Introduction
Non-alcoholic fatty liver disease (NAFLD) is a type
of liver disease that is becoming increasingly common due to the
global obesity epidemic (1). It is
estimated that approximately one-third of the world's population
suffers from this condition (2).
Typically, NAFLD is associated with obesity, but it also affects
non-obese individuals (3).
Notably, NAFLD is often linked to increased mortality rates in
individuals with other health conditions, such as cardiovascular
complications, type 2 diabetes, chronic kidney disease,
hypothyroidism, polycystic ovarian syndrome and psoriasis (4,5).
Understanding its pathogenesis is crucial for
developing effective treatment strategies. Of note, two theories,
namely the ‘two-hit’ (6) and
‘multiple parallel hit’ (7), have
been proposed to explain the development of NAFLD/NASH. The two-hit
theory suggests that the accumulation of fat in the liver (hepatic
steatosis) occurs initially, followed by NASH due to subsequent
‘second hits’. On the other hand, the multiple parallel hit theory
suggests that the development of steatosis and inflammation occurs
simultaneously due to various risk factors such as obesity, insulin
resistance, and dyslipidemia. Both theories provide insight into
the intricate pathogenesis of NAFLD, which involves the interaction
between hepatic fat, inflammation, oxidative stress and insulin
resistance (7).
An imbalance in the accumulation and clearance of
fat in the liver due to overnutrition causes hepatic steatosis
(Fig. 1). This condition is
closely related to insulin resistance, which is commonly observed
in obese individuals and affects nutrient metabolism and tissue
nutrient distribution (8). In
cases of peripheral insulin resistance, the liver experiences an
inflow of free fatty acids, which leads to the accumulation of fat
in the liver in the form of triglycerides. This process is often
accompanied by increased levels of lipotoxicity resulting from high
levels of free fatty acids, free cholesterol and other lipid
metabolites. Consequently, the liver experiences mitochondrial
dysfunction, oxidative stress, the production of reactive oxygen
species (ROS) and endoplasmic reticulum (ER) stress-associated
mechanisms (9) This triggers the
activation of the pro-inflammatory transcription factor, nuclear
factor-κB (NF-κB), playing a pivotal role in the regulation of
pro-inflammatory cytokines, such as interleukin (IL)-1β, IL-6 and
tumor necrosis factor-α (TNF-α) in NAFLD (10), causing both liver damage and an
increase in the numbers of inflammatory cells (11). All chronic liver diseases progress
over a long period of time, with severe liver disease being more
common among older populations (2). Currently, the most reliable method
used for the diagnosis of NAFLD is the through histopathological
assessment of a liver biopsy (12).
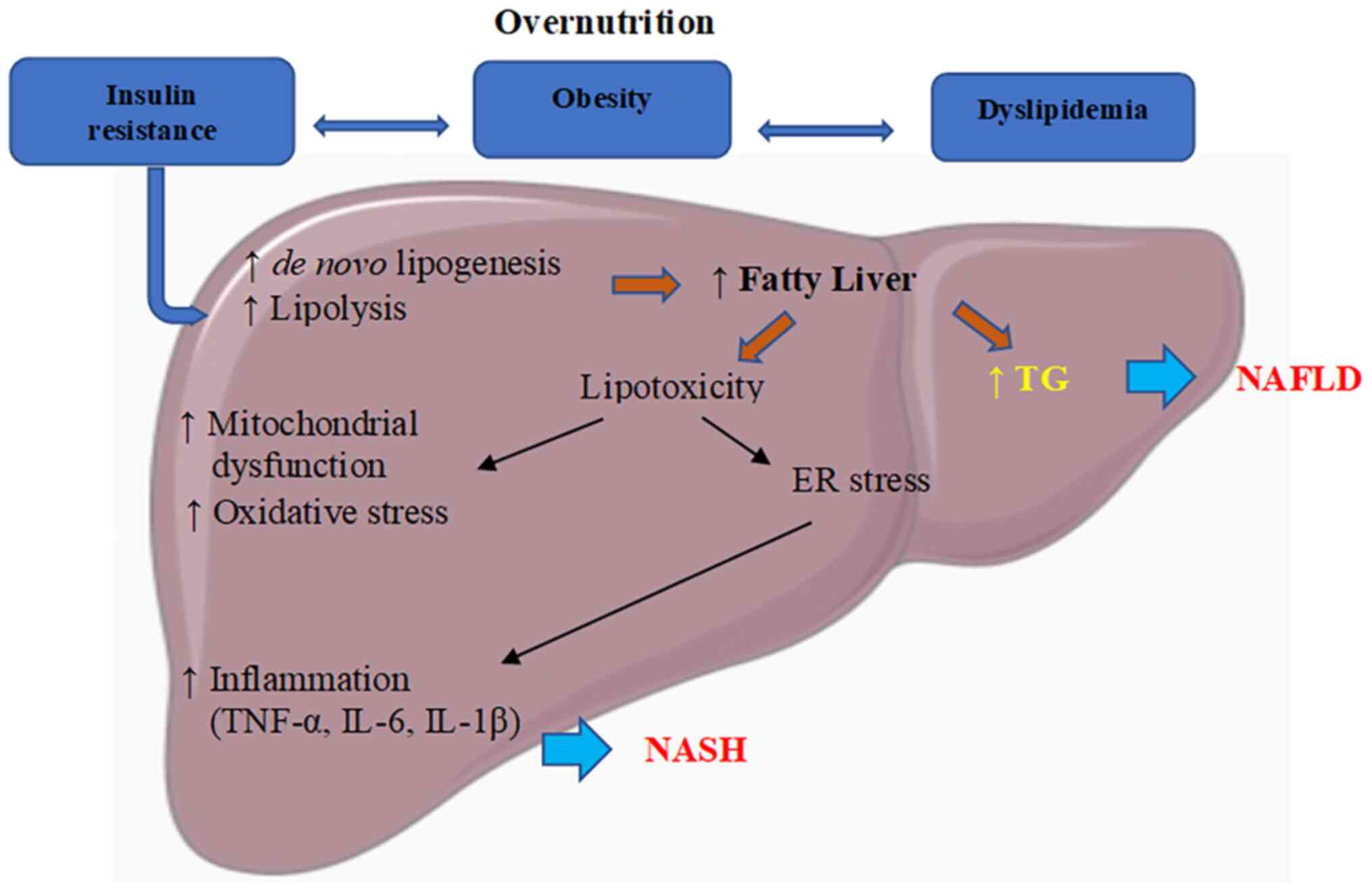 | Figure 1Pathophysiology of NAFLD.
Overnutrition causes dyslipidemia, obesity, and IR. IR activates
de novo lipogenesis and lipolysis, resulting in free fatty
acid and triglyceride accumulation in the liver, leading to the
development of NAFLD. Free fatty acids induce lipotoxicity,
activate mitochondrial dysfunction, and oxidative and ER stress.
Subsequently, this induces inflammatory signaling and stimulates
the production of TNF-α, IL-6 and IL-1β, causing NASH. ER,
endoplasmic reticulum; NAFLD, non-alcoholic fatty liver disease;
IR, insulin resistance; TNF, tumor necrosis factor; IL,
interleukin; TG, triglycerides. |
The management of patients with NAFLD involves
lifestyle changes, such as weight loss through diet and exercise.
Recently, the US Food and Drug Administration approved Rezdiffra
(resmetirom) for NASH treatment (13). In addition, lipid-lowering
medications, insulin sensitizers and antioxidants have been used
for treatment (14). Herbal
remedies have been used as an alternative to the current
medications.
Herbs have been used in traditional medicine for the
treatment of various diseases since ancient times. Some herbs, such
as milk thistle or Silybum marianum, have been evaluated in
clinical trials to assess their efficacy as a treatment for NAFLD.
Silymarin, which is extracted from milk thistle and contains a
mixture of flavonolignans, is the most extensively studied plant
for liver disease (15). In a
previous meta-analysis of eight randomized clinical trials of 622
patients (16), silymarin was
shown to reduce fasting blood glucose levels, insulin resistance,
and triglyceride, alanine aminotransferase (ALT) and aspartate
aminotransferase (AST) levels (Table
I).
 | Table IClinical trial data for silymarin,
berberine and resveratrol. |
Table I
Clinical trial data for silymarin,
berberine and resveratrol.
| No. | Phytochemical | Glucose | Insulin | Lipids | Liver biochemical
properties | Histopathological
scores | (Refs.) |
|---|
| 1 | Silymarin | FBG↓ HbA1c↓ | Insulin↓ IR↓
HOMA-IR↓ | TG↓ TC and HDL (no
effect) | ALT & AST↓ (no
clinical relevance) | No data | (16,22) |
| 2 | Berberine | FBG↓ | HOMA-IR↓ | TG↓ TC↓ LDL↓
HDL↓ | ALT & AST (No
changes) | No data | (17,23) |
| 3 | Resveratrol | FBG↓ | Insulin &
HOMA-IR (no changes) | TC↓ LDL↑ HDL (no
effect) | ALT & AST (no
changes) | Fibrosis ↑, NAFLD
activity score↓ | (21,24,25) |
Berberine, an isoquinoline alkaloid isolated from
the traditional Chinese medicinal herb, Coptis chinensis,
has also been studied in numerous clinical trials. In 18 randomized
clinical trial results selected from 1,660 studies related to
berberine (17), berberine alone
in patients with metabolic disorders was shown to lower lipid and
sugar levels, and to ameliorate insulin resistance (Table I). This effect becomes evident with
a treatment time >3 months. The lipid-lowering effect of
berberine was used as an alternative treatment for patients who do
not tolerate statins (18).
Statins are widely used as a lipid-lowering drug; however, they can
cause side-effects such as high blood glucose levels and cannot be
used by diabetic patients (19).
Other side-effects included memory and cognitive impairment, which
can cause unusual swelling in the neurons of patients taking
statins (20).
Resveratrol, a polyphenol found in a variety of
plant species, including grapes, peanuts and berries, has been
evaluated in some clinical trials; however, the results obtained
were rather mixed from the four randomized, double-blinded,
placebo-controlled trials involving 156 patients (21). Although some positive effects of
resveratrol were observed on metabolic parameters, the improvement
in liver function and fatty liver for silymarin (22), berberine (23) and resveratrol (24,25)
was less apparent than was expected (Table I). Given the controversial results,
larger scale and well-designed population-based clinical studies
are recommended to fully elucidate the efficacy of resveratrol.
Although a number of herbs that are traditionally
used for the treatment of liver diseases have not undergone
clinical trials, they are still used to treat diseases. Since the
development of NAFLD takes a considerable amount of time, the
long-term consumption of herbs may provide an alternative treatment
strategy with which to prevent NAFLD. The present systematic review
aimed to identify plants used in the management of NAFLD, and to
determine their mechanisms of action and obtain data on their
safety.
