1. Introduction
Following the coronavirus disease 2019 (COVID-19)
pandemic caused by severe acute respiratory syndrome coronavirus 2
(SARS-CoV-2), the incidence of mucormycosis has increased. This, in
conjunction with the pandemic has caused devastating human health
issues globally, particularly among South Asian and other Asian
countries, particularly in individuals with major predisposing
conditions, such as uncontrolled diabetes mellitus, comorbidity
effects from steroid therapy with elevated iron levels (1). Mucormycosis can be defined as an
angio-invasive fungal infection with relatively high morbidity and
mortality rates. Broadly the species of the phylum Zygomycota cause
mucormycosis, particularly Mucorales and Entomophthorales. In
Mucorales, four genera that are most closely associated are
Cunninghamella, Rhizopus, Mucor, Absidia, etc.
Conidiobolus and Basidiobolus are the two key genera
belonging to the Entomophthorales order and tend to cause
infections in human beings (2).
The etiologic agents of mucormycosis are cosmopolitan in
distribution. The mucormycosis condition is a classical
opportunistic invasion and typically affects immunocompromised
individuals, particularly those with conditions, such as
ketoacidosis, burn or trauma, or those under iron chelation
treatment and some individuals who are severely immunocompromised
due to malignancy or even chemotherapy (3). The case incidence of mucormycosis is
underrated possibly due to laborious diagnostic procedures and
mostly depends on histopathalogical analysis or the culturing
process and remains under-reported (4).
Among all mucormycetes, Rhizopus oryzae is
the most widespread strain and contributes for 60% of human cases
and is also responsible for 90% of rhinocerebral mucormycosis cases
(5). With a devastating and
multifaceted clinical symptomatology, mucormycosis has emerged as
an infectious disease worldwide. Mucor moulds are generally
found in soil, plants, manure or even in fruit and vegetable
compost, and are rarely found in air as a transient existence.
Depending on the site of infection, mucormycosis differentiates
into a rhinocerebral, pulmonary, cutaneous and gastrointestinal
infection (6). The pathogenicity
of infection ranges from mild to fatal, depending on the incubation
period of the moulds. The incubation period of this fungus is 5 to
6 days; individuals can become infected by inhaling spores of
moulds. Mucormycosis is generally non-contagious, and due to
potential innate immunity, the majority of individuals exposed to
the spores do not develop infection (7).
Once affected by Mucorales, the disease progresses
rapidly. Moreover, opportunistic fungi, such as Mucor
irregularis or Rhizomucor variabilis reported from
China, tend to cause diverse epidemiological and clinical
manifestations (8).
2. Parallelism of COVID-19 and
mucormycosis
In India, ~31 million individuals were affected by
the COVID-19 pandemic, and the number of COVID-19-related
mucormycosis cases also simultaneously increased, particularly
during the second wave, which occurred in June, 2021. During 2021,
mucormycosis was observed to be prevalent in India, and its
estimated incidence was 14 in every 100,000 individuals compared to
other cases (9). A sudden
emergence of mucormycosis cases along with COVID-19 was observed;
although rare, mucormycosis was a serious and rapid complication
associated with COVID-19(9). The
major cause of this condition was Mucorales spore inhalation by
patients with COVID-19 with a low oxygen (hypoxia), hyperglycemic
index or even steroid-induced hyperglycaemia, acidic conditions
such as metabolic acidosis, diabetic ketoacidosis and high iron
levels (increased ferritins), with a decreased phagocytic activity
of white blood cells (WBCs) due to immunosuppression
(SARS-CoV-2-mediated or other comorbidities). This was coupled with
prolonged hospitalization with or without COVID-19 infection or
sometimes after a few weeks of recovery (5-9).
However, during the COVID-19 pandemic, the number of mucormycosis
cases increase in India perhaps due to improper hygienic
maintenance in hospital linens, medications and packaged foods.
This increase may also be due to the following reasons: Following
infection with COVID-19 patients have a low immune status due to a
decrease in WBCs. In addition, during the viral infection, patients
are medicated with corticosteroids and tocilizumab to reduce lung
inflammation, which often worsens the immune status, hence leading
patients to become prone to fungal infection (9). The extent of acute Mucor
infection is dependent upon the overall immunological status and
overall health status of an individual. As COVID-19 can damage
respiratory tissues and blood vessels, this increases the
susceptibility to fungal infection; black fungus invades
(angioinvasive mycosis) rapidly and multiplies in blood vessel
walls where it effectively reduces and tears blood vessels and
tissues, thereby resulting in tissue damage (10). The infection can have adverse
affects in oxygen-dependent patients with poor sanitary or aseptic
practices.
3. Clinical classification of
mucormycosis
Depending on the site of infection, mucormycosis may
be categorised into the following types (Table I).
 | Table ITypes of mucormycosis and the
associated risk factors and symptoms. |
Table I
Types of mucormycosis and the
associated risk factors and symptoms.
| Clinical forms of
mucormycosis | Risk factors | Symptoms | (Refs.) |
|---|
|
Rhino-orbito-cerebral mucormycosis
(ROCM) | Diabetes, solid
organ transplant, corticoster -oid therapy, chronic kidney disease
and intravenou -s drug usage | Fever, Headache,
Facial swelling, Facial pain, Nasal discharge, Epistaxis,
Sinusitis, Hemipleagia | (1,23-28) |
| Pulmonary
mucormycosis (PM) | Haematological
malignancy, diabetes mellitus, haematopoietic stem cell transplant
or organ transplant, renal disease in PM, post pulmonary
tuberculosis | High fever,
persistent cough, pleuritic chest pain, dyspnoea and
haemoptysis. | (26-31) |
| Cutaneous
mucormycosis (CM) | Immunocompetent
patients, diabetes mellitus, SOT, penetrating trauma, open wound
trauma/motor vehicle accident/surgery, contaminated surgical
dressings/burns/natural disasters/animal bites or scratches | Localized
infections restricted to cutaneous and subcutaneous infections,
Fungal invasion to muscles, bones and tendons/ necrotising
fasciitis | (26,27,32,33) |
| Gastrointestinal
mucormycosis (GM) | Patients with
malnutrition or undergoing peritoneal dialysis, solid organ
transplant patients, haematological malignancies and neutropenia,
diabetes mellitus, chronic alcoholism, the administration of
broad-spectrum antibiotics | Abdominal pain,
gastrointestinal bleeding, abdominal distension and diarrhoea | (27,35,36) |
| Renal mucormycosis
(RM) | Kidney-associated
diseases, dialysis. | Fever, flank pain,
haematuria or anuria | (33,37-40) |
| Disseminated
mucormycosis (DM) | Solid organ
transplant and haematological malignancy patients | Spreading through
blood, leading to brain/sinus/lung/central nervous system/liver and
or kidney infection | (26,41) |
Rhino-orbito-cerebral mucormycosis
(ROCM)
ROCM is the most common clinical manifestation of
mucormycosis. The infection begins with the inhalation of spores,
spreading into the paranasal sinuses (11). The primary etiological agent of
ROCM is an aseptate fungus (Rhizopus oryzae) which is
associated with a 50% mortality rate. The fungus proliferates to
adjacent tissues such as the palate, sphenoid sinuses, orbits or
cavernous sinuses and enters the central nervous system. Black
eschar is the necrotised tissue patches due to the local extension
of fungal invasion (12).
