Introduction
Prostate cancer (PCa) is the second most frequently
diagnosed malignancy (excluding non-melanoma skin cancer) and is
the fifth leading cause of cancer-related mortality in men
worldwide (1,2). According to the Global Cancer
Observatory statistics for 2020, >1.4 million men were newly
diagnosed with PCa and 375,304 associated deaths were recorded in
2020(2). However, the ability to
diagnose and determine the PCa stage is restricted and
insufficiently specific when pre-screening methods such as
prostate-specific antigen are used (3).
Radiation therapy or radiotherapy (RT) is a standard
treatment provided to patients with locally advanced PCa (4,5),
with 50-60% of the total patients relying on this treatment
(6). However, patients may
experience off-target adverse effects of RT-induced toxicity,
harming the surrounding normal tissues (7). RT toxicity is classified as acute or
early if it occurs within 3 months of RT completion and is usually
resolved within 4-6 weeks post-treatment (8,9).
Chronic or late toxicities last several months and even years
post-completion of RT and may induce permanent tissue changes
(8,10,11).
In PCa, symptoms associated with genitourinary (GU) and
gastrointestinal (GI) toxicity are common in early RT-induced
toxicity and are validated through scoring criteria based on Common
Terminology Criteria for Adverse Events (12). The most frequent and acute effect
following RT is inflammation, leading to tissue damage and late
side effects such as fibrosis (13). Patients with PCa often experience
fatigue, prostate atrophy, physiological complications of the
urogenital tract, such as bladder and/or erectile dysfunction,
urinary incontinence, infertility, diarrhoea, and rectal bleeding,
and rarely secondary tumour development, which influences their
quality of life (14). However,
current pre-treatment assessments cannot be used to predict acute
or late RT-induced toxicity (15).
Therefore, novel biomarkers are required in RT oncology to predict
RT-induced toxicity and improve decision-making, treatment and
therapy monitoring of patients with PCa.
There have been some investigations on the possible
use of microRNAs (miRNAs/miRs) as biomarkers to predict RT-induced
toxicity, including RT-induced dermatitis for breast cancer and
esophagitis for non-small cell lung cancer (16,17).
miRNAs are a class of small non-coding RNA molecules (21-25
nucleotides) involved in post-transcriptional regulation. miRNAs
are important during the DNA damage response and regulate the
expression of various genes (18).
Thus, they serve a vital role in physiological cellular processes
such as cell cycle regulation (19), apoptosis (20) and cancer metastasis (21). In addition, they are stable in
different biological samples, such as plasma and serum, under
appropriate storage conditions and can be used as an efficient
diagnostic marker from liquid biopsies (22). Therefore, in numerous types of
cancer (23), including PCa
(24), they are available for
sampling from bodily fluid-liquid biopsies (25). These inherent features of miRNAs
make them attractive candidates for minimally invasive biomarkers
in PCa. Several studies have used quantitative PCR (qPCR) or RNA
array methodologies to investigate miRNA expression before and
after RT exposure in patients with PCa (26-31).
Several previous studies have reported that there could be
variations in miRNA expression levels in response to RT (32-35).
However, few prospective studies clinically investigate miRNAs in
blood samples to predict the severity of RT-induced toxicity based
on miRNA expression in patients with PCa (26,29-31).
The identification of circulating miRNAs induced by
RT may aid in the development of a radiation biomarker for use in
clinical diagnostic procedures in the future. Therefore, the
present study aimed to examine the latest literature on the impact
of RT on the circulating miRNA profile in the blood of patients
with PCa. In addition, the present study aimed to demonstrate the
association of miRNA expression levels with RT-induced toxicity,
and to provide a valuable understanding of carefully selected
miRNAs.
Materials and methods
Search strategy
The clinical studies investigating miRNA expression
and the association with RT-induced toxicity in PCa were identified
using electronic databases [PubMed (https://pubmed.ncbi.nlm.nih.gov/), Science Direct
(https://www.sciencedirect.com/) and
Google Scholar (https://scholar.google.com.au/)]. Furthermore,
reference lists of relevant studies were assessed to identify
further appropriate studies. The systematic search for miRNA
studies was carried out using the following key words: Prostate
cancer, plasma, serum, miRNA expression, side effects of RT,
RT-induced toxicity, genitourinary and gastrointestinal
toxicity.
Selection (inclusion and exclusion)
criteria
Titles and abstracts of relevant studies were
evaluated for their contents, ensuring adherence to both inclusion
and exclusion criteria for the systematic review. The inclusion
criteria were: i) Studies investigating miRNA expression in
patients with PCa only; ii) studies investigating the patient's
blood plasma or serum and peripheral blood mononuclear cells
(PBMCs) for miRNAs; iii) the study recorded the sample size,
sampling methods, diagnostic methods, patient characteristics and
clinicopathological outcome; and iv) studies analysed the
association of miRNA expression levels with RT-induced toxicity.
The exclusion criteria for the systematic review were: i) Studies
investigating miRNA expression in other types of cancer; ii)
editorials, commentaries and review articles; iii) studies
investigating miRNA expression in animal samples and in
vitro cell lines; and iv) non-English language published
studies.
Study review methods and outcome
measure
The relevant published articles were retrieved in
January 2023 and June 2023 and imported into an Endnote X21
database (36). Analogous articles
were identified and deleted using the duplicate function in
Endnote. Furthermore, the article titles and abstracts were
carefully screened to avoid irrelevant studies. Only studies
describing multivariable-adjusted hazard ratios were considered.
Studies that reported crude or unadjusted outcome measures among
patients treated with RT were excluded.
Data extraction
Two reviewers independently extracted the following
data from eligible studies related to PCa: i) General information
(first author, publication year, method of patient recruitment and
sampling methods); ii) clinical characteristics such as T-stage,
age, treatment option, number of patients and follow-up period;
iii) clinical outcomes: Biochemical recurrence, side effects of RT
or RT-induced toxicity; and iv) diagnostic methods: miRNA array and
reverse transcription-qPCR (RT-qPCR).
Quality assessment
The Quality Assessment of Diagnostic Accuracy
Studies-2 tool was used to evaluate the quality of the included
studies. Every assessment question received a score of ‘yes’, ‘no’
or ‘unclear’ (37). The case
selection process, index test, reference standard, case procedure
and progress are all included in this assessment. ‘Yes (1)’, ‘No (-1)’ and ‘Unclear (0)’ are the
scores. Lastly, the overall score indicates the calibre of the
research in the following ways: 8-14 denotes high-quality
literature, while 0-7 denotes low-quality literature with a high
likelihood of bias.
Furthermore, to further assess the quality of the
retrieved studies, the articles were evaluated based on the
following principles: i) Studies included the clinical
characteristics of participants and blood samples in a detailed
description; ii) studies that met the inclusion and exclusion
criteria for participants; iii) studies reported disease course
stage and starting point among all the participants; iv) studies
described the association between clinical characteristics and
outcomes; and v) studies considered other factors that influence
the predictive result.
Meta-analysis
The Comprehensive Meta-Analysis programme was used
to compare the effect sizes of selected miRNA studies with the
groups of RT-induced PCa toxicity. ‘Hedge's g’ was used to
determine the effect size due to the difference in sampling and
measurement tools in the calculations (38). In meta-analysis studies, fixed
effects or random effects models are used according to
heterogeneity (39). The fixed
effects model is applied when the effect sizes of the studies
included in the meta-analysis do not change, whereas the random
effects model is applied when the effect sizes differ between
studies (39).
The effect size can be classified as a strong effect
size if it is >0.80 and a weak effect size if it is <0.20.