Data and methods
Search strategy
In order to explore the potential use of natural
medicinal plants and plant extracts for the treatment of NAFLD,
NASH and metabolic-associated fatty liver disease, a comprehensive
search was conducted using relevant key words, such as ‘medicinal
plants’, ‘plant extracts’, ‘non-alcoholic fatty liver disease’ and
‘non-alcoholic steatohepatitis’. The search was performed across
various databases, such as Scopus, PubMed, Springer, NCBI, Google
Scholar, ScienceDirect and Web of Science. For this search, studies
conducted between January, 2016 and November, 2023 were considered,
with a focus on in vivo studies that evaluated the
effectiveness of natural medicinal plants for the treatment of
NAFLD. The mechanisms of action were supported by either in
vitro or in vivo models.
Study selection
The present systematic review was conducted to
explore the therapeutic potential of medicinal plants and herbal
medicine in the treatment of NAFLD and steatohepatitis. The records
for the review were collected from various scientific databases,
such as Scopus, PubMed, Springer, NCBI, Google Scholar, Science
Direct and Web of Science. The search was conducted using key
words, such as ‘fatty liver’, ‘NAFLD’, ‘plants’, ‘medicinal’,
‘herbal medicine’ and ‘therapeutic uses’, and the data were limited
to the period from 2016 to 2023. Inclusion criteria for the study
were articles written in the English language, basic research
studies and non-clinical studies. Conference abstracts, theses,
case reports, reviews, commentaries and editorials were excluded.
The author NEJ extracted the data, and both NEJ and SMJ
independently screened all the retrieved abstracts using the
inclusion and exclusion criteria. Any disagreements regarding
inclusion were resolved through extensive discussion with the other
two authors (RMS and CYC). Articles without liver histopathological
assessment or positive drug and single-dose studies were excluded
from the screening process.
Data extraction
After carefully applying the inclusion and exclusion
criteria, a total of 55 articles were deemed relevant and selected
for further analysis (as illustrated in Fig. 2). The information extracted from
these articles was then organized and tabulated in an Excel
spreadsheet. The key findings were subsequently summarized in three
tables as follows: One detailing the traditional uses of the plants
under investigation (Table II),
the animal dietary model (Table
III) and the other presenting the effects of these plants on
NAFLD (Table IV).
 | Table IITraditional usage of herbs. |
Table II
Traditional usage of herbs.
| No. | Plants | Family | Location | Part(s) used | Preparation | Traditional
usage | (Refs.) |
|---|
| 1 | Abroma
augusta L. | Sterculiaceae | India | Root, leaf | Infusions | Diabetes,
amenorrhea, dysmenorrhea, urinary system, nourish the liver | (28,29) |
| 2 | Antidesma
bunius | Euphorbiaceae | Bangladesh | Leaves, fruits,
bark, roots seeds | Decoction
Juices | Cough, stomachache,
hepatoprotective | (30) |
| 3 | Aralia
elata | Araliaceae | China | Root, stem bark,
leaves | Decoction | Joint pain,
bruises,lumps, abscess, hepatitis | (31,32) |
| 4 | Cassia
obtusifolia | Leguminosae | Korea | Seeds | Pounded seeds | Diuretics,
laxatives, tonics, dizziness, nourish the liver, constipation | (33,34) |
| 5 | Citrus
aurantium | Rutaceae | China | Peel | Decoction | Laxatives,
stomachic, emmenagogue, and dyspepsia, liver tonic | (35,36) |
| 6 | Curcuma
longa Linn. | Zingiberaceae | India | Roots | Decoction Pounded
roots | Asthma, liver
disorders, anorexia, rheumatism, diabetic wounds, sinusitis | (37) |
| 7 | Crocus
sativus L. | Iridaceae | Turkey | Flower stigma | Decoction | Insomnia, head,
heart, asthma, menstrual conditions, liver disease | (38,39) |
| 8 | Cyclosorus
terminans |
Thelypteridaceae | Thailand | Leaves, trunk | Decoction | Cough, burn,
malaria, edema, inflammation, and external bleeding, liver
damage | (40,41) |
| 9 | Glossogyne
tenuifolia | Asteraceae | Taiwan | Whole plant | Decoction | Acute tonsillitis,
bronchitis, diarrhea, urinary tract infection, antipyretic,
anti-inflammatory, hepatoprotective | (42,43) |
| 10 | Hibiscus
sabdariffa L. | Malvaceae | Africa | Leaves,
calyces | Infusions | Diuretic,
hypertension, pyrexia, and liver damage. | (44,45) |
| 11 | Moringa
oleifera | Moringaceae | Arabian | Whole plant | Decoction | Fever, headache,
constipation, labor pain, liver disease | (46,47) |
| 12 | Morus
latifolia | Moraceae | China | Leaves | Decoction | Coughing up
catarrh, fever, dizziness, vertigo, diabetes, liver diseases, blood
pressure | (48,49) |
| 13 | Panax
notoginseng | Araliaceae | China | Roots | Pounded roots | cardiovascular,
pain, inflammation, hepatitis, and liver cancer | (50,51) |
| 14 | Phyllanthus
emblica | Phyllanthaceae | India | Fruit | Juices | cold and fever,
liver tonic, ulcer and dyspepsia | (52) |
| 15 | Picrorhiza
kurroa | | India | Leaves | Infusions | Liver and upper
respiratory tract, fever, dyspepsia, diarrhea | (53) |
| 16 | Pimpinella
anisum L. | Apiaceae | Italy | Seeds | Pounded seeds | Diuretic, mild
expectorant, antifungal, antibacterial, liver disorders | (54,55) |
| 17 | Pluchea
indica | Asteraceae | Indonesia | Leaves | Infusions | Antipyretic,
diarrhea, antitussive, nourish the liver | (56,57) |
| 18 | Rosmarinus
offcinalis L. | Lamiaceae | Italy | Leaves | Pounded leaves | Headache,
dysmenorrhea, epilepsy, rheumatic pain, spasms, nourish the
liver | (58,59) |
| 19 | Rubus ideaus
L. | Rosaceae | Europe | Leaves, Fruit | Decoction | Stomatitis, sore
throats, coughs, tonsillitis, fevers, nourish the liver | (60,61) |
| 20 | Trigonella
foenum- graecum L. | Fabaceae | India | Seeds | Pounded seeds |
Anti-cholesterolemic, anti-tumor,
anti-inflammatory, expectorant, hypoglycemic, nourish the
liver | (62) |
 | Table IIIStages of NAFLD in animal dietary
models. |
Table III
Stages of NAFLD in animal dietary
models.
| No. | Model | IR | Obese | Steatosis | NASH | Fibrosis | HCC | (Refs.) |
|---|
| 1. | High-fat diet
(HFD) | Yes >10
weeks | Yes >10
weeks | Yes | Yes >12
weeks | Yes (minimal) 36-50
weeks | Yes 1 year | (70) |
| 2. | Methionine and
choline-deficient diet (MCD) | No | No | Yes | Yes 2-8 weeks | Yes 8-10 weeks | No | (71-73) |
| 3. | Choline deficient
L-amino acid-defined HFD | No | No | Yes | Yes (6-9
weeks) | Yes (6-9
weeks) | Yes | (74) |
| 4. | STZ + HFD | Yes | Yes | Yes 6 weeks | Yes 8 weeks | Yes 8-12 weeks | Yes>20
weeks | (75) |
| 5. | CCl4 +
HFD | Yes | Yes | Yes | Yes >4
weeks | Yes | - | (76) |
| 6. | High fructose
diet | Yes >8
weeks | Yes >8
weeks | Yes >8
weeks | No | - | - | (77) |
| 7. | High fat-high
fructose diet (HFHFD)/high-sugar and sigh-fat diet (HSHFD) | Yes >8
weeks | Yes >8
weeks | Yes | Yes >16
weeks | - | - | (77,78) |
 | Table IVHerbal plants and their mechanism of
actions in NAFLD. |
Table IV
Herbal plants and their mechanism of
actions in NAFLD.
| | Mechanism of
Actions |
|---|
| No. | Plants | Chemical
constituents | Subject | Dose tested | Effective dose
(mg/kg) | Toxicity study | Lipid
Metabolism | Insulin Resistance
(IR) | Inflammatory
Markers | Oxidative Stress
Markers | Other | (Refs.) |
|---|
| 1 | Abroma augusta
L. | Leaf ethanol
extract contains alkaloids, tannins, phenols and flavonoids. | Male Sprague-Dawley
(SD) rats on MCD, HFD, CCD, STZ + HFD. | 250 and 500 mg/kg,
orally, 24 weeks. Positive control (PC): Silymarin (100 mg/kg) | Ethanolic extract
at 500 mg/kg | Does not show any
sign of toxicity up to 2,000 mg/kg | ↓TC, ↓TG, ↓LDL,
↓HDL, ↓FFA, | ↓IR | - | ↓MDA ↑SOD | ↓Steatosis | (28,81) |
| 2. | Antidesma
bunius | Aqueous fruit
extract contain polyphenol, flavonoids, ascorbic acid, gallic acid,
(+)-catechin. | HFD, male SD
rats | 0.38, 0.76, 1.52
g/kg, oral, 12 weeks PC: Statin 10 mg/kg | 12 weeks, orally,
1.52 g/kg of extract | 500, 1,000, 1,500
and 2,000 mg/kg given orally reported non-toxic in Wistar rats | ↓GPAT-1, ↓ACC,
↓SREBP-1c ↓TG | - | ↓ TNF-a | ↓ MDA | ↓Steatosis | (82,83) |
| 3. | Aralia
elata | Aqueous extract of
roots contain flavonoid, total saponins, phenolics. | HFD, C57BL/6
mice | 100 and 300 mg/kg
for 4 weeks. PC: Resveratrol 300 mg/kg | Ethanol extract of
300 mg/kg | 5,000 mg/kg for 14
days reported non-toxic in rats | ↓TG ↓SREBP-1c ↓FAS
↑ACC1 ↓ACC2 ↑PPARα ↑CPT1 | ↓Glucose ↓Insulin
↓Akt2 ↓GLUT4 ↑PI3K | - | - | ↓Steatosis | (84-86) |
| 4. | Cassia
obtusifolia L. | Seeds ethanol
extract contain anthraquinones | HFD, male Wistar
Albino rats | 0.5, 1 and 2 g/kg,
6 weeks, oral PC-metformin 0.2 g/kg | 1 and 2 g/kg
ethanol extract | 10 g/kg for 14 days
reported non-toxic in rats | ↓TG ↓TC | - | ↓TNF-α ↓IL-6
↓IL-8 | ↑SOD ↑GSH ↓MDA | ↓MASH | (87-89) |
| 5 | Citrus
aurantium L. | Ethanol extract
peel contain flavonoids, limonoids, and alkaloids. | HFD, male C57BL/6
mice | 50 and 100 mg/kg of
ethanol, 8weeks, PC: Silymarin (200 mg/kg) | 100 mg/kg of
ethanol extract | 400, 2,000 and
4,000 mg/kg, 28 days, extract showed no signs of toxicity. | ↓TG ↓TC ↓PPARy
↓SREBP-1c ↓FAS ↑AMPK ↑NRF2 | - | ↓TNF-α ↓IL-6
↓IL-1α | - | ↓MASH | (90-93) |
| 6 | Curcuma
longa Linn. | Aqueous extracts of
C. longa roots contained curcumin. | HFD, C57BL/6
mice | 300 and 900 mg/kg,
oral 8 weeks PC: silymarin 50 mg/kg per oral | Aqueous extracts of
900 mg/kg | 250, 500, 1,000
mg/kg for 90 days did not show any signs of toxicity | ↓TG, ↓TC ↓SREBP-1c,
↓FAS, ↓ACC ↑AMPK ↑PPAR-α ↑CPT-1 | - | ↓CD36 ↓FATP5
↓FATP2 | ↑CAT ↑SOD ↑GST ↑GPx
↑GR ↑GSH ↓MDA | ↓Steatosis ↓ER
stress ↓p-mTOR ↓p-S6K ↓p-4-EBP-1 | (94-96) |
| 7 | Crocus
sativus | Aqueous C.
