of anaemia. An abdomen ultrasound performed during the
hospitalisation period was negative for liver, biliary tract and
spleen alterations, and a reduction in haptoglobin levels was
observed, which is more suggestive of intravascular haemolysis.
Haemolysis and other blood disorders secondary to viral infections
are common findings, and it is known that COVID-19 infection is
associated with immune dysregulation and that specific HLA antigens
are associated with a more severe disease (17-20);
thus, it was suspected that the patient had haemolytic anaemia
secondary to COVID-19, as it probably induced the selection of
anti-globulin antibodies, causing the haemolysis observed in this
case.
 | Table ICases of AIHA concomitant to
SARS-CoV-2 infection reported in the literature. |
Table I
Cases of AIHA concomitant to
SARS-CoV-2 infection reported in the literature.
| Authors (year of
publication) | Sex | Age, years | Comorbidities | DAT
specificity | Ab Optimum
Temp | Hb (g/dl) |
Reticulocytosis | Haptoglobin
(mg/dl) | Total bilirubin
level(s) mg/dl | AIHA treatment | Outcome | (Refs.) |
|---|
| Maslov et al
(2020) | M | 48 | Hypertension,
insulin-dependent diabetes, obesity, end-stage renal disease | / | Cold | 4.5 | / | 21 | 2.5 | | Exitus | (26) |
| Liput et al
(2021) | F | 33 | Iron deficiency
anaemia | IgG+C3 | Warm | 6.5 | Yes | <10 | 3 | Prednisone 1
mg/kg/day, blood transfusion | Recovery | (28) |
| Zama et al
(2021) | M | 15 | / | IgG+C3d | Cold | 3.7 | / | / | 3.51 | Prednisone 2 mg/kg,
ivig | Recovery | (34) |
| Ahmed et al
(2021) | M | 70 | CAD, gout, chronic
viral hepatitis B | C3d | Cold | 3.4 | Yes | <0.08 | 3.86 | Dexamethasone 6
mg/day, blood transfusion | Recovery | (35) |
| Moonla et al
(2020) | F | 24 | / | C3d | Cold | 10.9 | / | / | NR | | Recovery | (36) |
| Raghuwanshi
(2020) | M | 45 | / | / | Cold | 6.9 | Yes | / | 7.76 | Blood
transfusion | / | (37) |
| Jacobs et al
(2020) | F | 33 | Hypothyroidism | IgG+C3d | Cold | 1.3 | Yes | <8 | 2 | Blood transfusions,
prednisone 1 mg/kg, daily, rituximab 600 mg single dose | Recovery | (38) |
| Zagorski et
al (2020) | F | 46 | Immune
thrombocytopenic purpura (ITP), status post splenectomy, iron
deficiency anaemia, asthma | IgG+C3d | Cold | 5.3 | Yes | Undetectable | 9.2 | | Exitus | (39) |
| Capes et al
(2020) | M | 62 | Hypertension,
smoking, oropharyngeal squamous cell carcinoma in treatment with
radiochemotherapy | IgG+C3d | Cold | 6.9 | Yes | 0.13 | 1.3 | Blood
transfusion | Recovery | (40) |
| Kaur et al
(2021) | M | 61 | Hypertension, type
2 diabetes mellitus, hypercholesterolemia, end-stage renal disease
(ESRD), haemodialysis- dependent, anaemia of chronic disease,
coronary artery disease, paroxysmal atrial fibrillation,
obesity | C3d | Cold | 4.5 | Yes | / | 3.1 | Methylprednisolone
60 mg, blood transfusion | Recovery | (41) |
| Patil et al
(2020) | F | 51 | Breast ductal
carcinoma in situ post-mastectomy on chemoradiation | C3d | Cold | 5.1 | Yes | / | NR | / | Recovery | (42) |
| D'Aloisio et
al (2020) | M | 46 | Hypertension,
hereditary spherocytosis | / | Cold | 6.2 | / | 244 | 1.8 | Blood
transfusion | Recovery | (43) |
| Huscenot et
al (2020) | F | 43 | Obesity and
untreated multiple sclerosis | / | Cold | 6.1 | / | / | NR | / | Recovery | (44) |
| Huscenot et
al (2020) | M | 63 | Hypertension | IgG+C3 | Warm | 8.2 | / | < 0.08 | NR | / | Recovery | (44) |
| Gupta et al
(2021) | M | 77 | G6PD
deficiency | C3d | Cold | 8.8 | / | / | 1.9 | Methylprednisolone,
blood transfusion | Exitus | (45) |
| Nair et al
(2021) | M | 23 | Bronchial
asthma | IgG | Warm | 3.6 | Yes | / | 6.95 | Methylprednisolone
