Introduction
Rare diseases, identified as an incidence of <5
out of 100,000 individuals, are difficult to diagnose and treat. An
unknown response to treatment can often manifest in such cases. The
analysis of the responsiveness to therapy could aid in the
understanding of these uncommon diseases. Etiological studies allow
us to associate some risk factors to a specific outcome, including
the differential response to treatment, and regression analysis
computes the slope of this association. Linear regression is
subjected to the assumption of independence, where each case should
be not related to a cluster, such as repeated measures (1). This assumption does not exclude the
analysis of repeated measures when all the analyzed measures refer
to one patient, as in this case, the patients represents the cohort
and the measures represent the cases. Repeated measures are
analyzed through mixed models, which relax the independence
assumption and take into account more complex grouped or clustered
data. Similar to a single center study, where a single center
represents one cluster and this analysis does not need to be
analyzed for repeated measures, a single patient represents a
cluster in which all the measures do not depend from the
independence assumption. Indeed, adding the patient as a random
variable in the mixed model, would not change the results due to
this being a constant. Each patient has a different response to one
therapy and sometimes, mostly in uncommon diseases, this response
is not clear. For this reason, the present study describes the case
of a patient with hypocalcemia, hypomagnesemia and hypokalemia. It
also presents a statistical approach using a single patient which
may help solve the not understandable physio-pathological
pathways.
Case report
A 71-year-old male patient presented with
paresthesia and motor deficits in all four limbs, at the University
Hospital ‘G. Martino’ of Messina. The results tests for
hematochemical parameters revealed metabolic alkalosis and severe
hypokalemia-hypocalcemia-hypomagnesemia, for which infusion therapy
with calcium gluconate (2 g/die i.v.) and magnesium sulfate (2
g/die i.v.) was necessary, in order to achieve the normalization of
serum magnesium and calcium levels. However, the severe hypokalemia
persisted, despite the progressive increase in potassium chloride
(KCl) supplementation up to 120 mEq/day. Each administration was
performed with a speed between 40 and 200 ml/h. Potassium, calcium
and magnesium were administered in different periods of the day.
Serum potassium levels were measured at the central laboratory of
the aforementioned hospital, in the morning with a sample obtained
via the peripheral vein. The urinary potassium/creatinine ratio
>1.5 mmol/mmol excluded extrarenal loss, for which the
hypothesis of hyperaldosteronism was posed. This hypothesis was
rejected based on a serum concentration of aldosterone <15
pg/ml, repeated several times during the week, without alterations
in renin, cortisol, adrenocorticotropic hormone (ACTH) and
dehydroepiandrosterone-sulfate (DHEA-S). Upon the suspicion of an
apparent mineralcorticoid excess (AME), as described in the study
by Monnens and Levtchenko (2),
potassium canreonate was added in increasing doses up to 400
mg/day, with the gradual normalization of kalemia observed.
The lack of response to the intravenous potassium
supplement led the authors to perform a statistical analysis to
evaluate the impact of the therapy on kalemia, in an attempt to
identify possible confounders that could explain the lack of a
therapeutic response. The serum potassium concentration was
considered as an outcome and the dose of potassium canreonate
administered in the previous 48 h (according to the mechanism of
action of the canreonate), the amount of daily KCl administered in
the 24 h preceding the kalemia dosage, and, similarly to the
latter, the quantity of NaCL0.9% physiological solution, as
independent variables.
