Introduction
The most common cause of radicular pain and one of
the most common reasons for spinal surgery is intervertebral disc
herniation (IDH). This condition is caused by the displacement of
the nucleus pulposus (NP) to the outside of the intervertebral disc
(IVD) space. The progressive degeneration of a disc is considered
to cause IDH (1). The degenerative
process is influenced by various factors, including mechanical,
behavioral and genetic factors. The IVD allows flexibility and
transmits physiological loads across the spine. By sending signals
to cells that control appropriate matrix homeostasis, the
mechanical load plays a critical role in preserving a healthy IVD
(2,3). Conversely, ongoing exposure to high
loading is associated with disc degeneration. Degenerative
alterations in the annulus lead to IDH. A weakness caused by
annulus fissures makes it possible for disc material to expand or
move outside the annulus margins (1,4). A
recent trend in the surgical management of degenerative disc
disease is to preserve the spinal mobility segment and reduce
soft-tissue dissection. The intradiscal replacement of the NP or
artificial discs may be used instead of performing spinal fusion
(2,4,5).
This strategy aims to restore the NP, while maintaining the
integrity of the cartilaginous endplate and the biomechanics of the
anulus fibrosis. The objectives of using NP implants are to
stabilize spinal ligamentous structures, improve disc space height,
relieve or reduce transmission of shear pressures on the remaining
annulus, and stabilize motion (4,6,7).
The ideal implant for the NP must have the same
biomechanical characteristics and bioavailability as the human NP.
Polyvinyl alcohol (PVA) hydrogel materials for use as implants are
designed to have the characteristics of structural integrity,
biocompatibility, biodegradability, safety, viscosity and
mechanical strength. PVA hydrogel compounds, which have specific
material properties, can be used to replace a disc artificially
(6,8,9). The
present study systematically reviews the biomechanical
characterization of PVA hydrogel materials resembling the NP and
evaluates these materials as an NP replacement in IDH.
Data and methods
The present systematic review was performed
according to the Preferred Reporting Items for Systematic Reviews
and Meta-Analyses (PRISMA) guideline (10).
Search strategies
The PubMed, Google Scholar, Cochrane (CENTRAL) and
Science Direct databases were searched for relevant systematic
reviews, original articles and randomized clinical trials (RCTs) of
PVA hydrogel materials as NP replacements. The key words used for
the search were herniated NP, IVD herniation, NP replacement, IVD
substitution, PVA hydrogel and injectable hydrogel. The
corresponding author will provide the detailed search strategy upon
request. Additional studies were found by searching the references
of the retrieved publications and relevant overview articles.
Inclusion criteria
All reviews, original articles and/or RCTs written
in the English language were considered eligible for inclusion if
they fulfilled all of the following criteria: i) The study
population consisted of patients with IDH and NP replacement or IVD
substitution; ii) the study population (in vitro, in
vivo and ex vivo) had undergone NP replacement or IVD
substitution with PVA hydrogel materials; iii) PVA hydrogel
material biomechanics, including stress, strain and Young's
modulus, had been characterized. Studies that matched the inclusion
criteria for any groups were included, as were subgroups provided
that the subgroup findings were presented separately.
Study selection
The inclusion criteria were applied to the
references found by the literature search independently by two
reviewers to select relevant studies from the titles and abstracts
or, if necessary, from the whole publication. A third reviewer was
engaged to settle disputes, if necessary.
Categorization of the relevant
literature
Relevant literature was categorized with biomaterial
testing of the PVA hydrogel as an NP replacement. The literature
could be in the form of RCTs, reviews, original articles and
material biomechanical testing. All of the selected literature
contained stress, strain and Young's modulus as the biomechanical
properties of the PVA hydrogel materials.
Data extraction
The data were extracted independently by two
reviewers. Information was collected on the PVA hydrogel compound,
biomechanical characterization, stress, strain and Young's modulus.
Biomechanical testing in reports was required to have been
performed on a testing machine. The materials used to replace the
NP in IDH cases fulfilled all of the criteria described above.