1 g/day, prednisolone 1 mg/kg/day, blood transfusion | Recovery | (46) |
| Hindilerden et
al (2020) | M | 56 | Hypertension | IgG+C3d | Warm | 4.3 | Yes | 11.5 | 2.95 | IVIG, blood
transfusion, prednisolone 1 mg/kg/ day | Recovery | (47) |
| Lazarian et
al (2020) | M | 61 | Hypertension,
chronic renal failure | IgG+C3d | Warm | 6 | Yes | <0.1 | NR | Steroids | / | (48) |
| Lazarian et
al (2020) | F | 89 | Hypertension,
chronic renal failure, atrial fibrillation | IgG+C3d | Warm | 8.4 | Yes | <0.1 | NR | Steroids | / | (48) |
| Lazarian et
al (2020) | F | 62 | Hypertension,
cirrhosis | C3d | Cold | 10.8 | Yes | <0.1 | NR | SteroidS,
rituximab | / | (48) |
| Lazarian et
al (2020) | F | 69 | Obesity | IgG+C3d | Cold | 3.8 | Yes | <0.1 | NR | Steroids | / | (48) |
| Lazarian et
al (2020) | M | 61 | Hypertension,
chronic renal failure, diabetes, hypercholesterolaemia | C3d | Cold | 7.2 | Yes | 0.8 | NR | Blood
transfusion | / | (48) |
| Lazarian et
al (2020) | M | 61 | Diabetes | IgG | Warm | 7 | Yes | <0.1 | NR | Steroids,
rituximab | / | (48) |
| Lazarian et
al (2020) | M | 75 | Diabetes,
hypercholesterolaemia, cardiopathy, obesity, chronic obstructive
bronchopneumopathy | IgG | Warm | 7.1 | Yes | <0.1 | NR | Blood
transfusion | / | (48) |
| Brazel et al
(2021) | M | 51 | / | IgG+C3d | Mixed | 3.1 | Yes | <30 | 5.3 | Prednisone 1 mg/kg,
blood transfusion | Recovery | (49) |
| Rosenzweig et
al (2020) | F | 14 | / | IgG+C3b C3d | Mixed | 4 | Yes | <10 | NR | Blood transfusion,
rituximab | Recovery | (50) |
| Al-Mashdali et
al (2021) | M | 39 | / | IgG | NR | 8.2 | Yes | / | NR | Prednisolone 60 mg
daily | Recovery | (51) |
| Al-Mashdali et
al (2021) | M | 2 | β thalassemia major
(transplant + GvHD) | IgA+/IgG +/IgM+/C
3c+/C3d+ | Cold | 2.3 | / | / | 2.94 | Prednisone 2 mg/kg,
blood transfusions | Recovery | (51) |
| Mausoleo et
al (2021) | F | 53 | Autoimmune
thyroiditis | IgA | NR | 7 | Yes | <0.07 | NR | Methylprednisolone
500 mg, prednisone 1 mg/kg, blood transfusion, rituximab | Recovery | (52) |
| Huda et al
(2021) | M | 54 | Diabetes
mellitus | IgG | NR | 9 | Yes | 30 | 1.4 | Prednisone 80
mg | Recovery | (53) |
| Georgy et al
(2021) | M | 33 | / | / | NR | 7.5 | Yes | | 1.23 | Dexamethasone 40
mg/ day | Exitus | (54) |
| Jawed et al
(2020) | M | 50 | Obstructive sleep
apnoea and hypertension | C3d | NR | 7.9 | Yes | <30 | 7.84 | / | Recovery | (55) |
| Lopez et al
(2020) | F | 46 | Congenital
thrombocytopenia | IgG+C3d | NR | 9.7 | Yes | / | NR | IVIG, blood
transfusion, prednisone 60 mg/day | Recovery | (56) |
| Hernández et
al (2020) | F | 13 | Psoriasis | IgG | NR | 6.3 | Yes | <7.38 | 1.9 | Methylprednisolone
pulses 250 mg/daily, prednisolone 1 mg/kg daily | Recovery | (57) |
| Ramos- Ruperto
et al (2021) | M | 53 | / | IgG | NR | 6.5 | / | <7.75 | 1 |
Methylprednisolone | Recovery | (58) |
| Ramos- Ruperto
et al (2021) | F | 73 | / | C3d | NR | 7.4 | / | <7.75 | 3.5 | Blood
transfusion | Recovery | (58) |
| Ramos-Ruperto et
al (2021) | F | 76 | Hypertension,
hypothyroidism and chronic lymphocytic leukaemia (CLL) | IgG | NR | 8 | / | <7.75 | 0.35 |
Methylprednisolone | Recovery | (58) |
| Woldie et al
(2020) | M | 24 | AIHA | IgG+C3 | NR | 7.5 | / | / | 2.4 | Prednisone (1.5
mg/kg), cyclophosphamide | Recovery | (59) |
In acute inflammation, there are numerous factors
that contribute to the decrease in Hb levels, the most known being
the cytokine-induced iron metabolism dysregulation and the
inhibition of Epo formation. In COVID-19, there is the added risk
of iatrogenic anticoagulation and the cross-mimicry between
Spike-protein and some RBC surface proteins (21).