sativus flower stigma extract contained crocetin. | HFD, male SD
rats | 250 and 500 mg/kg,
orally, 4 weeks. PC: Standard botanical mixture 35 mg/kg | 4 weeks, 500
mg/kg | 1 g/kg, 14 days,
showed no mortality or any signs of toxicity. | ↓TG, ↓TC, ↓ LDL, ↓
VLDL, ↑ HDL | ↓Glucose
↓Insulin | ↓TNF-α | ↑CAT ↑SOD ↑GST ↑GPx
↑GSH ↓MDA AOPPs ↓NO2 | ↓MASH ↓Uric
acid | (97-99) |
| 8 | Cyclosorus
terminans | Aerial parts of
n-hexane extract contained coumarin, furanocou-marins, and
dioxocane | HFD, male Wistar
rats | 100 and 200 mg/kg,
oral, 2 weeks. PC: pioglitazone 20 mg/kg | 200 mg/kg, | Acute toxicity
study of 2 g/kg showed no signs of toxicity for 14 days. | cTG,↓TC ↓LDL, ↑HDL
↓SREBP1c, ↓Fasn ↑PPARα ↑PPARg ↑CPT2 | ↓Glucose ↓Insulin
↓HOMA-IR, ↑glycogen ↑Slc2a2 ↑Pparg ↑Irs1 &2s ↑Slc2a4 | ↓TNF-α ↓IL-6 | - | ↓MASH | (100-102) |
| 9 | Glossogyne
tenuifolia | Aqueous root and
whole plant extract contained phenolics, CGA, and
luteolin-7-glucoside. | HFD, male Wistar
rats | 50 and 150 mg/kg, 4
weeks PC : 20 mg/kg acarbose. | 150 mg/kg of
aqueous extract | Chronic toxicity
study in male mice rats with 5 g/kg for 28 days showed no signs of
toxicity | ↓TC ↑HDL | ↓Insulin | ↓IL-6 ↓STAT3 ↓MEK5
↓ERK5 ↓NFATc3 ↓ANP ↓BNP ↓p-p38 ↓p-JNK, ↓FGF2, ↓p-ERK ½, ↓UPA, ↓MMP2
&9 | - | ↓Steatosis
↓Apoptosis | (80,103) |
| 10 | Hibiscus
sabdariffa | Aqueous extract
contains total phenolic, flavonoid, carotenoid, and
anthocyanin | HFD SD rats | 250, 500 mg/kg
oral, 8 weeks. PC: Simvastatin 40 mg/kg | Aqueous extract of
500 mg/kg. | Acute 2 g/kg and
oral 125, 250, 500 mg/kg for 28 days were safe doses. | ↓FAS, ↓ACC, ↓MTP,
↓LDLR, ↑IRS-1, ↑Nrt2, ↑p-Akt | - | ↓TNF-α ↓IL6, | ↑CAT, ↑SOD,
↑GPx | ↓Fibrosis
↓MASH | (104,105) |
| 11 | Moringa oleifera
Lam | Seed ethanol
extract, contains alkaloids, flavonoid, phenolic acids
sterols. | HFCS, male SD
rats | 50 and 500 mg/kg,
orally, 12 weeks. PC: Fenofibrate (100 mg/kg) | 500 mg/kg seed
ethanol extracts | 30, 100, 300 and
1,000 mg/kg, no mortality | ↓Liver lipids | - | - | - | ↓MASH | (46,106) |
| 12 | Morus
latifolia | Ethanol leaf
contained chlorogenic acid, rutin, quercetin, caffeic acid and
coumaric acid | HFCS, f Wistar
Albino rats. | 120, 250, 500
mg/kg, orally, 21 days, PC: Orlistat 120 mg/kg | 120 mg/kg of leaf
extract | Sub-chronic
toxicity of 7.5 g/kg and genotoxicity of 10 g/kg showed no
mutagenic activity. | ↓TC, ↓TG, ↓ LDL,
↓VLDL ↑ HDL | ↓Glucose | - | - | ↓Steatosis | (107,108) |
| 13 | Panax notogin-
seng | Ethanol extract
roots has ginsenoside Rb1, Rg1, Rg2, and Rh | HSHFD of SD
rats | 30 and 60 mg/kg,
oral 8weeks, PC : Simvastatin 1 mg/kg | 30 mg/kg of
ethanolic extrac | 1.2 g/kg for 28
days show no sign of toxicity. | ↓TC, ↓TG ↓ PPAR-α
↑CPT-1A ↑CPT-2 ↓SREBP-1c ↑CYP-7A | ↓Glucose
↓Insulin | ↓TNF-α ↓IL-6 ↓IL-8
↓IL-1 ↓IL-1β | - | ↓Steatosis | (109) |
| 14 | Phyllan- thus
emblica | Fruit aqueous
extract contained gallic acid, corilagin, and ellagic acid | CDAHFD of C57BL/6J
mice | 0.9, 1.8, 3.6 g/kg,
6 weeks. PC: Silymarin (84 mg/kg) | Oral admistration
of 3.6 g/kg extract for 6 weeks | 5 g/kg for 14 days
showed no mortality or any signs of toxicity. | ↑HDL-C, ↓TC,
↓LDL-C, ↓Lipid droplet | - | - | - | ↓Steatosis | (110,111) |
| 15 | Picrorhiza
kurroa | Leaves contained
iridoid glycosides. | HFD of male Wistar
rats | 200 and 400 mg/kg,
oral, 4 weeks. PC: Silymarin (50 mg/kg) | Treatment of 400
mg/kg for 4 weeks | No mortality at 2
g/kg after 14 days observation. | ↓TC, ↓TG,
↑HDL-C, | - | - | - | ↓Steatosis | (53,112) |
| 16 | Pimpinella
anisum L. | Aqueous seeds
extract contains phenolic, ellagic and syringic acids. | CDD with lard of
male Wistar Albino rats | 25, 50, 100 and 200
mg/kg, 4 weeks. PC: Simvastatin 10 mg/kg | 200 mg/kg; 4
weeks | 400 and 800 mg/kg,
3 months were safe. | ↓TG, ↓TC, ↓LDL-C,
↑HDL-C | - | - | - | ↓Steatosis | (113) |
| 17 | Pluchea
indica | Ethanolic extract
leaves contains tannic acid, rutin, quercetin, gallic acid,
isoquercetin, catechin, and apigenin. | HFFD, SD rats | 100 and 3 00 mg/kg,
oral, 6 weeks. PC: Pioglitazone 10 mg/kg | Ethanolic extract
at 300 mg/kg | Acute oral toxicity
study with up to 2,000 mg/kg for 14 days reported being reasonably
non-toxic in rats | ↓TG, ↓VLDL-C,
↓LDL-C, ↑HDL-C, ↓FFA ↓SREBP-1c ↓FAS ↑PPARα ↑CPT1 ↑ACOX1 ↓leptin
↓adipocytes | ↓Glucose ↓Insulin
↓HOMA-IR | - | - | ↓Steatosis | (114) |
| 18 | Rosmarinus
officinalis Linn | Ethanol extract
whole grass contain triterpenes, phenolic & diterpenes | HSD, male SD
rats | 100,200 and 400
mg/kg, oral, 21 days PC: Fenofibrate (50 mg/kg) | 400 mg/kg | Does not show any
mortality in rats at 2,000 mg/kg given orally | ↓TG, ↓TC, ↓FFA,
↓SREBP-1c ↓AMPK ↓FAS ↓GAPDH | - | - | - | ↓Steatosis | (115,116) |
| 19 | Rubus ideaus
L | Ethanol extract of
red raspberry fruit contained flavonoids and phenolic acids | HFD, Male Wistar
Albino rats | 200, 100 and 500
mg/kg, oral, 28 days, PC: Metformin 150 mg/kg | 200 mg/kg | Acute toxicity,
2,000 mg/kg, orally was non-toxic. | ↓TC ↑HDL ↓TG ↓LDL
↓FFA | ↓Glucose
↓Insulin | ↓TNF-a | ↓MDA ↑SOD ↑GPx
↑GSH | ↓Steatosis | (117,118) |
| 20 | Trigonella
foenum- graecum L | Aqueous extract
seeds contain galactomannan, phenolic flavonoid, and amino
acids | HFD, Wistar
rats | 0.5 and 1.0 g/kg,
28 days. PC: Orlistat 10 mg/kg | 1.0 g/kg of aqueous
extract | 2 and 5 g/kg, 90
days showed no signs of toxicity. | ↓TG, ↓TC, ↓LDL,
↑HDL, ↓VLDL, ↓AI, ↓CRI, ↓leptin ↓Adiponectin ↓FAS, ↓LDH ↓G6PD
↓Lipase, | ↓Glucose ↓ Insulin
↓ HOMA-IR ↓apo-B | - | ↑SOD ↑GSH-Px
↓MDA | ↓Steatosis | (119-121) |
Results and Discussion
Traditional usage of plants
It is noteworthy that >60% of the world's
population, particularly in developing nations, relies mainly on
medicinal plants for their healthcare needs. This renders
traditional medicine a preferred healthcare system in a number of
communities (26) due to its
affordability, accessibility and low cost (27). As demonstrated in the present
study, all the 20 herbs identified that were found to lead to a
reduction in steatosis in liver histopathological analyses were
traditionally used as herbal medicines for liver ailments, liver
tonics, or for nourishing the liver (Table II) (28-62).