To perform the statistical analysis, the
distribution of each variable was evaluated using the Kolmogorov
Smirnov test and graphical evaluation of the distributions. Thus,
linear regression models were performed using kalemia as dependent
variables, and NaCl 0.9% (l/die), KCl infusion (mmol/die) and
potassium canreonate (mg/die) as independent variables, in
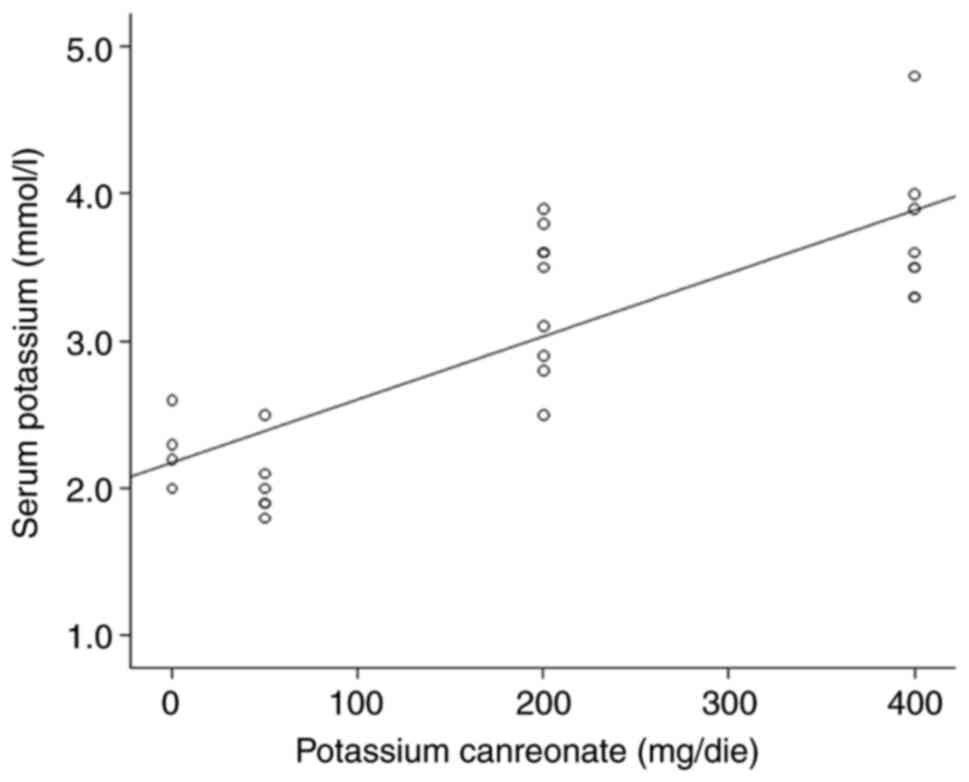
univariate and multivariate models. The analysis revealed a
statistically significant positive association between serum
potassium and potassium canreonate (β=4.3x10-3,
P<0.05) (Fig. 1), contrary to
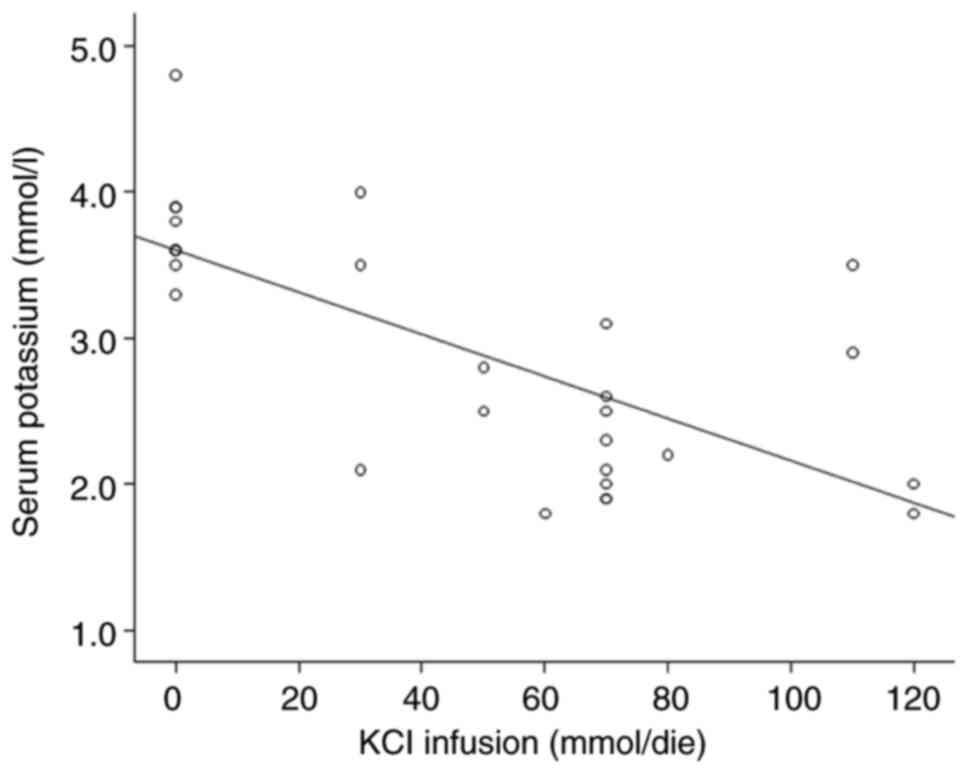
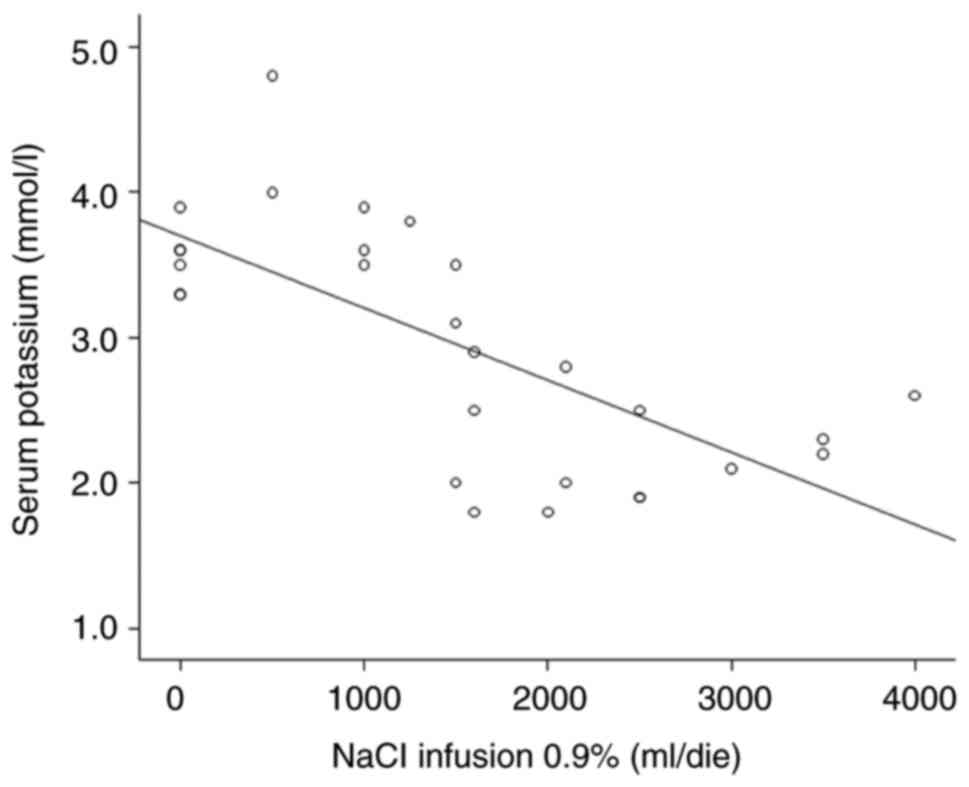
the association between kalemia and KCl infusion (mmol/day) and
NaCl0.9% (l/day), which exhibited a negative association
(β=-1x10-2, P<0.05 and β=-4.98x10-4,
P<0.05, respectively) (Figs. 2
and 3). All these three were added
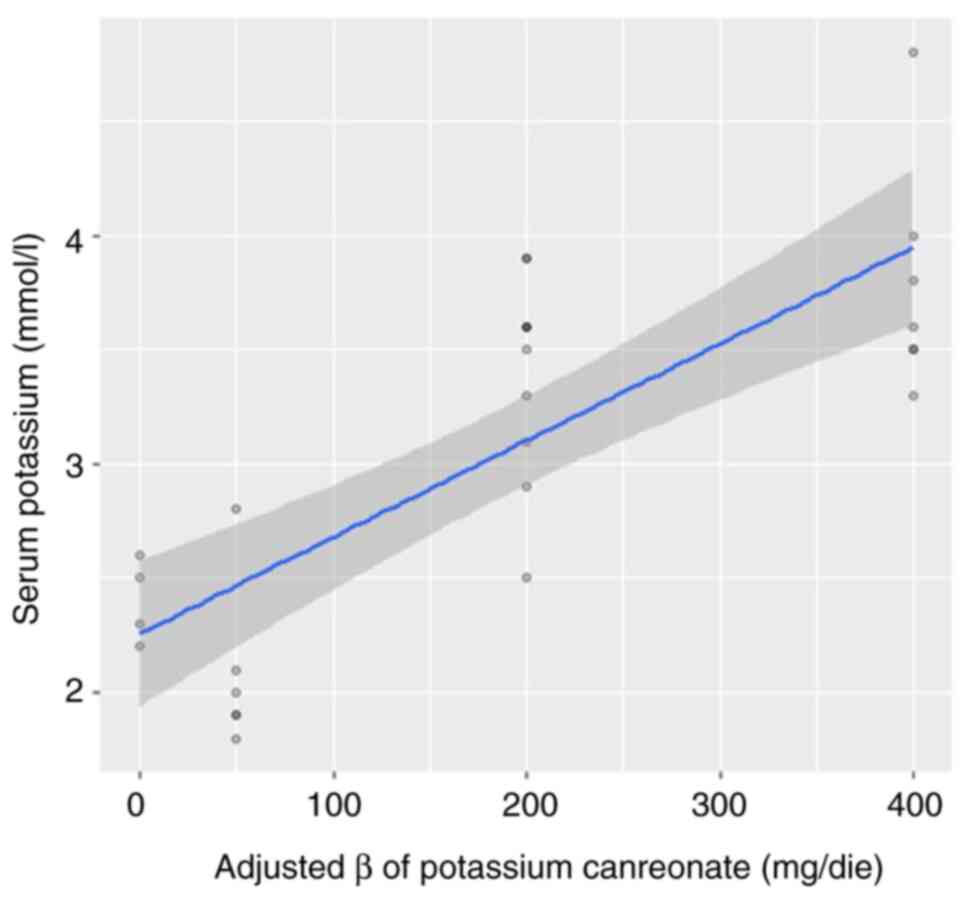
in a multivariate model, and only potassium canreonate was found to