The results of a study conducted by the Reference
Centre in Northern Italy for autoimmune cytopenia (AIC) suggested a
lower than expected incidence of COVID-19 in patients previously
diagnosed with AIC (22).
Even though anaemia is a common and very well-known
consequence of inflammation caused from the dysregulation of iron
homeostasis and the suppression of Epo, in SARS-CoV-2 infection,
there is the added risk of iatrogenic bleeding or bleeding
secondary to disseminated intravascular coagulation. Therefore, the
patient in the present study was treated with a prophylactic dose
of LMWH.
Molecular mimicry, which may be the cause of other
autoimmune SARS-CoV-2-related diseases, should be considered as a
potential trigger for AIHA, consisting of antibodies elicited
against viral proteins that cross-react with self-antigens.
Ankyrin-1, a protein present on erythrocyte membranes, appears to
have structural similarities with the viral spike protein, sharing
an immunogenic-antigenic epitope with the SARS-CoV-2 surface
glycoprotein known as Spike-protein (20). These factors contribute to the
plethora of evidence that suggest that autoimmune diseases are
potentially induced by SARS-CoV-2; therefore, it can be assumed
that anaemia in COVID-19 can be sustained both from the viral
infection itself and from the selection of auto-antibodies induced
by SARS-CoV-2(23).
In the case described herein, weakness and other
anaemia-related symptoms were the first clinical manifestations
that led the woman to seeking medical attention; at the time, she
did not present any respiratory symptoms that are commonly
associated with COVID-19, even though she had a cough and throat
ache the month prior to her admission. The authors found very few
other cases in literature in which there were only late pulmonary
symptoms or no pulmonary manifestations (24,25).
In other cases, there were coryzal symptoms prior to anaemia
(23,26), the most common being dyspnoea,
fever, cough, muscle aches, anosmia, ageusia. Regardless of the
first symptoms, it is proven that a decrease in Hb levels during
COVID-19 may lead to a worse prognosis (27,28),
since the already compromised respiratory system has to deal with a
significant reduction in Hb that cannot guarantee an adequate
peripheral oxygenation.
Most typical COVID-19 symptoms were usually absent
or manifested late in the cases found in the literature; therefore,
testing for SARS-CoV-2 should be considered for patients that
present anaemia-related features, along with the other common
viruses. DAT needs to also be performed to reveal a possible
autoimmune origin of anaemia. If possible, when AIHA is diagnosed,
a titration of antiglobulin-Abs should be performed to test the
type of autoantibodies involved.
The diverse types of AIHA, in fact, differ based on
the optimal reactivity temperatures of the autoantibodies.
Serologically AIHA cases are divided into the warm type (65%) known
as wAIHA, the cold type (29%) which is cold hemagglutinin disease
(CAD), 1% paroxysmal cold haemoglobinuria or mixed AIHA (mAIHA, 5%)
(29,30). The concomitant or sequential
association of AIHA with immune thrombocytopenia and sometimes
neutropenia define Evans syndrome, a rare syndrome that represents
7% of AIHA cases (31). Among the
different types, wAIHA is the most common, accounting for 70 to 80%
of all adult cases and ~50% of paediatric cases (32).
Due to laboratory limitations, the authors were not
able to perform a titration of antiglobulin-Ab to determine whether
the case was a CAD or wAIHA case, even though it is known that cold
agglutinins are often a complication of specific infections and
malignancies. In the literature search, a marked prevalence of CAD
was observed, with 17 cases out of 39 being CAD (44%) (24,27,33-45),
8 cases of wAIHA (20%) (28,46-48),
2 cases of mAIHA (5%) (49,50)
and 12 cases (31%) where titration was not performed (51-59).
In the majority of cases, a DAT was performed and a
marked prevalence of both the IgG and C3 type was observed. A
peculiar case is the second one reported by Zama et al
(34), regarding a 2-year old male
patient who had a history of β-thalassemia major, who underwent an
allogeneic haematopoietic stem cell transplantation and
subsequently manifested graft vs. host disease (GvHD). At 6 months
following transplantation, the patient in that study manifested
fever and weakness and tested positive for SARS-CoV-2, with blood
tests revealing severe anaemia (Hb, 2.3 g/dl); DAT tested positive
for cold agglutinins
IgA+/IgG+/IgM+/C3e+/C3d+,
which may be interpreted as the result of alloimmunization due to
the patient's recent history of transplantation and GvHD (34).