This highlights the significance of traditional medicine in
promoting liver health.
Animal dietary models
NAFLD is a condition characterized by the
accumulation of excessive fat in the liver. This disease progresses
from a simple state of liver steatosis to NASH and ultimately, into
liver fibrosis, cirrhosis, and in severe cases, hepatocellular
carcinoma (HCC). A review of the pathogenesis and histopathology of
the disease in animals revealed that mice and rats are the commonly
used models for the study of NAFLD (Table III). The C57BL/6 strain in mice,
and the Wistar and Sprague-Dawley (SD) strains in rats are
frequently used due to their inherent propensity to develop
obesity, type 2 diabetes and NAFLD (63,64).
The different stages of fatty liver are induced by altering the
diet and chemicals used, including steatosis [confirmed by
increased liver triglyceride levels, hepatocyte ballooning and
Mallory bodies, also known as Mallory-Denk bodies (MDBs)], NASH,
fibrosis and HCC, which are all dependent on the induction
period.
When hepatocellular steatosis occurs with concurrent
necro inflammatory reactions of the liver and hepatocellular
ballooning with or without fibrosis and/or cirrhosis, it is
diagnosed as NASH. Lobular inflammation and portal inflammation are
both present in NASH, along with other histological lesions, such
as hepatocellular ballooning, fibrosis, apoptotic bodies,
sinusoidal collagen formation, MDBs, megamitochondria, glycogenated
nuclei and iron deposition (64-66).
The time of onset, as well as the degree of both NAFLD and
accompanying metabolic features, are dependent on species, strain,
sex, composition of the gut microbiota and the employed dietary
intervention (67,68). Therefore, liver histology from
animal models is crucial for elucidating the mechanisms and
pathways involved in the pathogenesis of the NAFLD spectrum during
the non-clinical stage. In clinical studies, regulatory agencies in
the USA require liver histological endpoints in phase 3 studies
(69). The ethnopharmacological
usage of herbs may provide a reference with which to identify the
appropriate animal dietary model. The simple steatosis or NASH
observation from a high-fat diet (HFD) or methionine and choline
deficiency (MCD) model may be suitable for this purpose.
HFD. The HFD model is the most frequently
used dietary model for research in NASH. Research has shown that
rats fed a HFD (70) containing
45-75 kcal% develop NASH after 12 weeks. These rats exhibit a
phenotype similar to that of humans, characterized by obesity after
10 weeks, insulin resistance indicated by hyperinsulinemia and
hyperlipidemia after 10 weeks, and glucose intolerance after 12
weeks (Table III). It is
noteworthy that minimal fibrosis is only observable after 36-50
weeks of HFD (70).
MCD diet. The MCD diet is characterized by a
high sucrose content and moderate fat content. This means that it
typically contains 40% sucrose and 10% fat. However, this diet is
deficient in two essential nutrients, choline and methionine. As a
result, the ability of the body to oxidize fats and produce very
low-density lipoprotein particles is impaired (71). This leads to the accumulation of
fat in the liver, which can cause oxidative stress, liver cell
death, inflammation and fibrosis after 8-10 weeks.
Notably, mice fed a MCD diet do not exhibit obesity,
peripheral insulin resistance, or dyslipidemia (Table III), unlike humans with NASH.
Instead, they experience significant weight loss, cachexia and low
levels of serum insulin, fasting glucose, leptin, and triglycerides
(72). The NASH phenotype with
lobular inflammation and metabolic features, as well as ballooning,
develops rapidly in these mice within 2-8 weeks (72).
Therefore, this model is suitable for studying NASH
and its pharmacological treatment, but inadequate for studying
NAFLD due to its multisystemic nature (73). Mouse strains exhibit varying
responsiveness to an MCD diet (73).
Choline deficient L-amino acid-defined HFD. A
HFD that is deficient in choline and amino acids can lead to the
development of NASH with fibrosis in merely 6-9 weeks, even in the
absence of significant weight loss (Table III). However, this diet does not
fully replicate the metabolic syndrome observed in humans (74).
Streptozotocin (STZ) + HFD. When administered
to mice, STZ has been found to damage the pancreatic islets and
decrease insulin production. Additionally, a HFD diet beginning at
4 weeks of age, combined with the administration of neonatal STZ,
has been shown to cause simple steatosis at 6 weeks (Table III), NASH at 8 weeks, and
progressive pericellular fibrosis starting at 8-12 weeks, leading
to HCC after 20 weeks (75).
Carbon tetrachloride (CCl4) +
HFD. Exposure to CCl4 can trigger a response in the
liver that leads to an accumulation of harmful lipid and protein
peroxidation products, which can in turn, cause necrosis. When
combined with a HFD, CCl4 can exacerbate the development
of NASH and fibrosis. In a previous study conducted with mice, it
was found that multiple peritoneal injections of CCl4
over a period of 4 weeks induced not only steatosis, but also
hepatocellular ballooning, centrilobular fibrosis and
hypertriglyceridemia; weight loss was also observed in the mice
(76). Furthermore, the
histological features worsened progressively with each
administration of CCl4 (76).
HFD, high-sugar diet and high-fat, high-fructose
diet. Consuming fructose can significantly affect glucose and
lipid metabolism, leading to several health issues, such as
obesity, insulin resistance and lipid accumulation in the liver.
Research conducted on rats and mice has demonstrated that drinking
fructose-supplemented water for 8 weeks results in simple steatosis
without NASH and contributes to obesity and insulin resistance (IR)
(77). Additionally, rats that
were administered a high-fat, high-fructose diet experienced
hepatic inflammation after 16 weeks. Similar results were observed
in rats that were fed a high-fat, high-sucrose diet (77). Of note, rats that were fed with
glucose and sucrose exhibited a greater weight gain, but lesser
hepatic fat accumulation as compared to a high fructose-fed diet
(78).
Mechanisms of action
Since NAFLD is associated with IR or obesity, the
majority of the pathophysiological observations of the effect of
these herbs listed in Table IV
were shown to improve: i) lipid metabolism; ii) insulin resistance;
iii) inflammation; iv) oxidative stress; and v) endoplasmic
reticulum stress, in addition to the reduction of steatosis in the
liver histological assessment. However, all rats with steatosis had
elevated levels of liver injury markers, such as ALT and AST.
Lipid metabolism
The research results from the 20 plants (Table IV) (28-121)
related to markers for liver lipid metabolism revealed marked
decreases in triglyceride levels ranging from 11.6 to 72.4%, total
cholesterol levels from 13.1 to 50%, low-density lipoprotein levels
ranging from 10.8-60.9%, and increases in high-density lipoprotein
levels ranging from 13.2-58.2%. These plant extracts exert
anti-hyperlipidemic effects that have the potential to reverse or
reduce liver steatosis. In hyperlipidemia, the accumulation of high
levels of cholesterol and triglycerides in the blood causes fat to
accumulate in the liver, resulting in inflammation and oxidative
stress (79). The highest dose
evaluated and that was found to be effective in reducing liver fat
ranged from 100-400 mg/kg body weight in rats (Table IV). Some of these plant extracts,
such as Glossogyne tenuifolia (80) and Picrorhiza kurroa
(53) leaves, have been found to
be effective at a dose of 150 and 300 mg/kg, respectively.
Moreover, neither of these extracts exhibited any signs of toxicity
at the highest dose of 5 and 2 g/kg, respectively (Table IV). The extracts of Antidesma
bunius (82,83), Aralia elata (84-86),
Citrus aurantium (90-93),
Curcuma longa Linn. (94-96),
Cyclosorus terminans (101,102), Panax notoginseng (109), Pluchea indica (114), and Rosmarinus officinalis
Linn (115,116) have also been shown to reduce the
accumulation of lipids in rats fed a HFD through sterol regulatory
element-binding transcription factor 1c (SREBP-1c), peroxisome
proliferator-activated receptor-α (PPAR-α), fatty acid synthase
(FAS), acetyl-CoA carboxylase (ACC), and carnitine
palmitoyltransferase (CPT)2 regulation.
The adenosine monophosphate-activated protein kinase
(AMPK) pathway plays a crucial role in regulating hepatic
lipogenesis and β-oxidation and serves as a vital energy sensor for
intracellular energy metabolism. It helps in regulating free fatty
acids, de novo lipogenesis and hepatic lipid accumulation.
The extracts of C. longa Linn. (94,95)
and R. officinalis Linn. (115) can activate AMPK in NASH rats,
leading to reduced dyslipidemia and hyperglycemia. Moreover,
triggering AMPK signaling, affects adipocytokine production
(122), which further highlights
the potential of these extracts in regulating metabolic
disorders.
Several plant extracts, including Aralia
elata (84-86),
Curcuma longa Linn. (94,95),
Cyclosorus terminans (100-102),
Panax notoginseng (109),
and Pluchea indica (114)
(Table IV), enhanced fatty acid
β-oxidation by activating lipid antioxidant enzymes, such as CPT1
and peroxidation reduction through the activation of AMPK and
PPAR-α.
Several plant extracts, including those derived from
Hibiscus sabdariffa, Panax notoginseng, Antidesma
bunius, Curcuma longa Linn., Aralia elata, Cyclosorus
terminans, Pluchea indica, and R. officinalis
Linn. (Table IV), upregulated the
expression leels of SREBP-1c, FAS, ACC, and CPT-1, as well as
PPAR-α, a regulator of β-oxidation, in rats that were fed a HFD
(83,84,94,95,101,105,109,114,115). This suggests that AMPK activation
by these herbal compounds is associated with de novo lipid
synthesis, which is linked to the suppression of SREBP-1c, PPAR-α,
FAS, ACC and CPT-2 expression. Moreover, these plant extracts
promote the defense mechanism of β-oxidation, which leads to
hepatic fatty acid depletion, by modulating CPT-1 and PPAR-α
production.
IR
Carbohydrate metabolism plays a crucial role in IR,
as it is regulated by insulin hormone. When the insulin signal
fails to prompt glucose absorption in cells due to IR, it leads to
hyperglycemia, where glucose levels in the blood remain elevated.