have a significant impact (β=0.03, P<0.01), whereas no
significant associations were found with potassium infusion
(β=-0.004, P=0.28) and NaCl infusion (β=1.2x10-4,
P=0.38). The adjusted impact of potassium canreonate is presented
in Fig. 4. Thus, only potassium
carneonate significantly affected kalemia. This may be explained by
the highest association between NaCl infusion and K infusion,
related with a Pearson's coefficient of 0.68 (P<0.01). These
results demonstrated that neither NaCl infusion nor KCl infusion
affected kalemia in this patient, conversely to potassium
canreonate.
The literature reports association between AME and
some glomerulonephritis, such as IgA nephropathy (IgAN) and focal
and segmental glomerulosclerosis (3).
Discussion
Aldosterone-related diseases
Aldosterone is a steroid hormone synthesized in the
glomerular part of the adrenal gland that acts on the
mineralocorticoid receptor (MR) by increasing the reabsorption of
sodium and the excretion of potassium at the level of the
collecting tubule. It also activates Na/K pumps in myocardial cells
(PKC), probably without interaction with MR and, through Na/H
pumps, activates MAPKs and modulates intracellular Ca2+.
Its dysregulation causes two pathological features:
Hypoaldosteronism and hyperaldosteronism (4).
Hypoaldosteronism is a condition caused by a serum
aldosterone concentration <15 pg/ml. It may occur due to the
impaired surrealist gland function, with a high ACTH level to
compensate this gap, or a reduced central ACTH secretion, which
does not stimulate hormone secretion (5). The clinical manifestations often
include arterial hypotension, hyponatremia and hyperkalemia. It may
be related to a low renin production, causing hyporeninemic
hypoaldosteronism (6). Conversely,
the excess serum concentration of aldosterone causes hypernatremia
with hypokalemia and hypertension (5). However, a similar clinical
manifestation may be due to a defective receptor function, which
impairs or improves aldosterone function.