Excluding a case of macular haemorrhage (43), which could not justify the decrease
in Hb levels, there was no evidence of overt bleeding in the other
cases identified in the literature, similarly to the case of the
patient in the present study. In the other cases in the literature,
a high rate of hypertension as a comorbidity was found, followed in
frequency by type II diabetes and hypercholesterolemia. The patient
described herein also suffered from hypertension. Haematological
disorders were present in 8 cases in the literature among the ones
identified (27,38,40,42,50,55,57,58).
Only another 2 patients had a clinical history of hypothyroidism,
as in the case of the patient described herein (37,57).
Another 2 cases had a history of solid cancer, with one being still
in treatment during the episode of AIHA (39,41).
First-line therapy for AIHA involves the
administration of corticosteroids. Treatment with low-dose
corticosteroids for >6 months results in a lower incidence of
relapse than in patients who discontinue the administration at an
earlier stage (60-62).
If the patient is not responsive to treatment after 3 weeks,
treatment failure should considered, and therapy should be switched
to intravenous immunoglobulin, if not contraindicated. In the case
of a first-line failure, the second-line treatment consists of
immunosuppression and rituximab, a chimeric human IgG1-κ monoclonal
antibody against the protein, CD20, a therapeutic option for
thrombocytopenia, AIHA and Evans syndrome that usually yields a
response rate of 70-80% as the second-line treatment for AIHA; some
cases of hypogammaglobulinemia have been observed consequentially
to the use of rituximab (63).
Corticosteroid therapy was the first-line treatment
in a number of cases in the literature. In 25 of the identified
cases, steroids were administered following the failure of other
treatments. Among the most commonly used there were
methylprednisolone, prednisone and prednisolone. I the present
study, it was decided not to perform a blood transfusion after
consulting the haematologists.
The outcome of COVID-19 in patients with underlying
AIHA has yet to be studied, even though studies concerning other
underlying pathologies are already available (14,64,65).
Hb levels, assessed in patients with COVID-19 at the time of
hospital admission, indicate that patients with anaemia admitted to
the intensive care unit (ICU) have a higher mortality rate than
non-anaemic patients not in the ICU (22,66).
Moreover, SARS-CoV-2-infected patients with AIHA appear to have had
a longer hospitalisation period than COVID-19 patients with
non-AIHA anaemia (27). Of course,
SARS-CoV-2-targeted therapies and eventual O2 therapy
have to be administered along with treatment for AIHA, if not
contraindicated for the anaemia, to treat the viral infection as
well (67,68).
In the case in the present study, COVID-19 specific
treatment was not administered, such as monoclonal antibodies,
mostly due to the fact that they were not available at the time.
However, they could have improved the cytokine storm caused by
SARS-CoV-2 (69,70).
In 27 (69,2%) out of the 39 cases identified in the
literature, the patients, including the one described herein, had a
full recovery from both COVID-19 and AIHA (28,34-36,38,40-44,46,47,49-59).
Of note, 4 patients (10,2%) succumbed (26,39,45,54).
It has to be mentioned that some of the patients visited the
hospitals for observation at a time when their condition was
already at an advanced or severe stage; thus, effective therapy
could not be administered and the patient succumbed. The outcome in
8 cases (20,5%) was not reported (37,48).
Even in the more severe cases, there appears to be a
prevalence of a full recovery, which may be due to the early
commencement of treatment. Among the various steroid regimens
adopted, there does not appear to be a more effective one, while it
is controversial the role played by IV-Ig, considering the
autoimmune nature of AHIA, and rituximab, added just in a few of
the cases taken into account.
However, even if they are not among the most common
predictors of mortality in COVID-19 cases, AIHA and anaemia induced
by SARS-CoV-2 need to be considered one of the most concerning
aspects, as they could lead to severe consequences in combination
with the other features of the viral infection (71).
In conclusion, the clinical case described herein
has allowed for the re-evaluation of the complex associations that
exist between autoimmune and infectious diseases, with reference to
COVID-19. In particular, it is interesting to note that in the case
described herein, there was a complete absence of respiratory
symptoms at the time of admission and anaemia appeared to be the
only presentation of COVID-19, excluding the mild coughing and
throat ache the patient had manifested 1 month prior to her
admission. While thrombocytopenia remains the most common
autoimmune disease associated with SARS-CoV-2 infection, the
increasing number of reported cases of AIHA has become a cause for
concern and interest, particularly considering the still increasing
number of cases of other haematological disorders, such as
thrombocytopenia associated with COVID-19 and, in addition, how
SARS-CoV-2 may affect patients with an underlying haematological
disorder (9). Although the
mechanisms of the interaction between SARS-CoV-2 and haemolytic
anaemia are not yet fully understood and further studies are
certainly required to evaluate these, the possible association
between COVID-19 and AIHA cannot be ignored.
