1. Introduction
The skin, being the largest organ of the human body,
has critical functions, which include the synthesis of vitamin D,
controlling temperature, regulating water homeostasis, and
protecting from hazardous chemicals and pathogens (1,2). As
a part of homeostasis, the skin undergoes the complete substitution
of damaged tissues with new ones through a continuous processes of
regeneration. A high turnover of cell differentiation determines
the mechanisms of regeneration in normal processes. Skin tissue is
regenerated through the extensive proliferation and migration of a
massive number of new cells (3).
The skin has three layers. The epidermis is the
first layer and has no vascularization. This stratum is intricately
stratified, which constitutes an external barrier. Consisting of
lipid-rich structures and non-viable cells, this layer serves as a
protective boundary. The epidermis is generated from the basal
stratum germinativum via keratinocyte proliferation and the
differentiation process. The dermis, which is the second layer,
comprises connective tissue, primarily of fibroblasts. These cells
are mesenchymal, which function as a skin scaffold. These cells
secrete the extracellular matrix (ECM), which is characterized as a
fibrous and elastic component. The main components of the ECM are
collagen types I and III, which confer elasticity and strength.
From the total amount of collagen, 80-90% is represented by type 1
collagen and 8-12% by collagen type III. The hypodermis, which is
the third layer, is a subcutaneous tissue, composed of blood
vessels, lymph, and adipose tissue. This layer secretes various
cytokines and chemokines. These layers play roles in certain
important homeostasis processes, which include thermoregulation,
metabolism and immune functions (4).
Proteins play a crucial role in maintaining skin
homeostasis, which serve as essential substrates for key biological
components, including enzymes, hormones, cytokines and growth
factors. Protein deficiency can result in a decrease in the
expression of certain genes and the production of certain cytokines
and growth factors. Cytokines and growth factors are essential
substances in skin homeostasis. Therefore, protein malnutrition can
occur, which causes skin malfunction, leading to the thinning of
the epidermis and decreasing collagen production in the dermis.
Moreover, it can affect the healing process of wounds, as it
impairs angiogenesis and fibroblast proliferation. Tissue
regeneration begins with a proliferation of fibroblasts and then
continues with a synthesis of collagen from fibroblasts. These two
processes use proteins as substrates; hence, protein deficiency
causes impairment of these stages. Some research has examined the
effects of protein deficiency on skin homeostasis and wound
healing. The study by Sugiyama et al (5) revealed that protein deficiency
impaired the structure and functions of the skin epidermis. Their
study found a decrease in the thickness of the epidermis and in the
proliferative activity of epidermal cells. Another conclusion is
that protein deficiency affects both the synthesis and degradation
of skin collagen.
The status of dermal collagen is also affected by
protein deficiency. Protein deprivation significantly reduces skin
collagen (6). A previous study
demonstrated that insufficient protein intake causes a decrease in
collagen and elastin synthesis; the efficiency of enzymes, such as
matrix metalloproteinases (MMPs) that degrade old proteins is also
affected (7). The study by Alves
et al (8) examined open
acute wound healing and demonstrated that malnutrition impaired
regeneration by decreasing the mRNA levels of transforming growth
factor-β1 (TGF-β1).
As a complementary medicine, exercise is an activity
that has benefits on health. This function takes place in all body
systems, which include the skin. Additionally, exercise has been
employed as a complementary therapy to accelerate skin wound
healing. Although exercise has been proven to be beneficial for
skin health, the mechanisms of exercise in skin homeostasis and
regeneration caused by protein deficiency remain poorly understood.
The present review highlights the potential mechanisms of exercise
in maintaining skin homeostasis disrupted by protein deficiency,
placing particular emphasis on the processes that occur in the
epidermis and dermis.
2. Epidermal and dermal homeostasis and
healing
The main cellular component of the epidermis
includes keratinocytes, which comprise almost 95% of the epidermal
cell population. Epidermal homeostasis is associated with
keratinocyte regulation. Moreover, epidermal homeostasis is tightly
regulated. Through a regulated proliferation and differentiation
program, the epidermis maintains tissue homeostasis. In this
program, stem cell self-renewal and the differentiation process
take place and interact. During this process, the undifferentiated
keratinocytes from the basal layer are regenerated to form the
stratum spinosum, stratum granulosum, and stratum corneum. Those
cells move suprabasally to permanently withdraw from the cell
cycle, and alter their biological activity and morphology. The
outermost epidermal layer is comprised fo corneocytes. The
corneocytes consist of dead, flattened, non-nucleated keratinocytes
(9).
Mitotic cell types of keratinocytes and epidermal
stem cells (EpiSCs) are in the basal layer. Keratinocyte function
is regulated by growth factors that trigger intracellular signaling
pathways. Several types of growth factors have been explored, which
include the epidermal growth factor family and the TGF-β family,
which are considered to be key regulators (10). Keratinocyte stem cells are
characterized by their normally slow-cycle mitotic cells in
vivo; they can self-renew and are involved in maintaining
tissue (11). EpiSCs located in
the basal layer of the epidermis have been shown to be key sources
of cells for regeneration, metabolism and wound healing of the
skin. The sources of EpiSCs may be derived from the regeneration of
mesenchymal cells and the migration of other stem cells to the skin
tissue. When the skin is damaged, the niches affect the regulation
of the migration, proliferation and differentiation of stem cells,
by activating multiple signaling pathways. EpiSC differentiation
processes occur, which include changes in the number of repair
cells, the concentration of cytokines and components of the ECM
(12).