According to this classification, d≤0.20 is considered a weak
effect size, 0.20<d<0.80 is considered a medium effect size
and d≥0.80 is considered a strong effect size (40). Cochran's Q statistics, P-value and
I2 tests were used to test the heterogeneity of effect
sizes. In the heterogeneity assessment, if the heterogeneity rate
(I2) is <25%, it is absent; 25-50% is considered low;
51-75% is considered moderate; and >75% is considered high
(41).
The asymmetry of the funnel plot was tested using
linear regression to assess publication bias. The funnel plot did
not exhibit any noticeable asymmetry. The closer the regression
line is to 90˚, and the smaller the angle between it and the
diagnostic odds ratio (DOR) axis, the less likely it is to exhibit
bias. The angle in this figure is extremely near to 90˚, which
suggests that there is no discernible publication bias and that the
findings of the meta-analysis are trustworthy.
Results
Study search
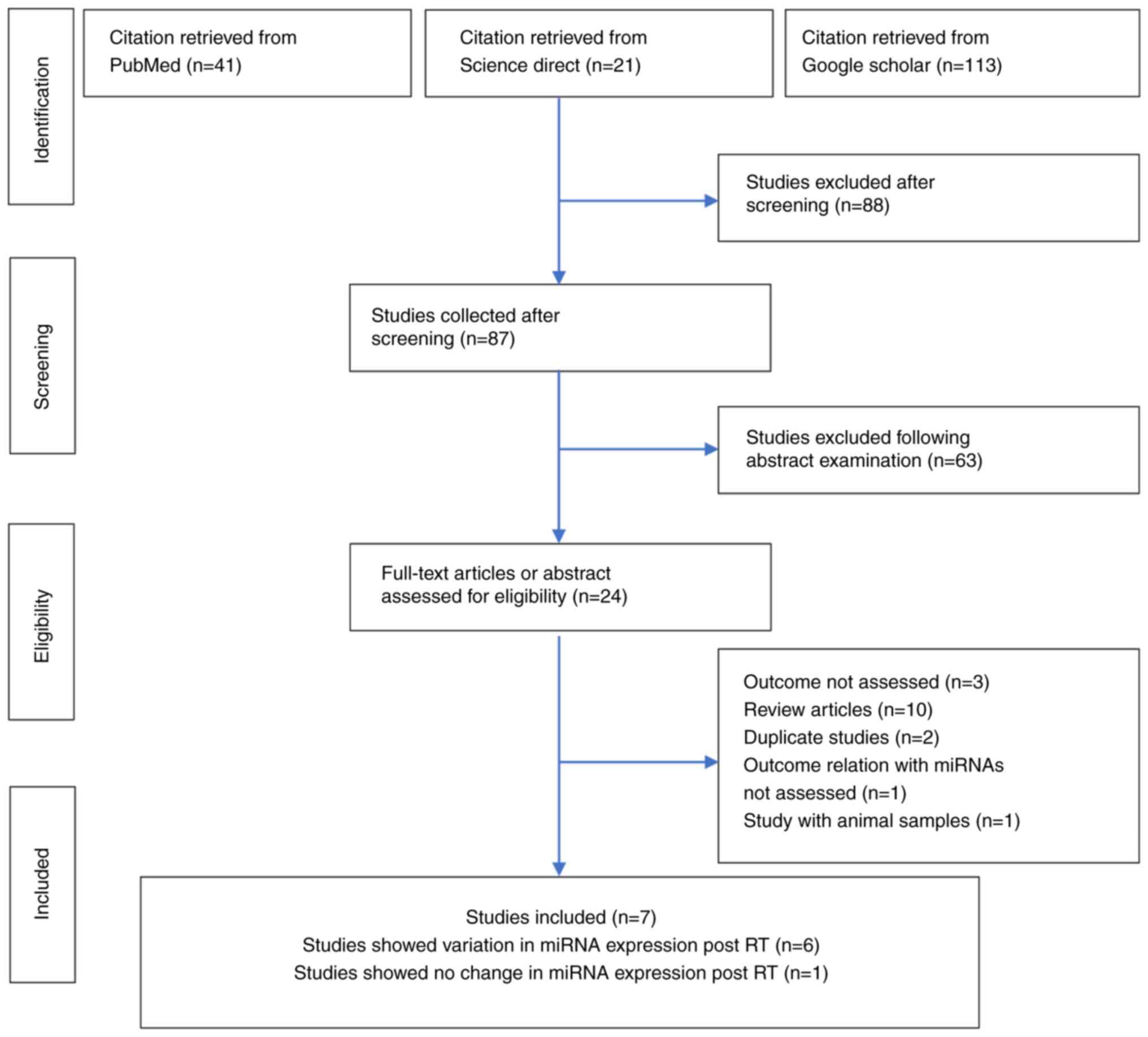
The literature search identified 175 studies: 41
from PubMed, 21 from Science Direct and 113 from Google Scholar. Of
these 175 studies, 88 were excluded following the title review and
87 studies were selected at the first screening stage. At the
second screening stage, 63 studies were removed following abstract
examination and 24 were selected. At the eligibility criteria
stage, 17 studies were removed for the following reasons: Outcomes
not evaluated (n=3), systematic review articles (n=10), duplication
of study groups (n=2), a relationship of RT with miRNA levels was
not considered (n=1) and a study on animal samples (n=1).
Ultimately, after eligibility consideration, seven articles were
selected, and Fig. 1 shows the
literature search and selection strategy as a flowchart.
miRNA expression levels in response to
RT
Few clinical studies have evaluated miRNA levels of
interest in blood samples (serum or plasma) collected from pre-RT
baseline, during a fractionated RT course, and through to follow-up
(Table I) (26-31,42).
Of the seven studies, six highlighted modified peripheral blood
lymphocyte, plasma and serum miRNA expression levels in the group
of patients with PCa post-RT (26-31,42).
Zedan et al (42) observed
significantly lower miRNA-93 and miRNA-221 levels in the follow-up
samples compared with baseline samples (P=0.006 and P≤0.001,
respectively). Furthermore, miRNA-93 downregulation was more
significant in the RT subgroup (P=0.018) than in the radical
prostatectomy (RP) subgroup (P=0.030). Conversely, miRNA-221 plasma
levels were more downregulated in the RP subgroup (P≤0.001) than in
the RT subgroup (P=0.028) (42).
 | Table IObservational clinical studies
investigating miRNA expression levels following RT in patients with
PCa. |
Table I
Observational clinical studies
investigating miRNA expression levels following RT in patients with
PCa.
| Author/s, year | No. of
patients | T-stage | Treatment | Type of sample | miRNA detection
methods | miRNAs | Results of
collected studies | (Refs.) |
|---|
| Kopcalic et
al, 2019 | 15 | Localized PCa | RT | PBLs | RT-qPCR | miRNA-21,
miRNA-146a and miRNA-155 | Significantly
higher levels of miRNA-21 in the post-RT samples compared with the
baseline samples (P=0.043). | (26) |
| Bahtiyar et
al, 2018 | 25 | Localized PCa | RT | Blood plasma | RT-qPCR | miRNA-223 and
miRNA-126 | No significant
differences in expression levels of miRNA-223 and miRNA-126 were
observed between the RT treated patients and control groups. | (27) |
| Malla et al,
2018 | 11 | Localized PCa | RT | Blood serum | RT-qPCR | hsa-let-7a-5p,
hsa-miRNA-141-3p, hsa-miRNA-145-5p, hsa-miR-21-5p and
hsa-miRNA-99b-5p | Upregulation of
hsa-let-7a-5p and hsa-miRNA-21-5p was identified after RT; the
difference was significant only in the high-risk group (P=0.037).