Outcome measurements
The literature was selected if it reported the test
method, crosslinking materials, stress (MPa), strain (%) and
Young's modulus (MPa). The ASTM standard test method and
crosslinking materials were those used to measure the composition
of the PVA hydrogel material mixture. The stress (MPa), strain (%)
and Young's modulus (MPa) were of the biomechanical properties of a
material were collected. These biomechanical properties were then
compared with the value of human NP biomechanical properties with
the goal of identifying an ideal PVA hydrogel compound biomaterial
similar to the NP.
Results
Study selection
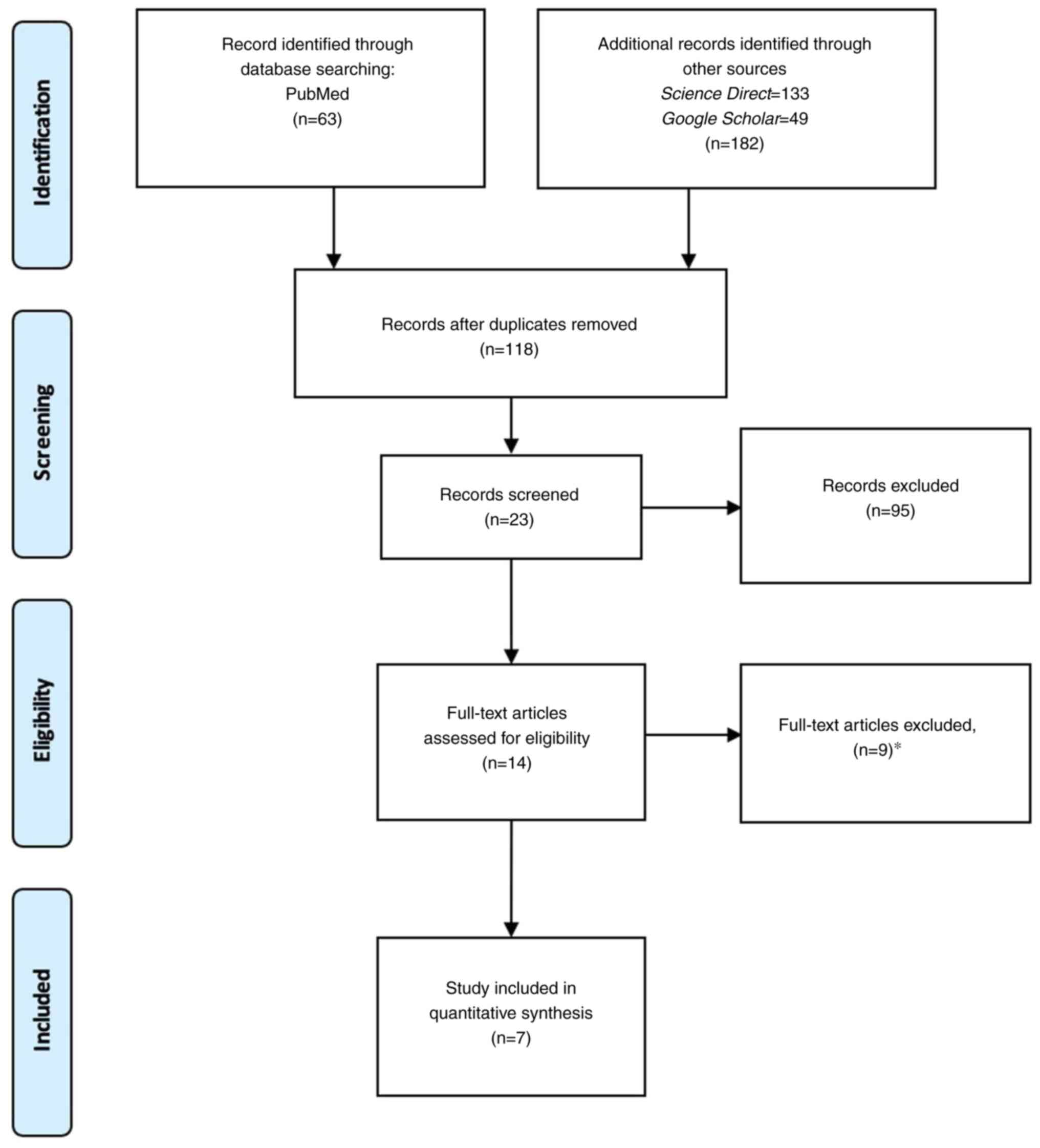
The initial search identified 245 references. A
total of 222 articles were excluded on the basis of duplication
and/or the abstract, title and key words. After reading the
complete articles, 16 articles were excluded for the following
reasons: The reports did not describe PVA hydrogel as the main
material (nine studies), no biomechanical properties (stress,
strain and Young's modulus) were reported, no outcome results were
provided (four studies), and there was no explanation of the
mechanical testing procedure (three studies). As a result, only
seven articles were included in the present systematic review
(Fig. 1).