Several studies on the factors that influence the
epidermis have been carried out. Aging and nutritional status are
regarded as two factors that can influence epidermal homeostasis.
The study by Lintzeri et al (13) concluded that the epidermis was
thinner in aged skin. The epidermis tends to become thinner with
aging and does not appear to be influenced by sex (13). The study by Leite et al
(14) concluded that a significant
difference existed in mean dermal thickness values in the
malnourished group in comparison to the well-nourished group.
Nonetheless, no difference was found in mean epidermal thickness
between these groups (14).
Skin injury stimulates re-epithelialization, which
involves the migration and proliferation of keratinocytes. It
begins with the migration of keratinocytes and continues with
proliferation. Several growth factors stimulate this process; two
such growth factors are TGF-β and fibroblast growth factor (FGF).
To enhance proliferation, growth factors are produced to stimulate
keratinocyte proliferation. Proliferation is required in order to
supply keratinocytes, which may be sufficient to cover the wound
surface. The proliferation of keratinocytes takes place in basal
EpiSCs. This process continues even following epithelial wound
closure (15).
Dermal homeostasis is related to collagen production
and degradation process. The amount of gross protein content in the
skin is ~22%. To maintain the collagen framework that has been
established, as well as the synthesis of the new collagen, the
amino acid intake must be sufficient (7). Collagen has a triple-helix structure.
This substance can initiate and be responsible for the interaction
between cells and the matrix. To date, almost 30 different types of
collagen have been identified. One of the most common types of
protein is collagen type I (Col-I), which is commonly found in the
skin. Col-I is comprised of the genes COL1A1 and COL1A2, known as
α1 and α2, respectively (16).
A decrease or loss in the amount of collagen in the
skin can have an unfavorable effect. Collagen is the main type of
fibrous connective tissue of the dermis. Types I and III collagen
are primarily responsible for the tensile strength of the skin.
Types I and III collagen are degraded by the collagenases, from the
MMP family, which are produced by skin fibroblasts. Collagenases
are the main enzymes in the breakdown process of collagen (6). Collagenases degrade collagen I, II
and III at distinct sites. MMPs are produced by several cell types,
and their production is regulated by cytokines. They participate in
cell migration, growth and differentiation, tissue remodeling and
angiogenesis (17). The inhibitors
that control the activity of these enzymes are tissue inhibitors of
metalloproteinases (TIMPs), which comprise of four members (TIMP-1,
TIMP-2, TIMP-3 and TIMP-4) (6).
Growth factor production is involved in skin
homeostasis and wound healing. Naturally, cells secrete growth
factor proteins, and the protein then interacts with cell surface
receptors. The binding triggers various processes, which stimulate
cell signal transduction pathways. Skin healing involves these
growth factor-stimulated cellular responses (18).
TGF-β1 is considered to play an essential role in
wound healing. TGF-β1 mediates fibroblast proliferation, collagen
production, ECM deposition and myofibroblast differentiation in
wound healing (19-21).
During this process, TGF-β is secreted by fibroblasts and is
released from storage sites in the disrupted ECM. TGF-β1 signaling
has some effects, including promoting the proliferation of
fibroblasts, the synthesis of ECM components such as Col-I and
fibronectin by fibroblasts, and the transition of fibroblasts to a
myofibroblast wound phenotype. TGF-β signaling also involves
crosstalk between the dermis and epidermis (22-25).
Several studies have concluded that other than
TGF-β, under physiological conditions, fibrosis keratinocytes
stimulate fibroblasts through the production of interleukin (IL)-1,
inducing keratinocyte growth factor and metalloproteinases.
Fibroblasts have been shown to modulate keratinocyte proliferation
and differentiation (26). As high
metabolic rates affect tissues, mesenchymal stem cells (MSCs) are
the first to be affected by protein deficiency (1). MSCs can differentiate into
keratinocytes in the human epidermis (27). Protein deprivation increases
collagenase activity by suppressing the gene expression of two of
the TIMPs. Protein deprivation decreases the active form of
collagenase. This process also decreases the levels of dermal
collagen (6).
3. Importance of exercise in skin alteration
following several diseases
Exercise is considered to play a crucial role in
skin health. Some reviews and research have suggested that several
skin disorders are related to exercise. Exercise plays crucial
roles in certain skin disorders, which include aging-related skin
disorders (cancer), inflammatory disorders (psoriasis and
dermatitis) and nutritional disorders, such as protein deficiency
(delayed wound healing) (28).