Pulmonary mucormycosis
This infection is typically associated with
haematological malignancies [Centers for Disease Control and
Prevention (CDC) guidelines] (13), mainly associated with the lungs and
is the dominant form of mucormycosis observed in patients with
transplantation or in immunocompromised individuals (14). In the majority of cases, symptoms
are generally non-specific and may include fever, cough, dyspnoea
and chest pain. The infection presents with typical lesions
involving parenchyma cells may extend to various sites in the
cardiac regions (11,14) with the causative agent is
Rhizopus or Mucor.
Cutaneous mucormycosis (CM)
CM may be classified into primary and secondary.
Primary infection occurs by the direct introduction of fungal
spores to damaged skin, whereas secondary infection occurs through
dissemination from previously infected regions, such as through
rhinocerebral infection (15).
Apophysomyces elegans, Lichthemia and Mucor spp. are
the main Mucorales species involved in primary CM, which accounts
for necrosis, redness, swelling, purulent discharge and a mouldy
appearance over the skin. The secondary infection is acute with a
high mortality rate. Initial symptoms include sinusitis with
necrotic eschar and the further loss of vision, as well as other
neurological deficits (16).
Gastrointestinal mucormycosis
(GIM)
Primary GIM is the less frequent form of the
disease. GIM results due to the consumption of contaminated food,
such as dried contaminated bread or bakery products in addition to
contaminated medical devices (11-17).
In the gastrointestinal tract, the stomach is the first target site
of infection and this may lead to infection in the colon and later
to the ileum, as well as to the duodenum and jejunum (17). Gastrointestinal bleeding with
altered bowel habits and severe abdominal pain are the typical
symptoms (18). The causative
agent of GIM is typically Mucor and precipitated with
Aspergillus and even Salmonella infection.
Disseminated mucormycosis (DM)
DM involves at least two non-contiguous sites,
commonly infecting the lungs/sinus/soft tissues/central nervous
system/liver/kidneys (19) mainly
by Mucor and other zygomycetes. A high iron concentration
and profound immunosuppression are the main predisposing factors
for DM. Shirane et al (20), through a case study analysis on a
58-year-old male patient revealed that the autopsy result of the
patient's body disclosed the presence of Mucor in heart,
liver, right kidney, right adrenal gland and cerebellum, which
resulted in thromboangiitis and infarction in these organs.
Uncommon presentations
Uncommon presentations include endocarditis/bone or
and joint infections/peritonitis/pyelonephritis. Osteoarticular
mucormycosis may affect after the trauma/surgical process.
Peritonitis occurs due to continuous ambulatory peritoneal
dialysis, while isolated renal mucormycosis is commonly observed in
patients with intravenous drug usage or renal transplant patients.
Another significantly rare manifestation is isolated cerebral
mucormycosis, involving the central nervous system following
proliferation from the paranasal sinus (21).
Health-care associated mucormycosis
(HCM)
HCM is a matter of utmost concern, particularly in
neonatal units, haematology, the transplantation of grafts or even
in intensive care units, diabetes and severe prematurity (3). Surgical intervention is associated
with 41% of HCM cases, while other cases are linked to the use of
contaminated medical devices, such as adhesive bandages/tongue
depressors/ostomy bags or others (21).
4. Epidemiology
The number of COVID-19 pandemic-associated black
fungus infection cases has increased globally and a similar
situation was observed in India. Furthermore, In India, The
National COVID-19 task force has issued an advisory notice and the
Union Health Ministry has instructed the states/UTs to declare
black fungus as an epidemic. Infection with Mucorales, particularly
in immunocompromised individuals can be quite rampant and
progressive with the etiological agent being the opportunistic
fungus, Mucor irregularis (22). Generally, the infection is chronic,
occurring in immunocompetent patients, involving the skin and
subcutaneous tissues, leading to severe complications. In India,
the state of Rajasthan first declared a mucormycosis epidemic,
while the city of Surat noted that 8 out of 40 COVID-19 survivors
developed this infection in the eyes and lost their vision
(information obtained from the India Today web desk during the
second lockdown in 2021) (22).
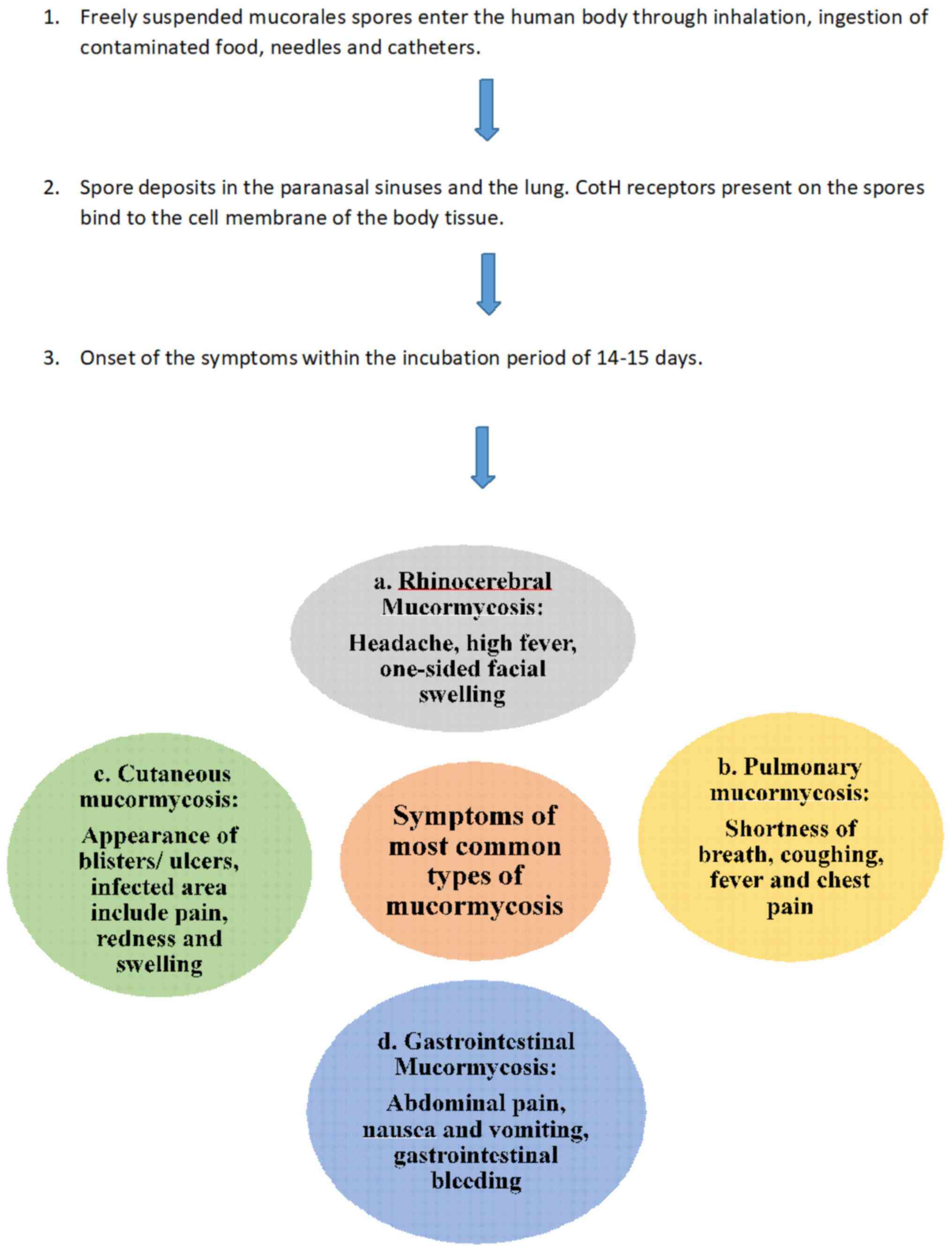
5. Symptoms
Mucormycosis symptoms generally begin with
sinusitis, nasal blockage, congestion with blackish or bloody nasal
discharge, pain in the cheek bone or one-sided facial pain,
numbness, swelling, blackish discoloration on nose palate,
loosening of the teeth, blurred or double vision with pain,
thrombosis, necrosis, chest pain, pleural effusion with difficulty
in respiratory functions (21-41)
(Fig. 1).