Description of study
characteristics
No reviews were found in the search. A total of
seven articles on biomechanical testing from PVA hydrogel were
found. All seven articles were on the PVA hydrogel used as the base
material that was crosslinked with other materials, described
unconfined uni-axial compression mechanical testing, and reported
the biomechanical properties results.
Data extraction
The relevant information from the studies selected
according to the inclusion criteria described above is presented in
Table I. The information explained
the mechanical testing procedure, crosslinking materials, and
biomechanical properties. The literature search identified the
materials with the biomechanical properties most similar to those
of the NP. The study by Binetti et al (2012) (11) demonstrated that a PVA hydrogel with
polyethylene glycol-diglycidyl ether (PEG-DGE) had a Young's
modulus of 2 MPa. This result is similar to the Young's modulus of
human NP (1.43-32.85 MPa) (12).
 | Table ICharacteristics of the primary studies
included in the present systematic review. |
Table I
Characteristics of the primary studies
included in the present systematic review.
| No. | Author(s), year of
publication | Test method | Crosslinking | Stress (MPa) | Strain (%) | Young's modulus
(MPa) | (Refs.) |
|---|
| 1. | Kita, 2010 | Unconfined uni-axial
compression test | PVP | 0.01 | 15 | 0.05 | (21) |
| 2. | Binetti et al,
2012 | Unconfined uni-axial
compression test | PEG-DGE | 5.71 | 35 | 2 | (11) |
| 3. | Mahanta et al,
2013 | Unconfined uni-axial
compression test | FeCl3 | 0.000006 | 47 | 0.000012 | (22) |
| 4. | Binetti et al,
2014 | Unconfined uni-axial
compression test | PVP, PEG-DGE | 0.18 | 30 | 0.6 | (23) |
| 5. | Neo et al,
2014 | Unconfined uni-axial
compression test | Silk | 0.06 | 45 | 0.13 | (19) |
| 6. | Charron et al,
2017 | Unconfined uni-axial
compression test | PEG 1% (w/v)
gelatin | 0.02 | 25 | 0.1 | (20) |
| 7. | Heo and Park,
2022 | Unconfined uni-axial
compression test | Phosphate-buffered
saline | 0.17 | 25 | 0.7 | (24) |
Discussion
New approaches supporting IVD regeneration that are
clinically practical and can enhance the quality of life of
patients need to be developed. Since the NP is the location at
which early IVD degeneration is most commonly observed, the NP is a
prospective target for future treatments. Tissue engineering with
NP bioinstructive materials is an alternative to currently
available therapies (7,13,14).