The evaluation of has-let-7a-5p and hsa- miRNA-21-5p revealed
different expression levels in both risk groups. Upregulation of
two miRNAs, hsa-let-7a-5p (fold change, 2.24) and hsa-miRNA-21-5p
(fold change, 1.77), was observed to be potentially induced by
RT. | (28) |
| Someya et
al, 2018 | 69 | Localized PCa | RT | PBLs | RT-qPCR | miRNA-410,
miRNA-221 and miRNA-99a | Expression levels
of miRNA-410 and miRNA-221 (P=0.020 and P=0.013, respectively) were
altered in post-RT blood samples compared with pre-RT blood
samples. | (29) |
| Someya et
al, 2015 | 48 | Localized PCa | RT | PBLs | miRNA array and
RT-qPCR | miRNA-99a | Statistically
significant differences in the expression of miRNA-199a in post-RT
samples. | (30) |
| Rana et al,
2019 | 12 | Localized PCa | RT | Blood plasma | RT-qPCR | miRNA-132-5p,
miRNA-23a-3p, miRNA-1-3p, miRNA-197-3p, miRNA-151a-5p and
miRNA-18b-5p | Six miRNAs
exhibited differential expression in post-RT samples compared with
pre-RT samples: miRNA-132-5p (upregulated; P=0.001), miRNA-23a-3p
(downregulated; P=0.020), miRNA-1-3p (upregulated; P=0.047),
miRNA-197-3p (upregulated; P=0.017), miRNA-151a-5p (upregulated;
P=0.031) and miRNA-18b-5p (upregulated; P=0.020). Significantly
higher levels of miRNA-21 in the post-RT group compared with the
control group (P=0.043). | (31) |
| Zedan et al,
2019 | 149 | Local or locally
advanced cancer | Radical
prostatectomy and RT | Blood plasma | RT-qPCR | miRNA-21, miRNA-93,
miRNA-125b and miRNA-221 | Levels of miRNA-93
and miRNA-221 were significantly lower in the follow-up samples
compared with the baseline samples (P=0.006 and P<0.001,
respectively). The same observation was recorded for miRNA- 125b in
the observational cohort (P=0.008). Both miRNA-125b and miRNA-221
were correlated with risk assessment (r=0.23, P=0.015, and r=0.203,
P=0.016, respectively) while miRNA-93 showed a tendency towards a
significant correlation with the prostatectomy Gleason score
(r=0.276; P=0.0576). | (42) |
Similarly, a pilot study also observed the effect of
RT on miRNAs and identified elevated levels of miRNA-21 in the
post-RT group compared with the pre-RT group (P=0.043) (26). Rana et al (31) reported that six miRNAs, including
miRNA-132-5p (upregulated; P=0.001), miRNA-23a-3p (downregulated;
P=0.020), miRNA-1-3p (upregulated; P=0.047), miRNA-197-3p
(upregulated; P=0.017), miRNA-151a-5p (upregulated; P=0.031) and
miRNA-18b-5p (upregulated; P=0.020), showed variation in expression
post-RT compared with pre-RT. In an additional study, miRNA-410 and
miRNA-221 expression levels were also altered in post-RT compared
with pre-RT blood samples (29).
Another study by Someya et al (30) also found statistically significant
differences in the expression of miRNA-199a in post-RT samples.
Upregulation of hsa-let-7a-5p and hsa-miRNA-21-5p was identified
after RT, and the difference was significant only in the high-risk
group (P=0.037) (28).
Upregulation of two miRNAs, hsa-let-7a-5p (fold change, 2.24) and
hsa-miRNA-21-5p (fold change, 1.77), was observed to be potentially
induced by RT (28).
Out of seven studies, one study indicated no
significant variation (P>0.05) in plasma miR-223 and miR-126
expression levels between the RT-treated and control groups
(27). The lack of variation and
significant differences in miRNA expression may indicate that these
miRNAs are not tumour-specific in serum/plasma.
miRNAs as biomarkers for RT-induced
toxicity
The included studies reported the possible
association between miRNA expression levels and RT-induced toxicity
in patients with PCa. Out of the seven studies, four indicated an
association between miRNA expression levels and RT-induced toxicity
(26,29-31).
The studies investigating blood-based miRNA biomarkers and
RT-induced toxicity are summarised in Table II.
 | Table IIStudies investigating blood-based
miRNA expression levels following RT and RT-induced toxicity. |
Table II
Studies investigating blood-based
miRNA expression levels following RT and RT-induced toxicity.
| Author/s, year | No. of
patients | Tumour stage | Treatment | miRNAs detection
methods | miRNAs | Results and
comments | (Refs.) |
|---|
| Kopcalic et
al, 2019 | 15 | Localized PCa | RT | RT-qPCR | miRNA-21,
miRNA-146a and miRNA-155 | Higher levels of
miRNA-21 were observed in patients with acute GU RT-toxicity than
in the group without GU RT-toxicity (P=0.068); however, this
difference was not statistically significant. Furthermore, within
the group of patients who experienced GU RT-toxicity, significantly
higher levels of miRNA-21 were identified in the post-RT group
compared with the control group (P=0.046). | (26) |
| Someya et
al, 2018 | 69 | Localised PCa | RT | RT-qPCR | Low-toxicity
patients miRNA-410 and miRNA-221 | miRNA-410 and
miRNA-221 expression was significantly associated with grade 1-2
gastrointestinal toxicity. Furthermore, miRNA-99a and miRNA-221
expression levels were elevated in the high-toxicity group (P=0.006
and P=0.050, respectively). | (29) |
| High-toxicity
patients: miRNA-99a and miRNA-221 |
| Someya et
al, 2015 | 48 | Localised PCa | RT | miRNA array and
RT-qPCR | miRNA-99a | In the RT-induced
grade 2-3 rectal bleeding group, miRNA-99a expression was
significantly higher (P=0.013) after RT. Thus, high miRNA-99a
expression could be used as a promising marker for predicting
rectal bleeding after RT. | (30) |
| Rana et al,
2019 | 12 | Localised PCa | RT | RT-qPCR | Low-toxicity
patients: miRNA-132-5p, miRNA-23a-3p and miRNA-1-3p | In the low-toxicity
group, three miRNAs exhibited differential expression at post-RT
compared with pre-RT: miRNA-132-5p (upregulated; P=0.001),
miRNA-23a-3p (downregulated; P=0.020) and miRNA-1-3p (upregulated;
P=0.047). | (31) |
| | | | | | High-toxicity
patients: miRNA-132-5p, miRNA-197-3p, miRNA-151a-5p and
miRNA-18b-5p | In the
high-toxicity group, four miRNAs exhibited differential expression
at post-RT compared with pre-RT: miRNA-132-5p (downregulated;
P=0.003), miRNA-197-3p (upregulated; P=0.017), miRNA-151a-5p
(upregulated; P=0.031) and miRNA-18b-5p (upregulated;
P=0.020). | |
In the low-toxicity group, miRNA-410 and miRNA-221
expression levels were significantly increased after RT and
associated with grade 1-2 acute GI toxicity (P=0.020 and P=0.013,
respectively) (29). In addition,
three miRNAs exhibited variation in expression post-RT compared
with pre-RT. miRNA-132-5p (upregulated; P=0.001) and miRNA-1-3p
(upregulated; P=0.047) were associated with low RT-induced
toxicity, while the expression levels of miRNA-23a-3p
(downregulated; P=0.020) were decreased in the low RT-induced
toxicity group (31).