6. Aetiology and pathophysiology of the
fungus
The word mycosis stands for the lethal fungal
disease caused by infection and direct interaction of fungal spores
with the body tissues. Mucormycosis, dermatophytoses, yeast
infections, systemic mycoses and mycetoma are the pathogenic fungi
involved in mycosis disease (42).
Prakash et al (43)
documented the pathogenic Mucorales fungi, Rhizopus,
Lichtheimia, Cunninghamella, Rhizomucor and
Apophysomyces as the causitive agents of mucormycosis, which
were isolated from Indian soils and similarly from air samples.
Among the Rhizopus species, Rhizopus arrhizus and
Rhizopus homothallicus are the most common agents causing
ROCM. Apophysomyces variabilis is the second highest
causative agent and accounts for 60% of the total cases of
mucormycosis in the population (44). During the COVID-19 pandemic, the
main factors responsible for the development of COVID-19-associated
mucormycosis included the high rate of diabetes mellitus,
unsanitary/poor hygienic conditions and high ferritin levels in the
body (45). The disease sets with
the entry of Mucor or other strain spores via the air tract
either through the nose, mouth or skin lacerations (46). Upon the entry of the spores,
individuals who are compromised with cellular and humoral immunity
are unable to provide a defence against the pathogen (47). Thus, the fungus can then spread to
the paranasal sinuses, and later to the orbit, meninges or brain.
Angiotensin-converting enzyme 2 (ACE2) and transmembrane serine
protease 2 (TMPRSS-2) are the two receptors through which COVID-19
enters the cell. ACE2 is the receptor for the majority of cells in
the body and has a higher rate of expression in the respiratory and
renal tract, and gastrointestinal epithelium. TMPRSS receptors are
similar to ACE2, but are present only in respiratory and
gastrointestinal epithelial cells. TMPRSS-2 along with ACE2
receptors has a tendency to attack lymphocytes, thus reducing
CD4+ and CD8+ T-cell counts, resulting in a
weaker immunity, also inducing lymphopenia. The reduction in the
T-cell number consequently increases interleukin levels and
effectively achieves the state of the cytokine storm (48). The cytokine storm weakens the
defence system reserve pool, causes the atrophy of lymphoid tissue,
and prevents the further production, differentiation and
proliferation of protective lymphocytes. The weakened immune system
paves the way for the entry of Mucorales into the body. Another key
condition which fuels the fungal growth is lactic acidosis; this
eventually destroys type II alveolar cells, leading to excessive
respiratory disabilities that intensify acid-base levels. The
resulting hypoperfusion and hypoxemia worsens the condition of the
body by increasing acidic conditions. The coupled reaction of the
cytokine storm and lacto/keto acidosis aggravates the state of the
patient; in this case, there is an urgent need for treatment
through immunosuppressive steroids. These criteria promote an ideal
environment for the fungus to grow (49). In addition to this, increased
ferritin level due haemolysis and an increased body temperature
promote the growth and development of the fungus in
immunosuppressed individuals (50). The ACE2 receptor nourishes the
growth of Mucorales by damaging pancreatic β-cells, which results
in elevated plasma glucose levels, and the consequent increase in
glucose levels feeds the fungus. This explains the increased rate
of mucormycosis in diabetic patients (6,47,48).
These irreversible consequences eventually deteriorate the overall
health status of the patient, often leading to fatal results
(51).
7. Conditions such as diabetes and the
incidence of mucormycosis
It has been reported that during 2013-2015, in four
major tertiary care hospital in India, there were 388 incidents of
mucormycosis, and 56% of these cases had unregulated diabetes,
which demonstrates that the existence of underlying conditions
predisposes the proliferation of the fungus. In addition, trauma
was reported in 10% of these cases, which signifies the linkage of
this fungal infection to diabetes (39,41,52).
India has a prevalence of diabetes of 9% in the adult population
(44). Conversely, coronavirus
infects the pancreas and can disrupt blood sugar levels perhaps due
to the infection or due to clinical treatment. In this context,
host immunity appears to be compromised; consequently, elevated
sugar levels provide an ideal pabulum for mucormycosis development
and in individuals with uncontrolled diabetes, this enables the
highest replication of SARS CoV-2, which produces mitochondrial
reactive oxygen species and activates hypoxia-inducible factor 1α
(18,19). In fact, the uncontrolled diabetes
condition enhances acidic media, which is an ideal condition for
the proliferation of the fungus. Thus, mycelial invasion and
proliferation are promoted by the hyperglycaemic index and acidosis
(53) followed by the enhanced
release of iron from ferritin (due to acidosis). Therefore, it is
critical to maintain blood sugar levels under control during the
course of antifungal treatment.
In a case study conducted among 95 patients with
COVID-19-associated mucormycosis (CAM) who were admitted to the
Bowring and Lady Curzon Hospital from June to September, 2021 (70
males and 25 females), 69% of the patients had type 2 diabetes
mellitus with mean serum ferritin levels of 537.38±468.88 ng/ml.
The patients were positive for Mucorales and the KOH test, while
serum ferritin levels were markedly elevated and identified as
Aspergillus, Mucor, Rhizopus and Candida
spp. (54).