The body compensates for this by producing more insulin, which can
cause hormonal imbalances and cell damage, particularly in liver
cells. Excess insulin production can also lead to liver fat
accumulation, which is a common occurrence in IR. Moreover,
hyperglycemia and hormonal imbalances can cause inflammation and
oxidative stress on liver cells, thus aggravating liver fat
accumulation. Therefore, it is essential to manage IR through a
healthy diet, regular exercise, and avoiding excessive alcohol
consumption to prevent and treat NAFLD. IR leads to the
accumulation of free fatty acids in the liver, which triggers de
novo lipogenesis and causes NAFLD (123,124). Thus, the following studies have
shown that certain natural extracts can help manage IR and its
associated complications.
It is noteworthy that all the studies cited in
Table IV (28-121),
examining the effects of herbal plant extracts on IR, found
significant decreases in glucose and insulin levels, as well as IR
with the oral administration of these herbal extracts, namely,
Abroma augusta (28,81).
Aralia elata (84-86),
Crocus sativus (97-99),
Cyclosorus terminans (100-102),
Glossogyne tenuifolia (80,103), Morus latifolia (107,108), Panax notoginseng (109), Pluchea indica (114), and Rubus ideaus (117,118), Trigonella foenum-graecum
(119-121).
For instance, 200 mg/kg hexane extract of
Cyclosorus terminans administered orally in rats fed a HFD
was shown to reduce blood glucose levels, insulin and the
homeostatic model assessment of insulin resistance (HOMA-IR) over a
period of 2 weeks. This extract increased the expression of genes
solute carrier family 2 member 4 (Slc2a4) and solute carrier family
2 member 2 (Scl2a2), thereby stimulating IR in the liver cells and
soleus muscle. It also promoted the expression of insulin receptor
substrate-1 (IRS1) and insulin receptor substrate-2(IRS2) genes,
promoting hepatic and soleus muscle glycogen production (100).
Similarly, treatment with 1 g/kg aqueous
Trigonella foenum-graecum bark extract for 28 days in rats
reversed the effects of IR and lowered the HOMA-IR and
apolipoprotein B (apoB) levels in the blood (119). IR is associated with increased
secretion and decreased clearance of ApoB, which reduces
low-density lipoprotein clearance (125). Trigonella foenum-graecum
managed to reduce the effects of IR and ApoB, and increase LDL
clearance (119).
Inflammation
The oral administration of extracts from various
herbs, such as Antidesma bunius (83), Cassia obtusifolia (87), Crocus sativus (97), Cyclosorus terminans
(101), Glossogyne
tenuifolia (103),
Hibiscus sabdariffa (105), Panax notoginseng (109) and Rubus ideaus (117), were found to lead to an
improvement in the levels of inflammatory markers linked to liver
damage. These herbal extracts have been found to reduce the levels
of pro-inflammatory cytokines and inflammation. The effective
dosages of these extracts range from 100-500 mg/kg body weight in
rats, and no signs of toxicity were observed even at the highest
evaluated dose of 2,000 mg/kg.
According to the study by Park et al
(126), hepatic adiponectin
induction reduced NASH-associated necro-inflammation and fibrosis
by antagonizing TNF and regulating each other's secretion. Another
study demonstrated that the oral administration of 500 mg/kg
Hibiscus sabdariffa for 8 weeks reduced the release of
pro-inflammatory cytokines, such as IL-6 and TNF-α, which inhibited
the development of NASH (105).
Liver inflammation causes inflammatory damage by
increasing lipid accumulation and redistribution from adipose
tissue to the liver. Hepatic steatosis, steatohepatitis, and
fibrosis are the first steps in the evolution of NAFLD caused by
liver inflammation (127). Herbal
extracts have been found to protect against the advancement of
hepatic steatosis to steatohepatitis by reducing liver
inflammation. This is achieved by the suppression of inflammatory
signaling pathways, controlling dyslipidemia, and enhancing liver
function in patients with NAFLD (127). Herbal remedies, such as
Antidesma bunius (83),
Cassia obtusifolia (87),
Crocus sativus (97),
Cyclosorus terminans (101), Glossogyne tenuifolia
(103), Hibiscus
sabdariffa (105), Panax
notoginseng (109) and
Rubus ideaus L. (117),
and (Table IV) have been shown to
reduce the expression levels of hepatic inflammatory cytokines
(TNF-α, IL-6, IL-8 and IL-1β) and to further ameliorate liver
fibrosis. Hou et al (109)
demonstrated that 30 mg/kg Panax notoginseng ethanol extract
reduced the levels of inflammatory markers, namely TNF-α, IL-6,
IL-8, IL-1 and IL-1β, and no signs of toxicity were observed at the
highest chronic dose evaluated at 1,200 mg/kg for 28 days. From the
results presented in Table IV,
some of these herbs reduced the biomarker for insulin resistance
and lipid metabolism in addition to steatosis reduction. As insulin
resistance and lipid accumulation induced steatosis, most likely
these herbs will ameliorate IR and lipid accumulation before the
development of steatosis. An earlier study on Hibiscus
sabdariffa aqueous extract at a lower dose of 300 mg/kg for 10
weeks, demonstrated reduced weight gain or obesity in rats fed a
HFD through the inhibition of adipogenesis (128), indicating a close link to
metabolic syndrome.
Oxidative stress
A total of eight herbal plants, namely Abroma
augusta (28,81), Antidesma bunius (82,83),
Cassia obtusifolia (87-89),
Curuma longa (94-96),
Crocus sativus (97-99),
Hibiscus sabdariffa (104,105), Rubus ideaus (117,118), and Trigonella
foenum-graecum (119-121)_
were found to be safe at the highest toxicity dose evaluated and
significantly reduce the levels of pro-oxidants, namely,
malondialdehyde, ER stress, ROS and oxidative end products, such as
4-hydroxynonenal (Table IV).
Visceral fat accumulation can lead to oxidative
stress, which is a common characteristic of NAFLD. This, in turn,
triggers lipid peroxidation, causing oxidative damage throughout
the body (105). The development
of NAFLD can result in liver damage due to an imbalance between the
production of reactive species and antioxidant defense. NAFLD
affects lipid metabolism, leading to the production of ROS through
fatty acid oxidation. The effective extract dosages ranged from
200-1,500 mg/kg body weight in experimental rats, and all of these
plant extracts did not lead to any signs of toxicity at the highest
evaluated dose of 2,000 mg/kg (Table
IV). The ethanol extract of 200 mg/kg Rubus ideaus has
been shown to significantly decrease the level of malondialdehyde,
while increasing the levels of the antioxidants, superoxide
dismutase, glutathione and glutathione peroxidase, thereby reducing
liver oxidative stress (117).
The extracts of Aralia elata (84-86),
Curcuma longa (94-96),
Cyclosorus terminans (100-102),
Panax notoginseng (109),
and Pluchea indica (114)
have been found to significantly promote fatty acid oxidation
through the activation of PPAR-α and PPAR-γ.
ER stress
The pathological disorders associated with obesity
and NAFLD include the ER stress response as one of the primary
characteristics (42). One of the
primary characteristics of these disorders is the activation of the
ER stress axis (42). However,
Curcuma longa Linn. has been found to block this axis by
activating AMPK, a physiological regulator of the mTOR signaling
pathway that helps lower lipid metabolism. By activating AMPK,
Curcuma longa Linn. can reduce the ER stress response
(94). This natural herb has also
been found to prevent hepatic dyslipidemia by downregulating the
levels of phosphorylated mammalian target of rapamycin (p-mTOR),
phosphorylated ribosomal protein s6 kinase (p-S6K) and
phosphorylated eukaryotic translation initiation factor 4E-binding
protein 1 (p-4-EBP-1), while alleviating ER stress (95). Curcuma longa Linn. has been
proven to inhibit overnutrition-induced hepatic lipid accumulation,
by controlling SREBP-1 and FAS through the protein endoplasmic
reticulum kinase/eukaryotic translation initiation factor 2, which
regulates lipid metabolism (95).
Additionally, Curcuma longa Linn. has been found to modulate
ER stress response and redox imbalance by impacting mTORC1,
demonstrating the role of the mechanistic target of rapamycin
complex 1 (mTORC1) activation and protein folding in the pathogenic
process of hepatic dyslipidemia (94).
Limitations
The limitations of the studies identified in the
present systematic review were the usage of non-standardized
extracts, which could affect the reproducibility of their
therapeutic effects. Variation in the bioactive synthesis is
expected with herbs collected or planted at different locations as
the biosynthesis of these bioactive compounds is dependent on the
quality of the soil, altitude, environment and the time of
harvest.
In conclusion, in the present systematic review, 20
herbs were found to reduce steatosis in histopathological
assessment. Of these, 15 herbs, namely A. augusta (28,81),
A. bunius (82,83), A. elata (84-86),
C. obtusifolia (87-89),
C. aurantium (90-93),
C. longa (94-96),
C. sativus (97-99),
C. terminans (100-102),
G. tenuifolia (80,103), H. sabdariffa (104,105), P. notoginseng (109), P. indica (114), R. officinalis (115,116), R. ideaus (117,118) and T. foenum-graecum
(119-121)
exhibited a mode of action involving lipid metabolism, insulin
resistance and/or inflammatory markers. The remaining five herbs,
namely M. oleifera (46,106), M. latifolia (107,108), P. emblica (110,111), P. kurroa (53,112) and P. anisum (113) warrant further investigations to
establish their cross-link mode of action. These herbs exhibited no
signs of toxicity. The herbs were found to exert a positive effect
against NAFLD, and understanding its mechanism of action is
probably useful for the treatment of other metabolic diseases
associated with NAFLD, namely dyslipidemia, diabetes, or
hypertension. Further studies are required to identify the
bioactive compounds and standardize the extracts. Planning for
either in vivo or in vitro experimental studies is
essential to identify the bioactive compounds present in herbs. The
traditional usage of these herbs can provide a useful reference for
such studies. This process helps in understanding the bioactive
components of the herbs and standardizing the extracts to ensure
reproducible therapeutic effects. An understanding of the mechanism
of action of these herbs is useful for planning more successful
clinical trials. The development of NAFLD is time-consuming and
herbs may provide alternative treatment to its prevention.
Acknowledgements
NEJ is grateful to Wiyata Husada Samarinda for
approving her study leave to pursue her PhD.
Funding
Funding: The present study was funded by the UiTM Grant (P3077),
600-RMC/GPK 5/3/116/2020, 600-RMC/GSS 5/3(061/2022) and the ITKES
Wiyata Husada Samarinda grant.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
NEJ extracted the information from the studies in
the databases and prepared the initial draft of the manuscript. RMS
and SMJ screened and analyzed the extracted information, and edited
the manuscript. CYC conceptualized the study, screened the
extracted information, and edited the final draft of the
manuscript. The authenticity of the raw data was confirmed by NEJ
and CYC. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools
(Microsoft Word installed with Grammarly and Generative AI) were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Mertens J, Weyler J, Dirinck E, Vonghia L,
Kwanten WJ, Mortelmans L, Peleman C, Chotkoe S, Spinhoven M,
Vanhevel F, et al: Prevalence, risk factors and diagnostic accuracy
of non-invasive tests for NAFLD in people with type 1 diabetes.