In addition, to helping to restore NP functionality by increasing
its disc height, biologically suitable materials to repair injured
tissue can also serve as a delivery system for cells and/or
biomolecules to help regenerate healthy tissue. This distinction
between NP restoration and regeneration imposes various critical
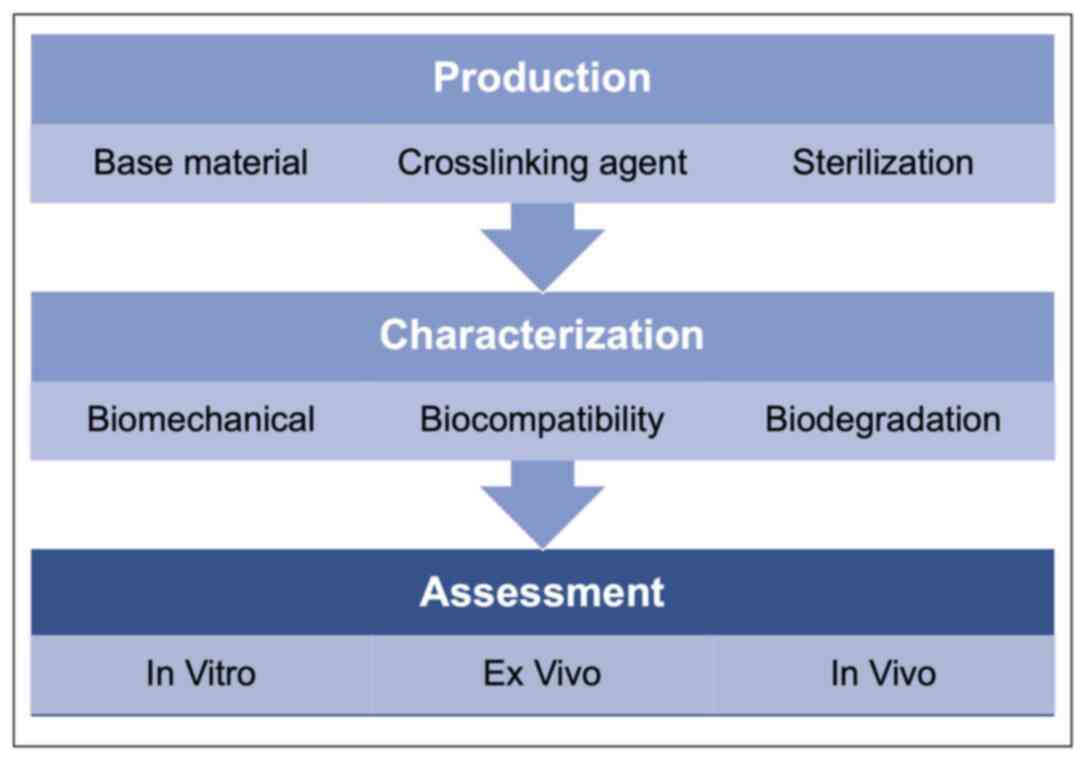
constraints on the relevant biomaterials (Fig. 2) (4,11,13,15).
Ideally, biomaterials need to restore the height and biomechanical
properties similar to those of the undamaged NP and be able to
withstand typical physiological loads on the disc (Table II). From a mechanical point of
view, mostly unconfined compression tests and rheology are
performed to understand a biomaterial's behavior under
physiologically relevant stresses. Consequently, a broad range of
parameters regarding their effects on the material's efficacy and
performance over time within the disc need to be considered during
development (Fig. 3) (4,9,13,16).
 | Table IIIntradiscal pressure values for
different positions and exercises. |
Table II
Intradiscal pressure values for
different positions and exercises.
| Position | Stress (MPa) |
|---|
| Lying | 0.10-0.12 |
| Standing | 0.5 |
| Sitting | 0.46-0.55 |
| Walking | 0.53-0.65 |
| Lifting 20 kg
(rounded back) | 2.3 |
| Lifting 20 kg
(straight back) | 1.7 |
In the present systematic review, the literature was
systematically selected to evaluate the biomechanical properties of
PVA hydrogels with various crosslinking materials. All the selected
literature described similar mechanical testing procedures for the
study materials. The biomechanical properties results were for
stress (MPa), strain (%) and Young's modulus (MPa) (9,17,18).
A total of seven articles were identified for the qualitative
analysis. The analysis revealed that knowledge on NP replacement is
limited, particularly with PVA hydrogel material, which is an ideal
substitute as its biomechanical properties are similar to those of
the NP. This condition may be affected by several factors, such as
the crosslinking materials, mechanical testing procedures and
material composition ratio.
In the literature search, the PVA hydrogels were
described as being crosslinked with polyvinyl pyrrolidone (PVP),
PEG-DGE, FeCl3, polyvinyl pyrrolidone (PVP)/PEG-DGE,
silk, PEG 1% (w/v) gelatin and phosphate-buffered saline (PBS).