Cortisol is a glucocorticoid hormone with an
affinity for MRs, such as aldosterone; however, its concentration
in plasma is 100-fold greater than aldosterone. The enzyme
11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) is expressed in
the distal nephron and collecting duct, and metabolizes cortisol to
cortisone, which does not affect MR. HSD2 deficiency results in the
reduced inactivation of cortisol, with a high ratio of urinary
tetrahydrocortisol to tetrahydrocortisone (7,8).
Other enzymes, including HSD1, 20β-oxidoreductase, 6β-hydroxylase,
5β-reductase, 5α6-reductase and 3α-HSD affect the metabolism of
cortisol (9).
Hyperaldosteronism, but with low or normal serum
concentrations of aldosterone, is observed in
pseudo-hyperaldosteronism (Liddle's syndrome or other receptor
genetic mutations) and the syndrome of an apparent excess of
mineralocorticoids (11β-HSD2 defect).
AME
In AME, the enzyme, 11β-HSD2, co-expressed in the
kidneys with the MR, plays a critical role: Cortisone is converted
from cortisol, as its inactive metabolite. In the case of AME,
mutations in the 11β-HSD2 gene result in a lack of the enzyme with
consequent failure to metabolize the cortisol that binds to MRI and
causes sodium, hypokalemia, the suppression of Plasmatic renin
activity (PRA) and hypertension. AME is a genetic disease, but
several exogenous factors can determine the function of the
11β-HSD2 enzyme.
The inhibition of the 11β-HSD2 gene can also be
caused by the ingestion of bioflavonoids, licorice and
carbenoxolone; thus, a correct medical history is critical for a
good differential diagnosis. Furthermore, some studies have
documented an association between the reduction of 11β-HSD2
activity with preeclampsia in renal disease and liver cirrhosis
(due to increased sodium retention) and with Cushing's syndrome,
due to enzyme saturation, excess of corticoid minerals and ectopic
ACTH production (3). In the latter
case, it is the excess substrate that overwhelms the conversion
capacity of 11β-HSD2. Furthermore, the reduced expression of
11β-HSD2 can already manifest itself at the placental level with
the reduction of birth weight and hypertension in adults (10).
In 2006, the study by Sechi et al found a
more rapid progression of chronic kidney disease and a higher
albumin/creatinine ratio in urine in patients with primary
aldosteronism (11). Similar
result were shown in the systematic review of 2020(12).
It is not yet clear whether the cause of this
worsening is due to the concentration of aldosterone itself or the
activation of its receptor. In this regard, the research published
by Bantis et al (3,13) in 2011 revealed an association
between the aldosterone receptor C-344T polymorphism and the
progression of renal disease in patients with IgAN and focal and
segmental glomerulosclerosis, probably due to the interaction with
the MAPK system.
In the present study, statistics was only a tool for
providing an answer, and a clinical explanation is warranted. The
analysis revealed a negative association between kalemia and KCl
infusion, as well as between kalemia and NaCl infusion. KCl doses
could be considered both as a confounding factor by indication, on
account of the higher prescribed doses in lower kalemia. The
positive association between KCl and NaCl infusions may be
explained by the administration of KCl with NaCl. Thus, the KCl
doses were strongly related to the NaCl doses, that could affect
the negative association revealed at the univariate regression.
Indeed, at the multivariate model, their association lost the
significance. Thus, the negative association between KCl and
kalemia may be explained by these two mechanisms.
As regards the association between NaCL and kalemia,
the reduction in serum potassium levels related to higher doses of
NaCl may be due to the probable effect of the increase in the
tubular flow of sodium on the kir1.1 channels, which increases
potassium excretion, in accordance with the findings of Palmer and
Clegg (14). This mechanism is
known as the aldosterone paradox, and allows for the compensation
of light hormonal variation, ensuring potassium homeostasis.
The effect of potassium canreonate on the
augmentation of kalemia was confirmed by multivariate analysis in
our analysis as the only variable related to the kalemia,
highlighting the hyperstimulation of the MR.
The main limitations of the present study are
represented by the possible unknown diseases, due to the worse
compliance of the patient, and by the ‘observational design’ of the
study. However, this may allow us to improve the diagnosis of AME
and its consequent possible association with the diagnosis of
IgAN.