Furthermore, in the high-toxicity group, miRNA-21
expression levels were higher among patients with acute GU
RT-induced toxicity than among those without GU radiotoxicity
(P=0.068); however, this difference was not statistically
significant (26). Furthermore,
miRNA-99a and miRNA-221 expression levels were elevated in the
high-toxicity group (P=0.006 and P=0.050, respectively) (29). In the RT-induced grade 2-3 rectal
bleeding group, miRNA-99a expression was higher (P=0.013) after RT
(30). Another study reported that
miRNA-197-3p (upregulated; P=0.017), miRNA-151a-5p (upregulated;
P=0.031) and miRNA-18b-5p (upregulated; P=0.020) expression levels
were elevated in post-RT samples compared with pre-RT samples and
showed significant association with high RT-induced toxicity
(Table III) (31). The study also reported that
miRNA-132-5p (downregulated; P=0.003) expression levels were
decreased and were associated with the high-toxicity group
(31).
 | Table IIImiRNAs dysregulated following RT in
prostate cancer based on RT-induced toxicity severity. |
Table III
miRNAs dysregulated following RT in
prostate cancer based on RT-induced toxicity severity.
| | miRNA expression
post-RT | |
|---|
| Author/s, year | RT-induced
toxicity | Increased
miRNAs | Decreased
miRNAs | (Refs.) |
|---|
| Someya et
al, 2018; Rana et al, 2019 | Low toxicity | miRNA-132-5p
(P=0.001), miRNA-1-3p (P=0.047), miRNA-410 (P=0.020) and miRNA-221
(P=0.013) | miRNA-23a-3p
(P=0.020) | (29,31) |
| Kopcalic et
al, 2019; Someya et al, 2015; Rana et al,
2019 | High toxicity | miRNA-197-3p
(P=0.017), miRNA-151a-5p (P=0.031), miRNA-18b-5p (P=0.020),
miRNA-99a (P=0.013) and miRNA-21 (P=0.068) | miRNA-132-5p
(P=0.003) | (26,30,31) |
Meta-analysis results
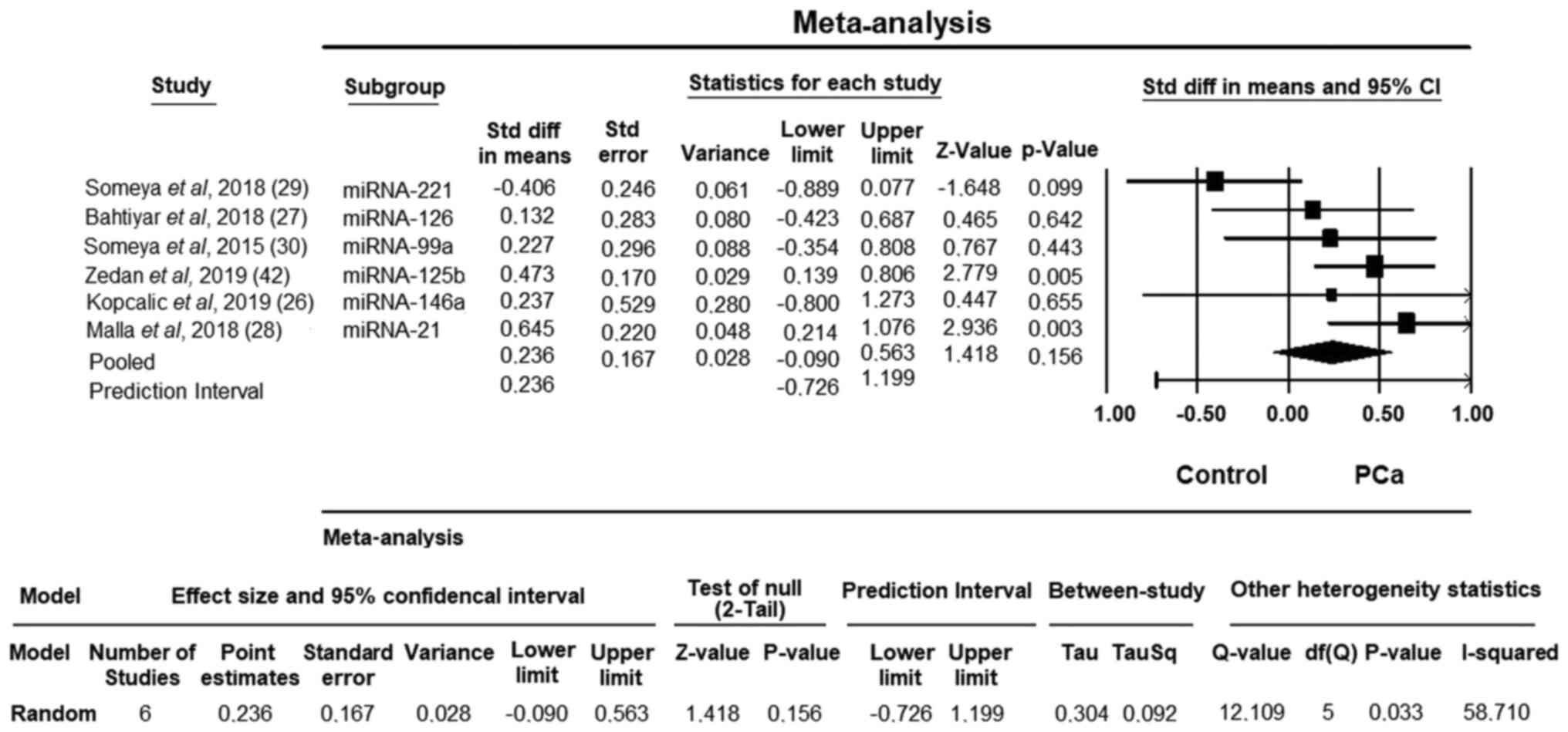
Fig. 2 shows the
summary results for RT-induced toxicity in PCa. Some miRNAs
exhibited altered expression in patients with PCa, including
miRNA-221, miRNA-126, miRNA-99a, miRNA-146a, miRNA-125b and
miRNA-21. Using the meta-analysis method, a statistically
significant signature of two upregulated miRNAs (miRNA-21 and
miRNA125b) in RT-induced toxicity in PCa compared with healthy
controls was identified. Good performance for RT-induced toxicity
in PCa was observed for miRNA21 (95% CI, 0.214-1.076; P=0.003) and
miRNA125b (95% CI, 0.139-0.806; P=0.005) expression.
The effect size obtained in the meta-analysis was
0.236 for the random-effects model. As a result of the
heterogeneity test, the Q value was estimated as 12.109 and the
obtained value was statistically significant (P=0.033). The data
obtained in this study were found to be heterogeneous based on the
Q test. The I2 value, which is another indicator for
heterogeneity, was 58.710%. This value was high, also indicating
heterogeneity. As a result of heterogeneity, the average effect
size (point estimate) estimated according to the random effects
model was 0.236, and it was determined that there was a moderate
effect in the present study according to the Cohen (1988)
classification (Fig. 2) (40).
For this, the results of three publication bias
tests (Orwin error protection coefficient, Kendall's tau and Egger
regression) should also be reported. Orwin's fail-safe N value was
found to be 1693 when trivial value was taken as 0.001, that is, in
order to make the relevant Fisher's Z value insignificant.