Notably, the studies by Neo et al (2014) (19) and Charron et al (2017)
(20) demonstrated that the PVA
hydrogel could be crosslinked with natural materials, such as silk
and gelatin. Hydrogels crosslinked with natural materials are very
interesting to study due to their biocompatibility,
biodegradability and safety (7,14).
The studies by Kita (2010) (21),
Binetti et al (2012) (11),
Mahanta et al (2013) (22),
Binetti et al (2014) (23),
and Heo and Park (2022) (24)
demonstrated that PVA hydrogels crosslinked with synthetic
materials, such as PVP, PEG-DGE, FeCl3, PVP/PGE-DGE and
PBS, lacked bioactivity. However, those materials have the
advantages of being able to be engineered and formed to generate
the appropriate mechanical properties. The present systematic
review found that the biomechanical properties of natural
crosslinking agents were less similar than those of synthetic
crosslinking agents to those of NP (14,15,25).
Synthetic hydrogel crosslinking has exhibited improved Young's
moduli values than those of natural materials. Young's modulus is a
metric that evaluates resistance to changing the shape of a
material in response to an applied force, which is essentially a
measurement of a material's stiffness; the higher the value of
Young's modulus, the more inelastic the material is, making it more
difficult to deform (14,18,15,25).
The ratio of stress to strain yields Young's modulus. Due to its
association with the amount of load that a material can withstand
while keeping its shape, Young's modulus is crucial in defining the
biomechanical characterization of a material. The study by Binetti
et al (2012) (11)
demonstrated that the PVA hydrogel with PEG-DGE possessed the
omptimal Young's modulus value among other materials and that value
was similar to that of the NP. PVA hydrogel with PEG-DEG
crosslinking is an ideal candidate for NP replacement.
In conclusion, the biomechanical properties of the
NP and of PVA hydrogel are similar. The PVA hydrogel can be
combined with various substances to improve its biomechanical
properties. The most promising materials for NP replacement were
found to be PVA and PEG-DGE hydrogel. The materials were tested
in vivo to determine their resistance and mechanical
strength in the body. The biocompatibility of PVA hydrogel needs to
be further evaluated.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
EAS, GIP, AHB and MF were involved in the conception
and design of the study, in data collection and analysis, as well
as in the writing, revising and reviewing of the manuscript. NSS,
AR and BU were involved in the conception and design of the study,
and in the revising and reviewing of the manuscript. EAS, GIP, AHB,
MF, NSS, AR and BU confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Amin RM, Andrade NS and Neuman BJ: Lumbar
disc herniation. Curr Rev Musculoskelet Med. 10:507–516.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Newell N, Little JP, Christou A, Adams MA,
Adam CJ and Masouros SD: Biomechanics of the human intervertebral
disc: A review of testing techniques and results. J Mech Behav
Biomed Mater. 69:420–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang X, Zhao Z, Niu C, Ma Z, Hou J, Wang
G and Tang M: Spinal biomechanical modelling in the process of
lumbar intervertebral disc herniation in middle-aged and elderly. J
Healthc Eng. 2021(2869488)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Iatridis JC, Nicoll SB, Michalek AJ,
Walter BA and Gupta MS: Role of biomechanics in intervertebral disc
degeneration and regenerative therapies: What needs repairing in
the disc and what are promising biomaterials for its repair? Spine
J. 13:243–262. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fenton DS: CHAPTER 23-Disc Herniation:
Recurrent vs. Postoperative Scarring. In: Czervionke LF, Fenton DS,
eds. Imaging Painful Spine Disorders. W.B. Saunders; 2011: 174-179.
https://www.sciencedirect.com/science/article/abs/pii/B9781416029045000239.
|
|
6
|
Lewis G: Nucleus pulposus replacement and
regeneration/repair technologies: present status and future
prospects. J Biomed Mater Res Part B Appl Biomater. 100:1702–1720.