8. Diagnosis
Since the mortality, morbidity and haematological
defects are prominent features, the diagnosis of mucormycosis poses
a tough challenge. Rapid diagnosis from invasive aspergillosis is
of top priority as antifungal treatment would differ, while the
underlying clinical conidtions are almost similar (6,55).
Basically, direct microscopy was the gold standard for diagnosis
until recently; however, the process was cumbersome. In the case of
invasive aspergillosis, the assay for circulating antigens, such as
galactomannan/β-D-1,3-glucan is ideal, although it provides no
evidence of Mucormycosis (56).
Hence, for mucormycosis, direct microscopy, histopathology for
hyphal detection and invasion along with the analysis of cultural
characteristics on Sabouraud dextrose agar or potato dextrose agar
are optimal diagnostic tools. With the advent of molecular biology
tools and applications, the rapid detection of fungal infection has
become a reality. A quantitative multiplex polymerase chain
reaction (qPCR)-based 18S rRNA targeting Mucor/Rhizopus,
Lichtheimia and Rhizomucor has recently become a
hallmark of detection, particularly during the early stages and
even within 3 days of the disease onset using blood or serum with
90% authenticity (52). In the
case of post-burn infection, this can be detected 11 days before
standard diagnosis. RT-PCR analysis of Mucorales in tissue/biopsy
for haematological malignancies can detect probable mucormycosis
infection (57).
9. Prophylaxis and treatment
The effective treatment of mucormycosis is generally
based on a multifaceted strategy, which may include early
medication at the optimal dosage, the complete evacuation of the
fungus and use of diverse adjunctive therapies (58). The majority of Mucorales exhibit
resistance to most antifungal agents in vitro
(voriconazole). Amphotericin B is an effective drug, with the
exception of some species of Cunninghamella and
Apophysomyces (6). In
addition, posaconazole and isavuconazole are also effective, and
itraconazole and terbinafine exhibit some activity against certain
species.
Amphotericin B
The recommendations from the European Conference on
Infections in Leukemia (ECIL-6) and the European Society of
Clinical Microbiology and Infectious Diseases (ESCMID)/European
Confederation of Medical Mycology (ECMM) guidelines suggest the
usage of a lipid formulation of amphotericin B as a frontline
therapy (59).
The liposomal amphotericin B suggested dose is 5
mg/kg/day and the maximum 10 mg/kg/day (central nervous system
infection). Amphotericin B is the polyene antifungal agent (highly
protein bound and poorly dialyzable) and binds to sterols
(ergosterol) on the fungal cell membrane surface. This antifungal
drug change in membrane permeability leading to cell cytoplasmic
leakage. The high dosage of this antifungal drug can result in
chills, fever, phlebitis, renal damage and anaphylaxis as
side-effects. Dosage may gradually increase from 5 to 10 mg/day, up
to a total dose of 0.5 to 0.7 mg/kg/day, depending on the
cardiorenal status of the patient. An important risk factor while
using amphotericin B is that the dosage must not exceed 1.5 mg/kg;
an overdose can result in cardiorespiratory arrest (60). A new formulation, namely a lipid
complex (amphotericin B) was designed, as it is less nephrotoxic
than the existing amphotericin B. This lipid-based formulation
increases the retention time during circulation and alters the
biodistribution. The amphotericin B complexed with lipid
concentrations induces capillary permeability in body tissues, as
normal tissue is purely impermeable to lipid-complex drugs. This
method of increasing the localization of drugs in the targeted
sites is termed passive targeting. This method enhances drug
delivery to the fungi in infected organs and phagocytes with a
lower toxicity, while maintaining antifungal efficacy with drug
levels sustained in tissues with the action of lipase for drug
release (61). The suggested dose
for liposomal amphotericin B is 5 mg/kg/day minimum to as high as
10 mg/kg/day (central nervous system infection).
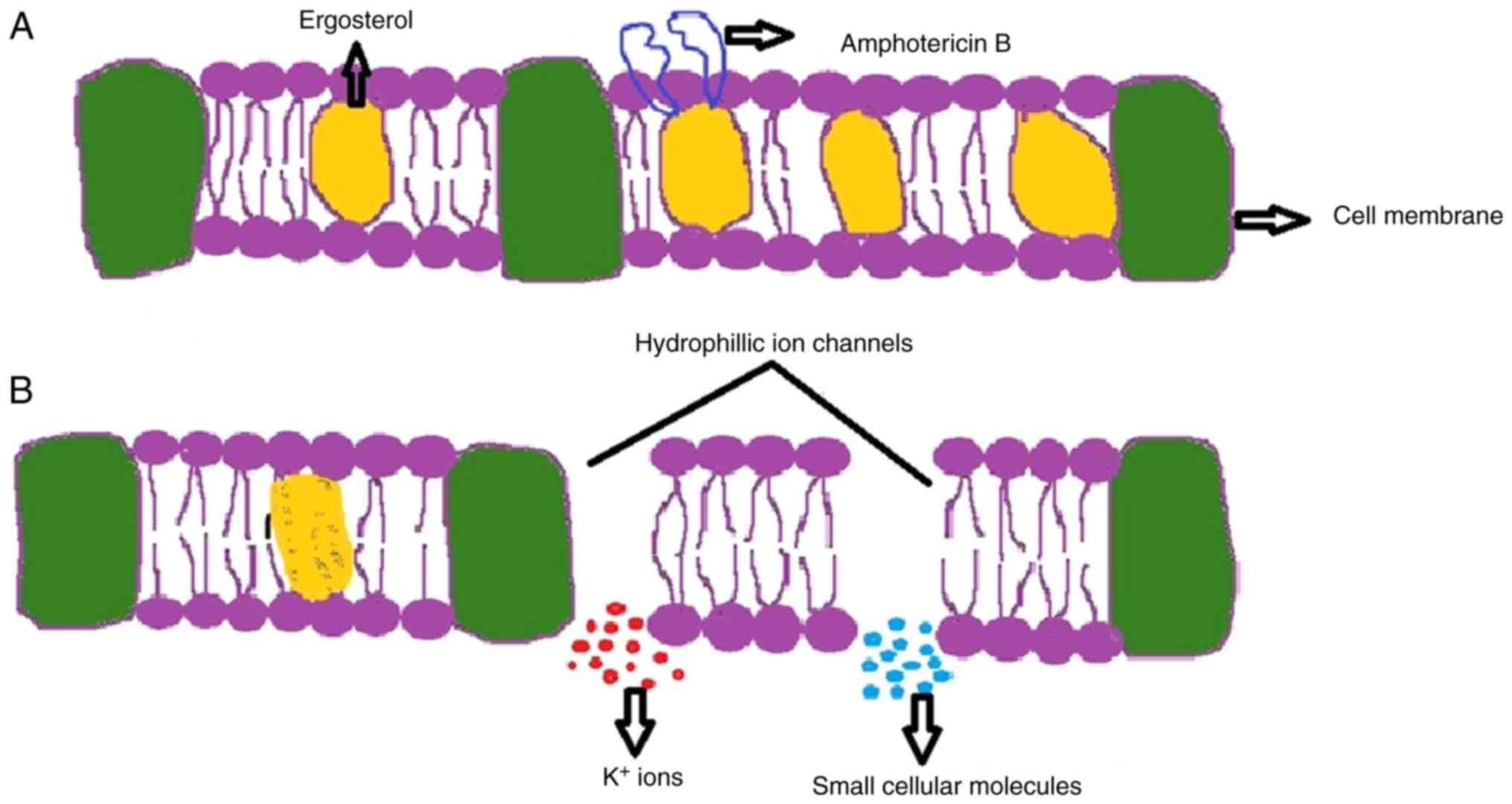
Mechanisms of Amphotericin B
The antifungal drug amphotericin B, a macrolide,
derived from Streptomyces nodosus which acts upon the plasma
membrane of the fungi (62). This
antibiotic molecule binds to the sterols present in the plasma
membranes, such as ergosterol of fungal cells and cholesterol of
mammalian cells. It has a higher affinity towards ergosterol than
cholesterol (63). Amphotericin B
enters through the cell wall of the fungus and binds to the
ergosterol present on the plasma membrane. This creates an ionic
imbalance by forming pores, and leads to the leakage of potassium
ions from the hydrophilic ion channels created inside the fungal
membrane. Amphotericin B has a high binding affinity towards
potassium ions and is hence known as an ionophore. The leakage of
potassium ions causes the loss of membrane rigidity, resulting in
the discharge of essential small molecules from the membrane;
through this manner, amphotericin b exhibits its fungicidal
activity (64). The mechanisms of
action of amphotericin B are illustrated in Fig. 2.