Kendall's tau z value was found to be 0.001 and one-way P-value was
found to be 0.500. This is an indication that there is no
publication bias. According to the Egger regression intercept
results, the intercept value is (β0)=-1.59, t=0.707 and the P-value
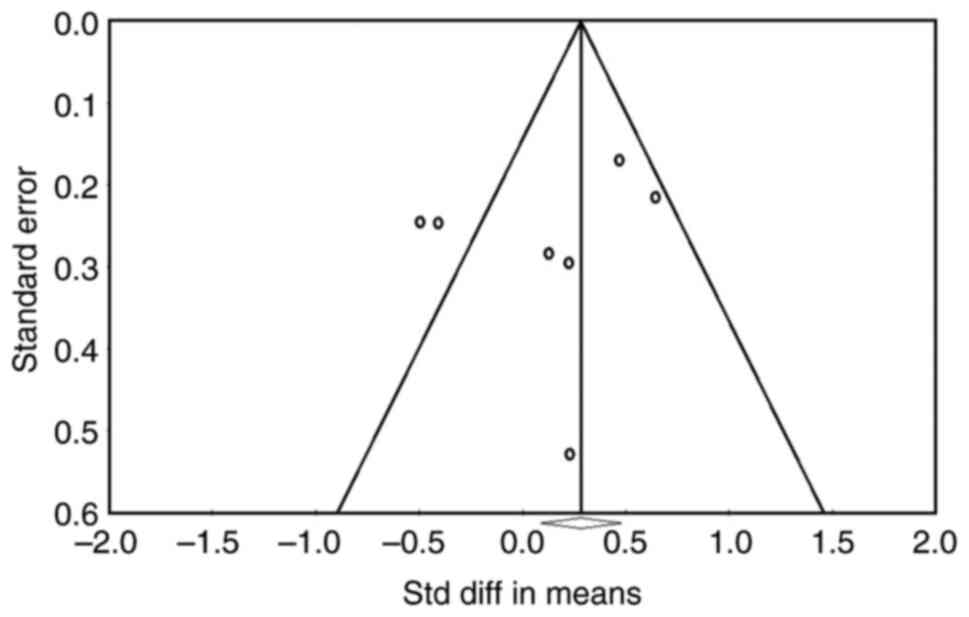
is 0.259. As demonstrated in Fig.
3, the asymmetry of the funnel plot and Deeks' funnel plot was
tested using linear regression in order to assess publication bias.
The funnel plot and Deeks' funnel plot did not exhibit any
noticeable asymmetry, as shown by a P-value of >0.05 in Fig. 3. The likelihood of a bias is
reduced, the closer the regression line is to 90˚, and the smaller
the angle between it and the DOR axis. The angle in this figure is
extremely near to 90˚, which suggests that there is no discernible
publication bias and that the meta-analysis findings are
trustworthy. These three tests for publication bias, along with the
funnel plot and Deeks's funnel plot results, demonstrated that the
results were trustworthy and devoid of publication bias (P>0.05;
Fig. 3).
Discussion
In clinical practice, when RT is performed to treat
localized PCa, a high dose needs to be delivered to the prostate,
while reducing the damage to the surrounding normal tissues. The
advancement of therapeutic procedures such as intensity-modulated
RT has permitted the escalation of the dose delivered to the
prostate, improving local tumour control without markedly
increasing RT-induced toxicity (43). At present, researchers are trying
to understand the important mechanisms involved in RT-induced
toxicity and identify possible molecular biomarkers that could
predict RT-induced toxicity (44).
Some previous studies have stated that mechanisms such as
inflammation and chronic oxidative stress, reduction of tissue stem
and progenitor cells, and damage to the microenvironment are
involved in RT-induced toxicity (45,46).
In PCa, miRNA expression is dysregulated, and this
can modulate the expression of oncogenes and tumour suppressor
genes (47-50).
Treatment resistance is still a great challenge; in this case,
miRNAs could be a novel therapeutic target and predict response to
treatments, such as chemotherapy and RT in patients with cancer
(47-49,51).
Therefore, miRNA expression provides novel perceptions of what
treatment is the most appropriate, and if treatment must be changed
or adjusted. Furthermore, regarding side effects, changes in miRNA
expression can be used to overcome these toxicities or to
understand their signs before the need to interrupt the therapy
with possible impairment in therapeutic results (26,33,52).
For the treatment of patients with PCa with RT, dose-escalation has
been established to improve biochemical recurrence control;
however, an increased RT dose increases the risk of late GU and GI
toxicity (53,54). When considering doses of ≥60 Gy,
the majority of dose-volume parameters are linked to late rectal
toxicity (55). In addition, grade
≥2 rectal toxicity rates are considerably higher for dose-volume
histograms passing above these thresholds than those passing below
(55).
miRNA-155 expression can increase or decrease
depending on the type of RT, the dose of RT and the rates of RT
(32,56). These essential factors contribute
to the cellular response to RT as reflected by miRNA expression
levels (56). For example, Korpela
et al (57) reported that
miRNA-21, miRNA-146a and miRNA-155 expression levels were increased
post-RT compared with pre-RT. In addition, another study
demonstrated that miRNA-21 levels varied during and after RT
(58). Stepanović et al
(59) also reported that miR-34a
expression was elevated at the 15 and 30th fraction of RT compared
with pre-RT samples. However, none of these biomarkers have shown
encouraging results that could be applied clinically (44).
Several characteristics of miRNAs make them
appropriate candidates for molecular biomarker development
(60), including the high
stability of miRNAs in the blood and urine (60,61).
Furthermore, miRNAs can also remain stable after incubation at room
temperature and after undergoing repeat freeze-thaw cycles
(61). miRNAs can easily be
detected with a standard RT-qPCR (60). However, there are still some
drawbacks to using miRNAs reliably as predictive biomarkers of
RT-induced toxicity. First, there is a shortage of miRNA studies
investigating RT-induced toxicity in PCa; therefore, determining
miRNA as a potential biomarker in RT oncology is necessary for
additional clinical investigations. Second, more research on blood
samples is necessary for consistent prospective study protocols.
This research should include controlled sample sizes, the
interpretation of statistical results, and the use of plasma or
serum. Third, in the future, prospective studies should consider
blood sampling before, during and after RT to evaluate miRNA
expression levels and follow-up to quantify acute and late
RT-induced toxicity.
The present study included published data from
previous clinical studies regarding the influence of RT on miRNA
expression levels and their association with the severity of
RT-induced toxicity in patients with PCa. After considering the
evidence indicating excellent stability and less difficulty in
quantifying miRNAs in liquid biopsies, miRNA could be used as
RT-induced toxicity biomarkers. Transcription of miRNA in
lymphocytes is active and responsive to various environmental
signals and irradiation (62).
Therefore, to study biomarkers in RT oncology, a systematic review
and meta-analysis of miRNA expression levels and their association
with the severity of RT-induced toxicity is an appropriate and
acceptable method.
One of the aims of the present study was to perform
a meta-analysis of miRNA expression profiling studies investigating
RT-induced toxicity in PCa to identify novel candidate biomarkers
and/or therapeutic targets. To the best of our knowledge, the
present study was the first meta-analysis to focus on the role of
miRNAs in RT-induced PCa toxicity, with a systematically quantified
evaluation of the diagnostic value. A total of 10 candidate miRNAs
(hsa-let-7a-5p, miRNA-21, miRNA-93, miRNA-99a, miRNA-125b,
miRNA-146a, miRNA-155, miRNA-210, miRNA-221 and miRNA-410) from six
articles were identified using electronic databases (26-30,42).
These findings suggested that identifying miRNAs with altered
expression in PCa may help identify novel biomarkers for PCa that
can be used to track and influence disease progression. A
shortcoming of the interpretation of the miRNA expression profile
is the lack of consistency between study results. The diversity of
the study population may result from various study designs,
variations in expression profiling platforms, and genetic,
environmental and clinicopathological variations among organ and/or
tissue donors. Further validation in large patient cohorts is
required to confirm the significance of these miRNAs as PCa
biomarkers and therapeutic targets.
Meta-analysis of the European ancestry cohorts
identified three genomic signals: Single nucleotide polymorphism
rs17055178 with rectal bleeding (Pmeta=6.2x10-10),
rs10969913 with decreased urinary stream
(Pmeta=2.9x10-10) and rs11122573 with hematuria
(Pmeta=1.8x10-8), and association with RT-induced
toxicity events such as rectal bleeding, lower urinary stream and
higher urinary frequency (63).