Exercise habits have been shown to have several beneficial effects
on skin health. Exercise can enhance the structure, moisturizing
and hydration function of the skin (29). Briefly, the known skin benefits of
exercise include improving blood flow to nourish cells and remove
toxins from the skin; preventing the signs of aging by boosting
collagen, elasticity, tone/turgor and the skin barrier; inhibiting
the anti-inflammatory actions of oxidative stress; decreasing
stress by increasing dermal resilience; and maintaining improved
overall skin well-being (30).
Exercise may be an effective preventive strategy
against psoriasis. A joint guideline review between the Journal of
the Academy of Dermatology and the National Psoriasis Foundation
published in 2019 recommended that dermatologists should give
regular exercise advice to patients with psoriasis to reduce the
risk of associated comorbidities, including metabolic syndrome
(31). The study by Lai et
al (32) demonstrated that
moderate and vigorous levels of physical activity appeared to be
beneficial for dermatitis. Hence, patients with active hand
dermatitis must be advised to remain physically active (32).
The study by Nishikori et al (29) demonstrated that resistance exercise
exerted effects on skin aging and skin rejuvenation. Some studies
have reported that exercise accelerates wound healing (29,33-35).
The review by Yeh et al (36) examined the preventive and
therapeutic roles of exercise in various skin diseases. Their
review demonstrated that exercise was recommended for the
attenuation of skin aging, the prevention of psoriasis and the
improvement of venous leg ulcers (36).
The aforementioned findings indicate that exercise
has several benefits on skin health. Nevertheless, the mechanisms
through which exercise exerts its effects are not completely
understood. The present review discusses how exercise contributes
to the maintenance of skin homeostasis, particularly under
conditions of protein deficiency.
4. Mechanisms of exercise in maintaining
skin homeostasis disrupted by protein deficiency
In the present review, as demonstrated in Table I, 12 articles on the mechanisms of
exercise in maintaining skin homeostasis were identified. Exercise
has been explored in various research to prove its effects on skin
health, which include effects on wound healing and the aging
process. Below, various studies on the effects of exercise on skin
health are presented and the findings from these are discussed.
Exercise is classified as acute or chronic; aerobic or anaerobic;
endurance or resistance type; and mild, moderate and high
intensity. The mechanisms include the role of cytokines, growth
factors, oxygenation and angiogenesis.
 | Table IStudies on exercise related to skin
health. |
Table I
Studies on exercise related to skin
health.
| Author(s), year of
publication | Title | Type of study and
method | Conclusion | (Refs.) |
|---|
| Coletti et
al, 2013 | Restoration vs.
reconstruction: Cellular mechanisms of skin, nerve and muscle
regeneration compared | Review | Moderate-intensity
exercise improves tissue oxygen to accelerate the wound healing
process, and mild hypoxia stimulates angiogenesis, collagen
formation, and cell survival | (3) |
| Lo Presti et
al, 2017 | Gelatinases and
physical exercise: A systematic review of evidence from human
studies | Systematic
review | MMP-9 gene
transcription occurs as a result of acute exercise, whereas MMP-2
and TIMP transcription result from the regular repetition of
exercise over time; a single bout of exercise and regular training
appear to have opposite effects on blood MMP-9 levels | (17) |
| Hoffmann and
Weigert, 2017 | Skeletal Muscle as
an Endocrine Organ: The Role of Myokines in Exercise
Adaptations | Review | After acute
exercise or training, the mRNA abundance of TGF-b1 and TGF-b
receptor 2 is increased | (49) |
| Ishihara, 2019 | Mild hyperbaric
oxygen: mechanisms and effects. | Review | Keratinocyte
proliferation and epidermal cell regeneration may be effective for
damage repair in the epidermis and are activated by exposure to
mild hyperbaric oxygen. | (45) |
| Riyahi et
al, 2021 | Reviewing the
Physiology of Cutaneous Wound Healing and Evaluating the Effect of
Exercise on It | Review | Exercise increases
circulatory IL-15 levels, which increases keratinocytes and
fibroblasts; exercise affects inflammation in the skin; the effect
of exercise on inflammation depends on its length and intensity;
regular exercise improves angiogenesis and increases local blood
flow providing oxygen and nutrients to wound tissue, which is
important in the synthesis of connective tissue; regular exercise
can prevent oxidative stress and accelerate healing by potentiating
the body's systemic antioxidative defense | (2) |
| Han et al,
2021 | Increase in Free
and Total Plasma TGF-β1 Following Physical Activity | Experimental study,
which measured plasma concentrations of free and total TGF-β1,
TGF-β2 and TGF-β3 in 40 subjects | Light physical
activity was associated with a significant increase in free and
total TGF-β1 and TGF-β2 plasma concentrations; the function of
TGF-β was indicated in several processes of skin process such as
angiogenesis, inflammation, fibroblast proliferation, and collagen
synthesis in wound healing | (22) |
| Ishikawa et
al, 2021 | Hot yoga increases
SIRT6 gene expression, inhibits ROS generation, and improves skin
condition | Original
article | Hot yoga increases