Triazoles
Triazoles are currently in clinical use and are the
largest class of antifungal drugs. Out of the 40 second-generation
triazoles, posaconazole and isavuconazole are two key antifungals
that possess good activity against Mucorales. The mechanism of drug
action involves the inhibition of the 14-α demethylation of
lanosterol present in the ergosterol biosynthetic pathway. The
demethylation of lanosterol results in the depletion and the
replacement of ergosterol with toxic 14-α-methylsterols, thus
altering membrane permeability and inhibiting membrane-bound
enzymes (65).
Posaconazole
Posaconazole is similar to itraconazole in its
structure, considered as second-line or salvage therapy for
patients who are intolerant to amphotericin B (66). Isavuconazole is structurally
resembles fluconazole, and is the only antifungal drug approved for
use in the treatment of invasive mucormycosis. At present,
isavuconazole is available in the market in its prodrug form known
as isavuconazonium sulfate, which is rapidly metabolized by serum
butylcholinesterase to its active form. The recommended dosage for
the intake of isavuconazonium sulfate is 372 mg every 8 h for six
doses, and 372 mg daily (67).
In vitro, isavuconazole exhibits activity against
Lichtheimia, Rhizopus, Mucor and
Cunninghamella spp. (68).
When the fungal infection is at very severe stage,
surgical intervention is the only alternative. In this context,
necrotic tissues have to be removed along with surrounding infected
healthy-looking tissues to prevent the further proliferation of
Mucorales hyphae. Surgery is compulsory during rhino-orbitocerebral
infection and soft tissue infection, even in the case of a single
localized pulmonary lesion, but has to be carefully considered when
infection is disseminated or reaches difficult-to-reach organs.
Ayurvedic and Unani system for the
treatment of mucormycosis
Some patients who have recovered from COVID-19
continue to have post-COVID-associated conditions, including
mucormycosis. The shortage of medicine creates a vital problem for
the treatment of the disease. Hence the research experts from the
Ministry of Ayush have asserted the implementation of the Ayurvedic
and Unani systems of medicine to prevent the spreading of black
fungus (69). Adluri and Perugu
(70), following the decision made
by the Telangana Government to implement Ayurvedic medicine in the
treatment of mucormycosis, assessed the safety and efficacy of the
Ayurvedic regime. In Ayurvedic medicine, mucormycosis was termed as
Vataja viradhi, and to cure the disease they used Pancha Tkita
Ghrita Guggullu (PTGG) as an Ayurvedic adjuvant therapy. They
performed controlled palcebo trials for patients with post-COVID
mucormycosis in the Gandhi Hospital, a large Government tertiary
centre in Telangana. In their case control study, they included 77
patients with post-COVID mucormycosis. A total of 65 patients
received PTGG: 36 patients received PTGG for 34.1 days (group 1)
and group 2 (n=29), used as the control (dropouts) received PTGG
only for 2.1 days. All patients were examined for disease
progression, disease recurrence, the persistence of symptoms and
mortality before and after treatment (70). The outcomes of the treatment were
fruitful with a zero mortality rate in group 1, whereas 13.8%
severity was noted in group 2; that case study reported that the
use of PTGG was helpful, safe and well-tolerated with concomitant
antifungal usage (70). Mohsina
et al (69) listed some of
the Ayurvedic medicine rituals, such as kasaya tikta rasa prayoga,
rikta prasadanam, ojo vrddhi krmighna, kapha pitta haram and ruksa.
They used many aragvadadi kasayam, amrtottaram kasayam, guducyadi
kasayam, nimbadi kasayam, sonitamrtam kasayam and katakakadiradi
kasayam to treat patients with post-COVID mucormycosis. Visa
vilvadi gulika comprised of Bilva, Tulasi, Karanja, Tagara,
Devadaru, Marica, Daruharidra, Ajamutra, Haritaki, Vbhitaki,
Amalaki, Sunti, Pippali, Haridra, Pathya, Nilini and Isvari. They
offered these kashayam at 50, 50, 50 ml on an empty stomach, after
food in the morning and before food and finally concluded that
these Ayurvedic medicines boost the immunity of patients with
post-COVID-associated mucormycosis (69). The leaf extract of Catharanthus
roseus, Lantana camara, Nerium indicum, Sida
cordifolia and Ziziphus mauritiana was examined against
Mucor circinelloides in in vitro antimycotic studies. The
highest antimycotic activity was exhibited by the ethanol leaf
extract of Catharanthus roseus, followed by Nerium
indicum and Lantana camara. Ziziphus mauritiana
exhibited moderate activity against Mucor circinelloides
(71). Balakrishna et al
(72) implemented Anu taila to
cure mucormycosis; Anu taila is comprised of tej patra, vidang,
nagkesar, chandan, tavak, bala, yeshtimadhu and daru haldi. The
consumption of Anu taila improved the immune response against Mucor
spores by activating pre-treated human THP-1 cells and TNF-α. The
repeated application of Anu taila significantly reduced the
ergosterol content in the Mucor biomass and was more effective than
amphotericin B, where the replacement of hyphae, sporangiophores
and sporangia with the fused biomass was evidently proven in SEM
images. Anu taila downregulated the sterol-c5-desaturase coding
ERG3 gene, crucial for maintaining structural integrity in
Mucor spp. and also blocks ergosterol biosynthesis (72).