Whole transcriptome and pathway analysis of liquid biopsies might
reveal mechanisms underlying the pathogenesis of acute or late
radiotoxicity. There is a lack of meta-analyses investigating
associations between miRNAs and side effects in patients with PCa
who have undergone RT, and the present study may be among the first
ones.
According to the studies reported, miRNAs have been
linked to significant events such as DNA damage repair, oxidative
stress, cell cycle regulation, inflammation, cell death and
apoptosis, and hypoxia (64-71).
For example, miRNA-21 is associated with apoptosis, targeting PTEN,
programmed cell death protein 4 and BCL2(64). miR-99a is associated with cell
cycle regulation (65). miR-221 is
also associated with apoptosis via targeting of PTEN (66). miRNA-18b is downregulated in LnCaP
cells after RT (67). miR-132-5p
is associated with fibrosis, so it may be closely related to
toxicity (68). miR-197-3p and
miR-23a-3p are associated with inflammation and liver fibrosis, and
apoptosis in diabetic kidney disease (69,70).
miRNA-410 can inhibit cytokine release, indicating its involvement
in the inflammatory response by targeting NF-κB (71).
Lymphocyte models for the investigation of responses
to radiation in terms of genetics and epigenetics are especially
informative and important. When exposed to radiation, quickly
dividing cells such as hematopoietic cells react first (72). Lymphocytes from circulation are
radiosensitive (73-75).
It has also been demonstrated that the transcriptome of lymphocytes
changes after irradiation (3 h after ex vivo irradiation
with 2-Gy ɣ-rays) (76).
Furthermore, genetic/epigenetic information can be transferred to
distant cells and organs by circulation and miRNA trafficking (via
exosomes which enter and exit lymphocytes) (77). Therefore, miRNA changes in response
to RT are noteworthy and should be utilized as adjunctive factors
for the prediction of therapy response, aside from information
obtained from serum or plasma samples. The studies described
indicate that miRNA changes in plasma/serum and PBMCs/peripheral
blood mononuclear lymphocytes may have the potential for use in
clinical practice (26-31,42).
Additionally, it should be noted that events in the cells, which
are the repercussion of radiation exposure, may increase or
decrease miRNA levels (67). These
miRNA level changes should also be considered as predictive
parameters of response to therapy, regardless of their absolute
values or targets and genes/pathways they are silencing.
The present study highlighted the importance of
transcriptome and non-coding transcript changes. The changes in the
transcriptome (coding and non-coding) may be used for prediction of
not only response to RT but also chemotherapy, as well as for other
types of malignancies. Furthermore, changes in miRNA levels during
therapy may be used in the future to modulate therapy, providing
information ranging from how to alter the course of treatment and
avoiding surrounding tissue damage, to lowering the incidence of RT
side effects (78). The
differential expression of the miRNA transcriptome between normal
and malignant tissues may be the key feature for miRNA utilization
as radioprotectors. In the present systematic review and
meta-analysis, miRNAs (miRNA-132-5p, miRNA-1-3p, miRNA-410,
miRNA-221, miRNA-23a-3p, miRNA-197-3p, miRNA-151a-5p, miRNA-18b-5p,
miRNA-99a and miRNA-21) are listed, which are potential candidates
for panels of radiotoxicity prediction. It is important to
determine which miRNA molecule is the best candidate to be
evaluated from a particular sample type (liquid biopsy, blood,
serum, plasma, lymphocytes, exosomes or tissue specimens), and if a
miRNA is associated with RT-induced toxicities. The next step to
verify specificity and sensitivity of these miRNAs as biomarkers is
to conduct extensive validation studies.
According to the present systematic review, miR-21,
miR-99a, miR-221, miR-18b, miR-132-5p, miR-197-3p, miR-23a-3p and
miR-410, miRNA 1-3p and miRNA-151a-5p are radiosensitive, and
directly involved in inflammation, fibrosis and apoptosis of the GI
and GU tract. Therefore, they might be utilised in the future for
prediction and modulation of the radiation response of individual
patients to increase the quality of life of patients with PCa. The
meta-analysis identified that miRNA-21 and miRNA-125b were
significant PCa-associated miRNAs differentially expressed in
RT-induced toxicity in PCa. However, further extensive validation
is required to determine the association between miRNA expression
levels and RT-induced toxicity in PCa and to prove their predictive
value.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JS, NP, TT, GS and SSS contributed to the
conception, design of the study and critically revised the
manuscript. JS, NP, DUA, SOY, KDM, MSE and SM prepared the
materials, collected the data and performed the analysis. JS, TT,
DUA, SOY, GS, MSE and KDM critically revised the the manuscript.
SSS, NP, TT, and SM confirm the authenticity of all the raw data.
All authors revised the manuscript. SSS supervised the over all
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
JS ORCID, 0000-0002-0457-2650.
References
|
1
|
Mattiuzzi C and Lippi G: Current cancer
epidemiology. J Epidemiol Glob Health. 9:217–222. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ferlay JEM, Lam F, Colombet M, Mery L,
Piñeros M, Znaor A, Soerjomataram I and Bray F: Global cancer
observatory: Cancer today. Lyon, France. International Agency for
Research on Cancer, 2020.
|
|
3
|
Nichol AM, Warde P and Bristow RG: Optimal
treatment of intermediate-risk prostate carcinoma with
radiotherapy: Clinical and translational issues. Cancer.
104:891–905. 2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Langhe S, De Ruyck K, Ost P, Fonteyne
V, Werbrouck J, De Meerleer G, De Neve W and Thierens H: Acute
radiation-induced nocturia in prostate cancer patients is
associated with pretreatment symptoms, radical prostatectomy, and
genetic markers in the TGFβ1 gene. Int J Radiat Oncol Biol Phys.
85:393–399. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Redmond KJ, Robertson S, Lo SS, Soltys SG,
Ryu S, McNutt T, Chao ST, Yamada Y, Ghia A, Chang EL, et al:
Consensus contouring guidelines for postoperative stereotactic body
radiation therapy for metastatic solid tumor malignancies to the
spine. Int J Radiat Oncol Biol Phys. 97:64–74. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Baskar R, Lee KA, Yeo R and Yeoh KW:
Cancer and radiation therapy: Current advances and future
directions. Int J Med Sci. 9:193–199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Furst CJ: Radiotherapy for cancer. Quality
of life. Acta Oncol. 35 (Suppl 7):S141–S148. 1996.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Berkey FJ: Managing the adverse effects of
radiation therapy. Am Fam Physician. 82:381–388, 394.
2010.PubMed/NCBI
|
|
9
|
Jereczek-Fossa BA, Zerini D, Fodor C,
Santoro L, Serafini F, Cambria R, Vavassori A, Cattani F, Garibaldi
C, Gherardi F, et al: Correlation between acute and late toxicity
in 973 prostate cancer patients treated with three-dimensional
conformal external beam radiotherapy. Int J Radiat Oncol Biol Phys.
78:26–34. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zelefsky MJ, Levin EJ, Hunt M, Yamada Y,
Shippy AM, Jackson A and Amols HI: Incidence of late rectal and
urinary toxicities after three-dimensional conformal radiotherapy
and intensity-modulated radiotherapy for localized prostate cancer.
Int J Radiat Oncol Biol Phys. 70:1124–1129. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ohri N, Dicker AP and Showalter TN: Late
toxicity rates following definitive radiotherapy for prostate
cancer. Can J Urol. 19:6373–6380. 2012.PubMed/NCBI
|
|
12
|
Christensen E, Pintilie M, Evans KR,
Lenarduzzi M, Ménard C, Catton CN, Diamandis EP and Bristow RG:
Longitudinal cytokine expression during IMRT for prostate cancer
and acute treatment toxicity. Clin Cancer Res. 15:5576–5583.