blood SIRT6 gene expression and inhibits plasma ROS production | (37) |
| Oizumi et
al, 2021 | The association
between activity levels and skin moisturising function in
adults | A cross-sectional,
observational study was conducted in Japan with 86 participants;
the study analyzed two skin moisturising function parameters based
on data from self-administered questionnaires concerning
participant exercise habits | Higher-intensity
exercise may promote skin-moisturising function; endurance exercise
induces IL-5, and IL-5 promotes the biosynthesis of the
mitochondria; thus, skin construction improves | (48) |
| Ishiuchi-Sato and
Nedachi, 2021 | Possible
involvement of CXC motif chemokine ligand 10 in exercise-induced
collagen production of mouse dermal fibroblasts | Experimental study
on mice were subjected to forced treadmill running. (15 cm/sec for
30 min) | Exercise reduces
the expression of myokine CXCL10 so that the skin apoptosis process
is reduced | (50) |
| Barzegari et
al, 2022 | The effect of three
different exercise methods HIIT, HIT and MIT on the expression of
FGF and TGF genes in liver tissue of male Wistar rats | Experimental study
on 32 male Wistar rats that participated in moderate-intensity
training, high-intensity training, and high-intensity intermittent
training; the amount of TGF and FGF gene changes was determined
using real-time PCR | MIT, HIT and HIIT
increase the expression of TGF and FGF | (52) |
| Chen et al,
2022 | Molecular
mechanisms of exercise contributing to tissue regeneration | Review | Exercise strongly
induces the overexpression of PGC-1α. PGC-1α in both human and
rodent muscle and may trigger the remodeling of the satellite cell
niche by altering the extracellular matrix composition | (53) |
| Nishikori et
al, 2023 | Resistance training
rejuvenates aging skin by reducing circulating inflammatory factors
and enhancing dermal extracellular matrices | An experimental
study to compare the effects of acute training and regular training
on skin aging in a 16-week intervention in 61 healthy middle-aged
Japanese women with a sedentary lifestyle | Regular training
counteracts skin aging such as deteriorations in skin elasticity,
upper dermal structure, and dermal thickness. Acute training also
had positive effects on skin elasticity and upper dermal structure,
but it did not improve dermal thickness | (29) |
The study by Riyahi et al (2) on the elderly concluded that exercise
has some positive effects on skin regeneration in wound healing.
These effects are mediated by circulating IL-15. IL-15 is an
essential mitochondrial signal, that increases the growth of
keratinocytes and fibroblasts (2).
These results are supported by the study by Nishikori et al
(29), which demonstrated that
aerobic exercise stimulated the release of IL-15, which reduced
skin aging by supporting mitochondrial biogenesis in the skin.
Regular training can inhibit skin aging, such as deteriorations in
skin elasticity, upper dermal structure and dermal thickness.
Nevertheless, acute training enhances skin elasticity and upper
dermal structure, but not dermal thickness (29).
Exercise affects inflammation in the skin. Length
and intensity are two factors that determine the effects of
exercise on inflammation. Aerobic exercise with moderate intensity
can reduce oxidative stress by increasing antioxidant enzyme
activities. Regular exercise potentiates the systemic antioxidative
defense of the body to prevent oxidative stress and accelerate
healing. Exercise supports vascular growth and increases tissue
vascularization by regulating reactive oxygen species (ROS).
Regular exercise improves angiogenesis and increases local blood
flow, providing oxygen and nutrients to wound tissue. Sufficient
oxygen and nutrients support the synthesis of skin connective
tissue (2).
The study by Ishikawa et al (37) reported that continuous hot yoga
exercise protected against ROS-induced senescence by modulating
sirtuin (SIRT) 6 expression. Several studies have provided evidence
that the SIRT family is involved in the regulation of longevity
(38-43).
Hot yoga may enhance the activation of SIRT6 via a mechanism
mediated by shear stress. The study by Ishikawa et al
(37) also reported that
continuous hot yoga exercise can suppress catecholamines and thus
promote blood flow. Exercise under appropriate heat stress and high
humid conditions is expected to increase cutaneous blood flow with
skin temperature (37).
Moderate-intensity exercise has been proven to
improve tissue oxygen to accelerate the wound healing process.
Tissue oxygenation is an essential factor in the healing process;
angiogenesis, collagen formation and cell survival can be
stimulated by mild hypoxia. Oxygen is critical for the synthesis of
connective tissue. Exercise provides adequate oxygen supply to
wound tissue and helps in healing (3). Hypoxia can occur physiologically by
exercise. This condition stimulates cellular adaptation, which is
mediated by key oxygen sensors, namely, hypoxia-inducible factors
(HIFs). HIFs respond acutely and induce the production of
endogenous metabolites and proteins to promptly regulate metabolic
pathways that maintain oxygen supply (44). Keratinocyte proliferation and
epidermal cell regeneration may be effective for the regeneration
of the epidermis. Keratinocyte proliferation and epidermal cell
regeneration are activated by exposure to mild hyperbaric oxygen
(45). Hyperbaric oxygen has the
same mechanism as exercise in enhancing oxidative metabolism.