Acknowledgements
The authors would like to thank Dr Cristina Di
Pietro, Unit of Infectious Diseases, University of Messina,
Messina, Italy, for her precious clinical point of view on the
manuscript and the Immunometric Laboratory of ‘Gaetano Martino’
Hospital for the contribution in providing information about the
examinations performed.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EVR, MC, GC, YR, GN, AM and CM were involved in the
conceptualization of the study. YR, CM, EVR, MC, GC and GN were
involved in acquiring, analysing and interpreting the patient's
data. YR, CM, EVR, GN, MC, GC and AM were involved the drafting,
writing, review and editing of the manuscript. All authors have
read and agreed to the published version of the manuscript. YR and
CM confirm the authenticity of all the raw data. All the authors
agree to be accountable for all aspects of the work. None of the
authors have performed any of the biochemical and blood test
reported in the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient whose case is described herein.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of her case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ceccarelli M, Berretta M, Venanzi Rullo E,
Nunnari G and Cacopardo B: ‘Differences and similarities between
Severe Acute Respiratory Syndrome (SARS)-CoronaVirus (CoV) and
SARS-CoV-2. Would a rose by another name smell as sweet?’. Eur Rev
Med Pharmacol Sci. 24:2781–2783. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vella F, Senia P, Ceccarelli M, Vitale E,
Maltezou H, Taibi R, Lleshi A, Venanzi Rullo E, Pellicanò GF,
Rapisarda V, et al: Transmission mode associated with coronavirus
disease 2019: A review. Eur Rev Med Pharmacol Sci. 24:7889–7904.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pavone P, Ceccarelli M, Marino S, Caruso
D, Falsaperla R, Berretta M, Rullo EV and Nunnari G: SARS-CoV-2
related paediatric acute-onset neuropsychiatric syndrome. Lancet
Child Adolesc Health. 5:e19–e21. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Meduri A, Oliverio GW, Mancuso G,
Giuffrida A, Guarneri C, Venanzi Rullo E, Nunnari G and Aragona P:
Ocular surface manifestation of COVID-19 and tear film analysis.
Sci Rep. 10(20178)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Guarneri C, Rullo EV, Pavone P, Berretta
M, Ceccarelli M, Natale A and Nunnari G: Silent COVID-19: What your
skin can reveal. Lancet Infect Dis. 21:24–25. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pirisi M, Rigamonti C, D'Alfonso S,
Nebuloni M, Fanni D, Gerosa C, Orrù G, Venanzi Rullo E, Pavone P,
Faa G, et al: Liver infection and COVID-19: The electron microscopy
proof and revision of the literature. Eur Rev Med Pharmacol Sci.
25:2146–2151. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guarneri C, Venanzi Rullo E, Gallizzi R,
Ceccarelli M, Cannavò S and Nunnari G: Diversity of clinical
appearance of cutaneous manifestations in the course of COVID-19. J
Eur Acad Dermatol Venereol. 34:e449–e450. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ceccarelli M, Marino A, Cosentino F,
Moscatt V, Celesia BM, Gussio M, Bruno R, Rullo EV, Nunnari G and
Cacopardo BS: Post-infectious ST elevation myocardial infarction
following a COVID-19 infection: A case report. Biomed Rep.
16(10)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lassandro G, Palladino V, Palmieri VV,
Amoruso A, Del Vecchio GC and Giordano P: Covid-19 and children
with immune thrombocytopenia: Emerging issues. Mediterr J Hematol
Infect Dis. 12(e2020028)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lai A, Bergna A, Menzo S, Zehender G,
Caucci S, Ghisetti V, Rizzo F, Maggi F, Cerutti F, Giurato G, et
al: Circulating SARS-CoV-2 variants in Italy, October 2020-March
2021. Virol J. 18(168)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lai A, Bergna A, Caucci S, Clementi N,
Vicenti I, Dragoni F, Cattelan AM, Menzo S, Pan A, Callegaro A, et
al: Molecular Tracing of SARS-CoV-2 in Italy in the first three
months of the epidemic. Viruses. 12(798)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Marietta M, Ageno W, Artoni A, De Candia
E, Gresele P, Marchetti M, Marcucci R and Tripodi A: COVID-19 and
haemostasis: A position paper from Italian Society on Thrombosis
and Haemostasis (SISET). Blood Transfus. 18:167–169.
2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hill A and Hill AQA: Autoimmune hemolytic
anemia. Hematology Am Soc Hematol Educ Program. 2018:382–389.