In conclusion, as demonstrated in the present study,
although unusual, conceiving over time the measurements of
variables on a single patient as ‘cases’ and the set of
measurements of the patient himself (single patient) as a ‘cohort’
may be an aid in quantitatively analyzing the impact of one or more
treatments and in identifying unknown causes that may influence the
therapeutic response, particularly in cases with a paradoxical
response.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FZ and VC conceptualized the study. FZ and RS
examined the patient. AL, FZ and FGV were involved in the search
for relevant literature. RS, FZ and VC were involved in the writing
and preparation of the original draft of the manuscript. VC and DS
were involved in data analysis. AL and RS were involved in the
writing, reviewing and editing of the manuscript. RS was involved
in the processing of images. DS and VC confirm the authenticity of
all the raw data. DS supervised the study. All authors have read
and agreed to the published version of the manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
participant included in the study. All procedures performed in
studies involving human participants were in accordance with the
ethical standards of the institutional and/or national research
committee and with the 1964 Declaration of Helsinki and its later
amendments or comparable ethical standards.
Patient consent for publication
Written informed consent was obtained from the
participant included in the study for the publication of his
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tripepi G, Jager KJ, Dekker FW and Zoccali
C: Linear and logistic regression analysis. Kidney Int. 73:806–810.
2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Monnens L and Levtchenko E: Distinction
between Liddle syndrome and apparent mineralocorticoid excess.
Pediatr Nephrol. 19:118–119. 2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bantis C, Heering PJ, Stangou M, Kouri NM,
Schwandt C, Memmos D, Rump LC and Ivens K: Influence of aldosterone
synthase gene C-344T polymorphism on focal segmental
glomerulosclerosis. Nephrology (Carlton). 16:730–735.
2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
White PC: 11beta-hydroxysteroid
dehydrogenase and its role in the syndrome of apparent
mineralocorticoid excess. Am J Med Sci. 322:308–315.
2001.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rajkumar V and Waseem M:
Hypoaldosteronism. In: StatPearls. StatPearls Publishing, Treasure
Island, FL, 2023.
|
|
6
|
Arai K, Papadopoulou-Marketou N and
Chrousos GP: Aldosterone Deficiency and Resistance. In: Endotext.
Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E,
de Herder WW, Dhatariya K, Dungan K, Hofland J, et al (eds):
MDText.com, Inc. South Dartmouth, MA, 2000.
|
|
7
|
Dammann C, Stapelfeld C and Maser E:
Expression and activity of the cortisol-activating enzyme
11β-hydroxysteroid dehydrogenase type 1 is tissue and
species-specific. Chem Biol Interact. 303:57–61. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Morineau G, Sulmont V, Salomon R,
Fiquet-Kempf B, Jeunemaître X, Nicod J and Ferrari P: Apparent
mineralocorticoid excess: Report of six new cases and extensive
personal experience. J Am Soc Nephrol. 17:3176–3184.
2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gathercole LL, Lavery GG, Morgan SA,
Cooper MS, Sinclair AJ, Tomlinson JW and Stewart PM:
11β-Hydroxysteroid dehydrogenase 1: Translational and therapeutic
aspects. Endocr Rev. 34:525–555. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Palermo M, Quinkler M and Stewart PM:
Apparent mineralocorticoid excess syndrome: An overview. Arq Bras
Endocrinol Metabol. 48:687–696. 2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sechi LA, Novello M, Lapenna R, Baroselli
S, Nadalini E, Colussi GL and Catena C: Long-term renal outcomes in
patients with primary aldosteronism. JAMA. 295:2638–2645.
2006.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Monticone S, Sconfienza E, D'Ascenzo F,
Buffolo F, Satoh F, Sechi LA, Veglio F and Mulatero P: Renal damage
in primary aldosteronism: A systematic review and meta-analysis. J
Hypertens. 38:3–12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bantis C, Heering PJ, Siekierka-Harreis M,
Kouri NM, Schwandt C, Rump LC and Ivens K: Impact of aldosterone
synthase gene C-344T polymorphism on IgA nephropathy. Ren Fail.
33:393–397. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Palmer BF and Clegg DJ: Physiology and
pathophysiology of potassium homeostasis: Core curriculum 2019. Am
J Kidney Dis. 74:682–695. 2019.PubMed/NCBI View Article : Google Scholar
|