Previous studies have revealed that the
moderate-intensity training effect is superior to that of high
intensity and strenuous intensity in the skin healing process. The
study by Heinen et al (46)
on patients with diabetes reported that moderate-intensity exercise
was the best option for wound healing. The study by
Amatriain-Fernández et al (47) indicated that regular
moderate-intensity exercise exerts anti-inflammatory and
antioxidant effects, and can stimulate several signaling pathways
(47). Aerobic exercise is more
effective than anaerobic exercise in the skin healing process,
although both exercises are effective. Nonetheless, anaerobic
exercises may increase ROS production in wound tissue and delay
regeneration (2).
Another potential mechanism of exercise in the skin
regeneration process is by inducing MMPs. MMPs are involved in
biological processes, which include angiogenesis. A previous study
demonstrated that acute intense exercise caused an increase in
circulating MMP-9 levels. MMP-9 is involved in the processes of
angiogenesis, including proteolysis of the capillary basement
membrane; ECM degradation induces the release of proangiogenic and
antiangiogenic factors. Angiogenesis is a key factor in
regeneration, including in skin tissue (17).
In their study, Oizumi et al (48) examined the association between the
activity level and skin-moisturizing function. The
skin-moisturizing function is a key physiological parameter of the
skin. Their study used an exercise habits questionnaire and
examined the types of skin moisturizing. The results revealed that
activity levels were associated with skin moisture levels. The
moderate- and high-activity-level habit groups had higher skin
moisture levels than the low-activity-level habit group. Moreover,
the results suggested that higher-intensity exercise had a
beneficial effect on increasing skin-moisturizing function.
Endurance exercise enhanced skin health by inducing IL-5, which
promoted the biosynthesis of the mitochondria (48).
Exercise induces the production of certain myokines
from muscle contraction (49). The
C-X-C motif chemokine ligand 10 (CXCL10) secretion from skeletal
muscles can control collagen production in mouse dermal
fibroblasts. CXCL10 is a myokine whose expression is reduced due to
muscle contraction. CXCL10 promotes the apoptosis of endothelial
cells. The reduction in CXCL10 expression in skeletal muscle
through exercise can be considered to have a wide range of effects
on the whole body, including the skin (50).
In another study, light physical activity was shown
to be associated with a significant increase in free and total
TGF-β1 and TGF-β2 plasma concentrations. TGF-β was involved in
several skin processes, such as angiogenesis, fibroblast
proliferation and collagen synthesis (22). Following acute exercise or
training, an increase in the mRNA levels of TGF-b1 and TGF-b
receptor 2 was observed. The release of TGF-β at the early stages
of the healing process recruits inflammatory cells from the
circulation into the wounded area. This process then continues with
angiogenesis and collagen synthesis. TGF-β stimulates the cells to
increase the synthesis of ECM proteins and simultaneously decreases
the levels of collagen proteases (49).
The effects of exercise on skin regeneration also
may be mediated by the production of growth factors. Previous
research has compared the effects of exercise on the expression of
TGF and FGF in liver tissue, demonstrating that moderate-intensity
training (MIT), high-intensity training (HIT) and high-intensity
interval training (HIIT) increases the expression of TGF and FGF.
However, it appears that the expression of these genes increases to
a greater extent with MIT than with HIT and HIIT. The duration of
MIT can be concluded to have been an effective factor in the
expression of both genes; among the most critical factors in the
expression of TGF and FGF are the intensity of training and its
duration. This effect is very likely to occur in any organ in the
body, including the skin, as the effects of growth factors are not
limited to a specific organ (51,52).
In the body, particularly in muscle tissue, exercise
strongly induces the overexpression of peroxisome
proliferator-activated receptor (PPAR)-γ coactivator-1α (PGC-1α).