JHEP Rep. 5(100753)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wong VWS, Ekstedt M, Wong GL and Hagström
H: Changing epidemiology, global trends and implications for
outcomes of NAFLD. J Hepatol. 79:842–852. 2023.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Patel AH, Peddu D, Amin S, Elsaid MI,
Minacapelli CD, Chandler TM, Catalano C and Rustgi VK: Nonalcoholic
fatty liver disease in lean/nonobese and obese individuals: A
comprehensive review on prevalence, pathogenesis, clinical
outcomes, and treatment. J Clin Transl Hepatol. 11:502–515.
2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Adams LA, Anstee QM, Tilg H and Targher G:
Non-alcoholic fatty liver disease and its relationship with
cardiovascular disease and other extrahepatic diseases. Gut.
66:1138–1153. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Li AA, Ahmed A and Kim D: Extrahepatic
manifestations of nonalcoholic fatty liver disease. Gut Liver.
14:168–178. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Day CP and James OF: Steatohepatitis: A
tale of two ‘hits’? Gastroenterology. 114:842–845. 1998.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tilg H and Moschen AR: Evolution of
inflammation in nonalcoholic fatty liver disease: The multiple
parallel hits hypothesis. Hepatology. 52:1836–1846. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Tomeno W, Yoneda M, Imajo K, Ogawa Y,
Kessoku T, Saito S, Eguchi Y and Nakajima A: Emerging drugs for
non-alcoholic steatohepatitis. Expert Opin Emerg Drugs. 18:279–290.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pessayre D and Fromenty B: NASH: A
mitochondrial disease. J Hepatol. 42:928–940. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lian B, Cai L, Zhang Z, Lin F, Li Z, Zhang
X and Jiang F: The anti-inflammatory effect of Pien Tze Huang in
non-alcoholic fatty liver disease. Biomed Pharmacother.
151(113076)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jurisic V, Terzic T, Colic S and Jurisic
M: The concentration of TNF-alpha correlate with number of
inflammatory cells and degree of vascularization in radicular
cysts. Oral Dis. 14:600–607. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sanyal AJ, Friedman SL, McCullough AJ and
Dimick-Santos L: American Association for the Study of Liver
Diseases; United States Food and Drug Administration. Challenges
and opportunities in drug and biomarker development for
nonalcoholic steatohepatitis: Findings and recommendations from an
American association for the study of liver diseases-U.S. food and
drug administration joint workshop. Hepatology. 61:1392–1405.
2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
U.S. Food and Drug Administration (FDA):
FDA Approves First Treatment for Patients with Liver Scarring Due
to Fatty Liver Disease. FDA, Silver Spring, MD, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-liver-scarring-due-fatty-liver-disease.
|
|
14
|
Takahashi Y, Sugimoto K, Inui H and
Fukusato T: Current pharmacological therapies for nonalcoholic
fatty liver disease/nonalcoholic steatohepatitis. World J
Gastroenterol. 21:3777–3785. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Flora K, Hahn M, Rosen H and Benner K:
Milk thistle (Silybum marianum) for the therapy of liver
disease. Am J Gastroenterol. 93:139–143. 1998.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kalopitas G, Antza C, Doundoulakis I,
Siargkas A, Kouroumalis E, Germanidis G, Samara M and Chourdakis M:
Impact of Silymarin in individuals with nonalcoholic fatty liver
disease: A systematic review and meta-analysis. Nutrition.
83(111092)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ye Y, Liu X, Wu N, Han Y, Wang J, Yu Y and
Chen Q: Efficacy and safety of berberine alone for several
metabolic disorders: A systematic review and meta-analysis of
randomized clinical trials. Front Pharmacol.
12(653887)2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Banach M, Patti AM, Giglio RV, Cicero AFG,
Atanasov AG, Bajraktari G, Bruckert E, Descamps O, Djuric DM, Ezhov
M, et al: The role of nutraceuticals in statin intolerant patients.
J Am Coll Cardiol. 72:96–118. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sukhija R, Prayaga S, Marashdeh M, Bursac
Z, Kakar P, Bansal D, Sachdeva R, Kesan SH and Mehta JL: Effect of
statins on fasting plasma glucose in diabetic and nondiabetic
patients. J Investing Med. 57:495–499. 2009.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Liu QZ, Lyu WT, Cai MX, Wu HL and Shang J:
Effect of metabolic diseases on emotional and cognitive functions
and its potential mechanisms research progress. Chin J Pharmacol
Toxicol. 29:847–858. 2015.
|
|
21
|
Zhang C, Yuan W, Fang J, Wang W, He P, Lei
J and Wang C: Efficacy of resveratrol supplementation against
non-alcoholic fatty liver disease: A meta-analysis of
placebo-controlled clinical trials. PLoS One.
11(e0161792)2016.PubMed/NCBI View Article : Google Scholar
|
|
22
|
de Avelar CR, Pereira EM, de Farias Costa
PR, de Jesus RP and de Oliveira LPM: Effect of silymarin on
biochemical indicators in patients with liver disease: Systematic
review with meta-analysis. World J Gastroenterol. 23:5004–5017.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Asbaghi O, Ghanbari N, Shekari M, Reiner
Z, Amirani E, Hallajzadeh J, Mirsafaei L and Asemi Z: The effect of
berberine supplementation on obesity parameters, inflammation and
liver function enzymes: A systematic review and meta-analysis of
randomized controlled trials. Clin Nutr ESPEN. 38:43–49.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Faghihzadeh F, Adibi P, Rafiei R and
Hekmatdoost A: Resveratrol supplementation improves inflammatory
biomarkers in patients with nonalcoholic fatty liver disease. Nutr
Res. 34:837–843. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Berman AY, Rachel A, Motechin RA,
Wiesenfeld MY and Holz MK: The therapeutic potential of
resveratrol: A review of clinical trials. NPJ Precis Oncol.
1(35)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shrestha PM and Dhillion SS: Medicinal
plant diversity and use in the highlands of Dolakha district,
Nepal. J Ethnopharmacol. 86:81–96. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Asase A, Kokubun T, Grayer RJ, Kite G,
Simmonds MS, Oteng Yeboah AA and Odamtten GT: Chemical constituents
and antimicrobial activity of medicinal plants from Ghana:
Cassia sieberiana, Haematostaphis barteri,
Mitragyna inermis and Pseudocedrela kotschyi.
Phytother Res. 22:1013–1016. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Sunitha P, Sathyanarayana N, Suresh VC,
Sreeramanan S, Annie JS and Xavier R: Phytochemical and antioxidant
analysis of the leaf extract of Malaysian medicinal plant Abroma
augusta L. Indian J Pharm Sci. 80:192–198. 2018.
|
|
29
|
Hazra K, Dutta S, Mandal AK, Ravte RK,
Mitra A and Hazra J: Pharmacognostical and phytochemical blueprint
of Abroma augusta L. stem bark. Indian J Nat Prod Resour.
12:271–280. 2021.
|
|
30
|
Islam MS, Ahammed MS, Sukorno FI, Koly SF,
Biswas MM and Hossain S: A review on phytochemical and
pharmacological potentials of Antidesma bunius. J Anal Pharm
Res. 7:602–604. 2018.
|
|
31
|
Xu Y, Liu J, Zeng Y, Jin S, Liu W, Li Z,
Qin X and Bai Y: Traditional uses, phytochemistry, pharmacology,
toxicity and quality control of medicinal genus Aralia: A review. J
Ethnopharmaco. 284(114671)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Z, Hu J, Su Z, Li C, Li R, Tang H and
Yi Y: Liver-protective activity of Aralia taibaiensis Z.Z.
Wang et H.C. Zheng. Zhongguo Zhong Yao Za Zhi. 22:307–308.
1997.PubMed/NCBI(In Chinese).
|
|
33
|
Hao Y, Sang Y and Zhao Y: The advancement
of the studies on the seeds of Cassia obtusifolia. Chin
Tradit Herb Drugs. 32:858–859. 2001.
|
|
34
|
Chen Y, Chen X, Yang X, Gao P, Yue C, Wang
L, Wu T, Jiang T, Wu H, Tang L and Wang Z: Cassiae semen: A
comprehensive review of botany, traditional use, phytochemistry,
pharmacology, toxicity, and quality control. J Ethnopharmacol.
306(116199)2023.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Memariani Z, Gorji N, Moeini R and Farzaei
MH: Chapter two: Traditional uses: In Phytonutrients in Food.
Woodhead Publishing, pp23-66, 2020.
|
|
36
|
Paul A and Cox PA: An ethnobotanical
survey of the use for Citrus auranticum (Rutaceae) in Haiti.
Econ Bot. 49:249–256. 1995.
|
|
37
|
Araujo CC and Leon LL: Biological
activities of Curcuma longa L. Mem Inst Oswaldo Cruz.
96:723–728. 2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yildirim MU, Sarihan EO and Khawar KM:
Ethnomedicinal and traditional usage of saffron (Crocus sativus L.)
in Turkey: In Saffron. Academic Press, pp21-31, 2020.
|
|
39
|
Hausenblas HA, Heekin K, Mutchie HL and
Anton S: A systematic review of randomized controlled trials
examining the effectiveness of saffron (Crocus sativus L.)
on psychological and behavioral outcome. J Integr Med. 13:231–240.
2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Webb L: Some new records of medicinal
plants used by the aborigines of tropical Queensland and New
Guinea. Proc R Soc Qld. 71:103–110. 1960.
|
|
41
|
Holdsworth D and Rali T: A survey of
medicinal plants of the Southern Highlands, Papua New Guinea. Int J
Crude Drug Res. 27:1–8. 1989.
|
|
42
|
Li TS: Taiwanese native medicinal plants:
Phytopharmacology and therapeutic values. Routledge and CRC Press.
110(183)2006.
|
|
43
|
Wu MJ, Weng CY, Ding HY and Wu PJ:
Anti-inflammatory and antiviral effects of Glossogyne
tenuifolia. Life Sci. 76:1135–1146. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Morton JF: Fruits of warm climates. J.F.
Morton, 1987.
|
|
45
|
Hou DX, Tong X, Terahara N, Luo D and Fuji
M: Delphinidin 3-sambubioside, a Hibiscus anthocyanin, induces
apoptosis in human leukemia cells through reactive oxygen
species-mediated mitochondrial pathway. Arch Biochem Biophys.