10. Comorbidity effects of mucormycosis
Mucormycosis may result in the loss of the upper jaw
or even sometimes the eyes. Due to this loss of the jaw, patients
may have difficulty with chewing, swallowing, facial aesthetics and
can suffer a disrupted self-confidence (73).
11. Preventive measures
The early, effective and rapid
diagnosis/administration of suitable effective drugs, the
application of hyperbaric oxygen, recombinant cytokines, the
transfusion of granulocytes, surgical intervention, prosthetic
obturator are the typical and crucial methods in successful
management (16,74). Patients with uncontrolled diabetes
require rapid corrective measures for metabolic abnormalities; the
use of sodium bicarbonate (with insulin) is mandatory to reverse
ketoacidosis, as it reduces the ability of Mucorales invasion
(74). However, the use of
immunosuppressive drugs/corticosteroids need to be reduced to the
lowest possible level with epidemiological knowledge (75).
12. Conclusion and future perspectives
The COVID-19 pandemic has increased the risk of
infections worldwide due to the lack of specific treatments
available for the most devastating viral infections. During the
second wave of the pandemic, the number of deaths rapidly increased
and reached uncontrollable levels. The management of COVID-19 led
to the continuous use of steroids, antibiotics and breath
supportive sources, such as oxygen carriers and ventilators; these
worsened the conditions of patients by increasing comorbidities.
Co-morbidities, such as diabetes and cardiovascular diseases
intensified during the management of COVID-19, which led to the
development of secondary infections, such as mucormycosis.
Mucormycosis is an invasive fungal infection accompanied by
ketoacidosis, high glucose and high ferritin levels, neutropenia
and a lower immunity. All these parameters make patients
immunocompromised, with decreased levels of WBCs, T-cells and other
immune cells, leading to a cytokine storm in the body and the
impairment of cellular organs. As the management of the disease is
critical, novel diagnostic and treatment strategies are required
for mucormycosis in order to prevent higher morbidity and mortality
rates. Hence, additional extensive research and investigations to
elucidate the root cause of mucormycosis are warranted in order to
provide clinicians with the tools to combat infection in
association with the pandemic.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
DG conceived the study and was also involved in the
editing, reviewing and revising of the manuscript, and also
communicated the manuscript to the journal. RS drafted the
manuscript, obtained the data acquired for the review and processed
the figures. Both the authors have read and approved the final
manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pushparaj K, Bhotla HK, Arumugam VA,
Pappusamy M, Easwaran M and Balasubramanian B: Mucormycosis (black
fungus) ensuing COVID-19 and comorbidity meets-Magnifying global
pandemic grieve and catastrophe begins. Sci Total Environ.
805(150355)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Hernandez-Ramirez G, Barber D, Tome-Amat
J, Garrido-Arandia M and Diaz-Perales A: Alternaria as an inducer
of allergic sensitization. J Fungi (Basel). 7(838)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ibrahim AS, Spellberg B, Walsh TJ and
Kontoyiannis DP: Pathogenesis of mucormycosis. Clin Infect Dis. 54
(Suppl 1):S16–S22. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Skiada A, Pavleas I and
Drogari-Apiranthitou M: Epidemiology and diagnosis of mucormycosis:
An update. J Fungi (Basel). 6(265)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Singh AK, Singh R, Joshi SR and Misra A:
Mucormycosis in COVID-19: A systematic review of cases reported
worldwide and in India. Diabetes Metab Syndr.
15(102146)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Skiada A, Lass-Floerl C, Klimko N, Ibrahim
A, Roilides E and Petrikkos G: Challenges in the diagnosis and
treatment of mucormycosis. Med Mycol. 56 (Suppl 1):S93–S101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Borkar SG: Mucormycosis: A surge in
mucorales fungal infection in post-covid patients in Indian States
and insight into known and unknown factors. Int J Global Health.
1:26–60. 2021.
|
|
8
|
Lu XL, Najafzadeh MJ, Dolatabadi S, Ran
YP, Gerrits van den Ende AH, Shen YN, Li CY, Xi LY, Hao F, Zhang
QQ, et al: Taxonomy and epidemiology of Mucor irregularis, agent of
chronic cutaneous mucormycosis. Persoonia. 30:48–56.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aranjani JM, Manuel A, Abdul Razack HI and
Mathew ST: COVID-19-associated mucormycosis: Evidence-based
critical review of an emerging infection burden during the
pandemic's second wave in India. PLoS Negl Trop Dis.
15(e0009921)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Alom S, Ali F and Md ZK: A comprehensive
review on mucormycosis (Black fungus) and its association with
covid-19. Curr Trends Pharm Res. 8:11–40. 2021.
|
|
11
|
Serris A, Danion F and Lanternier F:
Disease entities in mucormycosis. J Fungi (Basel).
5(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ghosh D, Dey S, Chakraborty H, Mukherjee
S, Halder A, Sarkar A and Sarkar J: Mucormycosis: A new threat to
Coronavirus disease 2019 with special emphasis on India. Clin
Epidemiol Glob Health. 15(101013)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dulski TM, DeLong M, Garner K, Patil N,
Cima MJ, Rothfeldt L and Kothari A: Notes from the field:
COVID-19-Associated Mucormycosis-Arkansas, July-September 2021.
MMWR Morb Mortal Wkly Rep. 70:1750–1751. 2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Bourcier J, Heudes PM, Morio F, Gastinne
T, Chevallier P, Rialland-Battisti F and Peterlin P: Prevalence of
the reversed halo sign in neutropenic patients compared with
non-neutropenic patients: Data from a single-centre study involving
27 patients with pulmonary mucormycosis (2003-2016). Mycoses.
60:526–533. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pérez ADC and Welsh EC: Cutaneous
mucormycosis. An Bras Dermatol. 92:304–311. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Huang SF, Ying-Jung Wu A, Shin-Jung Lee S,
Huang YS, Lee CY, Yang TL, Wang HW, Chen HJ, Chen YC, Ho TS, et al:
Review of COVID-19 associated pulmonary aspergillosis and
mucormycosis. J Microbiol Immunol Infect. 56:442–454.
2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Spellberg B: Gastrointestinal
mucormycosis: An evolving disease. Gastroenterol Hepatol (N Y).
8:140–142. 2012.PubMed/NCBI
|
|
18
|
Suresh S and Radha KV: Effect of a mixed
substrate on phytase production by Rhizopus oligosporus MTCC 556
using solid state fermentation and determination of dephytinization
activities in food grains. Food Sci Biotechnology. 24:551–559.