PGC-1α is a transcription coactivator, which in muscle, can alter
the ECM composition and subsequently results in remodeling of the
satellite cell niche; PGC-1α promotes exercise-induced tissue
regeneration and is involved in mitochondrial signaling. Thus,
PGC-1α functions as the key regulator of the crosstalk between
mitochondrial biogenesis and exercise-induced regeneration. This
mechanism potentially occurs in the skin regeneration process
(53). The various mechanisms in
the epidermis and dermis that could potentially explain the effects
of exercise on skin homeostasis disrupted by protein deficiency are
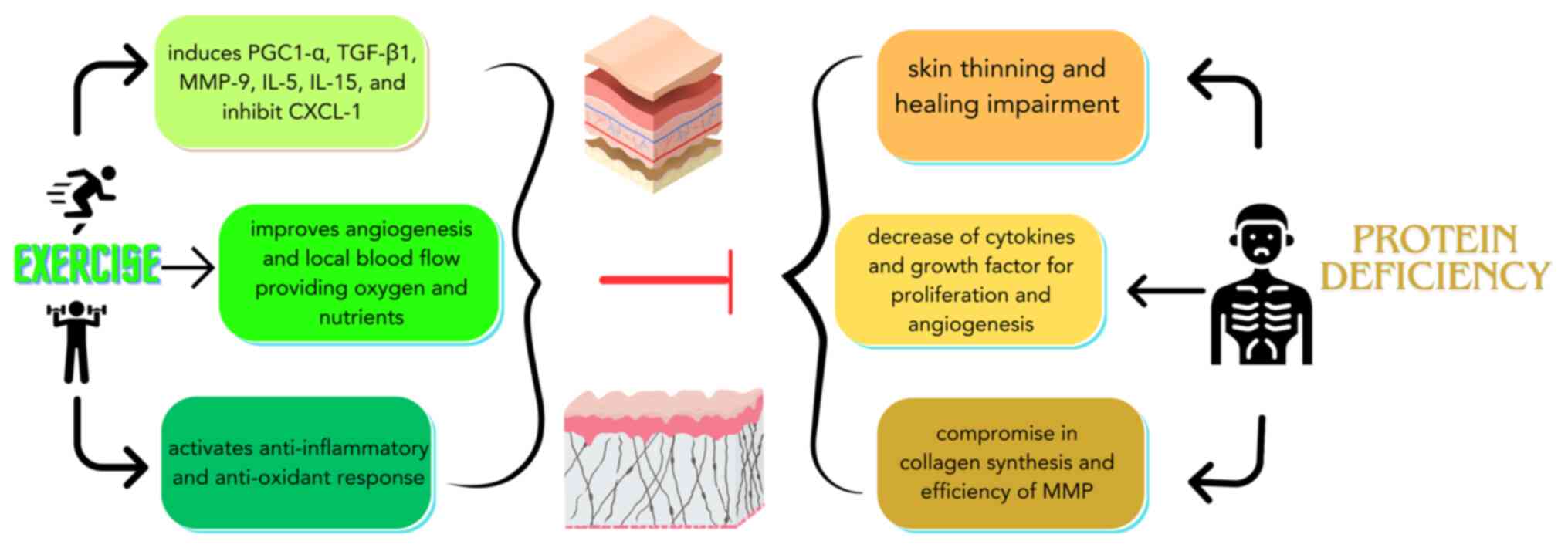
illustrated in Fig. 1.
A limitation of the present review is that
references were not distinguished in terms of the type of exercise,
research methods and subjects. In addition, the present review did
not separate the mechanisms that occur physiologically
(homeostasis) and pathologically (wound healing).
5. Conclusion and future perspectives
Exercise is beneficial for skin health, both under
normal and wound conditions, by affecting the processes that take
place in the epidermis and dermis. The mechanisms involved are
complex and comprise process that include various cytokines and
growth factors. Further research is warranted in order to determine
the effects and mechanisms of exercise on the skin with more
specific parameters, including distinguishing the types of exercise
and various skin conditions, and examining any mechanisms,
cytokines and growth factors involved in skin tissue hemostasis and
regeneration.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by scheme B internal
grant from Universitas Kristen Maranatha (grant no.
013/SK/ADD/UKM/III/2023) and the Academic Leadership Grant from
Universitas Padjadjaran (grant no. 1549/UN6.3.1/PT.00/2023).
Availability of data and materials
Not applicable.
Authors' contributions
DG, DKJ and FK conceived the study and searched the
literature for studies to be included in the review. JWG, DKJ, HG
and VMT performed the screening and selection of articles to be
included in the review. DG and FK drafted the manuscript. RL and IS
confirmed the findings from the included studies and reviewed the
drafted manuscript. All authors worked together in completing the
final draft of the review article. All authors have read and
approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
dos Santos GG, Batool S, Hastreiter A,
Sartori T, Nogueira-Pedro A, Borelli P and Fock RA: The influence
of protein malnutrition on biological and immunomodulatory aspects
of bone marrow mesenchymal stem cells. Clin Nutr. 36:1149–1157.
2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Riyahi F, Riahy S and Yousefpour M:
Reviewing the Physiology of Cutaneous Wound Healing and Evaluating
the Effect of Exercise on It: A Narrative Review Article. Ann Mil
Health Sci Res. 19(e115926)2021.
|
|
3
|
Coletti D, Teodori L, Lin Z, Beranudin JF
and Adamo S: Restoration versus reconstruction: Cellular mechanisms
of skin, nerve and muscle regeneration compared. Regen Med Res.
1(4)2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mazini L, Rochette L, Hamdan Y and Malka
G: Skin immunomodulation during regeneration: Emerging new targets.
J Pers Med. 11(85)2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sugiyama A, Fujita Y, Kobayashi T, Ryu M,
Suzuki Y, Masuda A, Ochi T and Takeuchi T: Effect of protein
malnutrition on the skin epidermis of hairless mice. J Vet Med Sci.