440:101–109. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Mapfumo M, Lembede BW, Nkomozepi P,
Ndhlala AR and Chivandi E: Crude Moringa oleifera Lam. seed
extract attenuates non-alcoholic fatty liver disease in growing
Sprague-Dawley rats. S Afr J Bot. 129:191–197. 2020.
|
|
47
|
Biswas SK, Chowdhury A, Das J, Roy A and
Hosen SZ: Pharmacological potentials of Moringa oleifera Lam
A review. Int J Pharm Sci Res. 3:305–310. 2012.
|
|
48
|
Sastri B: The wealth of India, a
dictionary of raw material and industrial products. Publ Inf Dir.
5:285–293. 1950.
|
|
49
|
Hussain F, Rana Z, Shafique H, Malik A and
Hussain Z: Phytopharmacological potential of different species of
Morus alba and their bioactive phytochemicals: A review.
Asian Pac J Trop Biomed. 7:950–956. 2017.
|
|
50
|
Wang T, Guo R, Zhou G, Zhou X, Kou Z, Sui
F, Li C, Tang L and Wang Z: Traditional uses, botany,
phytochemistry, pharmacology and toxicology of Panax
notoginseng (Burk.) F.H. Chen: A review. J Ethnopharmacol.
188:234–258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Tung NH, Uto T, Morinaga O, Kim YH and
Shoyama Y: Pharmacological effects of ginseng on liver functions
and diseases: A minireview. Evid Based Complement Alternat Med.
2012(173297)2012.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Mirunalini S and Krishnaveni M:
Therapeutic potential of Phyllanthus emblica (amla): The
ayurvedic wonder. J Basic Clin Physio Pharmacol. 21:93–105.
2010.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Shetty SN, Mengi S, Vaidya R and Vaidya
ADB: A study of standardized extracts of Picrorhiza kurroa
Royle ex Benth in experimental nonalcoholic fatty liver disease. J
Ayurveda Integr Med. 1:203–210. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hajou RMK, Afifi FU and Battah AH:
Comparative determination of multi-pesticide residues in
Pimpinella anisum using two different AOAC methods. Food
Chem. 88:469–478. 2004.
|
|
55
|
Jonas WB: Dictionary of complementary and
alternative medicine. J Altern Complement Med. 11:739–740.
2005.
|
|
56
|
Andarwulan N, Batari R, Sandrasari DA,
Bolling B and Wijaya H: Flavonoid content and antioxidant activity
of vegetables from Indonesia. Food Chem. 121:1231–1235.
2010.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Suriyaphan O: Nutrition, health benefits
and applications of Pluchea indica (L.) less leaves. Mahidol
Univ J Pharm Sci. 41:1–10. 2014.
|
|
58
|
Duke JA: Handbook of medicinal herbs. 2nd
edition. CRC press, 2002.
|
|
59
|
Heinrich M, Kufer J, Leonti M and
Pardo-de-Santayana M: Ethnobotany and
ethnopharmacology-interdisciplinary links with the historical
sciences. J Ethnopharmacol. 107:157–160. 2006.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Ody P: The complete medicinal herbal.
Dorling Kindersley, 1993.
|
|
61
|
Tao Y, Bao J, Zhu F, Pan M, Liu Q and Wang
P: Ethnopharmacology of Rubus idaeus Linnaeus: A critical
review on ethnobotany, processing methods, phytochemicals,
pharmacology and quality control. J Ethnopharmacol.
302(115870)2023.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Sun W, Shahrajabian MH and Cheng Q:
Fenugreek cultivation with emphasis on historical aspects and its
uses in traditional medicine and modern pharmaceutical science.
Mini Rev Med Chem. 21:724–730. 2021.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Kohli R and Feldstein AE: NASH animal
models: Are we there yet? J Hepatol. 55:941–943. 2011.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Takahashi Y, Soejima Y and Fukusato T:
Animal models of nonalcoholic fatty liver disease/nonalcoholic
steatohepatitis. World J Gastroenterol. 18:2300–2308.
2012.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Ganz M and Szabo G: Immune and
inflammatory pathways in NASH. Hepatol Int. 7 (Suppl 2):S771–S781.
2013.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Brunt EM and Tiniakos DG: Histopathology
of non-alcoholic fatty liver disease. World J Gastroenterol.
16:5286–5296. 2010.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kirsch R, Clarkson V, Shephard EG, Marais
DA, Jaffer MA, Woodburne VE, Kirsch RE and Hall Pde L: Rodent
nutritional model of non-alcoholic steatohepatitis: species, strain
and sex difference studies. J Gastroenterol Hepatol. 18:1272–1282.
2003.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Le Roy T, Llopis M, Lepage P, Bruneau A,
Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et
al: Intestinal microbiota determines development of non-alcoholic
fatty liver disease in mice. Gut. 62:1787–1794. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Harrison SA, Loomba R, Dubourg J, Ratziu V
and Noureddin M: Clinical trial landscape in NASH. Clin
Gastroenterol Hepatol. 21:2001–2014. 2023.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Ito M, Suzuki J, Tsujioka S, Sasaki M,
Gomori A, Shirakura T, Hirose H, Ito M, Ishihara A, Iwaasa H and
Kanatani A: Longitudinal analysis of murine steatohepatitis model
induced by chronic exposure to high-fat diet. Hepatol Res.
37:50–57. 2007.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Ibrahim SH, Hirsova P, Malhi H and Gores
GJ: Animal models of nonalcoholic steatohepatitis: Eat, delete, and
inflame. Dig Dis Sci. 61:1325–1336. 2016.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Rinella ME and Green RM: The
methionine-choline deficient dietary model of steatohepatitis does
not exhibit insulin resistance. J Hepatol. 40:47–51.
2004.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Van Herck MA, Vonghia L and Francque SM:
Animal models of nonalcoholic fatty liver disease-A starter's
guide. Nutrients. 9(1072)2017.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Matsumoto M, Hada N, Sakamaki Y, Uno A,
Shiga T, Tanaka C, Ito T, Katsume A and Sudoh M: An improved mouse
model that rapidly develops fibrosis in non-alcoholic
steatohepatitis. Int J Exp Pathol. 94:93–103. 2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Clapper JR, Hendricks MD, Gu G, Wittmer C,
Dolman CS, Herich J, Athanacio J, Villescaz C, Ghosh SS, Heilig JS,
et al: Diet-induced mouse model of fatty liver disease and
nonalcoholic steatohepatitis reflecting clinical disease
progression and methods of assessment. Am J Physiol Gastrointest
Liver Physiol. 305:G483–G495. 2013.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Kubota N, Kado S, Kano M, Masuoka N,
Nagata Y, Kobayashi T, Miyazaki K and Ishikawa F: A high-fat diet
and multiple administration of carbon tetrachloride induces liver
injury and pathological features associated with non-alcoholic
steatohepatitis in mice. Clin Exp Pharmacol Physiol. 40:422–430.
2013.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Mamikutty N, Thent ZC and Haji Suhaimi F:
Fructose-drinking water induced nonalcoholic fatty liver disease
and ultrastructural alteration of hepatocyte mitochondria in male
wistar rat. Biomed Res Int. 2015(895961)2015.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Bergheim I, Weber S, Vos M, Krämer S,
Volynets V, Kaserouni S, McClain CJ and Bischoff SC: Antibiotics
protect against fructose-induced hepatic lipid accumulation in
mice: Role of endotoxin. J Hepatol. 48:983–992. 2008.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Arroyave-Ospina JC, Wu Z, Geng Y and
Moshage H: Role of oxidative stress in the pathogenesis of
non-alcoholic fatty liver disease: Implications for prevention and
therapy. Antioxidants (Basel). 10(174)2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Asokan SM, Wang RY, Hung TH and Lin WT:
Hepato-protective effects of Glossogyne tenuifolia in
Streptozotocin-nicotinamide-induced diabetic rats on high fat diet.
BMC Complement Altern Med. 19(117)2019.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Yadav AK and Singh A: Hepatoprotective and
antioxidant activity of ethanolic leaves extract of abroma augusta
against non-alcoholic fatty liver disease (Nafld) in SD rats. Int J
Pharm Sci Res. 12:316–326. 2022.
|
|
82
|
Ngamlerst C, Udomkasemsab A,
Kongkachuichai R, Kwanbunjan K, Chupeerach C and Prangthip P: The
potential of antioxidant-rich Maoberry (Antidesma bunius)
extract on fat metabolism in liver tissues of rats fed a high-fat
diet. BMC Complement Altern Med. 19(294)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Chowtivannakul P, Srichaikul B and
Talubmook C: Hypoglycemic and hypolipidemic effects of seed extract
from Antidesma bunius (L.) spreng in streptozotocin-induced
diabetic rats. Pak J Biol Sci. 19:211–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Hwang KA, Hwang YJ, Kim GR and Choe JS:
Extracts from Aralia elata (Miq) Seem alleviate
hepatosteatosis via improving hepatic insulin sensitivity. BMC
Complement Altern Med. 15(347)2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Yang HK, Jin JY, Kim JM, Ko MS, Hong HJ,
Kim SC and Lee YJ: Single Oral Dose Toxicity Study of the Extract
of Aralia elata in Mice. J Toxical Pub Health. 22:439–443.
2006.
|
|
86
|
Qi M, Hua X, Peng X, Yan X and Lin J:
Comparison of chemical composition in the buds of Aralia
elata from different geographical origins of China. R Soc Open
Sci. 5(180676)2018.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Meng Y, Liu Y, Fang N and Guo Y:
Hepatoprotective effects of Cassia semen ethanol extract on
non-alcoholic fatty liver disease in experimental rat. Pharm Biol.
57:98–104. 2019.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Dong X, Fu J, Yin X, Yang C, Zhang X, Wang
W, Du X, Wang Q and Ni J: Cassiae semen: A review of its
phytochemistry and pharmacology (Review). Mol Med Rep.
16:2331–2346. 2017.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Zhang HF, Bai JP, Yu JZ, Yang LZ, Feng L
and Ma CG: Long-term toxicity test of semen cassiae hawthorn oat
capsules. Chin J Nat Med. 12:412–414. 2010.
|
|
90
|
Han HY, Lee SK, Choi BK, Lee DR, Lee HJ
and Kim TW: Preventive effect of Citrus aurantium peel
extract on high-fat diet-induced non-alcoholic fatty liver in mice.
Biol Pharm Bull. 42:255–260. 2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Suntar I, Khan H, Patel S, Celano R and
Rastrelli L: An overview on Citrus aurantium L.: Its
functions as food ingredient and therapeutic agent. Oxid Med Cell
Longev. 2018(7864269)2018.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Arbo MD, Schmitt GC, Limberger MF, Charão
MF, Moro AM, Ribeiro GL, Dallegrave E, Garcia SC, Leal MB and
Limberger RP: Subchronic toxicity of Citrus aurantium L.