2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Purkayastha A, Sharma N, Sarin A,
Bhatnagar S, Chakravarty N, Mukundan H, Suhag V and Singh S:
Radiation fibrosis syndrome: The evergreen menace of radiation
therapy. Asia Pac J Oncol Nurs. 6:238–245. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schaake W, Wiegman EM, de Groot M, van der
Laan HP, van der Schans CP, van den Bergh AC and Langendijk JA: The
impact of gastrointestinal and genitourinary toxicity on health
related quality of life among irradiated prostate cancer patients.
Radiother Oncol. 110:284–290. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singh J, Sohal SS, Ahuja K, Lim A, Duncan
H, Thachil T and De Ieso P: Investigation of circulatory cytokines
in patients undergoing intensity-modulated radiotherapy (IMRT) for
adenocarcinoma of the prostate and association with acute
RT-induced toxicity: A prospective clinical study. Cytokine.
131(155108)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Xu T, Liao Z, O'Reilly MS, Levy LB, Welsh
JW, Wang LE, Lin SH, Komaki R, Liu Z, Wei Q and Gomez DR: Serum
inflammatory miRNAs predict radiation esophagitis in patients
receiving definitive radiochemotherapy for non-small cell lung
cancer. Radiother Oncol. 113:379–384. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Isomura M, Oya N, Tachiiri S, Kaneyasu Y,
Nishimura Y, Akimoto T, Hareyama M, Sugita T, Mitsuhashi N,
Yamashita T, et al: IL12RB2 and ABCA1 genes are associated with
susceptibility to radiation dermatitis. Clin Cancer Res.
14:6683–6689. 2008.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu H and Gatti RA: MicroRNAs: new players
in the DNA damage response. J Mol Cell Biol. 3:151–158.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Bueno MJ, Pérez de Castro I and Malumbres
M: Control of cell proliferation pathways by microRNAs. Cell Cycle.
7:3143–3148. 2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Xu P, Vernooy SY, Guo M and Hay BA: The
Drosophila microRNA Mir-14 suppresses cell death and is required
for normal fat metabolism. Curr Biol. 13:790–795. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Croce CM: Causes and consequences of
microRNA dysregulation in cancer. Nat Rev Genet. 10:704–714.
2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Egidi MG, Cochetti G, Serva MR, Guelfi G,
Zampini D, Mechelli L and Mearini E: Circulating microRNAs and
kallikreins before and after radical prostatectomy: Are they really
prostate cancer markers? Biomed Res Int.
2013(241780)2013.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Matsuzaki J and Ochiya T: Circulating
microRNAs and extracellular vesicles as potential cancer
biomarkers: A systematic review. Int J Clin Oncol. 22:413–420.
2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Song CJ, Chen H, Chen LZ, Ru GM, Guo JJ
and Ding QN: The potential of microRNAs as human prostate cancer
biomarkers: A meta-analysis of related studies. J Cell Biochem.
119:2763–2786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Weber JA, Baxter DH, Zhang S, Huang DY,
Huang KH, Lee MJ, Galas DJ and Wang K: The microRNA spectrum in 12
body fluids. Clin Chem. 56:1733–1741. 2010.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kopcalic K, Petrovic N, Stanojkovic TP,
Stankovic V, Bukumiric Z, Roganovic J, Malisic E and Nikitovic M:
Association between miR-21/146a/155 level changes and acute
genitourinary radiotoxicity in prostate cancer patients: A pilot
study. Pathol Res Pract. 215:626–631. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Bahtiyar N, Onaran İ, Aydemir B, Baykara
O, Toplan S, Agaoglu FY and Akyolcu MC: Monitoring of platelet
function parameters and microRNA expression levels in patients with
prostate cancer treated with volumetric modulated arc radiotherapy.
Oncol Lett. 16:4745–4753. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Malla B, Aebersold DM and Dal Pra A:
Protocol for serum exosomal miRNAs analysis in prostate cancer
patients treated with radiotherapy. J Transl Med.
16(223)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Someya M, Hori M, Gocho T, Nakata K,
Tsuchiya T, Kitagawa M, Hasegawa T, Fukushima Y and Sakata KI:
Prediction of acute gastrointestinal and genitourinary radiation
toxicity in prostate cancer patients using lymphocyte microRNA. Jpn
J Clin Oncol. 48:167–174. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Someya M, Yamamoto H, Nojima M, Hori M,
Tateoka K, Nakata K, Takagi M, Saito M, Hirokawa N, Tokino T and
Sakata K: Relation between Ku80 and microRNA-99a expression and
late rectal bleeding after radiotherapy for prostate cancer.
Radiother Oncol. 115:235–239. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Rana P, Ghosh P, Anscher MS, Mikkelsen RB
and Yakovlev VA: Abstract 1802: Exosomal miRNA as a non-invasive
prediction marker of normal tissue toxicity after radiotherapy for
prostate cancer. Cancer Res. 79 (13 Suppl)(S1802)2019.
|
|
32
|
Metheetrairut C and Slack FJ: MicroRNAs in
the ionizing radiation response and in radiotherapy. Curr Opin
Genet Dev. 23:12–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cellini F, Morganti AG, Genovesi D,
Silvestris N and Valentini V: Role of microRNA in response to
ionizing radiations: Evidences and potential impact on clinical
practice for radiotherapy. Molecules. 19:5379–5401. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Konoshenko MY, Bryzgunova OE and Laktionov
PP: miRNAs and radiotherapy response in prostate cancer. Andrology.
9:529–545. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Singh VK and Pollard HB: Ionizing
radiation-induced altered microRNA expression as biomarkers for
assessing acute radiation injury. Expert Rev Mol Diagn. 17:871–874.
2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Oliveira MA: BJCVS/RBCCV and endnote. Rev
Bras Cir Cardiovasc. 30(127)2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Whiting P, Rutjes AWS, Reitsma JB, Bossuyt
PMM and Kleijnen J: The development of QUADAS: A tool for the
quality assessment of studies of diagnostic accuracy included in
systematic reviews. BMC Med Res Methodol. 3(25)2003.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Cooper H: Research synthesis and
meta-analysis: A step-by-step approach. Vol. 2. Sage publications,
2015.
|
|
39
|
Dettori JR, Norvell DC and Chapman JR:
Fixed-effect vs random-effects models for meta-analysis: 3 Points
to consider. Global Spine J. 12:1624–1626. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cohen J: Statistical power analysis for
the behavioral sciences. 2nd edition. Hillsdale, NJ: Lawrence
Erlbaum Associates, 1988.
|
|
41
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Zedan AH, Hansen TF, Assenholt J, Madsen
JS and Osther PJS: Circulating miRNAs in localized/locally advanced
prostate cancer patients after radical prostatectomy and
radiotherapy. Prostate. 79:425–432. 2019.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Weg ES, Pei X, Kollmeier MA, McBride SM
and Zelefsky MJ: Dose-escalated intensity modulated radiation
therapy for prostate cancer: 15-Year outcomes data. Adv Radiat
Oncol. 4:492–499. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Barnett GC, West CML, Dunning AM, Elliott
RM, Coles CE, Pharoah PDP and Burnet NG: Normal tissue reactions to
radiotherapy: Towards tailoring treatment dose by genotype. Nat Rev
Cancer. 9:134–142. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Barker HE, Paget JT, Khan AA and
Harrington KJ: The tumour microenvironment after radiotherapy:
Mechanisms of resistance and recurrence. Nat Rev Cancer.