2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Longhitano E, Nardi C, Calabrese V,
Messina R, Mazzeo G, Venanzi Rullo E, Ceccarelli M, Chatrenet A,
Saulnier P, Torreggiani M, et al: Hypernatraemia and low eGFR at
hospitalization in COVID-19 patients: A deadly combination. Clin
Kidney J. 14:2227–2233. 2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Buccafusca M, Micali C, Autunno M, Versace
AG, Nunnari G and Musumeci O: Favourable course in a cohort of
Parkinson's disease patients infected by SARS-CoV-2: A
single-centre experience. Neurol Sci. 42:811–816. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kruhonja Galic Z, Jagnjic S,
Bingulac-Popovic J, Planinc Peraica A, Hecimovic A, Strauss Patko M
and Jukic I: Warm red blood cell autoantibodies and clinical
diagnoses in patients with or without autoimmune hemolysis.
Transfus Clin Biol. 27:25–29. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ceccarelli M, Venanzi Rullo E and Nunnari
G: Risk factors of venous thrombo-embolism during cytomegalovirus
infection in immunocompetent individuals. A systematic review. Eur
J Clin Microbiol Infect Dis. 37:381–390. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Brodsky RA: Warm Autoimmune Hemolytic
Anemia. N Engl J Med. 381:647–654. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bonaccorsi I, Carrega P, Venanzi Rullo E,
Ducatelli R, Falco M, Freni J, Miceli M, Cavaliere R, Fontana V,
Versace A, et al: HLA-C*17 in COVID-19 patients: Hints for
associations with severe clinical outcome and cardiovascular risk.
Immunol Lett. 234:44–46. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Novelli L, Motta F, De Santis M, Ansari
AA, Gershwin ME and Selmi C: The JANUS of chronic inflammatory and
autoimmune diseases onset during COVID-19-A systematic review of
the literature. J Autoimmun. 117(102592)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Angileri F, Légaré S, Marino Gammazza A,
Conway de Macario E, Macario AJL and Cappello F: Is molecular
mimicry the culprit in the autoimmune haemolytic anaemia affecting
patients with COVID-19? Br J Haematol. 190:e92–e93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Barcellini W, Giannotta JA and Fattizzo B:
Are patients with autoimmune cytopenias at higher Risk of COVID-19
Pneumonia? The experience of a reference center in Northern Italy
and review of the literature. Front Immunol.
11(609198)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu
Y, Zhang L, Fan G, Xu J, Gu X, et al: Clinical features of patients
infected with 2019 novel coronavirus in Wuhan, China. Lancet.
395:497–506. 2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pelle MC, Tassone B, Ricchio M, Mazzitelli
M, Davoli C, Procopio G, Cancelliere A, La Gamba V, Lio E, Matera
G, et al: Late-onset myocardial infarction and autoimmune
haemolytic anaemia in a COVID-19 patient without respiratory
symptoms, concomitant with a paradoxical increase in inflammatory
markers: A case report. J Med Case Rep. 14(246)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Bader F, Manla Y, Atallah B and Starling
RC: Heart failure and COVID-19. Heart Fail Rev. 26:1–10.
2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Maslov DV, Simenson V, Jain S and Badari
A: COVID-19 and cold agglutinin hemolytic anemia. s TH Open.
04:e175–e177. 2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Algassim AA, Elghazaly AA, Alnahdi AS,
Mohammed-Rahim OM, Alanazi AG, Aldhuwayhi NA, Alanazi MM, Almutairi
MF, Aldeailej IM, Kamli NA and Aljurf MD: Prognostic significance
of hemoglobin level and autoimmune hemolytic anemia in SARS-CoV-2
infection, Ann. Hematol. 100:37–43. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Liput JR, Jordan K, Patadia R and Kander
E: Warm Autoimmune Hemolytic Anemia Associated With Asymptomatic
SARS-CoV-2 Infection. Cureus. 13(e14101)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Bass GF, Tuscano ET and Tuscano JM:
Diagnosis and classification of autoimmune hemolytic anemia.
Autoimmun Rev. 13:560–564. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Berentsen S, Randen U and Tjønnfjord GE:
Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol
Clin North Am. 29:455–471. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Audia S, Grienay N, Mounier M, Michel M
and Bonnotte B: Evans' Syndrome: From diagnosis to treatment. J
Clin Med. 9(3851)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kalfa TA: Warm antibody autoimmune
hemolytic anemia. Hematology Am Soc Hematol Educ Program.
2016:690–697. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Taherifard E, Taherifard E, Movahed H and
Mousavi MR: Hematologic autoimmune disorders in the course of
COVID-19: A systematic review of reported cases. Hematology.