(Rutaceae) extract and p-synephrine in mice. Regul Toxicol
Pharmacol. 54:114–117. 2009.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Deshmukh NS, Stohs SJ, Magar CC and Kadam
SB: Citrus aurantium (bitter orange) extract: Safety
assessment by acute and 14-day oral toxicity studies in rats and
the Ames Test for mutagenicity. Regul Toxicol Pharmacol.
90:318–327. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Mun J, Kim S, Yoon HG, You Y, Kim OK, Choi
KC, Lee YH, Lee J, Park J and Jun W: Water extract of Curcuma
longa L. Ameliorates non-alcoholic fatty liver disease.
Nutrients. 11(2536)2019.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Ahmad RS, Hussain MB, Sultan MT, Arshad
MS, Waheed M, Shariati MA, Plygun S and Hashempur MH: Biochemistry,
Safety, pharmacological activities, and clinical applications of
turmeric: A mechanistic review. Evid Based Complementary Altern
Med. 2020(7656919)2020.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Murugan S, Solanki H, Purusothaman D,
Bethapudi B, Ravalji M and Mundkinajeddu D: Safety evaluation of
standardized extract of Curcuma longa (NR-INF-02): A 90-day
subchronic oral toxicity study in rats. Biomed Res Int.
2021(6671853)2021.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Sabir U, Irfan HM, Alamgeer A, Ullah YS,
Althobaiti MH and Asim MF: Reduction of hepatic steatosis,
oxidative stress, inflammation, ballooning and insulin resistance
after therapy with safranal in NAFLD animal model: A new approach.
J Inflamm Res. 15:1293–1316. 2022.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Hosseinzadeh H, Sadeghi Shakib S, Khadem
Sameni A and Taghiabadi E: Acute and subacute toxicity of safranal
a constituent of saffron in mice and rats. Iran J Pharm Res.
12:93–99. 2013.PubMed/NCBI
|
|
99
|
El Daly ES: Protective effect of cysteine
and vitamin E, Crocus sativus and Nigella sativa
extracts on cisplatin-induced toxicity in rats. J Islamic Acad Sci.
9:105–118. 1996.PubMed/NCBI
|
|
100
|
Songtrai S, Dejyong K and Kaewsuwan S:
Acute oral toxicological evaluation in Wistar rats of
interruptin-rich extract from Cyclosorus terminans and its
in vitro antidiabetic potential. J Pharm Pharmacogn Res.
10:800–811. 2022.
|
|
101
|
Songtrai S, Pratchayasakul W, Arunsak B,
Chunchai T, Kongkaew A, Chattipakorn N, Chattipakorn SC and
Kaewsuwan S: Cyclosorus terminans extract ameliorates
insulin resistance and non-alcoholic fatty liver disease (NAFLD) in
high-fat diet (HFD)-induced obese rats. Nutrients.
14(4895)2022.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Quadri Spinelli T, Heilmann J, Rali T and
Sticher O: Bioactive coumarin derivatives from the fern
Cyclosorus interruptus. Planta Med. 66:728–733.
2000.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Tien YH, Chen BH, Wang Hsu GS, Lin WT,
Huang JH and Lu YF: Hepatoprotective and anti-oxidant activities of
Glossogyne tenuifolia against acetaminophen-induced
hepatotoxicity in mice. Am J Chin Med. 42:1385–1398.
2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Njinga NS, Kola-Mustapha AT, Quadri AL,
Atolani O, Ayanniyi RO, Buhari MO, Amusa TO, Ajani EO, Folaranmi
OO, Bakare-Odunola MT, et al: Toxicity assessment of sub-acute and
sub-chronic oral administration and diuretic potential of aqueous
extract of Hibiscus sabdariffa calyces. Heliyon.
6(e04853)2020.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Prasomthong J, Limpeanchob N, Daodee S,
Chonpathompikunlert P and Tunsophon S: Hibiscus sabdariffa
extract improves hepatic steatosis, partially through IRS-1/Akt and
Nrf2 signaling pathways in rats fed a high fat diet. Sci Rep.
12(7022)2022.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Kim Y, Jaja-Chimedza A, Merrill D, Mendes
O and Raskin I: A 14-day repeated-dose oral toxicological
evaluation of an isothiocyanate-enriched hydro-alcoholic extract
from Moringa oleifera Lam. seeds in rats. Toxicol Rep.
5:418–426. 2018.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Mary SV and Jibu T: The effect of M.
latifolia leaf extract on high-fructose corn syrup
(HFCS)-induced non-alcoholic fatty liver disease in rat models in
nonalcoholic fatty liver disease. IntechOpen Rijeka P Ch 2,
2019.
|
|
108
|
Li Y, Zhang X, Liang C, Hu J and Yu Z:
Safety evaluation of mulberry leaf extract: Acute, subacute
toxicity and genotoxicity studies. Regul Toxicol Pharmacol.
95:220–226. 2018.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Hou Y, Gu D, Peng J, Jiang K, Li Z, Shi J,
Yang S, Li S and Fan X: Ginsenoside Rg1 regulates liver lipid
factor metabolism in NAFLD model rats. ACS Omega. 5:10878–10890.
2020.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Luo X, Zhang B, Pan Y, Gu J, Tan R and
Gong P: Phyllanthus emblica aqueous extract retards hepatic
steatosis and fibrosis in NAFLD mice in association with the
reshaping of intestinal microecology. Front Pharmacol.
13(893561)2022.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Anto EJ, Syahputra RA, Silitonga HA,
Situmorang PC and Nugaraha SE: Oral acute toxicity study extract
ethanol of balakka fruit (Phyllanthus emblica). Pharmacia.
69:187–194. 2022.
|
|
112
|
Krishna AB, Manikyam HK, Sharma VK and
Sharma N: Single dose oral toxicity study of Picrorhiza
kurroa rhizome extract in Wistar rats. Fundam Toxicol Sci.
3:9–12. 2016.
|
|
113
|
Asadollahpoor A, Abdollahi M and Rahimi R:
Pimpinella anisum L. fruit: Chemical composition and effect
on rat model of nonalcoholic fatty liver disease. J Res Med Sci.
22(37)2017.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Singdam P, Naowaboot J, Senggunprai L,
Boonloh K and Pannangpetch P: Pluchea indica leaf extract
alleviates dyslipidemia and hepatic steatosis by modifying the
expression of lipid metabolism-related genes in rats fed a high
fat-high fructose diet. Prev Nutr Food Sci. 27:384–398.
2022.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Wang SJ, Chen Q, Liu MY, Yu HY, Xu JQ, Wu
JQ and Wang T: Regulation effects of rosemary (Rosmarinus
officinalis Linn.) on hepatic lipid metabolism in OA induced
NAFLD rats. Food Funct. 10:7356–7365. 2019.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Anadón A, Martínez-Larrañaga MR, Martínez
MA, Ares I, García-Risco MR, Señoráns FJ and Reglero G: Acute oral
safety study of rosemary extracts in rats. J Food Prod. 71:790–795.
2008.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Ma Y, Xiu W, Wang X and Yang Q: Extraction
of raspberry ketone from red raspberry and its intervention in the
non-alcoholic fatty liver disease. Appl Biol Chem. 65(78)2022.
|
|
118
|
Frías-Moreno MN, Parra-Quezada RA,
González-Aguilar G, Ruíz-Canizales J, Molina-Corral FJ, Sepulveda
DR, Salas-Salazar N and Olivas GI: Quality, bioactive compounds,
antioxidant capacity, and enzymes of raspberries at different
maturity stages, effects of organic vs. conventional fertilization.
Foods. 10(953)2021.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Kumar P, Bhandari U and Jamadagni S:
Fenugreek seed extract inhibit fat accumulation and ameliorates
dyslipidemia in high fat diet-induced obese rats. Biomed Res Int.
2014(606021)2014.PubMed/NCBI View Article : Google Scholar
|
|
120
|
Al-Ashban R, Abou-Shaaban R and Shah A:
Toxicity studies on Trigonella foenum-graecum L. seeds used
in spices and as a traditional remedy for diabetes. Adv Trad Med.
10:66–78. 2010.
|
|
121
|
Muralidhara Narasimhamurthy K, Viswanatha
S and Ramesh BS: Acute and subchronic toxicity assessment of
debitterized fenugreek powder in the mouse and rat. Food Chem
Toxicol. 37:831–838. 1999.PubMed/NCBI View Article : Google Scholar
|
|
122
|
Jeon SH, Jang E, Park G, Lee Y, Jang YP,
Lee KT, Inn KS, Lee JK and Lee JH: Beneficial activities of
Alisma orientale extract in a western diet-induced murine
non-alcoholic steatohepatitis and related fibrosis model via
Regulation of the hepatic adiponectin and farnesoid X receptor
pathways. Nutrients. 14(695)2022.PubMed/NCBI View Article : Google Scholar
|
|
123
|
Utzschneider KM and Kahn SE: Review: The
role of insulin resistance in nonalcoholic fatty liver disease. J
Clin Endocrinol Metab. 91:4753–4761. 2006.PubMed/NCBI View Article : Google Scholar
|
|
124
|
Loomba R, Abraham M, Unalp A, Wilson L,
Lavine J, Doo E and Bass NM: Nonalcoholic Steatohepatitis Clinical
Research Network. Association between diabetes, family history of
diabetes, and risk of nonalcoholic steatohepatitis and fibrosis.
Hepatology. 56:943–951. 2012.PubMed/NCBI View Article : Google Scholar
|
|
125
|
Haas ME, Attie AD and Biddinger SB: The
regulation of ApoB metabolism by insulin. Trends Endocrinol Metab.
24:391–397. 2013.PubMed/NCBI View Article : Google Scholar
|
|
126
|
Park PH, Sanz-Garcia C and Nagy LE:
Adiponectin as an anti-fibrotic and anti-inflammatory adipokine in
the liver. Curr Pathobiol Rep. 3:243–252. 2015.PubMed/NCBI View Article : Google Scholar
|
|
127
|
Marra F and Lotersztajn S: Pathophysiology
of NASH: Perspectives for a targeted treatment. Curr Pharm Des.
19:5250–5269. 2013.PubMed/NCBI View Article : Google Scholar
|
|
128
|
Jamal N, Zainol NI, Zakaria NA, Jofrry SM,
Mohamed R, Man F and Choo CY: Safety, efficacy, and mechanism of
action of herbs used for obesity management: A thematic review.
Obes Med. 32(100415)2022.
|