73:831–835. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oishi Y, Fu Z, Ohnuki Y, Kato H and
Noguchi T: Effects of protein deprivation on alpha1(I) and
alpha1(III) collagen and its degrading system in rat skin. Biosci
Biotechnol Biochem. 66:117–126. 2002.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garg S and Sangwan A: Dietary protein
deficit and deregulated autophagy: A new Clinico-diagnostic
perspective in pathogenesis of early aging, skin, and hair
disorders. Indian Dermatol Online J. 10:115–124. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Alves CC, Torrinhas RS, Giorgi R, Brentani
MM, Logullo AF and Waitzberg DL: TGF-β1 expression in wound healing
is acutely affected by experimental malnutrition and early enteral
feeding. Int Wound J. 11:533–539. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Scieglinska D, Krawczyk Z, Sojka DR and
Gogler-Pigłowska A: Heat shock proteins in the physiology and
pathophysiology of epidermal keratinocytes. Cell Stress Chaperones.
24:1027–1044. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hashimoto K: Regulation of keratinocyte
function by growth factors. J Dermatol Sci. 24 (Suppl 1):S46–S50.
2000.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lavker RM and Sun TT: Epidermal stem
cells: Properties, markers, and location. Proc Natl Acad Sci USA.
97:13473–13475. 2000.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Yang R, Wang J, Chen X, Shi Y and Xie J:
Epidermal stem cells in wound healing and regeneration. Stem Cells
Int. 2020(9148310)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lintzeri DA, Karimian N, Blume-Peytavi U
and Kottner J: Epidermal thickness in healthy humans: A systematic
review and meta-analysis. J Eur Acad Dermatol Venereol.
36:1191–1200. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Leite SN, Jordão Júnior AA, de Andrade
TAM, Masson Ddos S and Frade MAC: Experimental models of
malnutrition and its effect on skin trophism. An Bras Dermatol.
86:681–688. 2011.PubMed/NCBI View Article : Google Scholar : (In English,
Portuguese).
|
|
15
|
Wang Y and Graves DT: Keratinocyte
function in normal and diabetic wounds and modulation by FOXO1. J
Diabetes Res. 2020(3714704)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Naomi R, Ridzuan PM and Bahari H: Current
insights into collagen type I. Polymers (Basel).
13(2642)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lo Presti R, Hopps E and Caimi G:
Gelatinases and physical exercise: A systematic review of evidence
from human studies. Medicine (Baltimore). 96(e8072)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
de Araújo R, Lôbo M, Trindade K, Silva DF
and Pereira N: Fibroblast growth factors: A controlling mechanism
of skin aging. Skin Pharmacol Physiol. 32:275–282. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yin L, Zhao X, Ji S, He C, Wang G, Tang C,
Gu S and Yin C: The use of gene activated matrix to mediate
effective SMAD2 gene silencing against hypertrophic scar.
Biomaterials. 35:2488–2498. 2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu S, Jiang L, Li H, Shi H, Luo H, Zhang
Y, Yu C and Jin Y: Mesenchymal stem cells prevent hypertrophic scar
formation via inflammatory regulation when undergoing apoptosis. J
Invest Dermatol. 134:2648–2657. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang R, Ghahary A, Shen Q, Scott PG, Roy K
and Tredget EE: Hypertrophic scar tissues and fibroblasts produce
more transforming growth factor-beta1 mRNA and protein than normal
skin and cells. Wound Repair Regen. 8:128–137. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Han AJ, Alexander LC Jr, Huebner JL, Reed
AB and Kraus VB: Increase in free and total plasma TGF-β1 following
physical activity. Cartilage. 13 (1 Suppl):1741S–1748S.
2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shi A, Hillege MMG, Wüst RCI, Wu G and
Jaspers RT: Synergistic short-term and long-term effects of TGF-β1
and 3 on collagen production in differentiating myoblasts. Biochem
Biophys Res Commun. 547:176–182. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hata S, Okamura K, Hatta M, Ishikawa H and
Yamazaki J: Proteolytic and non-proteolytic activation of
keratinocyte-derived latent TGF-β1 induces fibroblast
differentiation in a wound-healing model using rat skin. J
Pharmacol Sci. 124:230–243. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Konstantinou E, Zagoriti Z, Pyriochou A
and Poulas K: Microcurrent stimulation triggers MAPK signaling and
TGF-β1 release in fibroblast and osteoblast-like cell lines. Cells.
9(1924)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Russo B, Brembilla NC and Chizzolini C:
Interplay between keratinocytes and fibroblasts: A systematic
review providing a new angle for understanding skin fibrotic
disorders. Front Immunol. 11(648)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Dos Santos JF, Borçari NR, da Silva Araújo
M and Nunes VA: Mesenchymal stem cells differentiate into
keratinocytes and express epidermal kallikreins: Towards an in
vitro model of human epidermis. J Cell Biochem. 120:13141–13155.
2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kaimal S and Thappa DM: Diet in
dermatology: Revisited. Indian J Dermatol Venereol Leprol.
76:103–115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nishikori S, Yasuda J, Murata K, Takegaki
J, Harada Y, Shirai Y and Fujita S: Resistance training rejuvenates
aging skin by reducing circulating inflammatory factors and
enhancing dermal extracellular matrices. Sci Rep.