2015.
|
|
19
|
Lin CY, Wang IT, Chang CC, Lee WC, Liu WL,
Huang YC, Chang KW, Huang HY, Hsiao HL, Kao KC, et al: Comparison
of clinical manifestation, diagnosis, and outcomes of invasive
pulmonary aspergillosis and pulmonary mucormycosis. Microorganisms.
7(531)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shirane S, Watanabe D, Sekiya N, Horiguchi
SI and Najima Y: Paraplegia via hematogenous dissemination of
Cunninghamella elegans (mucormycosis) after hematopoietic
stem cell transplantation. Int J Infect Dis. 113:210–212.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rammaert B, Lanternier F, Zahar JR,
Dannaoui E, Bougnoux ME, Lecuit M and Lortholary O:
Healthcare-associated mucormycosis. Clin Infect Dis. 54 (Suppl
1):S44–S54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
India Today: Black fungus detected in
Covid-19 survivors, 8 lose eyesight in Surat. India Today TV, New
Delhi, 2021. https://www.indiatoday.in/coronavirus-outbreak/story/black-fungus-mucormycosis-detected-covid19-survivors-8-lose-eyesight-surat-fungal-infection-symptoms-1799971-2021-05-07?utm_source=washare&utm_medium=socialicons&utm_campaign=shareurltracking.
Updated: May 20, 2021.
|
|
23
|
Reed C, Bryant R, Ibrahim AS, Edwards J
Jr, Filler SG, Goldberg R and Spellberg B: Combination
polyene-caspofungin treatment of rhino-orbital-cerebral
mucormycosis. Clin Infect Dis. 47:364–371. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Vaughan C, Bartolo A, Vallabh N and Leong
SC: A meta-analysis of survival factors in rhino-orbital-cerebral
mucormycosis-has anything changed in the past 20 years. Clin
Otolaryngol. 43:1454–1464. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yohai RA, Bullock JD, Aziz AA and Markert
RJ: Survival factors in rhino-orbital-cerebral mucormycosis. Surv
Ophthalmol. 39:3–22. 1994.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jeong W, Keighley C, Wolfe R, Lee WL,
Slavin MA, Kong DCM and Chen SA: The epidemiology and clinical
manifestations of mucormycosis: A systematic review and
meta-analysis of case reports. Clin Microbiol Infect. 25:26–34.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Roden MM, Zaoutis TE, Buchanan WL, Knudsen
TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH,
et al: Epidemiology and outcome of zygomycosis: A review of 929
reported cases. Clin Infect Dis. 41:634–653. 2005.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Prakash H and Chakrabarti A: Global
epidemiology of mucormycosis. J Fungi (Basel). 5(26)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tedder M, Spratt JA, Anstadt MP, Hegde SS,
Tedder SD and Lowe JE: Pulmonary mucormycosis: Results of medical
and surgical therapy. Ann Thor Surg. 57:1044–1050. 1994.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee FY, Mossad SB and Adal KA: Pulmonary
mucormycosis: The last 30 years. Arch Internal Med. 159:1301–1309.
1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Feng J and Sun X: Characteristics of
pulmonary mucormycosis and predictive risk factors for the outcome.
Infection. 46:503–512. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Skiada A, Rigopoulos D, Larios G,
Petrikkos G and Katsambas A: Global epidemiology of cutaneous
zygomycosis. Clin Dermatol. 30:628–632. 2012.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Chakrabarti A, Das A, Mandal J,
Shivaprakash MR, George VK, Tarai B, Rao P, Panda N, Verma SC and
Sakhuja V: The rising trend of invasive zygomycosis in patients
with uncontrolled diabetes mellitus. Med Mycol. 44:335–342.
2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Simbli M, Hakim F, Koudieh M and Tleyjeh
IM: Nosocomial post-traumatic cutaneous mucormycosis: A systematic
review. Scand J Infectious Dis. 40:577–582. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kaur H, Ghosh A, Rudramurthy SM and
Chakrabarti A: Gastrointestinal mucormycosis in apparently
immunocompetent hosts-A review. Mycoses. 61:898–908.
2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dioverti MV, Cawcutt KA, Abidi M, Sohail
MR, Walker RC and Osmon DR: Gastrointestinal mucormycosis in
immunocompromised hosts. Mycoses. 58:714–718. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Guinea J, Escribano P, Vena A, Muñoz P,
Martínez-Jiménez MDC, Padilla B and Bouza E: Increasing incidence
of mucormycosis in a large Spanish hospital from 2007 to 2015:
Epidemiology and microbiological characterization of the isolates.
PLoS One. 12(e0179136)2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Chakrabarti A, Chatterjee SS, Das A, Panda
N, Shivaprakash MR, Kaur A, Varma SC, Singhi S, Bhansali A and
Sakhuja V: Invasive zygomycosis in India: Experience in a tertiary
care hospital. Postgraduate Med J. 85:573–581. 2009.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Jianhong L, Xianliang H and Xuewu J:
Isolated renal mucormycosis in children. J Urol. 171:387–388.
2004.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Bhadauria D, Etta P, Chelappan A, Gurjar
M, Kaul A, Sharma RK, Gupta A, Prasad N, Marak RS, Jain M, et al:
Isolated bilateral renal mucormycosis in apparently immunocompetent
patients-a case series from India and review of the literature.
Clin Kidney J. 11:769–776. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Skiada A, Pagano L, Groll A, Zimmerli S,
Dupont B, Lagrou K, Lagrou K, Lass-Florl C, Bouza E, Klimko N, et
al: Zygomycosis in Europe: Analysis of 230 cases accrued by the
registry of the European Confederation of Medical Mycology (ECMM)
Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol
Infect. 17:1859–1867. 2011.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Anjum NA: Mucormycosis: Botanical insights
into the major causative agents, 8 June, 2021.
|
|
43
|
Prakash H, Singh S, Rudramurthy SM, Singh
P, Mehta N, Shaw D and Ghosh AK: An aero mycological analysis of
Mucormycetes in indoor and outdoor environments of northern India.
Med Mycol. 58:118–123. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Prakash H and Chakrabarti A: Epidemiology
of mucormycosis in India. Microorganisms. 9(523)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ravindra K and Ahlawat A: Five probable
factors responsible for the COVID-associated mucormycosis outbreak
in India. Int J Infectious Dis. 112:278–280. 2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Alim A, Pati BK, Sarfraz A and Bharti B:
Rhinocerebral mucormycosis in a diabetic patient: A case report
with brief review. Int J Med Res Prof. 5:150–154. 2019.
|
|
47
|
Mohindra S, Mohindra S, Gupta R, Bakshi J
and Gupta SK: Rhinocerebral mucormycosis: The disease spectrum in
27 patients. Mycoses. 50:290–296. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Almas T, Nazar W, Khedro T, Kanawati MA,
Adnan A, Almuhaileej M, Alshamlan A, Abdulhadi A, Manamperi KT and
Sarfraz S: COVID-19 and mucormycosis superinfection: Exploring the
missing pathophysiological links. Ann Med Surg (Lond).