2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Allen MJ, Schoonmaker JE, Bauer TW,
Williams PF, Higham PA and Yuan HA: Preclinical evaluation of a
poly (vinyl alcohol) hydrogel implant as a replacement for the
nucleus pulposus. Spine (Phila Pa 1976). 29:515–523.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Thomas JD, Lowman A and Marcolongo M:
Novel associated PVA/PVP hydrogels for nucleus pulposus
replacement. J. Biomed. Mater. Res. 67:1329–1337. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Di Martino A, Vaccaro AR, Lee JY, Denaro V
and Lim MR: Nucleus pulposus replacement: Basic science and
indications for clinical use. Spine (Phila Pa 1976). 30(Suppl
16):S16–S22. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. Int J Surg. 88(105906)2021.PubMed/NCBI View
Article : Google Scholar
|
|
11
|
Binetti VR, Marcolongo M and Lowman AM:
Development of a chemically crosslinked poly (vinyl alcohol)
hydrogel for injectable nucleus pulposus replacement. In: 2012 38th
annual northeast bioengineering conference (NEBEC). IEEE; 2012:
378-379.
|
|
12
|
Wilke HJ, Neef P, Caimi M, Hoogland T and
Claes LE: New in vivo measurements of pressures in the
intervertebral disc in daily life. Spine (Phila Pa 1976).
24:755–762. 1999.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schmitz TC, Salzer E, Crispim JF, Fabra
GT, LeVisage C, Pandit A, Tryfonidou M, Maitre CL and Ito K:
Characterization of biomaterials intended for use in the nucleus
pulposus of degenerated intervertebral discs. Acta Biomater.
114:1–15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leckie S and Kang J: Recent advances in
nucleus pulposus replacement technology. Curr Orthop Pract.
20:222–226. 2009.
|
|
15
|
Yang X and Li X: Nucleus pulposus tissue
engineering: A brief review. Eur Spine J. 18:1564–1572.
2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Carl A, Ledet E, Yuan H and Sharan A: New
developments in nucleus pulposus replacement technology. Spine J.
4(Suppl 6):S325–S329. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia H, Lin X, Wang D, Wang J, Shang Q, He
X, Wu K, Zhao B, Peng P, Wang H, et al: Injectable hydrogel with
nucleus pulposus-matched viscoelastic property prevents
intervertebral disc degeneration. J Orthop Transl. 33:162–173.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Joshi A, Fussell G, Thomas J, Hsuan A,
Lowman A, Karduna A, Vresilovic E and Marcolongo M: Functional
compressive mechanics of a PVA/PVP nucleus pulposus replacement.
Biomaterials. 27:176–184. 2006.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Neo PY, Shi P, Goh JCH and Toh SL:
Characterization and mechanical performance study of silk/PVA
cryogels: Towards nucleus pulposus tissue engineering. Biomed
Mater. 9(65002)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Charron PN, Blatt SE, McKenzie C and
Oldinski RA: Dynamic mechanical response of polyvinyl
alcohol-gelatin theta-gels for nucleus pulposus tissue replacement.
Biointerphases. 12(02C409)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kita BK: Characterization of in-situ
curing PVA-PEG hydrogels for nucleus pulposus replacement. Thesis.
1:125–142. 2010.
|
|
22
|
Mahanta N, Teow Y and Valiyaveettil S:
Viscoelastic hydrogels from poly (vinyl alcohol)-Fe (iii) complex.
Biomater Sci. 1:519–527. 2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Binetti VR, Fussell GW and Lowman AM:
Evaluation of two chemical crosslinking methods of poly (vinyl
alcohol) hydrogels for injectable nucleus pulposus replacement. J
Appl Polym Sci. 131:2014.
|
|
24
|
Heo M and Park S: Biphasic properties of
PVAH (polyvinyl alcohol hydrogel) reflecting biomechanical behavior
of the nucleus pulposus of the human intervertebral disc. Materials
(Basel). 15(1125)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Cloyd JM, Malhotra NR, Weng L, Chen W,
Mauck RL and Elliott DM: Material properties in unconfined
compression of human nucleus pulposus, injectable hyaluronic
acid-based hydrogels and tissue engineering scaffolds. Eur spine J.
16:1892–1898. 2007.PubMed/NCBI View Article : Google Scholar
|