15:409–425. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Kim JH, Jenrow KA and Brown SL: Mechanisms
of radiation-induced normal tissue toxicity and implications for
future clinical trials. Radiat Oncol J. 32:103–115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Staedel C, Tran TPA, Giraud J, Darfeuille
F, Di Giorgio A, Tourasse NJ, Salin F, Uriac P and Duca M:
Modulation of oncogenic miRNA biogenesis using functionalized
polyamines. Sci Rep. 8(1667)2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Di Giorgio A, Tran TPA and Duca M:
Small-molecule approaches toward the targeting of oncogenic miRNAs:
Roadmap for the discovery of RNA modulators. Future Med Chem.
8:803–816. 2016.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Iorio MV and Croce CM: microRNA
involvement in human cancer. Carcinogenesis. 33:1126–1133.
2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Gu LQ, Wanunu M, Wang MX, McReynolds L and
Wang Y: Detection of miRNAs with a nanopore single-molecule
counter. Expert Rev Mol Diagn. 12:573–584. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Rothschild SI: microRNA therapies in
cancer. Mol Cell Ther. 2(7)2014.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Balázs K, Antal L, Sáfrány G and Lumniczky
K: Blood-derived biomarkers of diagnosis, prognosis and therapy
response in prostate cancer patients. J Pers Med.
11(296)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Beckendorf V, Guerif S, Le Prisé E, Cosset
JM, Bougnoux A, Chauvet B, Salem N, Chapet O, Bourdain S, Bachaud
JM, et al: 70 Gy versus 80 Gy in localized prostate cancer: 5-Year
results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys.
80:1056–1063. 2011.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dearnaley DP, Sydes MR, Graham JD, Aird
EG, Bottomley D, Cowan RA, Huddart RA, Jose CC, Matthews JH, Millar
J, et al: Escalated-dose versus standard-dose conformal
radiotherapy in prostate cancer: First results from the MRC RT01
randomised controlled trial. Lancet Oncol. 8:475–487.
2007.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Michalski JM, Gay H, Jackson A, Tucker SL
and Deasy JO: Radiation dose-volume effects in radiation-induced
rectal injury. Int J Radiat Oncol Biol Phys. 76 (3
Suppl):S123–S129. 2010.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Chaudhry MA, Omaruddin RA, Brumbaugh CD,
Tariq MA and Pourmand N: Identification of radiation-induced
microRNA transcriptome by next-generation massively parallel
sequencing. J Radiat Res. 54:808–822. 2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Korpela E, Vesprini D and Liu SK: MicroRNA
in radiotherapy: miRage or miRador? Br J Cancer. 112:777–782.
2015.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Xu S, Ding N, Pei H, Hu W, Wei W, Zhang X,
Zhou G and Wang J: MiR-21 is involved in radiation-induced
bystander effects. RNA Biol. 11:1161–1170. 2014.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Stepanović A, Nikitović M, Stanojković TP,
Grujičić D, Bukumirić Z, Srbljak I, Ilić R, Milošević S,
Arsenijević T and Petrović N: Association between microRNAs
10b/21/34a and acute toxicity in glioblastoma patients treated with
radiotherapy and temozolomide. Sci Rep. 12(7505)2022.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Schwarzenbach H, Nishida N, Calin GA and
Pantel K: Clinical relevance of circulating cell-free microRNAs in
cancer. Nat Rev Clin Oncol. 11:145–156. 2014.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mitchell PS, Parkin RK, Kroh EM, Fritz BR,
Wyman SK, Pogosova-Agadjanyan EL, Peterson A, Noteboom J, O'Briant
KC, Allen A, et al: Circulating microRNAs as stable blood-based
markers for cancer detection. Proc Natl Acad Sci USA.
105:10513–10518. 2008.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Kabacik S, Manning G, Raffy C, Bouffler S
and Badie C: Time, dose and ataxia telangiectasia mutated (ATM)
status dependency of coding and noncoding RNA expression after
ionizing radiation exposure. Radiat Res. 183:325–337.
2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kerns SL, Fachal L, Dorling L, Barnett GC,
Baran A, Peterson DR, Hollenberg M, Hao K, Narzo AD, Ahsen ME, et
al: Radiogenomics consortium genome-wide association study
meta-analysis of late toxicity after prostate cancer radiotherapy.
J Natl Cancer Inst. 112:179–190. 2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Buscaglia LEB and Li Y: Apoptosis and the
target genes of microRNA-21. Chin J Cancer. 30:371–380.
2011.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Chen C, Zhao Z, Liu Y and Mu D:
microRNA-99a is downregulated and promotes proliferation, migration
and invasion in non-small cell lung cancer A549 and H1299 cells.
Oncol Lett. 9:1128–1134. 2015.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang Q, Song LR, Huo XL, Wang L, Zhang
GB, Hao SY, Jia HW, Kong CL, Jia W, Wu Z, et al: MicroRNA-221/222
inhibits the radiation-induced invasiveness and promotes the
radiosensitivity of malignant meningioma cells. Front Oncol.
10(1441)2020.PubMed/NCBI View Article : Google Scholar
|
|
67
|
John-Aryankalayil M, Palayoor ST, Makinde
AY, Cerna D, Simone CB II, Falduto MT, Magnuson SR and Coleman CN:
Fractionated radiation alters oncomir and tumor suppressor miRNAs
in human prostate cancer cells. Radiat Res. 178:105–117.
2012.PubMed/NCBI View Article : Google Scholar
|
|
68
|
O'Reilly S: MicroRNAs in fibrosis:
Opportunities and challenges. Arthritis Res Ther.
18(11)2016.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Cabral BCA, Hoffmann L, Bottaro T, Costa
PF, Ramos ALA, Coelho HSM, Villela-Nogueira CA, Ürményi TP, Faffe
DS and Silva R: Circulating microRNAs associated with liver
fibrosis in chronic hepatitis C patients. Biochem Biophys Rep.
24(100814)2020.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Sheng S, Zou M, Yang Y, Guan M, Ren S,
Wang X, Wang L and Xue Y: miR-23a-3p regulates the inflammatory
response and fibrosis in diabetic kidney disease by targeting early
growth response 1. In Vitro Cell Dev Biol Anim. 57:763–774.
2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang Y, Xu N, Zhao S, Jiao T, Fu W, Yang L
and Zhang N: miR-410-3p suppresses cytokine release from
fibroblast-like synoviocytes by regulating NF-κB signaling in
rheumatoid arthritis. Inflammation. 42:331–341. 2019.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Wagner RH, Boles MA and Henkin RE:
Treatment of radiation exposure and contamination. Radiographics.
14:387–396. 1994.PubMed/NCBI View Article : Google Scholar
|
|
73
|
McBride WH and Schaue D: Radiation-induced
tissue damage and response. J Pathol. 250:647–655. 2020.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chiba M: Radiation-responsive
transcriptome analysis in human lymphoid cells. Radiat Prot
Dosimetry. 152:164–167. 2012.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Mittelbrunn M and Sánchez-Madrid F:
Intercellular communication: Diverse structures for exchange of
genetic information. Nat Rev Mol Cell Biol. 13:328–335.
2012.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Moreno-Villanueva M, Zhang Y, Feiveson A,
Mistretta B, Pan Y, Chatterjee S, Wu W, Clanton R, Nelman-Gonzalez
M, Krieger S, et al: Single-Cell RNA-sequencing identifies
activation of TP53 and STAT1 pathways in human T lymphocyte
subpopulations in response to ex vivo radiation exposure. Int J Mol
Sci. 20(2316)2019.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Zhang J, Li S, Li L, Li M, Guo C, Yao J
and Mi S: Exosome and exosomal microRNA: Trafficking, sorting, and
function. Genomics Proteomics Bioinformatics. 13:17–24.
2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Petrović N, Stanojković TP and Nikitović
M: MicroRNAs in prostate cancer following radiotherapy: Towards
predicting response to radiation treatment. Curr Med Chem.
29:1543–1560. 2022.PubMed/NCBI View Article : Google Scholar
|