13(10214)2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Knaggs H and Lephart ED: Enhancing skin
anti-aging through healthy lifestyle factors. Cosmetics.
10(142)2023.
|
|
31
|
Yeroushalmi S, Hakimi M, Chung M,
Bartholomew E, Bhutani T and Liao W: Psoriasis and exercise: A
review. Psoriasis (Auckl). 12:189–197. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lai YC and Yew YW: A relationship between
physical activities and hand dermatitis: An epidemiology study of
the USA population. Indian J Dermatol. 60:584–587. 2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Emery CF, Kiecolt-Glaser JK, Glaser R,
Malarkey WB and Frid DJ: Exercise accelerates wound healing among
healthy older adults: A preliminary investigation. J Gerontol A
Biol Sci Med Sci. 60:1432–1436. 2005.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pence BD and Woods JA: Exercise, obesity,
and cutaneous wound healing: Evidence from rodent and human
studies. Adv Wound Care (New Rochelle). 3:71–79. 2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kawanishi M, Kami K, Nishimura Y, Minami
K, Senba E, Umemoto Y, Kinoshita T and Tajima F: Exercise-induced
increase in M2 macrophages accelerates wound healing in young mice.
Physiol Rep. 10(e15447)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Yeh C, Flatley E, Elkattawy O, Berger L
and Rao B: Exercise in dermatology: Exercise's influence on skin
aging, skin cancer, psoriasis, venous ulcers, and androgenetic
alopecia. J Am Acad Dermatol. 87:183–184. 2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ishikawa T, Ito E, Okada T and Sumi T: Hot
yoga increases SIRT6 gene expression, inhibits ROS generation, and
improves skin condition. Glycative Stress Res. 8:123–135. 2021.
|
|
38
|
Sharples AP, Hughes DC, Deane CS, Saini A,
Selman C and Stewart CE: Longevity and skeletal muscle mass: The
role of IGF signalling, the sirtuins, dietary restriction and
protein intake. Aging Cell. 14:511–523. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Giblin W, Skinner ME and Lombard DB:
Sirtuins: Guardians of mammalian healthspan. Trends Genet.
30:271–286. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Satoh A and Imai S: Systemic regulation of
mammalian ageing and longevity by brain sirtuins. Nat Commun.
5(4211)2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Schmeisser K, Mansfeld J, Kuhlow D, Weimer
S, Priebe S, Heiland I, Birringer M, Groth M, Segref A, Kanfi Y, et
al: Role of sirtuins in lifespan regulation is linked to
methylation of nicotinamide. Nat Chem Biol. 9:693–700.
2013.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kanfi Y, Naiman S, Amir G, Peshti V,
Zinman G, Nahum L, Bar-Joseph Z and Cohen HY: The sirtuin SIRT6
regulates lifespan in male mice. Nature. 483:218–221.
2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Imai S and Guarente L: It takes two to
tango: NAD+ and sirtuins in aging/longevity control. NPJ
Aging Mech Dis. 2(16017)2016.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kumar H and Choi DK: Hypoxia inducible
factor pathway and physiological adaptation: A cell survival
pathway? Mediators Inflamm. 2015(584758)2015.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ishihara A: Mild hyperbaric oxygen:
Mechanisms and effects. J Physiol Sci. 69:573–580. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Heinen M, Borm G, van der Vleuten C, Evers
A, Oostendorp R and van Achterberg T: The Lively Legs
self-management programme increased physical activity and reduced
wound days in leg ulcer patients: Results from a randomized
controlled trial. Int J Nurs Stud. 49:151–161. 2012.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Amatriain-Fernández S, Gronwald T,
Murillo-Rodríguez E, Imperatori C, Solano AF, Latini A and Budde H:
Physical exercise potentials against viral diseases like COVID-19
in the elderly. Front Med (Lausanne). 7(379)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Ryosuke O, Yoshie S and Hiromi A: The
association between activity levels and skin moisturising function
in adults. Dermatol Reports. 13(8811)2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Hoffmann C and Weigert C: Skeletal muscle
as an endocrine organ: The role of myokines in exercise
adaptations. Cold Spring Harb Perspect Med.
7(a029793)2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ishiuchi-Sato Y and Nedachi T: Possible
involvement of CXC motif chemokine ligand 10 in exercise-induced
collagen production of mouse dermal fibroblasts. Endocr J.
68:1359–1365. 2021.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yadav H and Rane SG: TGF-β/Smad3 signaling
regulates brown adipocyte induction in white adipose tissue. Front
Endocrinol (Lausanne). 3(35)2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Barzegari A, Naghibi S, Dashty MH and
Zafarabadi FK: The effect of three different exercise methods HIIT,
HIT and MIT on the expression of FGF and TGF genes in liver tissue
of male Wistar rats. Appl Health Stud Sport Phys. 9:100–113.
2022.
|
|
53
|
Chen J, Zhou R, Feng Y and Cheng L:
Molecular mechanisms of exercise contributing to tissue
regeneration. Signal Transduct Target Ther. 7(383)2022.PubMed/NCBI View Article : Google Scholar
|