68(102655)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Aggarwal S, Gollapudi S, Yel L, Gupta AS
and Gupta S: TNF-α-induced apoptosis in neonatal lymphocytes:
TNFRp55 expression and downstream pathways of apoptosis. Genes
Immunity. 1:271–279. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Fischer K, Hoffmann P, Voelkl S,
Meidenbauer N, Ammer J, Edinger M, Gottfried E, Schwarz S, Rothe G,
Hoves S, et al: Inhibitory effect of tumor cell-derived lactic acid
on human T cells. Blood. 109:3812–3819. 2007.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Johnson AK, Ghazarian Z, Cendrowski KD and
Persichino JG: Pulmonary aspergillosis and mucormycosis in a
patient with COVID-19. Med Mycol Case Rep. 32:64–67.
2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Patel A, Kaur H, Xess I, Michael JS, Savio
J, Rudramurthy S, Singh R, Shastri P, Umabala P, Sardana R, et al:
A multicentre observational study on the epidemiology, risk
factors, management and outcomes of mucormycosis in India. Clin
Microbiol Infect. 26:944.e9–944.e15. 2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Khanna M, Challa S, Kabeil AS, Inyang B,
Gondal FJ, Abah GA, Minnal Dhandapani M, Manne M and Mohammed L:
Risk of mucormycosis in diabetes mellitus: A systematic review.
Cureus. 13(e18827)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Indu DP, Yadhav MLK and Chetana GS: Is
serum ferritin an early marker for COVID-19-associated
mucormycosis? Cureus. 15(e36734)2023.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Pilmis B, Alanio A, Lortholary O and
Lanternier F: Recent advances in the understanding and management
of mucormycosis. F1000Research. 7(F1000 Faculty
Rev-1429)2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Millon L, Larosa F, Lepiller Q, Legrand F,
Rocchi S, Daguindau E, Scherer E, Bellanger AP, Leroy J and
Grenouillet F: Quantitative polymerase chain reaction detection of
circulating DNA in serum for early diagnosis of mucormycosis in
immunocompromised patients. Clin Infect Dis. 56:e95–e101.
2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Guegan H, Iriart X, Bougnoux ME, Berry A,
Robert-Gangneux F and Gangneux JP: Evaluation of
MucorGenius® mucorales PCR assay for the diagnosis of
pulmonary mucormycosis. J Infect. 81:311–317. 2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Muley P, Chitguppi R and Jambure R:
Proposal for a novel grading system for rhino-maxillary
mucormycosis based on the analysis of 30 cases. (May 27, 2021).
Available at SSRN: https://ssrn.com/abstract=3854282 or http://dx.doi.org/10.2139/ssrn.3854282.
|
|
59
|
Tissot F, Agrawal S, Pagano L, Petrikkos
G, Groll AH, Skiada A, Lass-Flörl C, Calandra T, Viscoli C and
Herbrecht R: ECIL-6 guidelines for the treatment of invasive
candidiasis, aspergillosis and mucormycosis in leukemia and
hematopoietic stem cell transplant patients. Haematologica.
102:433–444. 2017.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Meyer RD: Current role of therapy with
amphotericin B. Clinical infectious diseases. 14 (Suppl
1):S154–S160. 1992.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Dash AK, Patro S, Patro SK, Gupta AK and
Biswal RN: Amphotericin B emulsion in rhino-orbital mucormycosis:
Is it most effective? Clinical Rhinol An Inter J. 9:40–42.
2016.
|
|
62
|
Torrado JJ, Espada R, Ballesteros MP and
Torrado-Santiago S: Amphotericin B formulations and drug targeting.
J Pharm Sci. 97:2405–2425. 2008.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Liu T, Wang L and Liu CT: Cavitary
pulmonary mucormycosis caused by Cunninghamella in a patient with
diabetes. Am J Med Sci. 364:245–247. 2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Fernández-García R, Muñoz-García JC,
Wallace M, Fabian L, González-Burgos E, Gómez-Serranillos MP,
Raposo R, Bolás-Fernández F, Ballesteros MP, Healy AM, et al:
Self-assembling, supramolecular chemistry and pharmacology of
amphotericin B: Poly-aggregates, oligomers and monomers. J Control
Release. 341:716–732. 2022.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Kim MJ, Park PW, Ahn JY, Kim KH, Seo JY,
Jeong JH, Park MJ, Jung JW and Seo YH: Fatal pulmonary mucormycosis
caused by Rhizopus microsporus in a patient with diabetes. Ann Lab
Med. 34:76–79. 2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Odds FC, Brown AJ and Gow NA: Antifungal
agents: Mechanisms of action. Trends Microbiol. 11:272–279.
2003.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Pettit NN and Carver PL: Isavuconazole: A
new option for the management of invasive fungal infections. Ann
Pharmacother. 49:825–842. 2015.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Marty FM, Ostrosky-Zeichner L, Cornely OA,
Mullane KM, Perfect JR, Thompson GR III, Alangaden GJ, Brown JM,
Fredricks DN, Heinz WJ, et al: Isavuconazole treatment for
mucormycosis: A single-arm open-label trial and case-control
analysis. Lancet Infect Dis. 16:828–837. 2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Mohsina FP, Faheem IP, Tabassum S, Shah I
and Ahmad A: An insight of mucormycosis (black fungus) in ayurveda.
Open J Pharmacol Pharmacother. 6:13–17. 2021.
|
|
70
|
Adluri USP and Perugu S: Evaluation of
efficacy and safety of adjuvant Ayurvedic therapy in patients with
severe post-covid mucor-mycosis at a Government tertiary care
hospital-A Case-Control study. J Ayurveda Integr Med.
13(100585)2022.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Jangid R and Begum T: Antimycotic activity
of some medicinal plants against Mucor circinelloides. Biomed Res
Int. 2022(3523920)2022.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Balkrishna A, Rastogi S, Kharayat B, Tomer
M, Varshney Y, Singh K, Kumari P, Dev R, Srivastava J, Haldar S and
Varshney A: Anu taila, an herbal nasal drop, suppresses
mucormycosis by regulating host TNF-α response and fungal
ergosterol biosynthesis. J Appl Microbiol. 132:3355–3374.
2022.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Sharma S, Grover M, Bhargava S, Samdani S
and Kataria T: Post coronavirus disease mucormycosis: A deadly
addition to the pandemic spectrum. J Laryngol Otol. 135:442–447.
2021.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Szebenyi C, Gu Y, Gebremariam T, Kocsubé
S, Kiss-Vetráb S, Jáger O, Patai R, Spisák K, Sinka R, Binder U, et
al: CotH genes are necessary for normal spore formation and
virulence in Mucor lusitanicus. mBio. 14(e0338622)2023.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Chaudhary P and Singh P: Isolation,
identification and molecular characterization of microflora
obtained from spices and spice mixes. World J Pharmaceutical Res.
3:2020–2030. 2014.
|