26:225–239. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zama D, Pancaldi L, Baccelli F, Guida F,
Gottardi F, Dentale N, Esposito F, Masetti R, Viale P and Pession
A: Autoimmune hemolytic anemia in children with COVID-19. Pediatr
Blood Cancer. 69(e29330)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ahmed Y, Khandelwal A and Walker L: Cold
agglutinin disease and COVID-19 requiring therapeutic plasma
exchange. BMJ Case Rep. 14(e244227)2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Moonla C, Watanaboonyongcharoen P,
Suwanpimolkul G, Paitoonpong L, Jantarabenjakul W, Chanswangphuwana
C, Polprasert C, Rojnuckarin P and Putcharoen O: Cold agglutinin
disease following SARS-CoV-2 and Mycoplasma pneumoniae
co-infections. Clin Case Rep. 8:2402–2405. 2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Raghuwanshi B: Serological blood group
discrepancy and cold agglutinin autoimmune hemolytic anemia
associated with novel coronavirus. Cureus.
12(e11495)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Jacobs J and Eichbaum Q: COVID-19
associated with severe autoimmune hemolytic anemia. Transfusion.
61:635–640. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zagorski E, Pawar T, Rahimian S and Forman
D: Cold agglutinin autoimmune haemolytic anaemia associated with
novel coronavirus (COVID-19). Br J Haematol. 190:e183–e184.
2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Capes A, Bailly S, Hantson P, Gerard L and
Laterre PF: COVID-19 infection associated with autoimmune hemolytic
anemia. Ann Hematol. 99:1679–1680. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kaur J, Mogulla S, Khan R, Krishnamoorthy
G and Garg S: Transient Cold Agglutinins in a Patient With
COVID-19. Cureus. 13(e12751)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Patil NR, Herc ES and Girgis M: Cold
agglutinin disease and autoimmune hemolytic anemia with pulmonary
embolism as a presentation of COVID-19 infection. Hematol Oncol
Stem Cell Ther. 15:213–216. 2022.PubMed/NCBI View Article : Google Scholar
|
|
43
|
D'Aloisio R, Nasillo V, Gironi M and
Mastropasqua R: Bilateral macular hemorrhage in a patient with
COVID-19. Am J Ophthalmol Case Rep. 20(100958)2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Huscenot T, Galland J, Ouvrat M, Rossignol
M, Mouly S and Sène D: APHP Lariboisière COVID Group.
SARS-CoV-2-associated cold agglutinin disease: A report of two
cases. Ann Hematol. 99:1943–1944. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gupta R, Singh S, Anusim N, Gupta S, Gupta
S, Huben M, Howard G and Jaiyesimi I: Coronavirus Disease 2019 and
Cold Agglutinin Syndrome: An Interesting Case. Eur J Case Rep
Intern Med. 8(002387)2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Nair LJ, Regukumar A and Baalamurugan KT:
COVID-19-Associated severe autoimmune hemolytic anemia: A rare case
report. Saudi J Med Med Sci. 9:276–279. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Hindilerden F, Yonal-Hindilerden I, Akar
E, Yesilbag Z and Kart-Yasar K: Severe Autoimmune Hemolytic Anemia
in COVID-19 infection, safely treated with steroids. Mediterr J
Hematol Infect Dis. 12(e2020053)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lazarian G, Quinquenel A, Bellal M,
Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj
G, et al: Autoimmune haemolytic anaemia associated with COVID-19
infection. Br J Haematol. 190:29–31. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Brazel D, Eid T and Harding C: Warm and
cold autoimmune hemolytic anemia in the setting of COVID-19
Disease. Cureus. 13(e18127)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rosenzweig JD, McThenia SS and Kaicker S:
SARS-CoV-2 infection in two pediatric patients with immune
cytopenias: A single institution sexperience during the pandemic.
Pediatr Blood Cancer. 67(e28503)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Al-Mashdali AF, Ata YM and Yassin MA:
Concomitant autoimmune hemolytic anemia and pulmonary embolism
associated with mild COVID-19: A case report. Clin Case Rep.
9(e04952)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mausoleo A, Henriquez S, Goujard C,
Roque-Afonso AM, Noel N and Lambotte O: Severe IgA-mediated
autoimmune hemolytic anemia triggered by SARS-CoV-2 infection. Leuk
Lymphoma. 62:2037–2039. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huda Z and Jahangir A, Sahra S, Rafay Khan
Niazi M, Anwar S, Glaser A and Jahangir A: A Case of
COVID-19-Associated autoimmune hemolytic anemia with
hyperferritinemia in an immunocompetent host. Cureus.
13(e16078)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Georgy JT, Jayakaran JAJ, Jacob AS,
Gunasekaran K, Korula PJ, Devasia AJ and Iyadurai R: Evans syndrome
and immune thrombocytopenia in two patients with COVID-19. J Med
Virol. 93:2642–2644. 2021.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Jawed M, Hart E and Saeed M: Haemolytic
anaemia: A consequence of COVID-19. BMJ Case Rep.
13(e238118)2020.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lopez C, Kim J, Pandey A, Huang T and
DeLoughery TG: Simultaneous onset of COVID-19 and autoimmune
haemolytic anaemia, Br J. Haematol. 190:31–32. 2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Vega Hernández P, Borges Rivas Y, Ortega
Sánchez E, Marqués Cabrero A, Remedios Mateo L, Silvera Roig P,
Infante Quintanar A, Díaz-Delgado Peñas R, Sánchez Escudero V and
García-García ML: Autoimmune hemolytic anemia in a pediatric
patient with severe acute respiratory Syndrome coronavirus 2
infection. Pediatr Infect Dis J. 39(e288)2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Ramos-Ruperto L, García-Pérez E,
Hernández-Maraver D, Kerguelén-Fuentes A, Viejo-Llorente A,
Robles-Marhuenda Á and Busca-Arenzana C: A 3-case series of
autoimmune haemolytic anaemia and COVID-19: Is plasma exchange an
alternative? SN Compr Clin Med. 3:1420–1423. 2021.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Woldie IL, Brown IG, Nwadiaro NF, Patel A,
Jarrar M, Quint E, Khokhotva V, Hugel N, Winger M and Briskin A:
Autoimmune Hemolytic Anemia in a 24-Year-Old Patient With COVID-19
complicated by secondary cryptococcemia and acute necrotizing
encephalitis: A case report and review of literature. J Med Cases.
11:362–365. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Zanella A and Barcellini W: Treatment of
autoimmune hemolytic anemias. Haematologica. 99:1547–1554.
2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jäger U, Barcellini W, Broome CM, Gertz
MA, Hill A, Hill QA, Jilma B, Kuter DJ, Michel M, Montillo M, et
al: Diagnosis and treatment of autoimmune hemolytic anemia in
adults: Recommendations from the First International Consensus
Meeting. Blood Rev. 41(100648)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Barcellini W, Zaninoni A, Giannotta JA and
Fattizzo B: New insights in autoimmune hemolytic anemia: From
pathogenesis to therapy stage 1. J Clin Med. 9(3859)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Ottaviano G, Marinoni M, Graziani S,
Sibson K, Barzaghi F, Bertolini P, Chini L, Corti P, Cancrini C,
D'Alba I, et al: Rituximab unveils hypogammaglobulinemia and
immunodeficiency in children with autoimmune cytopenia. J Allergy
Clin Immunol Pract. 8:273–282. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ceccarelli M, Nunnari G, Celesia BM,
Pellicanò GF, Venanzi Rullo E, Berretta M and Santi Cacopardo B:
Editorial-Coronavirus disease 2019 and people living with HIV:
clinical considerations. Eur Rev Med Pharmacol Sci. 24:7534–7539.
2020.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Campanella E, Marino A, Ceccarelli M,
Gussio M, Cosentino F, Moscatt V and Cacopardo B: Pain crisis
management in a patient with sickle cell disease during SARS-CoV-2
infection: A case report and literature review. World Acad Sci J.
4(14)2022.
|
|
66
|
Barcellini W, Giannotta J and Fattizzo B:
Autoimmune hemolytic anemia in adults: Primary risk factors and
diagnostic procedures, Expert Rev. Hematol. 13:585–597.
2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Ceccarelli M, Pavone P, Venanzi Rullo E
and Nunnari G: Comment on safety and efficacy of oral
lopinavir/ritonavir in pediatric patients with coronavirus disease:
A nationwide comparative analysis. Eur Rev Med Pharmacol Sci.
25:2473–2474. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Marino A, Pampaloni A, Scuderi D,
Cosentino F, Moscatt V, Ceccarelli M and Cacopardo B: High-flow
nasal cannula oxygenation and tocilizumab administration in
patients critically ill with COVID-19: A report of three cases and
a literature review. World Acad Sci. 2(23)2020.
|
|
69
|
Marino A, Campanella E, Ceccarelli M,
Larocca L, Bonomo C, Micali C and Cacopardo B: Sarilumab
administration in patients with severe COVID-19: A report of four
cases and a literature review. World Acad Sci. 4(24)2022.
|
|
70
|
Marino A, Munafò A, Augello E, Bellanca
CM, Bonomo C, Ceccarelli M, Musso N, Cantarella G, Cacopardo B and
Bernardini R: Sarilumab Administration in COVID-19 Patients:
Literature review and considerations. Infect Dis Rep. 14:360–371.
2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Cosentino F, Moscatt V, Marino A,
Pampaloni A, Scuderi D, Ceccarelli M, Benanti F, Gussio M, Larocca
L, Boscia V, et al: Clinical characteristics and predictors of
death among hospitalized patients infected with SARS-CoV-2 in
Sicily, Italy: A retrospective observational study. Biomed Rep.
16(34)2022.PubMed/NCBI View Article : Google Scholar
|